Gap Junction Channel Regulation: A Tale of Two Gates—Voltage Sensitivity of the Chemical Gate and Chemical Sensitivity of the Fast Voltage Gate
Abstract
:1. Introduction
2. Gap Junction Channel Gates
- Cx32: hepatocytes, exocrine pancreas, kidney, myelinated Schwann cells.
- Cx38: Xenopus cells.
- Cx43: heart ventricular and atrial cardiomyocytes, endothelial cells, smooth muscle, and fibroblasts.
- Cx26: liver.
- Cx40: cardiomyocytes of atria and cardiac conduction system (His bundle and upper bundle branches).
- Cx50: lens epithelium, retina (horizontal cells and Müller cells), spinal cord tissue.
- Cx37: brain, uterus, ovary, endothelial cells of blood vessels.
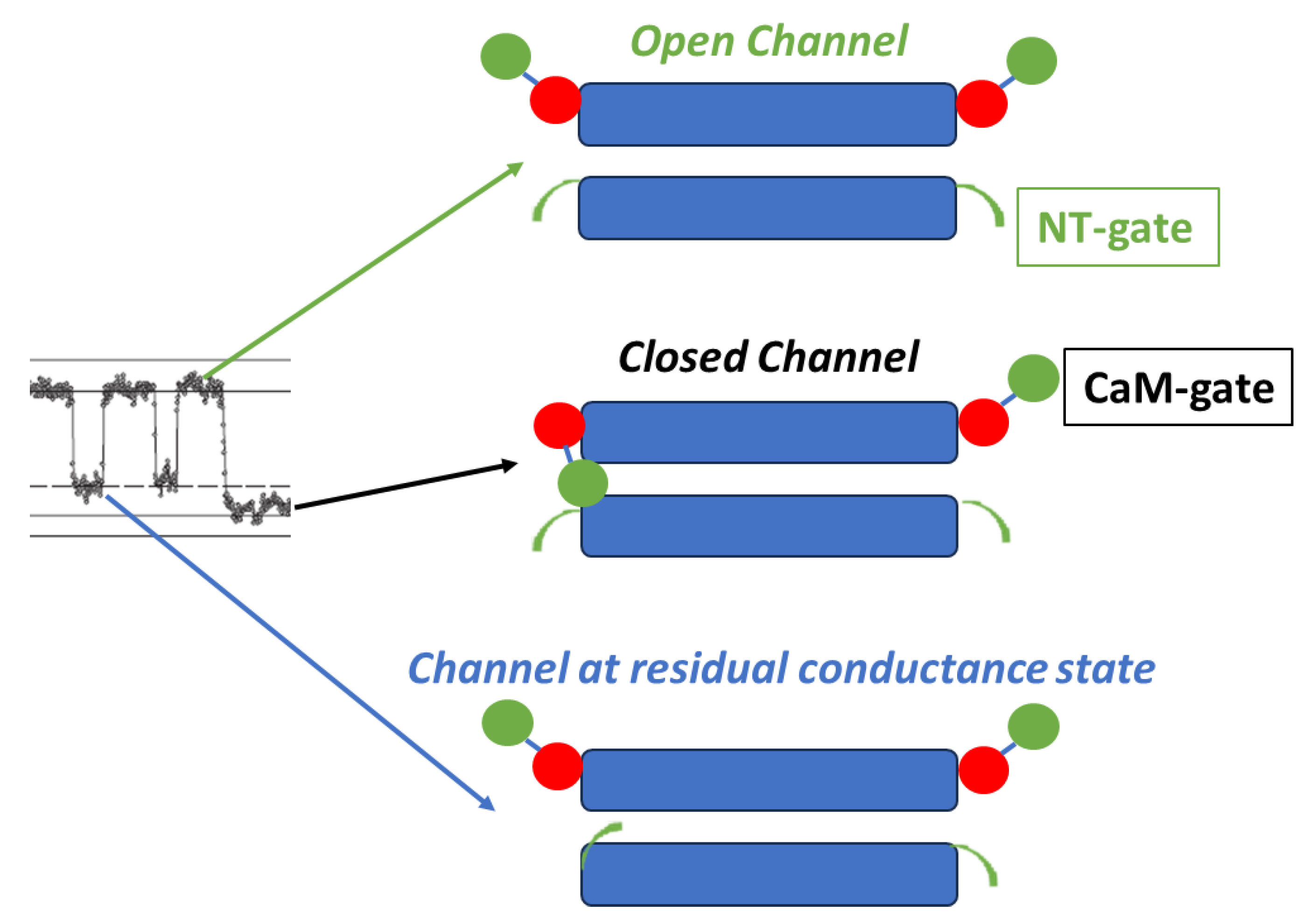
3. Cork Model of Chemical Gating
3.1. Ca-CaM-Cork Gating Model
3.2. CaM-Cork Gating Model
4. The Chemical Gate Is Voltage Sensitive
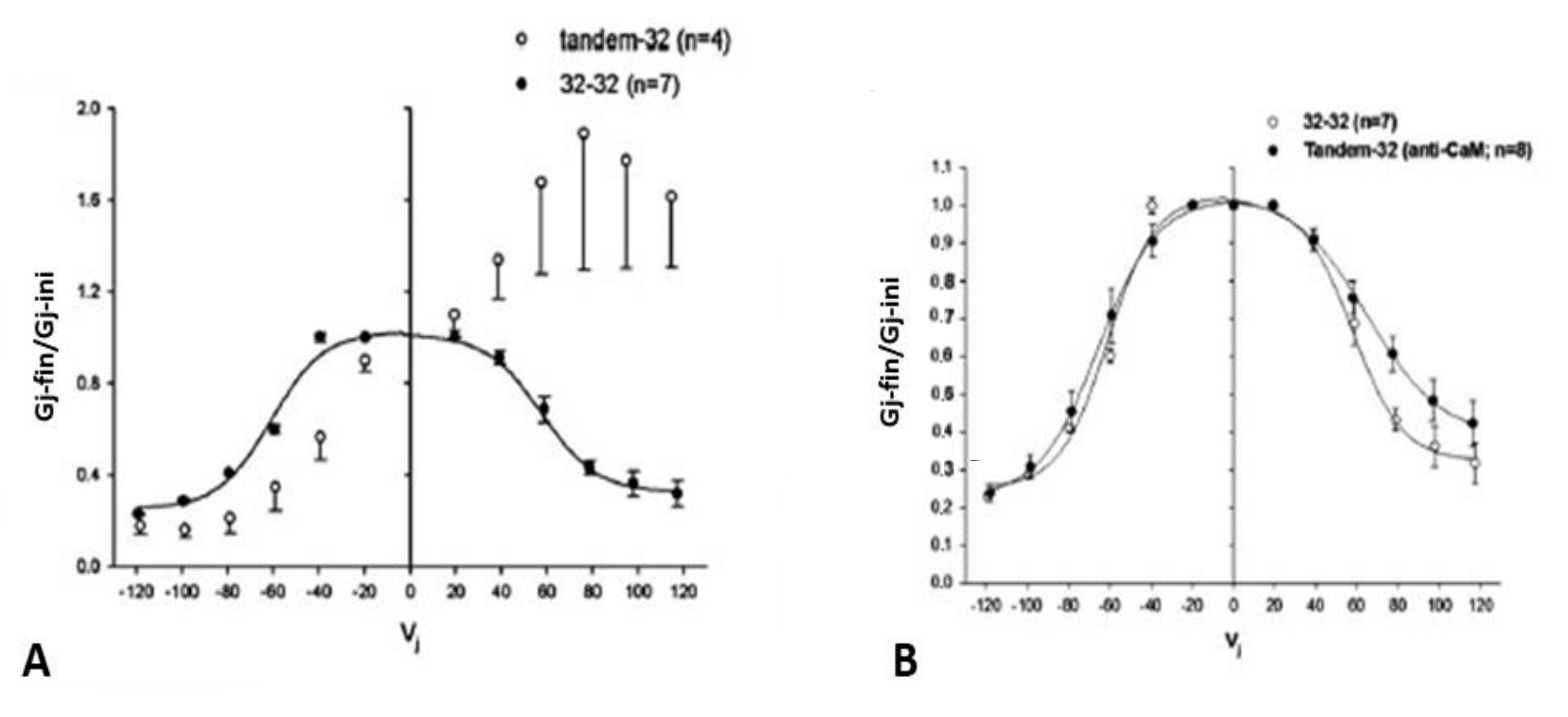
4.1. Vj-Ij Behavior of Heterotypic Tandem-32 Channels
4.2. Behavior of Channels Made of Tandem-32 Subjected to 60 mV Vj Pulses
4.3. Behavior of Channel Made of Tandem-32 Subjected to Steady State Vj
4.4. The Chemical-Gate of Homotypic Cx32 Channels Is Also Sensitive to Vj
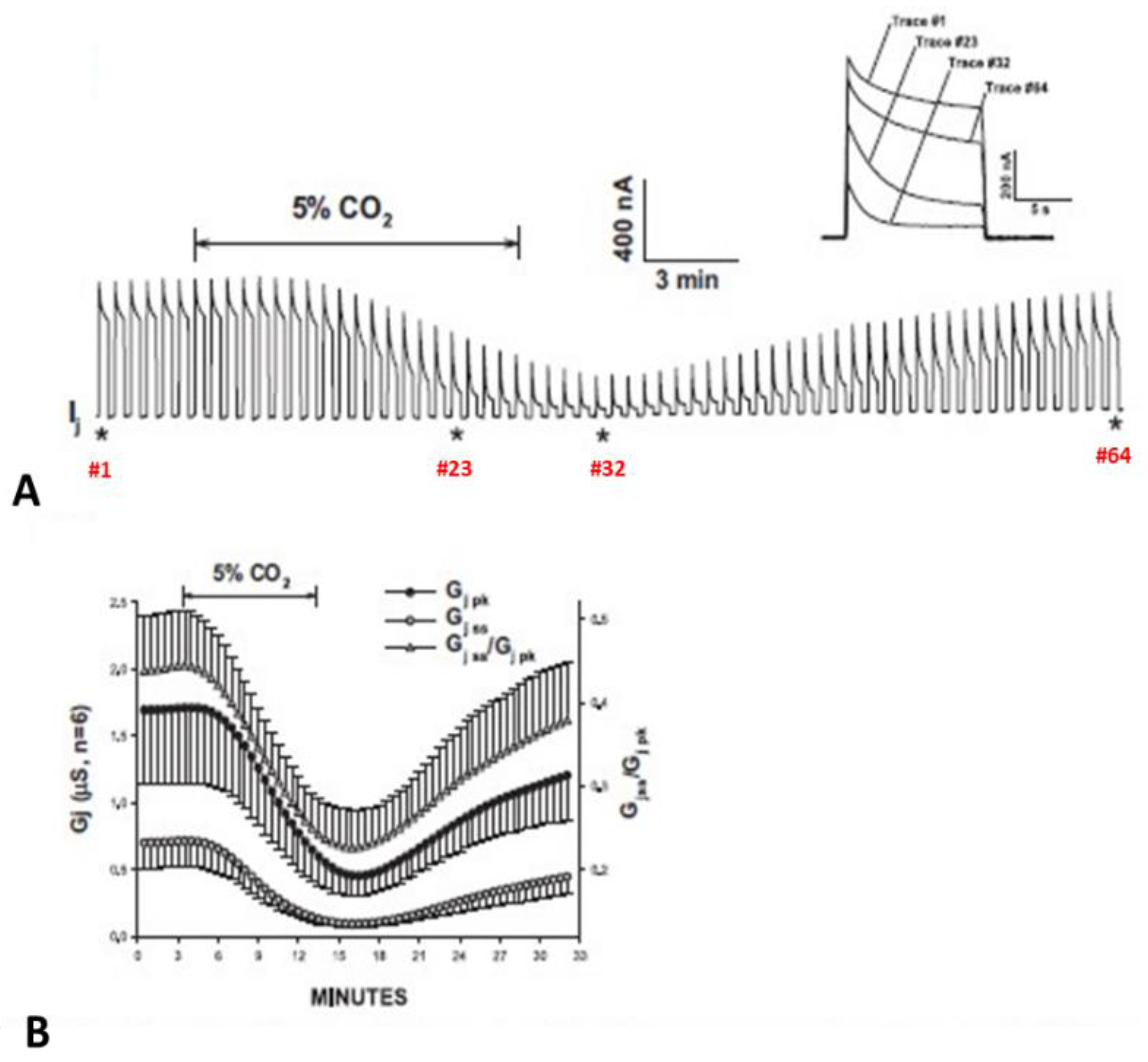
4.5. Effect of Vj Steady-State on Gj during Uncoupling by CO2
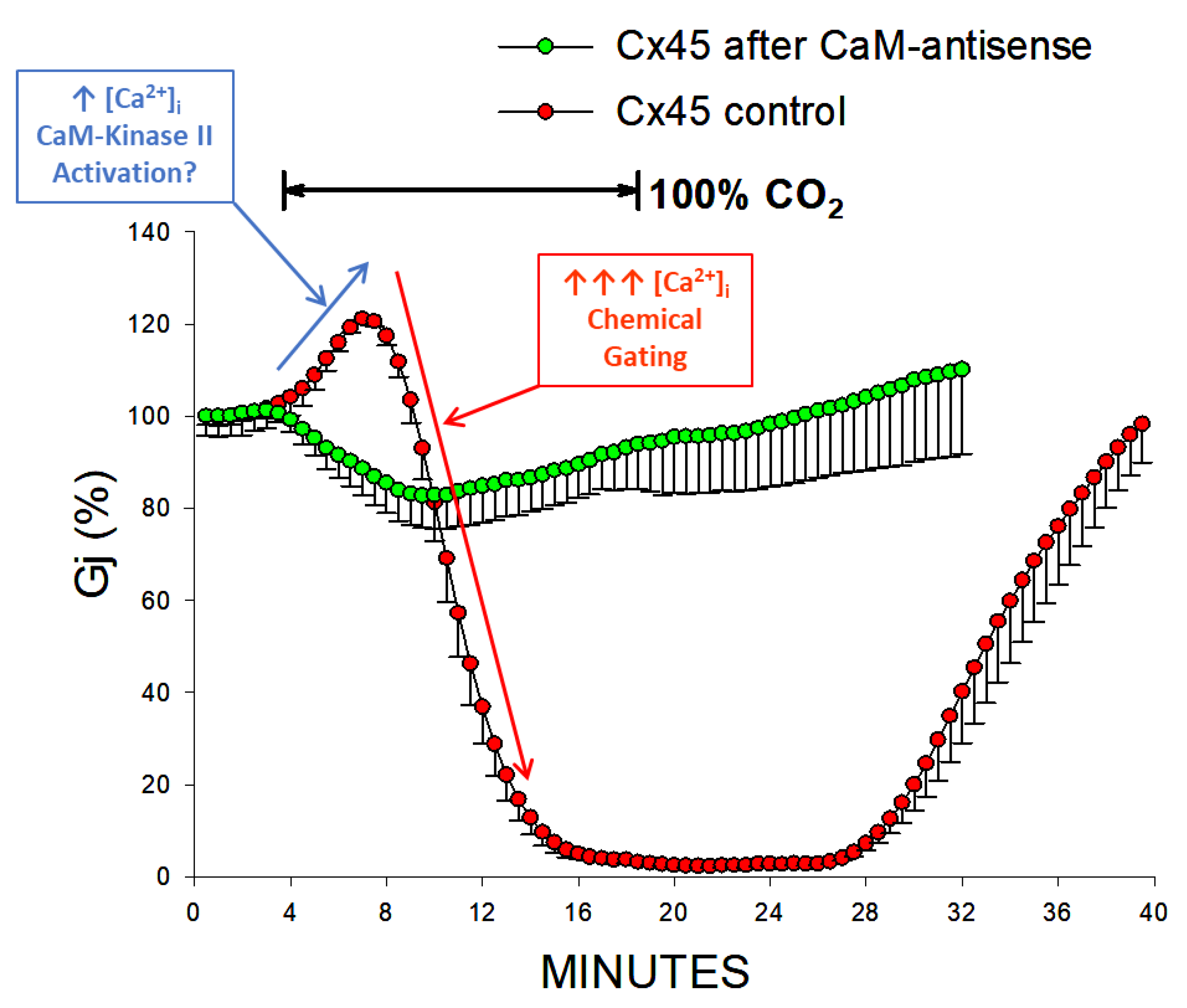
4.6. CaM-Expression Inhibition Affects the Vj-Sensitivity of the Chemical Gate of Tandem-32 Channels
4.7. CaM-Expression Inhibition Affects the Sensitiviti to Vj of the Chemical Gate in Cx45 Channels
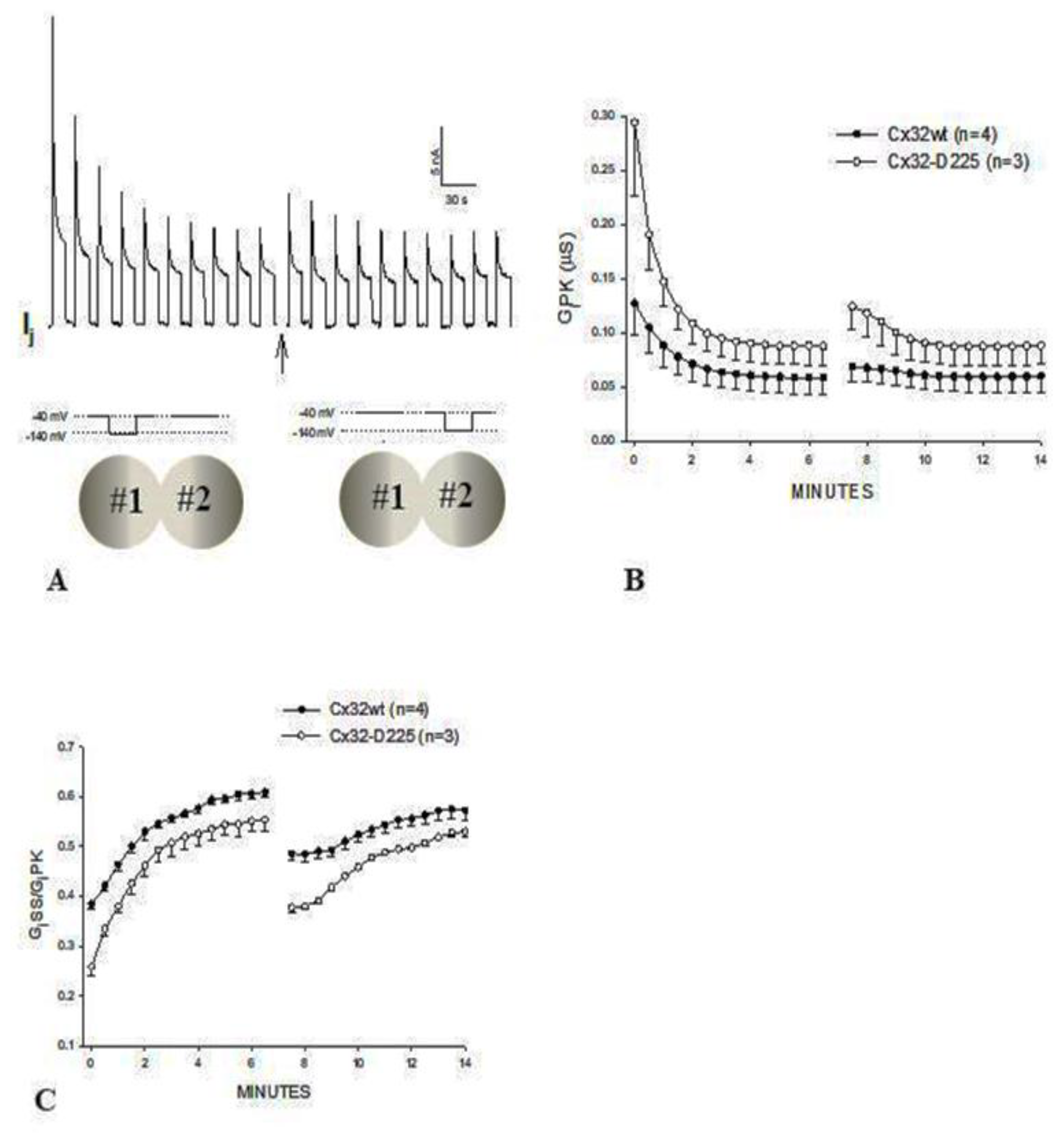
4.8. CaM Mutants Affect Gating Sensitivity and Expression of Cx32 Channels
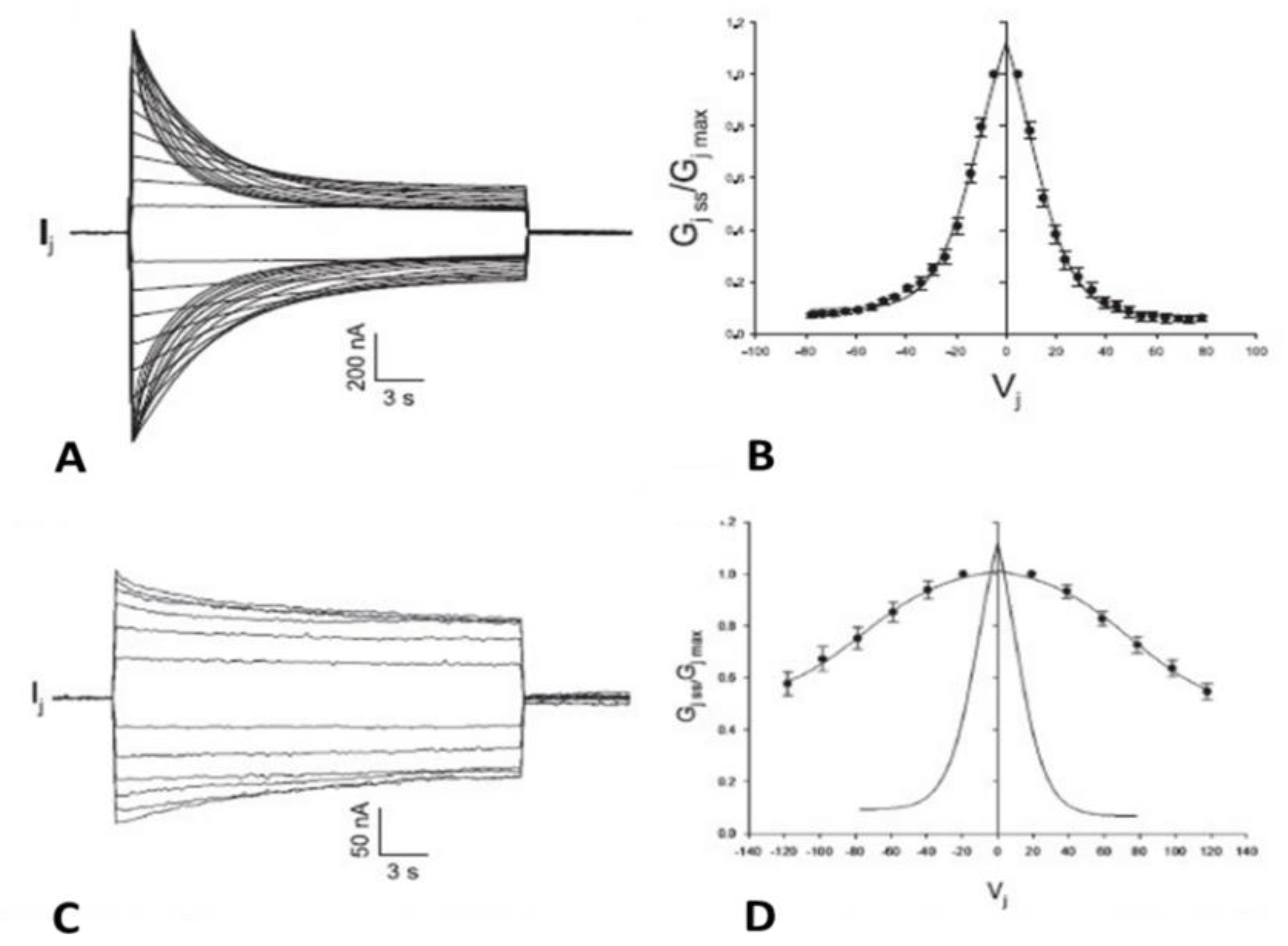
5. Chemical Sensitivity of the Fast-Vj Gate)
5.1. Cytosolic Acidification Inhibits the Vj-Sensitivity of the Fast-Vj Gate of Cx50 (Positive Gater) and Enhances That of the Mutant Cx50-D3N (Negative Gater)
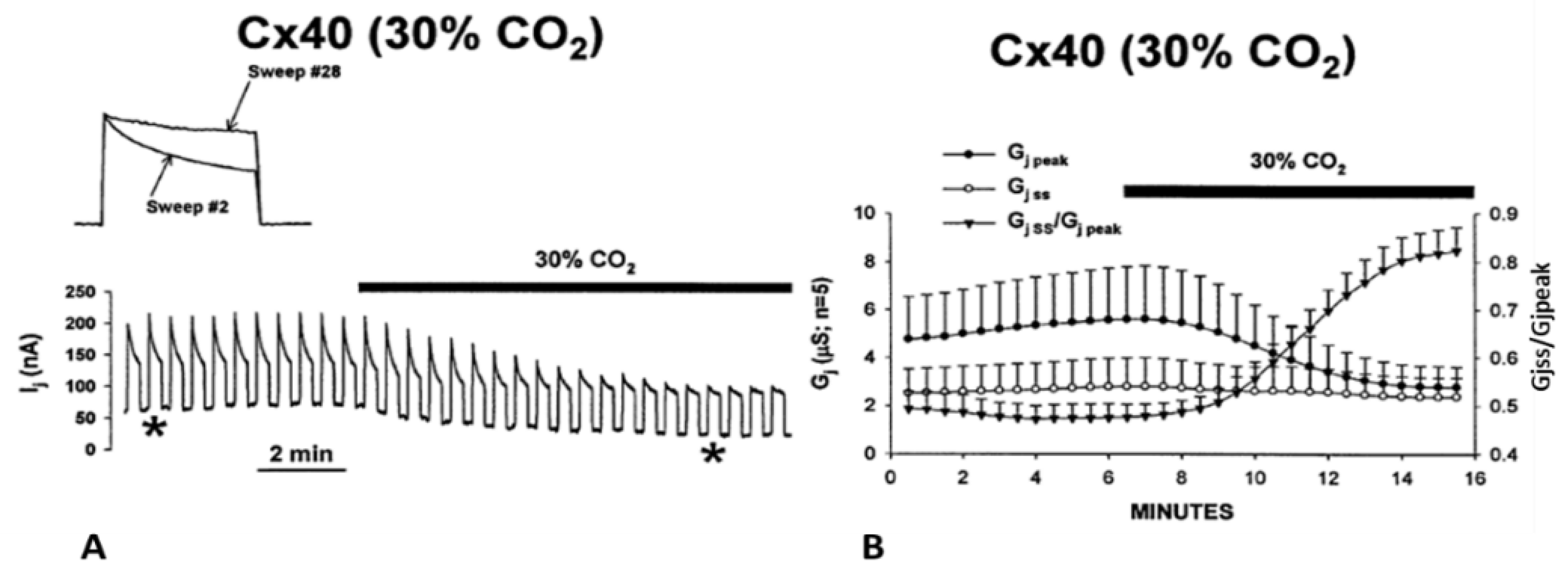
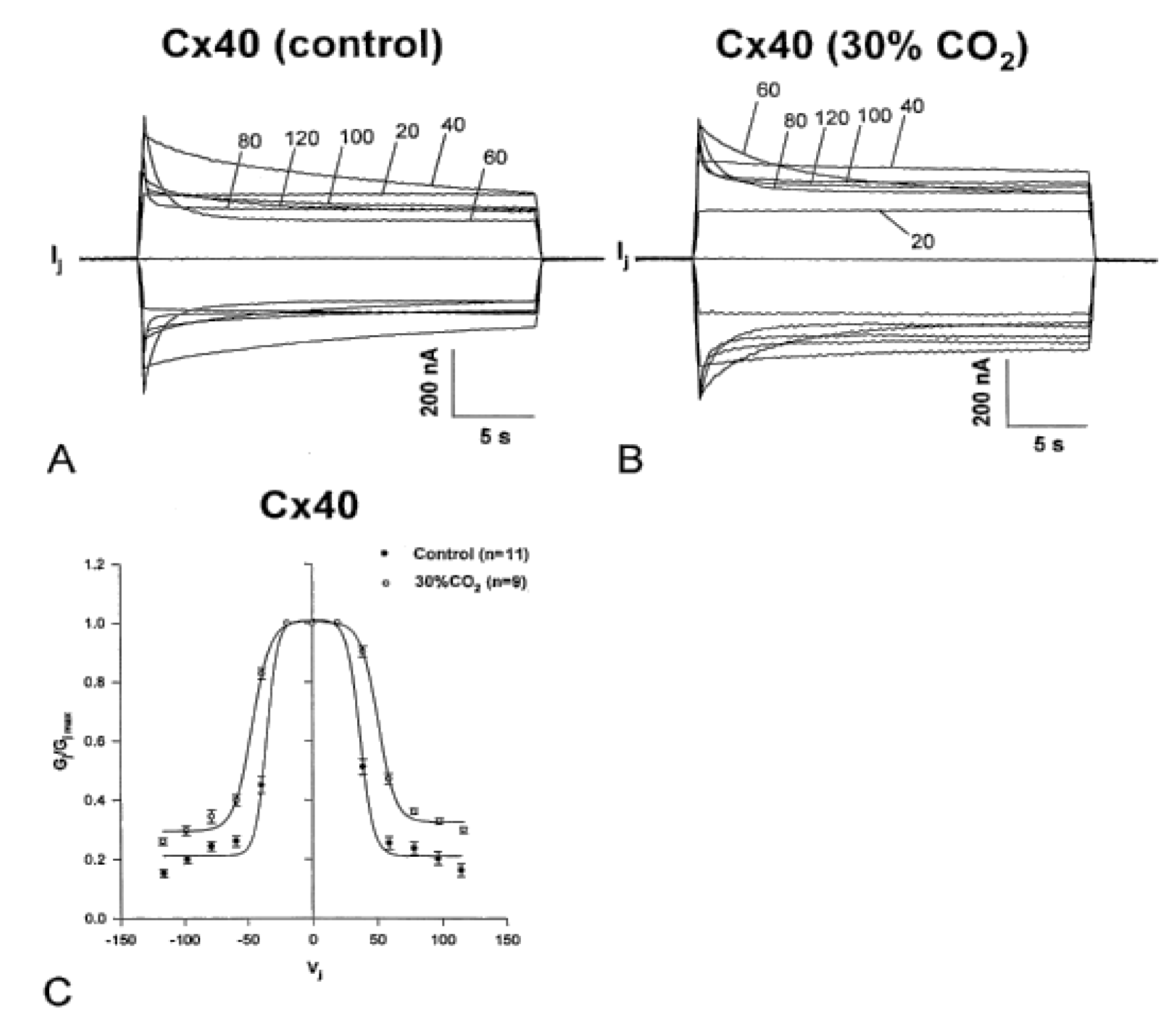
5.2. Cytosolic Acidification Decreases the Vj-Sensitivity of the Fast-Vj Gate of Cx40 (Positive Gater)
5.3. With Acidification the Vj-Sensitivity of the Fast-Vj gate of Cx32 (Negative Gater) Increases and That of Cx26 (Positive Gater) Decreases
5.3.1. Cx32
5.3.2. Cx26
5.3.3. CX32-N2D
6. Summary and Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Engelmann, T.W. Vergleichende Untersuchungen zur Lehre von der Muskel und Nervenelektricität. Pflügers Arch. 1877, 15, 116–148. [Google Scholar] [CrossRef]
- Engelmann, T.W. Hüber die leitung der erregung im herzmuskel. Pflügers Arch. 1875, 11, 465–480. [Google Scholar] [CrossRef]
- Rothschuh, K.E. Über den aufbau des herzmuskels aus “elektrophysiologischen elementen”. Verhandlungen Dtsch. Ges. Pathol. 1950, 16, 226–231. [Google Scholar]
- Rothschuh, K.E. Über den funktionellen aufbaudes herzens aus elektrophysiologischen Elementen und üiber den mechanismus der erregungsleitung im herzen. Pflügers Arch. 1951, 253, 238–251. [Google Scholar] [CrossRef] [PubMed]
- Weidmann, S. The electrical constants of Purkinje fibres. J. Physiol. 1952, 118, 348–360. [Google Scholar] [CrossRef] [PubMed]
- De Mello, W.C.; Motta, G.E.; Chapeau, M. A study on the healing-over of myocardial cells of toads. Circ. Res. 1969, 24, 475–487. [Google Scholar] [CrossRef]
- Loewenstein, W.R. Permeable junctions. In Cold Spring Harbor Symposia on Quantitative Biology; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1975; Volume 40, pp. 49–63. [Google Scholar]
- Bennett, M.V.L. Junctional permeability. In Intercellular Junctions and Synapses; Feldman, J., Gilula, N.B., Pitts, J.D., Eds.; John Wiley and Sons: New York, NY, USA, 1978; pp. 25–36. [Google Scholar]
- De Mello, W.C. Intercellular communication in cardiac muscle. Circ. Res. 1982, 51, 1–9. [Google Scholar] [CrossRef]
