Marker-Assisted Selection of Jacalin-Related Lectin Genes OsJRL45 and OsJRL40 Derived from Sea Rice 86 Enhances Salt Tolerance in Rice
Abstract
1. Introduction
2. Results
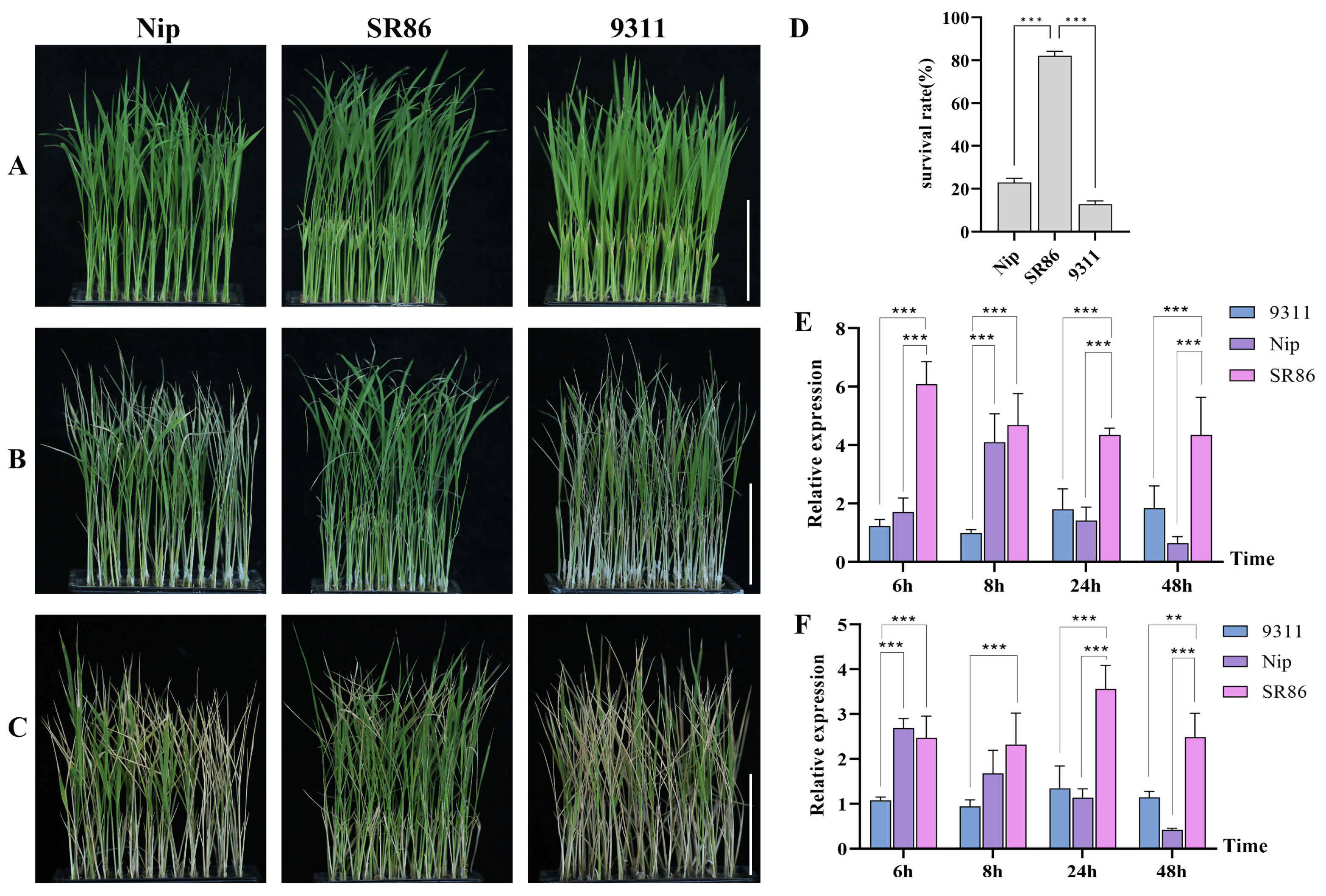
2.1. Analysis of OsJRL45 and OsJRL40 Expression under Salt Stress
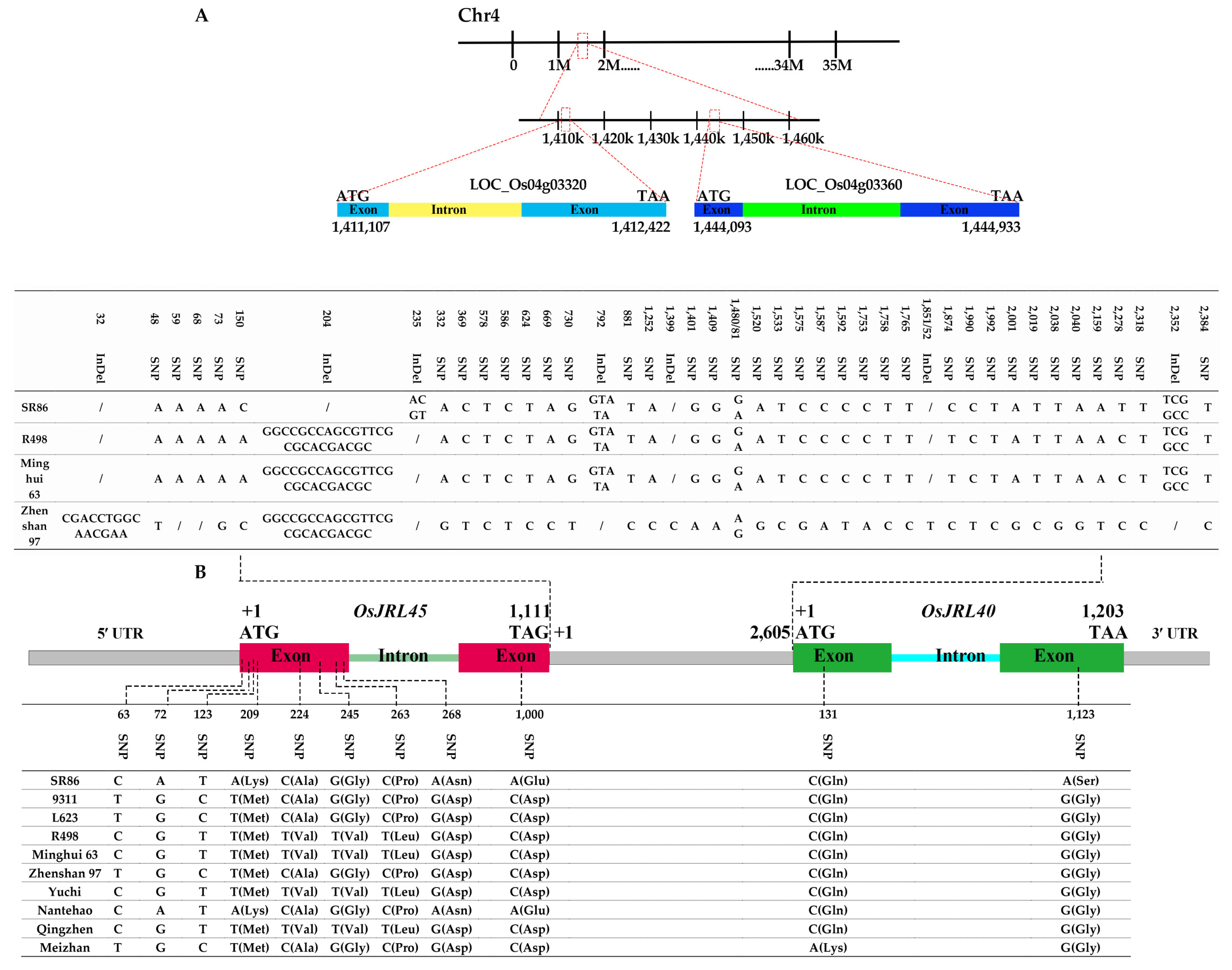
2.2. Analysis of Sequence Variation in OsJRL45 and OsJRL40
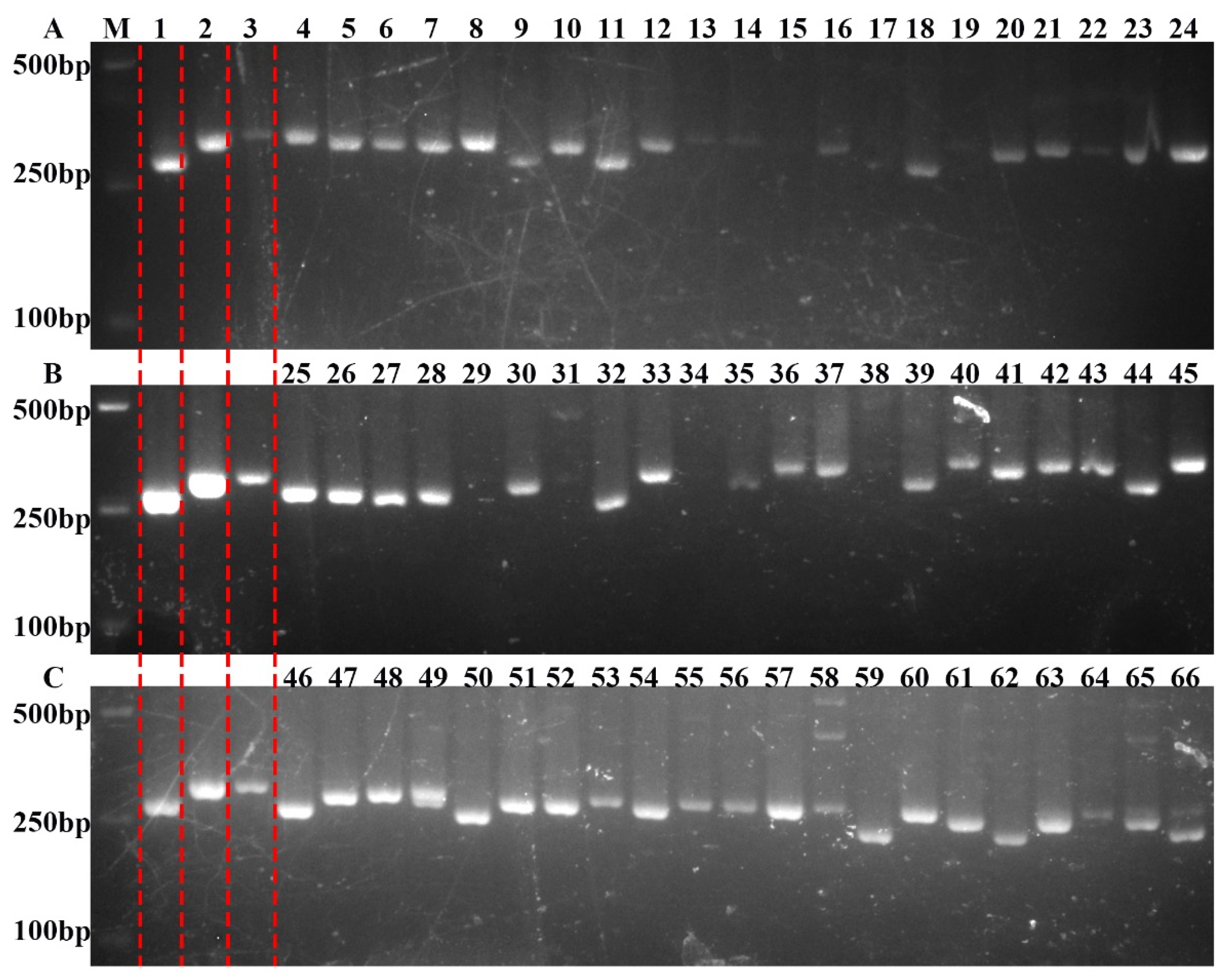
2.3. Validation of Molecular Markers in Populations
2.4. Validation of Molecular Markers for Salt Tolerance
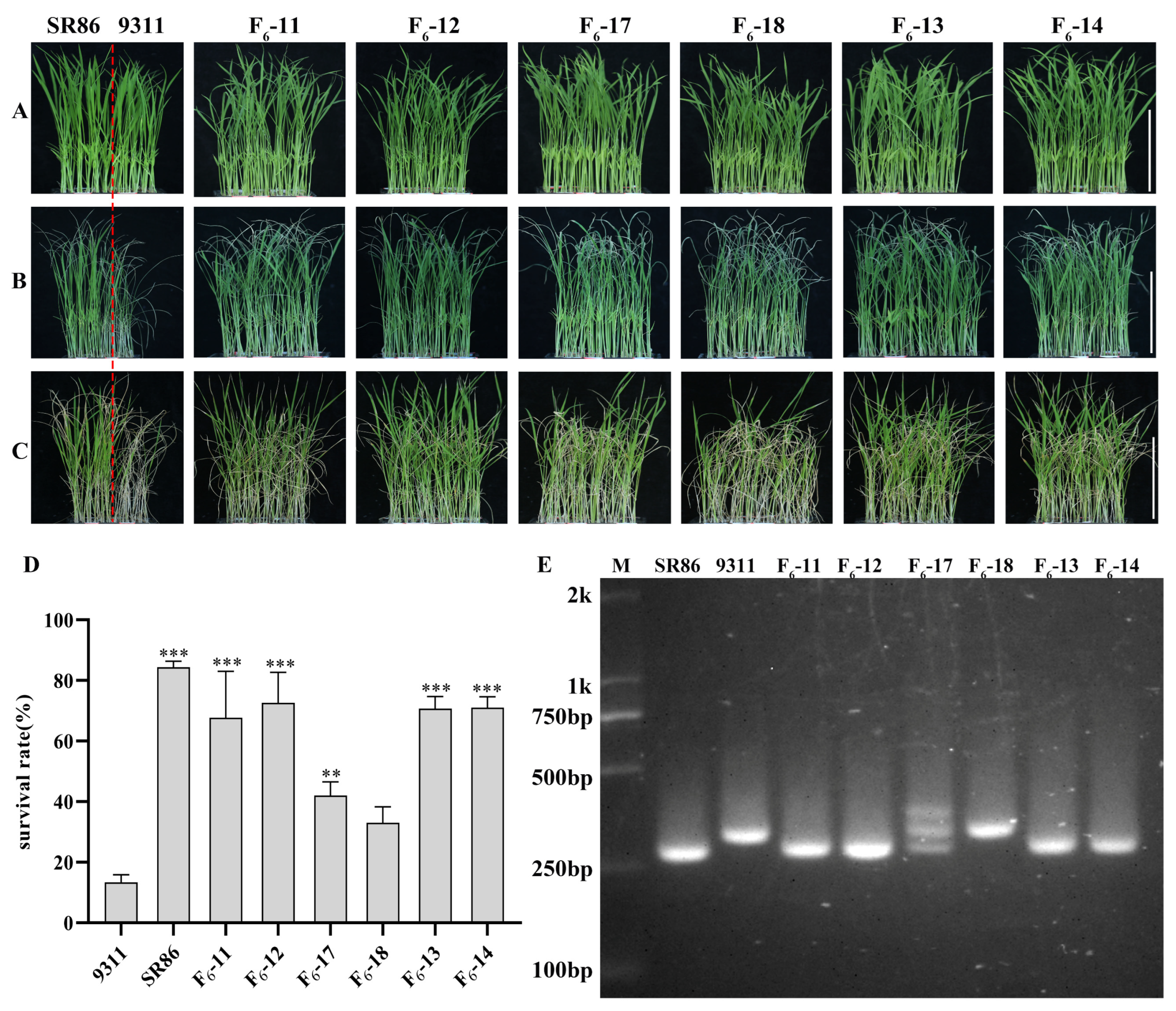
2.5. Validation of MAS for Salt Tolerance in RILs Derived from SR86 × 9311
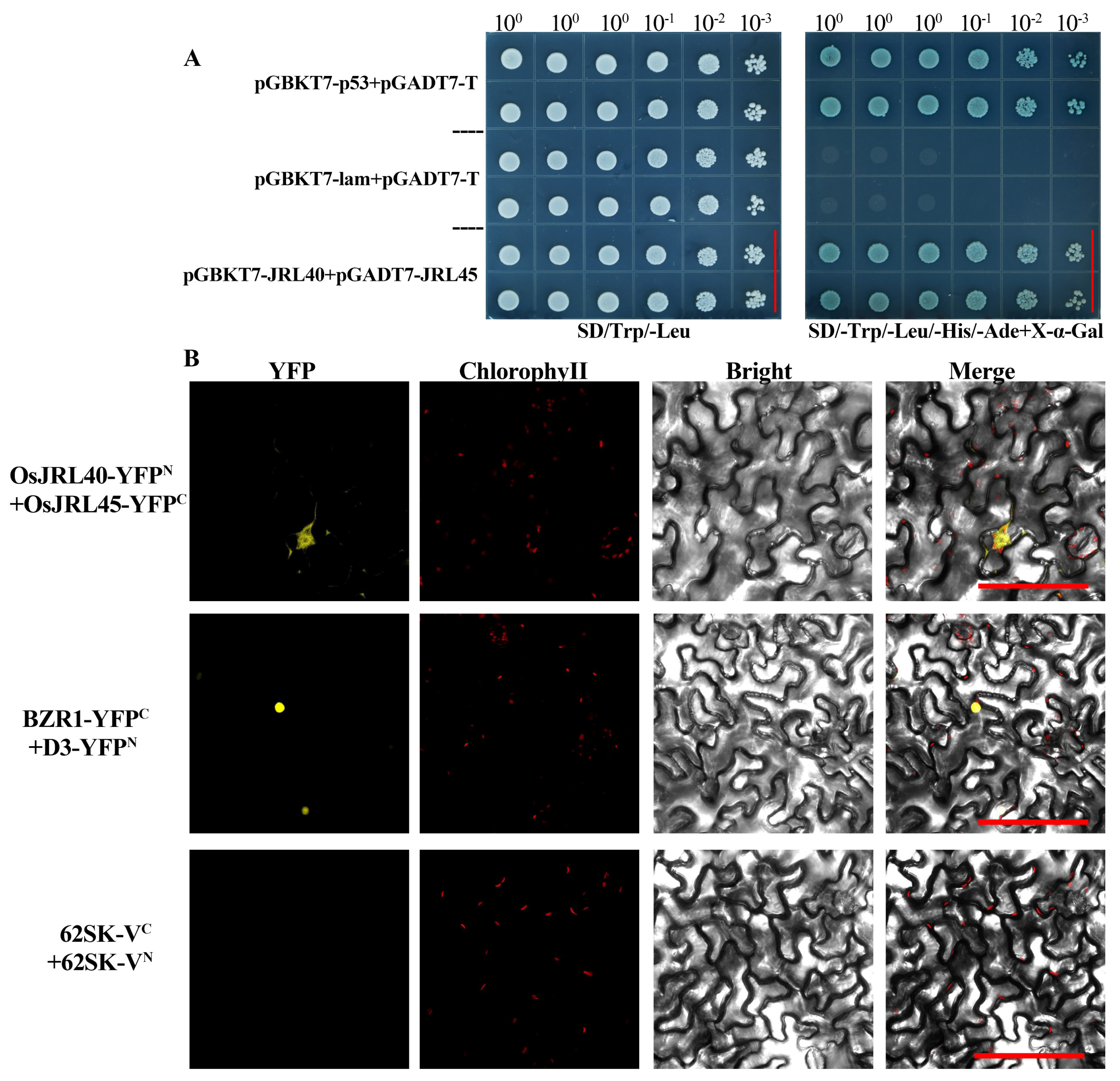
2.6. Analysis of the Interaction between OsJRL45 and OsJRL40
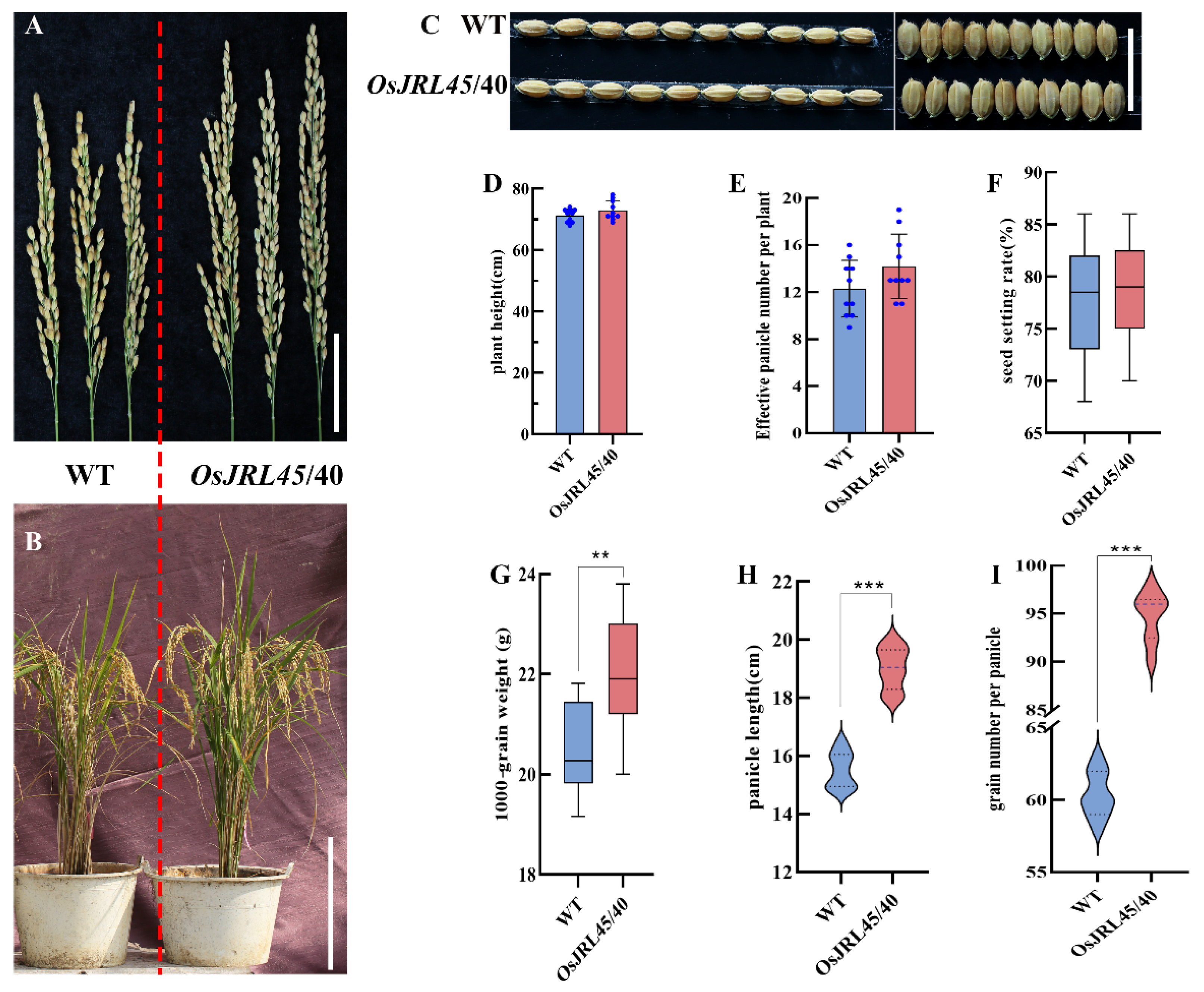
2.7. Analysis of Agronomic Traits in Mature Transgenic Plants Harboring OsJRL45/40 from SR86
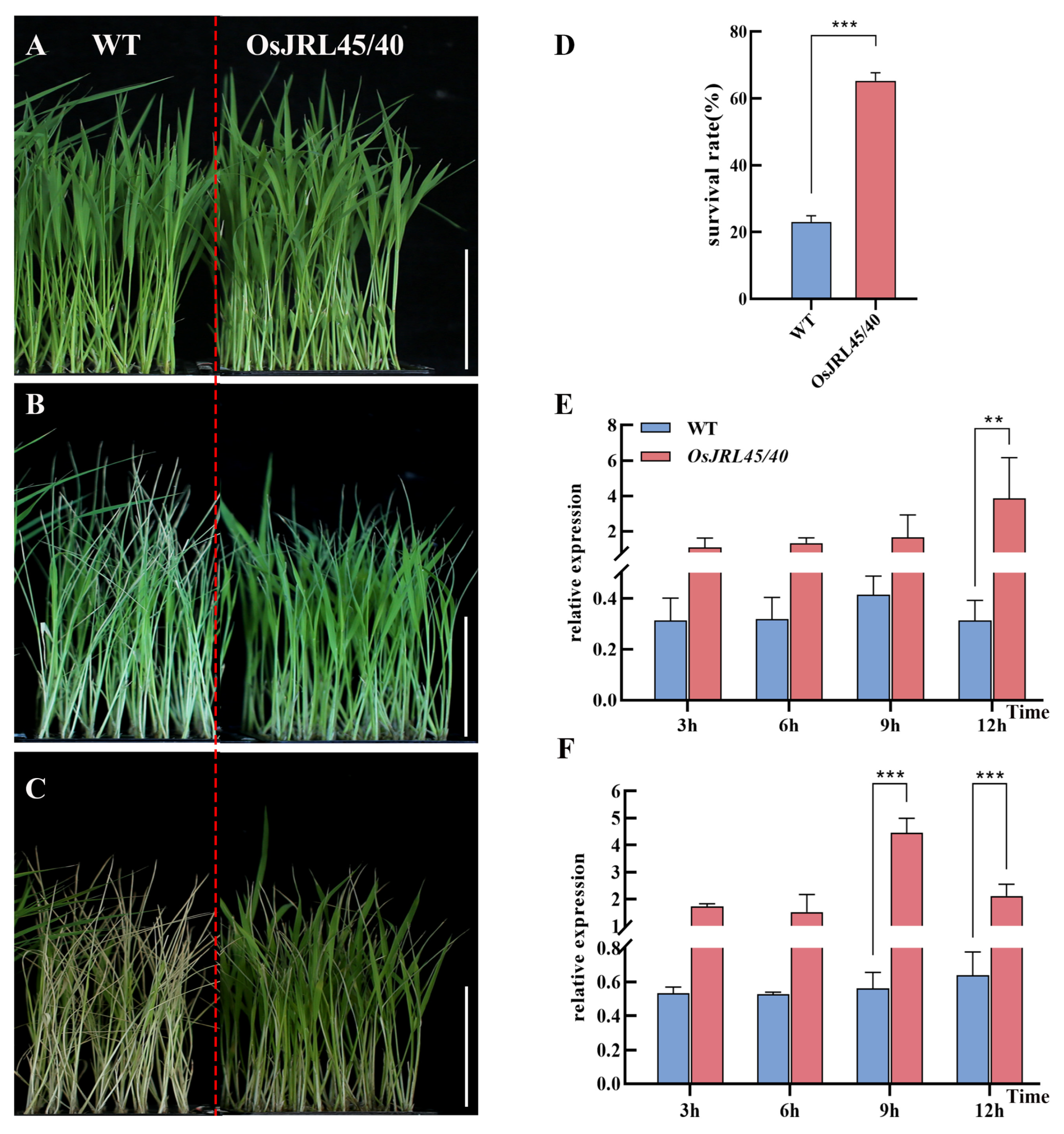
2.8. Co-Transformation with OsJRL45/40 from SR86 Enhances Tolerance of Salt Stress at the Three-Leaf Stage
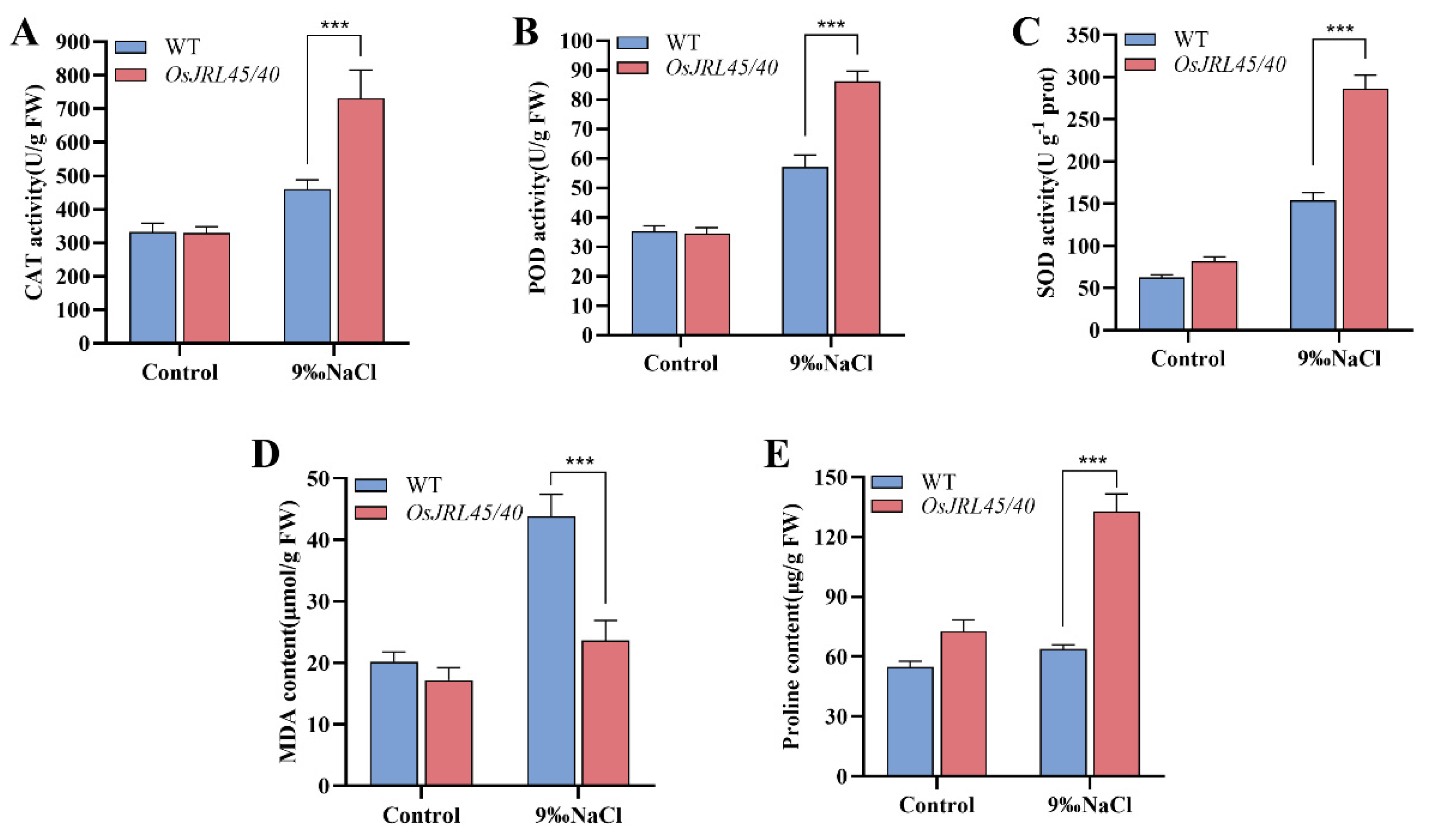
2.9. Co-Transformation with OsJRL45/40 Derived from SR86 Enhances Antioxidant Enzyme Activities under Salt Stress
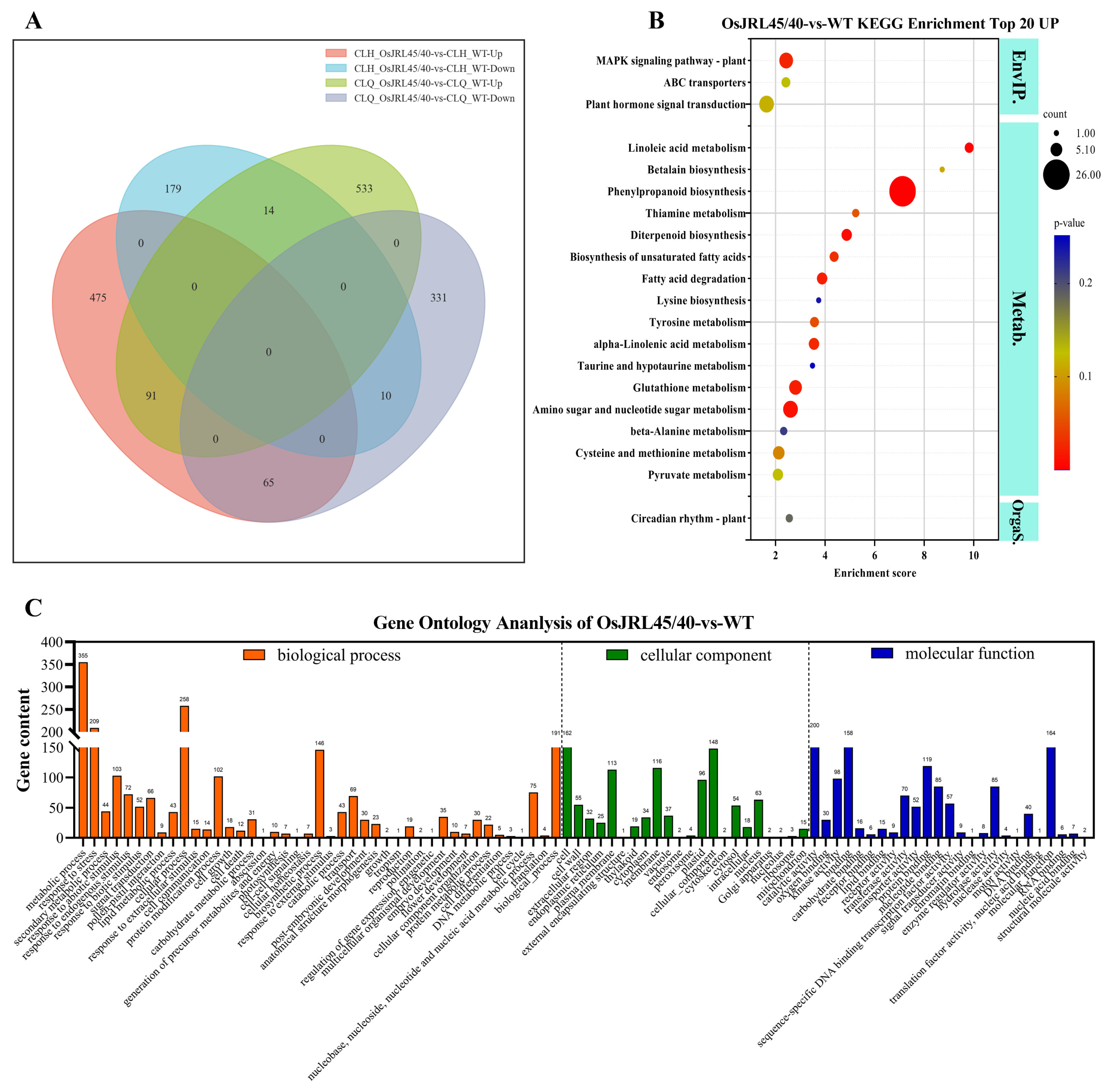
2.10. Analysis of Differentially Expressed Genes
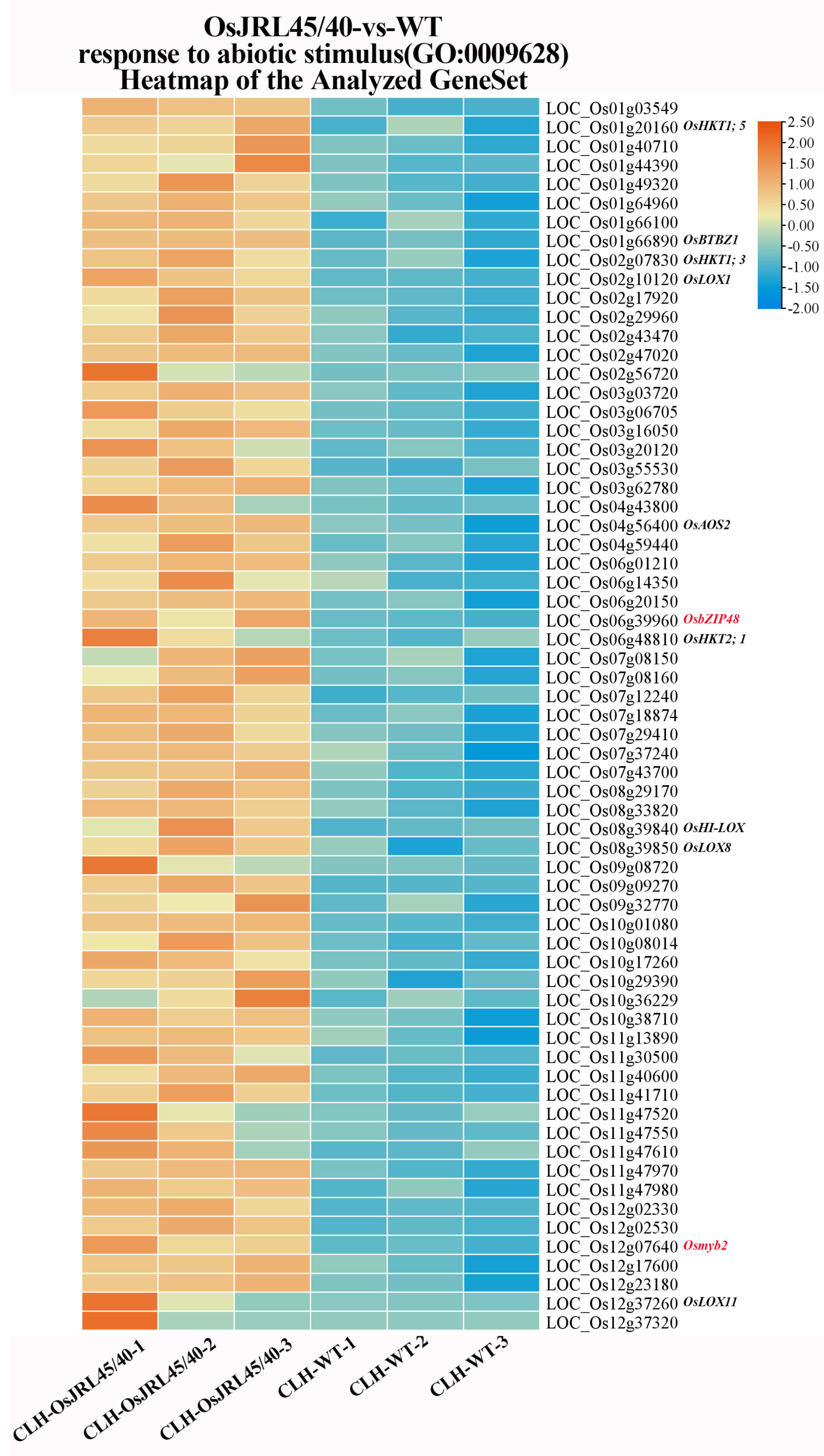
2.11. Gene Set Enrichment Analysis (GSEA) of the Response to Salt Stress
3. Discussion
3.1. OsJRL45 and OsJRL40 Interact Together to Enhance the Salt Tolerance of Plants
3.2. Transcriptome Analysis of OsJRL45 and OsJRL40 Transgenic Seedlings under Salt Stress
3.3. Sequence Differences between OsJRL45 and OsJRL40: Marker-Assisted Selection (MAS) Analysis for the Selection of Salt-Tolerant Rice
4. Materials and Methods
4.1. Experimental Plants
4.2. Construction of Segregating Populations
4.3. Marker-Assisted Selection
4.4. Salt Stress Treatment of Transgenic Plants
4.5. Measurement of Physiological Parameters
4.6. RNA Extraction and Quantitative Real-Time PCR (qRT-PCR)
4.7. Transcriptomic Data Analysis
4.8. Yeast Two-Hybrid (Y2H) Assays
4.9. Bimolecular Fluorescence Complementation (BiFC) Assay
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wing, R.A.; Purugganan, M.D.; Zhang, Q. The rice genome revolution: From an ancient grain to Green Super Rice. Nat. Rev. Genet. 2018, 19, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Butcher, K.; Wick, A.F.; DeSutter, T.; Chatterjee, A.; Harmon, J. Soil Salinity: A Threat to Global Food Security. Agron. J. 2016, 108, 2189–2200. [Google Scholar] [CrossRef]
- Godoy, F.; Olivos-Hernandez, K.; Stange, C.; Handford, M. Abiotic Stress in Crop Species: Improving Tolerance by Applying Plant Metabolites. Plants 2021, 10, 186. [Google Scholar] [CrossRef]
