Manganese Oxide-Doped Hierarchical Porous Carbon Derived from Tea Leaf Waste for High-Performance Supercapacitors
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Preparation of Tie Guan Yin Tea Leaf Carbon (TGC)
3.3. Characterization
3.4. Preparation of Electrode
3.5. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bang, J.H.; Lee, B.H.; Choi, Y.C.; Lee, H.M.; Kim, B.J. A study on superior mesoporous activated carbons for ultra power density supercapacitor from biomass precursors. Int. J. Mol. Sci. 2022, 23, 8537. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, C.; Cao, X.; Wang, Q.; Yang, G.; Chen, J. Porous carbon spheres derived from hemicelluloses for supercapacitor application. Int. J. Mol. Sci. 2022, 23, 7101. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, B.J.; Kim, S.J.; Park, Y.K.; Jung, S.C. Enhanced electrochemical performance of carbon nanotube with nitrogen and iron using liquid phase plasma process for supercapacitor applications. Int. J. Mol. Sci. 2018, 19, 3830. [Google Scholar] [CrossRef] [PubMed]
- De Silva, T.; Damery, C.; Alkhaldi, R.; Karunanithy, R.; Gallaba, D.H.; Patil, P.D.; Wasala, M.; Sivakumar, P.; Migone, A.; Talapatra, S. Carbon nanotube based robust and flexible solid-state supercapacitor. ACS Appl. Mater. Interfaces 2021, 13, 56004–56013. [Google Scholar] [CrossRef]
- Mo, Y.; Du, J.; Lv, H.; Zhang, Y.; Chen, A. N-doped mesoporous carbon nanosheets for supercapacitors with high performance. Diam. Relat. Mater. 2021, 111, 108206. [Google Scholar] [CrossRef]
- Li, B.; Yu, M.; Li, Z.; Yu, C.; Li, Q.; Wang, H. Three-dimensional activated carbon nanosheets modified by graphitized carbon dots: One-step alkali pyrolysis preparation and supercapacitor applications. J. Energy Storage 2022, 51, 104515. [Google Scholar] [CrossRef]
- Liu, C.; Yu, Z.; Neff, D.; Zhamu, A.; Jang, B.Z. Graphene-based supercapacitor with an ultrahigh energy density. Nano Lett. 2010, 10, 4863–4868. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Ni, M.; Ren, X.; Tian, Y.; Li, N.; Su, Y.; Zhang, X. Graphene in supercapacitor applications. Curr. Opin. Colloid Interface Sci. 2015, 20, 416–428. [Google Scholar] [CrossRef]
