Phylogenetic Analysis of Porcine Epidemic Diarrhea Virus (PEDV) during 2020–2022 and Isolation of a Variant Recombinant PEDV Strain
Abstract
:1. Introduction
2. Results
2.1. Prevalence of PEDV in Clinical Samples from Diarrheal Pigs
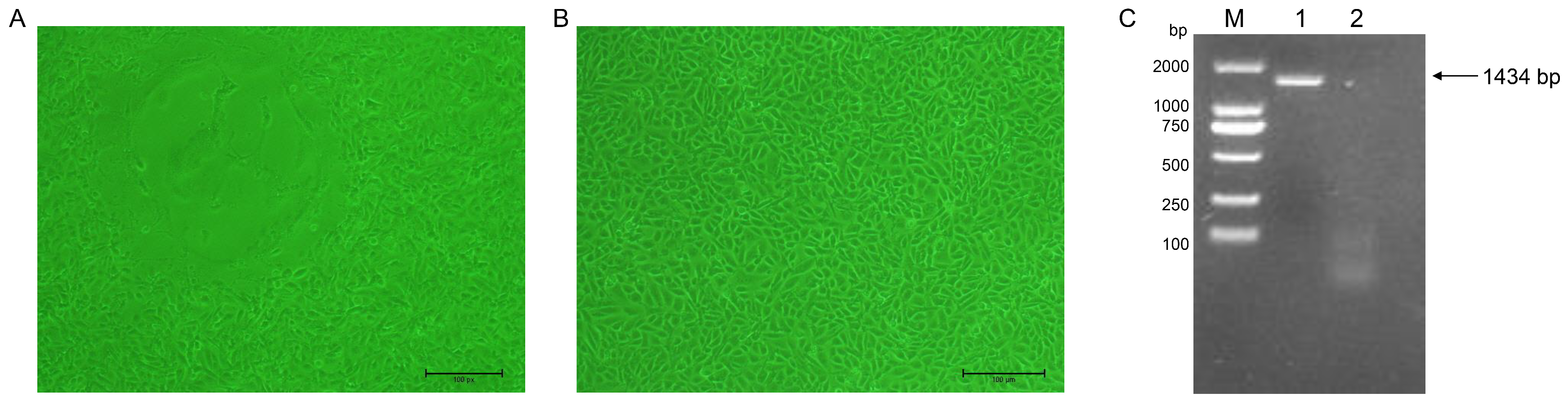
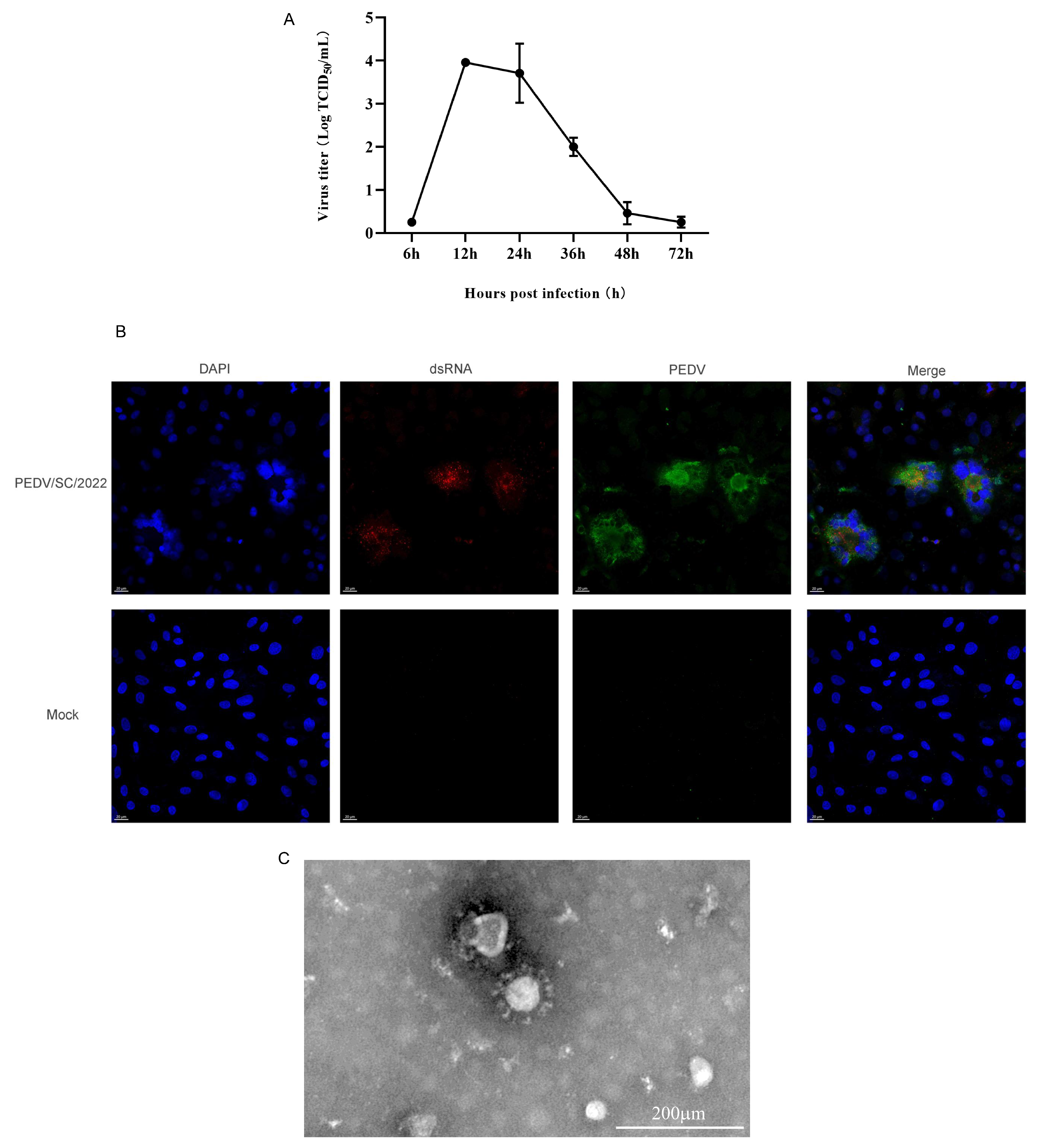
2.2. Virus Isolation and Identification
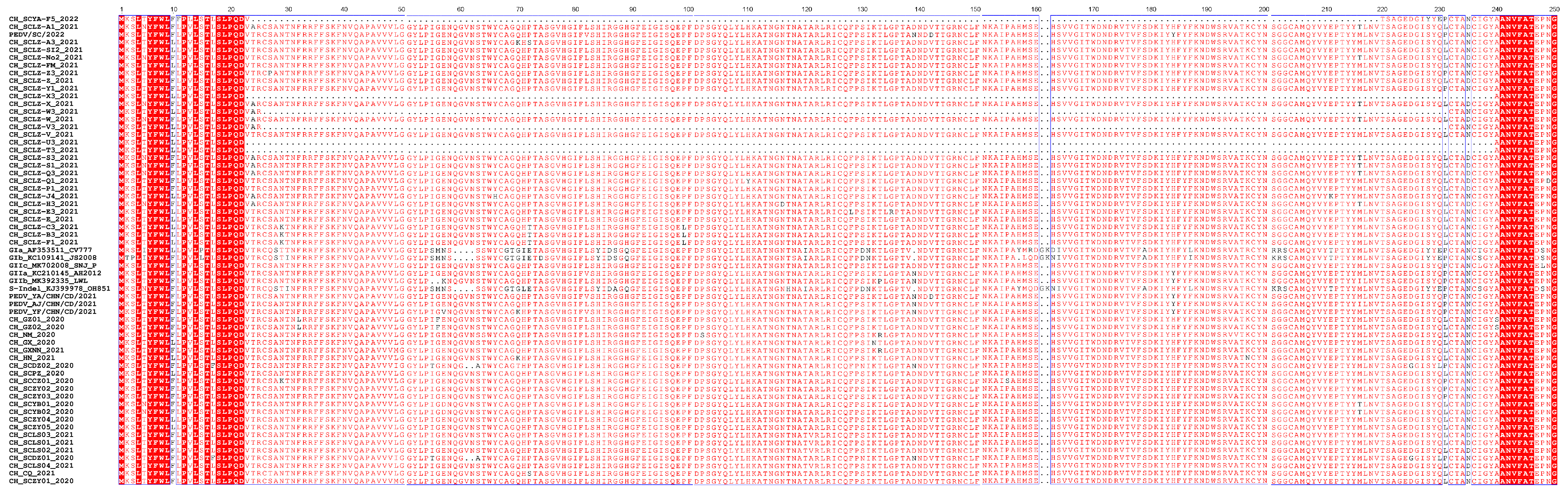
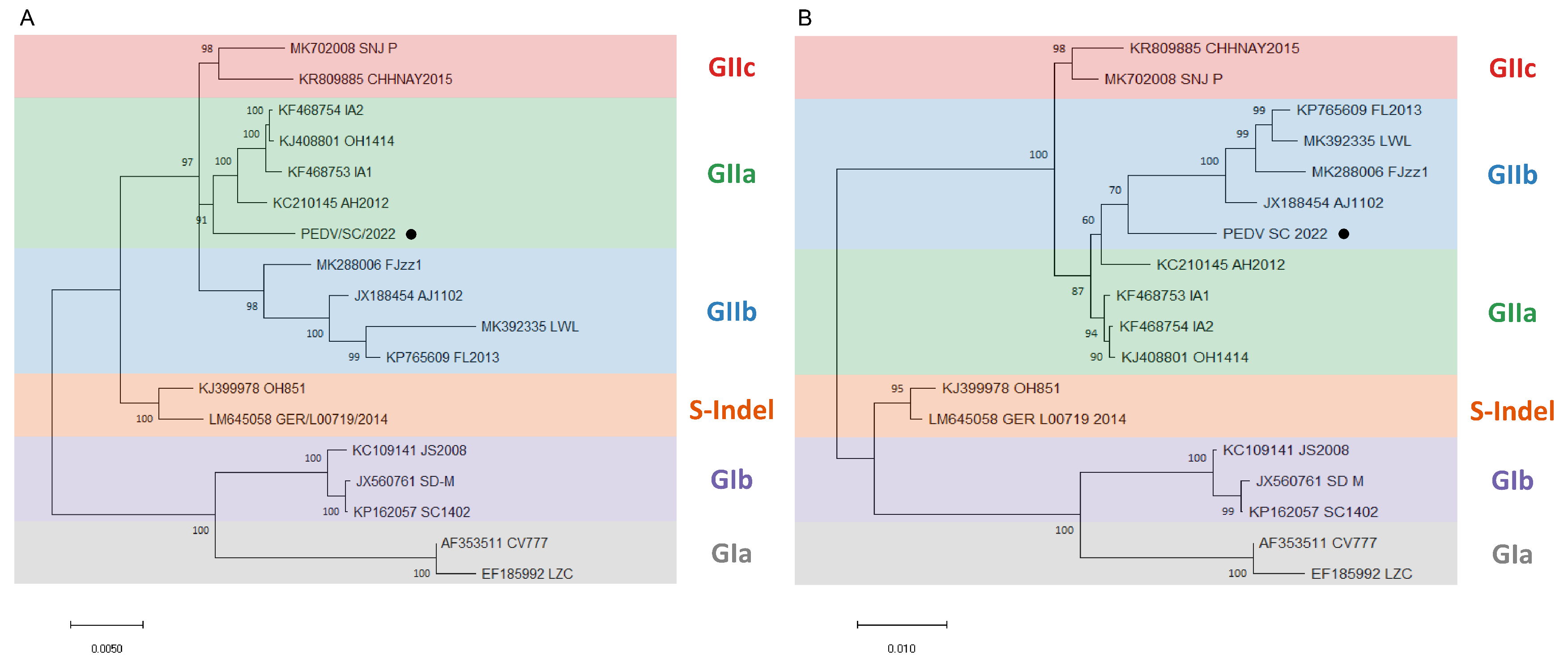
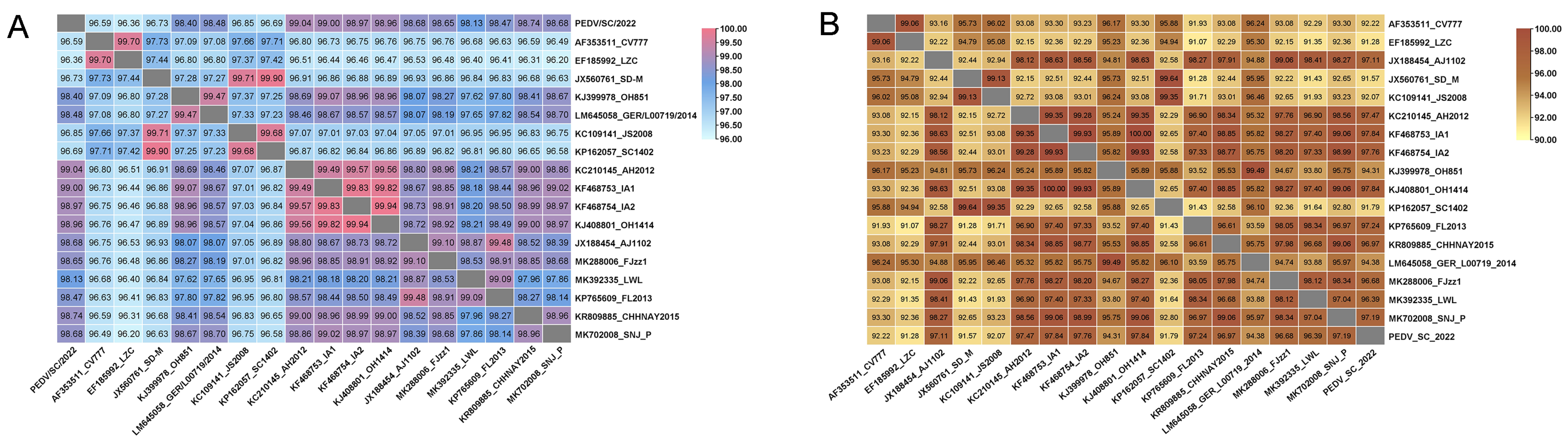
2.3. Phylogenetic Analysis and Alignment of the Genome and S Gene of PEDV/SC/2022
2.4. Recombination within the PEDV/SC/2022 ORF1b Gene
3. Discussion
4. Materials and Methods
4.1. Cells, Antibodies, and Clinical Samples
4.2. RNA Extraction and Detection of PEDV
4.3. Amplification and Sequencing of PEDV S Gene
4.4. Virus Isolation and RT-PCR Identification
4.5. TCID50 Assay
4.6. IFA
4.7. Electron Microscopy Analysis
4.8. Phylogenetic and Recombination Analysis
4.9. Growth Kinetics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wood, E.N. An apparently new syndrome of porcine epidemic diarrhoea. Vet. Rec. 1977, 100, 243–244. [Google Scholar] [CrossRef] [PubMed]
- Lee, C. Porcine epidemic diarrhea virus: An emerging and re-emerging epizootic swine virus. Virol. J. 2015, 12, 193. [Google Scholar] [CrossRef] [PubMed]
- Woo, P.C.Y.; de Groot, R.J.; Haagmans, B.; Lau, S.K.P.; Neuman, B.W.; Perlman, S.; Sola, I.; van der Hoek, L.; Wong, A.C.P.; Yeh, S.H. ICTV Virus Taxonomy Profile: Coronaviridae 2023. J. Gen. Virol. 2023, 104, 001843. [Google Scholar] [CrossRef] [PubMed]
- González, J.M.; Gomez-Puertas, P.; Cavanagh, D.; Gorbalenya, A.E.; Enjuanes, L. A comparative sequence analysis to revise the current taxonomy of the family Coronaviridae. Arch. Virol. 2003, 148, 2207–2235. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.P.; Hutchings, L.M. A transmissible gastroenteritis in pigs. J. Am. Vet. Med. Assoc. 1946, 108, 257–259. [Google Scholar]
- Tang, G.; Liu, Z.; Chen, D. Human coronaviruses: Origin, host and receptor. J. Clin. Virol. 2022, 155, 105246. [Google Scholar] [CrossRef]
- Licitra, B.N.; Duhamel, G.E.; Whittaker, G.R. Canine enteric coronaviruses: Emerging viral pathogens with distinct recombinant spike proteins. Viruses 2014, 6, 3363–3376. [Google Scholar] [CrossRef]
- Malone, B.; Urakova, N.; Snijder, E.J.; Campbell, E.A. Structures and functions of coronavirus replication-transcription complexes and their relevance for SARS-CoV-2 drug design. Nat. Rev. Mol. Cell Biol. 2022, 23, 21–39. [Google Scholar] [CrossRef]
- Kocherhans, R.; Bridgen, A.; Ackermann, M.; Tobler, K. Completion of the porcine epidemic diarrhoea coronavirus (PEDV) genome sequence. Virus Genes 2001, 23, 137–144. [Google Scholar] [CrossRef]
