Clerodendrum chinense Stem Extract and Nanoparticles: Effects on Proliferation, Colony Formation, Apoptosis Induction, Cell Cycle Arrest, and Mitochondrial Membrane Potential in Human Breast Adenocarcinoma Breast Cancer Cells
Abstract
1. Introduction
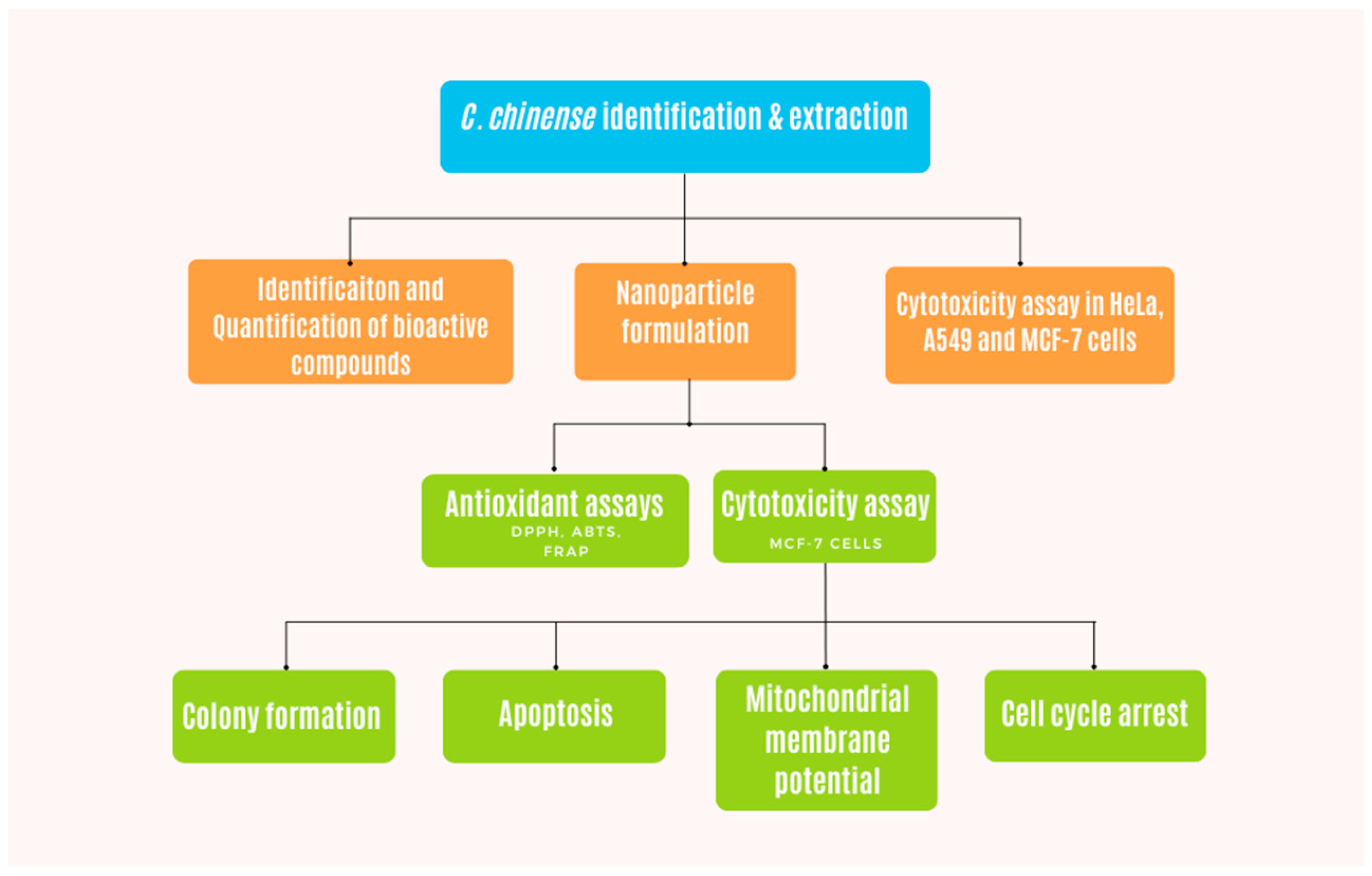
2. Results
2.1. Yield of C. chinense Stem Extract
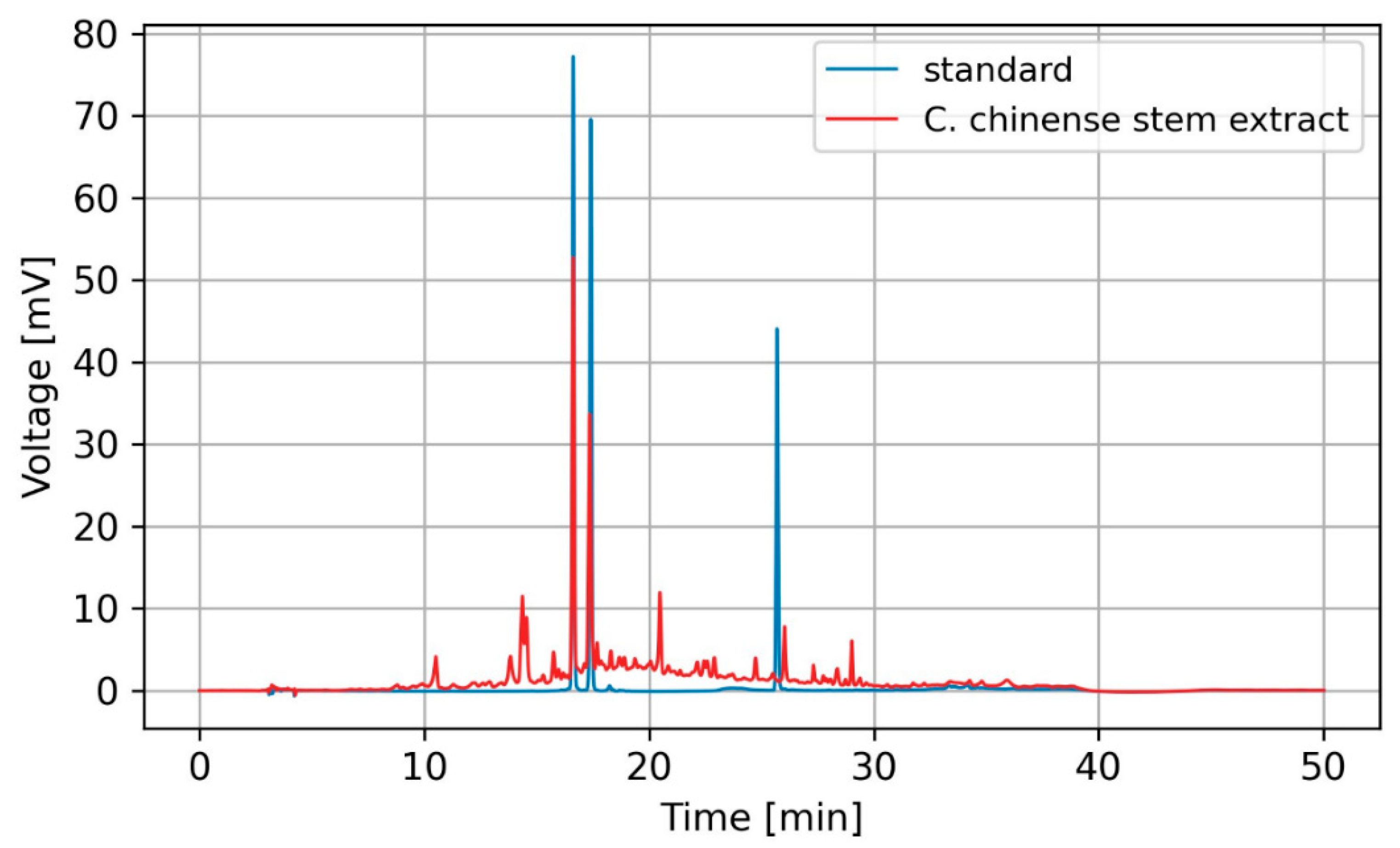
2.2. High-Performance Liquid Chromatography (HPLC) Profiles of Phytochemicals in C. chinense Stem Extract
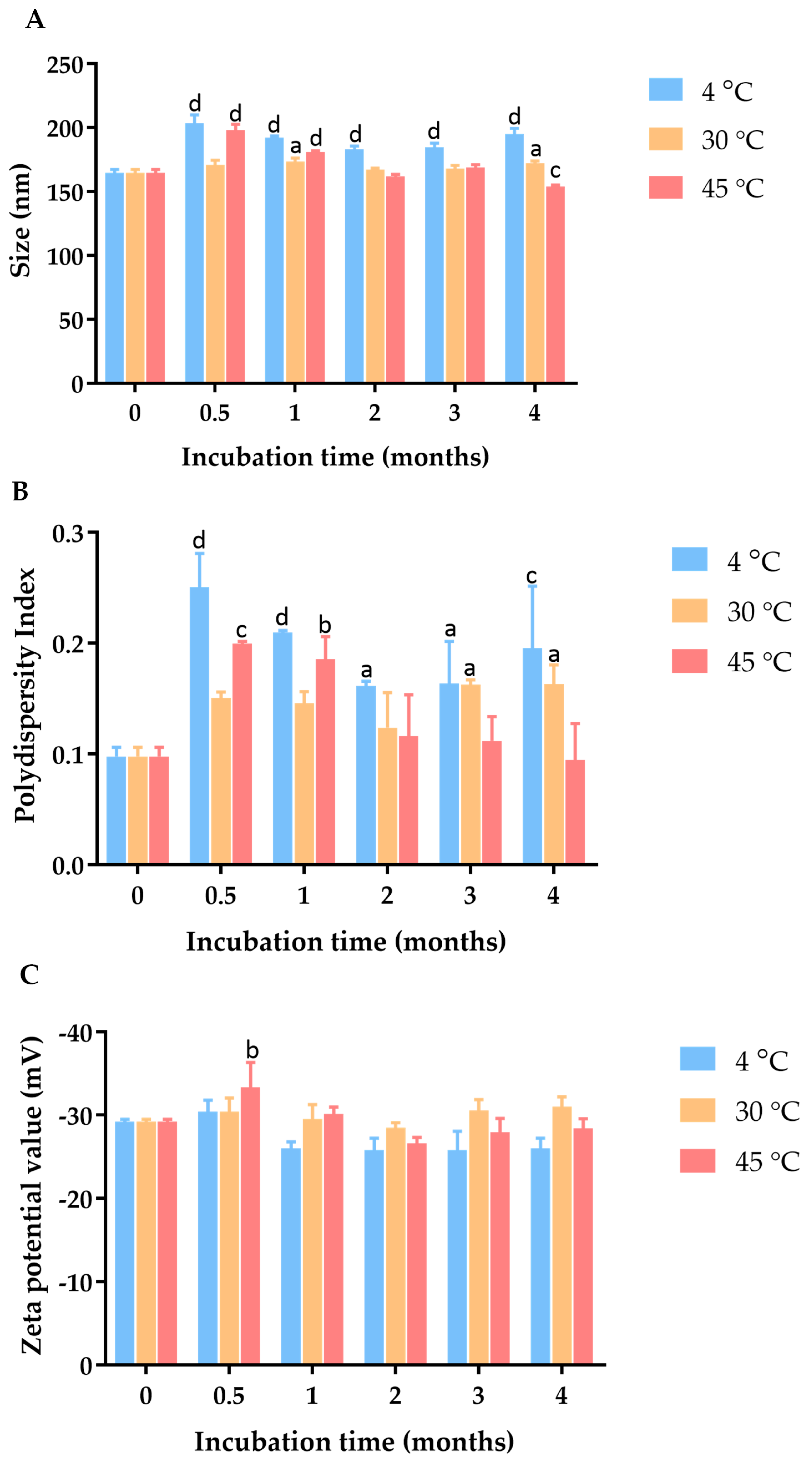
2.3. Characterization and Physical Stability Study of C. chinense Stem Extract Nanoparticles (NPs): Particle Size, Polydispersity Index (PDI), and Zeta Potential Value
2.4. Total Phenolic Content and Total Flavonoid Content in C. chinense Stem Extract and NPs
2.5. Antioxidant Activities of C. chinense Stem Extract Nanoparticles and NPs
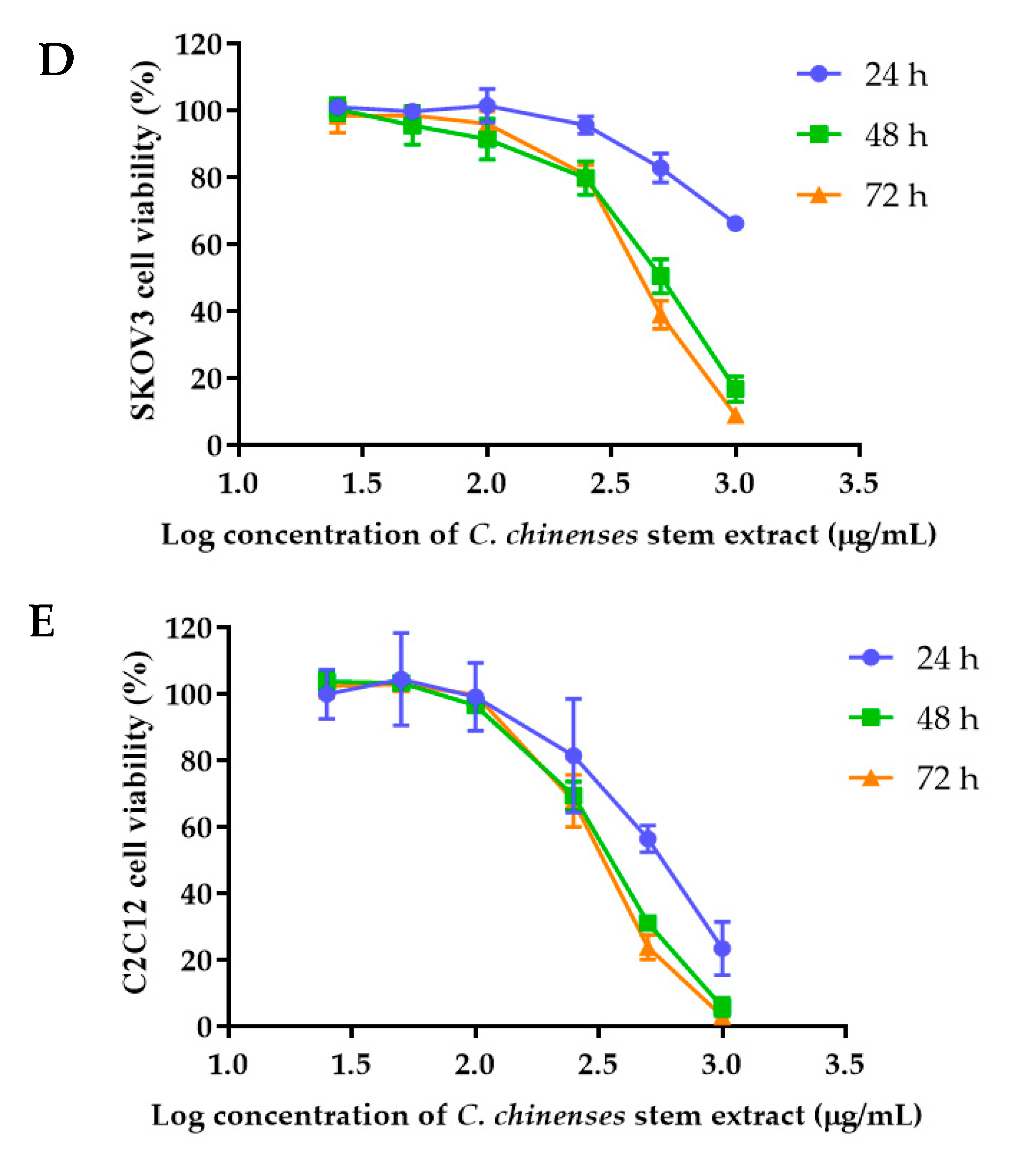
2.6. Cytotoxicity of C. chinense Stem Extract and NPs against Cancer Cell Lines
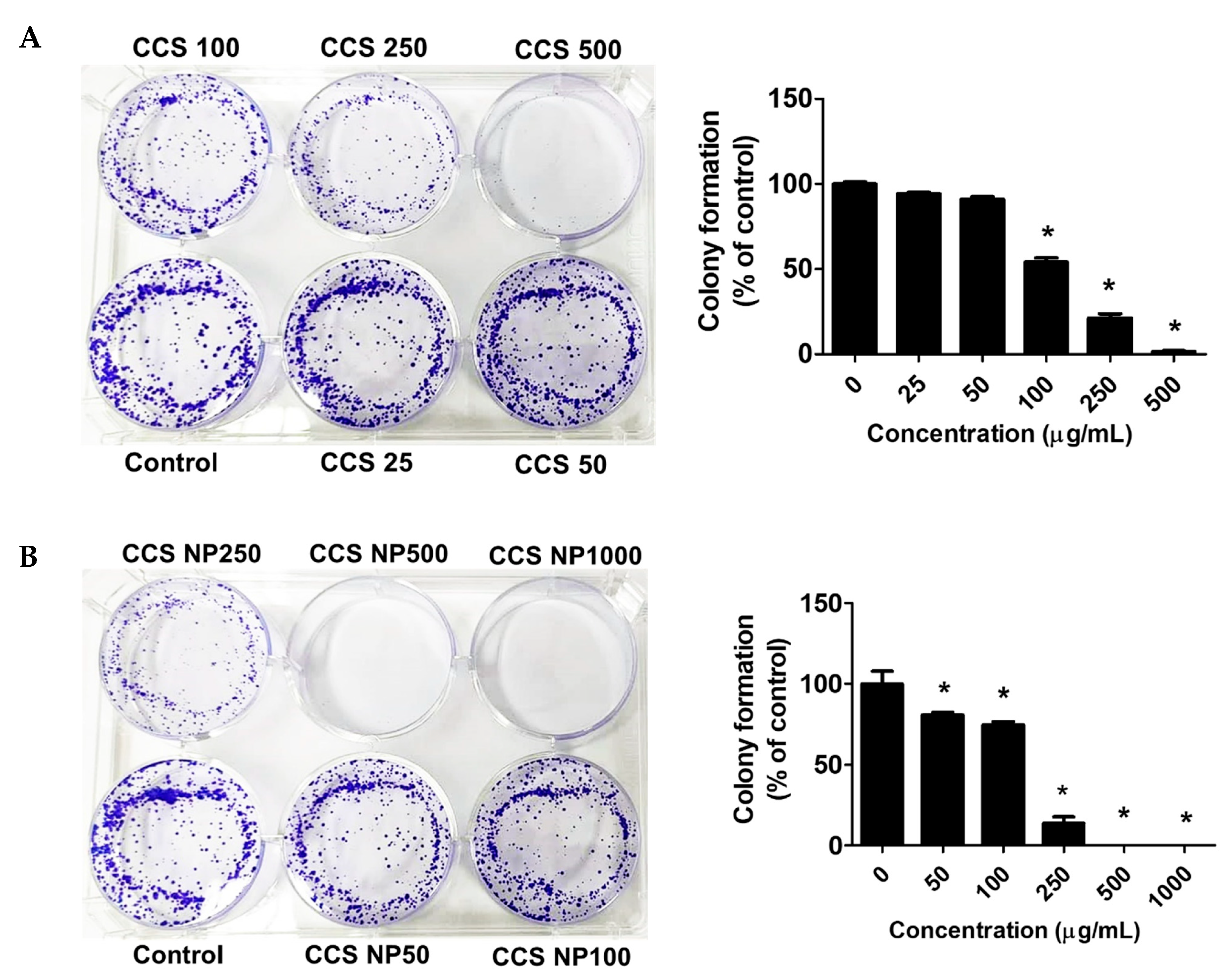
2.7. C. chinense Stem Extract and NPs Inhibited Colony Formation of MCF-7 Cells
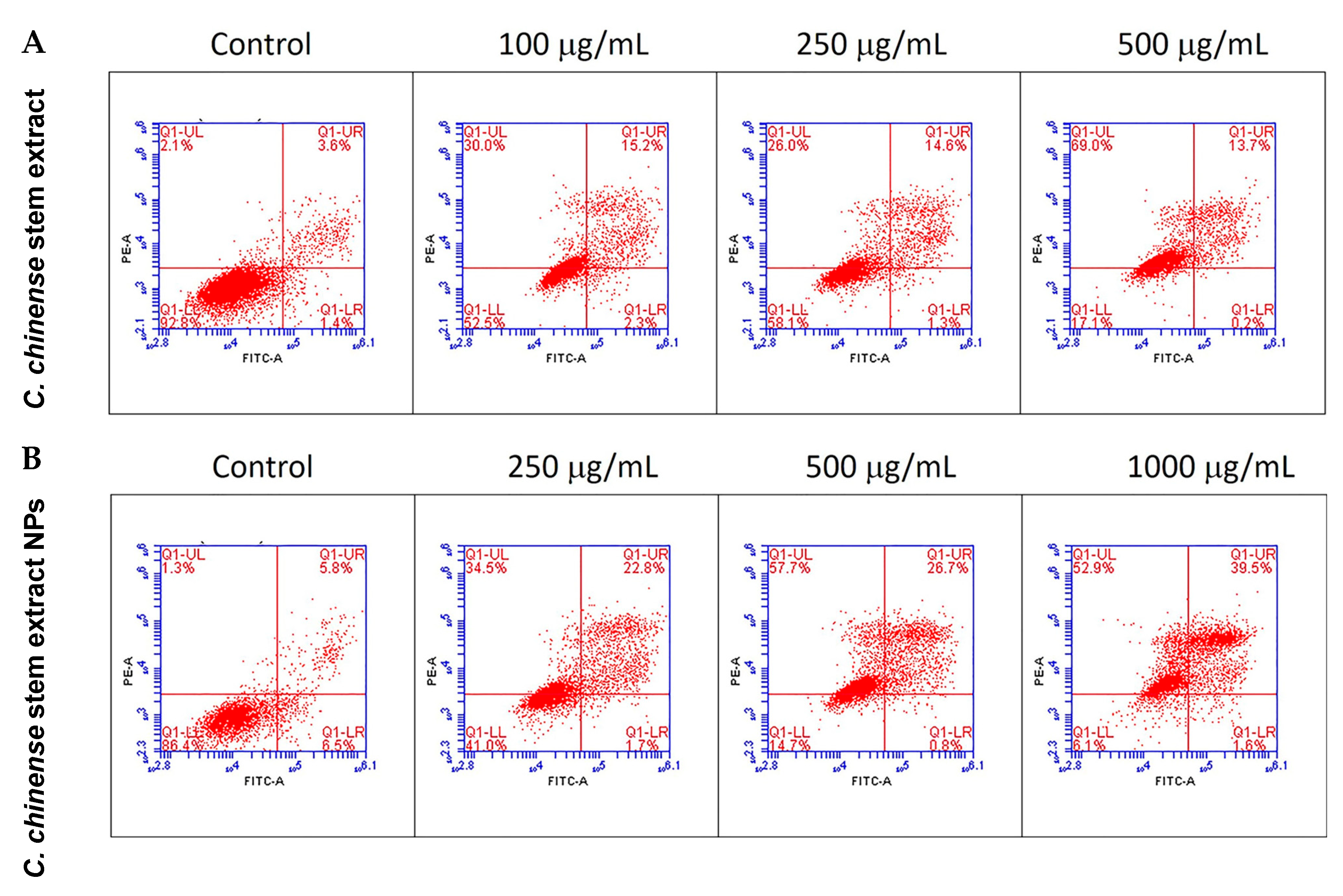
2.8. C. chinense Stem Extract and NPs Promoted Apoptosis in MCF-7 Cells
2.9. C. chinense Stem Extract and NPs Decreased Mitochondrial Membrane Potential (MMP)
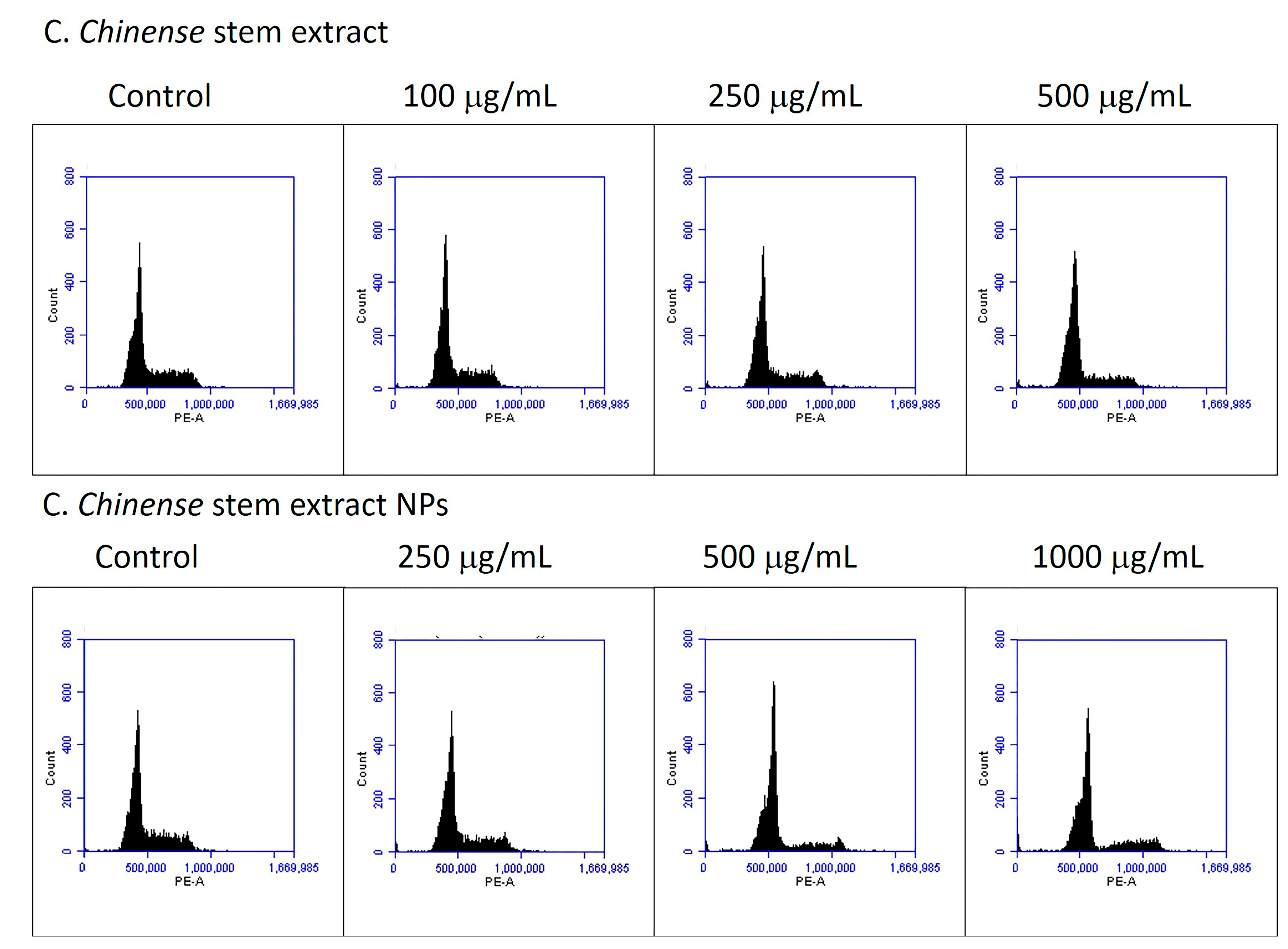
2.10. C. chinense Stem Extract and NP-Induced Cell Cycle Arrest
3. Discussion
4. Materials and Methods
4.1. Preparation of C. chinense Stem Extract
4.2. Identification and Quantification of Bioactive Compounds in C. chinense Using High-Performance Liquid Chromatography
4.3. Formulation of C. chinense Stem Extract Nanoparticles
4.4. Determination of Total Phenolic Content (TPC) in C. chinense Stem Extract and NPs
4.5. Determination of Total Flavonoid Content (TFC) in C. chinense Stem Extract and NPs
4.6. Antioxidant Activities of C. chinense Stem Extract and Nanoparticles
4.6.1. DPPH Free Radical Scavenging Activity Assay
4.6.2. ABTS Free Radical Scavenging Activity Assay
4.6.3. Ferric-Reducing Antioxidant Power (FRAP) Assay
4.7. Cell Proliferation Assay Using a Sulforhodamine B (SRB) Assay
4.8. Colony Formation Assay
4.9. Annexin V/PI Apoptosis Detection Assay
4.10. Mitochondrial Membrane Potential (MMP) Assay
4.11. Cell Cycle Arrest Assay