- Peracchia, C. Chemical gating of gap junction channels; roles of calcium, pH and calmodulin. Biochim. Biophys. Acta 2004, 1662, 61–80. [Google Scholar] [CrossRef]
- Délèze, J. Calcium ions and the healing over of heart fibers. In Electrophysiology of the Heart; Taccardi, B., Marchetti, C., Eds.; Pergamon Press: Oxford, UK, 1965; pp. 147–148. [Google Scholar]
- Noma, A.; Tsuboi, N. Dependence of junctional conductance on proton, calcium and magnesium ions in cardiac paired cells of guinea-pig. J. Physiol. 1987, 382, 193–211. [Google Scholar] [CrossRef]
- Noma, A.; Tsuboi, N. Direct measurement of the gap junctional conductance under the influence of Ca2+ in dissociated paired myocytes of guinea-pig. Jpn. Heart J. 1986, 27 (Suppl. 1), 161–166. [Google Scholar]
- Kanno, Y.; Loewenstein, W.R. Low-resistance coupling between gland cells. Some observations on intercellular contact membranes and intercellular space. Nature 1964, 201, 194–195. [Google Scholar] [CrossRef] [PubMed]
- Loewenstein, W.R.; Kanno, Y. Studies on an epithelial (gland) cell junction. i. modifications of surface membrane permeability. J. Cell Biol. 1964, 22, 565–586. [Google Scholar] [CrossRef] [PubMed]
- Rose, B.; Loewenstein, W.R. Permeability of cell junction depends on local cytoplasmic calcium activity. Nature 1975, 254, 250–252. [Google Scholar] [CrossRef] [PubMed]
- Rose, B.; Loewenstein, W.R. Permeability of a cell junction and the local cytoplasmic free ionized calcium concentration: A study with aequorin. J. Membr. Biol. 1976, 28, 87–119. [Google Scholar] [CrossRef] [PubMed]
- Turin, L.; Warner, A. Carbon dioxide reversibly abolishes ionic communication between cells of early amphibian embryo. Nature 1977, 270, 56–57. [Google Scholar] [CrossRef]
- Turin, L.; Warner, A.E. Intracellular pH in early Xenopus embryos: Its effect on current flow between blastomeres. J. Physiol. 1980, 300, 489–504. [Google Scholar] [CrossRef]
- Spray, D.C.; Harris, A.L.; Bennett, M.V. Gap junctional conductance is a simple and sensitive function of intracellular pH. Science 1981, 211, 712–715. [Google Scholar] [CrossRef]
- Arellano, R.O.; Ramón, F.; Rivera, A.; Zampighi, G.A. Lowering of pH does not directly affect the junctional resistance of crayfish lateral axons. J. Membr. Biol. 1986, 94, 293–299. [Google Scholar] [CrossRef]
- Lazrak, A.; Peracchia, C. Gap junction gating sensitivity to physiological internal calcium regardless of pH in Novikoff hepatoma cells. Biophys. J. 1993, 65, 2002–2012. [Google Scholar] [CrossRef]
- Trexler, E.B.; Bukauskas, F.F.; Bennett, M.V.; Bargiello, T.A.; Verselis, V.K. Rapid and direct effects of pH on connexins revealed by the connexin46 hemichannel preparation. J. Gen. Physiol. 1999, 113, 721–742. [Google Scholar] [CrossRef]
- Peracchia, C.; Bernardini, G.; Peracchia, L.L. Is calmodulin involved in the regulation of gap junction permeability? Pflügers Arch. 1983, 399, 152–154. [Google Scholar] [CrossRef] [PubMed]
- Hertzberg, E.L.; Gilula, N.B. Liver gap junctions and lens fiber junctions: Comparative analysis and calmodulin interaction. In Cold Spring Harbor Symposia on Quantitative Biology; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1981; Volume 46, pp. 639–645. [Google Scholar]
- Van Eldik, L.J.; Hertzberg, E.L.; Berdan, R.C.; Gilula, N.B. Interaction of calmodulin and other calcium-modulated proteins with mammalian and arthropod junctional membrane proteins. Biochem. Biophys. Res. Commun. 1985, 126, 825–832. [Google Scholar] [CrossRef]
- Eitelmann, S.E.K.; Petersilie, L.; Rose, C.R.; Stephan, J. Ca2+-dependent rapid uncoupling of astrocytes upon brief metabolic stress. Front. Cell. Neurosci. 2023, 17, 1151608. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Cassara, C.; Lin, X.; Veenstra, R.D. Calcium-calmodulin gating of a pH-insensitive isoform of connexin43 gap junctions. Biochem. J. 2019, 476, 1137–1148. [Google Scholar] [CrossRef]