- Sun, D.; Wang, X.; Wei, S.; Chen, J.; Feng, L. Epidemiology and vaccine of porcine epidemic diarrhea virus in China: A mini-review. J. Vet. Med. Sci. 2016, 78, 355–363. [Google Scholar] [CrossRef]
- Sun, M.; Ma, J.; Wang, Y.; Wang, M.; Song, W.; Zhang, W.; Lu, C.; Yao, H. Genomic and epidemiological characteristics provide new insights into the phylogeographical and spatiotemporal spread of porcine epidemic diarrhea virus in Asia. J. Clin. Microbiol. 2015, 53, 1484–1492. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Fang, L.; Xiao, S. Porcine epidemic diarrhea in China. Virus Res. 2016, 226, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.Q.; Cai, R.J.; Chen, Y.Q.; Liang, P.S.; Chen, D.K.; Song, C.X. Outbreak of porcine epidemic diarrhea in suckling piglets, China. Emerg. Infect. Dis. 2012, 18, 161–163. [Google Scholar] [CrossRef]
- Li, W.; Li, H.; Liu, Y.; Pan, Y.; Deng, F.; Song, Y.; Tang, X.; He, Q. New variants of porcine epidemic diarrhea virus, China, 2011. Emerg. Infect. Dis. 2012, 18, 1350–1353. [Google Scholar] [CrossRef]
- Chen, J.; Liu, X.; Shi, D.; Shi, H.; Zhang, X.; Feng, L. Complete genome sequence of a porcine epidemic diarrhea virus variant. J. Virol. 2012, 86, 3408. [Google Scholar] [CrossRef]
- Lee, S.; Lee, C. Outbreak-Related Porcine Epidemic Diarrhea Virus Strains Similar to US Strains, South Korea, 2013. Emerg. Infect. Dis. 2014, 20, 1223–1226. [Google Scholar] [CrossRef] [PubMed]
- Vlasova, A.N.; Marthaler, D.; Wang, Q.; Culhane, M.R.; Rossow, K.D.; Rovira, A.; Collins, J.; Saif, L.J. Distinct characteristics and complex evolution of PEDV strains, North America, May 2013–February 2014. Emerg. Infect. Dis. 2014, 20, 1620–1628. [Google Scholar] [CrossRef]
- Chen, P.; Wang, K.; Hou, Y.; Li, H.; Li, X.; Yu, L.; Jiang, Y.; Gao, F.; Tong, W.; Yu, H.; et al. Genetic evolution analysis and pathogenicity assessment of porcine epidemic diarrhea virus strains circulating in part of China during 2011–2017. Infect. Genet. Evol. 2019, 69, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Fang, L.; Ye, X.; Chen, J.; Xu, S.; Zhu, X.; Miao, Y.; Wang, D.; Xiao, S. Evolutionary and genotypic analyses of global porcine epidemic diarrhea virus strains. Transbound. Emerg. Dis. 2019, 66, 111–118. [Google Scholar] [CrossRef]
- Li, F.; Zeng, Y.; Zhang, R.; Peng, K.; Jiang, C.; Xu, Z.; Zhu, L. Genetic variations in S gene of porcine epidemic diarrhoea virus from 2018 in Sichuan Province, China. Vet. Med. Sci. 2020, 6, 910–918. [Google Scholar] [CrossRef]
