GDF-15 and mtDNA Deletions Are Useful Biomarkers of Mitochondrial Dysfunction in Insulin Resistance and PCOS
Abstract
:1. Introduction
2. Results
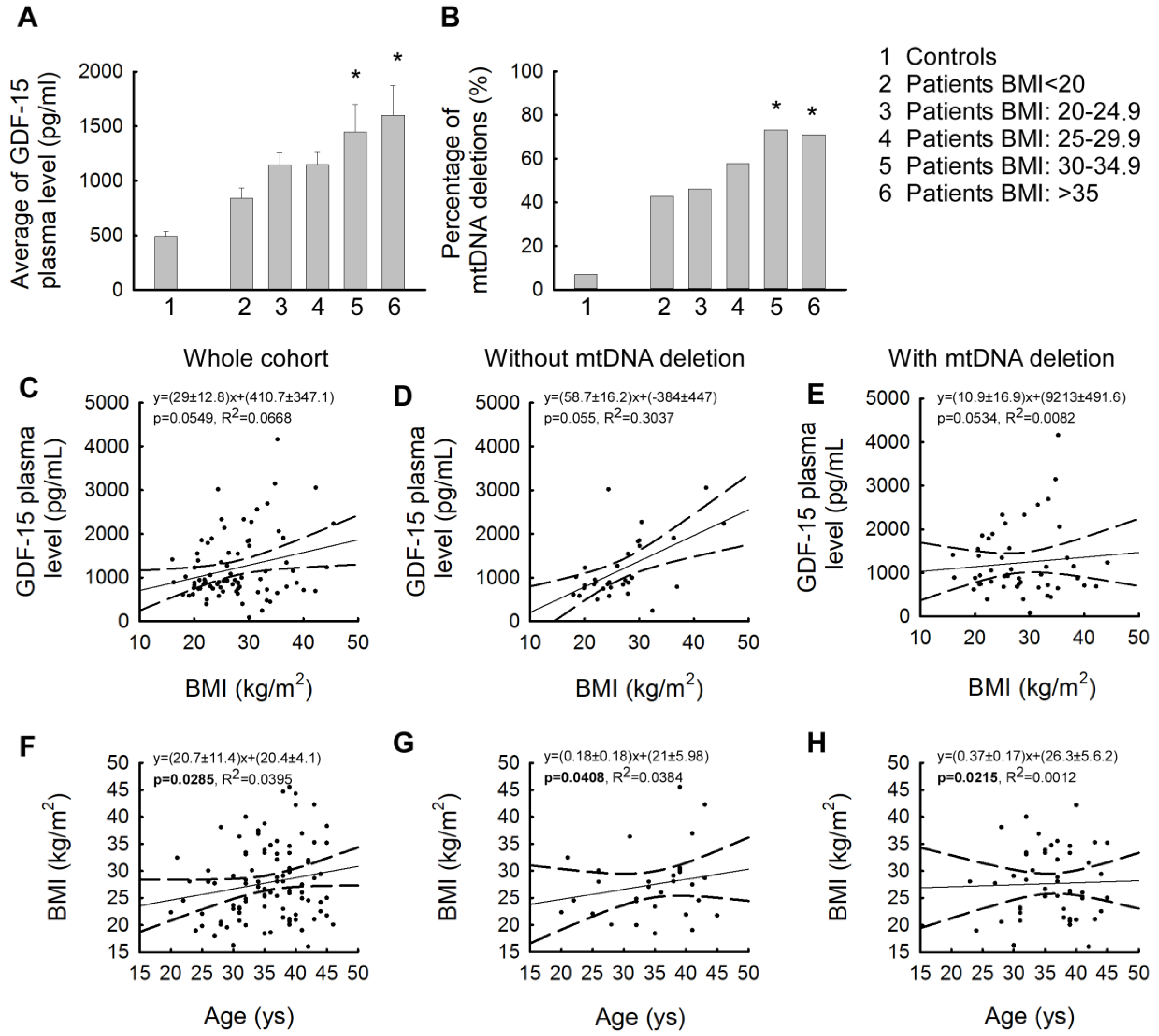
2.1. In IR Patients, Plasma GDF-15 Levels and the Presence of mtDNA Deletions Show a Significant Increase
2.2. Strong Correlation of Elevated GDF-15 with Reactive Hyperinsulinemia
2.3. Strong Correlation of Elevated GDF-15 Levels with High Body Mass Index
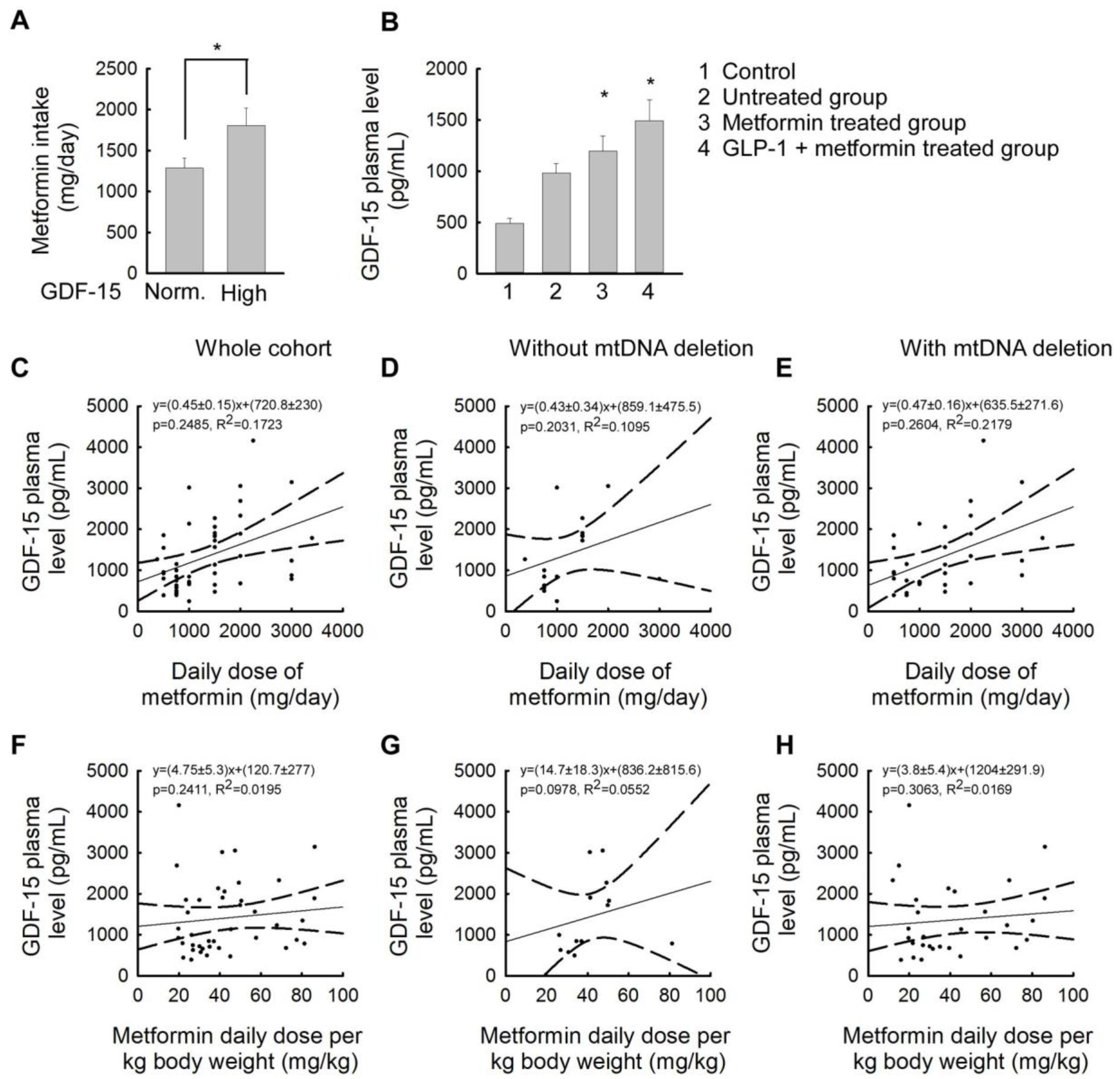
2.4. Association between Plasma GDF-15 Levels and Different Treatments for Insulin Resistance
3. Discussion
4. Materials and Methods
4.1. Cohort of the Study
4.2. Molecular Genetic Analysis
4.2.1. Sample Collection and DNA Analysis
4.2.2. Analysis of mtDNA Deletions
4.2.3. Measurement of GDF-15 Plasma Level
4.3. Statistical Analysis
5. Conclusions
6. Strength and Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fahed, M.; Abou Jaoudeh, M.G.; Merhi, S.; Mosleh, J.M.B.; Ghadieh, R.; Al Hayek, S.; El Hayek Fares, J.E. Evaluation of Risk Factors for Insulin Resistance: A Cross Sectional Study among Employees at a Private University in Lebanon. BMC Endocr. Disord. 2020, 20, 85. [Google Scholar] [CrossRef]
- Friedrich, N.; Thuesen, B.; Jørgensen, T.; Juul, A.; Spielhagen, C.; Wallaschofksi, H.; Linneberg, A. The Association between IGF-I and Insulin Resistance: A General Population Study in Danish Adults. Diabetes Care 2012, 35, 768–773. [Google Scholar] [CrossRef]
- El-Mir, M.Y.; Nogueira, V.; Fontaine, E.; Avéret, N.; Rigoulet, M.; Leverve, X. Dimethylbiguanide Inhibits Cell Respiration via an Indirect Effect Targeted on the Respiratory Chain Complex I. J. Biol. Chem. 2000, 275, 223–228. [Google Scholar] [CrossRef]
- Carvalho, C.; Correia, S.; Santos, M.S.; Seiça, R.; Oliveira, C.R.; Moreira, P.I. Metformin Promotes Isolated Rat Liver Mitochondria Impairment. Mol. Cell. Biochem. 2008, 308, 75–83. [Google Scholar] [CrossRef]
- Vial, G.; Detaille, D.; Guigas, B. Role of Mitochondria in the Mechanism(s) of Action of Metformin. Front. Endocrinol. 2019, 10, 294. [Google Scholar] [CrossRef]
- Ding, Y.; Xia, B.-H.; Zhang, C.-J.; Zhuo, G.-C. Mutations in Mitochondrial TRNA Genes May Be Related to Insulin Resistance in Women with Polycystic Ovary Syndrome. Am. J. Transl. Res. 2017, 9, 2984–2996. [Google Scholar]
