Role of Cannabinoids in Oral Cancer
Abstract
:1. Introduction
2. Endogenous and Exogenous Cannabinoids and Their Mechanisms of Action
3. The Effects of Cannabinoids on Oral Mucosa
3.1. Cannabinoids and Oral Cancer
3.1.1. Reported Procarcinogenic Effects of Cannabinoids
| Cannabinoid (Pure/Mix) | Method of Administration | Dose/Concentration | Experimental Model | Target Factors/Molecules | Effect | Reference |
|---|---|---|---|---|---|---|
| Procarcinogenic effects | ||||||
| ACEA, Hu308, THC | Treatment of cell cultures | 1 μM (ACEA), 1 μM (Hu308), 1 μM (THC) | UD-SCC-2, UPCI:SCC090, UM-SCC-104, and UM-SCC-47 (HNSCC cell lines) | p38 MAPK pathway | Increased cell proliferation | [99] |
| THC | Subcutaneous injection | 3 mg/kg daily | HPV-positive HNSCC xenografts of nude mice | p38 MAPK pathway | Tumor progression | [99] |
| Cannabis | Smoking | N/A | Case-control cohort study (HNSCC) | N/A | Increased risk of primary SCC | [100] |
| Cannabis | Smoking | N/A | Ex vivo study (LSCC) | EGFR cascade | Increased expression of EGFR and downstream molecules | [97] |
| Marijuana | Smoking | More than 6 times ever | Case-control cohort study (HNSCC) | N/A | A nearly significant increase in the risk of cervical cancer | [101] |
| Marijuana | Smoking | 20 or more days in the past month | Cross-sectional study (OSCC) | Oral microbiome | Changes in the oral microbiome consistent with malignancy | [92] |
| Marijuana | Smoking | N/A Dose-dependent | Case-control cohort study (HNSCC) | N/A | Increased dose-dependent risk of HNSCC incidence | [78] |
| Anti-carcinogenic effects | ||||||
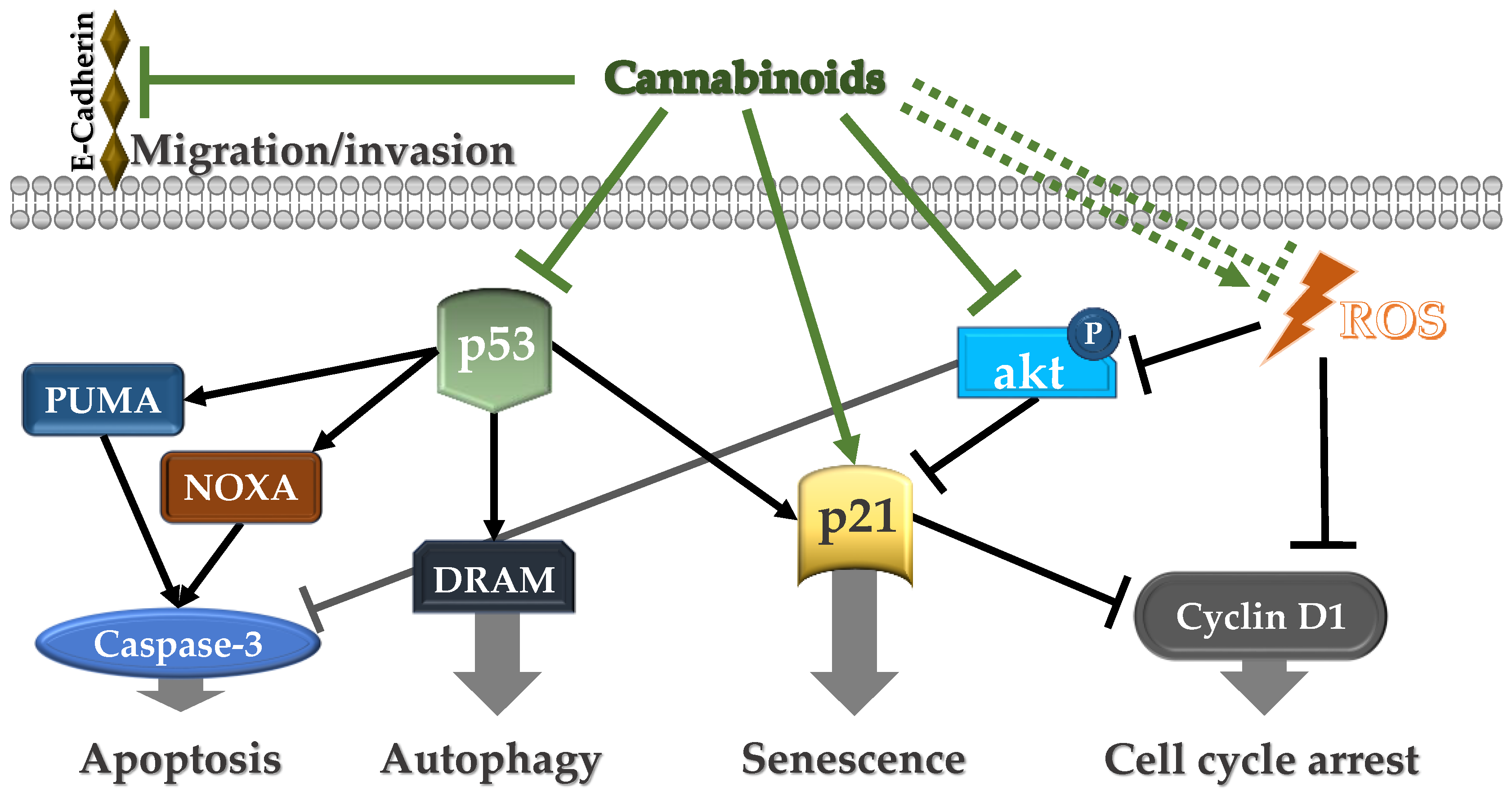
| Δ9, Δ8-THC | Treatment of cell cultures | IC50 = 10 μg/mL (Δ9-THC) and 13 μg/mL (Δ8-THC) | Ca9-22 oral cancer cells | Cyclin D1, p53, NOXA, PUMAα, DRAM, p21, H2AX | Decreased cell growth through apoptosis, autophagy, and oxidative stress | [102] |
| E-cadherin | Suppression of migration/invasion | |||||
| CBD | Treatment of cell cultures | 2 μM | Human oral squamous cell carcinoma, SAS, and human salivary gland cancer cells line ACCM | ID1, FOXM1, GDF15 | Inhibition of tumor progression, stimulation of autophagy, suppression of migration/invasion | [103] |
| DHEA, EPEA, NALA | Treatment of cell cultures | 10–30 µM | SNU-1041, SNU-1066 and SNU-1076 | Akt | Apoptosis induced by increased 5-LO-mediated ROS production | [104] |
| Δ9, Δ8-THC | Treatment of cell cultures | Up to 200 μmol/L | Tu183 cell line | Mitochondria | Inhibition of mitochondrial oxygen consumption | [105] |
| Marijuana | Smoking | Moderate weekly use | Case-control cohort study (OSCC) | N/A | Decreased risk of HNSCC, regardless of HPV status | [106] |
| Marijuana | Smoking | ≥50 hit-years | Case-control cohort study (OSCC) | N/A | Decreased risk of HPV-negative OSCC | [107] |
| Mixed or neutral effects on HNC carcinogenesis | ||||||
| Marijuana | Smoking | Loose-leaf usage at least weekly | Human trial (HPV-related OPSCC) | N/A | No difference in survival, tumor recurrence, or adverse effects | [108] |
| Cannabis | Smoking | Any dose, frequency | Case-control cohort study (HNSCC) | N/A | No significant increase in the risk of developing HNSCC | [109] |
| Marijuana | Smoking | Any dose, frequency | Case-control cohort study (OSCC) | N/A | No association with an increased risk of OSCC | [110] |
3.1.2. Inconclusive Ties
3.1.3. Anti-Carcinogenic Impact
3.2. The Relationship between Cannabinoids and HPV-Positive HNC
4. In Vitro Experimental Models on the Anti-Tumor Activity of Cannabinoid Compounds
5. Cannabinoids in the Management of Oral Cancer-Related Symptoms
| Cannabinoid (Pure/Mix) | Method of Administration | Dose/Concentration | Study Category (Cancer Type) | Population Size | Beneficial Effects | Adverse Effects | Reference |
|---|---|---|---|---|---|---|---|
| Marijuana | Smoking | Loose-leaf usage at least weekly | Cohort study (OPSCC, OC, HP, L) | 74 patients, 74 controls | Decrease in anxiety/depression, pain/discomfort, and tiredness. Increase in appetite and well-being | N/A | [161] |
| Medical Marijuana | N/A | N/A | Cohort study (HNC) | 63 patients | Decrease in headache, pain, nausea, loss of appetite, and reduced anxiety | N/A | [162] |
| Marijuana | Smoking | Loose-leaf usage at least weekly | Case-control (P16-positive OPSCC) | 47 subjects and 47 controls | There is no survival difference. | No effects | [108] |
| Nabilone | Ingested | 0.5 mg | Randomized Double-Blind (HNC) | 56 patients | No beneficial effects | No effects | [163] |
| Medical Marijuana | Inhalation, ingestion, and oil. | Various dosages; the majority used daily or more than once daily | cross-sectional survey (HNC RT) | 15 patients | Decrease in anxiety/depression, pain, and maintaining weight. Improving appetite, swallowing, xerostomia, and relieving muscle spasms | N/A | [164] |
| Marijuana | N/A | N/A | retrospective cohort study (OPSCC) | 74 patients | No beneficial effects | lower overall survival rates and greater weight loss during radiotherapy. | [165] |
6. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chin, D.; Boyle, G.M.; Porceddu, S.; Theile, D.R.; Parsons, P.G.; Coman, W.B. Head and neck cancer: Past, present and future. Expert Rev. Anticancer Ther. 2006, 6, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Montero, P.H.; Patel, S.G. Cancer of the oral cavity. Surg. Oncol. Clin. 2015, 24, 491–508. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Global Cancer Observatory. Available online: https://gco.iarc.fr/today/home (accessed on 19 November 2023).
- Oral and Oropharyngeal Cancer: Statistics. Available online: https://www.cancer.net/cancer-types/oral-and-oropharyngeal-cancer/statistics (accessed on 19 November 2023).
- Solomon, I.; Voiculescu, V.M.; Caruntu, C.; Lupu, M.; Popa, A.; Ilie, M.A.; Albulescu, R.; Caruntu, A.; Tanase, C.; Constantin, C.; et al. Neuroendocrine Factors and Head and Neck Squamous Cell Carcinoma: An Affair to Remember. Dis. Markers 2018, 2018, 9787831. [Google Scholar] [CrossRef]
- Semple, C.; Parahoo, K.; Norman, A.; McCaughan, E.; Humphris, G.; Mills, M. Psychosocial interventions for patients with head and neck cancer. Cochrane Database Syst. Rev. 2013, 7, CD009441. [Google Scholar] [CrossRef]
- Grafton-Clarke, C.; Chen, K.W.; Wilcock, J. Diagnosis and referral delays in primary care for oral squamous cell cancer: A systematic review. Br. J. Gen. Pract. 2019, 69, e112–e126. [Google Scholar] [CrossRef] [PubMed]
- Caruntu, A.; Caruntu, C. Recent Advances in Oral Squamous Cell Carcinoma. J. Clin. Med. 2022, 11, 6406. [Google Scholar] [CrossRef]
- Alfouzan, A.F. Review of surgical resection and reconstruction in head and neck cancer. Traditional versus current concepts. Saudi Med. J. 2018, 39, 971–980. [Google Scholar] [CrossRef]
- Kanazawa, T.; Sarukawa, S.; Fukushima, H.; Takeoda, S.; Kusaka, G.; Ichimura, K. Current Reconstructive Techniques Following Head and Neck Cancer Resection Using Microvascular Surgery. Ann. Vasc. Dis. 2011, 4, 189–195. [Google Scholar] [CrossRef]
- Singh, G.D.; Singh, M. Virtual Surgical Planning: Modeling from the Present to the Future. J. Clin. Med. 2021, 10, 5655. [Google Scholar] [CrossRef]
- FDA Approves Pembrolizumab for First-Line Treatment of Head and Neck Squamous Cell Carcinoma. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-first-line-treatment-head-and-neck-squamous-cell-carcinoma (accessed on 19 November 2023).
- Caruntu, A.; Moraru, L.; Lupu, M.; Vasilescu, F.; Dumitrescu, M.; Cioplea, M.; Popp, C.; Dragusin, A.; Caruntu, C.; Zurac, S. Prognostic Potential of Tumor-Infiltrating Immune Cells in Resectable Oral Squamous Cell Carcinoma. Cancers 2021, 13, 2268. [Google Scholar] [CrossRef]
- Lupu, M.; Caruntu, A.; Boda, D.; Caruntu, C. In Vivo Reflectance Confocal Microscopy-Diagnostic Criteria for Actinic Cheilitis and Squamous Cell Carcinoma of the Lip. J. Clin. Med. 2020, 9, 1987. [Google Scholar] [CrossRef] [PubMed]
- Ghiţă, M.A.; Constantin, C.; Rosca, A.E.; Căruntu, A.; Moraru, L.; Constantin, C.; Neagu, M.; Boda, D. Real-Time Investigation of Skin Blood Flow Changes Induced by Topical Capsaicin. Acta Dermatovenerol. Croat. 2017, 25, 223–227. [Google Scholar] [PubMed]
- Tampa, M.; Georgescu, S.R.; Mitran, M.I.; Mitran, C.I.; Matei, C.; Caruntu, A.; Scheau, C.; Nicolae, I.; Matei, A.; Caruntu, C.; et al. Current Perspectives on the Role of Matrix Metalloproteinases in the Pathogenesis of Basal Cell Carcinoma. Biomolecules 2021, 11, 903. [Google Scholar] [CrossRef]
- Kakabadze, M.Z.; Paresishvili, T.; Karalashvili, L.; Chakhunashvili, D.; Kakabadze, Z. Oral microbiota and oral cancer. Oncol. Rev. 2020, 14, 2. [Google Scholar] [CrossRef]
- Boda, D.; Neagu, M.; Constantin, C.; Voinescu, R.N.; Caruntu, C.; Zurac, S.; Spandidos, D.A.; Drakoulis, N.; Tsoukalas, D.; Tsatsakis, A.M. HPV strain distribution in patients with genital warts in a female population sample. Oncol. Lett. 2016, 12, 1779–1782. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Nanavati, R.; Modi, T.G.; Dobariya, C. Oral cancer: Etiology and risk factors: A review. J. Cancer Res. Ther. 2016, 12, 458–463. [Google Scholar] [CrossRef]
- Bagnardi, V.; Rota, M.; Botteri, E.; Tramacere, I.; Islami, F.; Fedirko, V.; Scotti, L.; Jenab, M.; Turati, F.; Pasquali, E.; et al. Light alcohol drinking and cancer: A meta-analysis. Ann. Oncol. 2013, 24, 301–308. [Google Scholar] [CrossRef]
- Beland, F.A.; Benson, R.W.; Mellick, P.W.; Kovatch, R.M.; Roberts, D.W.; Fang, J.-L.; Doerge, D.R. Effect of ethanol on the tumorigenicity of urethane (ethyl carbamate) in B6C3F1 mice. Food Chem. Toxicol. 2005, 43, 1–19. [Google Scholar] [CrossRef]
- Irani, S. Pre-cancerous lesions in the oral and maxillofacial region: A literature review with special focus on etiopathogenesis. Iran. J. Pathol. 2016, 11, 303. [Google Scholar]
- Scheau, C.; Mihai, L.G.; Bădărău, I.A.; Caruntu, C. Emerging applications of some important natural compounds in the field of oncology. Farmacia 2020, 68, 992–998. [Google Scholar] [CrossRef]