- Zhang, F.; Luo, Y.; Lin, C.; Tan, M.; Wan, P.; Xie, B.; Xiong, L.; Ji, H. Epidemiological monitoring and genetic variation analysis of pathogens associated with porcine viral diarrhea in southern China from 2021 to 2023. Front. Microbiol. 2024, 15, 1303915. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, H.; Sun, L.; Wang, X.; Xiao, M.; Zeng, L.; Wang, H.; Yang, H.; Lin, F.; Wang, C.; Qin, L.; et al. Molecular characterization and phylogenetic analysis of porcine epidemic diarrhea virus strains circulating in China from 2020 to 2021. BMC Vet. Res. 2022, 18, 392. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Lu, H.; Geng, C.; Yang, K.; Liu, W.; Liu, Z.; Yuan, F.; Gao, T.; Wang, S.; Wen, P.; et al. Epidemic and Evolutionary Characteristics of Swine Enteric Viruses in South-Central China from 2018 to 2021. Viruses 2022, 14, 1420. [Google Scholar] [CrossRef] [PubMed]
- Wen, F.; Yang, J.; Li, A.; Gong, Z.; Yang, L.; Cheng, Q.; Wang, C.; Zhao, M.; Yuan, S.; Chen, Y.; et al. Genetic characterization and phylogenetic analysis of porcine epidemic diarrhea virus in Guangdong, China, between 2018 and 2019. PLoS ONE 2021, 16, e0253622. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Liu, Y.; Wang, S.; Zhang, L.; Liang, P.; Wang, L.; Dong, J.; Song, C. Molecular Characteristics and Pathogenicity of Porcine Epidemic Diarrhea Virus Isolated in Some Areas of China in 2015–2018. Front. Vet. Sci. 2020, 7, 607662. [Google Scholar] [CrossRef]
- Park, J.E. Porcine Epidemic Diarrhea: Insights and Progress on Vaccines. Vaccines 2024, 12, 212. [Google Scholar] [CrossRef]
- Zhang, F.; Luo, S.; Gu, J.; Li, Z.; Li, K.; Yuan, W.; Ye, Y.; Li, H.; Ding, Z.; Song, D.; et al. Prevalence and phylogenetic analysis of porcine diarrhea associated viruses in southern China from 2012 to 2018. BMC Vet. Res. 2019, 15, 470. [Google Scholar] [CrossRef]
- Jia, S.; Feng, B.; Wang, Z.; Ma, Y.; Gao, X.; Jiang, Y.; Cui, W.; Qiao, X.; Tang, L.; Li, Y.; et al. Dual priming oligonucleotide (DPO)-based real-time RT-PCR assay for accurate differentiation of four major viruses causing porcine viral diarrhea. Mol. Cell Probes 2019, 47, 101435. [Google Scholar] [CrossRef]
- Chen, J.; Tian, L.; Liu, Y.; Sun, Y.; Li, Z.; Cai, X.; Meng, Q.; Qiao, J. Molecular characterization and phylogenetic analysis of porcine epidemic diarrhea virus in Xinjiang, China, from 2020 to 2022. Arch. Virol. 2024, 169, 96. [Google Scholar] [CrossRef]
- Ge, Y.; Jiang, F.; Wang, S.; Wu, H.; Liu, Y.; Wang, B.; Hou, W.; Yu, X.; Wang, H. Natural Evolution of Porcine Epidemic Diarrhea Viruses Isolated from Maternally Immunized Piglets. Animals 2023, 13, 1766. [Google Scholar] [CrossRef]
- Tian, Y.; Yang, X.; Li, H.; Ma, B.; Guan, R.; Yang, J.; Chen, D.; Han, X.; Zhou, L.; Song, Z.; et al. Molecular characterization of porcine epidemic diarrhea virus associated with outbreaks in southwest China during 2014–2018. Transbound Emerg. Dis. 2021, 68, 3482–3497. [Google Scholar] [CrossRef] [PubMed]