- Siemers, K.M.; Klein, A.K.; Baack, M.L. Mitochondrial Dysfunction in PCOS: Insights into Reproductive Organ Pathophysiology. Int. J. Mol. Sci. 2023, 24, 13123. [Google Scholar] [CrossRef]
- Sergi, D.; Naumovski, N.; Heilbronn, L.K.; Abeywardena, M.; O’Callaghan, N.; Lionetti, L.; Luscombe-Marsh, N. Mitochondrial (Dys)Function and Insulin Resistance: From Pathophysiological Molecular Mechanisms to the Impact of Diet. Front. Physiol. 2019, 10, 532. [Google Scholar] [CrossRef]
- Petersen, M.C.; Shulman, G.I. Roles of Diacylglycerols and Ceramides in Hepatic Insulin Resistance. Trends Pharmacol. Sci. 2017, 38, 649–665. [Google Scholar] [CrossRef]
- Sokolowska, E.; Blachnio-Zabielska, A. The Role of Ceramides in Insulin Resistance. Front. Endocrinol. 2019, 10, 577. [Google Scholar] [CrossRef]
- Elkanawati, R.; Sumiwi, S.; Levita, J. Impact of Lipids on Insulin Resistance: Insights from Human and Animal Studies. Drug Des. Devel. Ther. 2024, 18, 3337–3360. [Google Scholar] [CrossRef]
- Boenzi, S.; Diodato, D. Biomarkers for Mitochondrial Energy Metabolism Diseases. Essays Biochem. 2018, 62, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Hubens, W.H.G.; Vallbona-Garcia, A.; de Coo, I.F.M.; van Tienen, F.H.J.; Webers, C.A.B.; Smeets, H.J.M.; Gorgels, T.G.M.F. Blood Biomarkers for Assessment of Mitochondrial Dysfunction: An Expert Review. Mitochondrion 2022, 62, 187–204. [Google Scholar] [CrossRef]
- Finsterer, J.; Zarrouk-Mahjoub, S. Biomarkers for Detecting Mitochondrial Disorders. J. Clin. Med. 2018, 7, 16. [Google Scholar] [CrossRef]
- Mohd Khair, S.Z.N.; Abd Radzak, S.M.; Mohamed Yusoff, A.A. The Uprising of Mitochondrial DNA Biomarker in Cancer. Dis. Markers 2021, 2021, 7675269. [Google Scholar] [CrossRef]
- Davis, R.L.; Liang, C.; Sue, C.M. A Comparison of Current Serum Biomarkers as Diagnostic Indicators of Mitochondrial Diseases. Neurology 2016, 86, 2010–2015. [Google Scholar] [CrossRef]
- Pence, B.D. Growth Differentiation Factor-15 in Immunity and Aging. Front. Aging 2022, 3, 837575. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Filipovic, Z.; Brown, D.; Breit, S.N.; Vassilev, L.T. Macrophage Inhibitory Cytokine-1: A Novel Biomarker for P53 Pathway Activation. Mol. Cancer Ther. 2003, 2, 1023–1029. [Google Scholar]