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A549 | Human lung carcinoma |
| ABTS | 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| ANOVA | Analysis of variance |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| EGCG | Epigallocatechin gallate |
| FRAP | Ferric-Reducing Antioxidant Power |
| GAE | Gallic acid equivalent |
| HeLa | Human epithelial cervical cancer |
| HPLC | High-performance liquid chromatography |
| JC-1 | 5,5,6,6-Tetrachloro-1,1,3,3-tetraethylbenzimidazolylcarbocyanine iodide |
| MCF-7 | Human breast adenocarcinoma |
| MMP | Mitochondrial membrane potential |
| NAPIs | Natural active pharmaceutical ingredients |
| NPs | Nanoparticles |
| PDI | Polydispersity index |
| QE | Quercetin equivalent |
| RP | Reversed Phase |
| SRB | Sulforhodamine B |
| TEM | Transmission electron microscopy |
| TFC | Total flavonoid content |
| TPC | Total phenolic content |
| ZTP | Ziyang green tea |
References
- Debela, D.T.; Muzazu, S.G.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New approaches and procedures for cancer treatment: Current perspectives. SAGE Open Med. 2021, 9, 20503121211034366. [Google Scholar] [CrossRef]
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef]
- Williams, A.; Moo, T.-A. The Impact of Socioeconomic Status and Social Determinants of Health on Disparities in Breast Cancer Incidence, Treatment, and Outcomes. Curr. Breast Cancer Rep. 2023, 15, 30–36. [Google Scholar] [CrossRef]
- Geyer, F.C.; Lopez-Garcia, M.A.; Lambros, M.B.; Reis-Filho, J.S. Genetic characterization of breast cancer and implications for clinical management. J. Cell. Mol. Med. 2009, 13, 4090–4103. [Google Scholar] [CrossRef]
- Elenbaas, B.; Spirio, L.; Koerner, F.; Fleming, M.D.; Zimonjic, D.B.; Donaher, J.L.; Popescu, N.C.; Hahn, W.C.; Weinberg, R.A. Human breast cancer cells generated by oncogenic transformation of primary mammary epithelial cells. Genes Dev. 2001, 15, 50–65. [Google Scholar] [CrossRef]
- Dhyani, P.; Quispe, C.; Sharma, E.; Bahukhandi, A.; Sati, P.; Attri, D.C.; Szopa, A.; Sharifi-Rad, J.; Docea, A.O.; Mardare, I.; et al. Anticancer potential of alkaloids: A key emphasis to colchicine, vinblastine, vincristine, vindesine, vinorelbine and vincamine. Cancer Cell Int. 2022, 22, 206. [Google Scholar] [CrossRef] [PubMed]