- Abrams, D.I.; Guzman, M. Cannabis in cancer care. Clin. Pharmacol. Ther. 2015, 97, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, A.D.; Inchingolo, A.M.; Malcangi, G.; Avantario, P.; Azzollini, D.; Buongiorno, S.; Viapiano, F.; Campanelli, M.; Ciocia, A.M.; De Leonardis, N.; et al. Effects of Resveratrol, Curcumin and Quercetin Supplementation on Bone Metabolism—A Systematic Review. Nutrients 2022, 14, 3519. [Google Scholar] [CrossRef]
- Popescu, G.D.A.; Scheau, C.; Badarau, I.A.; Dumitrache, M.-D.; Caruntu, A.; Scheau, A.-E.; Costache, D.O.; Costache, R.S.; Constantin, C.; Neagu, M.; et al. The Effects of Capsaicin on Gastrointestinal Cancers. Molecules 2020, 26, 94. [Google Scholar] [CrossRef]
- Dumitrache, M.-D.; Jieanu, A.S.; Scheau, C.; Badarau, I.A.; Popescu, G.D.A.; Caruntu, A.; Costache, D.O.; Costache, R.S.; Constantin, C.; Neagu, M.; et al. Comparative effects of capsaicin in chronic obstructive pulmonary disease and asthma (Review). Exp. Ther. Med. 2021, 22, 917. [Google Scholar] [CrossRef] [PubMed]
- Caputo, M.P.; Rodriguez, C.S.; Padhya, T.A.; Mifsud, M.J. Medical cannabis as adjunctive therapy for head and neck cancer patients. Cureus 2021, 13, 9. [Google Scholar] [CrossRef] [PubMed]
- Castaneto, M.S.; Gorelick, D.A.; Desrosiers, N.A.; Hartman, R.L.; Pirard, S.; Huestis, M.A. Synthetic cannabinoids: Epidemiology, pharmacodynamics and clinical implications. Drug Alcohol Depend. 2014, 144, 12–41. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V. The endocannabinoid system: Its general strategy of action, tools for its pharmacological manipulation and potential therapeutic exploitation. Pharmacol. Res. 2009, 60, 77–84. [Google Scholar] [CrossRef]
- Castillo, P.E.; Younts, T.J.; Chávez, A.E.; Hashimotodani, Y. Endocannabinoid Signaling and Synaptic Function. Neuron 2012, 76, 70–81. [Google Scholar] [CrossRef]
- Parolaro, D.; Realini, N.; Vigano, D.; Guidali, C.; Rubino, T. The endocannabinoid system and psychiatric disorders. Exp. Neurol. 2010, 224, 3–14. [Google Scholar] [CrossRef]
- Marsicano, G.; Lafenêtre, P. Roles of the endocannabinoid system in learning and memory. Curr. Top. Behav. Neurosci. 2009, 1, 201–230. [Google Scholar] [CrossRef]
- Kesner, A.J.; Lovinger, D.M. Cannabinoids, Endocannabinoids and Sleep. Front. Mol. Neurosci. 2020, 13, 125. [Google Scholar] [CrossRef] [PubMed]
- Woodhams, S.G.; Sagar, D.R.; Burston, J.J.; Chapman, V. The role of the endocannabinoid system in pain. Handb. Exp. Pharmacol. 2015, 227, 119–143. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, O.; Ganguly, D. Endocannabinoids in immune regulation and immunopathologies. Immunology 2021, 164, 242–252. [Google Scholar] [CrossRef]
- Schulz, P.; Hryhorowicz, S.; Rychter, A.M.; Zawada, A.; Słomski, R.; Dobrowolska, A.; Krela-Kaźmierczak, I. What Role Does the Endocannabinoid System Play in the Pathogenesis of Obesity? Nutrients 2021, 13, 373. [Google Scholar] [CrossRef] [PubMed]
- The Endocannabinoid System: Essential and Mysterious. Available online: https://www.health.harvard.edu/blog/the-endocannabinoid-system-essential-and-mysterious-202108112569 (accessed on 27 November 2023).
- Crocq, M.-A. History of cannabis and the endocannabinoid system. Dialogues Clin. Neurosci. 2020, 22, 223–228. [Google Scholar] [CrossRef]
- Schurman, L.D.; Lu, D.; Kendall, D.A.; Howlett, A.C.; Lichtman, A.H. Molecular Mechanism and Cannabinoid Pharmacology. Subst. Use Disord. Etiol. Treat. 2019, 258, 323–353. [Google Scholar] [CrossRef]
- Lu, H.-C.; Mackie, K. An Introduction to the Endogenous Cannabinoid System. Biol. Psychiatry 2016, 79, 516–525. [Google Scholar] [CrossRef]
- Ye, L.; Cao, Z.; Wang, W.; Zhou, N. New Insights in Cannabinoid Receptor Structure and Signaling. Curr. Mol. Pharmacol. 2019, 12, 239–248. [Google Scholar] [CrossRef]
- Bisogno, T.; Melck, D.; Bobrov, M.Y.; Gretskaya, N.M.; Bezuglov, V.V.; DE Petrocellis, L.; DI Marzo, V. N-acyl-dopamines: Novel synthetic CB1 cannabinoid-receptor ligands and inhibitors of anandamide inactivation with cannabimimetic activity in vitro and in vivo. Biochem. J. 2000, 351, 817–824. [Google Scholar] [CrossRef]
- Hanuš, L.; Abu-Lafi, S.; Fride, E.; Breuer, A.; Vogel, Z.; Shalev, D.E.; Kustanovich, I.; Mechoulam, R. 2-Arachidonyl glyceryl ether, an endogenous agonist of the cannabinoid CB1 receptor. Proc. Natl. Acad. Sci. USA 2001, 98, 3662–3665. [Google Scholar] [CrossRef] [PubMed]
- Porter, A.C.; Sauer, J.-M.; Knierman, M.D.; Becker, G.W.; Berna, M.J.; Bao, J.; Nomikos, G.G.; Carter, P.; Bymaster, F.P.; Leese, A.B.; et al. Characterization of a Novel Endocannabinoid, Virodhamine, with Antagonist Activity at the CB1 Receptor. J. Pharmacol. Exp. Ther. 2002, 301, 1020–1024. [Google Scholar] [CrossRef]
- Sylantyev, S.; Jensen, T.P.; Ross, R.A.; Rusakov, D.A. Cannabinoid- and lysophosphatidylinositol-sensitive receptor GPR55 boosts neurotransmitter release at central synapses. Proc. Natl. Acad. Sci. USA 2013, 110, 5193–5198. [Google Scholar] [CrossRef] [PubMed]