- He, W.T.; Bollen, N.; Xu, Y.; Zhao, J.; Dellicour, S.; Yan, Z.; Gong, W.; Zhang, C.; Zhang, L.; Lu, M.; et al. Phylogeography Reveals Association between Swine Trade and the Spread of Porcine Epidemic Diarrhea Virus in China and across the World. Mol. Biol. Evol. 2022, 39, msab364. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Han, F.; Yan, X.; Liu, L.; Shu, X.; Hu, H. Prevalence and phylogenetic analysis of spike gene of porcine epidemic diarrhea virus in Henan province, China in 2015–2019. Infect. Genet. Evol. 2021, 88, 104709. [Google Scholar] [CrossRef]
- Cui, J.T.; Qiao, H.; Hou, C.Y.; Zheng, H.H.; Li, X.S.; Zheng, L.L.; Chen, H.Y. Characteristics of the spike and ORF3 genes of porcine epidemic diarrhea virus in Henan and Shanxi provinces of China. Arch. Virol. 2020, 165, 2323–2333. [Google Scholar] [CrossRef]
- Guo, Z.; Ruan, H.; Qiao, S.; Deng, R.; Zhang, G. Co-infection status of porcine circoviruses (PCV2 and PCV3) and porcine epidemic diarrhea virus (PEDV) in pigs with watery diarrhea in Henan province, central China. Microb. Pathog. 2020, 142, 104047. [Google Scholar] [CrossRef]
- Li, F.; Goff, S.P. Receptor recognition mechanisms of coronaviruses: A decade of structural studies. J. Virol. 2015, 89, 1954–1964. [Google Scholar] [CrossRef]
- Oh, J.; Lee, K.W.; Choi, H.W.; Lee, C. Immunogenicity and protective efficacy of recombinant S1 domain of the porcine epidemic diarrhea virus spike protein. Arch. Virol. 2014, 159, 2977–2987. [Google Scholar] [CrossRef]
- Rawal, G.; Yim-Im, W.; Aljets, E.; Halbur, P.G.; Zhang, J.; Opriessnig, T. Porcine Respiratory Coronavirus (PRCV): Isolation and Characterization of a Variant PRCV from USA Pigs. Pathogens 2023, 12, 1097. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, S.; Song, D.; Park, B. Novel porcine epidemic diarrhea virus variant with large genomic deletion, South Korea. Emerg. Infect. Dis. 2014, 20, 2089–2092. [Google Scholar] [CrossRef]
- Masuda, T.; Murakami, S.; Takahashi, O.; Miyazaki, A.; Ohashi, S.; Yamasato, H.; Suzuki, T. New porcine epidemic diarrhoea virus variant with a large deletion in the spike gene identified in domestic pigs. Arch. Virol. 2015, 160, 2565–2568. [Google Scholar] [CrossRef]
- Hou, Y.; Lin, C.M.; Yokoyama, M.; Yount, B.L.; Marthaler, D.; Douglas, A.L.; Ghimire, S.; Qin, Y.; Baric, R.S.; Saif, L.J.; et al. Deletion of a 197-Amino-Acid Region in the N-Terminal Domain of Spike Protein Attenuates Porcine Epidemic Diarrhea Virus in Piglets. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Hou, Y.; Wang, Q. The enhanced replication of an S-intact PEDV during coinfection with an S1 NTD-del PEDV in piglets. Vet. Microbiol. 2019, 228, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Shirato, K.; Matsuyama, S.; Ujike, M.; Taguchi, F. Role of proteases in the release of porcine epidemic diarrhea virus from infected cells. J. Virol. 2011, 85, 7872–7880. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, M.; Wyler, R. Propagation of the virus of porcine epidemic diarrhea in cell culture. J. Clin. Microbiol. 1988, 26, 2235–2239. [Google Scholar] [CrossRef]