- Greenwell, M.; Rahman, P.K. Medicinal Plants: Their Use in Anticancer Treatment. Int. J. Pharm. Sci. Res. 2015, 6, 4103–4112. [Google Scholar] [CrossRef]
- Ganesan, K.; Du, B.; Chen, J. Effects and mechanisms of dietary bioactive compounds on breast cancer prevention. Pharmacol. Res. 2022, 178, 105974. [Google Scholar] [CrossRef] [PubMed]
- Mileo, A.M.; Miccadei, S. Polyphenols as Modulator of Oxidative Stress in Cancer Disease: New Therapeutic Strategies. Oxid. Med. Cell. Longev. 2016, 2016, 6475624. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, P.B.; Ha, S.E.; Vetrivel, P.; Kim, H.H.; Kim, S.M.; Kim, G.S. Functions of polyphenols and its anticancer properties in biomedical research: A narrative review. Transl. Cancer Res. 2020, 9, 7619–7631. [Google Scholar] [CrossRef]
- Cháirez-Ramírez, M.H.; de la Cruz-López, K.G.; García-Carrancá, A. Polyphenols as Antitumor Agents Targeting Key Players in Cancer-Driving Signaling Pathways. Front. Pharmacol. 2021, 12, 710304. [Google Scholar] [CrossRef] [PubMed]
- Chojnacka, K.; Lewandowska, U. The Antiangiogenic Activity of Polyphenol-Rich Extracts and Its Implication on Cancer Chemoprevention. Food Rev. Int. 2020, 36, 77–103. [Google Scholar] [CrossRef]
- Bag, N.; Bag, A. Antimetastatic Properties of Tea Polyphenols. Nutr. Cancer 2020, 72, 365–376. [Google Scholar] [CrossRef]
- Azqueta, A.; Collins, A. Polyphenols and DNA Damage: A Mixed Blessing. Nutrients 2016, 8, 785. [Google Scholar] [CrossRef]
- Kar, P.; Goyal, A.; Das, A.; Sen, A. Antioxidant and pharmaceutical potential of Clerodendrum L.: An overview. Int. J. Green Pharm. 2014, 8, 210–216. [Google Scholar] [CrossRef]
- Qi, J.; Zhang, Y.; Liu, Q.; Liu, H.; Fan, Y.; Yue, J. Clerodenoids A—F: C-ring Aromatized and/or Rearranged Abietane Diterpenoids from Clerodendrum chinense var. simplex. Chin. J. Chem. 2021, 39, 1891–1897. [Google Scholar] [CrossRef]
- Barung, E.N.; Kalonio, D.E.; Banne, Y.; Kambuno, N.T. Anticancer Activities of Sesewanua Leaf Extracts (Clerodendrum fragrans (Vent.) Willd) Against A549 Lung Cancer Cell. Open Access Maced. J. Med. Sci. 2021, 9, 1226–1230. [Google Scholar] [CrossRef]
- Chittasupho, C.; Samee, W.; Tadtong, S.; Jittachai, W.; Managit, C.; Athikomkulchai, S. Cytotoxicity, Apoptosis Induction, Oxidative Stress, and Cell Cycle Arrest of Clerodendrum chinense Flower Extract Nanoparticles in HeLa Cells. Nat. Life Sci. Commun. 2023, 22, e2023057. [Google Scholar] [CrossRef]
