Genome-Wide Association Studies (GWAS) and Transcriptome Analysis Reveal Male Heterogametic Sex-Determining Regions and Candidate Genes in Northern Snakeheads (Channa argus)
Abstract
1. Introduction
2. Results
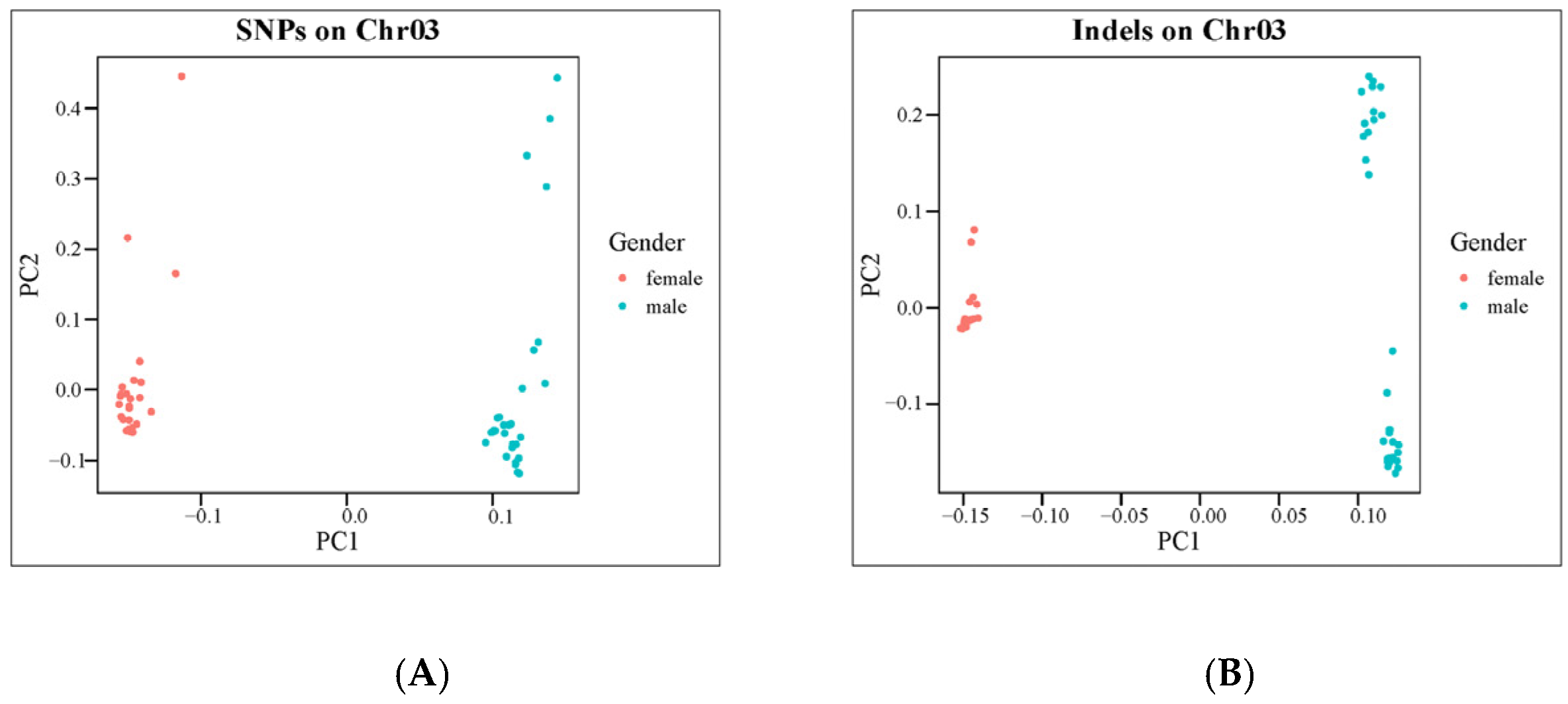
2.1. Resequencing, Variants Calling and Population Structure Analyses
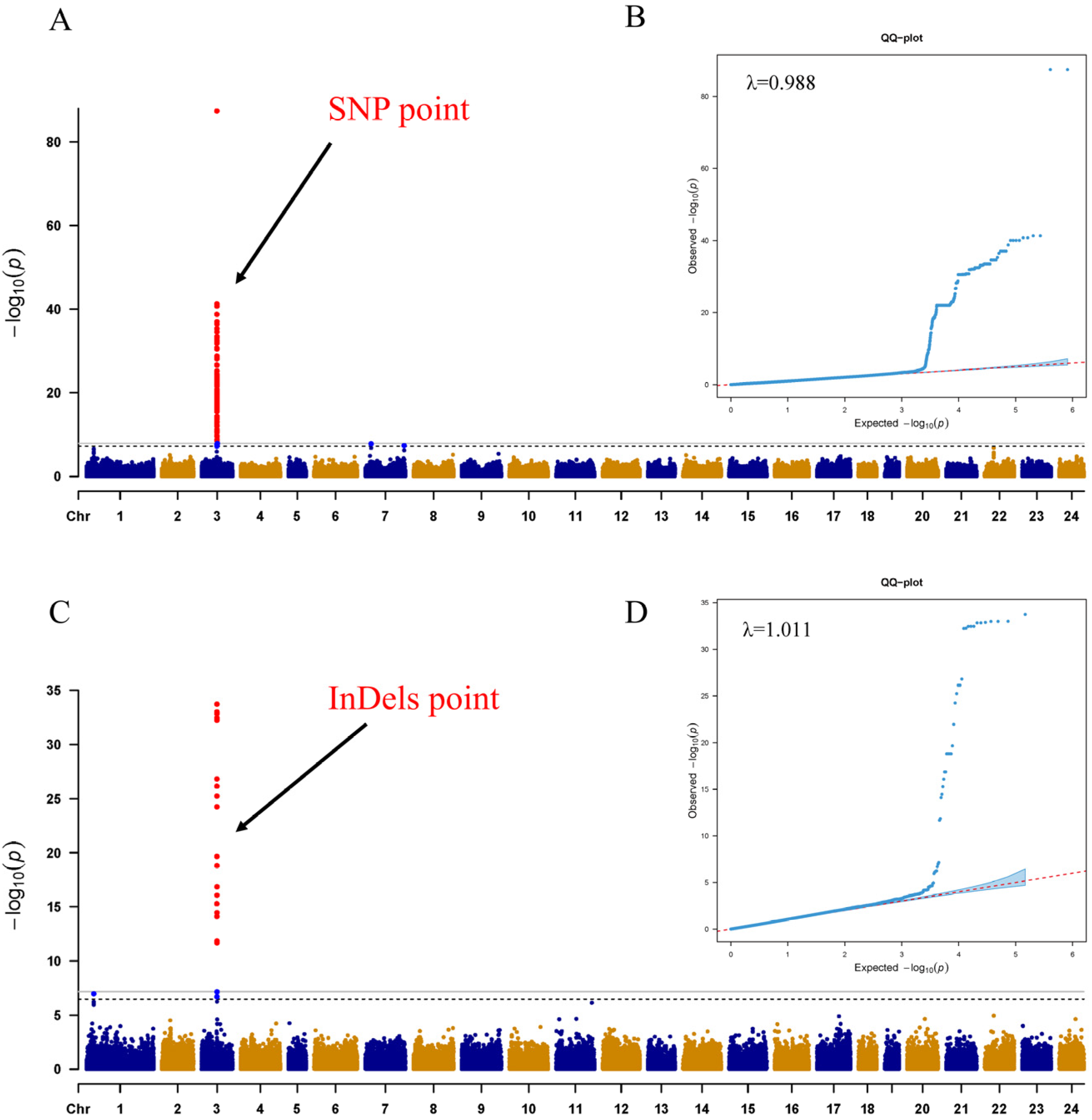
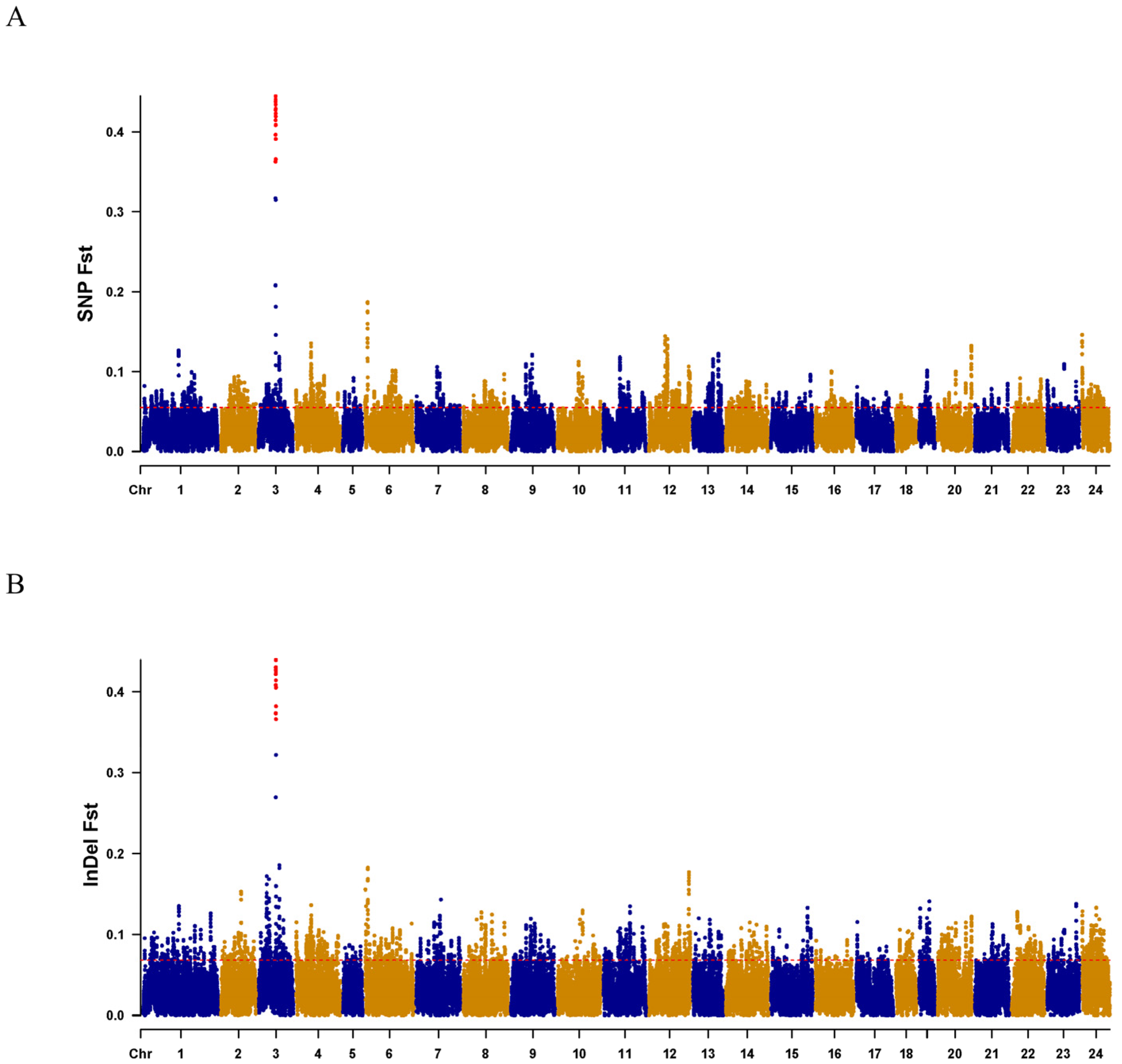
2.2. Sex-Linked Region and Candidate Gene of C. argus
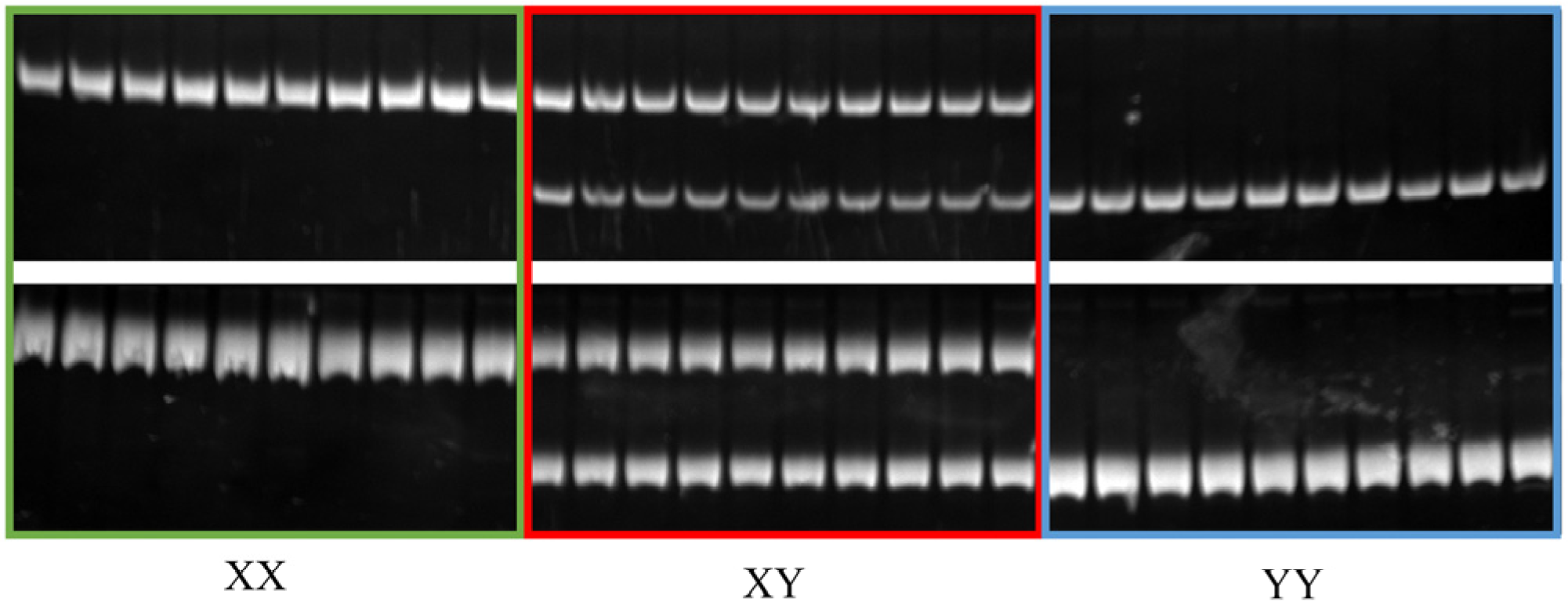
2.3. Selection and Test of the Sex-Specific Markers
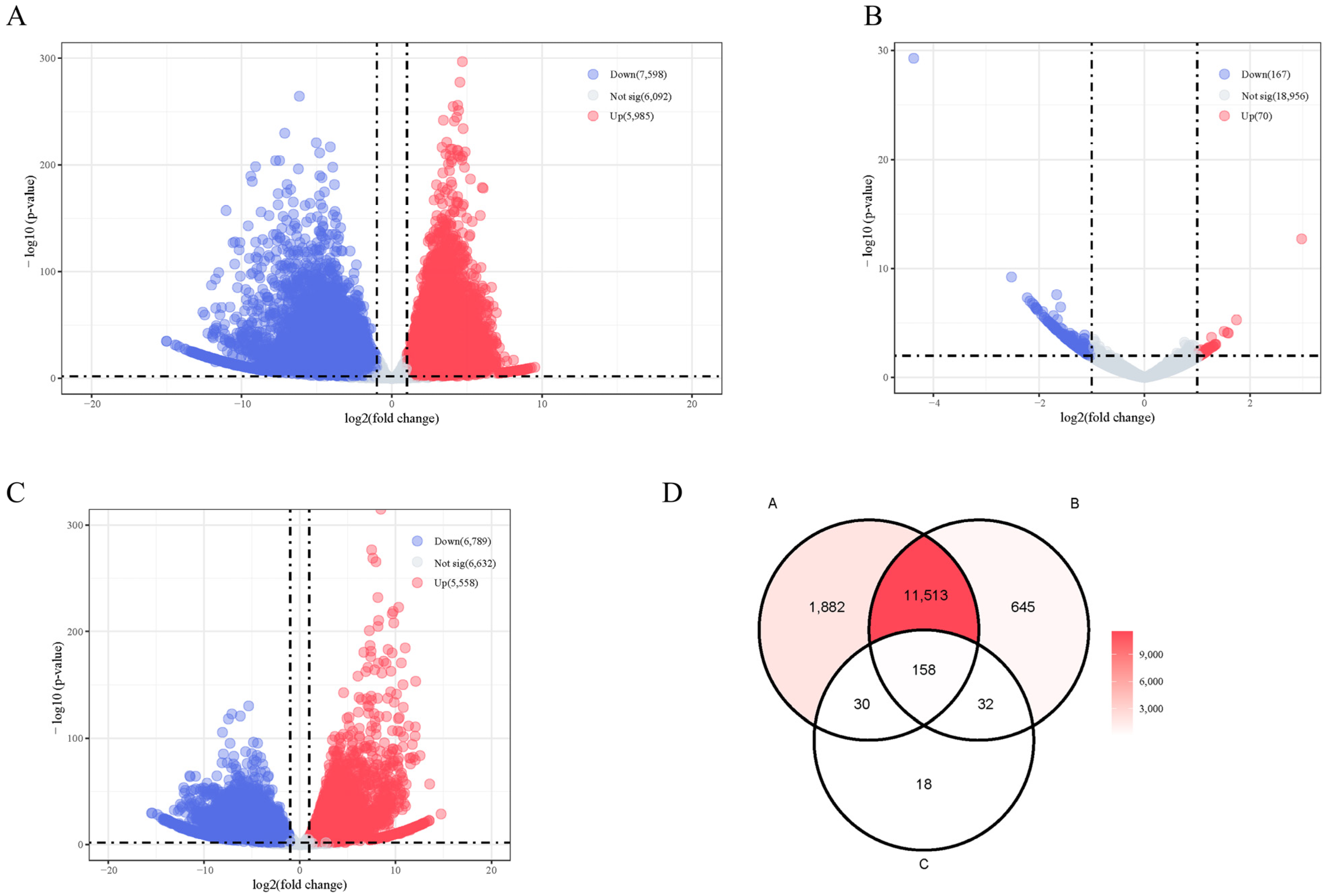
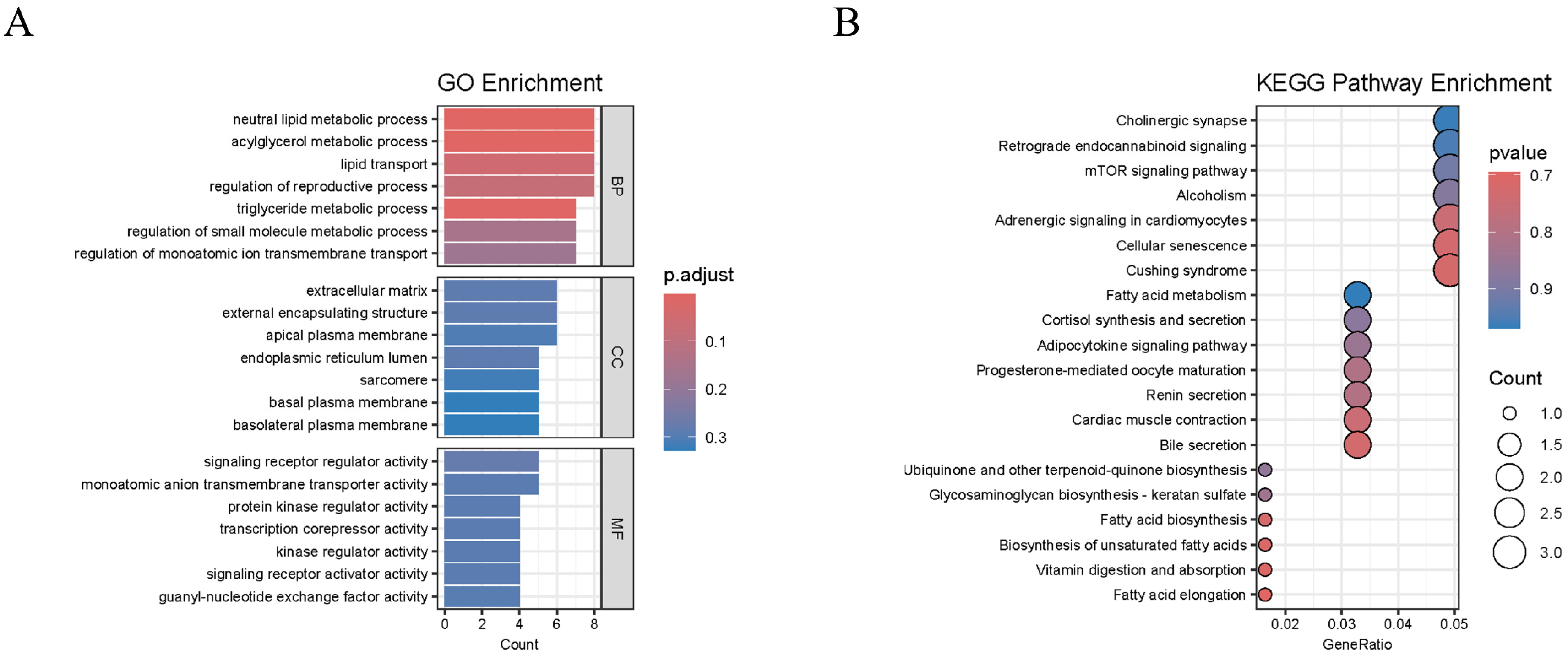
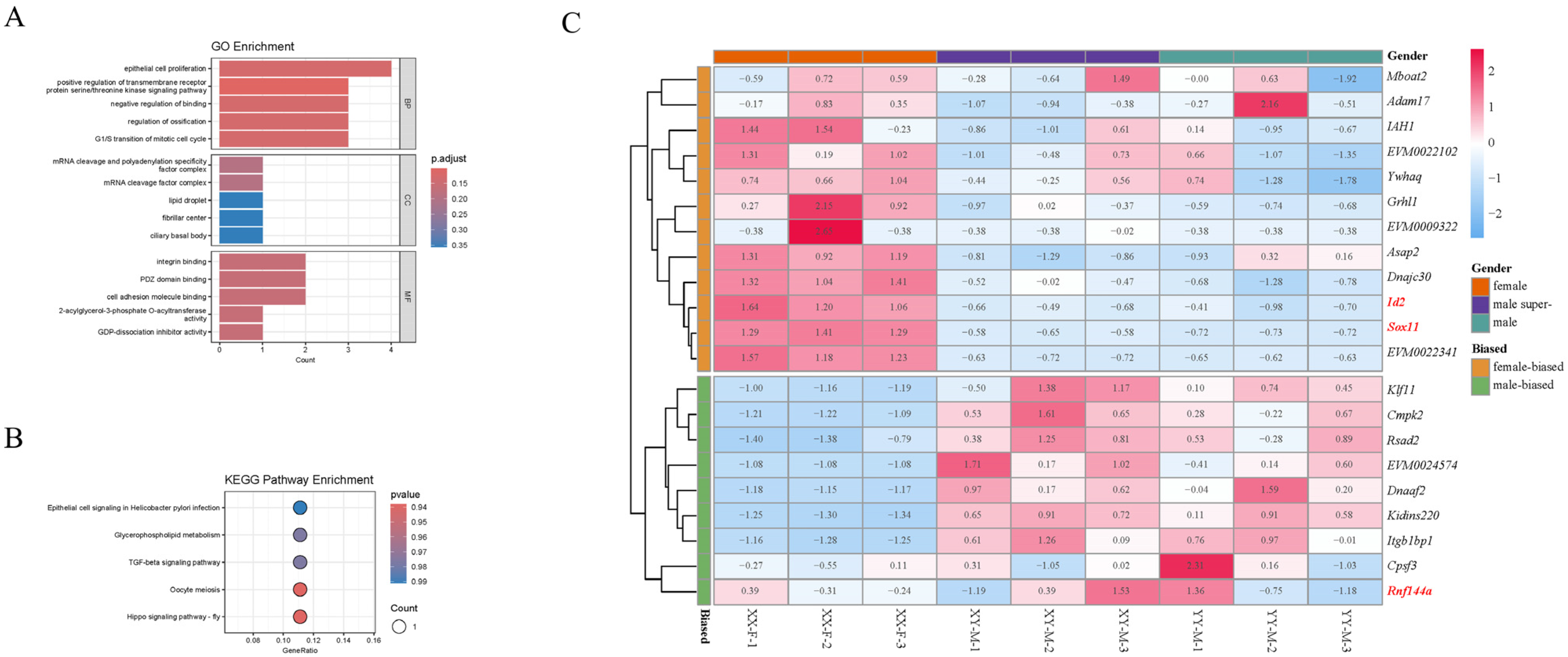
2.4. Transcriptome Analysis
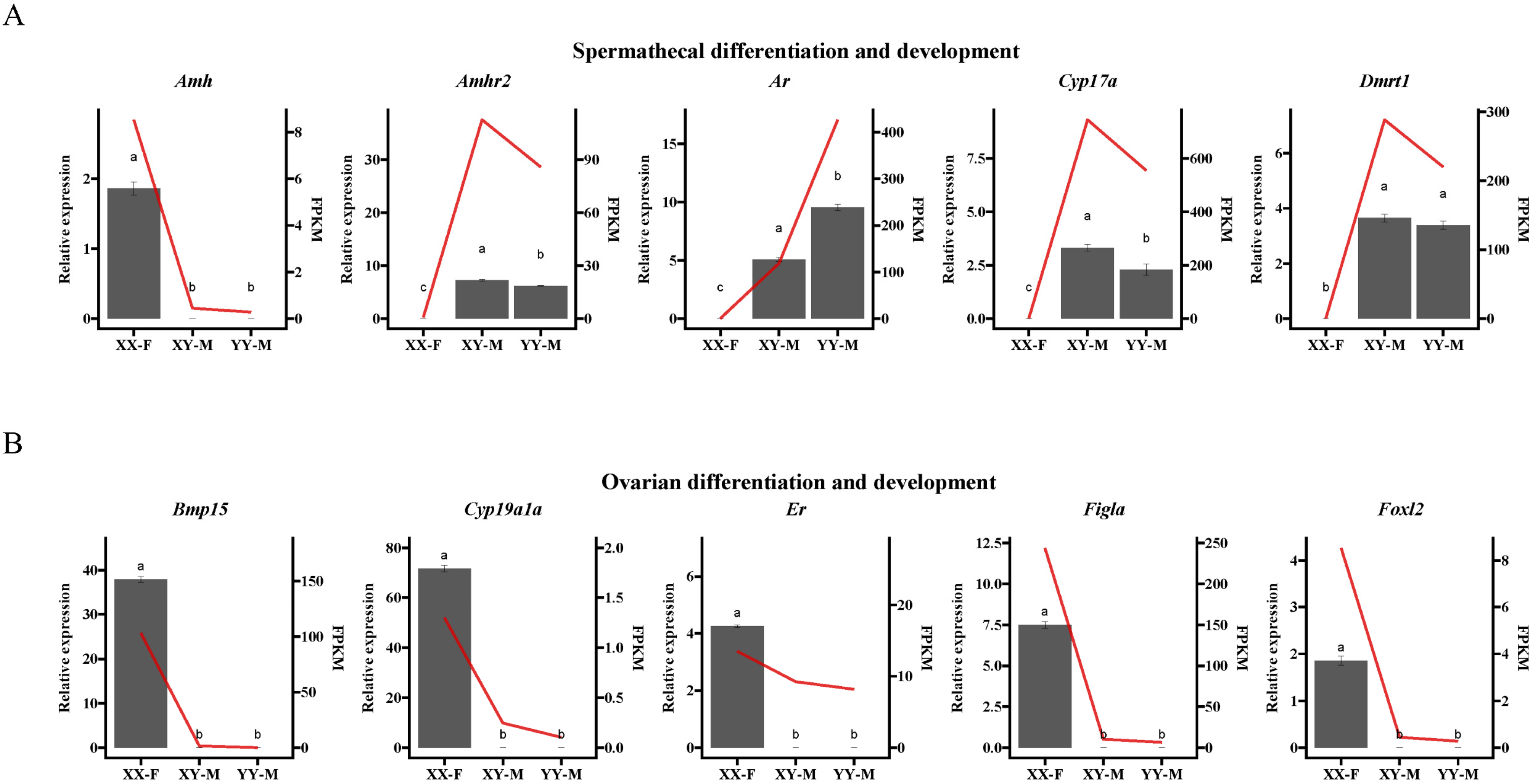
2.5. Identification of Candidate Genes by GWAS and RNA-Seq
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Sample Sources and DNA Extraction
4.3. Whole Genome Resequencing and Variation Detection
4.4. Sex-Determining Region and Loci Screening Using GWAS
4.5. Sex Candidate Gene and Region Identify by Fst
4.6. Male-Specific Markers Development and Validation through InDel Region
4.7. RNA Extraction, Sequencing, and Analysis of Sex-Biased Genes
4.8. Validation of the Candidate Genes in RNA-Seq by qPCR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guiguen, Y.; Fostier, A.; Herpin, A. Sex Determination and Differentiation in Fish. In Sex Control in Aquaculture; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 35–63. ISBN 978-1-119-12729-1. [Google Scholar]
- Kobayashi, Y.; Nagahama, Y.; Nakamura, M. Diversity and Plasticity of Sex Determination and Differentiation in Fishes. Sex. Dev. 2012, 7, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Bachtrog, D.; Mank, J.E.; Peichel, C.L.; Kirkpatrick, M.; Otto, S.P.; Ashman, T.-L.; Hahn, M.W.; Kitano, J.; Mayrose, I.; Ming, R.; et al. Sex Determination: Why so Many Ways of Doing It? PLoS Biol. 2014, 12, e1001899. [Google Scholar] [CrossRef] [PubMed]
- Sember, A.; Nguyen, P.; Perez, M.F.; Altmanová, M.; Ráb, P.; Cioffi, M.d.B. Multiple Sex Chromosomes in Teleost Fishes from a Cytogenetic Perspective: State of the Art and Future Challenges. Philos. Trans. R. Soc. B Biol. Sci. 2021, 376, 20200098. [Google Scholar] [CrossRef] [PubMed]
- Kitano, J.; Ansai, S.; Takehana, Y.; Yamamoto, Y. Diversity and Convergence of Sex-Determination Mechanisms in Teleost Fish. Annu. Rev. Anim. Biosci. 2024, 12, 233–259. [Google Scholar] [CrossRef]
- de Freitas, N.L.; Al-Rikabi, A.B.H.; Bertollo, L.A.C.; Ezaz, T.; Yano, C.F.; de Oliveira, E.A.; Hatanaka, T.; de Bello Cioffi, M. Early Stages of XY Sex Chromosomes Differentiation in the Fish Hoplias malabaricus (Characiformes, Erythrinidae) Revealed by DNA Repeats Accumulation. Curr. Genom. 2018, 19, 216–226. [Google Scholar] [CrossRef]
- Xue, L.; Gao, Y.; Wu, M.; Tian, T.; Fan, H.; Huang, Y.; Huang, Z.; Li, D.; Xu, L. Telomere-to-Telomere Assembly of a Fish Y Chromosome Reveals the Origin of a Young Sex Chromosome Pair. Genome Biol. 2021, 22, 203. [Google Scholar] [CrossRef]
- Nguyen, D.H.M.; Ponjarat, J.; Laopichienpong, N.; Panthum, T.; Singchat, W.; Ahmad, S.F.; Kraichak, E.; Muangmai, N.; Duengkae, P.; Peyachoknagul, S.; et al. Genome-Wide SNP Analysis of Hybrid Clariid Fish Reflects the Existence of Polygenic Sex-Determination in the Lineage. Front. Genet. 2022, 13, 789573. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, G.; Shao, C.; Huang, Q.; Liu, G.; Zhang, P.; Song, W.; An, N.; Chalopin, D.; Volff, J.-N.; et al. Whole-Genome Sequence of a Flatfish Provides Insights into ZW Sex Chromosome Evolution and Adaptation to a Benthic Lifestyle. Nat. Genet. 2014, 46, 253–260. [Google Scholar] [CrossRef]
- Li, Y.-L.; Xing, T.-F.; Liu, J.-X. Genome-Wide Association Analyses Based on Whole-Genome Sequencing of Protosalanx Hyalocranius Provide Insights into Sex Determination of Salangid Fishes. Mol. Ecol. Resour. 2020, 20, 1038–1049. [Google Scholar] [CrossRef]