- Cotrina, M.L.; Kang, J.; Lin, J.H.; Bueno, E.; Hansen, T.W.; He, L.; Liu, Y.; Nedergaard, M. Astrocytic gap junctions remain open during ischemic conditions. J. Neurosci. 1998, 18, 2520–2537. [Google Scholar] [CrossRef] [PubMed]
- Crow, J.M.; Atkinson, M.M.; Johnson, R.G. Micromolar levels of intracellular calcium reduce gap junctional permeability in lens cultures. Investig. Ophthalmol. Vis. Sci. 1994, 35, 3332–3341. [Google Scholar]
- Dahl, G.; Isenberg, G. Decoupling of heart muscle cells: Correlation with increased cytoplasmic calcium activity and with changes of nexus ultrastructure. J. Membr. Biol. 1980, 53, 63–75. [Google Scholar] [CrossRef]
- Dakin, K.; Zhao, Y.; Li, W.H. LAMP, a new imaging assay of gap junctional communication unveils that Ca2+ influx inhibits cell coupling. Nat. Methods 2005, 2, 55–62. [Google Scholar] [CrossRef]
- Dekker, L.R.; Fiolet, J.W.; VanBavel, E.; Coronel, R.; Opthof, T.; Spaan, J.A.; Janse, M.J. Intracellular Ca2+, intercellular electrical coupling, and mechanical activity in ischemic rabbit papillary muscle. Effects of preconditioning and metabolic blockade. Circ. Res. 1996, 79, 237–246. [Google Scholar] [CrossRef]
- Enkvist, M.O.; McCarthy, K.D. Astroglial gap junction communication is increased by treatment with either glutamate or high K+ concentration. J. Neurochem. 1994, 62, 489–495. [Google Scholar] [CrossRef]
- Giaume, C.; Venance, L. Characterization and regulation of gap junction channels in cultured astrocytes. In Gap Junctions in the Nervous System; Spray, D.C., Dermietzel, R., Eds.; R.G Landes Medical Pub.: Austin, TX, USA, 1996; pp. 135–157. [Google Scholar]
- Neyton, J.; Trautmann, A. Acetylcholine modulation of the conductance of intercellular junctions between rat lacrimal cells. J. Physiol. 1986, 377, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Veenstra, R.D.; Dehaan, R.L. Cardiac gap junction channel activity in embryonic chick ventricle cells. Am. J. Physiol. 1988, 254 Pt 2, H170–H180. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Kopp, R.F.; Chen, Y.; Yang, J.J.; Roe, M.W.; Veenstra, R.D. Gating of connexin 43 gap junctions by a cytoplasmic loop calmodulin binding domain. Am. J. Physiol. Cell Physiol. 2012, 302, C1548–C1556. [Google Scholar] [CrossRef] [PubMed]
- Lazrak, A.; Peres, A.; Giovannardi, S.; Peracchia, C. Ca-mediated and independent effects of arachidonic acid on gap junctions and Ca-independent effects of oleic acid and halothane. Biophys. J. 1994, 67, 1052–1059. [Google Scholar] [CrossRef] [PubMed]
- Ramón, F.; Arellano, R.O.; Rivera, A.; Zampighi, G.A. Effects of internally perfused calmodulin on the junctional resistance of crayfish lateral axons. In Gap Junctions; Hertzberg, E.L., Johnson, R.G., Eds.; Alan R. Liss, Inc.: New York, NY, USA, 1988; pp. 255–265. [Google Scholar]
- Arellano, R.O.; Ramón, F.; Rivera, A.; Zampighi, G.A. Calmodulin acts as an intermediary for the effects of calcium on gap junctions from crayfish lateral axons. J. Membr. Biol. 1988, 101, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Török, K.; Stauffer, K.; Evans, W.H. Connexin 32 of gap junctions contains two cytoplasmic calmodulin-binding domains. Biochem. J. 1997, 326 Pt 2, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Dodd, R.; Peracchia, C.; Stolady, D.; Török, K. Calmodulin association with connexin32-derived peptides suggests trans-domain interaction in chemical gating of gap junction channels. J. Biol. Chem. 2008, 283, 26911–26920. [Google Scholar] [CrossRef]