- Chittasupho, C.; Athikomkulchai, S.; Samee, W.; Na Takuathung, M.; Yooin, W.; Sawangrat, K.; Saenjum, C. Phenylethanoid Glycoside-Enriched Extract Prepared from Clerodendrum chinense Leaf Inhibits A549 Lung Cancer Cell Migration and Apoptosis Induction through Enhancing ROS Production. Antioxidants 2023, 12, 461. [Google Scholar] [CrossRef]
- Gavas, S.; Quazi, S.; Karpiński, T.M. Nanoparticles for Cancer Therapy: Current Progress and Challenges. Nanoscale Res. Lett. 2021, 16, 173. [Google Scholar] [CrossRef]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef]
- Payne, A.C.; Mazzer, A.; Clarkson, G.J.; Taylor, G. Antioxidant assays—Consistent findings from FRAP and ORAC reveal a negative impact of organic cultivation on antioxidant potential in spinach but not watercress or rocket leaves. Food Sci. Nutr. 2013, 1, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Funes, L.; Fernández-Arroyo, S.; Laporta, O.; Pons, A.; Roche, E.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Micol, V. Correlation between plasma antioxidant capacity and verbascoside levels in rats after oral administration of lemon verbena extract. Food Chem. 2009, 117, 589–598. [Google Scholar] [CrossRef]
- Tang, Y.; Zhao, D.Y.; Elliott, S.; Zhao, W.; Curiel, T.J.; Beckman, B.S.; Burow, M.E. Epigallocatechin-3 gallate induces growth inhibition and apoptosis in human breast cancer cells through survivin suppression. Int. J. Oncol. 2007, 31, 705–711. [Google Scholar] [CrossRef]
- Patel, V.A.; Longacre, A.; Hsiao, K.; Fan, H.; Meng, F.; Mitchell, J.E.; Rauch, J.; Ucker, D.S.; Levine, J.S. Apoptotic cells, at all stages of the death process, trigger characteristic signaling events that are divergent from and dominant over those triggered by necrotic cells: Implications for the delayed clearance model of autoimmunity. J. Biol. Chem. 2006, 281, 4663–4670. [Google Scholar] [CrossRef]
- Khan, H.; Zubair, H.; Ullah, M.F.; Ahmad, A.; Hadi, S. A Prooxidant Mechanism for the Anticancer and Chemopreventive Properties of Plant Polyphenols. Curr. Drug Targets 2012, 13, 1738–1749. [Google Scholar] [CrossRef] [PubMed]
- Rajashekar, C. Dual Role of Plant Phenolic Compounds as Antioxidants and Prooxidants. Am. J. Plant Sci. 2023, 14, 15–28. [Google Scholar] [CrossRef]