- de Oliveira, E.A.; Sember, A.; Bertollo, L.A.C.; Yano, C.F.; Ezaz, T.; Moreira-Filho, O.; Hatanaka, T.; Trifonov, V.; Liehr, T.; Al-Rikabi, A.B.H.; et al. Tracking the Evolutionary Pathway of Sex Chromosomes among Fishes: Characterizing the Unique XX/XY1Y2 System in Hoplias malabaricus (Teleostei, Characiformes). Chromosoma 2018, 127, 115–128. [Google Scholar] [CrossRef]
- Li, M.; Zhang, R.; Fan, G.; Xu, W.; Zhou, Q.; Wang, L.; Li, W.; Pang, Z.; Yu, M.; Liu, Q.; et al. Reconstruction of the Origin of a Neo-Y Sex Chromosome and Its Evolution in the Spotted Knifejaw, Oplegnathus Punctatus. Mol. Biol. Evol. 2021, 38, 2615–2626. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Zou, Y.; Xiao, S.; Li, W.; Han, Z.; Han, F.; Xiao, J.; Liu, F.; Wang, Z. Chromosome Assembly of Collichthys lucidus, a Fish of Sciaenidae with a Multiple Sex Chromosome System. Sci. Data 2019, 6, 132. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Zou, Y.; Xiao, S.; Chen, J.; Wang, Z.; Wang, Y.; Jie, X.; Cai, M. Development of a PCR-Based Genetic Sex Identification Method in Spinyhead Croaker (Collichthys lucidus). Aquaculture 2020, 522, 735130. [Google Scholar] [CrossRef]
- Araya-Jaime, C.; Mateussi, N.T.B.; Utsunomia, R.; Costa-Silva, G.J.; Oliveira, C.; Foresti, F. ZZ/Z0: The New System of Sex Chromosomes in Eigenmannia Aff. Trilineata (Teleostei: Gymnotiformes: Sternopygidae) Characterized by Molecular Cytogenetics and DNA Barcoding. Zebrafish 2017, 14, 464–470. [Google Scholar] [CrossRef]
- Mei, J.; Gui, J.-F. Genetic Basis and Biotechnological Manipulation of Sexual Dimorphism and Sex Determination in Fish. Sci. China Life Sci. 2015, 58, 124–136. [Google Scholar] [CrossRef]
- Liu, H.; Pang, M.; Yu, X.; Zhou, Y.; Tong, J.; Fu, B. Sex-Specific Markers Developed by next-Generation Sequencing Confirmed an XX/XY Sex Determination System in Bighead Carp (Hypophthalmichthys nobilis) and Silver Carp (Hypophthalmichthys molitrix). DNA Res. 2018, 25, 257–264. [Google Scholar] [CrossRef]
- Mu, J.; Hu, W.; Chen, R.; Yang, Y.; Li, H.; Li, W.; Yin, X.; Xu, D. Production of Neomale and Neofemale Large Yellow Croaker (Larimichthys Crocea) and Establishment of All-Female Populations. Aquaculture 2024, 590, 741010. [Google Scholar] [CrossRef]
- Niu, J.-S.; Wang, T.; Li, Z.; Wang, Z.-W.; Ding, M.; Wang, M.-T.; Lian, Z.-Q.; Mei, J.; Wang, Y.; Zhou, L.; et al. Efficient Breeding and Growth Advantage of All-Male Population in Lanzhou Catfish (Silurus Lanzhouensis). Aquaculture 2024, 578, 740023. [Google Scholar] [CrossRef]
- Zhao, J.; Ou, M.; Wang, Y.; Liu, H.; Luo, Q.; Zhu, X.; Chen, B.; Chen, K. Breeding of YY Super-Male of Blotched Snakehead (Channa maculata) and Production of All-Male Hybrid (Channa Argus ♀ × C. Maculata ♂). Aquaculture 2021, 538, 736450. [Google Scholar] [CrossRef]
- Ortega-Recalde, O.; Goikoetxea, A.; Hore, T.A.; Todd, E.V.; Gemmell, N.J. The Genetics and Epigenetics of Sex Change in Fish. Annu. Rev. Anim. Biosci. 2020, 8, 47–69. [Google Scholar] [CrossRef]
- Balogh, R.E.; Csorbai, B.; Guti, C.; Keszte, S.; Urbányi, B.; Orbán, L.; Kovács, B. Validation of a Male-Specific DNA Marker Confirms XX/XY-Type Sex Determination in Several Hungarian Strains of African Catfish (Clarias Gariepinus). Theriogenology 2023, 205, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Huang, W.; Peng, S.; Zhou, J.; Zhan, H.; Gui, L.; Li, W.; Li, Q. Screening and Characterization of Sex-Specific Markers by NGS Sequencing in Spinibarbus hollandi with Implication of XY Sex Determination System. Aquaculture 2023, 565, 739147. [Google Scholar] [CrossRef]
- Liu, H.; Xia, W.; Li, B.; Liu, L.; Wang, Y.; Luo, Q.; Ou, M.; Zhu, X.; Chen, K.; Zhao, J. Sex-Specific Markers Developed by 2b-RAD and Genome Sequencing Reveal an XX/XY Sex-Determination System in Mud Carp (Cirrhinus molitorella). Aquaculture 2023, 565, 739131. [Google Scholar] [CrossRef]
