Identification of Powdery Mildew Resistance-Related Genes in Butternut Squash (Cucurbita moschata)
Abstract
:1. Introduction
2. Results
2.1. Evaluation of Powdery Mildew Resistance of Cucurbita moschata
2.2. Variations in the Whole Genome and Transcriptome Sequencing
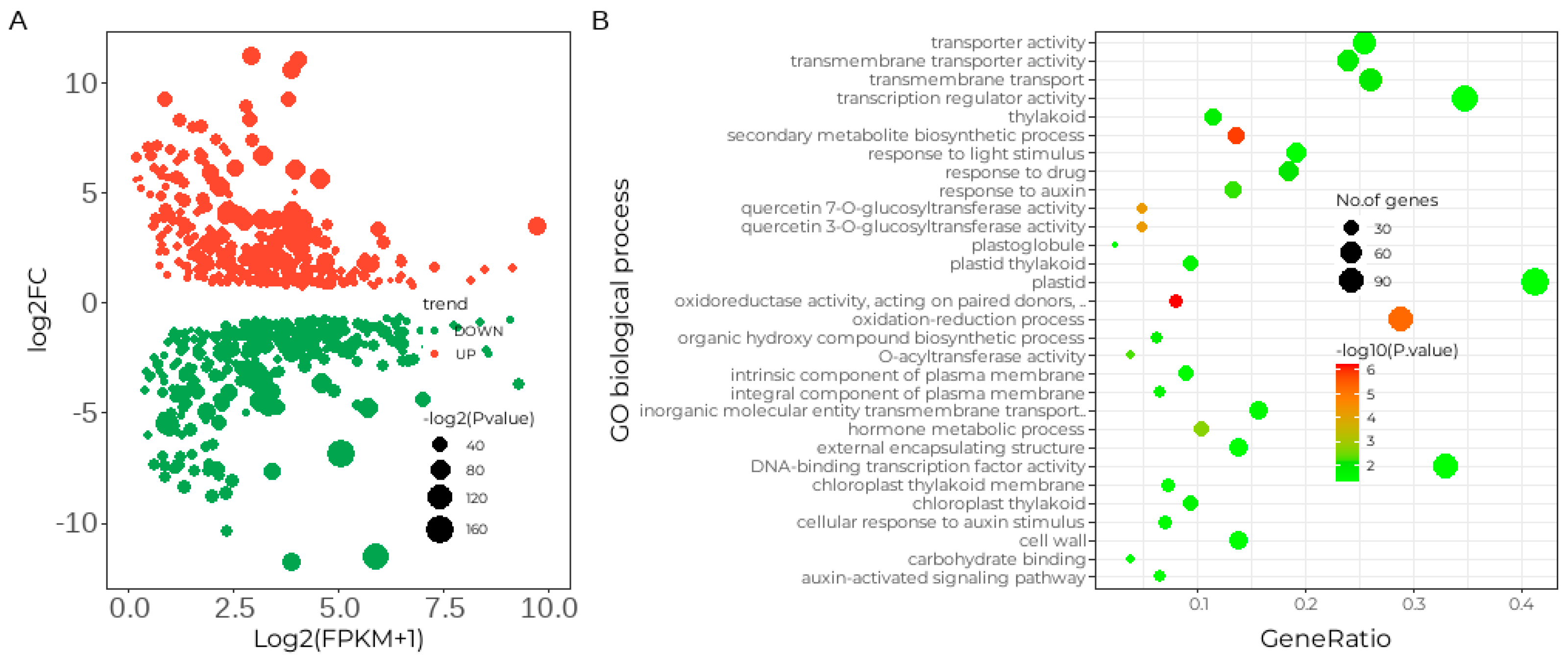
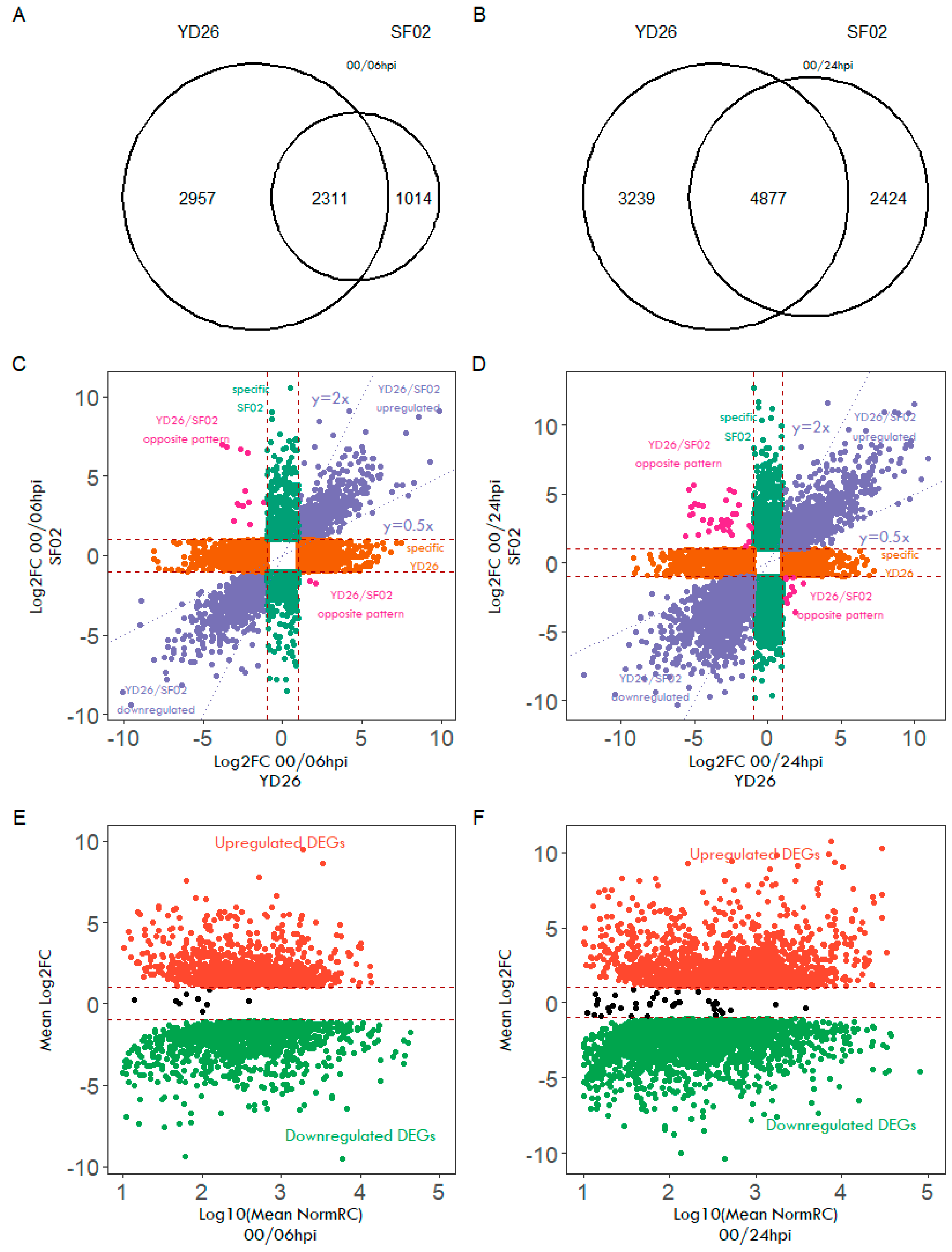
2.3. Differentially Expressed Disease Genes during Powdery Mildew Resistance
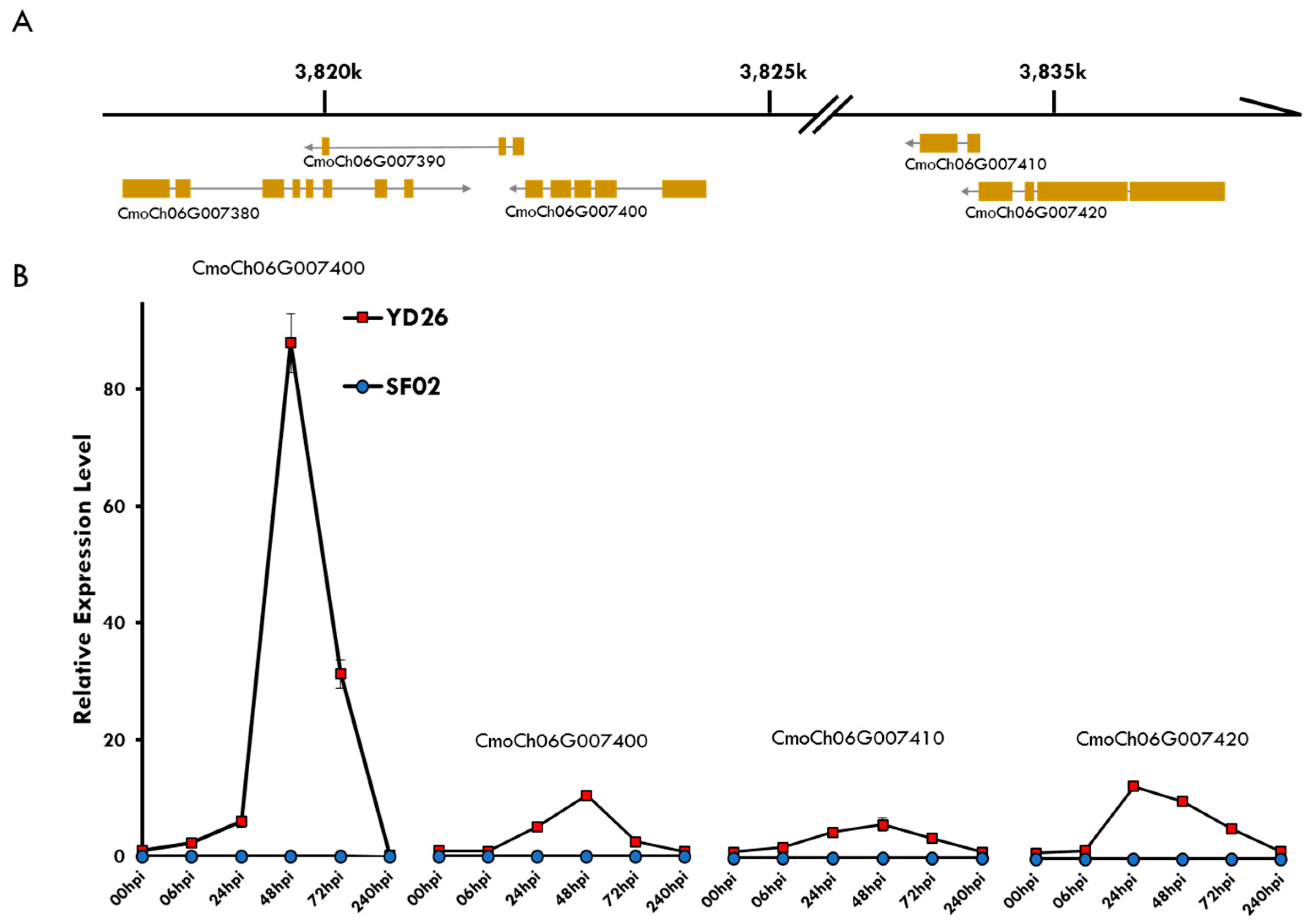
2.4. MLOs Family Clade V Genes in Butternut Squash
3. Discussion
3.1. Origin of Powdery Mildew Resistance of Squash
3.2. Co-Expressed R Gene Cluster for Powdery Mildew Resistance
3.3. The R and S Genes for Powdery Mildew Resistance
4. Materials and Methods
4.1. Plant Materials and Powdery Mildew Inoculation
4.2. Whole Genome Re-Sequencing and Variation Identification of Squash
4.3. Transcriptome Analysis after Powdery Mildew Inoculation
4.4. Differentially Expressed Genes (DEGs) and Disease-Related Gene Identification
4.5. Identification of Gene Expression Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agrios, G.N. Plant Pathology; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Aist, J.R.; Bushnell, W.R. Invasion of Plants by Powdery Mildew Fungi, and Cellular Mechanisms of Resistance. In The Fungal Spore and Disease Initiation in Plants and Animals; Cole, G.T., Hoch, H.C., Eds.; Springer: Boston, MA, USA, 1991; pp. 321–345. [Google Scholar]
- Xu, W.; Meng, Y.; Wise, R.P. Mla-and Rom1-mediated control of microRNA398 and chloroplast copper/zinc superoxide dismutase regulates cell death in response to the barley powdery mildew fungus. New Phytol. 2014, 201, 1396–1412. [Google Scholar] [CrossRef]
- Wei, F.; Wing, R.A.; Wise, R.P. Genome Dynamics and Evolution of the Mla (Powdery Mildew) Resistance Locus in Barley. Plant Cell 2002, 14, 1903–1917. [Google Scholar] [CrossRef]
