Which Gelatin and Antibiotic Should Be Chosen to Seal a Woven Vascular Graft?
Abstract
:1. Introduction
2. Results
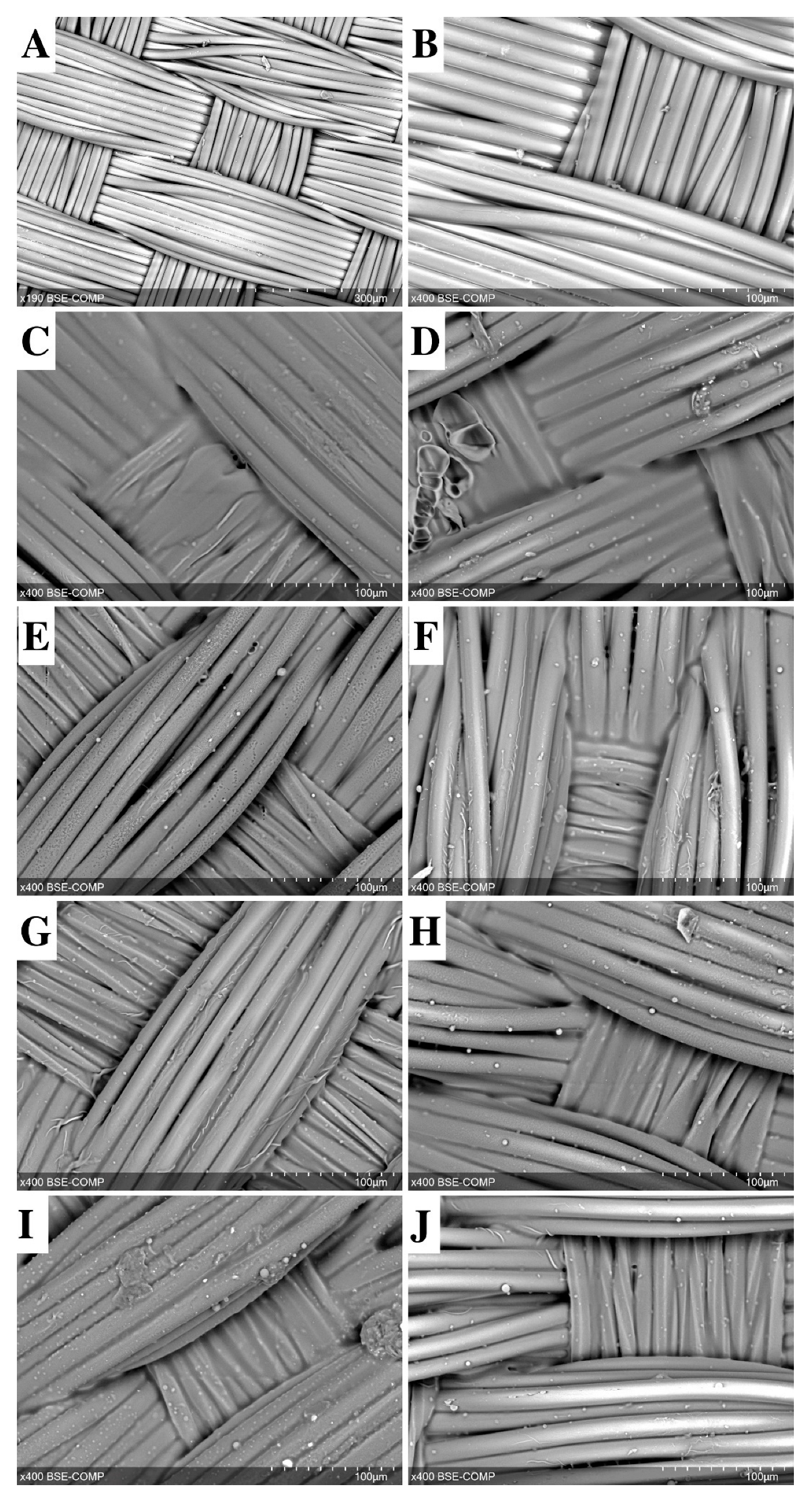
2.1. General Characteristics
2.1.1. Water Permeability
2.1.2. Kinking Radius
2.2. Antibacterial Properties
2.3. Cytocompatibility
2.3.1. Indirect Cytotoxicity
2.3.2. Cell Adhesion
2.3.3. Cell Viability
2.3.4. Cell Distribution
2.4. SEM and EDS Identification of Graft/Cell Interaction
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Treatment Groups
- (1)
- Vancomycin 3.3 g/L;
- (2)
- Ceftriaxone 3.3 g/L;
- (3)
- Rifampicin 1 g/L.