- Tran, O.; Kerruth, S.; Coates, C.; Kaur, H.; Peracchia, C.; Carter, T.; Török, K. Ca2+-Dependent and -Independent Calmodulin Binding to the Cytoplasmic Loop of Gap Junction Connexins. Int. J. Mol. Sci. 2023, 24, 4153. [Google Scholar] [CrossRef]
- Peracchia, C. Calmodulin-Mediated Regulation of Gap Junction Channels. Int. J. Mol. Sci. 2020, 21, 485. [Google Scholar] [CrossRef]
- Evans, W.H.; Martin, P.E. Gap junctions: Structure and function (Review). Mol. Membr. Biol. 2002, 19, 121–136. [Google Scholar] [CrossRef]
- Burr, G.S.; Mitchell, C.K.; Keflemariam, Y.J.; Heidelberger, R.; O’Brien, J. Calcium-dependent binding of calmodulin to neuronal gap junction proteins. Biochem. Biophys. Res. Commun. 2005, 335, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zou, T.; Liu, Y.; Qi, Y. The gating effect of calmodulin and calcium on the connexin50 hemichannel. Biol. Chem. 2006, 387, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Salarian, M.; Chen, Y.; Zhuo, Y.; Brown, N.E.; Hepler, J.R.; Yang, J. Direct Visualization of Interaction between Calmodulin and Connexin45. Biochem. J. 2017, 474, 4035–4051. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Riquelme, M.A.; Gu, S.; Jiang, J.X. Regulation of Connexin Gap Junctions and Hemichannels by Calcium and Calcium Binding Protein Calmodulin. Int. J. Mol. Sci. 2020, 21, 8194. [Google Scholar] [CrossRef] [PubMed]
- Zoidl, G.R.; Spray, D.C. The Roles of Calmodulin and CaMKII in Cx36 Plasticity. Int. J. Mol. Sci. 2021, 22, 4473. [Google Scholar] [CrossRef] [PubMed]
- Peracchia, C.; Sotkis, A.; Wang, X.G.; Peracchia, L.L.; Persechini, A. Calmodulin directly gates gap junction channels. J. Biol. Chem. 2000, 275, 26220–26224. [Google Scholar] [CrossRef] [PubMed]
- Siu, R.C.; Smirnova, E.; Brown, C.A.; Zoidl, C.; Spray, D.C.; Donaldson, L.W.; Zoidl, G. Structural and Functional Consequences of Connexin 36 (Cx36) Interaction with Calmodulin. Front. Mol. Neurosci. 2016, 9, 120. [Google Scholar] [CrossRef]
- Diez, J.A.; Elvira, M.; Villalobo, A. The epidermal growth factor receptor tyrosine kinase phosphorylates connexin32. Mol. Cell. Biochem. 1998, 187, 201–210. [Google Scholar] [CrossRef]
- Myllykoski, M.; Kuczera, K.; Kursula, P. Complex formation between calmodulin and a peptide from the intracellular loop of the gap junction protein connexin43: Molecular conformation and energetics of binding. Biophys. Chem. 2009, 144, 130–135. [Google Scholar] [CrossRef]
- Johnston, M.F.; Simon, S.A.; Ramón, F. Interaction of anaesthetics with electrical synapses. Nature 1980, 286, 498–500. [Google Scholar] [CrossRef]
- Bastide, B.; Herve, J.C.; Cronier, L.; Deleze, J. Rapid onset and calcium independence of the gap junction uncoupling induced by heptanol in cultured heart cells. Pflügers Arch. 1995, 429, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Bastiaanse, E.M.; Jongsma, H.J.; van der Laarse, A.; Takens-Kwak, B.R. Heptanol-induced decrease in cardiac gap junctional conductance is mediated by a decrease in the fluidity of membranous cholesterol-rich domains. J. Membr. Biol. 1993, 136, 135–145. [Google Scholar] [CrossRef]
- Rudisuli, A.; Weingart, R. Electrical properties of gap junction channels in guinea-pig ventricular cell pairs revealed by exposure to heptanol. Pflügers Arch. 1989, 415, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Burt, J.M.; Spray, D.C. Volatile anesthetics block intercellular communication between neonatal rat myocardial cells. Circ. Res. 1989, 65, 829–837. [Google Scholar] [CrossRef]
- Burt, J.M.; Spray, D.C. Single-channel events and gating behavior of the cardiac gap junction channel. Proc. Natl. Acad. Sci. USA 1988, 85, 3431–3434. [Google Scholar] [CrossRef] [PubMed]