- Hua, T.; Vemuri, K.; Pu, M.; Qu, L.; Han, G.W.; Wu, Y.; Zhao, S.; Shui, W.; Li, S.; Korde, A.; et al. Crystal Structure of the Human Cannabinoid Receptor CB(1). Cell 2016, 167, 750–762.e14. [Google Scholar] [CrossRef]
- Howlett, A.C.; Abood, M.E. CB(1) and CB(2) Receptor Pharmacology. Adv. Pharmacol. 2017, 80, 169–206. [Google Scholar] [CrossRef] [PubMed]
- Howlett, A.C. International Union of Pharmacology. XXVII. Classification of Cannabinoid Receptors. Pharmacol. Rev. 2002, 54, 161–202. [Google Scholar] [CrossRef]
- Mackie, K.; Lai, Y.; Westenbroek, R.; Mitchell, R. Cannabinoids activate an inwardly rectifying potassium conductance and inhibit Q-type calcium currents in AtT20 cells transfected with rat brain cannabinoid receptor. J. Neurosci. 1995, 15, 6552–6561. [Google Scholar] [CrossRef]
- Spanagel, R. Cannabinoids and the endocannabinoid system in reward processing and addiction: From mechanisms to interventions. Dialogues Clin. Neurosci. 2020, 22, 241–250. [Google Scholar] [CrossRef]
- Scheau, C.; Badarau, I.A.; Mihai, L.-G.; Scheau, A.-E.; Costache, D.O.; Constantin, C.; Calina, D.; Caruntu, C.; Costache, R.S.; Caruntu, A. Cannabinoids in the Pathophysiology of Skin Inflammation. Molecules 2020, 25, 652. [Google Scholar] [CrossRef]
- Scheau, C.; Caruntu, C.; Badarau, I.A.; Scheau, A.-E.; Docea, A.O.; Calina, D.; Caruntu, A. Cannabinoids and Inflammations of the Gut-Lung-Skin Barrier. J. Pers. Med. 2021, 11, 494. [Google Scholar] [CrossRef]
- Xing, C.; Zhuang, Y.; Xu, T.-H.; Feng, Z.; Zhou, X.E.; Chen, M.; Wang, L.; Meng, X.; Xue, Y.; Wang, J.; et al. Cryo-EM Structure of the Human Cannabinoid Receptor CB2-G(i) Signaling Complex. Cell 2020, 180, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Cohen, G.; Gover, O.; Schwartz, B. Phytocannabinoids Reduce Inflammation of Primed Macrophages and Enteric Glial Cells: An In Vitro Study. Int. J. Mol. Sci. 2023, 24, 14628. [Google Scholar] [CrossRef] [PubMed]
- Simard, M.; Rakotoarivelo, V.; Di Marzo, V.; Flamand, N. Expression and Functions of the CB2 Receptor in Human Leukocytes. Front. Pharmacol. 2022, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lutz, B. Neurobiology of cannabinoid receptor signaling. Dialogues Clin. Neurosci. 2020, 22, 207–222. [Google Scholar] [CrossRef]
- Ryberg, E.; Larsson, N.; Sjögren, S.; Hjorth, S.; Hermansson, N.; Leonova, J.; Elebring, T.; Nilsson, K.; Drmota, T.; Greasley, P.J. The orphan receptor GPR55 is a novel cannabinoid receptor. Br. J. Pharmacol. 2007, 152, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Járai, Z.; Wagner, J.A.; Varga, K.; Lake, K.D.; Compton, D.R.; Martin, B.R.; Zimmer, A.M.; Bonner, T.I.; Buckley, N.E.; Mezey, E.; et al. Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB1 or CB2 receptors. Proc. Natl. Acad. Sci. USA 1999, 96, 14136–14141. [Google Scholar] [CrossRef] [PubMed]
- Lauckner, J.E.; Jensen, J.B.; Chen, H.-Y.; Lu, H.-C.; Hille, B.; Mackie, K. GPR55 is a cannabinoid receptor that increases intracellular calcium and inhibits M current. Proc. Natl. Acad. Sci. USA 2008, 105, 2699–2704. [Google Scholar] [CrossRef]
- Kovalchuk, O.; Kovalchuk, I. Cannabinoids as anticancer therapeutic agents. Cell Cycle 2020, 19, 961–989. [Google Scholar] [CrossRef]
- Blal, K.; Besser, E.; Procaccia, S.; Schwob, O.; Lerenthal, Y.; Abu Tair, J.; Meiri, D.; Benny, O. The Effect of Cannabis Plant Extracts on Head and Neck Squamous Cell Carcinoma and the Quest for Cannabis-Based Personalized Therapy. Cancers 2023, 15, 497. [Google Scholar] [CrossRef]
- Small, E. Evolution and Classification of Cannabis sativa (Marijuana, Hemp) in Relation to Human Utilization. Bot. Rev. 2015, 81, 189–294. Available online: http://www.jstor.org/stable/45211992 (accessed on 20 November 2023). [CrossRef]
- Andre, C.M.; Hausman, J.-F.; Guerriero, G. Cannabis sativa: The Plant of the Thousand and One Molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Atakan, Z. Cannabis, a complex plant: Different compounds and different effects on individuals. Ther. Adv. Psychopharmacol. 2012, 2, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Dariš, B.; Verboten, M.T.; Knez, Ž.; Ferk, P. Cannabinoids in cancer treatment: Therapeutic potential and legislation. Bosn. J. Basic Med. Sci. 2019, 19, 14–23. [Google Scholar] [CrossRef]
- Morales, P.; Hurst, D.P.; Reggio, P.H. Molecular Targets of the Phytocannabinoids: A Complex Picture. Prog. Chem. Org. Nat. Prod. 2017, 103, 103–131. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, M.; Sánchez, C.; Galve-Roperh, I. Control of the cell survival/death decision by cannabinoids. J. Mol. Med. 2001, 78, 613–625. [Google Scholar] [CrossRef] [PubMed]
- Share of Frequency of Cannabis Use among Users in the Last Month in Europe in 2020. Available online: https://www.ptonline.com/articles/how-to-get-better-mfi-results (accessed on 6 December 2022).