- Peng, Y.-X.; Huang, Y.-Q.; Zhong, J.; Jiang, Z.-T.; Fan, S.; Shi, H.-J.; Chen, H.-P.; Deng, S.-P.; Li, G.-L.; Jiang, D.-N. Identification of Sex-Linked Marker and Candidate Sex Determination Gene in Ornamental Fish, African Scat (Scatophagus Tetracanthus). Aquaculture 2023, 563, 739023. [Google Scholar] [CrossRef]
- Degani, G. Russian Sturgeon Acclimatization in Northern Israel: Nutrition, Reproduction, and Growth. Mich. J. Anim. Sci. Technol. 2023, 1, 1–36. [Google Scholar]
- Colihueque, N.; Parraguez, M. Assessing the Effectiveness of Sex-Linked Molecular Markers to Identify Neomale Breeders for the Production of All-Female Progenies of Rainbow Trout. Mar. Biotechnol. 2024, 26, 199–204. [Google Scholar] [CrossRef]
- Tao, W.; Zhu, X.; Cao, J.; Xiao, H.; Dong, J.; Kocher, T.D.; Lu, M.; Wang, D. Screening and Characterization of Sex-Linked DNA Markers in Mozambique Tilapia (Oreochromis mossambicus). Aquaculture 2022, 557, 738331. [Google Scholar] [CrossRef]
- Beardmore, J.A.; Mair, G.C.; Lewis, R.I. Monosex Male Production in Finfish as Exemplified by Tilapia: Applications, Problems, and Prospects. In Reproductive Biotechnology in Finfish Aquaculture; Lee, C.-S., Donaldson, E.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2001; pp. 283–301. ISBN 978-0-444-50913-0. [Google Scholar]
- Simco, B.A.; Goudie, C.A.; Klar, G.T.; Parker, N.C.; Davis, K.B. Influence of Sex on Growth of Channel Catfish. Trans. Am. Fish. Soc. 1989, 118, 427–434. [Google Scholar] [CrossRef]
- Liu, H.; Guan, B.; Xu, J.; Hou, C.; Tian, H.; Chen, H. Genetic Manipulation of Sex Ratio for the Large-Scale Breeding of YY Super-Male and XY All-Male Yellow Catfish (Pelteobagrus fulvidraco (Richardson)). Mar Biotechnol 2013, 15, 321–328. [Google Scholar] [CrossRef]
- Wang, D.-W.; Li, Y.; Li, Z.-Q. Identification of a Male-Specific Amplified Fragment Length Polymorphism (AFLP) and a Sequence Characterized Amplified Region (SCAR) Marker in Eucommia ulmoides Oliv. Int. J. Mol. Sci. 2011, 12, 857–864. [Google Scholar] [CrossRef]
- Wang, L.; Sun, F.; Wan, Z.Y.; Ye, B.; Wen, Y.; Liu, H.; Yang, Z.; Pang, H.; Meng, Z.; Fan, B.; et al. Genomic Basis of Striking Fin Shapes and Colors in the Fighting Fish. Mol. Biol. Evol. 2021, 38, 3383–3396. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-L.; Li, J.; Deng, S.-P.; Tian, Y.-S.; Wang, Q.-Y.; Zhuang, Z.-M.; Sha, Z.-X.; Xu, J.-Y. Isolation of Female-Specific AFLP Markers and Molecular Identification of Genetic Sex in Half-Smooth Tongue Sole (Cynoglossus semilaevis). Mar. Biotechnol. 2007, 9, 273–280. [Google Scholar] [CrossRef]
- Bye, V.J.; Lincoln, R.F. Commercial Methods for the Control of Sexual Maturation in Rainbow Trout (Salmo gairdneri R.). Aquaculture 1986, 57, 299–309. [Google Scholar] [CrossRef]
- Saillant, E.; Fostier, A.; Menu, B.; Haffray, P.; Chatain, B. Sexual Growth Dimorphism in Sea Bass Dicentrarchus Labrax. Aquaculture 2001, 202, 371–387. [Google Scholar] [CrossRef]
- Beullens, K.; Eding, E.H.; Ollevier, F.; Komen, J.; Richter, C.J.J. Sex Differentiation, Changes in Length, Weight and Eye Size before and after Metamorphosis of European Eel (Anguilla anguilla L.) Maintained in Captivity. Aquaculture 1997, 153, 151–162. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, N.; Peng, K.; He, X.; Chen, C.X.; Liu, H.; Liu, K.; Jia, L.; Bao, B. A Combination of Genome-Wide Association Study Screening and SNaPshot for Detecting Sex-Related SNPs and Genes in Cynoglossus Semilaevis. Comp. Biochem. Physiol. Part D Genom. Proteom. 2020, 35, 100711. [Google Scholar] [CrossRef]
- Martínez, P.; Viñas, A.M.; Sánchez, L.; Díaz, N.; Ribas, L.; Piferrer, F. Genetic Architecture of Sex Determination in Fish: Applications to Sex Ratio Control in Aquaculture. Front. Genet. 2014, 5, 340. [Google Scholar] [CrossRef]
- Zheng, S.; Wang, X.; Zhang, S.; Long, J.; Tao, W.; Li, M.; Wang, D. Screening and Characterization of Sex-Linked DNA Markers and Marker-Assisted Selection in the Southern Catfish (Silurus meridionalis). Aquaculture 2020, 517, 734783. [Google Scholar] [CrossRef]
