Unveiling the Role of Gut Microbiota and Metabolites in Autoimmune Thyroid Diseases: Emerging Perspectives
Abstract
:1. Introduction
2. Clinical and Immune Characteristics of AITDs
3. Characteristics of Gut Microbiota in AITDs
4. Progress of Gut Microbiota in Animal Models of Autoimmune Diseases
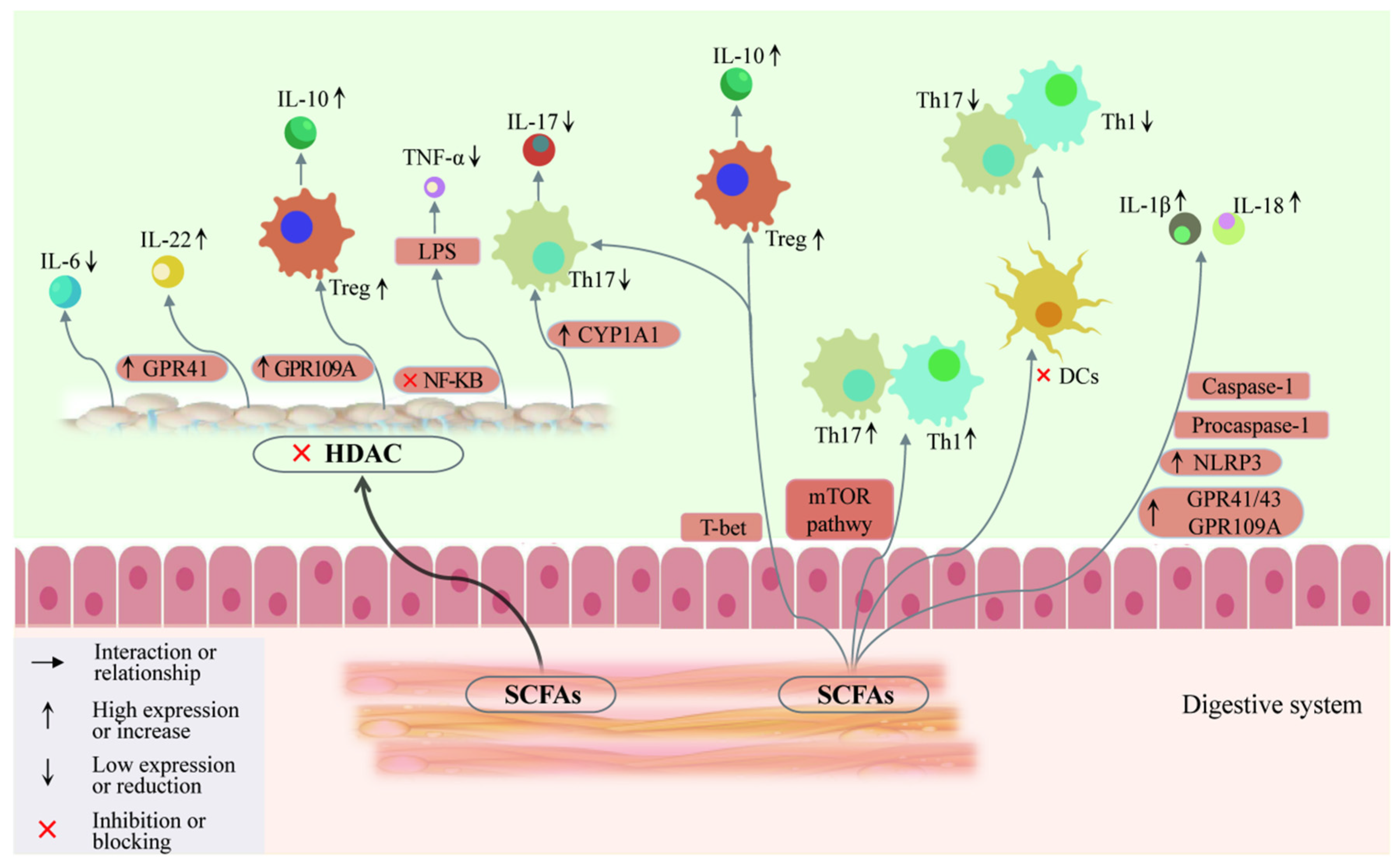
5. Involvement of Gut Microbiota and SCFAs in AITDs through Th17/Treg Balance
5.1. Gut Microbiota Is Significantly Associated with Immune Regulation
5.2. Gut Microbiota Regulates the Synthesis and Metabolism of SCFAs
5.3. Involvement of SCFAs in AITDs through Regulation of T Cell Differentiation
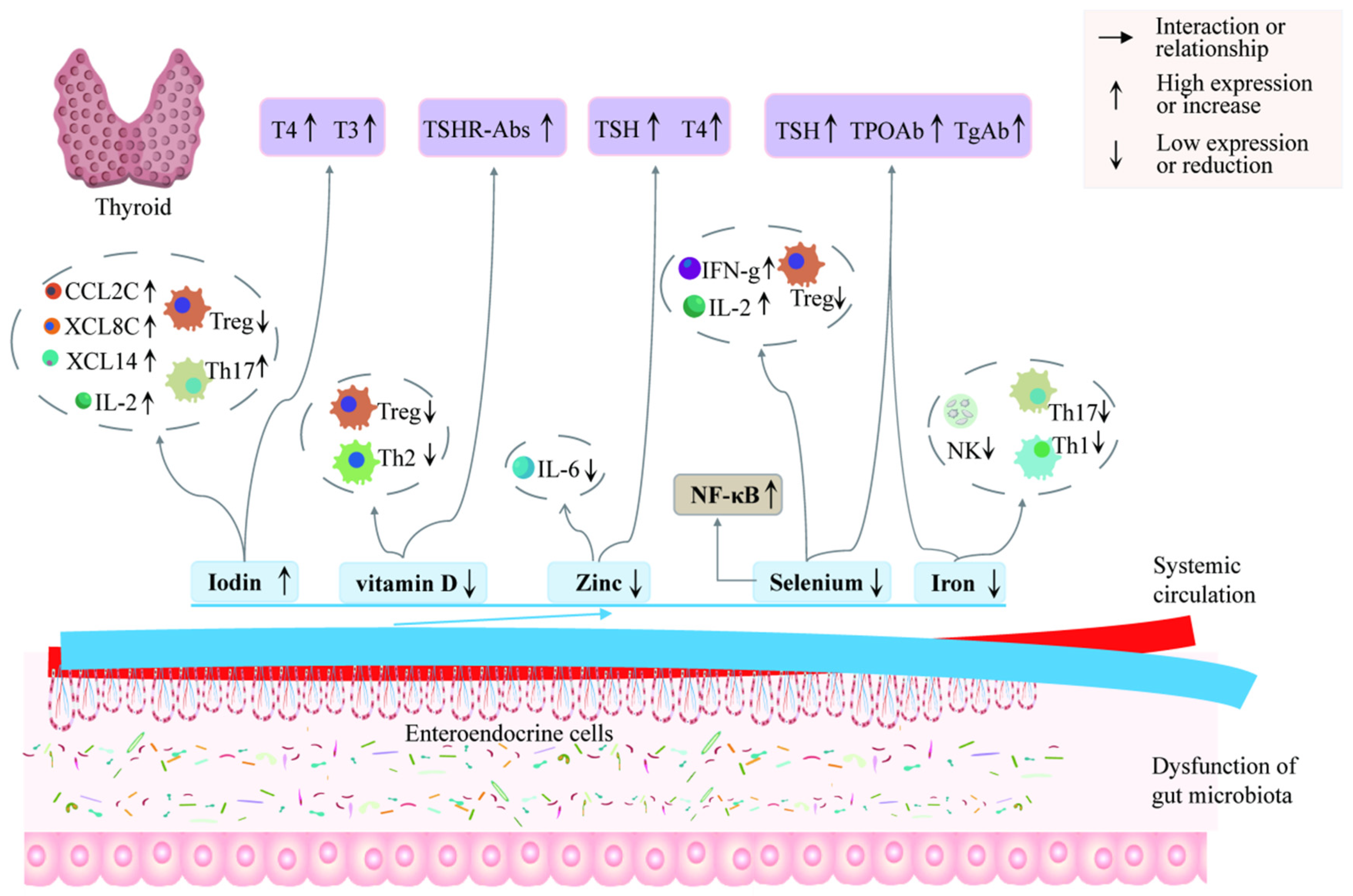
6. Involvement of Gut Microbiota and Metabolites in AITDs through Thyroid Hormones
6.1. Gut Microbiota Is Significantly Associated with Thyroid Hormones
6.2. Involvement in AITDs through Regulation of Thyroid Hormones
7. Summary and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jeong, C.; Baek, H.; Bae, J.; Hwang, N.; Ha, J.; Cho, Y.S.; Lim, D.J. Gut microbiome in the Graves’ disease: Comparison before and after anti-thyroid drug treatment. PLoS ONE 2024, 19, e0300678. [Google Scholar]
- Grimm, D. Cell and Molecular Biology of Thyroid Disorders 2.0. Int. J. Mol. Sci. 2021, 22, 1990. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Yu, X.; Kosik, R.O.; Song, Y.; Qiao, T.; Tong, J.; Liu, S.; Fan, S.; Luo, Q.; Chai, L.; et al. Gut Microbiota May Play a Significant Role in the Pathogenesis of Graves’ Disease. Thyroid 2021, 31, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Xi, Z.; Yang, T.; Huang, T.; Zhou, J.; Yang, P. Identification and Preliminary Clinical Validation of Key Extracellular Proteins as the Potential Biomarkers in Hashimoto’s Thyroiditis by Comprehensive Analysis. Biomedicines 2023, 11, 3127. [Google Scholar] [CrossRef]
- Hönes, G.S.; Sivakumar, R.G.; Hoppe, C.; König, J.; Führer, D.; Moeller, L.C. Cell-Specific Transport and Thyroid Hormone Receptor Isoform Selectivity Account for Hepatocyte-Targeted Thyromimetic Action of MGL-3196. Int. J. Mol. Sci. 2022, 23, 13714. [Google Scholar] [CrossRef] [PubMed]
- Beduleva, L.; Sidorov, A.; Fomina, K.; Terentiev, A.; Menshikov, I.; Shklyaeva, N.; Ivanov, P.; Varaksin, V. Experimental rat models for Hashimoto’s thyroiditis. J. Endocrinol. Investig. 2024, 47, 1205–1214. [Google Scholar]
- Zheng, H.; Xu, J.; Chu, Y.; Jiang, W.; Yao, W.; Mo, S.; Song, X.; Zhou, J. A Global Regulatory Network for Dysregulated Gene Expression and Abnormal Metabolic Signaling in Immune Cells in the Microenvironment of Graves’ Disease and Hashimoto’s Thyroiditis. Front. Immunol. 2022, 13, 879824. [Google Scholar]
- Stensland, Z.C.; Coleman, B.M.; Rihanek, M.; Baxter, R.M.; Gottlieb, P.A.; Hsieh, E.W.Y.; Sarapura, V.D.; Simmons, K.M.; Cambier, J.C.; Smith, M.J. Peripheral immunophenotyping of AITD subjects reveals alterations in immune cells in pediatric vs. adult-onset AITD. iScience 2022, 25, 103626. [Google Scholar]
- Chang, S.-C.; Lin, S.-F.; Chen, S.-T.; Chang, P.-Y.; Yeh, Y.-M.; Lo, F.-S.; Lu, J.-J. Alterations of Gut Microbiota in Patients with Graves’ Disease. Front. Cell. Infect. Microbiol. 2021, 11, 663131. [Google Scholar]
- Ni, Y.; Hu, Y.; Lou, X.; Rong, N.; Liu, F.; Yang, C.; Zheng, A.; Yang, S.; Bao, J.; Fu, Z. Spermidine Ameliorates Nonalcoholic Steatohepatitis through Thyroid Hormone-Responsive Protein Signaling and the Gut Microbiota-Mediated Metabolism of Bile Acids. J. Agric. Food Chem. 2022, 70, 6478–6492. [Google Scholar]