- European Monitoring Centre for Drugs and Drug Addiction. Trends and Developments; European Monitoring Centre for Drugs and Drug Addiction: Lisbon, Portugal, 2022. [Google Scholar]
- Hashibe, M.; Ford, D.E.; Zhang, Z.-F. The Journal of Clinical Marijuana Smoking and. J. Clin. Pharmacol. 2002, 42, 103–107. [Google Scholar] [CrossRef]
- Schauer, G.L.; King, B.A.; Bunnell, R.E.; Promoff, G.; McAfee, T.A. Toking, Vaping and Eating for Health or Fun: Marijuana Use Patterns in Adults, U.S., 2014. Am. J. Prev. Med. 2016, 50, 1–8. [Google Scholar] [CrossRef]
- Streck, J.M.; Hughes, J.R.; Klemperer, E.M.; Howard, A.B.; Budney, A.J. Modes of cannabis use: A secondary analysis of an intensive longitudinal natural history study. Addict Behav. 2019, 98, 106033. [Google Scholar] [CrossRef]
- Sheehan, T.J.; Hamnett, H.J.; Beasley, R.; Fitzmaurice, P.S. Chemical and physical variations of cannabis smoke from a variety of cannabis samples in New Zealand. Forensic Sci. Res. 2019, 4, 168–178. [Google Scholar] [CrossRef]
- Hoffmann, D.; Brunnemann, K.D.; Gori, G.B.; Wynder, E.L. On the Carcinogenicity of Marijuana Smoke. In Recent Advances in Phytochemistry; Springer: Boston, MA, USA, 1975; pp. 63–81. [Google Scholar] [CrossRef]
- Sargent, J.D.; Halenar, M.J.; Edwards, K.C.; Woloshin, S.; Schwartz, L.; Emond, J.; Tanski, S.; A Taylor, K.; Pierce, J.P.; Liu, J.; et al. Tobacco Use and Respiratory Symptoms Among Adults: Findings from the Longitudinal Population Assessment of Tobacco and Health (PATH) Study 2014–2016. Nicotine Tob. Res. 2022, 24, 1607–1618. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Morgenstern, H.; Spitz, M.R.; Tashkin, D.P.; Yu, G.P.; Marshall, J.R.; Hsu, T.C.; Schantz, S.P. Marijuana use and increased risk of squamous cell carcinoma of the head and neck. Cancer Epidemiol. Biomarkers Prev. 1999, 8, 1071–1078. [Google Scholar] [PubMed]
- Wu, T.-C.; Tashkin, D.P.; Djahed, B.; Rose, J.E. Pulmonary Hazards of Smoking Marijuana as Compared with Tobacco. N. Engl. J. Med. 1988, 318, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Harpreet, S.; Joseph, K.; Wafaa, S.; Seunghee, C. Impact of Cannabis on the Port of Entry-Oral Tissues: An Overview. Int. J. Oral Dent. Health 2019, 5, 10–14. [Google Scholar] [CrossRef]
- Shariff, J.A.; Ahluwalia, K.P.; Papapanou, P.N. Relationship between Frequent Recreational Cannabis (Marijuana and Hashish) Use and Periodontitis in Adults in the United States: National Health and Nutrition Examination Survey 2011 to 2012. J. Periodontol. 2017, 88, 273–280. [Google Scholar] [CrossRef]
- Rawal, S.Y.; Tatakis, D.N.; Tipton, D.A. Periodontal and oral manifestations of marijuana use. J. Tenn. Dent. Assoc. 2012, 92, 22–26. [Google Scholar]
- Darling, M.R.; Arendorf, T.M. Effects of cannabis smoking on oral soft tissues. Community Dent. Oral Epidemiol. 1993, 21, 78–81. [Google Scholar] [CrossRef]
- Cho, C.M.; Hirsch, R.; Johnstone, S. General and oral health implications of cannabis use. Aust. Dent. J. 2005, 50, 70–74. [Google Scholar] [CrossRef]
- Caruntu, A.; Scheau, C.; Tampa, M.; Georgescu, S.R.; Caruntu, C.; Tanase, C. Complex Interaction Among Immune, Inflammatory and Carcinogenic Mechanisms in the Head and Neck Squamous Cell Carcinoma. Adv. Exp. Med. Biol. 2021, 1335, 11–35. [Google Scholar] [CrossRef]
- Baldwin, G.C.; Tashkin, D.P.; Buckley, D.M.; Park, A.N.; Dubinett, S.M.; Roth, M.D. Marijuana and Cocaine Impair Alveolar Macrophage Function and Cytokine Production. Am. J. Respir. Crit. Care Med. 1997, 156, 1606–1613. [Google Scholar] [CrossRef]
- Sherman, M.P.; Aeberhard, E.E.; Wong, V.Z.; Simmons, M.S.; Roth, M.D.; Tashkin, D.P. Effects of smoking marijuana, tobacco or cocaine alone or in combination on dna damage in human alveolar macrophages. Life Sci. 1995, 56, 2201–2207. [Google Scholar] [CrossRef]
- Aujayeb, A.; Donald, C.; Doe, S. Breath-holding in a marijuana smoker. Respir. Med. Case Rep. 2012, 5, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Taylor, F.M., 3rd. Marijuana as a potential respiratory tract carcinogen: A retrospective analysis of a community hospital population. South. Med. J. 1988, 81, 1213–1216. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.; Zheng, H.J.; Zhang, C.P. The Oral Microbiota May Have Influence on Oral Cancer. Front. Cell. Infect. Microbiol. 2019, 9, 476. [Google Scholar] [CrossRef] [PubMed]
- Zeng, B.; Tan, J.; Guo, G.; Li, Z.; Yang, L.; Lao, X.; Wang, D.; Ma, J.; Zhang, S.; Liao, G.; et al. The oral cancer microbiome contains tumor space-specific and clinicopathology-specific bacteria. Front. Cell. Infect. Microbiol. 2022, 12, 942328. [Google Scholar] [CrossRef] [PubMed]
- Newman, T.M.; Krishnan, L.P.; Lee, J.; Adami, G.R. Microbiomic differences at cancer-prone oral mucosa sites with marijuana usage. Sci. Rep. 2019, 9, 12697. [Google Scholar] [CrossRef] [PubMed]
- Alhouayek, M.; Boldrup, L.; Fowler, C.J. Altered mRNA expression of genes involved in endocannabinoid signalling in squamous cell carcinoma of the oral tongue. Cancer Investig. 2019, 37, 327–338. [Google Scholar] [CrossRef]
- Hashibe, M.; Straif, K.; Tashkin, D.P.; Morgenstern, H.; Greenland, S.; Zhang, Z.-F. Epidemiologic review of marijuana use and cancer risk. Alcohol 2005, 35, 265–275. [Google Scholar] [CrossRef]
- Firth, N. Marijuana use and oral cancer: A review. Oral Oncol. 1997, 33, 398–401. [Google Scholar] [CrossRef]
- Reece, A.S.; Bennett, K.; Hulse, G.K. Cannabis- and Substance-Related Carcinogenesis in Europe: A Lagged Causal Inferential Panel Regression Study. J. Xenobiotics 2023, 13, 323–385. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Mandal, S.; Banerjee, S.; Mandal, G.K.; Bhowmick, A.K.; Murmu, N. Cannabis smoke can be a major risk factor for early-age laryngeal cancer—A molecular signaling-based approach. Tumor Biol. 2015, 36, 6029–6036. [Google Scholar] [CrossRef]