- Siddiqui, J.A.; Pothuraju, R.; Khan, P.; Sharma, G.; Muniyan, S.; Seshacharyulu, P.; Jain, M.; Nasser, M.W.; Batra, S.K. Pathophysiological Role of Growth Differentiation Factor 15 (GDF15) in Obesity, Cancer, and Cachexia. Cytokine Growth Factor Rev. 2022, 64, 71–83. [Google Scholar] [CrossRef]
- Hong, J.H.; Chung, H.K.; Park, H.Y.; Joung, K.-H.; Lee, J.H.; Jung, J.G.; Kim, K.S.; Kim, H.J.; Ku, B.J.; Shong, M. GDF15 Is a Novel Biomarker for Impaired Fasting Glucose. Diabetes Metab. J. 2014, 38, 472–479. [Google Scholar] [CrossRef]
- Schindler, K.; Vila, G.; Hoppichler, F.; Lechleitner, M.; Luger, A.; Anderwald, C.; Hoefler, J.; Tomasec, G.; Kautzky-Willer, A.; Ludvik, B. The Impact of Type 2 Diabetes on Circulating Adipokines in Patients with Metabolic Syndrome. Obes. Facts 2012, 5, 270–276. [Google Scholar] [CrossRef]
- Dabravolski, S.A.; Nikiforov, N.G.; Eid, A.H.; Nedosugova, L.V.; Starodubova, A.V.; Popkova, T.V.; Bezsonov, E.E.; Orekhov, A.N. Mitochondrial Dysfunction and Chronic Inflammation in Polycystic Ovary Syndrome. Int. J. Mol. Sci. 2021, 22, 3923. [Google Scholar] [CrossRef]
- Ofori, E.K.; Dziedzorm, W.; Buabeng, A.; Dogodzi, F.K.; Adusu-Donkor, L.X.; Bernard, S.K.; Amponsah, S.K.; Asare-Anane, H. Comparative Determination of Mitochondrial Biomarkers and Their Relationship with Insulin Resistance in Type 2 Diabetic Patients: An Observational Cross-Sectional Study. Endocrinol. Diabetes Metab. 2024, 7, e507. [Google Scholar] [CrossRef]
- Conte, M.; Martucci, M.; Mosconi, G.; Chiariello, A.; Cappuccilli, M.; Totti, V.; Santoro, A.; Franceschi, C.; Salvioli, S. GDF15 Plasma Level Is Inversely Associated with Level of Physical Activity and Correlates with Markers of Inflammation and Muscle Weakness. Front. Immunol. 2020, 11, 915. [Google Scholar] [CrossRef]
- Sarkar, S.; Melchior, J.T.; Henry, H.R.; Syed, F.; Mirmira, R.G.; Nakayasu, E.S.; Metz, T.O. GDF15: A Potential Therapeutic Target for Type 1 Diabetes. Expert Opin. Ther. Targets 2022, 26, 57–67. [Google Scholar] [CrossRef]
- Ackermann, K.; Bonaterra, G.A.; Kinscherf, R.; Schwarz, A. Growth Differentiation Factor-15 Regulates OxLDL-Induced Lipid Homeostasis and Autophagy in Human Macrophages. Atherosclerosis 2019, 281, 128–136. [Google Scholar] [CrossRef]