- van Dijk, M.; Morley, T.; Rau, M.L.; Saghai, Y. A meta-analysis of projected global food demand and population at risk of hunger for the period 2010–2050. Nat. Food 2021, 2, 494–501. [Google Scholar] [CrossRef]
- Ashraf, M.; Akram, N.A. Improving salinity tolerance of plants through conventional breeding and genetic engineering: An analytical comparison. Biotechnol. Adv. 2009, 27, 744–752. [Google Scholar] [CrossRef]
- Rasheed, A.; Li, H.; Nawaz, M.; Mahmood, A.; Hassan, M.U.; Shah, A.N.; Hussain, F.; Azmat, S.; Gillani, S.F.A.; Majeed, Y.; et al. Molecular tools, potential frontiers for enhancing salinity tolerance in rice: A critical review and future prospective. Front. Plant Sci. 2022, 13, 966749. [Google Scholar] [CrossRef]
- Bhatt, T.; Sharma, A.; Puri, S.; Minha, A.P. Salt Tolerance Mechanisms and Approaches: Future Scope of Halotolerant Genes and Rice Landraces. Rice Sci. 2020, 27, 368–383. [Google Scholar] [CrossRef]
- Hussain, S.; Zhang, J.H.; Zhong, C.; Zhu, L.F.; Cao, X.C.; Yu, S.M.; James, A.B.; Hu, J.J.; Jin, Q.Y. Effects of salt stress on rice growth, development characteristics, and the regulating ways: A review. J. Integr. Agric. 2017, 16, 2357–2374. [Google Scholar] [CrossRef]
- Wu, H.H. Plant salt tolerance and Na+ sensing and transport. Crop J. 2018, 6, 215–225. [Google Scholar] [CrossRef]
- Chen, G.; Liu, C.; Gao, Z.; Zhang, Y.; Zhang, A.; Zhu, L.; Hu, J.; Ren, D.; Yu, L.; Xu, G.; et al. Variation in the Abundance of OsHAK1 Transcript Underlies the Differential Salinity Tolerance of an indica and a japonica Rice Cultivar. Front. Plant Sci. 2017, 8, 2216. [Google Scholar] [CrossRef]
- Chen, G.; Hu, Q.; Luo, L.; Yang, T.; Zhang, S.; Hu, Y.; Yu, L.; Xu, G. Rice potassium transporter OsHAK1 is essential for maintaining potassium-mediated growth and functions in salt tolerance over low and high potassium concentration ranges. Plant Cell Environ. 2015, 38, 2747–2765. [Google Scholar] [CrossRef] [PubMed]
- Rai, H.; Yokoyama, S.; Satoh-Nagasawa, N.; Furukawa, J.; Nomi, T.; Ito, Y.; Fujimura, S.; Takahashi, H.; Suzuki, R.; Yousra, E.; et al. Cesium Uptake by Rice Roots Largely Depends Upon a Single Gene, HAK1, Which Encodes a Potassium Transporter. Plant Cell Physiol. 2017, 58, 1486–1493. [Google Scholar] [CrossRef]
- Wang, J.; Nan, N.; Li, N.; Liu, Y.; Wang, T.J.; Hwang, I.; Liu, B.; Xu, Z.Y. A DNA Methylation Reader-Chaperone Regulator-Transcription Factor Complex Activates OsHKT1;5 Expression during Salinity Stress. Plant Cell 2020, 32, 3535–3558. [Google Scholar] [CrossRef]
- Tian, Q.; Shen, L.; Luan, J.; Zhou, Z.; Guo, D.; Shen, Y.; Jing, W.; Zhang, B.; Zhang, Q.; Zhang, W. Rice shaker potassium channel OsAKT2 positively regulates salt tolerance and grain yield by mediating K+ redistribution. Plant Cell Environ. 2021, 44, 2951–2965. [Google Scholar] [CrossRef]