- Şenol, H.; Tulay, P.; Ergören, M.; Hanoğlu, A.; Çalış, İ.; Mocan, G. Cytotoxic Effects of Verbascoside on MCF-7 and MDA-MB-231. Turk. J. Pharm. Sci. 2021, 18, 637–644. [Google Scholar] [CrossRef]
- Delazar, A.; Asnaashari, S.; Nikkhah, E.; Asgharian, P. Phytochemical analysis and antiproliferative activity of the aerial parts of Scrophularia subaphylla. Res. Pharm. Sci. 2019, 14, 263–272. [Google Scholar] [CrossRef]
- Zhou, L.; Feng, Y.; Jin, Y.; Liu, X.; Sui, H.; Chai, N.; Chen, X.; Liu, N.; Ji, Q.; Wang, Y.; et al. Verbascoside promotes apoptosis by regulating HIPK2-p53 signaling in human colorectal cancer. BMC Cancer 2014, 14, 747. [Google Scholar] [CrossRef]
- Chen, R.C.; Su, J.H.; Yang, S.M.; Li, J.; Wang, T.J.; Zhou, H. Effect of isoverbascoside, a phenylpropanoid glycoside antioxidant, on proliferation and differentiation of human gastric cancer cell. Acta Pharmacol. Sin. 2002, 23, 997–1001. [Google Scholar]
- Zhang, Z.; Feng, Y.; Li, Z.Y.; Cao, X.Z. Antiproliferative and apoptotic activity of glycyrrhizinic acid in MCF-7 human breast cancer cells and evaluation of its effect on cell cycle, cell migration and m-TOR/PI3K/Akt signalling pathway. Arch. Med. Sci. 2019, 15, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Yu, J.L.; Sun, D.Q.; Kong, F.; Qu, X.J.; Zou, W.; Wu, J.; Wang, R.M. Curcumin induces apoptosis in SGC-7901 gastric adenocarcinoma cells via regulation of mitochondrial signaling pathways. Asian Pac. J. Cancer Prev. 2014, 15, 3987–3992. [Google Scholar] [CrossRef]
- Lin, J.W.; Chen, J.T.; Hong, C.Y.; Lin, Y.L.; Wang, K.T.; Yao, C.J.; Lai, G.M.; Chen, R.M. Honokiol traverses the blood-brain barrier and induces apoptosis of neuroblastoma cells via an intrinsic bax-mitochondrion-cytochrome c-caspase protease pathway. Neuro Oncol. 2012, 14, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Lu, J.L.; Liang, Y.R.; Li, Q.S. Suppressive Effects of EGCG on Cervical Cancer. Molecules 2018, 23, 2334. [Google Scholar] [CrossRef]
- Qanungo, S.; Das, M.; Haldar, S.; Basu, A. Epigallocatechin-3-gallate induces mitochondrial membrane depolarization and caspase-dependent apoptosis in pancreatic cancer cells. Carcinogenesis 2005, 26, 958–967. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Qu, H.; Wu, Y.; Wang, Z.; Wang, L. Study on antitumor, antioxidant and immunoregulatory activities of the purified polyphenols from pinecone of Pinus koraiensis on tumor-bearing S180 mice in vivo. Int. J. Biol. Macromol. 2017, 94, 735–744. [Google Scholar] [CrossRef]