- Veenstra, R.D.; DeHaan, R.L. Measurement of single channel currents from cardiac gap junctions. Science 1986, 233, 972–974. [Google Scholar] [CrossRef]
- Bennett, M.V. Physiology of electrotonic junctions. Ann. N. Y. Acad. Sci. 1966, 137, 509–539. [Google Scholar] [CrossRef]
- Harris, A.L. Emerging issues of connexin channels: Biophysics fills the gap. Q. Rev. Biophys. 2001, 34, 325–472. [Google Scholar] [CrossRef]
- Spray, D.C.; Harris, A.L.; Bennett, M.V. Equilibrium properties of a voltage-dependent junctional conductance. J. Gen. Physiol. 1981, 77, 77–93. [Google Scholar] [CrossRef]
- Brink, P. Gap junction voltage dependence. A clear picture emerges. J. Gen. Physiol. 2000, 116, 11–12. [Google Scholar] [CrossRef]
- Bukauskas, F.F.; Verselis, V.K. Gap junction channel gating. Biochim. Biophys. Acta 2004, 1662, 42–60. [Google Scholar] [CrossRef] [PubMed]
- Yeager, M.; Unger, V.M.; Falk, M.M. Synthesis, assembly and structure of gap junction intercellular channels. Curr. Opin. Struct. Biol. 1998, 8, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Peracchia, C.; Wang, X.G.; Peracchia, L.L. Is the chemical gate of connexins voltage sensitive? Behavior of Cx32 wild-type and mutant channels. Am. J. Physiol. 1999, 276 Pt 1, C1361–C1373. [Google Scholar] [CrossRef] [PubMed]
- Klee, C.B.; Vanaman, T.C. Calmodulin. Adv. Prot. Chem. 1982, 35, 213–321. [Google Scholar]
- Harris, A.L.; Locke, D. Connexins—A Guide; Humana Press: Totowa, NJ, USA; Springer: New York, NY, USA, 2009. [Google Scholar]
- Evans, W.H. Cell communication across gap junctions: A historical perspective and current developments. Biochem. Soc. Trans. 2015, 43, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Beyer, E.C.; Berthoud, V.M. Gap junction gene and protein families: Connexins, innexins, and pannexins. Biochim. Biophys. Acta 2018, 1860, 5–8. [Google Scholar] [CrossRef]
- Cruciani, V.; Mikalsen, S.O. The vertebrate connexin family. Cell. Mol. Life Sci. 2006, 63, 1125–1140. [Google Scholar] [CrossRef] [PubMed]
- Sohl, G.; Willecke, K. Gap junctions and the connexin protein family. Cardiovasc. Res. 2004, 62, 228–232. [Google Scholar] [CrossRef]
- Sohl, G.; Willecke, K. An update on connexin genes and their nomenclature in mouse and man. Cell Commun. Adhes. 2003, 10, 173–180. [Google Scholar] [CrossRef]
- Bukauskas, F.F.; Peracchia, C. Two distinct gating mechanisms in gap junction channels: CO2-Sensitive and voltage-sensitive. Biophys. J. 1997, 72, 2137–2142. [Google Scholar] [CrossRef]
- Snipas, M.; Kraujalis, T.; Paulauskas, N.; Maciunas, K.; Bukauskas, F.F. Stochastic Model of Gap Junctions Exhibiting Rectification and Multiple Closed States of Slow Gates. Biophys. J. 2016, 110, 1322–1333. [Google Scholar] [CrossRef] [PubMed]
- Snipas, M.; Gudaitis, L.; Kraujaliene, L.; Kraujalis, T.; Verselis, V.K. Modeling and analysis of voltage gating in gap jmunction channels at the single-channel level. Biophys. J. 2023, 122, 4176–4193. [Google Scholar] [CrossRef] [PubMed]
- Bukauskas, F.F.; Weingart, R. Voltage-dependent gating of single gap junction channels in an insect cell line. Biophys. J. 1994, 67, 613–625. [Google Scholar] [CrossRef] [PubMed]
- Bukauskas, F.F.; Elfgang, C.; Willecke, K.; Weingart, R. Biophysical properties of gap junction channels formed by mouse connexin40 in induced pairs of transfected human HeLa cells. Biophys. J. 1995, 68, 2289–2298. [Google Scholar] [CrossRef] [PubMed]
- Horn, R.; Lange, K. Estimating kinetic constants from single channel data. Biophys. J. 1983, 43, 207–223. [Google Scholar] [CrossRef] [PubMed]
- Paulauskas, N.; Pranevicius, H.; Mockus, J.; Bukauskas, F.F. Stochastic 16-state model of voltage gating of gap-junction channels enclosing fast and slow gates. Biophys. J. 2012, 102, 2471–2480. [Google Scholar] [CrossRef] [PubMed]