- Hao, S.H.; Wang, Y.R.; Yan, Y.X.; Liu, Y.H.; Wang, J.Y.; Chen, S. A Review on Plant Responses to Salt Stress and Their Mechanisms of Salt Resistance. Horticulturae 2021, 7, 132. [Google Scholar] [CrossRef]
- Liu, C.T.; Mao, B.G.; Yuan, D.Y.; Chu, C.C.; Duan, M.J. Salt tolerance in rice: Physiological responses and molecular mechanisms. Crop J. 2022, 10, 13–25. [Google Scholar] [CrossRef]
- Das, G.; Patra, J.K.; Baek, K.H. Insight into MAS: A Molecular Tool for Development of Stress Resistant and Quality of Rice through Gene Stacking. Front. Plant Sci. 2017, 8, 985. [Google Scholar]
- Tamiru, M.; Takagi, H.; Abe, A.; Yokota, T.; Kanzaki, H.; Okamoto, H.; Saitoh, H.; Takahashi, H.; Fujisaki, K.; Oikawa, K.; et al. A chloroplast-localized protein LESION AND LAMINA BENDING affects defence and growth responses in rice. New Phytol. 2016, 210, 1282–1297. [Google Scholar] [CrossRef]
- Nagata, K.; Ando, T.; Nonoue, Y.; Mizubayashi, T.; Kitazawa, N.; Shomura, A.; Matsubara, K.; Ono, N.; Mizobuchi, R.; Shibaya, T.; et al. Advanced backcross QTL analysis reveals complicated genetic control of rice grain shape in a japonica x indica cross. Breed. Sci. 2015, 65, 308–318. [Google Scholar] [CrossRef]
- Fekih, R.; Tamiru, M.; Kanzaki, H.; Abe, A.; Yoshida, K.; Kanzaki, E.; Saitoh, H.; Takagi, H.; Natsume, S.; Undan, J.R.; et al. The rice (Oryza sativa L.) LESION MIMIC RESEMBLING, which encodes an AAA-type ATPase, is implicated in defense response. Mol. Genet. Genom. 2015, 290, 611–622. [Google Scholar] [CrossRef]
- Fekih, R.; Ishimaru, Y.; Okada, S.; Maeda, M.; Miyagi, R.; Obana, T.; Suzuki, K.; Inamori, M.; Enoki, H.; Yamasaki, M. High-Density Linkage Maps from Japanese Rice japonica Recombinant Inbred Lines Using Genotyping by Random Amplicon Sequencing-Direct (GRAS-Di). Plants 2023, 12, 929. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.M.; Rafii, M.Y.; Ismail, M.R.; Mahmood, M.; Rahim, H.A.; Alam, M.A.; Ashkani, S.; Malek, M.A.; Latif, M.A. Marker-assisted backcrossing: A useful method for rice improvement. Biotechnol. Biotechnol. Equip. 2015, 29, 237–254. [Google Scholar] [CrossRef]
- Prabhu, A.S.; Filippi, M.C.; Silva, G.B.; Silva Lobo, V.L.; Morais, O.P. An Unprecedented Outbreak of Rice Blast on a Newly Released Cultivar BRS Colosso in Brazil. In Advances in Genetics, Genomics and Control of Rice Blast Disease; Springer: Dordrecht, The Netherlands, 2009. [Google Scholar]