- Bi, J.; Zeng, S.; Xiao, S.; Chen, H.; Fang, L. Complete genome sequence of porcine epidemic diarrhea virus strain AJ1102 isolated from a suckling piglet with acute diarrhea in China. J. Virol. 2012, 86, 10910–10911. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Sui, L.; Kong, D.; Liu, D.; Gao, Y.; Jiang, Y.; Cui, W.; Li, J.; Li, Y.; Wang, L. Porcine epidemic diarrhea virus strain CH/HLJ/18 isolated in China: Characterization and phylogenetic analysis. Virol. J. 2024, 21, 28. [Google Scholar] [CrossRef]
- Peng, Q.; Yang, Z.; Wu, L.; Yu, P.; Li, Q.; Lan, J.; Luo, L.; Zhao, S.; Yan, Q. Evaluation of the Inactivation Efficacy of Four Disinfectants for Feline Parvovirus Derived from Giant Panda. Microorganisms 2023, 11, 1844. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, F.; Jakovlić, I.; Zou, H.; Zhang, J.; Li, W.X.; Wang, G.T. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Resour. 2020, 20, 348–355. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Martin, D.P.; Varsani, A.; Roumagnac, P.; Botha, G.; Maslamoney, S.; Schwab, T.; Kelz, Z.; Kumar, V.; Murrell, B. RDP5: A computer program for analyzing recombination in, and removing signals of recombination from, nucleotide sequence datasets. Virus Evol. 2021, 7, veaa087. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.; Rybicki, E. RDP: Detection of recombination amongst aligned sequences. Bioinformatics 2000, 16, 562–563. [Google Scholar] [CrossRef]
- Lam, H.M.; Ratmann, O.; Boni, M.F. Improved Algorithmic Complexity for the 3SEQ Recombination Detection Algorithm. Mol. Biol. Evol. 2018, 35, 247–251. [Google Scholar] [CrossRef]
- Posada, D.; Crandall, K.A. Evaluation of methods for detecting recombination from DNA sequences: Computer simulations. Proc. Natl. Acad. Sci. USA 2001, 98, 13757–13762. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, M.J.; Armstrong, J.S.; Gibbs, A.J. Sister-scanning: A Monte Carlo procedure for assessing signals in recombinant sequences. Bioinformatics 2000, 16, 573–582. [Google Scholar] [CrossRef]
- Smith, J.M. Analyzing the mosaic structure of genes. J. Mol. Evol. 1992, 34, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Salminen, M.O.; Carr, J.K.; Burke, D.S.; McCutchan, F.E. Identification of breakpoints in intergenotypic recombinants of HIV type 1 by bootscanning. AIDS Res. Hum. Retroviruses 1995, 11, 1423–1425. [Google Scholar] [CrossRef]
- Samson, S.; Lord, É.; Makarenkov, V. SimPlot++: A Python application for representing sequence similarity and detecting recombination. Bioinformatics 2022, 38, 3118–3120. [Google Scholar] [CrossRef]
