Circulating Immune Complexes and Complement Activation in Sensitized Kidney Transplant Recipients
Abstract
:1. Introduction
2. Results
2.1. Donor-Specific Antibodies and Kidney Function
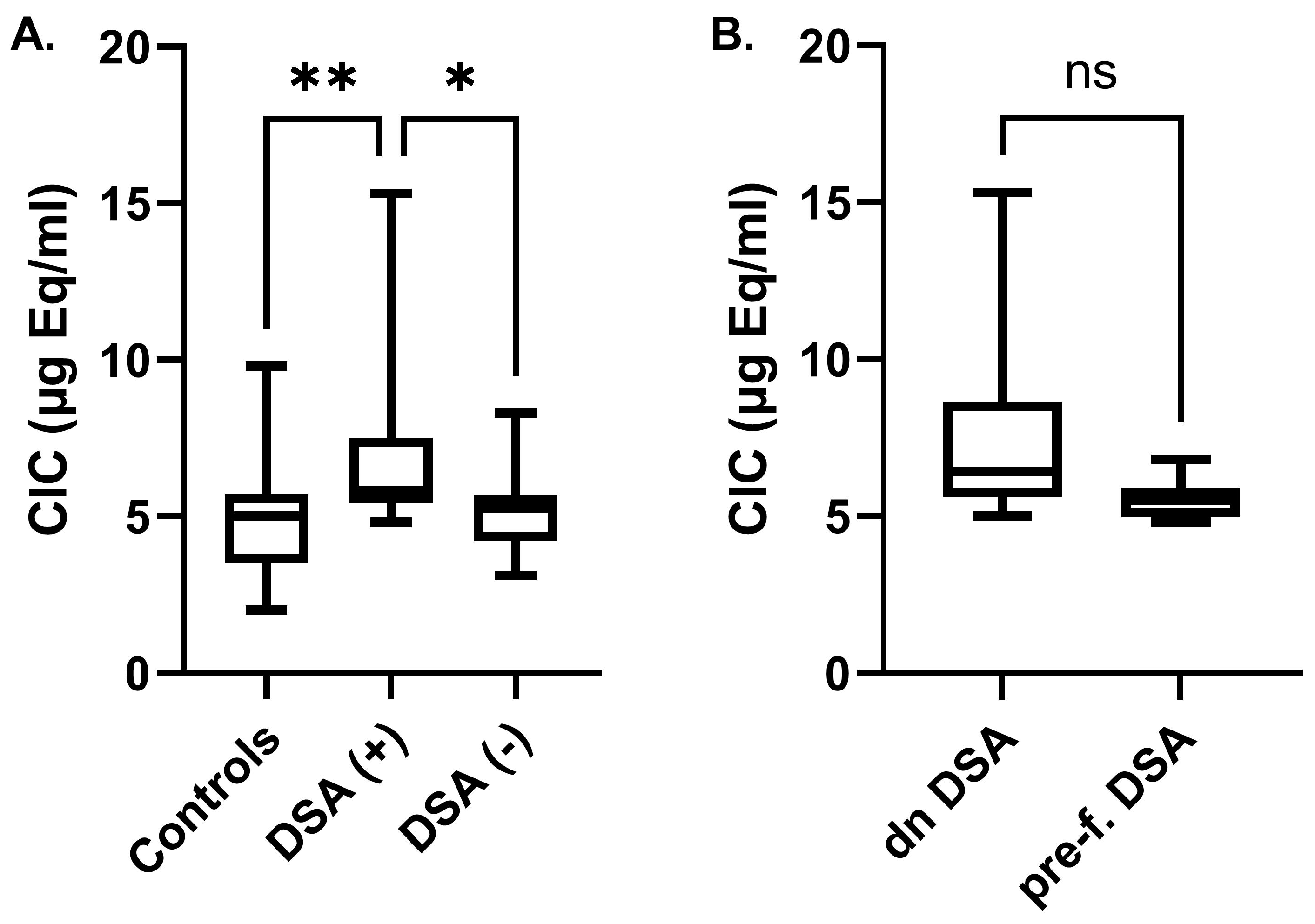
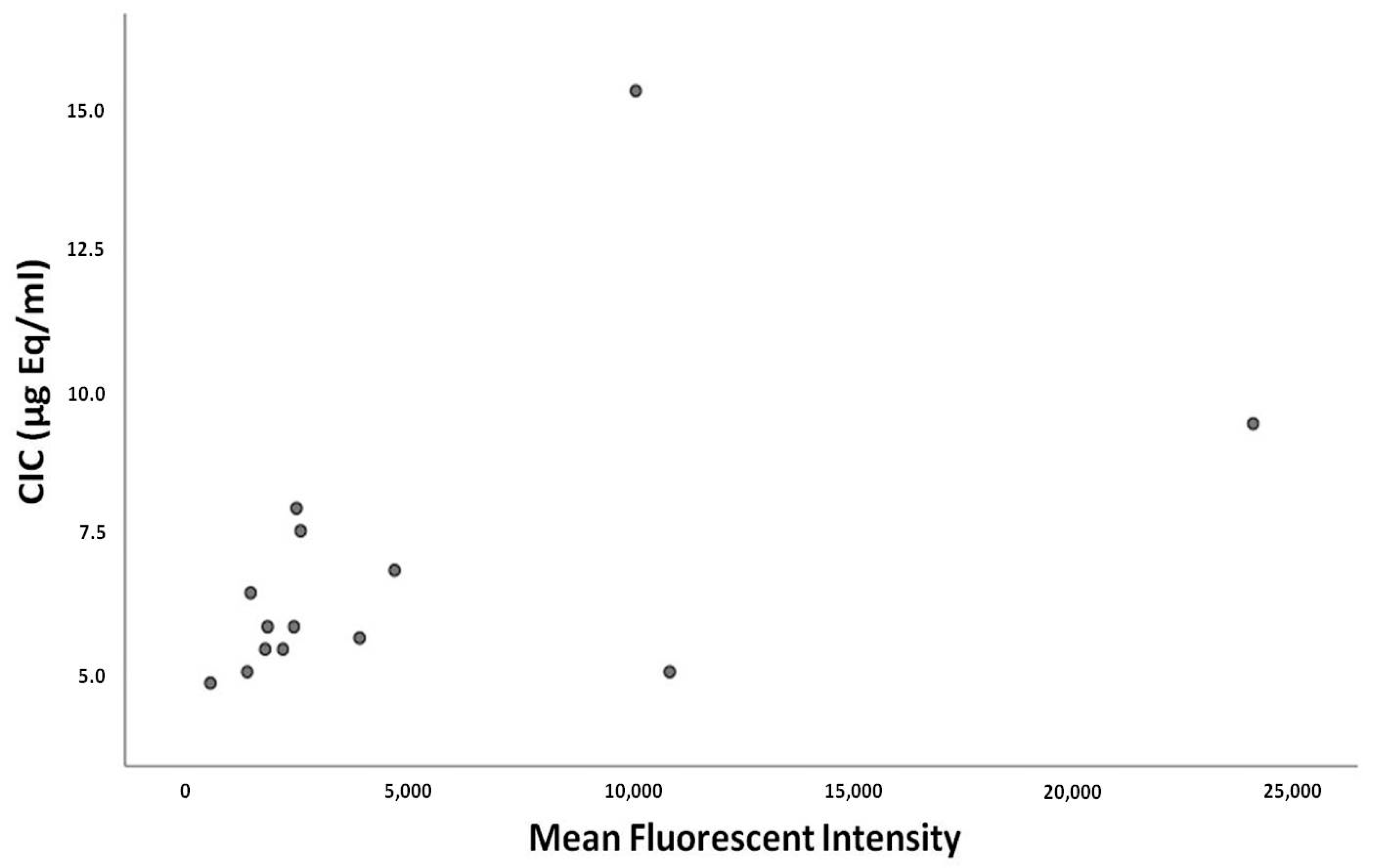
2.2. Circulating Immune Complexes Correlations
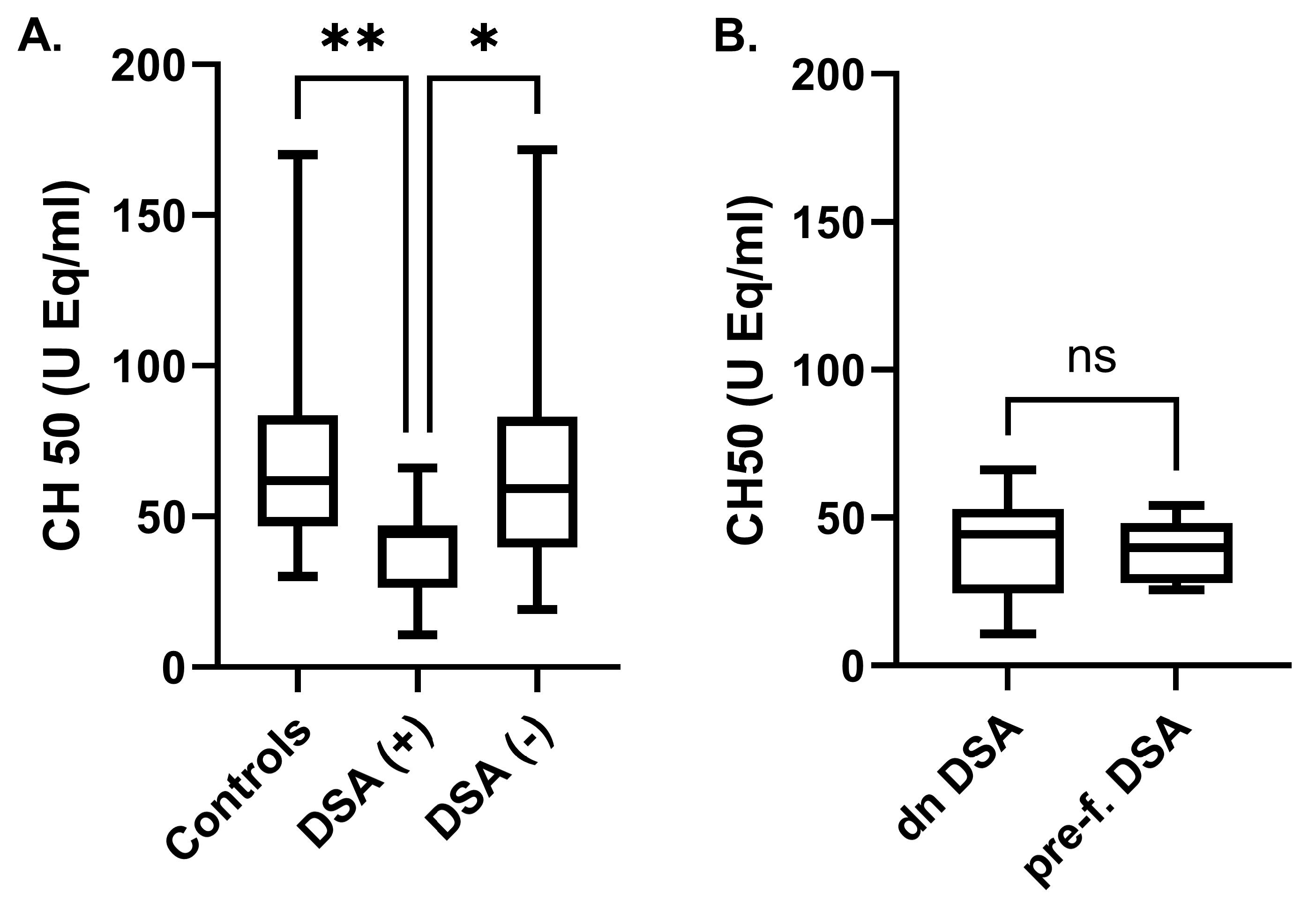
2.3. Complement-Activation Correlations
3. Discussion
4. Materials and Method
4.1. Patients
4.2. Ethics Approval and Consent to Participate
4.3. Immunosuppressive Treatment
4.4. Detection of Donor-Specific HLA Antibodies
4.5. Serum Creatinine Determination and eGFR Calculation
4.6. Enzyme Immunoassays
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DSA | Donor-specific antibodies |
| KTR | Kidney transplant recipient |
| AMR | Antibody-mediated rejection |
| CIC | Circulating immune complexes |
| CH50 | Complement hemolysis 50% |
| MFI | Mean fluorescent intensity |
| NLR | Neutrophil-to-lymphocyte ratio |
References
- Pascual, J.; Zamora, J.; Pirsch, J.D. A systematic review of kidney transplantation from expanded criteria donors. Am. J. Kidney Dis. 2008, 52, 553–586. [Google Scholar] [CrossRef]
- Mohan, S.; Palanisamy, A.; Tsapepas, D.; Tanriover, B.; Crew, R.J.; Dube, G.; Ratner, L.E.; Cohen, D.J.; Radhakrishnan, J. Donor-specific antibodies adversely affect kidney allograft outcomes. J. Am. Soc. Nephrol. 2012, 23, 2061–2071. [Google Scholar] [CrossRef]
- Malheiro, J.; Tafulo, S.; Dias, L.; Martins, S.; Fonseca, I.; Beirao, I.; Castro-Henriques, A.; Cabrita, A. Determining donor-specific antibody C1q-binding ability improves the prediction of antibody-mediated rejection in human leucocyte antigen-incompatible kidney transplantation. Transpl. Int. 2017, 30, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, S.M.; Chen, G.; Sequeira, F.A.; Lou, C.D.; Alexander, S.R.; Tyan, D.B. Complement-fixing donor-specific antibodies identified by a novel C1q assay are associated with allograft loss. Pediatr. Transplant. 2012, 16, 12–17. [Google Scholar] [CrossRef]