- Vannimenus, C.; The ALTAK Study Group; Bricout, H.; Le Rouzic, O.; Mouawad, F.; Chevalier, D.; Dansin, E.; Rotsaert, L.; Lefebvre, G.; Cottencin, O.; et al. Compared characteristics of current vs. past smokers at the time of diagnosis of a first-time lung or head and neck cancer: A cross-sectional study. BMC Cancer 2018, 18, 372. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Sadat, S.H.; Ebisumoto, K.; Sakai, A.; Panuganti, B.A.; Ren, S.; Goto, Y.; Haft, S.; Fukusumi, T.; Ando, M.; et al. Cannabinoids Promote Progression of HPV-Positive Head and Neck Squamous Cell Carcinoma via p38 MAPK Activation. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 2693–2703. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Hua, G.; Zhang, H.; Chan, T.J.; Xie, M.; Levin, M.; Farrokhyar, F.; Archibald, S.D.; Jackson, B.; E Young, J.; et al. Rate of Second Primary Head and Neck Cancer with Cannabis Use. Cureus 2020, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Sidney, S.; Quesenberry, C.P.J.; Friedman, G.D.; Tekawa, I.S. Marijuana use and cancer incidence (California, United States). Cancer Causes Control 1997, 8, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Semlali, A.; Beji, S.; Ajala, I.; Rouabhia, M. Effects of tetrahydrocannabinols on human oral cancer cell proliferation, apoptosis, autophagy, oxidative stress and DNA damage. Arch. Oral Biol. 2021, 129, 105200. [Google Scholar] [CrossRef]
- Desprez, P.-Y.; Murase, R.; Limbad, C.; Woo, R.W.; Adrados, I.; Weitenthaler, K.; Soroceanu, L.; Salomonis, N.; McAllister, S.D. Cannabidiol Treatment Results in a Common Gene Expression Response Across Aggressive Cancer Cells from Various Origins. Cannabis Cannabinoid Res. 2021, 6, 148–155. [Google Scholar] [CrossRef]
- Park, S.W.; Hah, J.H.; Oh, S.M.; Jeong, W.J.; Sung, M.W. 5-lipoxygenase mediates docosahexaenoyl ethanolamide and N-arachidonoyl-L-alanine-induced reactive oxygen species production and inhibition of proliferation of head and neck squamous cell carcinoma cells. BMC Cancer 2016, 16, 458. [Google Scholar] [CrossRef]
- Whyte, D.A.; Al-Hammadi, S.; Balhaj, G.; Brown, O.M.; Penefsky, H.S.; Souid, A.K. Cannabinoids inhibit cellular respiration of human oral cancer cells. Pharmacology 2010, 85, 328–335. [Google Scholar] [CrossRef]
- Liang, C.; McClean, M.D.; Marsit, C.; Christensen, B.; Peters, E.; Nelson, H.H.; Kelsey, K.T. A Population-Based Case-Control Study of Marijuana Use and Head and Neck Squamous Cell Carcinoma. Cancer Prev. Res. 2009, 2, 759–768. [Google Scholar] [CrossRef]
- Shewale, J.B.; Pickard, R.K.L.; Xiao, W.; Jiang, B.; Gillison, M.L. Independent association of marijuana use and poor oral hygiene with HPV–negative but not HPV–positive head and neck squamous cell carcinomas. Cancer 2021, 127, 2099–2110. [Google Scholar] [CrossRef]
- Zhang, H.; Xie, M.; Levin, M.; Archibald, S.D.; Jackson, B.S.; Young, J.E.M.; Gupta, M.K. Survival outcomes of marijuana users in p16 positive oropharynx cancer patients. J. Otolaryngol.-Head Neck Surg. J. D’oto-Rhino-Laryngologie Chir. Cervico-Faciale 2019, 48, 43. [Google Scholar] [CrossRef] [PubMed]
- Aldington, S.; Harwood, M.M.; Cox, B.; Weatherall, M.; Beckert, L.; Hansell, A.; Pritchard, A.; Robinson, G.; Beasley, R.; Aldington, B.S.; et al. Cannabis use and cancer of the head and neck: Case-control study. Otolaryngol.-Head Neck Surg. 2008, 138, 374–380. [Google Scholar] [CrossRef]
- Rosenblatt, K.A.; Daling, J.R.; Chen, C.; Sherman, K.J.; Schwartz, S.M. Marijuana use and risk of oral squamous cell carcinoma. Cancer Res. 2004, 64, 4049–4054. [Google Scholar] [CrossRef]
- Ghasemiesfe, M.; Barrow, B.; Leonard, S.; Keyhani, S.; Korenstein, D. Association between Marijuana Use and Risk of Cancer. JAMA Netw. Open 2019, 2, e1916318. [Google Scholar] [CrossRef] [PubMed]
- Berthiller, J.; Lee, Y.-C.A.; Boffetta, P.; Wei, Q.; Sturgis, E.M.; Greenland, S.; Morgenstern, H.; Zhang, Z.-F.; Lazarus, P.; Muscat, J.; et al. Marijuana smoking and the risk of head and neck cancer: Pooled analysis in the INHANCE consortium. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2009, 18, 1544–1551. [Google Scholar] [CrossRef]
- Gieringer, D.; St. Laurent, J.; Goodrich, S. Cannabis Vaporizer Combines Efficient Delivery of THC with Effective Suppression of Pyrolytic Compounds. J. Cannabis Ther. 2004, 4, 7–27. [Google Scholar] [CrossRef]
- Malouff, J.M.; Rooke, S.E.; Copeland, J. Experiences of Marijuana-Vaporizer Users. Subst. Abus. 2014, 35, 127–128. [Google Scholar] [CrossRef]
- Budney, A.J.; Sargent, J.D.; Lee, D.C. Vaping cannabis (marijuana): Parallel concerns to e-cigs? Addiction 2015, 110, 1699–1704. [Google Scholar] [CrossRef]
- Almeida-da-silva, C.; Matshik, H. Effects of electronic cigarette aerosol exposure on oral and systemic health. Biomed. J. 2020, 44, 252–259. [Google Scholar] [CrossRef]
- Andrikopoulos, G.I.; Farsalinos, K. Electronic Nicotine Delivery Systems (ENDS) and Their Relevance in Oral Health. Toxics 2019, 7, 61. [Google Scholar] [CrossRef]
- Blount, B.C.; Karwowski, M.P.; Shields, P.G.; Morel-Espinosa, M.; Valentin-Blasini, L.; Gardner, M.; Braselton, M.; Brosius, C.R.; Caron, K.T.; Chambers, D.; et al. Vitamin E Acetate in Bronchoalveolar-Lavage Fluid Associated with EVALI. N. Engl. J. Med. 2019, 382, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Lung Injury Response Epidemiology/Surveillance Task Force. Update: Product, Substance-Use and Demographic Characteristics of Hospitalized Patients in a Nationwide Outbreak of E-cigarette, or Vaping, Product Use–Associated Lung Injury—United States. Morb. Mortal. Wkly. Rep. 2020, 69, 44–49. [Google Scholar]
- Clark, T.M. Scoping Review and Meta-Analysis Suggests that Cannabis Use May Reduce Cancer Risk in the United States. Cannabis Cannabinoid Res. 2021, 6, 413–434. [Google Scholar] [CrossRef] [PubMed]
- Tampa, M.; Mitran, C.I.; Mitran, M.I.; Nicolae, I.; Dumitru, A.; Matei, C.; Manolescu, L.; Popa, G.L.; Caruntu, C.; Georgescu, S.R. The Role of Beta HPV Types and HPV-Associated Inflammatory Processes in Cutaneous Squamous Cell Carcinoma. J. Immunol. Res. 2020, 2020, 5701639. [Google Scholar] [CrossRef]
- Gillison, M.L.; Koch, W.M.; Capone, R.B.; Spafford, M.; Westra, W.H.; Wu, L.; Zahurak, M.L.; Daniel, R.W.; Viglione, M.; Symer, D.E.; et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J. Natl. Cancer Inst. 2000, 92, 709–720. [Google Scholar] [CrossRef]
- Maura, P.; Gillison, L.; Broutian, T.; Pickard, R.K.L.; Tong, Z.-Y.; Xiao, W.; Kahle, L.; Barry, B.A.; Graubard, I.; Anil, K. Chaturvedi Prevalence of Oral HPV Infection in the United States, 2009–2010 Dr. Physiol. Behav. 2018, 176, 100–106. [Google Scholar] [CrossRef]
- Miranda-Galvis, M.; Soares, C.C.; Carnielli, C.M.; Buttura, J.R.; de Sá, R.S.; Kaminagakura, E.; Marchi, F.A.; Leme, A.F.P.; Pinto, C.A.L.; Santos-Silva, A.R.; et al. New Insights into the Impact of Human Papillomavirus on Oral Cancer in Young Patients: Proteomic Approach Reveals a Novel Role for S100A8. Cells 2023, 12, 1323. [Google Scholar] [CrossRef] [PubMed]
- Santacroce, L.; Di Cosola, M.; Bottalico, L.; Topi, S.; Charitos, I.A.; Ballini, A.; Inchingolo, F.; Cazzolla, A.P.; Dipalma, G. Focus on HPV Infection and the Molecular Mechanisms of Oral Carcinogenesis. Viruses 2021, 13, 559. [Google Scholar] [CrossRef]