- Zhou, Z.J.; Qiu, Y.; Pu, Y.; Huang, X.; Ge, X.Y. BioAider: An efficient tool for viral genome analysis and its application in tracing SARS-CoV-2 transmission. Sustain. Cities Soc. 2020, 63, 102466. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef]
- Lei, C.; Yang, J.; Hu, J.; Sun, X. On the Calculation of TCID(50) for Quantitation of Virus Infectivity. Virol. Sin. 2021, 36, 141–144. [Google Scholar] [CrossRef] [PubMed]


| Total | Sample Type | Total | PEDV Positive Rate Positive/Sample (%) | |
|---|---|---|---|---|
| Small Intestine Tissue | Feces | |||
| Sichuan | 198 | 95 | 293 | 151/293 (51.54%) |
| Guizhou | 1 | 4 | 5 | 5/5 (100%) |
| Chongqing | 1 | 0 | 1 | 1/1 (100%) |
| Inner Mongolia | 0 | 16 | 16 | 10/16 (62.5%) |
| Henan | 0 | 14 | 14 | 14/14 (100%) |
| Jiangsu | 0 | 5 | 5 | 5/5 (100%) |
| Guangxi | 0 | 13 | 13 | 13/13 (100%) |
| Total | 200 | 147 | 347 | 199/347 (57.35%) |
| Strain | GenBank No. | Location | Year |
|---|---|---|---|
| CV777 | AF353511 | Belgium | 1977 |
| LZC | EF185992 | China | 2007 |
| JS2008 | KC109141 | China | 2013 |
| SD-M | JX560761 | China | 2012 |
| SC1402 | KP162057 | China | 2014 |
| CHHNAY2015 | KR809885 | China | 2015 |
| SNJ_P | MK702008 | China | 2018 |
| OH851 | KJ399978 | USA | 2014 |
| GER/L00719/2014 | LM645058 | Germany | 2014 |
| OH1414 | KJ408801 | USA | 2014 |
| IA1 | KF468753 | USA | 2013 |
| IA2 | KF468754 | USA | 2013 |
| AH2012 | KC210145 | China | 2012 |
| LWL | MK392335 | China | 2019 |
| FL2013 | KP765609 | China | 2014 |
| FJzz1 | MK288006 | China | 2011 |
| AJ1102 | JX188454 | China | 2011 |
| Primer | Sequence | Lenth (bp) |
|---|---|---|
| PEDV-N-F | 5′-CACAGATAGTGAGAAAGTGCTTCA-3′ | 1434 |
| PEDV-N-R | 5′-CAGTAATAACAGTGTAATGGCACT-3′ | |
| PEDV-S-1-F | 5′-TTTGTGGTTTTTCTAATCATTTGGTCAACG-3′ | 1659 |
| PEDV-S-1-R | 5′-GAACTAAACCCATTGATAGTAGTGTCA-3′ | |
| PEDV-S-2-F | 5′-GTCACAATTAATTTCACTGGTC-3′ | 1716 |
| PEDV-S-2-R | 5′-CTGTAGAACATCCGTCTGTAG-3′ | |
| PEDV-S-3-F | 5′-GCAGATATAGTCTGTGCAC-3′ | 1551 |
| PEDV-S-3-R | 5′-AGAAGTAGATAAAAACACTGGTG-3′ |
| Strain | GenBank No. | Location | Year |
|---|---|---|---|
| CV777 | AF353511 | Belgium | 1977 |
| DR13 | DQ862099 | South Korea | 1999 |
| LZC | EF185992 | China | 2006 |
| KNU-0802 | GU180143 | South Korea | 2008 |
| KNU-0902 | GU180145 | South Korea | 2009 |
| SM98 | GU937797 | South Korea | 2011 |
| CNU-091222-01 | JN184634 | South Korea | 2009 |
| CH/S | JN547228 | China | 1986 |
| BJ-2011-1 | JN825712 | China | 2011 |
| CHGD-01 | JN980698 | China | 2011 |
| virulent_DR13 | JQ023161 | South Korea | 2009 |
| attenuated_DR13 | JQ023162 | South Korea | 2002 |
| GD_B | JX088695 | China | 2012 |
| AJ1102 | JX188454 | China | 2011 |