- Wei, F.; Gobelman-Werner, K.; Morroll, S.M.; Kurth, J.; Mao, L.; Wing, R.; Leister, D.; Schulze-Lefert, P.; Wise, R.P. The Mla (Powdery Mildew) Resistance Cluster is Associated with Three NBS-LRR Gene Families and Suppressed Recombination Within a 240-kb DNA Interval on Chromosome 5S (1HS) of Barley. Genetics 1999, 153, 1929–1948. [Google Scholar] [CrossRef]
- Austin, M.J.; Muskett, P.; Kahn, K.; Feys, B.J.; Jones, J.D.G.; Parker, J.E. Regulatory Role of SGT1 in Early R Gene-Mediated Plant Defenses. Science 2002, 295, 2077–2080. [Google Scholar] [CrossRef]
- Shen, Q.-H.; Zhou, F.; Bieri, S.; Haizel, T.; Shirasu, K.; Schulze-Lefert, P. Recognition specificity and RAR1/SGT1 dependence in barley Mla disease resistance genes to the powdery mildew fungus. Plant Cell 2003, 15, 732–744. [Google Scholar] [CrossRef]
- Xiao, S.; Calis, O.; Patrick, E.; Zhang, G.; Charoenwattana, P.; Muskett, P.; Parker, J.E.; Turner, J.G. The atypical resistance gene, RPW8, recruits components of basal defence for powdery mildew resistance in Arabidopsis. Plant J. 2005, 42, 95–110. [Google Scholar] [CrossRef]
- Kang, Y.; Zhou, M.; Merry, A.; Barry, K. Mechanisms of powdery mildew resistance of wheat–a review of molecular breeding. Plant Pathol. 2020, 69, 601–617. [Google Scholar] [CrossRef]
- Huang, X.Q.; Röder, M.S. Molecular mapping of powdery mildew resistance genes in wheat: A review. Euphytica 2004, 137, 203–223. [Google Scholar] [CrossRef]
- Burdon, J.J.; Barrett, L.G.; Rebetzke, G.; hrall, P.H. Guiding deployment of resistance in cereals using evolutionary principles. Evol. Appl. 2014, 7, 609–624. [Google Scholar] [CrossRef]
- van Schie, C.C.N.; Takken, F.L.W. Susceptibility Genes 101: How to Be a Good Host. Annu. Rev. Phytopathol. 2014, 52, 551–558.13. [Google Scholar] [CrossRef]
- Jørgensen, I.H. Discovery, characterization and exploitation of Mlo powdery mildew resistance in barley. Euphytica 1992, 63, 141–152. [Google Scholar] [CrossRef]
- Piffanelli, P.; Ramsay, L.; Waugh, R.; Benabdelmouna, A.; D’Hont, A.; Hollricher, K.; Jørgensen, J.H.; Schulze-Lefert, P.; Panstruga, R. A barley cultivation-associated polymorphism conveys resistance to powdery mildew. Nature 2004, 430, 887–891. [Google Scholar] [CrossRef]