- Alkader, D.A.A.; Asadi, N.; Solangi, U.; Singh, R.; Rasuli, S.F.; Farooq, M.J.; Raheela, F.N.U.; Waseem, R.; Gilani, S.M.; Abbas, K.; et al. Exploring the role of gut microbiota in autoimmune thyroid disorders: A systematic review and meta-analysis. Front. Endocrinol. 2023, 14, 1238146. [Google Scholar] [CrossRef] [PubMed]
- El-Zawawy, H.T.; Ahmed, S.M.; El-Attar, E.A.; Ahmed, A.A.; Roshdy, Y.S.; Header, D.A. Study of gut microbiome in Egyptian patients with autoimmune thyroid diseases. Int. J. Clin. Pract. 2021, 75, e14038. [Google Scholar] [CrossRef] [PubMed]
- Miro, C.; Nappi, A.; Sagliocchi, S.; Di Cicco, E.; Murolo, M.; Torabinejad, S.; Acampora, L.; Pastore, A.; Luciano, P.; La Civita, E.; et al. Thyroid Hormone Regulates the Lipid Content of Muscle Fibers, Thus Affecting Physical Exercise Performance. Int. J. Mol. Sci. 2023, 24, 12074. [Google Scholar] [CrossRef] [PubMed]
- Fotakis, C.; Moros, G.; Kontogeorgou, A.; Iacovidou, N.; Boutsikou, T.; Zoumpoulakis, P. Uncontrolled Thyroid during Pregnancy Alters the Circulative and Exerted Metabolome. Int. J. Mol. Sci. 2022, 23, 4248. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Ly, D.D.; Nguyen, N.T.; Qi, X.F.; Yi, H.S.; Shong, M.; Cha, S.K.; Park, S.; Park, K.S. Thyroid Hormone Induces Ca(2+)-Mediated Mitochondrial Activation in Brown Adipocytes. Int. J. Mol. Sci. 2021, 22, 8640. [Google Scholar] [CrossRef]
- Han, Z.; Cen, C.; Ou, Q.; Pan, Y.; Zhang, J.; Huo, D.; Chen, K. The Potential Prebiotic Berberine Combined with Methimazole Improved the Therapeutic Effect of Graves’ Disease Patients Through Regulating the Intestinal Microbiome. Front. Immunol. 2021, 12, 826067. [Google Scholar] [CrossRef]
- Cornejo-Pareja, I.; Ruiz-Limón, P.; Gómez-Pérez, A.M.; Molina-Vega, M.; Moreno-Indias, I.; Tinahones, F.J. Differential Microbial Pattern Description in Subjects with Autoimmune-Based Thyroid Diseases: A Pilot Study. J. Pers. Med. 2020, 10, 192. [Google Scholar] [CrossRef]
- Wang, B.; Xu, Y.; Zhang, M.; Zhang, J.; Hou, X.; Li, J.; Cai, Y.; Sun, Z.; Ban, Y.; Wang, W. Oral and intestinal microbial features in pregnant women with hypothyroidism and their correlations with pregnancy outcomes. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E1044–E1052. [Google Scholar] [CrossRef]
- Achilla, C.; Chorti, A.; Papavramidis, T.; Angelis, L.; Chatzikyriakidou, A. Genetic and Epigenetic Association of FOXP3 with Papillary Thyroid Cancer Predisposition. Int. J. Mol. Sci. 2024, 25, 7161. [Google Scholar] [CrossRef]
- Guo, C.; Liu, Q.; Zong, D.; Zhang, W.; Zuo, Z.; Yu, Q.; Sha, Q.; Zhu, L.; Gao, X.; Fang, J.; et al. Single-cell transcriptome profiling and chromatin accessibility reveal an exhausted regulatory CD4+ T cell subset in systemic lupus erythematosus. Cell Rep. 2022, 41, 111606. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, Y.; Hu, S.; Zhang, M.; Shi, B.; Wang, Y. Decreased Treg Cell and TCR Expansion Are Involved in Long-Lasting Graves’ Disease. Front. Endocrinol. 2021, 12, 632492. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, W.; Guo, Z.; Huang, S.; Lei, H.; Zang, P.; Lu, B.; Shao, J.; Gu, P. Associations between gut microbiota and thyroidal function status in Chinese patients with Graves’ disease. J. Endocrinol. Investig. 2021, 44, 1913–1926. [Google Scholar] [CrossRef]
- Gong, B.; Wang, C.; Meng, F.; Wang, H.; Song, B.; Yang, Y.; Shan, Z. Association between Gut Microbiota and Autoimmune Thyroid Disease: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2021, 12, 774362. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Chang, L.C.; Chang, Y.C.; Chung, W.H.; Yang, S.F.; Su, S.C. Compositional Alteration of Gut Microbiota in Psoriasis Treated with IL-23 and IL-17 Inhibitors. Int. J. Mol. Sci. 2023, 24, 4568. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Zhao, F.; Liu, Y.; Wu, X.; Feng, J.; Jin, X.; Yan, W.; Guo, X.; Shi, S.; Li, Z.; et al. Randomized Clinical Trial: Probiotics Alleviated Oral-Gut Microbiota Dysbiosis and Thyroid Hormone Withdrawal-Related Complications in Thyroid Cancer Patients Before Radioiodine Therapy Following Thyroidectomy. Front. Endocrinol. 2022, 13, 834674. [Google Scholar] [CrossRef]