- Collard, B.C.; Mackill, D.J. Marker-assisted selection: An approach for precision plant breeding in the twenty-first century. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008, 363, 557–572. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Hu, F.; Adolf, A.; Ackah, M.; Justice, A.A.; Lin, Q.; Li, L.; Zhao, W.G. Cloning and abiotic stress expression analysis of galactose-binding lectin (GBL) gene from mulberry and its prokaryotic expression in E. coli. J. Hortic. Sci. Biotechnol. 2021, 96, 24–33. [Google Scholar] [CrossRef]
- Tsaneva, M.; Van Damme, E.J.M. 130 years of Plant Lectin Research. Glycoconj. J. 2020, 37, 533–551. [Google Scholar] [CrossRef]
- Witzel, K.; Matros, A.; Bertsch, U.; Aftab, T.; Rutten, T.; Ramireddy, E.; Melzer, M.; Kunze, G.; Mock, H.P. The Jacalin-Related Lectin HvHorcH Is Involved in the Physiological Response of Barley Roots to Salt Stress. Int. J. Mol. Sci. 2021, 22, 10248. [Google Scholar] [CrossRef]
- Zhang, H.; Deng, C.; Yao, J.; Zhang, Y.L.; Zhang, Y.N.; Deng, S.; Zhao, N.; Sa, G.; Zhou, X.; Lu, C.; et al. Populus euphratica JRL Mediates ABA Response, Ionic and ROS Homeostasis in Arabidopsis under Salt Stress. Int. J. Mol. Sci. 2019, 20, 815. [Google Scholar] [CrossRef]
- He, X.; Li, L.; Xu, H.; Xi, J.; Cao, X.; Xu, H.; Rong, S.; Dong, Y.; Wang, C.; Chen, R.; et al. A rice jacalin-related mannose-binding lectin gene, OsJRL, enhances Escherichia coli viability under high salinity stress and improves salinity tolerance of rice. Plant Biol. (Stuttg.) 2017, 19, 257–267. [Google Scholar] [CrossRef]
- Shekha, S.; Kuma, D. Ectopic Overexpression of Lectin in Transgenic Brassica juncea Plants Exhibit Resistance to Fungal Phytopathogen and Showed Alleviation to Salt and Drought Stress. J. Bioeng. Biomed. Sci. 2015, 5, 147. [Google Scholar] [CrossRef]
- Sun, Y.; Qiao, Z.; Muchero, W.; Chen, J.G. Lectin Receptor-Like Kinases: The Sensor and Mediator at the Plant Cell Surface. Front. Plant Sci. 2020, 11, 596301. [Google Scholar] [CrossRef]
- Passricha, N.; Saifi, S.K.; Kharb, P.; Tuteja, N. Rice lectin receptor-like kinase provides salinity tolerance by ion homeostasis. Biotechnol. Bioeng. 2020, 117, 498–510. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Prashanth, K.H.; Bhatti, M.S.; Pati, P.K. OsSalT gene cloned from rice provides evidence of its role in salinity and drought stress tolerance. Plant Sci. 2022, 320, 111306. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.M.; Yin, X.L.; Wang, F.; Zhang, C.Z.; Xiao, F.C.; Wang, H.Y.; Hu, S.C.; Liu, W.H.; Zhou, S.Q.; Chen, L.B.; et al. Jacalin-related lectin 45 (OsJRL45) isolated from ‘sea rice 86’ enhances rice salt tolerance at the seedling and reproductive stages. BMC Plant Biol. 2023, 23, 553. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.M.; Yin, X.L.; Wang, F.; Hu, S.C.; Liu, W.H.; Chen, L.B.; Dai, X.J.; Liang, M.Z. OsJRL40, a Jacalin-Related Lectin Gene, Promotes Salt Stress Tolerance in Rice. Int. J. Mol. Sci. 2023, 24, 7441. [Google Scholar] [CrossRef]