- You, X.; Shan, X.; Shi, Q. Research Advances in the Genomics and Applications for Molecular Breeding of Aquaculture Animals. Aquaculture 2020, 526, 735357. [Google Scholar] [CrossRef]
- Peralta, D.M.; Túnez, J.I.; Cruz, U.E.R.; Ceballos, S.G. A Rapid Approach for Sex Assignment by RAD-Seq Using a Reference Genome. PLoS ONE 2024, 19, e0297987. [Google Scholar] [CrossRef]
- Teal, C.N.; Coykendall, D.K.; Campbell, M.R.; Eardley, D.L.; Delomas, T.A.; Shira, J.T.; Schill, D.J.; Bonar, S.A.; Culver, M. Sex-Specific Markers Undetected in Green Sunfish Lepomis Cyanellus Using Restriction-Site Associated DNA Sequencing. J. Fish Biol. 2022, 100, 1528–1540. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Liu, H.; Cheng, L.; Pan, Z.; Chang, G.; Wu, N.; Wang, H.; Ding, H.; Fang, Y.; Wang, L. Identification of Sex-Specific Sequences through 2b-RAD Sequencing in Pseudobagrus ussuriensis. Aquaculture 2021, 539, 736639. [Google Scholar] [CrossRef]
- Huang, W.; Lai, J.; Liang, W.; Ye, S.; Li, J.; Zhou, J.; Zhang, Y.; Peng, S.; Zhan, H.; Zheng, P.; et al. Identification of Sex-Specific DNA Markers in the Army Fish (Spinibarbus hollandi) by Whole Genome Re-Sequencing Method. Aquaculture 2024, 583, 740605. [Google Scholar] [CrossRef]
- Wen, M.; Wang, S.; Zhu, C.; Zhang, Y.; Liu, Z.; Wu, C.; Wang, S.; Wang, Y.; Ren, L.; Tao, M.; et al. Identification of Sex Locus and a Male-Specific Marker in Blunt-Snout Bream (Megalobrama amblycephala) Using a Whole Genome Resequencing Method. Aquaculture 2024, 582, 740559. [Google Scholar] [CrossRef]
- Wen, M.; Zhang, Y.; Wang, S.; Hu, F.; Tang, C.; Li, Q.; Qin, Q.; Tao, M.; Zhang, C.; Zhao, R.; et al. Sex Locus and Sex Markers Identification Using Whole Genome Pool-Sequencing Approach in the Largemouth Bass (Micropterus salmoides L.). Aquaculture 2022, 559, 738375. [Google Scholar] [CrossRef]
- Luo, H.; Xiao, J.; Jiang, Y.; Ke, Y.; Ke, C.; Cai, M. Mapping and Marker Identification for Sex-Determining in the Pacific Abalone, Haliotis Discus Hannai Ino. Aquaculture 2021, 530, 735810. [Google Scholar] [CrossRef]
- Wang, M.; Li, L.; Lin, H.; Zhou, Z.; Liu, B.; Zhong, J.; Pu, F.; Shi, Y.; Zhou, T.; Xu, P. Genome-Wide Association Study Identifies Genomic Loci of Sex Determination, Gonadal Weight and Gonadosomatic Index Traits in Takifugu Bimaculatus. Aquaculture 2022, 546, 737389. [Google Scholar] [CrossRef]
- Wu, X.; Zhao, L.; Fan, Z.; Lu, B.; Chen, J.; Tan, D.; Jiang, D.; Tao, W.; Wang, D. Screening and Characterization of Sex-Linked DNA Markers and Marker-Assisted Selection in Blue Tilapia (Oreochromis aureus). Aquaculture 2021, 530, 735934. [Google Scholar] [CrossRef]
- Du, J.; Zhou, J.; Li, S.; Shao, J.; Jiang, P.; Dong, C.; Bai, J. A PCR-Based Method for Genetic Sex Identification and Evidence of the XX/XY Sex Determination System in Largemouth Bass (Micropterus salmoides L.). Aquaculture 2021, 545, 737220. [Google Scholar] [CrossRef]
- Martínez, P.; Robledo, D.; Taboada, X.; Blanco, A.; Moser, M.; Maroso, F.; Hermida, M.; Gómez-Tato, A.; Álvarez-Blázquez, B.; Cabaleiro, S.; et al. A Genome-Wide Association Study, Supported by a New Chromosome-Level Genome Assembly, Suggests Sox2 as a Main Driver of the Undifferentiatiated ZZ/ZW Sex Determination of Turbot (Scophthalmus maximus). Genomics 2021, 113, 1705–1718. [Google Scholar] [CrossRef]
- Liu, H.; Luo, Q.; Ou, M.; Zhu, X.; Zhao, J.; Chen, K. High-Density Genetic Linkage Map and QTL Fine Mapping of Growth and Sex in Snakehead (Channa argus). Aquaculture 2020, 519, 734760. [Google Scholar] [CrossRef]
- Ou, M.; Chen, K.-C.; Luo, Q.; Liu, H.-Y.; Wang, Y.-P.; Chen, B.-X.; Liang, X.-Q.; Zhao, J. Performance Evaluation of XY All-Male Hybrids Derived from XX Female Channa Argus and YY Super-Males Channa Maculate. Aquacult. Rep. 2021, 20, 100768. [Google Scholar] [CrossRef]