- Paulauskas, N.; Pranevicius, M.; Pranevicius, H.; Bukauskas, F.F. A stochastic four-state model of contingent gating of gap junction channels containing two “fast” gates sensitive to transjunctional voltage. Biophys. J. 2009, 96, 3936–3948. [Google Scholar] [CrossRef]
- Vogel, R.; Valiunas, V.; Weingart, R. Subconductance states of Cx30 gap junction channels: Data from transfected HeLa cells versus data from a mathematical model. Biophys. J. 2006, 91, 2337–2348. [Google Scholar] [CrossRef]
- Persechini, A.; Gansz, K.J.; Paresi, R.J. Activation of myosin light chain kinase and nitric oxide synthase activities by engineered calmodulins with duplicated or exchanged EF hand pairs. Biochemistry 1996, 35, 224–228. [Google Scholar] [CrossRef]
- Astegno, A.; La Verde, V.; Marino, V.; Dell’Orco, D.; Dominici, P. Biochemical and biophysical characterization of a plant calmodulin: Role of the N- and C-lobes in calcium binding, conformational change, and target interaction. Biochim. Biophys. Acta 2016, 1864, 297–307. [Google Scholar] [CrossRef]
- Peracchia, C.; Salim, M.; Peracchia, L.L. Unusual slow gating of gap junction channels in oocytes expressing connexin32 or its COOH-terminus truncated mutant. J. Membr. Biol. 2007, 215, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Fasciani, I.; Temperan, A.; Perez-Atencio, L.F.; Escudero, A.; Martinez-Montero, P.; Molano, J.; Gomez-Hernandez, J.M.; Paino, C.L.; Gonzalez-Nieto, D.; Barrio, L.C. Regulation of connexin hemichannel activity by membrane potential and the extracellular calcium in health and disease. Neuropharmacology 2013, 75, 479–490. [Google Scholar] [CrossRef]
- Gomez-Hernandez, J.M.; de Miguel, M.; Larrosa, B.; Gonzalez, D.; Barrio, L.C. Molecular basis of calcium regulation in connexin-32 hemichannels. Proc. Natl. Acad. Sci. USA 2003, 100, 16030–16035. [Google Scholar] [CrossRef] [PubMed]
- DeVries, S.H.; Schwartz, E.A. Hemi-gap-junction channels in solitary horizontal cells of the catfish retina. J. Physiol. 1992, 445, 201–230. [Google Scholar] [CrossRef] [PubMed]
- Spray, D.C.; Ye, Z.C.; Ransom, B.R. Functional connexin “hemichannels”: A critical appraisal. Glia 2006, 54, 758–773. [Google Scholar] [CrossRef] [PubMed]
- Saez, J.C.; Leybaert, L. Hunting for connexin hemichannels. FEBS Lett. 2014, 588, 1205–1211. [Google Scholar] [CrossRef]
- Lopez, W.; Ramachandran, J.; Alsamarah, A.; Luo, Y.; Harris, A.L.; Contreras, J.E. Mechanism of gating by calcium in connexin hemichannels. Proc. Natl. Acad. Sci. USA 2016, 113, E7986–E7995. [Google Scholar] [CrossRef]
- Lopez, W.; Gonzalez, J.; Liu, Y.; Harris, A.L.; Contreras, J.E. Insights on the mechanisms of Ca2+ regulation of connexin26 hemichannels revealed by human pathogenic mutations (D50N/Y). J. Gen. Physiol. 2013, 142, 23–35. [Google Scholar] [CrossRef]
- Syrjanen, J.; Michalski, K.; Kawate, T.; Furukawa, H. On the molecular nature of large-pore channels. J. Mol. Biol. 2021, 433, 166994. [Google Scholar] [CrossRef]
- Wang, X.G.; Peracchia, C. Connexin 32/38 chimeras suggest a role for the second half of inner loop in gap junction gating by low pH. Am. J. Physiol. 1996, 271 Pt 1, C1743–C1749. [Google Scholar] [CrossRef]
- Wang, X.G.; Peracchia, C. Molecular dissection of a basic COOH-terminal domain of Cx32 that inhibits gap junction gating sensitivity. Am. J. Physiol. 1998, 275 Pt 1, C1384–C1390. [Google Scholar] [CrossRef] [PubMed]
- Werner, R.; Levine, E.; Rabadan-Diehl, C.; Dahl, G. Gating properties of connexin32 cell-cell channels and their mutants expressed in Xenopus oocytes. Proc. R. Soc. Lond. B Biol. Sci. 1991, 243, 5–11. [Google Scholar]
- Trexler, E.B.; Bennett, M.V.; Bargiello, T.A.; Verselis, V.K. Voltage gating and permeation in a gap junction hemichannel. Proc. Natl. Acad. Sci. USA 1996, 93, 5836–5841. [Google Scholar] [CrossRef] [PubMed]