4.3. Integral Water Permeability
4.4. Kinking Radius Measurements
4.5. Antibacterial Properties
4.6. Cytocompatibility Evaluation
4.6.1. Cell Culture
4.6.2. Indirect Cytotoxicity of Samples
4.6.3. Cell Adhesion and Viability
4.6.4. Cell Distribution
4.7. Scanning Electron Microscopy (SEM) and Energy Dispersive Spectrometry (EDS) Analysis
4.8. Statistical Analysis
5. Conclusions
6. Study Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yee, J.; Kendle, A.P. Aortic Dissection Presenting as a STEMI. J. Educ. Teach. Emerg. Med. 2022, 7, 26–54. [Google Scholar] [CrossRef]
- Okita, Y. Current surgical results of acute type A aortic dissection in Japan. Ann. Cardiothorac. Surg. 2016, 5, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Malaisrie, S.C.; Szeto, W.Y.; Halas, M.; Girardi, L.N.; Coselli, J.S.; Sundt, T.M., 3rd; Chen, E.P.; Fischbein, M.P.; Gleason, T.G.; Okita, Y.; et al. 2021 The American Association for Thoracic Surgery expert consensus document: Surgical treatment of acute type A aortic dissection. J. Thorac Cardiovasc. Surg. 2021, 162, 735–758.e2. [Google Scholar] [CrossRef] [PubMed]
- White, A.; Broder, J.; Mando-Vandrick, J.; Wendell, J.; Crowe, J. Acute aortic emergencies—Part 2: Aortic dissections. Adv. Emerg. Nurs. J. 2013, 35, 28–52. [Google Scholar] [CrossRef]
- Borst, H.G.; Walterbusch, G.; Schaps, D. Extensive aortic replacement using “elephant trunk” prosthesis. J. Thorac Cardiovasc. Surg. 1983, 31, 37–40. [Google Scholar] [CrossRef]
- Karck, M.; Chavan, A.; Hagl, C.; Friedrich, H.; Galanski, M.; Haverich, A. The frozen elephant trunk technique: A new treatment for thoracic aortic aneurysms. J. Thorac. Cardiovasc. Surg. 2003, 125, 1550–1553. [Google Scholar] [CrossRef] [PubMed]
- Porterie, J.; Hostalrich, A.; Dagenais, F.; Marcheix, B.; Chaufour, X.; Ricco, J.B. hybrid treatment of complex diseases of the aortic arch and descending thoracic aorta by frozen elephant trunk technique. J. Clin. Med. 2023, 12, 5693. [Google Scholar] [CrossRef]
- Islam, M.S. Relationship between Textile Irregularities and Pre-mature Rupture of Polyester Vascular Graft Knitted Fabric. Master’s Thesis. 2017, 136p. Available online: https://mspace.lib.umanitoba.ca/handle/1993/32852 (accessed on 29 October 2023).