- Ou, M.; Yang, C.; Luo, Q.; Huang, R.; Zhang, A.-D.; Liao, L.-J.; Li, Y.-M.; He, L.-B.; Zhu, Z.-Y.; Chen, K.-C.; et al. An NGS-Based Approach for the Identification of Sex-Specific Markers in Snakehead (Channa argus). Oncotarget 2017, 8, 98733–98744. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Wen, H.; Qi, X.; Li, C.; Sun, C.; Wang, L.; Zhu, M.; Jiang, T.; Zhang, X.; Li, Y. Comparative Study of Candidate Sex Determination Regions in Snakeheads (Channa Argus and C. Maculata) and Development of Novel Sex Markers. Aquaculture 2023, 575, 739771. [Google Scholar] [CrossRef]
- Wang, L.; Xie, N.; Shen, Y.; Ye, B.; Yue, G.H.; Feng, X. Constructing High-Density Genetic Maps and Developing Sexing Markers in Northern Snakehead (Channa argus). Mar. Biotechnol. 2019, 21, 348–358. [Google Scholar] [CrossRef]
- Fu, B.; Zhou, Y.; Liu, H.; Yu, X.; Tong, J. Updated Genome Assembly of Bighead Carp (Hypophthalmichthys nobilis) and Its Differences Between Male and Female on Genomic, Transcriptomic, and Methylation Level. Front. Genet. 2021, 12, 728177. [Google Scholar] [CrossRef]
- Li, S.; Xu, L.; Shi, Y.; Chen, J. Male-Specific Markers Developed by next-Generation Sequencing Confirmed an XX/XY Sex-Determination System in Farmed Ayu (Plecoglossus altivelis). Aquaculture 2021, 541, 736822. [Google Scholar] [CrossRef]
- Felip, A.; Young, W.P.; Wheeler, P.A.; Thorgaard, G.H. An AFLP-Based Approach for the Identification of Sex-Linked Markers in Rainbow Trout (Oncorhynchus mykiss). Aquaculture 2005, 247, 35–43. [Google Scholar] [CrossRef]
- Chen, S.-L.; Ji, X.-S.; Shao, C.-W.; Li, W.-L.; Yang, J.-F.; Liang, Z.; Liao, X.-L.; Xu, G.-B.; Xu, Y.; Song, W.-T. Induction of Mitogynogenetic Diploids and Identification of WW Super-Female Using Sex-Specific SSR Markers in Half-Smooth Tongue Sole (Cynoglossus semilaevis). Mar. Biotechnol. 2012, 14, 120–128. [Google Scholar] [CrossRef]
- Liu, Z.J.; Cordes, J.F. DNA Marker Technologies and Their Applications in Aquaculture Genetics. Aquaculture 2004, 238, 1–37. [Google Scholar] [CrossRef]
- Dai, S.; Chen, M.; Zheng, S.; Su, J.; Wu, J.; Han, L.; Zhou, C.; Zou, Y.; Wang, D.; Li, M. Sex-Linked DNA Marker Screening and Characterization in Albino Northern Snakehead (Channa argus Var.) via Third-Generation Sequencing and Pool Resequencing. Aquaculture 2025, 594, 741449. [Google Scholar] [CrossRef]
- Matsuda, M.; Nagahama, Y.; Shinomiya, A.; Sato, T.; Matsuda, C.; Kobayashi, T.; Morrey, C.E.; Shibata, N.; Asakawa, S.; Shimizu, N.; et al. DMY Is a Y-Specific DM-Domain Gene Required for Male Development in the Medaka Fish. Nature 2002, 417, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Xiao, S.; Xu, S.; Ye, K.; Lin, X.; Sun, S.; Wang, Z. Identification of a Male-Specific DNA Marker in the Large Yellow Croaker (Larimichthys crocea). Aquaculture 2017, 480, 116–122. [Google Scholar] [CrossRef]
- Mustapha, U.F.; Jiang, D.-N.; Liang, Z.-H.; Gu, H.-T.; Yang, W.; Chen, H.-P.; Deng, S.-P.; Wu, T.-L.; Tian, C.-X.; Zhu, C.-H.; et al. Male-Specific Dmrt1 Is a Candidate Sex Determination Gene in Spotted Scat (Scatophagus argus). Aquaculture 2018, 495, 351–358. [Google Scholar] [CrossRef]
- Han, C.; Zhu, Q.; Lu, H.; Wang, C.; Zhou, X.; Peng, C.; Tang, L.; Han, L.; Chen, J.; Li, S.; et al. Screening and Characterization of Sex-Specific Markers Developed by a Simple NGS Method in Mandarin Fish (Siniperca chuatsi). Aquaculture 2020, 527, 735495. [Google Scholar] [CrossRef]
- Li, M.; Xu, H.; Xu, W.; Zhou, Q.; Xu, X.; Zhu, Y.; Zheng, W.; Li, W.; Pang, Z.; Chen, S. Isolation of a Male-Specific Molecular Marker and Development of a Genetic Sex Identification Technique in Spotted Knifejaw (Oplegnathus punctatus). Mar. Biotechnol. 2020, 22, 467–474. [Google Scholar] [CrossRef]
- Han, C.; Zhou, X.; Lu, H.; Zhu, Q.; Han, L.; Li, S.; Lin, H.; Zhang, Y. A Simple PCR-Based Genetic Sex Identification Method in the Blotched Snakehead (Channa maculata) Developed by High-Throughput Sequencing. Aquaculture 2021, 538, 736579. [Google Scholar] [CrossRef]