- Li, W.; He, N.; Tian, L.; Shi, X.; Yang, X. Inhibitory effects of polyphenol-enriched extract from Ziyang tea against human breast cancer MCF-7 cells through reactive oxygen species-dependent mitochondria molecular mechanism. J. Food Drug Anal. 2016, 24, 527–538. [Google Scholar] [CrossRef]
- Zhao, Q.; Huo, X.C.; Sun, F.D.; Dong, R.Q. Polyphenol-rich extract of Salvia chinensis exhibits anticancer activity in different cancer cell lines, and induces cell cycle arrest at the G0/G1-phase, apoptosis and loss of mitochondrial membrane potential in pancreatic cancer cells. Mol. Med. Rep. 2015, 12, 4843–4850. [Google Scholar] [CrossRef]
- Ou, Y.; Zhang, Y.; Zhu, X.; Zhu, D. Verbascoside induces the cell cycle arrest and apoptosis of breast cancer via suppression of the PI3K/AKT signaling pathway. Res. Sq. 2022. preprint. [Google Scholar] [CrossRef]
- Sciandra, F.; Bottoni, P.; De Leo, M.; Braca, A.; Brancaccio, A.; Bozzi, M. Verbascoside Elicits Its Beneficial Effects by Enhancing Mitochondrial Spare Respiratory Capacity and the Nrf2/HO-1 Mediated Antioxidant System in a Murine Skeletal Muscle Cell Line. Int. J. Mol. Sci. 2023, 24, 15276. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, L.; Yang, G.; Shi, H.; Jiang, C.; Liu, W.; Zhang, Y. Study on the determination of polyphenols in tobacco by HPLC coupled with ESI-MS after solid-phase extraction. J. Chromatogr. Sci. 2003, 41, 36–40. [Google Scholar] [CrossRef]
- Tunit, P.; Thammarat, P.; Okonogi, S.; Chittasupho, C. Hydrogel Containing Borassus flabellifer L. Male Flower Extract for Antioxidant, Antimicrobial, and Anti-Inflammatory Activity. Gels 2022, 8, 126. [Google Scholar] [CrossRef]
- Chittasupho, C.; Chaobankrang, K.; Sarawungkad, A.; Samee, W.; Singh, S.; Hemsuwimon, K.; Okonogi, S.; Kheawfu, K.; Kiattisin, K.; Chaiyana, W. Antioxidant, Anti-Inflammatory and Attenuating Intracellular Reactive Oxygen Species Activities of Nicotiana tabacum var. Virginia Leaf Extract Phytosomes and Shape Memory Gel Formulation. Gels 2023, 9, 78. [Google Scholar] [CrossRef] [PubMed]