- Zhang, R. Donor-Specific Antibodies in Kidney Transplant Recipients. Clin. J. Am. Soc. Nephrol. 2018, 13, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Filippone, E.J.; Farber, J.L. Humoral Immune Response and Allograft Function in Kidney Transplantation. Am. J. Kidney Dis. 2015, 66, 337–347. [Google Scholar] [CrossRef]
- Lee, P.C.; Zhu, L.; Terasaki, P.I.; Everly, M.J. HLA-specific antibodies developed in the first year posttransplant are predictive of chronic rejection and renal graft loss. Transplantation 2009, 88, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Stites, E.; Le Quintrec, M.; Thurman, J.M. The Complement System and Antibody-Mediated Transplant Rejection. J. Immunol. 2015, 195, 5525–5531. [Google Scholar] [CrossRef]
- Valenzuela, N.M.; McNamara, J.T.; Reed, E.F. Antibody-mediated graft injury: Complement-dependent and complement-independent mechanisms. Curr. Opin. Organ. Transplant. 2014, 19, 33–40. [Google Scholar] [CrossRef]
- Bohmig, G.A.; Exner, M.; Habicht, A.; Schillinger, M.; Lang, U.; Kletzmayr, J.; Saemann, M.D.; Horl, W.H.; Watschinger, B.; Regele, H. Capillary C4d deposition in kidney allografts: A specific marker of alloantibody-dependent graft injury. J. Am. Soc. Nephrol. 2002, 13, 1091–1099. [Google Scholar]
- Collins, A.B.; Schneeberger, E.E.; Pascual, M.A.; Saidman, S.L.; Williams, W.W.; Tolkoff-Rubin, N.; Cosimi, A.B.; Colvin, R.B. Complement activation in acute humoral renal allograft rejection: Diagnostic significance of C4d deposits in peritubular capillaries. J. Am. Soc. Nephrol. 1999, 10, 2208–2214. [Google Scholar] [CrossRef] [PubMed]
- Sellares, J.; de Freitas, D.G.; Mengel, M.; Reeve, J.; Einecke, G.; Sis, B.; Hidalgo, L.G.; Famulski, K.; Matas, A.; Halloran, P.F. Understanding the causes of kidney transplant failure: The dominant role of antibody-mediated rejection and nonadherence. Am. J. Transplant. 2012, 12, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Reed, E.F. Effect of antibodies on endothelium. Am. J. Transplant. 2009, 9, 2459–2465. [Google Scholar] [CrossRef]