- Bonaterra, G.A.; Zügel, S.; Thogersen, J.; Walter, S.A.; Haberkorn, U.; Strelau, J.; Kinscherf, R. Growth Differentiation Factor-15 Deficiency Inhibits Atherosclerosis Progression by Regulating Interleukin-6-Dependent Inflammatory Response to Vascular Injury. J. Am. Heart Assoc. 2012, 1, e002550. [Google Scholar] [CrossRef]
- Anand, I.S.; Kempf, T.; Rector, T.S.; Tapken, H.; Allhoff, T.; Jantzen, F.; Kuskowski, M.; Cohn, J.N.; Drexler, H.; Wollert, K.C. Serial Measurement of Growth-Differentiation Factor-15 in Heart Failure: Relation to Disease Severity and Prognosis in the Valsartan Heart Failure Trial. Circulation 2010, 122, 1387–1395. [Google Scholar] [CrossRef]
- Kempf, T.; von Haehling, S.; Peter, T.; Allhoff, T.; Cicoira, M.; Doehner, W.; Ponikowski, P.; Filippatos, G.S.; Rozentryt, P.; Drexler, H.; et al. Prognostic Utility of Growth Differentiation Factor-15 in Patients with Chronic Heart Failure. J. Am. Coll. Cardiol. 2007, 50, 1054–1060. [Google Scholar] [CrossRef]
- Nyárády, B.B.; Kiss, L.Z.; Bagyura, Z.; Merkely, B.; Dósa, E.; Láng, O.; Kőhidai, L.; Pállinger, É. Growth and Differentiation Factor-15: A Link between Inflammaging and Cardiovascular Disease. Biomed. Pharmacother. 2024, 174, 116475. [Google Scholar] [CrossRef]
- Tektonidou, M.G.; Papassotiriou, I.; Sfikakis, P.P. Growth Differentiation Factor 15 (GDF-15) as Potential Cardiovascular Risk Biomarker in Antiphospholipid Syndrome. Rheumatology 2021, 61, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.-D.; Huang, Q.; Yang, C.; Li, R.; Huang, A.-F. GDF-15: A Potential Biomarker and Therapeutic Target in Systemic Lupus Erythematosus. Front. Immunol. 2022, 13, 926373. [Google Scholar] [CrossRef] [PubMed]
- Lerner, L.; Hayes, T.G.; Tao, N.; Krieger, B.; Feng, B.; Wu, Z.; Nicoletti, R.; Chiu, M.I.; Gyuris, J.; Garcia, J.M. Plasma Growth Differentiation Factor 15 Is Associated with Weight Loss and Mortality in Cancer Patients. J. Cachexia Sarcopenia Muscle 2015, 6, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Runco, D.V.; DiMeglio, L.A.; Vanderpool, C.P.; Han, Y.; Daggy, J.; Kelley, M.M.; Mikesell, R.; Zimmers, T.A. Growth Differentiation Factor 15 (GDF15) Elevation in Children with Newly Diagnosed Cancer. Front. Oncol. 2023, 13, 1295228. [Google Scholar] [CrossRef]
- Johnen, H.; Lin, S.; Kuffner, T.; Brown, D.A.; Tsai, V.W.-W.; Bauskin, A.R.; Wu, L.; Pankhurst, G.; Jiang, L.; Junankar, S.; et al. Tumor-Induced Anorexia and Weight Loss Are Mediated by the TGF-Beta Superfamily Cytokine MIC-1. Nat. Med. 2007, 13, 1333–1340. [Google Scholar] [CrossRef]