- Bossowski, A.; Moniuszko, M.; Idźkowska, E.; Grubczak, K.; Singh, P.; Bossowska, A.; Diana, T.; Kahaly, G.J. Decreased proportions of CD4 + IL17+/CD4 + CD25 + CD127- and CD4 + IL17+/CD4 + CD25 + CD127 - FoxP3+ T cells in children with autoimmune thyroid diseases. Autoimmunity 2016, 49, 320–328. [Google Scholar]
- Cayres, L.C.F.; de Salis, L.V.V.; Rodrigues, G.S.P.; Lengert, A.V.H.; Biondi, A.P.C.; Sargentini, L.D.B.; Brisotti, J.L.; Gomes, E.; de Oliveira, G.L.V. Detection of Alterations in the Gut Microbiota and Intestinal Permeability in Patients with Hashimoto Thyroiditis. Front. Immunol. 2021, 12, 579140. [Google Scholar] [CrossRef]
- Fenneman, A.C.; Rampanelli, E.; van der Spek, A.H.; Fliers, E.; Nieuwdorp, M. Protocol for a double-blinded randomised controlled trial to assess the effect of faecal microbiota transplantations on thyroid reserve in patients with subclinical autoimmune hypothyroidism in the Netherlands: The IMITHOT trial. BMJ Open 2023, 13, e073971. [Google Scholar]
- Erdem, M.G.; Unlu, O.; Ates, F.; Karis, D.; Demirci, M. Oral Microbiota Signatures in the Pathogenesis of Euthyroid Hashimoto’s Thyroiditis. Biomedicines 2023, 11, 1012. [Google Scholar] [CrossRef]
- Su, X.; Zhao, Y.; Li, Y.; Ma, S.; Wang, Z. Gut dysbiosis is associated with primary hypothyroidism with interaction on gut-thyroid axis. Clin. Sci. 2020, 134, 1521–1535. [Google Scholar]
- Yao, Z.; Zhao, M.; Gong, Y.; Chen, W.; Wang, Q.; Fu, Y.; Guo, T.; Zhao, J.; Gao, L.; Bo, T. Relation of Gut Microbes and L-Thyroxine Through Altered Thyroxine Metabolism in Subclinical Hypothyroidism Subjects. Front. Cell Infect. Microbiol. 2020, 10, 495. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Jiang, W.; Kosik, R.O.; Song, Y.; Luo, Q.; Qiao, T.; Tong, J.; Liu, S.; Deng, C.; Qin, S.; et al. Gut microbiota changes and its potential relations with thyroid carcinoma. J. Adv. Res. 2022, 35, 61–70. [Google Scholar] [PubMed]
- Demir, E.; Önal, B.; Özkan, H.; Utku, İ.K.; Şahtiyancı, B.; Kumbasar, A.; Yenmiş, G.; Demir, B. The relationship between elevated plasma zonulin levels and Hashimoto’s thyroiditis. Turk. J. Med. Sci. 2022, 52, 605–612. [Google Scholar] [PubMed]
- Liu, X.; Yuan, J.; Liu, S.; Tang, M.; Meng, X.; Wang, X.; Li, Y.; Chai, Y.; Kou, C.; Yang, Q.; et al. Investigating causal associations among gut microbiota, metabolites and autoimmune hypothyroidism: A univariable and multivariable Mendelian randomization study. Front. Immunol. 2023, 14, 1213159. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Wang, J.; Xie, G.; Zou, G.; Li, S.; Zhang, J.; Cai, W.; Xu, J. Correlation between gut microbiota and the development of Graves’ disease: A prospective study. iScience 2023, 26, 107188. [Google Scholar] [CrossRef]
- Moshkelgosha, S.; Verhasselt, H.L.; Masetti, G.; Covelli, D.; Biscarini, F.; Horstmann, M.; Daser, A.; Westendorf, A.M.; Jesenek, C.; Philipp, S.; et al. Modulating gut microbiota in a mouse model of Graves’ orbitopathy and its impact on induced disease. Microbiome 2021, 9, 45. [Google Scholar]
- Liu, S.; An, Y.; Cao, B.; Sun, R.; Ke, J.; Zhao, D. The Composition of Gut Microbiota in Patients Bearing Hashimoto’s Thyroiditis with Euthyroidism and Hypothyroidism. Int. J. Endocrinol. 2020, 2020, 5036959. [Google Scholar] [CrossRef]