- Freialdenhoven, A.; Peterhansel, C.; Kurth, J.; Kreuzaler, F.; Schulze-Lefert, P. Identification of Genes Required for the Function of Non-Race-Specific mlo Resistance to Powdery Mildew in Barley. Plant Cell 1996, 8, 5–14. [Google Scholar] [CrossRef]
- Kusch, S.; Panstruga, R. mlo-based resistance: An apparently universal “weapon” to defeat powdery mildew disease. Mol. Plant-Microbe Interact. 2017, 30, 179–189. [Google Scholar] [CrossRef]
- Jacott, C.N.; Ridout, C.J.; Murray, J.D. Unmasking Mildew Resistance Locus O. Trends Plant Sci. 2021, 26, 1006–1013. [Google Scholar] [CrossRef]
- Devoto, A.; Hartmann, H.A.; Piffanelli, P.; Elliott, C.; Simmons, C.; Taramino, G.; Goh, C.S.; Cohen, F.E.; Emerson, B.C.; Schulze-Lefert, P.; et al. Molecular Phylogeny and Evolution of the Plant-Specific Seven-Transmembrane MLO Family. J. Mol. Evol. 2003, 56, 77–88. [Google Scholar] [CrossRef]
- Nie, J.; Wang, Y.; He, H.; Guo, C.; Zhu, W.; Pan, J.; Li, D.; Lian, H.; Pan, J.; Cai, R. Loss-of-Function Mutations in CsMLO1 Confer Durable Powdery Mildew Resistance in Cucumber (Cucumis sativus L.). Front. Plant Sci. 2015, 6, 1155. [Google Scholar] [CrossRef]
- Berg, J.A.; Appiano, M.; Santillán Martínez, M.; Hermans, F.W.; Vriezen, W.H.; Visser, R.G.; Bai, Y.; Schouten, H.J. A transposable element insertion in the susceptibility gene CsaMLO8 results in hypocotyl resistance to powdery mildew in cucumber. BMC Plant Biol. 2015, 15, 243. [Google Scholar] [CrossRef]
- Berg, J.A.; Appiano, M.; Bijsterbosch, G.; Visser, R.G.; Schouten, H.J.; Bai, Y. Functional characterization of cucumber (Cucumis sativus L.) Clade V MLO genes. BMC Plant Biol. 2017, 17, 80. [Google Scholar] [CrossRef]
- Cho, M.C.; Om, Y.H.; Heo, Y.C.; Kim, D.H.; Kim, J.S.; Park, H.G. Breeding for powdery mildew resistant varieties in Cucurbita moschata. Res. Plant Dis. 2005, 11, 106–114. [Google Scholar] [CrossRef]