- Chou, T.C.; Huang, C.H.; Doong, R.A.; Hu, C.C. Architectural design of hierarchically ordered porous carbons for high-rate electrochemical capacitors. J. Mater. Chem. A 2013, 1, 2886–2895. [Google Scholar] [CrossRef]
- Shang, T.; Xu, Y.; Li, P.; Han, J.; Wu, Z.; Tao, Y.; Yang, Q.H. A bio-derived sheet-like porous carbon with thin-layer pore walls for ultrahigh-power supercapacitors. Nano Energy 2020, 70, 104531. [Google Scholar] [CrossRef]
- Yin, Y.; Liu, Q.; Zhao, T.; Chen, T.; Wang, J.; Gui, L.; Lu, C. Recent progress and future directions of biomass-derived hierarchical porous carbon: Designing, preparation, and supercapacitor applications. Energy Fuels 2023, 37, 3523–3554. [Google Scholar] [CrossRef]
- Hu, H.; Yan, M.; Jiang, J.; Huang, A.; Cai, S.; Lan, L.; Ye, K.; Chen, D.; Tang, K.; Zuo, Q.; et al. A state-of-the-art review on biomass-derived carbon materials for supercapacitor applications: From precursor selection to design optimization. Sci. Total Environ. 2024, 912, 169141. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.; Singh, M.K.; Yadav, N.; Hashmi, S. High performance quasi-solid-state supercapacitors with peanut-shell-derived porous carbon. J. Power Sources 2018, 402, 133–146. [Google Scholar] [CrossRef]
- Liang, K.; Chen, Y.; Wang, S.; Wang, D.; Wang, W.; Jia, S.; Mitsuzakic, N.; Chen, Z. Peanut shell waste derived porous carbon for high-performance supercapacitors. J. Energy Storage 2023, 70, 107947. [Google Scholar] [CrossRef]
- Sasono, S.R.A.; Rois, M.F.; Widiyastuti, W.; Nurtono, T.; Setyawan, H. Nanofiber-enrich dispersed activated carbon derived from coconut shell for supercapacitor material. Results Eng. 2023, 18, 101070. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, Z.; Song, N.; Li, X. High-performance supercapacitors and batteries derived from activated banana-peel with porous structures. Electrochim. Acta 2016, 222, 1257–1266. [Google Scholar] [CrossRef]