- Liu, C.; Dai, J.; Wang, X.; Hu, X. The Influence of Textile Structure Characteristics on the Performance of Artificial Blood Vessels. Polymers 2023, 15, 3003. [Google Scholar] [CrossRef]
- Santos, I.C.; Rodrigues, A.; Figueiredo, L.; Rocha, L.A.; Tavares, J.M.R.S. Mechanical properties of stent–graft materials. Proc. IMechE Part L J. Mater. Des. Appl. 2012, 226, 330–341. [Google Scholar] [CrossRef]
- Shadanov, A.A.; Timchenko, T.P.; Vladimirov, S.V.; Lushchyk, P.E.; Zablotsky, A.V.; Kiselyov, S.O.; Zhuravleva, I.Y.; Sirota, D.A.; Chernyavskiy, A.M. The influence of weaving technologies on the integral characteristics of synthetic vascular prostheses. Sovrem. Tehnol. Med. 2022, 14, 5–13. [Google Scholar] [CrossRef]
- Hori, D.; Kusadokoro, S.; Shimizu, T.; Kimura, N.; Yamaguchi, A. Prosthetic Graft Dilation at the Aortic Arch in the Era of Hybrid Aortic Surgery. Ann. Vasc. Dis. 2020, 13, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, T.A.; Shambat, S.M.; Haunreiter, V.D.; Mestres, C.A.; Weber, A.; Maisano, F.; Zinkernagel, A.S.; Hasse, B. Polyester Vascular Graft Material and Risk for Intracavitary Thoracic Vascular Graft Infection. Emerg. Infect. Dis. 2020, 26, 2448–2452. [Google Scholar] [CrossRef]
- Claaßen, C.; Dannecker, M.; Grübel, J.; Kotzampasi, M.E.; Tovar, G.E.M.; Stanzel, B.V.; Borchers, K. The choice of biopolymer is crucial to trigger angiogenesis with vascular endothelial growth factor releasing coatings. J. Mater. Sci. Mater. Med. 2020, 31, 93. [Google Scholar] [CrossRef] [PubMed]
- Kurowiak, J.; Klekiel, T.; Będziński, R. Biodegradable Polymers in Biomedical Applications: A Review-Developments, Perspectives and Future Challenges. Int. J. Mol. Sci. 2023, 24, 16952. [Google Scholar] [CrossRef] [PubMed]
- Aziz, R.; Falanga, M.; Purenovic, J.; Mancini, S.; Lamberti, P.; Guida, M. A Review on the Applications of Natural Biodegradable Nano Polymers in Cardiac Tissue Engineering. Nanomaterials 2023, 13, 1374. [Google Scholar] [CrossRef]
- Kim, Y.W. Aortic endograft infection: Diagnosis and management. Vasc. Specialist. Int. 2023, 39, 26. [Google Scholar] [CrossRef]
- Moore, W.S.; Chvapil, M.; Seiffert, G.; Keown, K. Development of an infection-resistant vascular prosthesis. Arch. Surg. 1981, 116, 1403–1407. [Google Scholar] [CrossRef]
- Cao, H.; Qiao, S.; Qin, H.; Jandt, K.D. Antibacterial designs for implantable medical devices: Evolutions and challenges. J. Funct. Biomater. 2022, 13, 86. [Google Scholar] [CrossRef]
- Honig, S.; Seeger, P.; Rohde, H.; Kölbel, T.; Debus, E.S.; Diener, H. Efficacy of antiseptic impregnation of aortic endografts with rifampicin compared to silver against in vitro contamination with four bacteria that frequently cause vascular graft infections. JVS Vasc. Sci. 2020, 1, 181–189. [Google Scholar] [CrossRef]
- Galdbart, J.O.; Branger, C.; Andreassian, B.; Lambert-Zechovsky, N.; Kitzis, M. Elution of six antibiotics bonded to polyethylene vascular grafts sealed with three proteins. J. Surg. Res. 1996, 66, 174–178. [Google Scholar] [CrossRef]
- Goëau-Brissonnière, O.; Javerliat, I.; Koskas, F.; Coggia, M.; Pechère, J.C. Rifampin-bonded vascular grafts and postoperative infections. Ann. Vasc. Surg. 2011, 25, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Mufty, H.; Van Den Eynde, J.; Meuris, B.; Metsemakers, W.J.; Van Wijngaerden, E.; Vandendriessche, T.; Steenackers, H.P.; Fourneau, I. Pre-clinical in vivo models of vascular graft coating in the prevention of vascular graft infection: A systematic review. Eur. J. Vasc. Endovasc. Surg. 2021, 62, 99–118. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.W.; Sohn, M.J.; Cho, C.R.; Koo, H.W.; Yoon, S.W. Evaluation of cumulative and conditional antibiotic release from vancomycin-embedded fibrin sealant and its antibacterial activity: An in vitro study. J. Korean Neurosurg. Soc. 2020, 63, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Cyphert, E.L.; von Recum, H.A. Emerging technologies for long-term antimicrobial device coatings: Advantages and limitations. Exp. Biol. Med. 2017, 242, 788–798. [Google Scholar] [CrossRef] [PubMed]
- Herten, M.; Idelevich, E.A.; Sielker, S.; Becker, K.; Scherzinger, A.S.; Osada, N.; Torsello, G.B.; Bisdas, T. Vascular graft impregnation with antibiotics: The influence of high concentrations of rifampin, vancomycin, daptomycin, and bacteriophage endolysin hy-133 on viability of vascular cells. Med. Sci. Monit. Basic Res. 2017, 23, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.sigmaaldrich.com/RU/en/search/gelatin?focus=products&page=1&perpage=30&sort=relevance&term=gelatin&type=product) (accessed on 29 October 2023).