- Holdsworth, W.L.; LaPlant, K.E.; Bell, D.C.; Jahn, M.M.; Mazourek, M. Cultivar-Based Introgression Mapping Reveals Wild Species-Derived Pm-0, the Major Powdery Mildew Resistance Locus in Squash. PLoS ONE 2016, 11, e0167715. [Google Scholar] [CrossRef] [PubMed]
- Park, B.S.; Jang, S.; Yu, Y.; Choi, G.J.; Kang, B.C.; Seo, S. QTL Mapping and molecular markers of Powdery Mildew Resistance in Pumpkin (Cucurbita moschata). Hortic. Sci. Technol. 2020, 38, 717–729. [Google Scholar] [CrossRef]
- Cohen, R.; Hanan, A.; Paris, H.S. Single-gene resistance to powdery mildew in zucchini squash (Cucurbita pepo). Euphytica 2003, 130, 433–441. [Google Scholar] [CrossRef]
- Guo, W.L.; Chen, B.H.; Guo, Y.Y.; Chen, X.J.; Li, Q.F.; Yang, H.L.; Li, X.Z.; Zhou, J.G.; Wang, G.Y. Expression of Pumpkin CmbHLH87 Gene Improves Powdery Mildew Resistance in Tobacco. Front. Plant Sci. 2020, 11, 163. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qi, C.; Luo, Y.; Zhang, F.; Dai, Z.; Li, M.; Qu, S. Identification and mapping of CpPM10.1, a major gene involved in powdery mildew (race 2 France of Podosphaera xanthii) resistance in zucchini (Cucurbita pepo L.). Theor. Appl. Genet. 2021, 134, 2531–2545. [Google Scholar] [CrossRef]
- Zhu, L.; Li, Y.M.; Li, J.T.; Wang, Y.; Zhang, Z.L.; Wang, Y.J.; Wang, Z.L.; Hu, J.B.; Yang, L.M.; Sun, S.R. Genome-wide identification and analysis of the MLO gene families in three Cucurbita species. Czech J. Genet. Plant Breed. 2021, 57, 119–123. [Google Scholar] [CrossRef]
- Win, K.T.; Zhang, C.; Lee, S. Genome-wide identification and description of MLO family genes in pumpkin (Cucurbita maxima Duch.). Hortic. Environ. Biotechnol. 2018, 59, 397–410. [Google Scholar] [CrossRef]
- Lebeda, A.; Křístková, E.; Mieslerová, B.; Dhillon, N.P.S.; McCreight, J.D. Status, Gaps and Perspectives of Powdery Mildew Resistance Research and Breeding in Cucurbits. Crit. Rev. Plant Sci. 2024, 43, 211–290. [Google Scholar] [CrossRef]