- Verselis, V.K.; Ginter, C.S.; Bargiello, T.A. Opposite voltage gating polarities of two closely related connexins. Nature 1994, 368, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Young, K.C.; Peracchia, C. Opposite Cx32 and Cx26 voltage-gating response to CO2 reflects opposite voltage-gating polarity. J. Membr. Biol. 2004, 202, 161–170. [Google Scholar] [CrossRef]
- Oh, S.; Ri, Y.; Bennett, M.V.; Trexler, E.B.; Verselis, V.K.; Bargiello, T.A. Changes in permeability caused by connexin 32 mutations underlie X-linked Charcot-Marie-Tooth disease. Neuron 1997, 19, 927–938. [Google Scholar] [CrossRef]
- Peracchia, C.; Wang, X.G.; Peracchia, L.L. Slow gating of gap junction channels and calmodulin. J. Membr. Biol. 2000, 178, 55–70. [Google Scholar] [CrossRef]
- Klinke, J. Über die Bedingungen für das Auftreten eines färberischen Polarisationsbildes am Nerven. Pflugers Arch. 1931, 227, 715–726. [Google Scholar] [CrossRef]
- Barrio, L.C.; Capel, J.; Jarillo, J.A.; Castro, C.; Revilla, A. Species-specific voltage-gating properties of connexin-45 junctions expressed in Xenopus oocytes. Biophys. J. 1997, 73, 757–769. [Google Scholar] [CrossRef]
- Moreno, A.P.; Laing, J.G.; Beyer, E.C.; Spray, D.C. Properties of gap junction channels formed of connexin 45 endogenously expressed in human hepatoma (SKHep1) cells. Am. J. Physiol. 1995, 268 Pt 1, C356–C365. [Google Scholar] [CrossRef]
- Veenstra, R.D.; Wang, H.Z.; Westphale, E.M.; Beyer, E.C. Multiple connexins confer distinct regulatory and conductance properties of gap junctions in developing heart. Circ. Res. 1992, 71, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Bukauskas, F.F.; Angele, A.B.; Verselis, V.K.; Bennett, M.V. Coupling asymmetry of heterotypic connexin 45/ connexin 43-EGFP gap junctions: Properties of fast and slow gating mechanisms. Proc. Natl. Acad. Sci. USA 2002, 99, 7113–7118. [Google Scholar] [CrossRef] [PubMed]
- Peracchia, C.; Young, K.C.; Wang, X.G.; Peracchia, L.L. Is the voltage gate of connexins CO2-sensitive? Cx45 channels and inhibition of calmodulin expression. J. Membr. Biol. 2003, 195, 53–62. [Google Scholar] [CrossRef]
- Elenes, S.; Martinez, A.D.; Delmar, M.; Beyer, E.C.; Moreno, A.P. Heterotypic docking of Cx43 and Cx45 connexons blocks fast voltage gating of Cx43. Biophys. J. 2001, 81, 1406–1418. [Google Scholar] [CrossRef]
- Valiunas, V. Biophysical properties of connexin-45 gap junction hemichannels studied in vertebrate cells. J. Gen. Physiol. 2002, 119, 147–164. [Google Scholar] [CrossRef] [PubMed]
- Peracchia, C.; Wang, X.; Li, L.; Peracchia, L.L. Inhibition of calmodulin expression prevents low-pH-induced gap junction uncoupling in Xenopus oocytes. Pflugers Arch. 1996, 431, 379–387. [Google Scholar] [CrossRef]
- Tetenborg, S.; Yadav, S.C.; Hormuzdi, S.G.; Monyer, H.; Janssen-Bienhold, U.; Dedek, K. Differential Distribution of Retinal Ca2+/Calmodulin-Dependent Kinase II (CaMKII) Isoforms Indicates CaMKII-beta and -delta as Specific Elements of Electrical Synapses Made of Connexin36 (Cx36). Front. Mol. Neurosci. 2017, 10, 425. [Google Scholar] [CrossRef] [PubMed]
- De Vuyst, E.; Decrock, E.; Cabooter, L.; Dubyak, G.R.; Naus, C.C.; Evans, W.H.; Leybaert, L. Intracellular calcium changes trigger connexin 32 hemichannel opening. EMBO J. 2006, 25, 34–44. [Google Scholar] [CrossRef]
- De Vuyst, E.; Wang, N.; Decrock, E.; De, B.M.; Vinken, M.; Van, M.M.; Lai, C.; Culot, M.; Rogiers, V.; Cecchelli, R.; et al. Ca2+ regulation of connexin 43 hemichannels in C6 glioma and glial cells. Cell Calcium 2009, 46, 176–187. [Google Scholar] [CrossRef]