- Zhang, Q.; Tong, B.; Xie, Z.; Li, Y.; Li, Y.; Wang, L.; Luo, B.; Qi, X. Changes in the gut microbiota of patients with Graves’ orbitopathy according to severity grade. Clin. Exp. Ophthalmol. 2023, 51, 808–821. [Google Scholar]
- Yan, H.X.; An, W.C.; Chen, F.; An, B.; Pan, Y.; Jin, J.; Xia, X.P.; Cui, Z.J.; Jiang, L.; Zhou, S.J.; et al. Intestinal microbiota changes in Graves’ disease: A prospective clinical study. Biosci. Rep. 2020, 40, BSR20191242. [Google Scholar] [CrossRef]
- Biscarini, F.; Masetti, G.; Muller, I.; Verhasselt, H.L.; Covelli, D.; Colucci, G.; Zhang, L.; Draman, M.S.; Okosieme, O.; Taylor, P.; et al. Gut Microbiome Associated with Graves Disease and Graves Orbitopathy: The INDIGO Multicenter European Study. J. Clin. Endocrinol. Metab. 2023, 108, 2065–2077. [Google Scholar]
- Cao, J.; Wang, N.; Luo, Y.; Ma, C.; Chen, Z.; Chenzhao, C.; Zhang, F.; Qi, X.; Xiong, W. A cause–effect relationship between Graves’ disease and the gut microbiome contributes to the thyroid–gut axis: A bidirectional two-sample Mendelian randomization study. Front. Immunol. 2023, 14, 977587. [Google Scholar] [CrossRef]
- Zhao, H.; Yuan, L.; Zhu, D.; Sun, B.; Du, J.; Wang, J. Alterations and Mechanism of Gut Microbiota in Graves’ Disease and Hashimoto’s Thyroiditis. Pol. J. Microbiol. 2022, 71, 173–189. [Google Scholar]
- Yang, M.; Zheng, X.; Wu, Y.; Zhang, R.; Yang, Q.; Yu, Z.; Liu, J.; Zha, B.; Gong, Q.; Yang, B.; et al. Preliminary Observation of the Changes in the Intestinal Flora of Patients with Graves’ Disease Before and After Methimazole Treatment. Front. Cell. Infect. Microbiol. 2022, 12, 794711. [Google Scholar]
- Zhu, Q.; Hou, Q.; Huang, S.; Ou, Q.; Huo, D.; Vázquez-Baeza, Y.; Cen, C.; Cantu, V.; Estaki, M.; Chang, H.; et al. Compositional and genetic alterations in Graves’ disease gut microbiome reveal specific diagnostic biomarkers. ISME J. 2021, 15, 3399–3411. [Google Scholar] [PubMed]
- Zheng, D.; Liao, H.; Chen, S.; Liu, X.; Mao, C.; Zhang, C.; Meng, M.; Wang, Z.; Wang, Y.; Jiang, Q.; et al. Elevated Levels of Circulating Biomarkers Related to Leaky Gut Syndrome and Bacterial Translocation Are Associated with Graves’ Disease. Front. Endocrinol. 2021, 12, 796212. [Google Scholar]
- Li, A.; Li, T.; Gao, X.; Yan, H.; Chen, J.; Huang, M.; Wang, L.; Yin, D.; Li, H.; Ma, R.; et al. Gut Microbiome Alterations in Patients with Thyroid Nodules. Front. Cell. Infect. Microbiol. 2021, 11, 643968. [Google Scholar]
- Huo, D.; Cen, C.; Chang, H.; Ou, Q.; Jiang, S.; Pan, Y.; Chen, K.; Zhang, J. Probiotic Bifidobacterium longum supplied with methimazole improved the thyroid function of Graves’ disease patients through the gut-thyroid axis. Commun Biol 2021, 4, 1046. [Google Scholar]
- Cai, Y.; Xu, Y.; Ban, Y.; Li, J.; Sun, Z.; Zhang, M.; Wang, B.; Hou, X.; Hao, Y.; Ouyang, Q.; et al. Plasma Lipid Profile and Intestinal Microflora in Pregnancy Women with Hypothyroidism and Their Correlation with Pregnancy Outcomes. Front. Endocrinol. 2022, 12, 792536. [Google Scholar]
- Liu, J.; Qin, X.; Lin, B.; Cui, J.; Liao, J.; Zhang, F.; Lin, Q. Analysis of gut microbiota diversity in Hashimoto’s thyroiditis patients. BMC Microbiol. 2022, 22, 318. [Google Scholar] [CrossRef]
- Dong, T.; Zhao, F.; Yuan, K.; Zhu, X.; Wang, N.; Xia, F.; Lu, Y.; Huang, Z. Association between Serum Thyroid-Stimulating Hormone Levels and Salivary Microbiome Shifts. Front. Cell. Infect. Microbiol. 2021, 11, 603291. [Google Scholar]