- Suzuki, H.; Mitsunaga, S.; Ikeda, M.; Aoyama, T.; Yoshizawa, K.; Yoshimatsu, H.; Kawai, N.; Masuda, M.; Miura, T.; Ochiai, A. Clinical and Tumor Characteristics of Patients with High Serum Levels of Growth Differentiation Factor 15 in Advanced Pancreatic Cancer. Cancers 2021, 13, 4842. [Google Scholar] [CrossRef]
- Ling, T.; Zhang, J.; Ding, F.; Ma, L. Role of Growth Differentiation Factor 15 in Cancer Cachexia (Review). Oncol. Lett. 2023, 26, 462. [Google Scholar] [CrossRef]
- Ji, X.; Zhao, L.; Ji, K.; Zhao, Y.; Li, W.; Zhang, R.; Hou, Y.; Lu, J.; Yan, C. Growth Differentiation Factor 15 Is a Novel Diagnostic Biomarker of Mitochondrial Diseases. Mol. Neurobiol. 2017, 54, 8110–8116. [Google Scholar] [CrossRef]
- Montero, R.; Yubero, D.; Villarroya, J.; Henares, D.; Jou, C.; Rodríguez, M.A.; Ramos, F.; Nascimento, A.; Ortez, C.I.; Campistol, J.; et al. GDF-15 Is Elevated in Children with Mitochondrial Diseases and Is Induced by Mitochondrial Dysfunction. PLoS ONE 2016, 11, e0148709. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, M.; Nouraie, M.; Roth, M.G.; Tabib, T.; Winters, S.; Chen, X.; Sembrat, J.; Chu, Y.; Cardenes, N.; et al. GDF15 Is an Epithelial-Derived Biomarker of Idiopathic Pulmonary Fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019, 317, L510–L521. [Google Scholar] [CrossRef]
- Harb, H.; Stephen-Victor, E.; Crestani, E.; Benamar, M.; Massoud, A.; Cui, Y.; Charbonnier, L.-M.; Arbag, S.; Baris, S.; Cunnigham, A.; et al. A Regulatory T Cell Notch4-GDF15 Axis Licenses Tissue Inflammation in Asthma. Nat. Immunol. 2020, 21, 1359–1370. [Google Scholar] [CrossRef]
- Lowell, B.B.; Shulman, G.I. Mitochondrial Dysfunction and Type 2 Diabetes. Science 2005, 307, 384–387. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, D.; Chang, E.H.; Kim, J.H.; Hwang, J.W.; Kim, J.-Y.; Kyung, J.W.; Kim, S.H.; Oh, J.S.; Shim, S.M.; et al. GDF-15 Secreted from Human Umbilical Cord Blood Mesenchymal Stem Cells Delivered through the Cerebrospinal Fluid Promotes Hippocampal Neurogenesis and Synaptic Activity in an Alzheimer’s Disease Model. Stem Cells Dev. 2015, 24, 2378–2390. [Google Scholar] [CrossRef]