- Kesh, H.; Yadav, S. Recent advances in genetics and breeding of pumpkin (Cucurbita moschata Duch.). J. Hortic. Sci. Biotechnol. 2023, 98, 141–158. [Google Scholar] [CrossRef]
- Paris, H.S.; Brown, R.N. The Genes of Pumpkin and Squash. HortScience 2005, 40, 1620–1630. [Google Scholar] [CrossRef]
- Sun, H.; Wu, S.; Zhang, G.; Jiao, C.; Guo, S.; Ren, Y.; Zhang, J.; Zhang, H.; Gong, G.; Jia, Z.; et al. Karyotype stability and unbiased fractionation in the paleo-allotetraploid Cucurbita genomes. Mol. Plant 2017, 10, 1293–1306. [Google Scholar] [CrossRef] [PubMed]
- Vlot, A.C.; Liu, P.P.; Cameron, R.K.; Park, S.W.; Yang, Y.; Kumar, D.; Zhou, F. Identification of likely orthologs of tobacco salicylic acid-binding protein 2 and their role in systemic acquired resistance in Arabidopsis thaliana. Plant J. 2008, 56, 445–456. [Google Scholar] [CrossRef] [PubMed]
- An, C.; Mou, Z. Salicylic Acid and its Function in Plant Immunity. J. Integr. Plant Biol. 2011, 53, 412–428. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef]
- Zhang, P.; Zhu, Y.; Zhou, S. Comparative analysis of powdery mildew resistant and susceptible cultivated cucumber (Cucumis sativus L.) varieties to reveal the metabolic responses to Sphaerotheca fuliginea infection. BMC Plant Biol. 2021, 21, 24. [Google Scholar]
- Harth, J.E.; Ferrari, M.J.; Helms, A.M.; Tooker, J.F.; Stephenson, A.G. Zucchini Yellow Mosaic Virus Infection Limits Establishment and Severity of Powdery Mildew in Wild Populations of Cucurbita pepo. Front. Plant Sci. 2018, 9, 792. [Google Scholar]
- Calle Garcia, J.; Guadagno, A.; Paytuvi-Gallart, A.; Saera-Vila, A.; Amoroso, C.G.; D’Esposito, D.; Andolfo, G.; Aiese Cigliano, R.; Sanseverino, W.; Ercolano, M.R. PRGdb 4.0: An updated database dedicated to genes involved in plant disease resistance process. Nucleic Acids Res. 2021, 50, D1483–D1490. [Google Scholar] [CrossRef]