- Tripathy, A.; Mohanty, S.; Nayak, S.K.; Ramadoss, A. Renewable banana-peel-derived activated carbon as an inexpensive and efficient electrode material showing fascinating supercapacitive performance. J. Environ. Chem. Eng. 2021, 9, 106398. [Google Scholar] [CrossRef]
- Ramasahayam, S.K.; Clark, A.L.; Hicks, Z.; Viswanathan, T. Spent coffee grounds derived P, N co-doped C as electrocatalyst for supercapacitor applications. Electrochim. Acta 2015, 168, 414–422. [Google Scholar] [CrossRef]
- Yun, Y.S.; Park, M.H.; Hong, S.J.; Lee, M.E.; Park, Y.W.; Jin, H.J. Hierarchically porous carbon nanosheets from waste coffee grounds for supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 3684–3690. [Google Scholar] [CrossRef]
- Hossain, R.; Nekouei, R.K.; Mansuri, I.; Sahajwalla, V. In-situ O/N-heteroatom enriched activated carbon by sustainable thermal transformation of waste coffee grounds for supercapacitor material. J. Energy Storage 2021, 33, 102113. [Google Scholar] [CrossRef]
- Zhi, M.; Xiang, C.; Li, J.; Li, M.; Wu, N. Nanostructured carbon–metal oxide composite electrodes for supercapacitors: A review. Nanoscale 2013, 5, 72–88. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, J.; Wang, T.; Shao, J.; Wang, D.; Yang, Y.W. Mesoporous transition metal oxides for supercapacitors. Nanomater 2015, 5, 1667–1689. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Xing, B.; Zhang, C.; Huang, G.; Yu, J.; Jiang, Z.; Qu, X.; Wu, X.; Cao, Y.; Zhang, C. MnOX-modified corrugated carton-derived hierarchical porous carbon with ultrafast kinetics behaviour for high-performance symmetric supercapacitors. J. Alloys Compd. 2020, 848, 156423. [Google Scholar] [CrossRef]
- Yang, G.; Park, S.J. MnO2 and biomass-derived 3D porous carbon composites electrodes for high performance supercapacitor applications. J. Alloys Compd. 2018, 741, 360–367. [Google Scholar] [CrossRef]
- Ma, Z.W.; Liu, H.Q.; Lü, Q.F. Porous biochar derived from tea saponin for supercapacitor electrode: Effect of preparation technique. J. Energy Storage 2021, 40, 102773. [Google Scholar] [CrossRef]