- Jiang, W.-W.; Zhang, Z.-Z.; He, P.-P.; Jiang, L.-P.; Chen, J.-Z.; Zhang, X.-T.; Hu, M.; Zhang, Y.-K.; Ouyang, X.-P. Emerging Roles of Growth Differentiation Factor-15 in Brain Disorders (Review). Exp. Ther. Med. 2021, 22, 1270. [Google Scholar] [CrossRef]
- Berberoglu, Z.; Aktas, A.; Fidan, Y.; Canan Yazici, A.; Aral, Y. Plasma GDF-15 Levels and Their Association with Hormonal and Metabolic Status in Women with Polycystic Ovary Syndrome Aged 25–35. Minerva Endocrinol. 2014, 39, 89–97. [Google Scholar]
- Santoso, B.; Rahmawati, N.Y.; Sa’adi, A.; Dwiningsih, S.R.; Annas, J.Y.; Tunjungseto, A.; Widyanugraha, M.Y.A.; Mufid, A.F.; Ahsan, F. Elevated Peritoneal Soluble Endoglin and GDF-15 in Infertile Women with Severe Endometriosis and Pelvic Adhesion. J. Reprod. Immunol. 2021, 146, 103343. [Google Scholar] [CrossRef]
- Karczewska-Kupczewska, M.; Kowalska, I.; Nikolajuk, A.; Adamska, A.; Otziomek, E.; Gorska, M.; Straczkowski, M. Hyperinsulinemia Acutely Increases Serum Macrophage Inhibitory Cytokine-1 Concentration in Anorexia Nervosa and Obesity. Clin. Endocrinol. 2012, 76, 46–50. [Google Scholar] [CrossRef]
- Ruegsegger, G.N.; Creo, A.L.; Cortes, T.M.; Dasari, S.; Nair, K.S. Altered Mitochondrial Function in Insulin-Deficient and Insulin-Resistant States. J. Clin. Investig. 2018, 128, 3671–3681. [Google Scholar] [CrossRef]
- Schernthaner-Reiter, M.H.; Kasses, D.; Tugendsam, C.; Riedl, M.; Peric, S.; Prager, G.; Krebs, M.; Promintzer-Schifferl, M.; Clodi, M.; Luger, A.; et al. Growth Differentiation Factor 15 Increases Following Oral Glucose Ingestion: Effect of Meal Composition and Obesity. Eur. J. Endocrinol. 2016, 175, 623–631. [Google Scholar] [CrossRef]
- Escobar-Morreale, H.F. Polycystic Ovary Syndrome: Definition, Aetiology, Diagnosis and Treatment. Nat. Rev. Endocrinol. 2018, 14, 270–284. [Google Scholar] [CrossRef]
- Conte, M.; Giuliani, C.; Chiariello, A.; Iannuzzi, V.; Franceschi, C.; Salvioli, S. GDF15, an Emerging Key Player in Human Aging. Ageing Res. Rev. 2022, 75, 101569. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Taniguchi, Y.; Shinkai, S.; Tanaka, M.; Ito, M. Secreted Growth Differentiation Factor 15 as a Potential Biomarker for Mitochondrial Dysfunctions in Aging and Age-Related Disorders. Geriatr. Gerontol. Int. 2016, 16 (Suppl. S1), 17–29. [Google Scholar] [CrossRef]