- ISO 10993-5:2009; Biological Evaluation of Medical Devices. Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009.
- Harris, P.; Normand, V.; Norton, I.T. GELATIN. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Elsevier: Amsterdam, The Netherlands, 2003; pp. 2865–2871. [Google Scholar] [CrossRef]
- Hitchcock, D.I. The combination of gelatin with hydrochloric acid. J. Gen. Physiol. 1929, 12, 495–509. [Google Scholar] [CrossRef]
- Ward, A.G. The chemical structure and physical properties of gelatin. J. Photogr. Sci. 1955, 3, 60–67. [Google Scholar] [CrossRef]
- Iino, K.; Takago, S.; Saito, N.; Ueda, H.; Yamamoto, Y.; Kato, H.; Kimura, K.; Takemura, H. Total arch replacement and frozen elephant trunk for acute type A aortic dissection. J. Thorac. Cardiovasc Surg. 2022, 164, 1400–1409.e3. [Google Scholar] [CrossRef]
- Sewald, L.; Claaßen, C.; Götz, T.; Claaßen, M.H.; Truffault, V.; Tovar, G.E.M.; Southan, A.; Borchers, K. Beyond the modification degree: Impact of raw material on physicochemical properties of gelatin type A and type B methacryloyls. Macromol. Biosci. 2018, 18, e1800168. [Google Scholar] [CrossRef]
- Ji, F.; Zhou, W.; Zhang, Z.; Zhang, B. Effects of relative molecular weight distribution and isoelectric point on the swelling behavior of gelatin films. Front. Chem. 2022, 10, 857976. [Google Scholar] [CrossRef]
- Young, S.; Wong, M.; Tabata, Y.; Mikos, A.G. Gelatin as a delivery vehicle for the controlled release of bioactive molecules. J. Control. Release 2005, 109, 256–274. [Google Scholar] [CrossRef] [PubMed]
- Fukuba, S.; Akizuki, T.; Hoshi, S.; Matsuura, T.; Shujaa-Addin, A.; Okada, M.; Tabata, Y.; Matsui, M.; Tabata, M.J.; Sugiura-Nakazato, M.; et al. Comparison between different isoelectric points of biodegradable gelatin sponges incorporating β-tricalcium phosphate and recombinant human fibroblast growth factor-2 for ridge augmentation: A preclinical study of saddle-type defects in dogs. J. Periodontal. Res. 2019, 54, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Leu Alexa, R.; Iovu, H.; Ghitman, J.; Serafim, A.; Stavarache, C.; Marin, M.-M.; Ianchis, R. 3D-printed gelatin methacryloyl- based scaffolds with potential application in tissue engineering. Polymers 2021, 13, 727. [Google Scholar] [CrossRef] [PubMed]
- Boire, T.C.; Himmel, L.E.; Yu, F.; Guth, C.M.; Dollinger, B.R.; Werfel, T.A.; Balikov, D.A.; Duvall, C.L. Effect of pore size and spacing on neovascularization of a biodegradble shape memory polymer perivascular wrap. J. Biomed. Mater. Res. A 2021, 109, 272–288. [Google Scholar] [CrossRef]
- Braga, S.F.; Neves, J.R.; Ferreira, J.; Carrilho, C.; Simões, J.C.; Mesquita, A. Neointimal hyperplasia. Rev. Port Cir. Cardiotorac. Vasc. 2019, 26, 213–217. [Google Scholar]
- ISO 7198:2016; Cardiovascular Implants and Extracorporeal Systems. Vascular Prostheses: Tubular Vascular Grafts and Vascular Patches. ISO: Geneva, Switzerland, 2016.