- Chen, R.S.; Cheng, Y.F.; Han, S.Y.; Van Handel, B.; Dong, L.; Li, X.M.; Xie, X.Q. Whole genome sequencing and comparative transcriptome analysis of a novel seawater adapted, salt-resistant rice cultivar—Sea rice 86. BMC Genom. 2017, 18, 655. [Google Scholar] [CrossRef]
- Sun, Y.; Song, K.; Guo, M.; Wu, H.; Ji, X.; Hou, L.; Liu, X.; Lu, S. A NAC Transcription Factor from ‘Sea Rice 86’ Enhances Salt Tolerance by Promoting Hydrogen Sulfide Production in Rice Seedlings. Int. J. Mol. Sci. 2022, 23, 6435. [Google Scholar] [CrossRef]
- Wang, G.; Weng, L.; Huang, Y.; Ling, Y.; Zhen, Z.; Lin, Z.; Hu, H.; Li, C.; Guo, J.; Zhou, J.L.; et al. Microbiome-metabolome analysis directed isolation of rhizobacteria capable of enhancing salt tolerance of Sea Rice 86. Sci. Total Environ. 2022, 843, 156817. [Google Scholar] [CrossRef]
- Gao, Q.M.; Wang, H.Y.; Yin, X.L.; Wang, F.; Hu, S.C.; Liu, W.H.; Chen, L.B.; Dai, X.J.; Liang, M.Z. Identification of Salt Tolerance Related Candidate Genes in ‘Sea Rice 86’ at the Seedling and Reproductive Stages Using QTL-Seq and BSA-Seq. Genes 2023, 14, 458. [Google Scholar] [CrossRef]
- Liang, W.; Ma, X.; Wan, P.; Liu, L. Plant salt-tolerance mechanism: A review. Biochem. Biophys. Res. Commun. 2018, 495, 286–291. [Google Scholar] [CrossRef]
- Quan, X.Y.; Meng, C.; Xie, C.J.; Sun, H.F.; Xu, B.Y.; Bermudez, R.S.; He, W.X. Genome-Wide and Transcriptome Analysis of Jacalin-Related Lectin Genes in Barley and the Functional Characterization of HvHorcH in Low-Nitrogen Tolerance in Arabidopsis. Int. J. Mol. Sci. 2023, 24, 16641. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, B.; Chen, J.; Jiao, Y.; Guo, H.; Liu, S.; Ramakrishnan, M.; Qi, G. Genome-Wide Identification of JRL Genes in Moso Bamboo and Their Expression Profiles in Response to Multiple Hormones and Abiotic Stresses. Front. Plant Sci. 2021, 12, 809666. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.J.; Zhong, Z.H.; Song, L.L.; Stefan, O.; Wang, Z.H.; Lu, G.L. Evolutionary analysis of plant jacalin-related lectins (JRLs) family and expression of rice JRLs in response to Magnaporthe oryzae. J. Integr. Agric. 2018, 17, 1252–1266. [Google Scholar] [CrossRef]
- Song, M.; Xu, W.; Xiang, Y.; Jia, H.; Zhang, L.; Ma, Z. Association of jacalin-related lectins with wheat responses to stresses revealed by transcriptional profiling. Plant Mol. Biol. 2014, 84, 95–110. [Google Scholar] [CrossRef] [PubMed]
- Nagano, A.J.; Fukao, Y.; Fujiwara, M.; Nishimura, M.; Hara-Nishimura, I. Antagonistic jacalin-related lectins regulate the size of ER body-type beta-glucosidase complexes in Arabidopsis thaliana. Plant Cell Physiol. 2008, 49, 969–980. [Google Scholar] [CrossRef]