- Song, W.; Xie, Y.; Sun, M.; Li, X.; Fitzpatrick, C.K.; Vaux, F.; O’Malley, K.G.; Zhang, Q.; Qi, J.; He, Y. A Duplicated Amh Is the Master Sex-Determining Gene for Sebastes Rockfish in the Northwest Pacific. Open Biol. 2021, 11, 210063. [Google Scholar] [CrossRef]
- Wei, L.; Yang, C.; Tao, W.; Wang, D. Genome-Wide Identification and Transcriptome-Based Expression Profiling of the Sox Gene Family in the Nile Tilapia (Oreochromis niloticus). Int. J. Mol. Sci. 2016, 17, 270. [Google Scholar] [CrossRef]
- Zhang, X.; Ruan, Z.; Sun, C.; Hu, C.; Huang, Y.; You, X.; Wang, X.; Xu, J.; Liu, H.; Liu, X.; et al. Genome-Wide Identification, Sequence Alignment, and Transcription of Five Sex-Related Genes in Largemouth Bass (Micropterus salmoides). FBL 2024, 29, 63. [Google Scholar] [CrossRef]
- Yang, J.; Hu, Y.; Han, J.; Xiao, K.; Liu, X.; Tan, C.; Zeng, Q.; Du, H. Genome-Wide Analysis of the Chinese Sturgeon Sox Gene Family: Identification, Characterisation and Expression Profiles of Different Tissues. J. Fish Biol. 2020, 96, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lin, D.; Zhang, Y.; Ma, G.; Zhang, W. A Homologue of Sox11 Predominantly Expressed in the Ovary of the Orange-Spotted Grouper Epinephelus Coioides. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2008, 149, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhou, L.; Zhang, J.; Wang, Y.; Wang, Z.; Liu, X.; Cai, M. Genome-Wide Identification and Transcriptome-Based Expression Profiling of the Sox Gene Family in the Spinyhead Croaker (Collichthys lucidus). J. Fish Biol. 2022, 100, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Bertho, S.; Herpin, A.; Branthonne, A.; Jouanno, E.; Yano, A.; Nicol, B.; Muller, T.; Pannetier, M.; Pailhoux, E.; Miwa, M.; et al. The Unusual Rainbow Trout Sex Determination Gene Hijacked the Canonical Vertebrate Gonadal Differentiation Pathway. Proc. Natl. Acad. Sci. USA 2018, 115, 12781–12786. [Google Scholar] [CrossRef] [PubMed]
- Ayllon, F.; Solberg, M.F.; Besnier, F.; Fjelldal, P.G.; Hansen, T.J.; Wargelius, A.; Edvardsen, R.B.; Glover, K.A. Autosomal sdY Pseudogenes Explain Discordances Between Phenotypic Sex and DNA Marker for Sex Identification in Atlantic Salmon. Front. Genet. 2020, 11, 544207. [Google Scholar] [CrossRef]
- Bertho, S.; Herpin, A.; Schartl, M.; Guiguen, Y. Lessons from an Unusual Vertebrate Sex-Determining Gene. Philos. Trans. R. Soc. B Biol. Sci. 2021, 376, 20200092. [Google Scholar] [CrossRef]
- Yano, A.; Nicol, B.; Jouanno, E.; Quillet, E.; Fostier, A.; Guyomard, R.; Guiguen, Y. The Sexually Dimorphic on the Y-Chromosome Gene (sdY) Is a Conserved Male-Specific Y-Chromosome Sequence in Many Salmonids. Evol. Appl. 2013, 6, 486–496. [Google Scholar] [CrossRef]
- Bao, L.; Tian, C.; Liu, S.; Zhang, Y.; Elaswad, A.; Yuan, Z.; Khalil, K.; Sun, F.; Yang, Y.; Zhou, T.; et al. The Y Chromosome Sequence of the Channel Catfish Suggests Novel Sex Determination Mechanisms in Teleost Fish. BMC Biol. 2019, 17, 6. [Google Scholar] [CrossRef]
- Xie, Q.-P.; Zhan, W.; Shi, J.-Z.; Liu, F.; Niu, B.-L.; He, X.; Liu, M.; Wang, J.; Liang, Q.-Q.; Xie, Y.; et al. Whole-Genome Assembly and Annotation for the Little Yellow Croaker (Larimichthys polyactis) Provide Insights into the Evolution of Hermaphroditism and Gonochorism. Mol. Ecol. Resour. 2023, 23, 632–658. [Google Scholar] [CrossRef]
- Adolfi, M.C.; Du, K.; Kneitz, S.; Cabau, C.; Zahm, M.; Klopp, C.; Feron, R.; Paixão, R.V.; Varela, E.S.; de Almeida, F.L.; et al. A Duplicated Copy of Id2b Is an Unusual Sex-Determining Candidate Gene on the Y Chromosome of Arapaima (Arapaima gigas). Sci. Rep. 2021, 11, 21544. [Google Scholar] [CrossRef]