- DeClercq, V.; Nearing, J.T.; Langille, M.G.I. Investigation of the impact of commonly used medications on the oral microbiome of individuals living without major chronic conditions. PLoS ONE 2021, 16, e0261032. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Feng, J.; Li, J.; Zhao, L.; Liu, Y.; Chen, H.; Jin, Y.; Zhu, B.; Wei, Y. Alterations of the Gut Microbiota in Hashimoto’s Thyroiditis Patients. Thyroid 2018, 28, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xu, H.; Xu, J.; Guo, X.; Zhao, H.; Chen, Y.; Zhou, Y.; Nie, Y.F. prausnitzii and its supernatant increase SCFAs-producing bacteria to restore gut dysbiosis in TNBS-induced colitis. AMB Express 2021, 11, 33. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, B.; Luckey, D.; Bodhke, R.; Chen, J.; Marietta, E.; Jeraldo, P.; Murray, J.; Taneja, V. Prevotella histicola Protects From Arthritis by Expansion of Allobaculum and Augmenting Butyrate Production in Humanized Mice. Front. Immunol. 2021, 12, 609644. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Wu, W.; Chen, L.; Yang, W.; Huang, X.; Ma, C.; Chen, F.; Xiao, Y.; Zhao, Y.; Ma, C.; et al. Microbiota-derived short-chain fatty acids promote Th1 cell IL-10 production to maintain intestinal homeostasis. Nat. Commun. 2018, 9, 3555. [Google Scholar] [CrossRef]
- Haghikia, A.; Jörg, S.; Duscha, A.; Berg, J.; Manzel, A.; Waschbisch, A.; Hammer, A.; Lee, D.H.; May, C.; Wilck, N.; et al. Dietary Fatty Acids Directly Impact Central Nervous System Autoimmunity via the Small Intestine. Immunity 2015, 43, 817–829. [Google Scholar] [CrossRef]
- Sanchez, H.N.; Moroney, J.B.; Gan, H.; Shen, T.; Im, J.L.; Li, T.; Taylor, J.R.; Zan, H.; Casali, P. B cell-intrinsic epigenetic modulation of antibody responses by dietary fiber-derived short-chain fatty acids. Nat. Commun. 2020, 11, 60. [Google Scholar] [CrossRef]
- Luo, M.; Chen, Y.; Pan, X.; Chen, H.; Fan, L.; Wen, Y. E. coli Nissle 1917 ameliorates mitochondrial injury of granulosa cells in polycystic ovary syndrome through promoting gut immune factor IL-22 via gut microbiota and microbial metabolism. Front. Immunol. 2023, 14, 1137089. [Google Scholar] [CrossRef]
- Lou, K.; Liu, S.; Zhang, F.; Sun, W.; Su, X.; Bi, W.; Yin, Q.; Qiu, Y.; Zhang, Z.; Jing, M.; et al. The effect of hyperthyroidism on cognitive function, neuroinflammation, and necroptosis in APP/PS1 mice. J. Transl. Med. 2023, 21, 657. [Google Scholar] [CrossRef]
- Zhang, M.; Jiang, W.; Lu, G.; Wang, R.; Lv, Z.; Li, D. Insight Into Mouse Models of Hyperthyroidism. Front. Endocrinol. 2022, 13, 929750. [Google Scholar] [CrossRef]
- Zekri, Y.; Guyot, R.; Flamant, F. An Atlas of Thyroid Hormone Receptors’ Target Genes in Mouse Tissues. Int. J. Mol. Sci. 2022, 23, 11444. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wenhart, C.; Reimann, A.; Goebel, S.; Cho, Y.L.; Muench, G. Mapping Thyroid Changes in Size and Position During Enlargement in Adult Mice with Hyperthyroidism. Endocrinology 2024, 165, bqae062. [Google Scholar] [CrossRef]
- Shen, H.; Han, J.; Li, Y.; Lu, C.; Zhou, J.; Li, Y.; Su, X. Different host-specific responses in thyroid function and gut microbiota modulation between diet-induced obese and normal mice given the same dose of iodine. Appl. Microbiol. Biotechnol. 2019, 103, 3537–3547. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhao, Y.; Zhu, X.; Liu, X. Low regulatory T cell and high IL-17 mRNA expression in a mouse Graves’ disease model. J. Endocrinol. Investig. 2016, 40, 397–407. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, W.; Lu, G.; Qiao, T.; Gao, D.; Zhang, M.; Cai, H.; Chai, L.; Yi, W.; Lv, Z. The Potential Role of Pyrroloquinoline Quinone to Regulate Thyroid Function and Gut Microbiota Composition of Graves’ Disease in Mice. Pol. J. Microbiol. 2023, 72, 443–460. [Google Scholar] [CrossRef]