- Kaur, N.; Sagar, A.; Sharma, P.; Ashish; Pati, P.K. Structural insights into rice SalTol QTL located SALT protein. Sci. Rep. 2020, 10, 16589. [Google Scholar] [CrossRef]
- Akladious, S.A.; Mohamed, H.I. Ameliorative effects of calcium nitrate and humic acid on the growth, yield component and biochemical attribute of pepper (Capsicum annuum) plants grown under salt stress. Sci. Hortic. 2018, 236, 244–250. [Google Scholar] [CrossRef]
- Kohli, S.K.; Khanna, K.; Bhardwaj, R.; Abd Allah, E.F.; Ahmad, P.; Corpas, F.J. Assessment of Subcellular ROS and NO Metabolism in Higher Plants: Multifunctional Signaling Molecules. Antioxidants 2019, 8, 641. [Google Scholar] [CrossRef]
- Razzaq, A.; Ali, A.; Safdar, L.B.; Zafar, M.M.; Rui, Y.; Shakeel, A.; Shaukat, A.; Ashraf, M.; Gong, W.; Yuan, Y. Salt stress induces physiochemical alterations in rice grain composition and quality. J. Food Sci. 2020, 85, 14–20. [Google Scholar] [CrossRef] [PubMed]
- van Zelm, E.; Zhang, Y.; Testerink, C. Salt Tolerance Mechanisms of Plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef]
- Gao, R.; Jia, Y.; Xu, X.; Fu, P.; Zhou, J.; Yang, G. Structural insights into the Oryza sativa cation transporters HKTs in salt tolerance. J. Integr. Plant Biol. 2024, 66, 700–708. [Google Scholar] [CrossRef]
- Yu, J.; Zhu, C.; Xuan, W.; An, H.; Tian, Y.; Wang, B.; Chi, W.; Chen, G.; Ge, Y.; Li, J.; et al. Genome-wide association studies identify OsWRKY53 as a key regulator of salt tolerance in rice. Nat. Commun. 2023, 14, 3550. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Yang, H.; Qiao, C.; Zhu, C.; Bai, T.; Du, H.; Ma, S.; Wang, N.; Luo, C.; Zhang, Y.; et al. Natural variations of chlorophyll fluorescence and ion transporter genes influenced the differential response of japonica rice germplasm with different salt tolerances. Front. Plant Sci. 2023, 14, 1095929. [Google Scholar] [CrossRef]
- Muzaffar, A.; Chen, Y.S.; Lee, H.T.; Wu, C.C.; Le, T.T.; Liang, J.Z.; Lu, C.H.; Balasubramaniam, H.; Lo, S.F.; Yu, L.C.; et al. A newly evolved rice-specific gene JAUP1 regulates jasmonate biosynthesis and signalling to promote root development and multi-stress tolerance. Plant Biotechnol. J. 2024, 22, 1417–1432. [Google Scholar] [CrossRef]
- Shikha, D.; Jakhar, P.; Satbhai, S.B. Role of jasmonate signaling in the regulation of plant responses to nutrient deficiency. J. Exp. Bot. 2023, 74, 1221–1243. [Google Scholar] [CrossRef]
- Ndecky, S.; Nguyen, T.H.; Eiche, E.; Cognat, V.; Pflieger, D.; Pawar, N.; Betting, F.; Saha, S.; Champion, A.; Riemann, M.; et al. Jasmonate signaling controls negative and positive effectors of salt stress tolerance in rice. J. Exp. Bot. 2023, 74, 3220–3239. [Google Scholar] [CrossRef]