- Athikomkulchai, S.; Tunit, P.; Tadtong, S.; Jantrawut, P.; Sommano, S.R.; Chittasupho, C. Moringa oleifera Seed Oil For-mulation Physical Stability and Chemical Constituents for Enhancing Skin Hydration and Antioxidant Activity. Cosmetics 2021, 8, 2. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]







| Assay | Total Phenolic Content (Gallic Acid Equivalent) | Total Flavonoid Content (Quercetin Equivalent) | ||
|---|---|---|---|---|
| C. chinense Extract | C. chinense Extract NPs | C. chinense Extract | C. chinense Extract NPs | |
| DPPH assay | 0.9515 * | 0.9996 **** | 0.8734 | 0.9982 **** |
| ABTS assay | 0.9740 ** | 0.9824 **** | 0.9997 **** | 0.8877 * |
| FRAP assay | 0.9789 ** | 0.9992 **** | 0.9998 **** | 0.9983 **** |
| IC50 Value | Selectivity Index | |||||
|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | |
| C. chinense stem extract | ||||||
| MCF-7 | 430.4 | 210.3 | 109.2 | 1.3 | 1.7 | 3.0 |
| HeLa | 499.4 | 213.5 | 155.6 | 1.1 | 1.7 | 2.1 |
| A549 | 733.4 | 320.5 | 206.9 | 0.8 | 1.1 | 1.6 |
| SKOV-3 | 1501 | 486.4 | 423 | 0.4 | 0.7 | 0.8 |
| C2CL12 | 558.7 | 355.3 | 329.2 | - | - | - |
| C. chinense stem extract NPs | ||||||
| MCF-7 | 2329 | 2150 | 1664 | 4.1 | 1.3 | 1.1 |
| A549 | 4960 | 2673 | 1363 | 1.9 | 1.0 | 1.4 |
| SKOV-3 | 10,201 | 3079 | 2044 | 0.9 | 0.9 | 0.9 |
| C2CL12 | 9528 | 2799 | 1905 | - | - | - |
| Groups of Treatment | Viable Cells (%) | Late Apoptosis (%) | Necrosis (%) |
|---|---|---|---|
| Control | 93.30 ± 0.26 | 3.27 ± 0.17 | 2.17 ± 0.03 |
| Extract 100 µg/mL | 52.43 ± 0.07 * | 14.07 ± 0.57 * | 31.30 ± 0.66 * |
| Extract 250 µg/mL | 56.97 ± 0.58 * | 14.77 ± 0.44 * | 27.17 ± 0.80 * |
| Extract 500 µg/mL | 16.13 ± 0.50 * | 12.13 ± 0.80 * | 71.53 ± 1.27 * |
| NPs 250 µg/mL | 39.57 ± 0.87 * | 26.73 ± 3.89 * | 32.23 ± 4.73 * |
| NPs 500 µg/mL | 14.27 ± 0.26 * | 25.57 ± 0.57 * | 59.30 ± 0.81 * |
| NPs 1000 µg/mL | 6.10 ± 0.12 * | 40.13 ± 1.00 * | 52.07 ± 0.83 * |
| Groups of Treatment | JC-1 Aggregates | JC-1 Monomers | Ratio of Monomers to Aggregates |
|---|---|---|---|
| Control | 91.07 ± 0.19 | 6.07 ± 0.22 | 0.067 |
| Extract 100 µg/mL | 92.47 ± 0.35 | 5.83 ± 0.34 | 0.063 |
| Extract 250 µg/mL | 87.27 ± 3.03 * | 10.67 ± 2.75 * | 0.130 |
| Extract 500 µg/mL | 79.87 ± 4.82 * | 15.97 ± 4.09 * | 0.200 |
| NPs 250 µg/mL | 82.27 ± 3.82 * | 15.23 ± 3.82 * | 0.185 |
| NPs 500 µg/mL | 77.97 ± 0.61 * | 18.17 ± 0.38 * | 0.233 |
| NPs 1000 µg/mL | 38.37 ± 1.53 * | 52.90 ± 1.10 * | 1.379 |