- Su, X.; Yin, X.; Liu, Y.; Yan, X.; Zhang, S.; Wang, X.; Lin, Z.; Zhou, X.; Gao, J.; Wang, Z.; et al. Gut Dysbiosis Contributes to the Imbalance of Treg and Th17 Cells in Graves’ Disease Patients by Propionic Acid. J. Clin. Endocrinol. Metab. 2020, 105, dgaa511. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.L.U.; Sena-Evangelista, K.C.M.; de Azevedo, E.P.; Pinheiro, F.I.; Cobucci, R.N.; Pedrosa, L.F.C. Selenium in Human Health and Gut Microflora: Bioavailability of Selenocompounds and Relationship with Diseases. Front. Nutr. 2021, 8, 685317. [Google Scholar] [CrossRef]
- Misharin, A.; Hewison, M.; Chen, C.R.; Lagishetty, V.; Aliesky, H.A.; Mizutori, Y.; Rapoport, B.; McLachlan, S.M. Vitamin D deficiency modulates Graves’ hyperthyroidism induced in BALB/c mice by thyrotropin receptor immunization. Endocrinology 2009, 150, 1051–1060. [Google Scholar] [CrossRef]
- González-Ramos, S.; Paz-García, M.; Fernández-García, V.; Portune, K.J.; Acosta-Medina, E.F.; Sanz, Y.; Castrillo, A.; Martín-Sanz, P.; Obregon, M.J.; Boscá, L. NOD1 deficiency promotes an imbalance of thyroid hormones and microbiota homeostasis in mice fed high fat diet. Sci. Rep. 2020, 10, 12317. [Google Scholar] [CrossRef]
- Li, J.; Xu, Y.; Cai, Y.; Zhang, M.; Sun, Z.; Ban, Y.; Zhai, S.; Hao, Y.; Ouyang, Q.; Wu, B.; et al. Association of Differential Metabolites with Small Intestinal Microflora and Maternal Outcomes in Subclinical Hypothyroidism During Pregnancy. Front. Cell. Infect. Microbiol. 2022, 11, 779659. [Google Scholar] [CrossRef]
- Song, X.; Zhang, H.; Zhang, Y.; Goh, B.; Bao, B.; Mello, S.S.; Sun, X.; Zheng, W.; Gazzaniga, F.S.; Wu, M.; et al. Gut microbial fatty acid isomerization modulates intraepithelial T cells. Nature 2023, 619, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hu, S.; Yin, J.; Peng, X.; King, L.; Li, L.; Xu, Z.; Zhou, L.; Peng, Z.; Ze, X.; et al. Effect of synbiotic supplementation on immune parameters and gut microbiota in healthy adults: A double-blind randomized controlled trial. Gut Microbes 2023, 15, 2247025. [Google Scholar] [PubMed]
- Wu, B.; Xu, Y.; Ban, Y.; Zhang, M.; Sun, Z.; Cai, Y.; Li, J.; Hao, Y.; Ouyang, Q.; Hu, L.; et al. Correlation between the intestinal microflora and peripheral blood Th1/Th2 balance in hypothyroidism during the first half of pregnancy. Front. Cell. Infect. Microbiol. 2023, 13, 1159238. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Li, F.; Zhang, R.; Wu, Y.; Yang, Q.; Wang, F.; Yu, Z.; Liu, J.; Cha, B.; Gong, Q.; et al. Alteration of the Intestinal Microbial Flora and the Serum IL-17 Level in Patients with Graves’ Disease Complicated with Vitamin D Deficiency. Int. Arch. Allergy Immunol. 2022, 183, 225–234. [Google Scholar]
- Xu, B.; Fu, Y.; Yin, N.; Qin, W.; Huang, Z.; Xiao, W.; Huang, H.; Mei, Q.; Fan, J.; Zeng, Y.; et al. Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii served as key components of fecal microbiota transplantation to alleviate colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2024, 326, G607–G621. [Google Scholar]
- Wastyk, H.C.; Fragiadakis, G.K.; Perelman, D.; Dahan, D.; Merrill, B.D.; Yu, F.B.; Topf, M.; Gonzalez, C.G.; Van Treuren, W.; Han, S.; et al. Gut-microbiota-targeted diets modulate human immune status. Cell 2021, 184, 4137–4153.e14. [Google Scholar]
- Li, H.B.; Xu, M.L.; Xu, X.D.; Tang, Y.Y.; Jiang, H.L.; Li, L.; Xia, W.J.; Cui, N.; Bai, J.; Dai, Z.M.; et al. Faecalibacterium prausnitzii Attenuates CKD via Butyrate-Renal GPR43 Axis. Circ. Res. 2022, 131, e120–e134. [Google Scholar]