- He, Z.; Yan, R.-G.; Shang, Q.-B.; Yang, Q.-E. Elevated Id2 Expression Causes Defective Meiosis and Spermatogenesis in Mice. Dev. Dyn. 2024, 253, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.; Zhou, Z.; Zhou, T.; Zhao, J.; Chen, L.; Lin, H.; Pu, F.; Ke, Q.; Bai, H.; Xu, P. Genome-Wide Association Study of Body Shape-Related Traits in Large Yellow Croaker (Larimichthys crocea). Mar. Biotechnol. 2020, 22, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An Ultra-Fast All-in-One FASTQ Preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Durbin, R. Fast and Accurate Short Read Alignment with Burrows–Wheeler Transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. 1000 Genome Project Data Processing Subgroup The Sequence Alignment/Map Format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.W.; Daly, M.J.; et al. PLINK: A Tool Set for Whole-Genome Association and Population-Based Linkage Analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef]
- Zhou, X.; Stephens, M. Genome-Wide Efficient Mixed-Model Analysis for Association Studies. Nat. Genet. 2012, 44, 821–824. [Google Scholar] [CrossRef]
- Zhang, Z.; Ersoz, E.; Lai, C.-Q.; Todhunter, R.J.; Tiwari, H.K.; Gore, M.A.; Bradbury, P.J.; Yu, J.; Arnett, D.K.; Ordovas, J.M.; et al. Mixed Linear Model Approach Adapted for Genome-Wide Association Studies. Nat. Genet. 2010, 42, 355–360. [Google Scholar] [CrossRef]
- Ai, C.H.; Zhu, Z.X.; Huang, D.D.; Yang, G.; De Liu, T.; Bai, Y.; Liang, X.Y.; Xiong, Y.Y.; Lin, Y.L.; Lin, H.R.; et al. Identification of SNPs and Candidate Genes Associated with Early Growth in Orange-Spotted Grouper (Epinephelus coioides) by a Genome-Wide Association Study. Aquaculture 2023, 565, 739129. [Google Scholar] [CrossRef]
- Yin, L.; Zhang, H.; Tang, Z.; Xu, J.; Yin, D.; Zhang, Z.; Yuan, X.; Zhu, M.; Zhao, S.; Li, X.; et al. rMVP: A Memory-Efficient, Visualization-Enhanced, and Parallel-Accelerated Tool for Genome-Wide Association Study. Genom. Proteom. Bioinform. 2020, 19, 619–628. [Google Scholar] [CrossRef]
- Tanner, M.; Sharrard, M.; Rigby, A. Gene Polymorphisms and the Use of the Bonferroni Correction Factor: When and When Not to Apply? Arch. Dis. Child. 1997, 76, 385. [Google Scholar] [CrossRef][Green Version]
- Nei, M. Definition and Estimation of Fixation Indices. Evolution 1986, 40, 643–645. [Google Scholar] [CrossRef]
- Dong, S.-S.; He, W.-M.; Ji, J.-J.; Zhang, C.; Guo, Y.; Yang, T.-L. LDBlockShow: A Fast and Convenient Tool for Visualizing Linkage Disequilibrium and Haplotype Blocks Based on Variant Call Format Files. Brief. Bioinform. 2021, 22, bbaa227. [Google Scholar] [CrossRef]
- Ou, M.; Huang, R.; Yang, C.; Gui, B.; Luo, Q.; Zhao, J.; Li, Y.; Liao, L.; Zhu, Z.; Wang, Y.; et al. Chromosome-Level Genome Assemblies of Channa Argus and Channa Maculata and Comparative Analysis of Their Temperature Adaptability. GigaScience 2021, 10, giab070. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, M.-C.; Konaté, M.M.; Chen, L.; Das, B.; Karlovich, C.; Williams, P.M.; Evrard, Y.A.; Doroshow, J.H.; McShane, L.M. TPM, FPKM, or Normalized Counts? A Comparative Study of Quantification Measures for the Analysis of RNA-Seq Data from the NCI Patient-Derived Models Repository. J. Transl. Med. 2021, 19, 269. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Mao, X.; Cai, T.; Olyarchuk, J.G.; Wei, L. Automated Genome Annotation and Pathway Identification Using the KEGG Orthology (KO) as a Controlled Vocabulary. Bioinformatics 2005, 21, 3787–3793. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.-G.; Han, Y.; He, Q.-Y. clusterProfiler: An R Package for Comparing Biological Themes Among Gene Clusters. OMICS A J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, J.; Liu, C.; Luo, S.; Gao, X.; Wu, Y.; Wang, J.; Wang, X.; Wu, X.; Shen, W.; et al. Genetics Responses to Hypoxia and Reoxygenation Stress in Larimichthys Crocea Revealed via Transcriptome Analysis and Weighted Gene Co-Expression Network. Animals 2021, 11, 3021. [Google Scholar] [CrossRef]
- Raivo Kolde Pheatmap: Pretty Heatmaps 2010, 1.0.12. Available online: https://cran.r-project.org/web/packages/pheatmap/pheatmap.pdf (accessed on 6 October 2024).