- Aubert, V.; Venetz, J.P.; Pantaleo, G.; Pascual, M. Low levels of human leukocyte antigen donor-specific antibodies detected by solid phase assay before transplantation are frequently clinically irrelevant. Hum. Immunol. 2009, 70, 580–583. [Google Scholar] [CrossRef]
- Otten, H.G.; Verhaar, M.C.; Borst, H.P.; Hene, R.J.; van Zuilen, A.D. Pretransplant donor-specific HLA class-I and -II antibodies are associated with an increased risk for kidney graft failure. Am. J. Transplant. 2012, 12, 1618–1623. [Google Scholar] [CrossRef]
- Tsuji, T. Key Points of the Banff 2019 Classification of Renal Allograft Pathology. Nephron 2023, 147 (Suppl. S1), 6–8. [Google Scholar] [CrossRef]
- Loupy, A.; Lefaucheur, C.; Vernerey, D.; Prugger, C.; Duong van Huyen, J.P.; Mooney, N.; Suberbielle, C.; Fremeaux-Bacchi, V.; Mejean, A.; Desgrandchamps, F.; et al. Complement-binding anti-HLA antibodies and kidney-allograft survival. N. Engl. J. Med. 2013, 369, 1215–1226. [Google Scholar] [CrossRef]
- Thammanichanond, D.; Mongkolsuk, T.; Rattanasiri, S.; Kantachuvesiri, S.; Worawichawong, S.; Jirasiritham, S.; Kitpoka, P. Significance of C1q-fixing donor-specific antibodies after kidney transplantation. Transplant. Proc. 2014, 46, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Okabe, Y.; Noguchi, H.; Miyamoto, K.; Kaku, K.; Tsuchimoto, A.; Masutani, K.; Nakamura, M. Preformed C1q-binding Donor-specific Anti-HLA Antibodies and Graft Function After Kidney Transplantation. Transplant. Proc. 2018, 50, 3460–3466. [Google Scholar] [CrossRef]
- Thammanichanond, D.; Wiwattanathum, P.; Mongkolsuk, T.; Kantachuvesiri, S.; Worawichawong, S.; Vallipakorn, S.A.; Kitpoka, P. Role of Pretransplant Complement-fixing Donor-specific Antibodies Identified by C1q Assay in Kidney Transplantation. Transplant. Proc. 2016, 48, 756–760. [Google Scholar] [CrossRef]