- Robles-Vera, I.; de la Visitación, N.; Toral, M.; Sánchez, M.; Romero, M.; Gómez-Guzmán, M.; Yang, T.; Izquierdo-García, J.L.; Guerra-Hernández, E.; Ruiz-Cabello, J.; et al. Probiotic Bifidobacterium breve prevents DOCA-salt hypertension. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2020, 34, 13626–13640. [Google Scholar] [CrossRef]
- Sun, J.; Zhao, F.; Lin, B.; Feng, J.; Wu, X.; Liu, Y.; Zhao, L.; Zhu, B.; Wei, Y. Gut Microbiota Participates in Antithyroid Drug Induced Liver Injury Through the Lipopolysaccharide Related Signaling Pathway. Front. Pharmacol. 2020, 11, 598170. [Google Scholar]
- Yamamura, R.; Nakamura, K.; Kitada, N.; Aizawa, T.; Shimizu, Y.; Nakamura, K.; Ayabe, T.; Kimura, T.; Tamakoshi, A. Associations of gut microbiota, dietary intake, and serum short-chain fatty acids with fecal short-chain fatty acids. Biosci. Microbiota Food Health 2020, 39, 11–17. [Google Scholar]
- Kircher, B.; Woltemate, S.; Gutzki, F.; Schlüter, D.; Geffers, R.; Bähre, H.; Vital, M. Predicting butyrate- and propionate-forming bacteria of gut microbiota from sequencing data. Gut Microbes 2022, 14, 2149019. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Qin, Q.; Yan, S.; Yang, Y.; Yan, H.; Li, T.; Wang, L.; Gao, X.; Li, A.; Ding, S. Gut Microbiome Alterations in Patients with Carotid Atherosclerosis. Front. Cardiovasc. Med. 2021, 8, 739093. [Google Scholar] [CrossRef]
- Liang, L.; Liu, L.; Zhou, W.; Yang, C.; Mai, G.; Li, H.; Chen, Y. Gut microbiota-derived butyrate regulates gut mucus barrier repair by activating the macrophage/WNT/ERK signaling pathway. Clin. Sci. 2022, 136, 291–307. [Google Scholar] [CrossRef]
- Wang, G.; Qin, S.; Chen, L.; Geng, H.; Zheng, Y.; Xia, C.; Yao, J.; Deng, L. Butyrate dictates ferroptosis sensitivity through FFAR2-mTOR signaling. Cell Death Dis. 2023, 14, 292. [Google Scholar] [CrossRef]
- Tian, S.; Lei, Y.; Zhao, F.; Che, J.; Wu, Y.; Lei, P.; Kang, Y.E.; Shan, Y. Improving insulin resistance by sulforaphane via activating the Bacteroides and Lactobacillus SCFAs-GPR-GLP1 signal axis. Food Funct. 2024, 15, 8644–8660. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Xu, Z.; Huang, G.; Zhang, R.; Deng, M.; Huang, F.; Su, D. Lychee Pulp-Derived Dietary Fiber-Bound Phenolic Complex Upregulates the SCFAs-GPRs-ENS Pathway and Aquaporins in Loperamide-Induced Constipated Mice by Reshaping Gut Microbiome. J. Agric. Food Chem. 2023, 71, 15087–15096. [Google Scholar] [CrossRef]
- Phung, C.D.; Tran, T.H.; Nguyen, H.T.; Nguyen, T.T.; Jeong, J.H.; Ku, S.K.; Yong, C.S.; Choi, H.G.; Kim, J.O. Nanovaccines silencing IL-10 production at priming phase for boosting immune responses to melanoma. J. Control. Release Off. J. Control. Release Soc. 2021, 338, 211–223. [Google Scholar] [CrossRef]
- Antonelli, A.; Ferrari, S.M.; Frascerra, S.; Ruffilli, I.; Gelmini, S.; Minuto, M.; Pupilli, C.; Miccoli, P.; Sellari-Franceschini, S.; Ferrannini, E.; et al. Peroxisome proliferator-activated receptor-α agonists modulate CXCL9 and CXCL11 chemokines in Graves’ ophthalmopathy fibroblasts and preadipocytes. Mol. Cell. Endocrinol. 2012, 349, 255–261. [Google Scholar] [CrossRef]
- Tian, X.; Zeng, Y.; Tu, Q.; Jiao, Y.; Yao, S.; Chen, Y.; Sun, L.; Xia, Q.; Luo, Y.; Yuan, L.; et al. Butyrate alleviates renal fibrosis in CKD by regulating NLRP3-mediated pyroptosis via the STING/NF-κB/p65 pathway. Int. Immunopharmacol. 2023, 124 Pt B, 111010. [Google Scholar] [CrossRef]
- Klasson, C.L.; Sadhir, S.; Pontzer, H. Daily physical activity is negatively associated with thyroid hormone levels, inflammation, and immune system markers among men and women in the NHANES dataset. PLoS ONE 2022, 17, e0270221. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Li, X.; Ahmed, A.; Wu, D.; Liu, L.; Qiu, J.; Yan, Y.; Jin, M.; Xin, Y. Gut microbe analysis between hyperthyroid and healthy individuals. Curr. Microbiol. 2014, 69, 675–680. [Google Scholar] [CrossRef]