- Inal, I.I.G.; Holmes, S.M.; Banford, A.; Aktas, Z. The performance of supercapacitor electrodes developed from chemically activated carbon produced from waste tea. Appl. Surf. Sci. 2015, 357, 696–703. [Google Scholar] [CrossRef]
- Thirumal, V.; Yuvakkumar, R.; Ravi, G.; Dineshkumar, G.; Ganesan, M.; Alotaibi, S.H.; Velauthapillai, D. Characterization of activated biomass carbon from tea leaf for supercapacitor applications. Chemosphere 2022, 291, 132931. [Google Scholar] [CrossRef]
- Bhoyate, S.; Ranaweera, C.K.; Zhang, C.; Morey, T.; Hyatt, M.; Kahol, P.K.; Ghimire, M.; Mishra, S.R.; Gupta, R.K. Eco-friendly and high performance supercapacitors for elevated temperature applications using recycled tea leaves. Glob. Chall. 2017, 1, 1700063. [Google Scholar] [CrossRef]
- Gandla, D.; Chen, H.; Tan, D.Q. Mesoporous structure favorable for high voltage and high energy supercapacitor based on green tea waste-derived activated carbon. Mater. Res. Express 2020, 7, 085606. [Google Scholar] [CrossRef]
- Eom, H.; Kim, J.; Nam, I.; Bae, S. Recycling black tea waste biomass as activated porous carbon for long life cycle supercapacitor electrodes. Materials 2021, 14, 6592. [Google Scholar] [CrossRef]
- Qu, X.; Kang, W.; Lai, C.; Zhang, C.; Hong, S.W. A simple route to produce highly efficient porous carbons recycled from tea waste for high-performance symmetric supercapacitor electrodes. Molecules 2022, 27, 791. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Xi, H.; Cui, F.; Zhang, J.; Chen, P.; Cui, T. Self-templating synthesis of hierarchical porous carbon with multi-heteroatom co-doping from tea waste for high-performance supercapacitor. J. Energy Storage 2022, 45, 103509. [Google Scholar] [CrossRef]
- Fu, M.; Zhu, Z.; Zhang, Z.; Zhuang, Q.; Gao, F.; Chen, W.; Yu, H.; Liu, Q. Microwave assisted growth of MnO 2 on biomass carbon for advanced supercapacitor electrode materials. J. Mater. Sci. 2021, 56, 6987–6996. [Google Scholar] [CrossRef]
- Tian, Z.; Li, H.; Yang, W.; Jian, S.; Zhang, C.; Jiang, S. Fabrication of manganese oxides/carbon composites for high energy density asymmetric supercapacitor. Diam. Relat. Mater. 2023, 131, 109582. [Google Scholar] [CrossRef]