- Lachmann, N.; Todorova, K.; Schulze, H.; Schonemann, C. Systematic comparison of four cell- and Luminex-based methods for assessment of complement-activating HLA antibodies. Transplantation 2013, 95, 694–700. [Google Scholar] [CrossRef]
- Llorente, S.; Boix, F.; Eguia, J.; Lopez, M.; Bosch, A.; Martinez, H.; Gonzalez, M.J.; Lopez-Hernandez, R.; Salgado, G.; Moya-Quiles, M.R.; et al. C1q-fixing human leukocyte antigen assay in immunized renal patients: Correlation between Luminex SAB-C1q and SAB-IgG. Transplant. Proc. 2012, 44, 2535–2537. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, V.; Quan, M. SLE and Serum Complement: Causative, Concomitant or Coincidental? Open Rheumatol. J. 2017, 11, 113–122. [Google Scholar] [CrossRef]
- Mezo, B.; Heilos, A.; Bohmig, G.A.; Eskandary, F.; Wahrmann, M.; Bond, G.; Kozakowski, N.; Halloran, P.F.; Rusai, K.; Prohaszka, Z. Complement Markers in Blood and Urine: No Diagnostic Value in Late Silent Antibody-Mediated Rejection. Transplant. Direct 2019, 5, e470. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.M.; Moon, Y.J.; Jung, K.W.; Park, Y.S.; Jun, I.G.; Kim, S.O.; Song, J.G.; Hwang, G.S. Neutrophil-to-lymphocyte ratio is a predictor of early graft dysfunction following living donor liver transplantation. Liver Int. 2019, 39, 1545–1556. [Google Scholar] [CrossRef]
- Ommen, S.R.; Hammill, S.C.; Gibbons, R.J. The relative lymphocyte count predicts death in patients receiving implantable cardioverter defibrillators. Pacing Clin. Electrophysiol. 2002, 25, 1424–1428. [Google Scholar] [CrossRef]
- Cho, I.R.; Park, J.C.; Park, C.H.; Jo, J.H.; Lee, H.J.; Kim, S.; Shim, C.N.; Lee, H.; Shin, S.K.; Lee, S.K.; et al. Pre-treatment neutrophil to lymphocyte ratio as a prognostic marker to predict chemotherapeutic response and survival outcomes in metastatic advanced gastric cancer. Gastric Cancer 2014, 17, 703–710. [Google Scholar] [CrossRef]