| Groups of Treatment | G0/G1 Phase | S Phase | G2/M Phase |
|---|---|---|---|
| Control | 64.63 ± 0.44 | 15.57 ± 0.24 | 17.53 ± 0.55 |
| Extract 100 µg/mL | 66.53 ± 0.23 * | 14.97 ± 0.03 | 15.97 ± 0.19 * |
| Extract 250 µg/mL | 66.57 ± 0.24 * | 19.50 ± 0.15 * | 10.87 ± 0.09 * |
| Extract 500 µg/mL | 71.40 ± 0.56 * | 15.97 ± 0.34 | 8.90 ± 0.26 * |
| NPs 250 µg/mL | 66.57 ± 0.38 * | 13.87 ± 0.13 * | 15.47 ± 0.30 * |
| NPs 500 µg/mL | 74.63 ± 0.28 * | 11.77 ± 0.52 * | 9.63 ± 0.09 * |
| NPs 1000 µg/mL | 67.87 ± 0.03 * | 13.50 ± 0.15 * | 11.77 ± 0.29 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chittasupho, C.; Samee, W.; Na Takuathung, M.; Okonogi, S.; Nimkulrat, S.; Athikomkulchai, S. Clerodendrum chinense Stem Extract and Nanoparticles: Effects on Proliferation, Colony Formation, Apoptosis Induction, Cell Cycle Arrest, and Mitochondrial Membrane Potential in Human Breast Adenocarcinoma Breast Cancer Cells. Int. J. Mol. Sci. 2024, 25, 978. https://doi.org/10.3390/ijms25020978
Chittasupho C, Samee W, Na Takuathung M, Okonogi S, Nimkulrat S, Athikomkulchai S. Clerodendrum chinense Stem Extract and Nanoparticles: Effects on Proliferation, Colony Formation, Apoptosis Induction, Cell Cycle Arrest, and Mitochondrial Membrane Potential in Human Breast Adenocarcinoma Breast Cancer Cells. International Journal of Molecular Sciences. 2024; 25(2):978. https://doi.org/10.3390/ijms25020978
Chicago/Turabian StyleChittasupho, Chuda, Weerasak Samee, Mingkwan Na Takuathung, Siriporn Okonogi, Sathaporn Nimkulrat, and Sirivan Athikomkulchai. 2024. "Clerodendrum chinense Stem Extract and Nanoparticles: Effects on Proliferation, Colony Formation, Apoptosis Induction, Cell Cycle Arrest, and Mitochondrial Membrane Potential in Human Breast Adenocarcinoma Breast Cancer Cells" International Journal of Molecular Sciences 25, no. 2: 978. https://doi.org/10.3390/ijms25020978
APA StyleChittasupho, C., Samee, W., Na Takuathung, M., Okonogi, S., Nimkulrat, S., & Athikomkulchai, S. (2024). Clerodendrum chinense Stem Extract and Nanoparticles: Effects on Proliferation, Colony Formation, Apoptosis Induction, Cell Cycle Arrest, and Mitochondrial Membrane Potential in Human Breast Adenocarcinoma Breast Cancer Cells. International Journal of Molecular Sciences, 25(2), 978. https://doi.org/10.3390/ijms25020978