- Li, H.; Yang, H.; Sun, H.; Huang, Y.; An, P.; Yunhua, Y.; Zhao, H. A manganese oxide/biomass porous carbon composite for high-performance supercapacitor electrodes. Electrochim. Acta 2024, 473, 143514. [Google Scholar] [CrossRef]
- Peng, C.; Yan, X.B.; Wang, R.T.; Lang, J.W.; Ou, Y.J.; Xue, Q.J. Promising activated carbons derived from waste tea-leaves and their application in high performance supercapacitors electrodes. Electrochim. Acta 2013, 87, 401–408. [Google Scholar] [CrossRef]
- Zhao, N.; Deng, L.; Luo, D.; Zhang, P. One-step fabrication of biomass-derived hierarchically porous carbon/MnO nanosheets composites for symmetric hybrid supercapacitor. Appl. Surf. Sci. 2020, 526, 146696. [Google Scholar] [CrossRef]
- Radhakanth, S.; Singhal, R. In–situ synthesis of MnO dispersed carbon nanofibers as binder-free electrodes for high-performance supercapacitors. Chem. Eng. Sci. 2023, 265, 118224. [Google Scholar] [CrossRef]
- Chen, R.; Wang, Y.; Liu, Y.; Li, J. Selective electrochemical detection of dopamine using nitrogen-doped graphene/manganese monoxide composites. RSC Adv. 2015, 5, 85065–85072. [Google Scholar] [CrossRef]
- Guo, W.; Guo, X.; Yang, L.; Wang, T.; Zhang, M.; Duan, G.; Liu, X.; Li, Y. Synthetic melanin facilitates MnO supercapacitors with high specific capacitance and wide operation potential window. Polymer 2021, 235, 124276. [Google Scholar] [CrossRef]
- Xiao, N.; Zhang, X.; Liu, C.; Wang, Y.; Li, H.; Qiu, J. Coal-based carbon anodes for high-performance potassium-ion batteries. Carbon 2019, 147, 574–581. [Google Scholar] [CrossRef]
- Khan, A.; Senthil, R.A.; Pan, J.; Osman, S.; Sun, Y.; Shu, X. A new biomass derived rod-like porous carbon from tea-waste as inexpensive and sustainable energy material for advanced supercapacitor application. Electrochim. Acta 2020, 335, 135588. [Google Scholar] [CrossRef]
- Bedin, K.C.; Martins, A.C.; Cazetta, A.L.; Pezoti, O.; Almeida, V.C. KOH-activated carbon prepared from sucrose spherical carbon: Adsorption equilibrium, kinetic and thermodynamic studies for Methylene Blue removal. Chem. Eng. J. 2016, 286, 476–484. [Google Scholar] [CrossRef]