- Chandrashekara, S.; Rajendran, A.; Bai Jaganath, A.; Krishnamurthy, R. Neutrophil-lymphocyte ratio, pain perception, and disease activity score may serve as important predictive markers for sustained remission in rheumatoid arthritis. Reumatismo 2015, 67, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Xiao, D.; Guo, J.; Chahan, B.; Wang, Z. Neutrophil-lymphocyte count ratio as a diagnostic marker for acute kidney injury: A systematic review and meta-analysis. Clin. Exp. Nephrol. 2020, 24, 126–135. [Google Scholar] [CrossRef]
- Yuan, Q.; Wang, J.; Peng, Z.; Zhou, Q.; Xiao, X.; Xie, Y.; Wang, W.; Huang, L.; Tang, W.; Sun, D.; et al. Neutrophil-to-lymphocyte ratio and incident end-stage renal disease in Chinese patients with chronic kidney disease: Results from the Chinese Cohort Study of Chronic Kidney Disease (C-STRIDE). J. Transl. Med. 2019, 17, 86. [Google Scholar] [CrossRef]
- Gupta, A.; Sinnott, P. Clinical relevance of pretransplant human leukocyte antigen donor-specific antibodies in renal patients waiting for a transplant: A risk factor. Hum. Immunol. 2009, 70, 618–622. [Google Scholar] [CrossRef] [PubMed]
- Crespo, M.; Torio, A.; Mas, V.; Redondo, D.; Perez-Saez, M.J.; Mir, M.; Faura, A.; Guerra, R.; Montes-Ares, O.; Checa, M.D.; et al. Clinical relevance of pretransplant anti-HLA donor-specific antibodies: Does C1q-fixation matter? Transpl. Immunol. 2013, 29, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Wisse, B.W.; Kamburova, E.G.; Joosten, I.; Allebes, W.A.; van der Meer, A.; Hilbrands, L.B.; Baas, M.C.; Spierings, E.; Hack, C.E.; van Reekum, F.E.; et al. Toward a Sensible Single-antigen Bead Cutoff Based on Kidney Graft Survival. Transplantation 2019, 103, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef]

| Healthy Subjects | Kidney Transplant Recipients | ||
|---|---|---|---|
| DSA-Negative | DSA-Positive | ||
| Number of subjects per group | 22 | 28 | 15 |
| Gender (M/F) | 9/13 | 13/15 | 10/5 |