- Wang, X.; Li, G.; Gao, X.; Xiong, L.; Wang, W.; Han, R. Powdery mildew resistance gene (Pm-AN) located in a segregation distortion region of melon LGV. Euphytica 2011, 180, 421–428. [Google Scholar] [CrossRef]
- Yuste-Lisbona, F.J.; Capel, C.; Gómez-Guillamón, M.L.; Capel, J.; López-Sesé, A.I.; Lozano, R. Codominant PCR-based markers and candidate genes for powdery mildew resistance in melon (Cucumis melo L.). Theor. Appl. Genet. 2011, 122, 747–758. [Google Scholar] [CrossRef]
- Tobias, P.A.; Guest, D.I.; Külheim, C.; Hsieh, J.F.; Park, R.F. A curious case of resistance to a new encounter pathogen: Myrtle rust in Australia. Mol. Plant Pathol. 2016, 17, 783–788. [Google Scholar] [CrossRef]
- Sekhwal, M.K.; Li, P.; Lam, I.; Wang, X.; Cloutier, S.; You, F.M. Disease Resistance Gene Analogs (RGAs) in Plants. Int. J. Mol. Sci. 2015, 16, 19248–19290. [Google Scholar] [CrossRef]
- Bezerra-Neto, J.P.; Araújo, F.C.; Ferreira-Neto, J.R.; Silva, R.L.; Borges, A.N.; Matos, M.K.; Silva, J.B.; Silva, M.D.; Kido, E.A.; Benko-Iseppon, A.M. NBS-LRR genes—Plant health sentinels: Structure, roles, evolution and biotechnological applications. In Applied Plant Biotechnology for Improving Resistance to Biotic Stress; Poltronieri, P., Hong, Y., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 63–120. [Google Scholar]
- Leister, D. Tandem and segmental gene duplication and recombination in the evolution of plant disease resistance genes. Trends Genet. 2004, 20, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Yahiaoui, N.; Brunner, S.; Keller, B. Rapid generation of new powdery mildew resistance genes after wheat domestication. Plant J. 2006, 47, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Boissot, N.; Chovelon, V.; Rittener-Ruff, V.; Giovinazzo, N.; Mistral, P.; Pitrat, M.; Charpentier, M.; Troadec, C.; Bendahmane, A.; Dogimont, C. A highly diversified NLR cluster in melon contains homologs that confer powdery mildew and aphid resistance. Hortic. Res. 2023, 11, uhad256. [Google Scholar] [CrossRef] [PubMed]