- Zou, K.; Cai, P.; Wang, B.; Liu, C.; Li, J.; Qiu, T.; Zou, G.; Hou, H.; Ji, X. Insights into enhanced capacitive behavior of carbon cathode for lithium ion capacitors: The coupling of pore size and graphitization engineering. Nanomicro Lett. 2020, 12, 1–19. [Google Scholar] [CrossRef]
- Ke, Q.; Zhang, Y.; Fu, Y.; Yang, C.; Wu, F.; Li, Z.; Wei, Y.; Zhang, K. Study on electrochemical performance of MnO@rGO/carbon fabric-based wearable supercapacitors. Materials 2023, 15, 4687. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Liu, B.; Liu, Y.; Chen, H.; Yang, M.; Li, H. MnO2 nanostructures deposited on graphene-like porous carbon nanosheets for high-rate performance and high-energy density asymmetric supercapacitors. ACS Sustain. Chem. Eng. 2019, 7, 3101–3110. [Google Scholar] [CrossRef]
- Ratnaji, T.; Kennedy, L.J. Hierarchical porous carbon derived from tea waste for energy storage applications: Waste to worth. Diam. Relat. Mater. 2020, 110, 108100. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, X.S. A comparative study of electrocapacitive properties of manganese dioxide clusters dispersed on different carbons. Carbon 2013, 52, 1–9. [Google Scholar] [CrossRef]
- Sankar, S.; Ahmed, A.T.A.; Inamdar, A.I.; Im, H.; Im, Y.B.; Lee, Y.; Kim, D.Y.; Lee, S. Biomass-derived ultrathin mesoporous graphitic carbon nanoflakes as stable electrode material for high-performance supercapacitors. Mater. Des. 2019, 169, 107688. [Google Scholar] [CrossRef]
- Song, X.; Ma, X.; Li, Y.; Ding, L.; Jiang, R. Tea waste derived microporous active carbon with enhanced double-layer supercapacitor behaviors. Appl. Surf. Sci. 2019, 487, 189–197. [Google Scholar] [CrossRef]
- Wang, H.; Chen, H.; Hou, X.; Ye, H.; Guo, Z.; Chen, Z.; Jin, Y.; Du, Y.; Ren, P. MnO decorated biomass derived carbon based on hyperaccumulative characteristics as advanced electrode materials for high-performance supercapacitors. Diam. Relat. Mater. 2023, 136, 109888. [Google Scholar] [CrossRef]