- Carrer, A.; Leparulo, A.; Crispino, G.; Ciubotaru, C.D.; Marin, O.; Zonta, F.; Bortolozzi, M. Cx32 hemichannel opening by cytosolic Ca2+ is inhibited by the R220X mutation that causes Charcot-Marie-Tooth disease. Hum. Mol. Genet. 2018, 27, 80–94. [Google Scholar] [CrossRef]
- Geiser, J.R.; van Tuinen, D.; Brockerhoff, S.E.; Neff, M.M.; Davis, T.N. Can calmodulin function without binding calcium? Cell 1991, 65, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Martin, P.E.; Evans, W.H. Assembly of gap junction channels: Mechanism, effects of calmodulin antagonists and identification of connexin oligomerization determinants. Eur. J. Biochem. 2001, 268, 4544–4552. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Chenavas, S.; Kieken, F.; Trease, A.; Brownell, S.; Anbanandam, A.; Sorgen, P.L.; Spagnol, G. Calmodulin Directly Interacts with the Cx43 Carboxyl-Terminus and Cytoplasmic Loop Containing Three ODDD-Linked Mutants (M147T, R148Q, and T154A) that Retain alpha-Helical Structure, but Exhibit Loss-of-Function and Cellular Trafficking Defects. Biomolecules 2020, 10, 1452. [Google Scholar] [CrossRef] [PubMed]
- Peracchia, C.; Chen, J.T.; Peracchia, L.L. CO2 sensitivity of voltage gating and gating polarity of gapjunction channels--connexin40 and its COOH-terminus-truncated mutant. J. Membr. Biol. 2004, 200, 105–113. [Google Scholar] [CrossRef]
- Peracchia, C.; Peracchia, L.L. Inversion of both gating polarity and CO2 sensitivity of voltage gating with D3N mutation of Cx50. Am. J. Physiol. Cell Physiol. 2005, 288, C1381–C1389. [Google Scholar] [CrossRef]
- Sasaki, S.; Ishibashi, K.; Nagai, T.; Marumo, F. Regulation mechanisms of intracellular pH of Xenopus laevis oocyte. Biochim. Biophys. Acta 1992, 1137, 45–51. [Google Scholar] [CrossRef]
- Oh, S.; Rubin, J.B.; Bennett, M.V.; Verselis, V.K.; Bargiello, T.A. Molecular determinants of electrical rectification of single channel conductance in gap junctions formed by connexins 26 and 32. J. Gen. Physiol. 1999, 114, 339–364. [Google Scholar] [CrossRef]
- Oh, S.; Abrams, C.K.; Verselis, V.K.; Bargiello, T.A. Stoichiometry of transjunctional voltage-gating polarity reversal by a negative charge substitution in the amino terminus of a connexin32 chimera. J. Gen. Physiol. 2000, 116, 13–31. [Google Scholar] [CrossRef]
- Purnick, P.E.; Oh, S.; Abrams, C.K.; Verselis, V.K.; Bargiello, T.A. Reversal of the gating polarity of gap junctions by negative charge substitutions in the N-terminus of connexin 32. Biophys. J. 2000, 79, 2403–2415. [Google Scholar] [CrossRef]
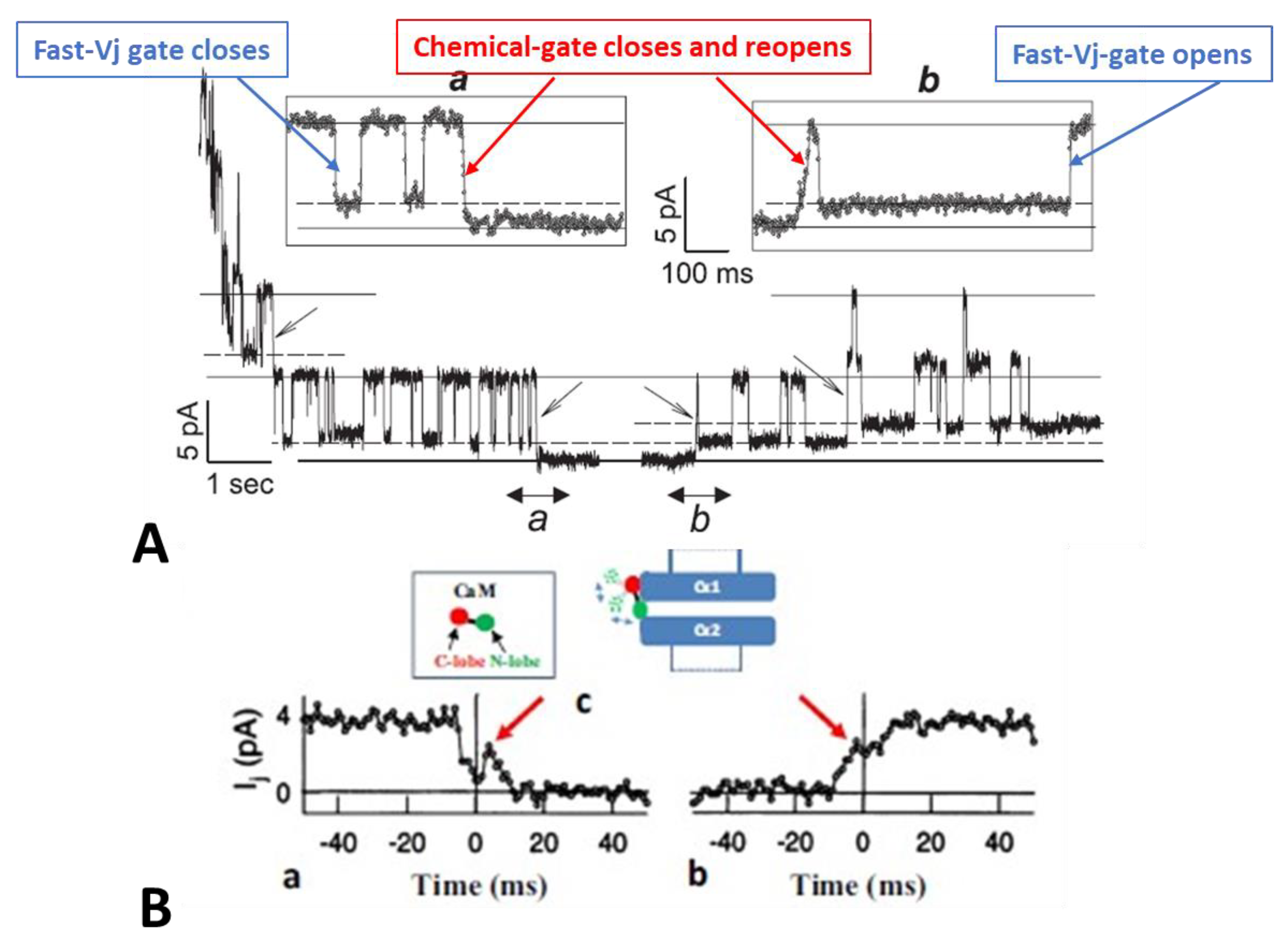


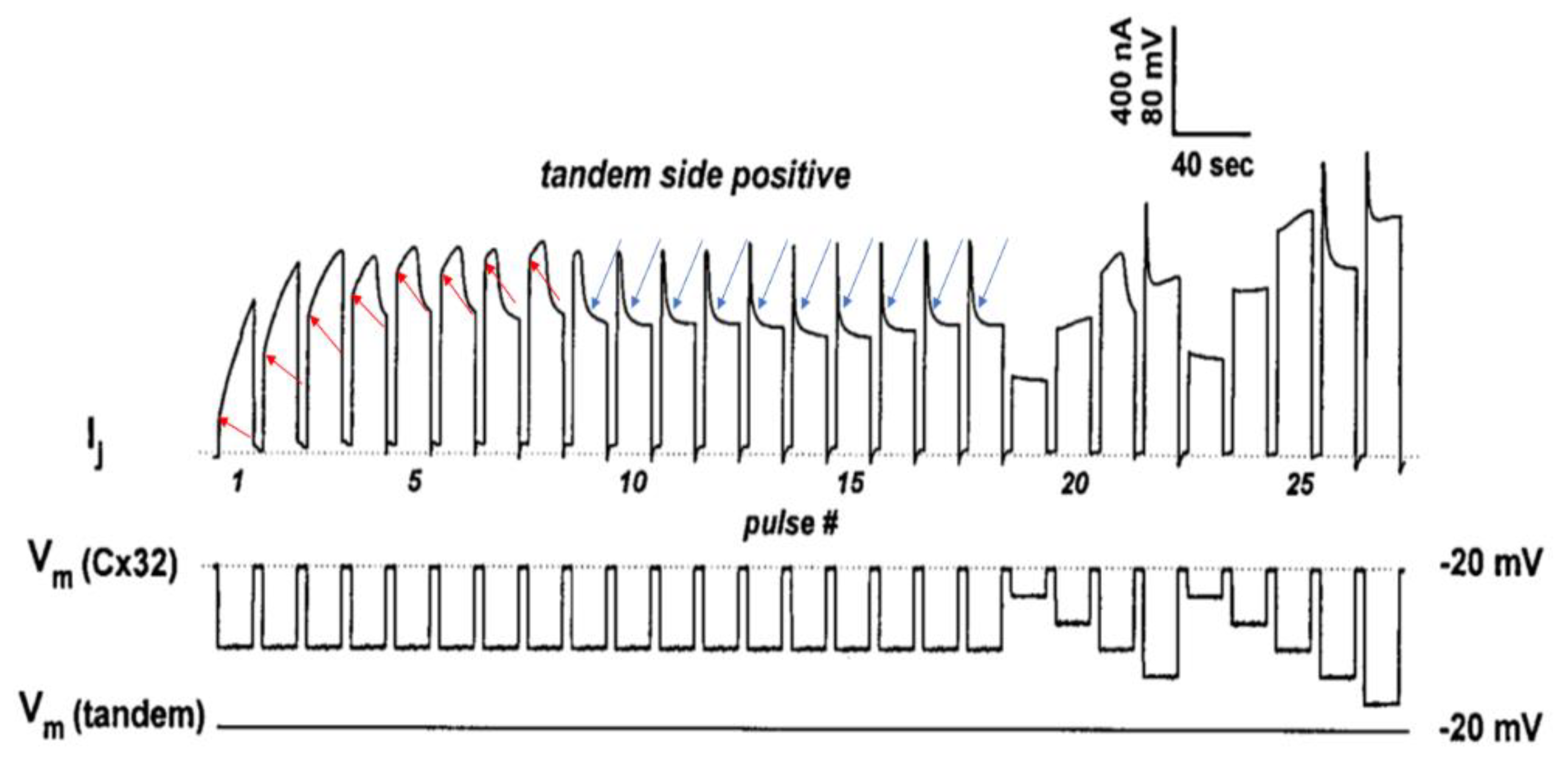
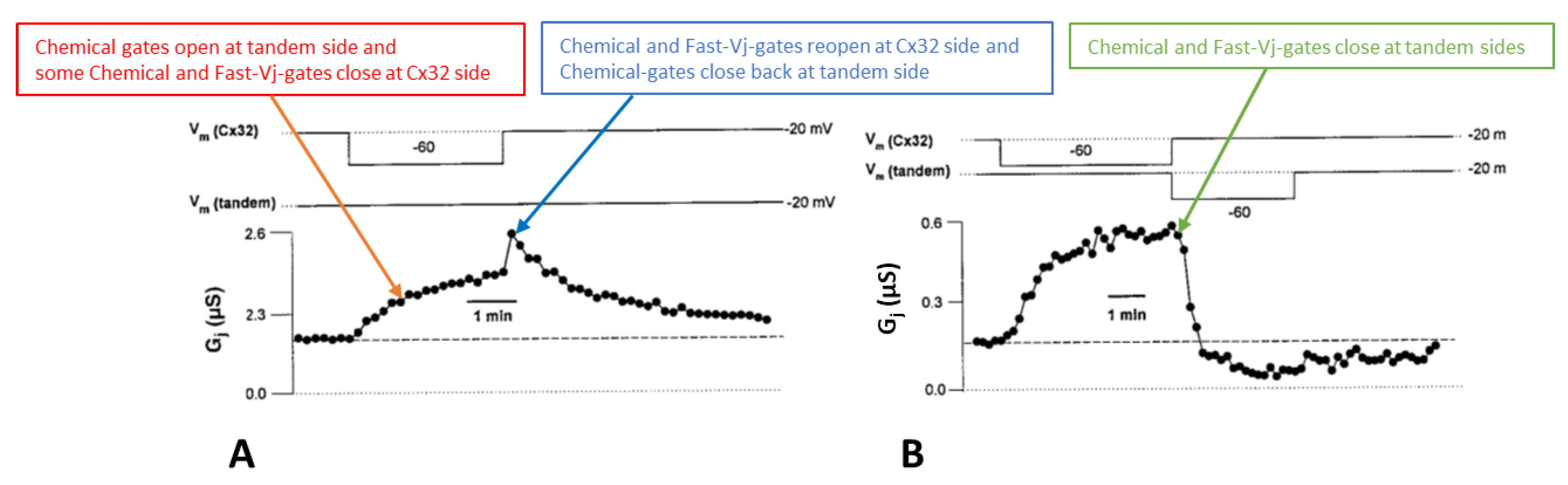
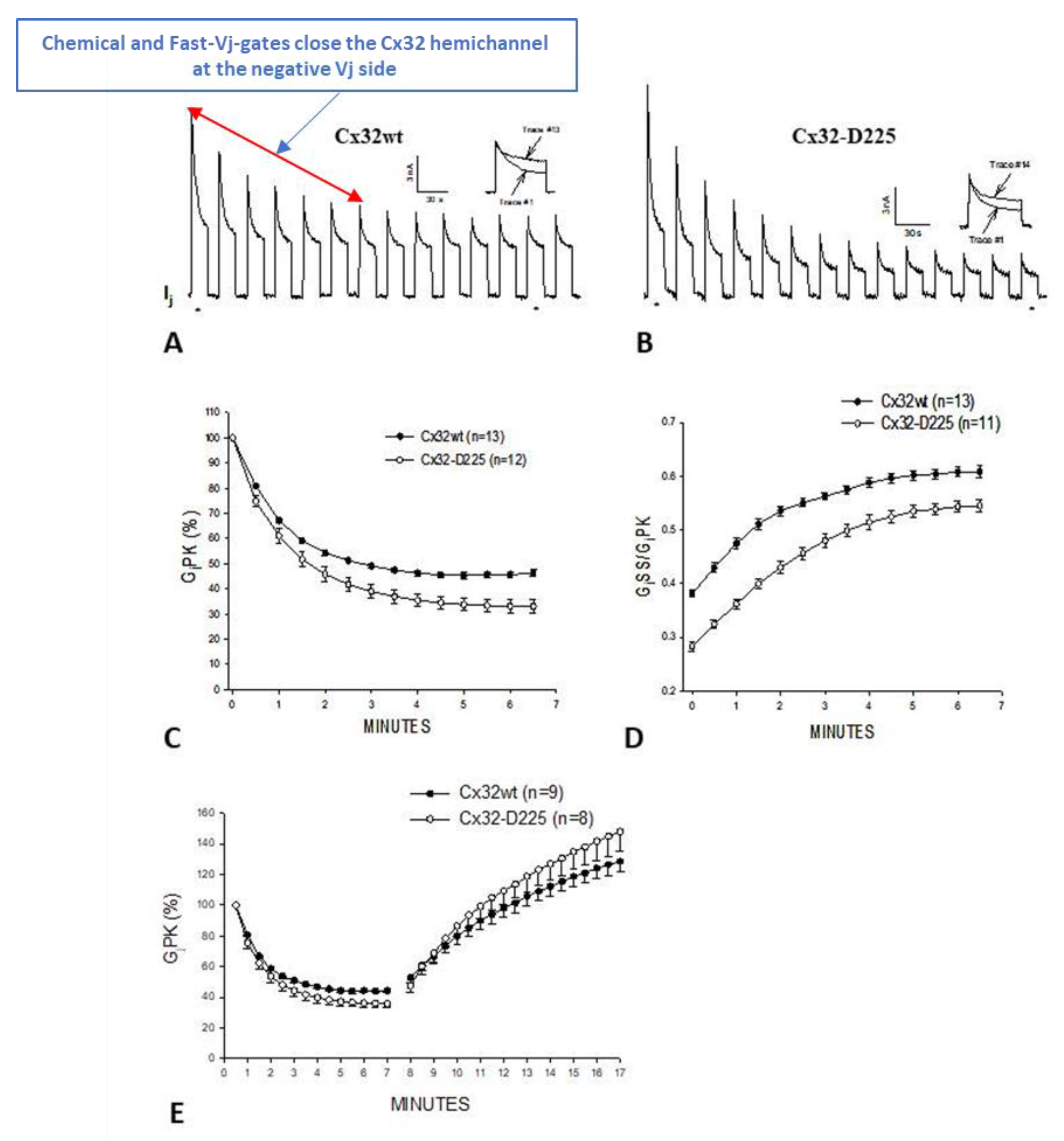





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peracchia, C. Gap Junction Channel Regulation: A Tale of Two Gates—Voltage Sensitivity of the Chemical Gate and Chemical Sensitivity of the Fast Voltage Gate. Int. J. Mol. Sci. 2024, 25, 982. https://doi.org/10.3390/ijms25020982
Peracchia C. Gap Junction Channel Regulation: A Tale of Two Gates—Voltage Sensitivity of the Chemical Gate and Chemical Sensitivity of the Fast Voltage Gate. International Journal of Molecular Sciences. 2024; 25(2):982. https://doi.org/10.3390/ijms25020982
Chicago/Turabian StylePeracchia, Camillo. 2024. "Gap Junction Channel Regulation: A Tale of Two Gates—Voltage Sensitivity of the Chemical Gate and Chemical Sensitivity of the Fast Voltage Gate" International Journal of Molecular Sciences 25, no. 2: 982. https://doi.org/10.3390/ijms25020982
APA StylePeracchia, C. (2024). Gap Junction Channel Regulation: A Tale of Two Gates—Voltage Sensitivity of the Chemical Gate and Chemical Sensitivity of the Fast Voltage Gate. International Journal of Molecular Sciences, 25(2), 982. https://doi.org/10.3390/ijms25020982