| LC | JX489155 | China | 2011 |
| ZJCZ4 | JX524137 | China | 2011 |
| SD-M | JX560761 | China | 2012 |
| GD-1 | JX647847 | China | 2011 |
| JS2008 | KC109141 | China | 2013 |
| CH/ZMDZY/11 | KC196276 | China | 2011 |
| AH2012 | KC210145 | China | 2012 |
| JS-HZ2012 | KC210147 | China | 2012 |
| USA/Colorado/2013 | KF272920 | USA | 2013 |
| NCH/GDGZ/2012 | KF384500 | China | 2012 |
| USA/Indiana/17846/2013 | KF452323 | USA | 2013 |
| MN | KF468752 | USA | 2013 |
| IA1 | KF468753 | USA | 2013 |
| IA2 | KF468754 | USA | 2013 |
| CH/JX-1/2013 | KF760557 | China | 2013 |
| CH/YNKM-8/2013 | KF761675 | China | 2013 |
| KPEDV-9 | KF898124 | South Korea | 1997 |
| SHQP/YM/2013 | KJ196348 | China | 2013 |
| OH851 | KJ399978 | USA | 2014 |
| OH1414 | KJ408801 | USA | 2014 |
| KNU-1303 | KJ451038 | South Korea | 2013 |
| KNU-1401 | KJ451047 | South Korea | 2014 |
| KNU-1402 | KJ451048 | South Korea | 2014 |
| CH/JX-2/2013 | KJ526096 | China | 2013 |
| K13JA12-1 | KJ539151 | South Korea | 2014 |
| K14JB01 | KJ539154 | South Korea | 2014 |
| USA/Kansas29/2013 | KJ645637 | USA: Kansas | 2013 |
| USA/Iowa107/2013 | KJ645696 | USA | 2013 |
| MEX/124/2014 | KJ645700 | Mexico | 2014 |
| USA/Minnesota52/2013 | KJ645704 | USA | 2013 |
| VN/VAP1113_1 | KJ960179 | Viet Nam | 2013 |
| GDS01 | KM089829 | China | 2014 |
| KNU-1406-1 | KM403155 | South Korea | 2014 |
| CHM2013 | KM887144 | China | 2013 |
| USA/IA/2013/19321 | KM975738 | USA | 2013 |
| SC1402 | KP162057 | China | 2014 |
| Hawaii/39249/2014 | KP688354 | USA | 2013 |
| SQ2014 | KP728470 | China | 2013 |
| FL2013 | KP765609 | China | 2014 |
| CH/HNYF/14 | KP890336 | China | 2015 |
| 15V010/BEL/2015 | KR003452 | Belgium | 2015 |
| FR/001/2014 | KR011756 | France | 2014 |
| PC21A | KR078299 | USA | 2013 |
| CH/HNQX-3/14 | KR095279 | China | 2015 |
| EAS1 | KR610991 | Thailand | 2015 |
| EAS2 | KR610992 | Thailand | 2014 |
| CBR1 | KR610993 | Thailand | 2014 |
| CH/HNAY/2015 | KR809885 | China | 2015 |
| YN1 | KT021227 | China | 2013 |
| YN15 | KT021228 | China | 2013 |
| CH/HNLH/2015 | KT199103 | China | 2015 |
| CV777 | KT323979 | China | 1998 |
| HUA-14PED96 | KT941120 | Viet Nam | 2014 |
| YC2014 | KU252649 | China | 2014 |
| SLO/JH-11/2015 | KU297956 | Slovenia | 2015 |
| ZJU/G1/2013 | KU664503 | China | 2013 |
| CH/SCCD/2014 | KU975389 | China | 2014 |
| JSLS-1/2015 | KX534205 | China | 2015 |
| KB2013-4 | KX580953 | China | 2013 |
| 85-7 | KX839246 | China | 2013 |
| CH/GX/2015/750A | KY793536 | China | 2015 |
| AVCT12 | LC053455 | Thailand | 2010 |
| IWT-1/JPN/2014 | LC063834 | Japan | 2014 |
| OKN-1/JPN/2013 | LC063836 | Japan | 2013 |
| MYG-1/JPN/2014 | LC063838 | Japan | 2014 |
| MYZ-1/JPN/2013 | LC063846 | Japan | 2013 |
| L00721/GER/2014 | LM645057 | Germany | 2014 |