- Yang, L.; Chang, C.-C.; Sun, Z.; Madsen, D.; Zhu, H.; Padkjær, S.B.; Wu, X.; Huang, T.; Hultman, K.; Paulsen, S.J.; et al. GFRAL Is the Receptor for GDF15 and Is Required for the Anti-Obesity Effects of the Ligand. Nat. Med. 2017, 23, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-Y.; Bruce, K.; Danaei, Z.; Li, R.J.W.; Barros, D.R.; Kuah, R.; Lim, Y.-M.; Mariani, L.H.; Cherney, D.Z.; Chiu, J.F.M.; et al. Metformin Triggers a Kidney GDF15-Dependent Area Postrema Axis to Regulate Food Intake and Body Weight. Cell Metab. 2023, 35, 875–886.e5. [Google Scholar] [CrossRef]
- Wang, D.; Townsend, L.K.; DesOrmeaux, G.J.; Frangos, S.M.; Batchuluun, B.; Dumont, L.; Kuhre, R.E.; Ahmadi, E.; Hu, S.; Rebalka, I.A.; et al. GDF15 Promotes Weight Loss by Enhancing Energy Expenditure in Muscle. Nature 2023, 619, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Emmerson, P.J.; Wang, F.; Du, Y.; Liu, Q.; Pickard, R.T.; Gonciarz, M.D.; Coskun, T.; Hamang, M.J.; Sindelar, D.K.; Ballman, K.K.; et al. The Metabolic Effects of GDF15 Are Mediated by the Orphan Receptor GFRAL. Nat. Med. 2017, 23, 1215–1219. [Google Scholar] [CrossRef]
- Hale, C.; Véniant, M.M. Growth Differentiation Factor 15 as a Potential Therapeutic for Treating Obesity. Mol. Metab. 2021, 46, 101117. [Google Scholar] [CrossRef]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Alexiou, A.; Papadakis, M.; Nadwa, E.H.; Albogami, S.M.; Alorabi, M.; Saad, H.M.; Batiha, G.E.-S. Metformin and Growth Differentiation Factor 15 (GDF15) in Type 2 Diabetes Mellitus: A Hidden Treasure. J. Diabetes 2022, 14, 806–814. [Google Scholar] [CrossRef]
- Mullican, S.E.; Lin-Schmidt, X.; Chin, C.-N.; Chavez, J.A.; Furman, J.L.; Armstrong, A.A.; Beck, S.C.; South, V.J.; Dinh, T.Q.; Cash-Mason, T.D.; et al. GFRAL Is the Receptor for GDF15 and the Ligand Promotes Weight Loss in Mice and Nonhuman Primates. Nat. Med. 2017, 23, 1150–1157. [Google Scholar] [CrossRef]
- Valenzuela-Vallejo, L.; Chrysafi, P.; Bello-Ramos, J.; Bsata, S.; Mantzoros, C.S. Circulating Total and Intact GDF-15 Levels Are Not Altered in Response to Weight Loss Induced by Liraglutide or Lorcaserin Treatment in Humans with Obesity. Metabolism 2022, 133, 155237. [Google Scholar] [CrossRef]
- Xiong, Y.; Walker, K.; Min, X.; Hale, C.; Tran, T.; Komorowski, R.; Yang, J.; Davda, J.; Nuanmanee, N.; Kemp, D.; et al. Long-Acting MIC-1/GDF15 Molecules to Treat Obesity: Evidence from Mice to Monkeys. Sci. Transl. Med. 2017, 9, eaan8732. [Google Scholar] [CrossRef] [PubMed]