| Age (years) | 46 ± 14 | 55 ± 15 | 50 ± 11 |
| Serum creatinine (mg/dL) | 0.8 ± 0.1 | 1.4 ± 0.6 | 1.7 ± 0.8 |
| eGFR (mL/min/1.73 m2) | 106 ± 13 | 61 ± 27 | 53 ± 24 |
| Neutrophil count (×103/μL) | 3.5 ± 0.8 | 4.8 ± 1.5 | 5.3 ± 2.6 |
| Lymphocyte count (×103/μL) | 2.3 ± 0.6 | 2.2 ± 0.9 | 2.0 ± 0.7 |
| Neutrophil/lymphocyte ratio | 1.6 ± 0.6 | 2.6 ± 1.4 | 3.0 ± 1.7 |
| Mean fluorescence intensity DSA (median, 25% and 75% percentile) | - | - | 2443 (1683–6039) |
| Serum IgG levels (mg/dL) | - | 966 ± 305 | 1087 ± 363 |
| Cause of ESKD | |||
| Glomerulonephritis | - | 7 | 6 |
| Diabetes mellitus | - | 3 | - |
| Hypertension | - | 1 | - |
| Alport syndrome | - | - | 2 |
| Tubulointerstitial disease | 8 | 1 | |
| Unknown | - | 9 | 6 |
| Immunosuppressant treatment | |||
| Glucocorticosteroids | - | 28 | 15 |
| Tacrolimus | - | 25 | 15 |
| Cyclosporine | - | 3 | |
| Mycophenolate | - | 25 | 15 |
| Everolimus | - | 3 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trivyza, M.S.; Stergiopoulou, C.; Tsakas, S.; Ntrinias, T.; Papasotiriou, M.; Karydis, N.; Papachristou, E.; Goumenos, D.S. Circulating Immune Complexes and Complement Activation in Sensitized Kidney Transplant Recipients. Int. J. Mol. Sci. 2024, 25, 10904. https://doi.org/10.3390/ijms252010904
Trivyza MS, Stergiopoulou C, Tsakas S, Ntrinias T, Papasotiriou M, Karydis N, Papachristou E, Goumenos DS. Circulating Immune Complexes and Complement Activation in Sensitized Kidney Transplant Recipients. International Journal of Molecular Sciences. 2024; 25(20):10904. https://doi.org/10.3390/ijms252010904
Chicago/Turabian StyleTrivyza, Maria Stella, Charikleia Stergiopoulou, Sotiris Tsakas, Theodoros Ntrinias, Marios Papasotiriou, Nikolaos Karydis, Evangelos Papachristou, and Dimitrios S. Goumenos. 2024. "Circulating Immune Complexes and Complement Activation in Sensitized Kidney Transplant Recipients" International Journal of Molecular Sciences 25, no. 20: 10904. https://doi.org/10.3390/ijms252010904