- Mao, H.; Chen, K.; Zhu, X.; Luo, Q.; Zhao, J.; Li, W.; Wu, X.; Xu, H. Identification of Suitable Reference Genes for Quantitative Real-Time PCR Normalization in Blotched Snakehead Channa Maculata. J. Fish Biol. 2017, 90, 2312–2322. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]

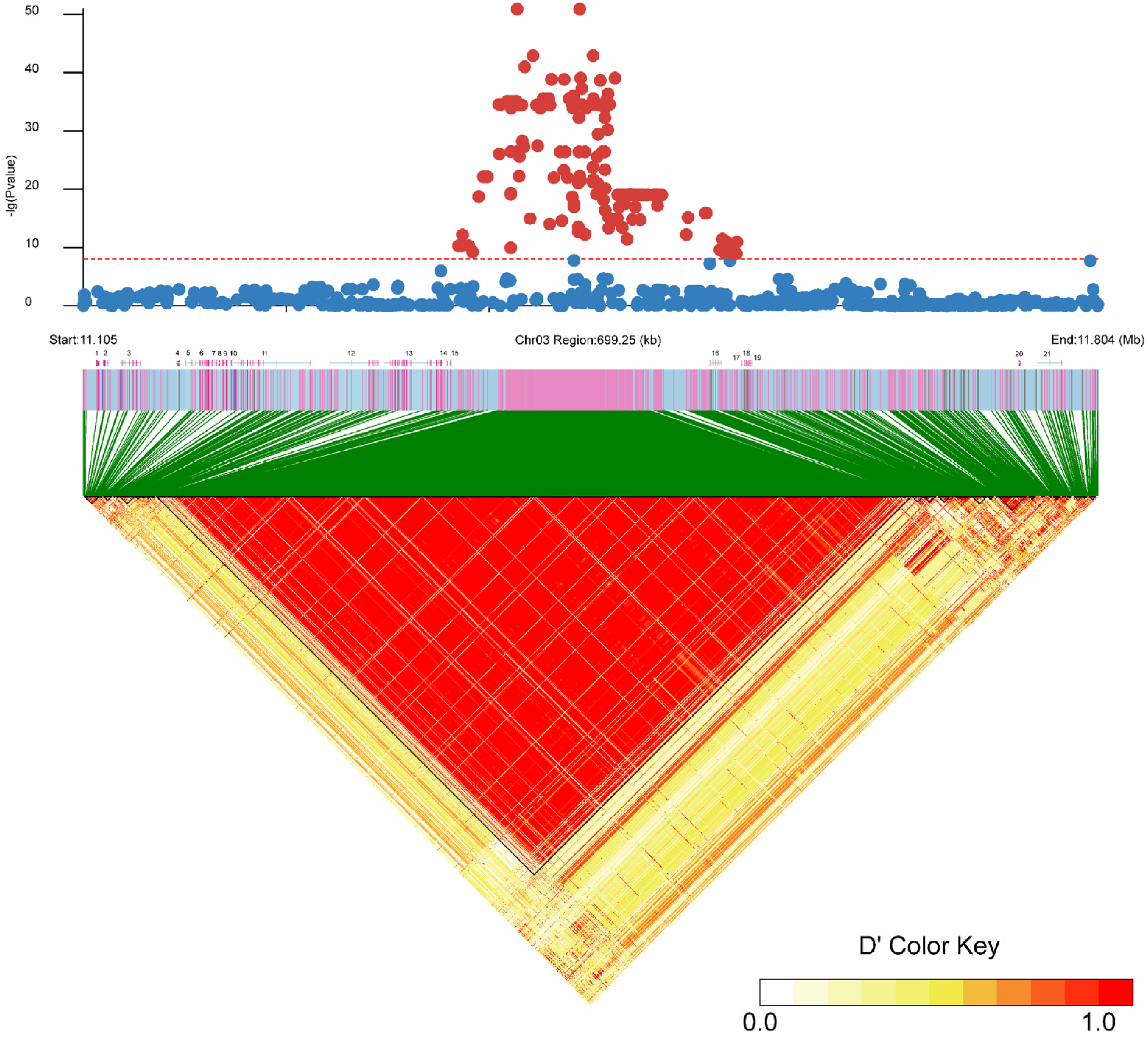

| ID | Gene Position | Description | Candidate Gene |
|---|---|---|---|
| 1 | 11,113,901~11,116,316 | Required for cytoplasmic pre-assembly of axonemal dyneins, thereby playing a central role in motility in cilia and flag | EVM0016285 |
| 2 | 11,117,840~11,123,073 | KLF transcription factor 11 | Klf11 |
| 3 | 11,130,447~11,144,557 | Grainyhead-like protein 1 homolog | Grhl1 |
| 4 | 11,169,451~11,171,481 | uncharacterized protein | EVM0024574 |
| 5 | 11,175,684~11,180,589 | Tyrosine 3-monooxygenase tryptophan 5-monooxygenase activation protein, theta polypeptide b | Ywhaq |
| 6 | 11,182,245~11,194,044 | Disintegrin and metalloproteinase | Adam17 |
| 7 | 11,195,427~11,197,648 | Isoamyl acetate-hydrolyzing esterase 1 homolog | Iah1 |
| 8 | 11,198,272~11,199,288 | Homolog subfamily C member | Dnajc30 |
| 9 | 11,200,437~11,204,673 | Cleavage and polyadenylation | Cpsf3 |
| 10 | 11,206,096~11,207,457 | Integrin beta 1 binding protein 1 | Itgb1bp1 |
| 11 | 11,208,746~11,262,323 | Arf-GAP with SH3 domain, ANK repeat and PH domain-containing protein | Asap2 |
| 12 | 11,275,093~11,308,850 | Membrane bound O-acyltransferase domain containing | Mboat2 |
| 13 | 11,312,296~11,352,898 | Kinase D-interacting substrate of | Kidins220 |
| 14 | 11,355,614~11,356,323 | Inhibitor of DNA binding 2, dominant Negative helix–loop–helix protein | Id2 |
| 15 | 11,356,961~11,358,844 | Uncharacterized protein | EVM0022341 |
| 16 | 11,537,084~11,544,994 | Ring finger protein | Rnf144a |
| 17 | 11,558,889~11,559,263 | Uncharacterized protein | EVM0022102 |
| 18 | 11,560,799~11,562,796 | Radical S-adenosyl methionine | Rsad2 |
| 19 | 11,563,209~11,566,190 | (UMP-CMP) kinase 2 | Cmpk2 |
| 20 | 11,749,994~11,751,085 | SRY (sex-determining region Y)-box | Sox11 |
| 21 | 11,762,407~11,780,316 | Uncharacterized protein | EVM0009322 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Zhang, J.; Cui, T.; Xia, W.; Luo, Q.; Fei, S.; Zhu, X.; Chen, K.; Zhao, J.; Ou, M. Genome-Wide Association Studies (GWAS) and Transcriptome Analysis Reveal Male Heterogametic Sex-Determining Regions and Candidate Genes in Northern Snakeheads (Channa argus). Int. J. Mol. Sci. 2024, 25, 10889. https://doi.org/10.3390/ijms252010889
Liu H, Zhang J, Cui T, Xia W, Luo Q, Fei S, Zhu X, Chen K, Zhao J, Ou M. Genome-Wide Association Studies (GWAS) and Transcriptome Analysis Reveal Male Heterogametic Sex-Determining Regions and Candidate Genes in Northern Snakeheads (Channa argus). International Journal of Molecular Sciences. 2024; 25(20):10889. https://doi.org/10.3390/ijms252010889
Chicago/Turabian StyleLiu, Haiyang, Jin Zhang, Tongxin Cui, Weiwei Xia, Qing Luo, Shuzhan Fei, Xinping Zhu, Kunci Chen, Jian Zhao, and Mi Ou. 2024. "Genome-Wide Association Studies (GWAS) and Transcriptome Analysis Reveal Male Heterogametic Sex-Determining Regions and Candidate Genes in Northern Snakeheads (Channa argus)" International Journal of Molecular Sciences 25, no. 20: 10889. https://doi.org/10.3390/ijms252010889
APA StyleLiu, H., Zhang, J., Cui, T., Xia, W., Luo, Q., Fei, S., Zhu, X., Chen, K., Zhao, J., & Ou, M. (2024). Genome-Wide Association Studies (GWAS) and Transcriptome Analysis Reveal Male Heterogametic Sex-Determining Regions and Candidate Genes in Northern Snakeheads (Channa argus). International Journal of Molecular Sciences, 25(20), 10889. https://doi.org/10.3390/ijms252010889