- Conte, M.; Ostan, R.; Fabbri, C.; Santoro, A.; Guidarelli, G.; Vitale, G.; Mari, D.; Sevini, F.; Capri, M.; Sandri, M.; et al. Human Aging and Longevity Are Characterized by High Levels of Mitokines. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Varhaug, K.N.; Nido, G.S.; de Coo, I.; Isohanni, P.; Suomalainen, A.; Tzoulis, C.; Knappskog, P.; Bindoff, L.A. Using Urine to Diagnose Large-Scale MtDNA Deletions in Adult Patients. Ann. Clin. Transl. Neurol. 2020, 7, 1318–1326. [Google Scholar] [CrossRef] [PubMed]
- Welsh, P.; Kimenai, D.M.; Marioni, R.E.; Hayward, C.; Campbell, A.; Porteous, D.; Mills, N.L.; O’Rahilly, S.; Sattar, N. Reference Ranges for GDF-15, and Risk Factors Associated with GDF-15, in a Large General Population Cohort. Clin. Chem. Lab. Med. 2022, 60, 1820–1829. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varhegyi, V.; Modos, A.; Trager, D.; Gerszi, D.; Horvath, E.M.; Sipos, M.; Acs, N.; Molnar, M.J.; Varbiro, S.; Gal, A. GDF-15 and mtDNA Deletions Are Useful Biomarkers of Mitochondrial Dysfunction in Insulin Resistance and PCOS. Int. J. Mol. Sci. 2024, 25, 10916. https://doi.org/10.3390/ijms252010916
Varhegyi V, Modos A, Trager D, Gerszi D, Horvath EM, Sipos M, Acs N, Molnar MJ, Varbiro S, Gal A. GDF-15 and mtDNA Deletions Are Useful Biomarkers of Mitochondrial Dysfunction in Insulin Resistance and PCOS. International Journal of Molecular Sciences. 2024; 25(20):10916. https://doi.org/10.3390/ijms252010916
Chicago/Turabian StyleVarhegyi, Vera, Anna Modos, Domonkos Trager, Dora Gerszi, Eszter Maria Horvath, Miklos Sipos, Nandor Acs, Maria Judit Molnar, Szabolcs Varbiro, and Aniko Gal. 2024. "GDF-15 and mtDNA Deletions Are Useful Biomarkers of Mitochondrial Dysfunction in Insulin Resistance and PCOS" International Journal of Molecular Sciences 25, no. 20: 10916. https://doi.org/10.3390/ijms252010916
APA StyleVarhegyi, V., Modos, A., Trager, D., Gerszi, D., Horvath, E. M., Sipos, M., Acs, N., Molnar, M. J., Varbiro, S., & Gal, A. (2024). GDF-15 and mtDNA Deletions Are Useful Biomarkers of Mitochondrial Dysfunction in Insulin Resistance and PCOS. International Journal of Molecular Sciences, 25(20), 10916. https://doi.org/10.3390/ijms252010916