- Chovelon, V.; Feriche-Linares, R.; Barreau, G.; Chadoeuf, J.; Callot, C.; Gautier, V.; Le Paslier, M.C.; Berad, A.; Faivre-Rampant, P.; Lagnel, J.; et al. Building a cluster of NLR genes conferring resistance to pests and pathogens: The story of the Vat gene cluster in cucurbits. Hortic. Res. 2021, 8, 72. [Google Scholar] [CrossRef] [PubMed]
- Kusch, S.; Pesch, L.; Panstruga, R. Comprehensive Phylogenetic Analysis Sheds Light on the Diversity and Origin of the MLO Family of Integral Membrane Proteins. Genome Biol. Evol. 2016, 8, 878–895. [Google Scholar] [CrossRef]
- Consonni, C.; Humphry, M.E.; Hartmann, H.A.; Livaja, M.; Durner, J.; Westphal, L.; Vogel, J.; Lipka, V.; Kemmerling, B.; Schulze-Lefert, P.; et al. Conserved requirement for a plant host cell protein in powdery mildew pathogenesis. Nat. Genet. 2006, 38, 716–720. [Google Scholar] [CrossRef]
- Assaad, F.F.; Qiu, J.L.; Youngs, H.; Ehrhardt, D.; Zimmerli, L.; Kalde, M.; Wanner, G.; Peck, S.C.; Edwards, H.; Ramonell, K.; et al. The PEN1 Syntaxin Defines a Novel Cellular Compartment upon Fungal Attack and Is Required for the Timely Assembly of Papillae. Mol. Biol. Cell 2004, 15, 5118–5129. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Y.; Hu, Y.; Yang, J.; Liao, D.; Liu, P.; Wen, C.; Yun, T. Identification of Powdery Mildew Resistance-Related Genes in Butternut Squash (Cucurbita moschata). Int. J. Mol. Sci. 2024, 25, 10896. https://doi.org/10.3390/ijms252010896
Fu Y, Hu Y, Yang J, Liao D, Liu P, Wen C, Yun T. Identification of Powdery Mildew Resistance-Related Genes in Butternut Squash (Cucurbita moschata). International Journal of Molecular Sciences. 2024; 25(20):10896. https://doi.org/10.3390/ijms252010896
Chicago/Turabian StyleFu, Yiqian, Yanping Hu, Jingjing Yang, Daolong Liao, Pangyuan Liu, Changlong Wen, and Tianhai Yun. 2024. "Identification of Powdery Mildew Resistance-Related Genes in Butternut Squash (Cucurbita moschata)" International Journal of Molecular Sciences 25, no. 20: 10896. https://doi.org/10.3390/ijms252010896