- Dahlstrom, K.R.; Bell, D.; Hanby, D.; Li, G.; Wang, L.-E.; Wei, Q.; Williams, M.D.; Sturgis, E.M. Socioeconomic characteristics of patients with oropharyngeal carcinoma according to tumor HPV status, patient smoking status and sexual behavior. Oral Oncol. 2015, 51, 832–838. [Google Scholar] [CrossRef]
- Mith, E.M.S.; Itchie, J.M.R.; Ummersgill, K.F.S.; Lussmann, J.P.K.; Ee, J.H.L.; Ang, D.W. Age, sexual behavior and human papillomavirus infection in oral cavity and oropharyngeal cancers. Int. J. Cancer 2004, 108, 766–772. [Google Scholar] [CrossRef]
- Boscolo-Rizzo, P.; Del Mistro, A.; Bussu, F.; Lupato, V.; Baboci, L.; Almadori, G.; Da Mosto, M.; Paludetti, G. New insights into human papillomavirus-associated head and neck squamous cell carcinoma. Acta Otorhinolaryngol. Ital. Organo Uff. Della Soc. Ital. Otorinolaringol. Chir. Cerv.-Facc. 2013, 33, 77–87. [Google Scholar]
- Sun, A.J.; Eisenberg, M.L. Association between Marijuana Use and Sexual Frequency in the United States: A Population-Based Study. J. Sex. Med. 2017, 14, 1342–1347. [Google Scholar] [CrossRef]
- Ortiz, P.A.P.; González, D.; Ramos, J.; Muñoz, C.; Reyes, J.C.; Cynthia, M. Pérez Association of marijuana use with oral HPV infection and periodontitis among Hispanic adults: Implications for oral cancer prevention. Physiol. Behav. 2018, 176, 139–148. [Google Scholar] [CrossRef]
- Paraskeva; Patsos, H.A.; Greenhough, A.; Hicks, D.J.; Al Kharusi, M.; Collard, T.J.; Lane, J.D.; Paraskeva, C.; Williams, A.C. The endogenous cannabinoid, anandamide, induces COX-2-dependent cell death in apoptosis-resistant colon cancer cells. Int. J. Oncol. 2010, 37, 187–193. [Google Scholar] [CrossRef]
- Contassot, E.; Tenan, M.; Schnüriger, V.; Pelte, M.-F.; Dietrich, P.-Y. Arachidonyl ethanolamide induces apoptosis of uterine cervix cancer cells via aberrantly expressed vanilloid receptor-1. Gynecol. Oncol. 2004, 93, 182–188. [Google Scholar] [CrossRef]
- Wiatrak, B.; Kubis-Kubiak, A.; Piwowar, A.; Barg, E. PC12 Cell Line: Cell Types, Coating of Culture Vessels, Differentiation and Other Culture Conditions. Cells 2020, 9, 958. [Google Scholar] [CrossRef]
- Sarker, K.P.; Biswas, K.K.; Yamakuchi, M.; Lee, K.-Y.; Hahiguchi, T.; Kracht, M.; Kitajima, I.; Maruyama, I. ASK1-p38 MAPK/JNK signaling cascade mediates anandamide-induced PC12 cell death. J. Neurochem. 2003, 85, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Voiculescu, V.M.; Lisievici, C.V.; Lupu, M.; Vajaitu, C.; Draghici, C.C.; Popa, A.V.; Solomon, I.; Sebe, T.I.; Constantin, M.M.; Caruntu, C. Mediators of Inflammation in Topical Therapy of Skin Cancers. Mediators Inflamm. 2019, 2019, 8369690. [Google Scholar] [CrossRef] [PubMed]
- Caruntu, A.; Moraru, L.; Surcel, M.; Munteanu, A.; Costache, D.O.; Tanase, C.; Constantin, C.; Scheau, C.; Neagu, M.; Caruntu, C. Persistent Changes of Peripheral Blood Lymphocyte Subsets in Patients with Oral Squamous Cell Carcinoma. Healthcare 2022, 10, 342. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Kunos, G. Modulating the endocannabinoid system in human health and disease—Successes and failures. FEBS J. 2013, 280, 1918–1943. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, S.B.; Lindgren, T.; Jonsson, M.; Jacobsson, S.O.P. Cannabinoid receptor-independent cytotoxic effects of cannabinoids in human colorectal carcinoma cells: Synergism with 5-fluorouracil. Cancer Chemother. Pharmacol. 2009, 63, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, M.-M.; Loron, A.G.; Sayyah, M. The ω-3 endocannabinoid docosahexaenoyl ethanolamide reduces seizure susceptibility in mice by activating cannabinoid type 1 receptors. Brain Res. Bull. 2021, 170, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Romano, M.; Claria, J. Cyclooxygenase-2 and 5-lipoxygenase converging functions on cell proliferation and tumor angiogenesis: Implications for cancer therapy. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2003, 17, 1986–1995. [Google Scholar] [CrossRef] [PubMed]
- McDougle, D.R.; Kambalyal, A.; Meling, D.D.; Das, A. Endocannabinoids anandamide and 2-arachidonoylglycerol are substrates for human CYP2J2 epoxygenase. J. Pharmacol. Exp. Ther. 2014, 351, 616–627. [Google Scholar] [CrossRef]
- Rezaei, S.; Darban, R.A.; Javid, H.; Hashemy, S.I. The Therapeutic Potential of Aprepitant in Glioblastoma Cancer Cells through Redox Modification. Biomed. Res. Int. 2022, 2022, 8540403. [Google Scholar] [CrossRef]
- Akbari, S.; Darban, R.A.; Javid, H.; Esparham, A.; Hashemy, S.I. The anti-tumoral role of Hesperidin and Aprepitant on prostate cancer cells through redox modifications. Naunyn. Schmiedebergs. Arch. Pharmacol. 2023, 396, 3559–3567. [Google Scholar] [CrossRef]
- Katso, R.; Okkenhaug, K.; Ahmadi, K.; White, S.; Timms, J.; Waterfield, M.D. Waterfield Cellular function of phosphoinositide 3-kinases: Implications for development, homeostasis and cancer. Annu. Rev. Cell Dev. Biol. 2001, 17, 615–675. [Google Scholar] [CrossRef] [PubMed]
- Bucurica, S.; Gaman, L.; Jinga, M.; Popa, A.A.; Ionita-Radu, F. Golgi Apparatus Target Proteins in Gastroenterological Cancers: A Comprehensive Review of GOLPH3 and GOLGA Proteins. Cells 2023, 12, 1823. [Google Scholar] [CrossRef]
- He, Y.; Sun, M.M.; Zhang, G.G.; Yang, J.; Chen, K.S.; Xu, W.W.; Li, B. Targeting PI3K/Akt signal transduction for cancer therapy. Signal Transduct. Target. Ther. 2021, 6, 425. [Google Scholar] [CrossRef]
- Tampa, M.; Georgescu, S.R.; Mitran, C.I.; Mitran, M.I.; Matei, C.; Scheau, C.; Constantin, C.; Neagu, M. Recent Advances in Signaling Pathways Comprehension as Carcinogenesis Triggers in Basal Cell Carcinoma. J. Clin. Med. 2020, 9, 3010. [Google Scholar] [CrossRef]
- Broek, R.V.; Mohan, S.; Eytan, D.F.; Chen, Z.; Van Waes, C. The PI3K/Akt/mTOR axis in head and neck cancer: Functions, aberrations, cross-talk, and therapies. Oral Dis. 2015, 21, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Sacks, P.G.; Parnes, S.M.; E Gallick, G.; Mansouri, Z.; Lichtner, R.; Satya-Prakash, K.L.; Pathak, S.; Parsons, D.F. Establishment and characterization of two new squamous cell carcinoma cell lines derived from tumors of the head and neck. Cancer Res. 1988, 48, 2858–2866. [Google Scholar] [PubMed]
- Bridgeman, M.B.; Abazia, D.T. Medicinal Cannabis: History, Pharmacology and Implications for the Acute Care Setting. Pharm. Ther. 2017, 42, 180–188. [Google Scholar]
- Cannabis Legalization World Map: UPDATED. Available online: https://www.cannabisbusinesstimes.com/article/cannabis-legalization-world-map/ (accessed on 20 November 2023).