- Huang, T.; Qiu, Z.; Wu, D.; Hu, Z. Bamboo-based activated carbon @ MnO2 nanocomposites for flexible high-performance supercapacitor electrode materials. Int. J. Electrochem. Sci. 2015, 10, 6312–6323. [Google Scholar] [CrossRef]
- Xuan, H.; Wang, Y.; Lin, G.; Wang, F.; Zhou, L.; Dong, X.; Chen, Z. Air-assisted activation strategy for porous carbon spheres to give enhanced electrochemical performance. RSC Adv. 2016, 6, 15313–15319. [Google Scholar] [CrossRef]



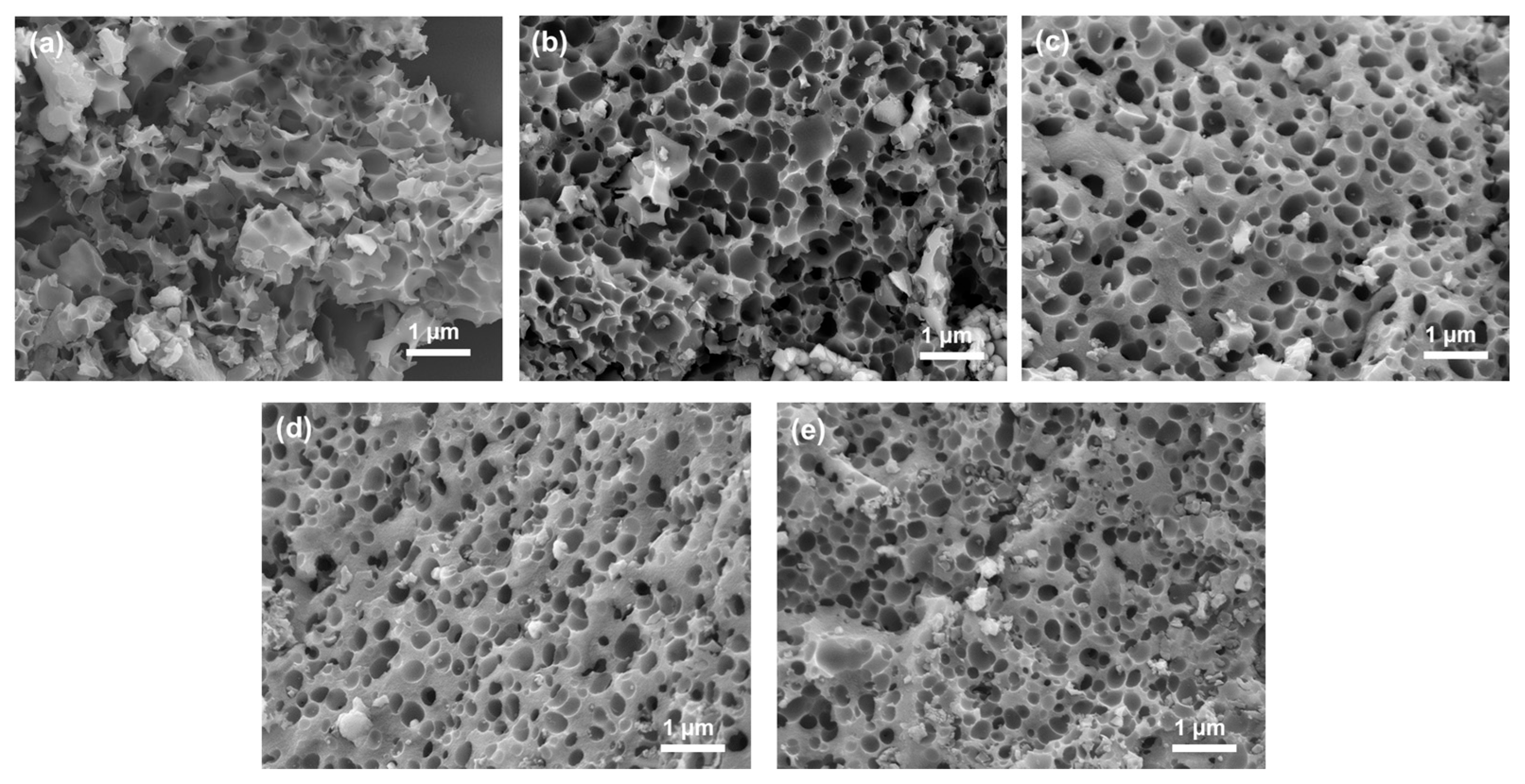
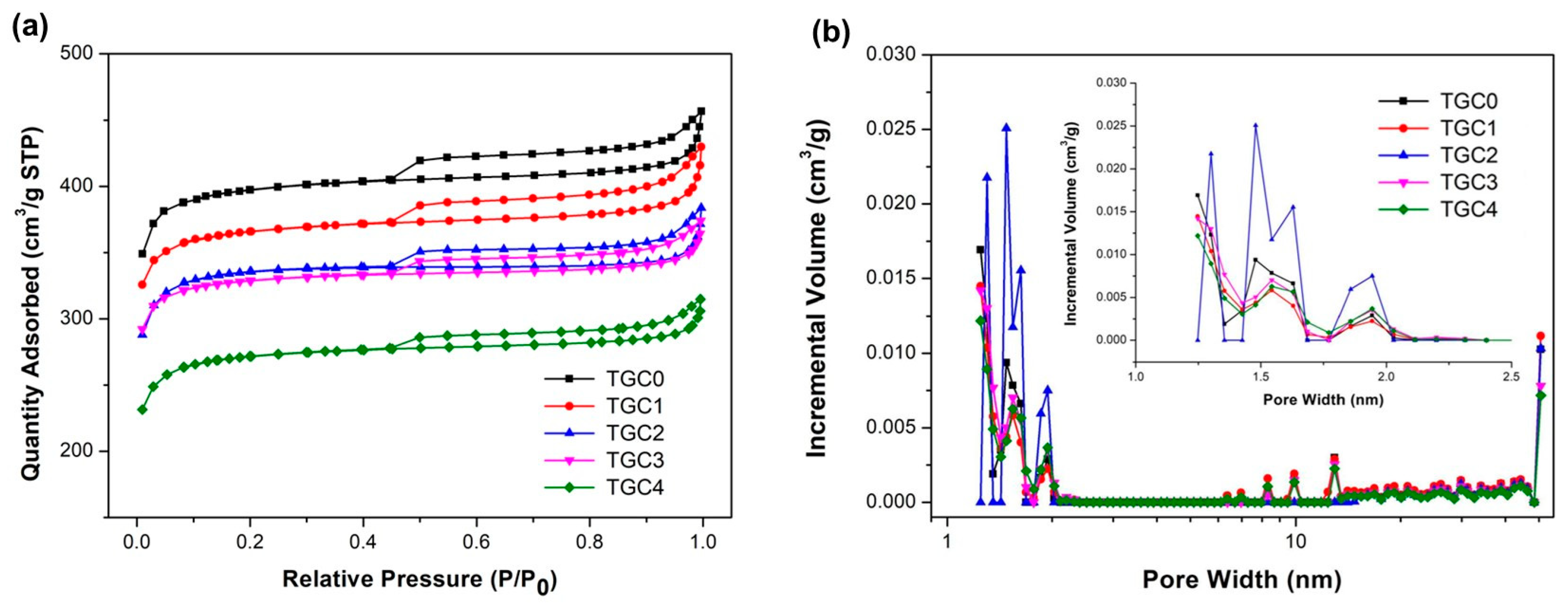

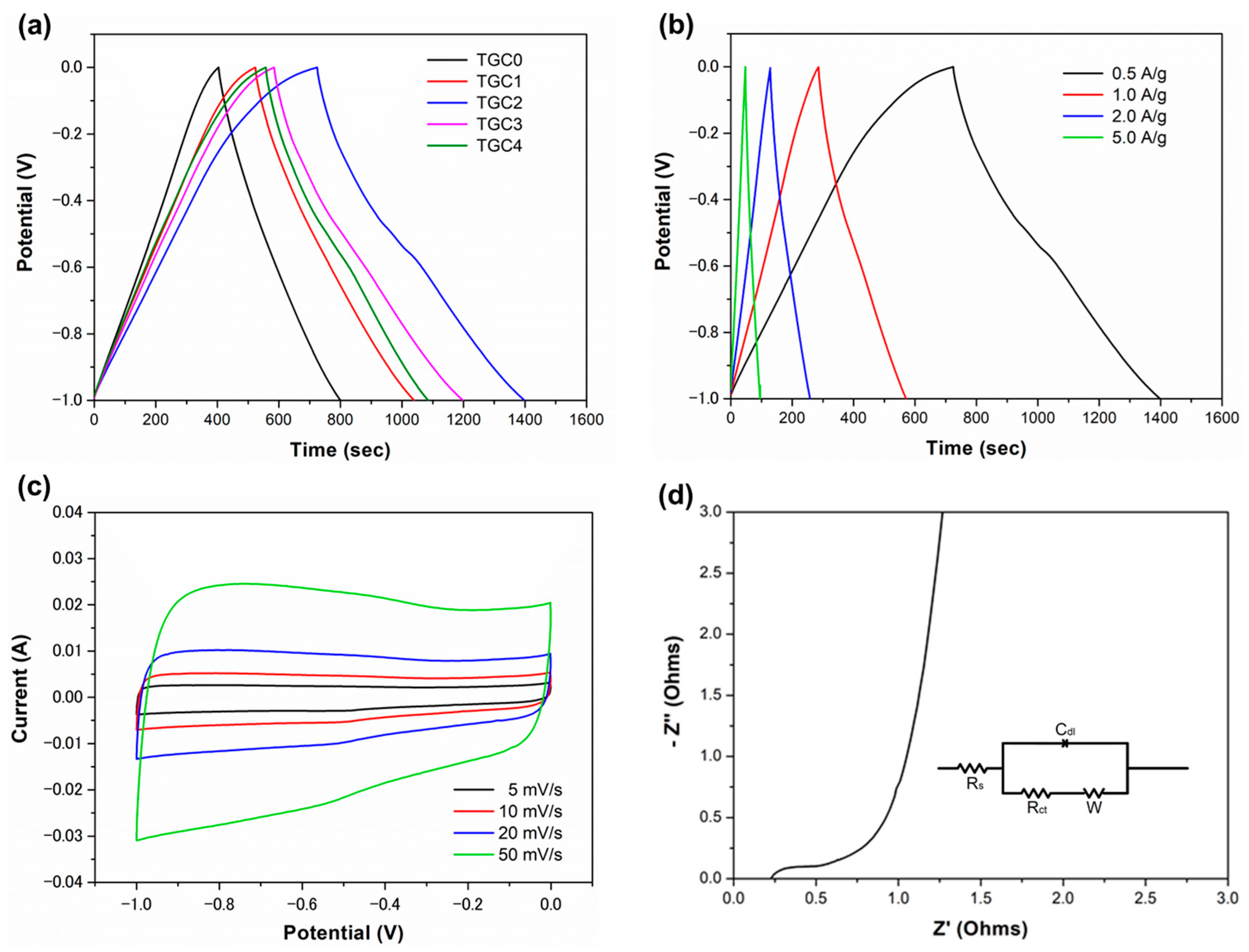
| Specimen | SBET m2/g | Smicro m2/g | Vtotal cm3/g | Vmicro cm3/g | Average Pore Diameter nm | Smicro/Stotal % | Vmicro/Vtotal % |
|---|---|---|---|---|---|---|---|
| TCG0 | 1483 | 1432 | 0.6882 | 0.5945 | 1.86 | 96.56 | 86.38 |
| TCG1 | 1367 | 1320 | 0.6431 | 0.5477 | 1.88 | 96.56 | 85.17 |
| TCG2 | 1259 | 1226 | 0.5747 | 0.5058 | 1.83 | 97.38 | 88.01 |
| TCG3 | 1229 | 1193 | 0.5633 | 0.4941 | 1.84 | 97.07 | 87.72 |
| TCG4 | 1017 | 976 | 0.4730 | 0.4043 | 1.87 | 95.97 | 85.48 |
| Specimen | C=C 284.8 eV | C-O 286.1 eV | C=O 288.9 eV | (C-O) + (C=O) |
|---|---|---|---|---|
| TCG1 | 75.04% | 17.81% | 7.15% | 24.96% |
| TCG2 | 62.85% | 31.72% | 5.43% | 37.15% |
| TCG3 | 63.68% | 29.94% | 6.38% | 36.32% |
| TCG4 | 76.13% | 18.97% | 4.90% | 23.87% |
| Specimen | Mn-O-Mn 530.4 eV | Mn-O-C 531.9 eV | (H-O-H)/C-O 532.7 eV | COOH 533.7 eV |
|---|---|---|---|---|
| TCG1 | 2.04% | 40.87% | 31.62% | 25.48% |
| TCG2 | 2.87% | 40.71% | 22.56% | 33.86% |
| TCG3 | 4.10% | 42.41% | 27.15% | 26.34% |
| TCG4 | 5.87% | 53.06% | 25.49% | 15.58% |
| Active Materials | Current Density (A/g) | Specific Capacitance (F/g) | References |
|---|---|---|---|
| Microporous and mesoporous K2CO3-activated carbons produced from tea waste. | 1.5 mA/cm2 | 203 | [26] |
| KOH-activated biomass carbon from tea leaves. | 0.5 | 132 | [27] |
| KOH-activated porous carbons derived from tea waste. | 0.05 | 256 | [31] |
| Hierarchical porous carbon with multi-heteroatom co-doping from tea waste. | 0.5 | 170 | [32] |
| KOH-activated carbons derived from Bi luo chun tea leaf waste. | 1 | 330 | [36] |