- Asif, S.; Jan, R.; Kim, N.; Asaf, S.; Lubna; Khan, M.A.; Kim, E.G.; Jang, Y.H.; Bhatta, D.; Lee, I.J.; et al. Halotolerant endophytic bacteria alleviate salinity stress in rice (Oryza sativa L.) by modulating ion content, endogenous hormones, the antioxidant system and gene expression. BMC Plant Biol. 2023, 23, 494. [Google Scholar] [CrossRef]
- Feng, Z.; Xu, Y.; Xie, Z.; Yang, Y.; Lu, G.; Jin, Y.; Wang, M.; Liu, M.; Yang, H.; Li, W.; et al. Overexpression of Abscisic Acid Biosynthesis Gene OsNCED3 Enhances Survival Rate and Tolerance to Alkaline Stress in Rice Seedlings. Plants 2024, 13, 1713. [Google Scholar] [CrossRef]
- Jin, X.; Li, X.; Xie, Z.; Sun, Y.; Jin, L.; Hu, T.; Huang, J. Nuclear factor OsNF-YC5 modulates rice seed germination by regulating synergistic hormone signaling. Plant Physiol. 2023, 193, 2825–2847. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.W.; Liu, S.B.; Feng, J.H.; Wang, W.; Wang, Y.W.; Yu, Q.; Liao, Y.R.; Mo, Y.H.; Xu, Z.J.; Li, L.H.; et al. Overexpression of a stress response membrane protein gene OsSMP1 enhances rice tolerance to salt, cold and heavy metal stress. Environ. Exp. Bot. 2021, 182, 104327. [Google Scholar] [CrossRef]
- Wang, A.; Shu, X.; Jing, X.; Jiao, C.; Chen, L.; Zhang, J.; Ma, L.; Jiang, Y.; Yamamoto, N.; Li, S.; et al. Identification of rice (Oryza sativa L.) genes involved in sheath blight resistance via a genome-wide association study. Plant Biotechnol. J. 2021, 19, 1553–1566. [Google Scholar] [CrossRef]
- Zhang, F.; Huang, J.; Guo, H.; Yang, C.; Li, Y.; Shen, S.; Zhan, C.; Qu, L.; Liu, X.; Wang, S.; et al. OsRLCK160 contributes to flavonoid accumulation and UV-B tolerance by regulating OsbZIP48 in rice. Sci. China Life Sci. 2022, 65, 1380–1394. [Google Scholar] [CrossRef]
- Liu, Y.; Li, M.; Yu, J.; Ma, A.; Wang, J.; Yun, D.J.; Xu, Z.Y. Plasma membrane-localized Hsp40/DNAJ chaperone protein facilitates OsSUVH7-OsBAG4-OsMYB106 transcriptional complex formation for OsHKT1;5 activation. J. Integr. Plant Biol. 2023, 65, 265–279. [Google Scholar] [CrossRef]
- Krishnamurthy, S.L.; Sharma, S.K.; Kumar, V.; Tiwari, S.; Singh, N.K. Analysis of genomic region spanning Saltol using SSR markers in rice genotypes showing differential seedlings stage salt tolerance. J. Plant Biochem. Biotechnol. 2015, 25, 331–336. [Google Scholar] [CrossRef]
- Dai, X.J.; He, C.; Zhou, L.; Liang, M.Z.; Fu, X.C.; Qin, P.; Yang, Y.Z.; Chen, L.B. Identification of a specific molecular marker for the rice blast-resistant gene Pigm and molecular breeding of thermo-sensitive genic male sterile leaf-color marker lines. Mol. Breed. 2018, 38, 72. [Google Scholar] [CrossRef]