| GER/L00719/2014 | LM645058 | Germany | 2014 |
| PEDV/GER/L01020-K01_15-10/2015 | LT898413 | Germany | 2015 |
| PEDV/GER/L01014-K01_15-04/2015 | LT898420 | Germany | 2015 |
| PEDV/GER/L00906-K16_14-01/2014 | LT898430 | Germany | 2014 |
| CH/JXJA/2017 | MF375374 | China | 2017 |
| NW8 | MF782687 | China | 2015 |
| PPC 14 | MG781192 | South Korea | 2014 |
| CH/SCZY44/2017 | MH061338 | China | 2017 |
| CH/SCDY523/2018 | MH593144 | China | 2018 |
| JS-A | MH748550 | China | 2017 |
| FJzz1 | MK288006 | China | 2011 |
| LW/L | MK392335 | China | 2010 |
| SNJ-P | MK702008 | China | 2018 |
| PEDV-1556-Valencia-Requena | MN692763 | Spain | 2014 |
| PEDV-1611-Murcia-Lorca | MN692768 | Spain | 2015 |
| PEDV-1613-Murcia-Fuentealamo | MN692769 | Spain | 2015 |
| CH-HNAY-2016 | MN893406 | China | 2016 |
| CH-HNAY-2017 | MN893407 | China | 2017 |
| CH-HNHB-1-2017 | MN893408 | China | 2017 |
| CH-HNHB-2-2017 | MN893409 | China | 2017 |
| CH-HNJY-2017 | MN893410 | China | 2017 |
| CH-HNLH-2016 | MN893411 | China | 2016 |
| CH-HNLH-2017 | MN893412 | China | 2017 |
| CH-HNNY-2017 | MN893413 | China | 2017 |
| CH-HNPDS-1-2018 | MN893414 | China | 2018 |
| CH-HNPDS-2-2018 | MN893415 | China | 2018 |
| CH-HNPY-2016 | MN893416 | China | 2016 |
| CH-HNSMX-2016 | MN893417 | China | 2016 |
| CH-HNXX-2017 | MN893418 | China | 2017 |
| CH-HNXY-1-2017 | MN893419 | China | 2017 |
| CH-HNXY-2-2017 | MN893420 | China | 2017 |
| CH-HNXY-3-2018 | MN893421 | China | 2018 |
| CH-HNZK-1-2017 | MN893422 | China | 2017 |
| CH-HNZK-2-2018 | MN893423 | China | 2018 |
| CH-HNZK-3-2018 | MN893424 | China | 2018 |
| CH-HNZK-4-2017 | MN893425 | China | 2017 |
| CH-HNZMD-2017 | MN893426 | China | 2017 |
| CH-HNZZ-2017 | MN893427 | China | 2017 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Q.; Fu, P.; Zhou, Y.; Lang, Y.; Zhao, S.; Wen, Y.; Wang, Y.; Wu, R.; Zhao, Q.; Du, S.; et al. Phylogenetic Analysis of Porcine Epidemic Diarrhea Virus (PEDV) during 2020–2022 and Isolation of a Variant Recombinant PEDV Strain. Int. J. Mol. Sci. 2024, 25, 10878. https://doi.org/10.3390/ijms252010878
Peng Q, Fu P, Zhou Y, Lang Y, Zhao S, Wen Y, Wang Y, Wu R, Zhao Q, Du S, et al. Phylogenetic Analysis of Porcine Epidemic Diarrhea Virus (PEDV) during 2020–2022 and Isolation of a Variant Recombinant PEDV Strain. International Journal of Molecular Sciences. 2024; 25(20):10878. https://doi.org/10.3390/ijms252010878
Chicago/Turabian StylePeng, Qianling, Ping Fu, Yutong Zhou, Yifei Lang, Shan Zhao, Yiping Wen, Yiping Wang, Rui Wu, Qin Zhao, Senyan Du, and et al. 2024. "Phylogenetic Analysis of Porcine Epidemic Diarrhea Virus (PEDV) during 2020–2022 and Isolation of a Variant Recombinant PEDV Strain" International Journal of Molecular Sciences 25, no. 20: 10878. https://doi.org/10.3390/ijms252010878