| KOH-activated rod-like porous carbon from tea-waste. | 1 | 332 | [42] |
| Green tea waste-derived ultrathin mesoporous graphitic carbon nanoflakes. | 0.5 | 165 | [50] |
| Tea waste-derived microporous KOH-activated carbon. | 1 | 170 | [51] |
| Manganese oxide-doped hierarchical porous carbon derived from tea leaf waste. | 0.5 | 337 | This work |
| MnOX-modified corrugated carton-derived hierarchical porous carbon. | 2.5 | 279 | [23] |
| MnO2 and banana peel-derived 3D porous carbon composites. | 0.3 | 140 | [24] |
| Manganese oxide/soybean straw porous carbon composite | 1 | 438 | [35] |
| litchi shell-derived hierarchically porous carbon/MnO nanosheets. | 1 | 496 | [37] |
| MnO-decorated Salvinia adnata-based carbon. | 1 | 437 | [52] |
| Bamboo-based activated carbon @ MnO2 nanocomposites. | 1 | 221 | [53] |
| Specimen | Tie Guan Yin Tea Hydrochar | KOH | KMnO4 |
|---|---|---|---|
| TGC0 | 1 | 3 | 0 |
| TCG1 | 1 | 3 | 0.01 |
| TCG2 | 1 | 3 | 0.02 |
| TCG3 | 1 | 3 | 0.03 |
| TCG4 | 1 | 3 | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, H.-Y.; Chang, H.-M.; Wang, C.-P. Manganese Oxide-Doped Hierarchical Porous Carbon Derived from Tea Leaf Waste for High-Performance Supercapacitors. Int. J. Mol. Sci. 2024, 25, 10884. https://doi.org/10.3390/ijms252010884
Chung H-Y, Chang H-M, Wang C-P. Manganese Oxide-Doped Hierarchical Porous Carbon Derived from Tea Leaf Waste for High-Performance Supercapacitors. International Journal of Molecular Sciences. 2024; 25(20):10884. https://doi.org/10.3390/ijms252010884
Chicago/Turabian StyleChung, Hsiu-Ying, Hong-Min Chang, and Chun-Pang Wang. 2024. "Manganese Oxide-Doped Hierarchical Porous Carbon Derived from Tea Leaf Waste for High-Performance Supercapacitors" International Journal of Molecular Sciences 25, no. 20: 10884. https://doi.org/10.3390/ijms252010884
APA StyleChung, H.-Y., Chang, H.-M., & Wang, C.-P. (2024). Manganese Oxide-Doped Hierarchical Porous Carbon Derived from Tea Leaf Waste for High-Performance Supercapacitors. International Journal of Molecular Sciences, 25(20), 10884. https://doi.org/10.3390/ijms252010884