| Group No. | Sample Treatment | Characteristics | |||
|---|---|---|---|---|---|
| Kinking Radius, mm | Water Permeability, mL/min/cm2 | m/o Inhibition Zone, mm * | |||
| St. Aureus | Ent. Faecalis | ||||
| 0 | Control (−) | 10.9 ± 2.8 | 78.8 ± 2.7 | 0 | 0 |
| 1 | GelA | 42.5 ± 4.0 | 0 | 0 | 0 |
| 2 | GelA + Vancomycin | 21.0 ± 0 | 2.1 ± 0.7 | 15.02 ± 0.73 | 12.52 ± 2.12 |
| 3 | GelA + Ceftriaxone | 25.0 ± 0 | 0 | 0 | 0 |
| 4 | GelA + Rifampicin | 51.0 ± 0 | 0 | 0 | 0 |
| 5 | GelB | 17.3 ± 0.4 | 0 | 0 | 0 |
| 6 | GelB + Vancomycin | 21.0 ± 0 | 10.8 ± 6.1 | 15.81 ± 0.45 | 12.38 ± 1.49 |
| 7 | GelB + Ceftriaxone | 34.9 ± 0.75 | 0.2 ± 0 | 0 | 0 |
| 8 | GelB + Rifampicin | 19.0 ± 0 | 6.1 ± 0.4 | 0 | 0 |
| Group No. | Sample Treatment | Kinking Radius | Water Permeability | Antibacterial Properties | Cytocom- Patibility | SEM and EDS |
|---|---|---|---|---|---|---|
| 0 | Control (−) | 12 | 6 | 20 | 24 | 8 |
| 1 | Gelatin A | 4 | 6 | 20 | 24 | 8 |
| 2 | Gelatin A + Vancomycin | 4 | 6 | 20 | 24 | 8 |
| 3 | Gelatin A + Ceftriaxone | 4 | 6 | 20 | 24 | 8 |
| 4 | Gelatin A + Rifampicin | 4 | 6 | 20 | 24 | 8 |
| 5 | Gelatin B | 4 | 6 | 20 | 24 | 8 |
| 6 | Gelatin B + Vancomycin | 4 | 6 | 20 | 24 | 8 |
| 7 | Gelatin B + Ceftriaxone | 4 | 6 | 20 | 24 | 8 |
| 8 | Gelatin B + Rifampicin | 4 | 6 | 20 | 24 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuravleva, I.Y.; Shadanov, A.A.; Surovtseva, M.A.; Vaver, A.A.; Samoylova, L.M.; Vladimirov, S.V.; Timchenko, T.P.; Kim, I.I.; Poveshchenko, O.V. Which Gelatin and Antibiotic Should Be Chosen to Seal a Woven Vascular Graft? Int. J. Mol. Sci. 2024, 25, 965. https://doi.org/10.3390/ijms25020965
Zhuravleva IY, Shadanov AA, Surovtseva MA, Vaver AA, Samoylova LM, Vladimirov SV, Timchenko TP, Kim II, Poveshchenko OV. Which Gelatin and Antibiotic Should Be Chosen to Seal a Woven Vascular Graft? International Journal of Molecular Sciences. 2024; 25(2):965. https://doi.org/10.3390/ijms25020965
Chicago/Turabian StyleZhuravleva, Irina Yu., Aldar A. Shadanov, Maria A. Surovtseva, Andrey A. Vaver, Larisa M. Samoylova, Sergey V. Vladimirov, Tatiana P. Timchenko, Irina I. Kim, and Olga V. Poveshchenko. 2024. "Which Gelatin and Antibiotic Should Be Chosen to Seal a Woven Vascular Graft?" International Journal of Molecular Sciences 25, no. 2: 965. https://doi.org/10.3390/ijms25020965
APA StyleZhuravleva, I. Y., Shadanov, A. A., Surovtseva, M. A., Vaver, A. A., Samoylova, L. M., Vladimirov, S. V., Timchenko, T. P., Kim, I. I., & Poveshchenko, O. V. (2024). Which Gelatin and Antibiotic Should Be Chosen to Seal a Woven Vascular Graft? International Journal of Molecular Sciences, 25(2), 965. https://doi.org/10.3390/ijms25020965