- Doyle, J. DNA Protocols for Plants. In Molecular Techniques in Taxonomy; Hewitt Godfrey, M., Johnston Andrew, W.B., Young, J., Peter, W., Eds.; Springer: Berlin/Heidelberg, Germany, 1991; pp. 283–293. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Zhang, K.; Su, J.; Xu, M.; Zhou, Z.; Zhu, X.; Ma, X.; Hou, J.; Tan, L.; Zhu, Z.; Cai, H.; et al. A common wild rice-derived BOC1 allele reduces callus browning in indica rice transformation. Nat. Commun. 2020, 11, 443. [Google Scholar] [CrossRef]
- Wang, J.; Nan, N.; Shi, L.; Li, N.; Huang, S.; Zhang, A.; Liu, Y.; Guo, P.; Liu, B.; Xu, Z.Y. Arabidopsis BRCA1 represses RRTF1-mediated ROS production and ROS-responsive gene expression under dehydration stress. New Phytol. 2020, 228, 1591–1610. [Google Scholar] [CrossRef]
- Ruan, W.; Guo, M.; Wang, X.; Guo, Z.; Xu, Z.; Xu, L.; Zhao, H.; Sun, H.; Yan, C.; Yi, K. Two RING-Finger Ubiquitin E3 Ligases Regulate the Degradation of SPX4, An Internal Phosphate Sensor, for Phosphate Homeostasis and Signaling in Rice. Mol. Plant 2019, 12, 1060–1074. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef]



| Number of SR86 × 9311 RILs | |
|---|---|
| SR86 | 228 |
| 9311 | 54 |
| Heterozygous | 36 |
| No product | 2 |
| Total | 320 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, X.; Gao, Q.; Wang, F.; Liu, W.; Luo, Y.; Zhong, S.; Feng, J.; Bai, R.; Chen, L.; Dai, X.; et al. Marker-Assisted Selection of Jacalin-Related Lectin Genes OsJRL45 and OsJRL40 Derived from Sea Rice 86 Enhances Salt Tolerance in Rice. Int. J. Mol. Sci. 2024, 25, 10912. https://doi.org/10.3390/ijms252010912
Yin X, Gao Q, Wang F, Liu W, Luo Y, Zhong S, Feng J, Bai R, Chen L, Dai X, et al. Marker-Assisted Selection of Jacalin-Related Lectin Genes OsJRL45 and OsJRL40 Derived from Sea Rice 86 Enhances Salt Tolerance in Rice. International Journal of Molecular Sciences. 2024; 25(20):10912. https://doi.org/10.3390/ijms252010912
Chicago/Turabian StyleYin, Xiaolin, Qinmei Gao, Feng Wang, Weihao Liu, Yiting Luo, Shuixiu Zhong, Jiahui Feng, Rui Bai, Liangbi Chen, Xiaojun Dai, and et al. 2024. "Marker-Assisted Selection of Jacalin-Related Lectin Genes OsJRL45 and OsJRL40 Derived from Sea Rice 86 Enhances Salt Tolerance in Rice" International Journal of Molecular Sciences 25, no. 20: 10912. https://doi.org/10.3390/ijms252010912
APA StyleYin, X., Gao, Q., Wang, F., Liu, W., Luo, Y., Zhong, S., Feng, J., Bai, R., Chen, L., Dai, X., & Liang, M. (2024). Marker-Assisted Selection of Jacalin-Related Lectin Genes OsJRL45 and OsJRL40 Derived from Sea Rice 86 Enhances Salt Tolerance in Rice. International Journal of Molecular Sciences, 25(20), 10912. https://doi.org/10.3390/ijms252010912