- de Souza, M.R.; Henriques, A.T.; Limberger, R.P. Medical cannabis regulation: An overview of models around the world with emphasis on the Brazilian scenario. J. Cannabis Res. 2022, 4, 33. [Google Scholar] [CrossRef] [PubMed]
- Medical Cannabis in the United States. Available online: https://en.wikipedia.org/wiki/Medical_cannabis_in_the_United_States (accessed on 23 November 2023).
- Elliot, H.L. Rolfe Medical Cannabis & Cannabinoid Regulation 2023. 2023. Available online: https://practiceguides.chambers.com/practice-guides/medical-cannabis-cannabinoid-regulation-2023 (accessed on 23 November 2023).
- Kramer, J.L. Medical marijuana for cancer. CA. Cancer J. Clin. 2015, 65, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Bostwick, J.M. Blurred boundaries: The therapeutics and politics of medical marijuana. Mayo Clin. Proc. 2012, 87, 172–186. [Google Scholar] [CrossRef] [PubMed]
- VanDolah, H.J.; Bauer, B.A.; Mauck, K.F. Clinicians’ guide to cannabidiol and hemp oils. Mayo Clin. Proc. 2019, 94, 1840–1851. [Google Scholar] [CrossRef]
- Vinette, B.; Côté, J.; El-Akhras, A.; Mrad, H.; Chicoine, G.; Bilodeau, K. Routes of administration, reasons for use and approved indications of medical cannabis in oncology: A scoping review. BMC Cancer 2022, 22, 319. [Google Scholar] [CrossRef]
- Bellocchio, L.; Inchingolo, A.D.; Inchingolo, A.M.; Lorusso, F.; Malcangi, G.; Santacroce, L.; Scarano, A.; Bordea, I.R.; Hazballa, D.; D’oria, M.T.; et al. Cannabinoids Drugs and Oral Health—From Recreational Side-Effects to Medicinal Purposes: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 8329. [Google Scholar] [CrossRef]
- Ligresti, A.; De Petrocellis, L.; Di Marzo, V. From Phytocannabinoids to Cannabinoid Receptors and Endocannabinoids: Pleiotropic Physiological and Pathological Roles Through Complex Pharmacology. Physiol. Rev. 2016, 96, 1593–1659. [Google Scholar] [CrossRef]
- Zhang, H.; Xie, M.; Archibald, S.D.; Jackson, B.S.; Gupta, M.K. Association of marijuana use with psychosocial and quality of life outcomes among patients with head and neck cancer. JAMA Otolaryngol. Neck Surg. 2018, 144, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.; Zhang, H.; Gupta, M.K. Attitudes toward and Acceptability of Medical Marijuana Use among Head and Neck Cancer Patients. Ann. Otol. Rhinol. Laryngol. 2023, 132, 13–18. [Google Scholar] [CrossRef]
- Côté, M.; Trudel, M.; Wang, C.; Fortin, A. Improving Quality of Life With Nabilone During Radiotherapy Treatments for Head and Neck Cancers: A Randomized Double-Blind Placebo-Controlled Trial. Ann. Otol. Rhinol. Laryngol. 2016, 125, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Elliott, D.A.; Nabavizadeh, N.; Romer, J.L.; Chen, Y.; Holland, J.M. Medical marijuana use in head and neck squamous cell carcinoma patients treated with radiotherapy. Support. Care Cancer 2016, 24, 3517–3524. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, A.; Schymick, M.; Khalil, R.; Williams, A.; Siddiqui, F. Does the use of Marijuana Impact Outcomes in Patients with Squamous Cell Carcinoma of the Oropharynx? Int. J. Radiat. Oncol. 2020, 108, E42. [Google Scholar] [CrossRef]
- Smith, L.A.; Azariah, F.; Lavender, V.T.; Stoner, N.S.; Bettiol, S. Cannabinoids for nausea and vomiting in adults with cancer receiving chemotherapy. Cochrane Database Syst. Rev. 2015, 2015, 11. [Google Scholar] [CrossRef]
- Caffarel, M.M.; Andradas, C.; Pérez-Gómez, E.; Guzmán, M.; Sánchez, C. Cannabinoids: A new hope for breast cancer therapy? Cancer Treat. Rev. 2012, 38, 911–918. [Google Scholar] [CrossRef]
- MacCallum, C.A.; Eadie, L.; Barr, A.M.; Boivin, M.; Lu, S. Practical Strategies Using Medical Cannabis to Reduce Harms Associated With Long Term Opioid Use in Chronic Pain. Front. Pharmacol. 2021, 12, 633168. [Google Scholar] [CrossRef]
- Neben-Wittich, M.A. Medical Cannabis to Treat Symptoms from Head and Neck Radiation Therapy. Pract. Radiat. Oncol. 2021, 11, 313–316. [Google Scholar] [CrossRef]
- Rosewall, T.; Feuz, C.; Bayley, A. Cannabis and Radiation Therapy: A Scoping Review of Human Clinical Trials. J. Med. Imaging Radiat. Sci. 2020, 51, 342–349. [Google Scholar] [CrossRef]
- WHäuser; Welsch, P.; Radbruch, L.; Fisher, E.; Bell, R.F.; Moore, R.A. Cannabis-based medicines and medical cannabis for adults with cancer pain. Cochrane Database Syst. Rev. 2023, 6, CD014915. [Google Scholar] [CrossRef]
- Cuba, L.F.; Salum, F.G.; Cherubini, K.; Figueiredo, M.A.Z. Cannabidiol: An alternative therapeutic agent for oral mucositis? J. Clin. Pharm. Ther. 2017, 42, 245–250. [Google Scholar] [CrossRef]
- Dominiak, H.S.H.; Hasselsteen, S.D.; Nielsen, S.W.; Andersen, J.R.; Herrstedt, J. Prevention of Taste Alterations in Patients with Cancer Receiving Paclitaxel- or Oxaliplatin-Based Chemotherapy—A Pilot Trial of Cannabidiol. Nutrients 2023, 15, 3014. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cretu, B.; Zamfir, A.; Bucurica, S.; Scheau, A.E.; Savulescu Fiedler, I.; Caruntu, C.; Caruntu, A.; Scheau, C. Role of Cannabinoids in Oral Cancer. Int. J. Mol. Sci. 2024, 25, 969. https://doi.org/10.3390/ijms25020969
Cretu B, Zamfir A, Bucurica S, Scheau AE, Savulescu Fiedler I, Caruntu C, Caruntu A, Scheau C. Role of Cannabinoids in Oral Cancer. International Journal of Molecular Sciences. 2024; 25(2):969. https://doi.org/10.3390/ijms25020969
Chicago/Turabian StyleCretu, Brigitte, Alexandra Zamfir, Sandica Bucurica, Andreea Elena Scheau, Ilinca Savulescu Fiedler, Constantin Caruntu, Ana Caruntu, and Cristian Scheau. 2024. "Role of Cannabinoids in Oral Cancer" International Journal of Molecular Sciences 25, no. 2: 969. https://doi.org/10.3390/ijms25020969
APA StyleCretu, B., Zamfir, A., Bucurica, S., Scheau, A. E., Savulescu Fiedler, I., Caruntu, C., Caruntu, A., & Scheau, C. (2024). Role of Cannabinoids in Oral Cancer. International Journal of Molecular Sciences, 25(2), 969. https://doi.org/10.3390/ijms25020969