- Jiang, W.; Lu, G.; Gao, D.; Lv, Z.; Li, D. The relationships between the gut microbiota and its metabolites with thyroid diseases. Front. Endocrinol. 2022, 13, 943408. [Google Scholar] [CrossRef]
- Wang, B.; Xu, Y.; Hou, X.; Li, J.; Cai, Y.; Hao, Y.; Ouyang, Q.; Wu, B.; Sun, Z.; Zhang, M.; et al. Small Intestinal Bacterial Overgrowth in Subclinical Hypothyroidism of Pregnant Women. Front. Endocrinol. 2021, 12, 604070. [Google Scholar] [CrossRef]
- Shen, H.; Xu, J.; Lu, C.; Han, J.; Zhou, J.; Ming, T.; Li, Y.; Su, X. Effects of the Sex Factor on Mouse Iodine Intake: Interactions between the Gut Microbiota Composition and Metabolic Syndromes. ACS Omega 2021, 6, 28569–28578. [Google Scholar] [CrossRef]
- Laurino, A.; Raimondi, L. Thyroid Homeostasis: An Intricate Network of Production, Transport, Metabolism and Receptors Interaction. Int. J. Mol. Sci. 2022, 23, 6751. [Google Scholar] [CrossRef]
- McDonald, C.M.; Brown, K.H.; Goh, Y.E.; Manger, M.S.; Arnold, C.D.; Krebs, N.F.; Westcott, J.; Long, J.M.; Gibson, R.S.; Jamwal, M.; et al. Quintuply-fortified salt for the improvement of micronutrient status among women of reproductive age and preschool-aged children in Punjab, India: Protocol for a randomized, controlled, community-based trial. BMC Nutr. 2022, 8, 98. [Google Scholar] [CrossRef]
- Henjum, S.; Groufh-Jacobsen, S.; Aakre, I.; Gjengedal, E.L.F.; Langfjord, M.M.; Heen, E.; Sele, V.; Andersson, M. Thyroid function and urinary concentrations of iodine, selenium, and arsenic in vegans, lacto-ovo vegetarians and pescatarians. Eur. J. Nutr. 2023, 62, 3329–3338. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, L.; Tang, L.; Huang, D.; Pan, D.; Guo, W.; He, S.; Huang, Y.; Chen, Y.; Xiao, X.; et al. Gut microbiota is associated with response to (131)I therapy in patients with papillary thyroid carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 1453–1465. [Google Scholar] [CrossRef]
- Mendoza-León, M.J.; Mangalam, A.K.; Regaldiz, A.; González-Madrid, E.; Rangel-Ramírez, M.A.; Álvarez-Mardonez, O.; Vallejos, O.P.; Méndez, C.; Bueno, S.M.; Melo-González, F.; et al. Gut microbiota short-chain fatty acids and their impact on the host thyroid function and diseases. Front. Endocrinol. 2023, 30, 1192216. [Google Scholar] [CrossRef]
- Huang, H.J.; Wang, S.S.; Jin, M.M.; Cheng, B.W.; Liu, Y.; Liu, X.C.; Yu, Q.Y.; Yang, X.J. Genetically predicted selenium concentrations and thyroid function: A two-sample Mendelian randomization study. Clin. Endocrinol. 2023, 98, 813–822. [Google Scholar] [CrossRef]
- Vaivode, I.; Zake, T.; Strele, I.; Upmale-Engela, S.; Gogins, D.; Gersone, G.; Skesters, A.; Dambrova, M.; Konrade, I. Stress-Related Immune Response and Selenium Status in Autoimmune Thyroid Disease Patients. Int. J. Mol. Sci. 2023, 24, 2440. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Oltra, E.; Dijck-Brouwer, D.A.J.; Chillon, T.S.; Seemann, P.; Asaad, S.; Demircan, K.; Espejo-Oltra, J.A.; Sánchez-Fito, T.; Martín-Martínez, E.; et al. Autoantibodies to selenoprotein P in chronic fatigue syndrome suggest selenium transport impairment and acquired resistance to thyroid hormone. Redox Biol. 2023, 65, 102796. [Google Scholar] [CrossRef] [PubMed]
- Lossow, K.; Renko, K.; Schwarz, M.; Schomburg, L.; Schwerdtle, T.; Kipp, A.P. The Nutritional Supply of Iodine and Selenium Affects Thyroid Hormone Axis Related Endpoints in Mice. Nutrients 2021, 13, 3773. [Google Scholar] [CrossRef] [PubMed]
- Zavros, A.; Andreou, E.; Aphamis, G.; Bogdanis, G.C.; Sakkas, G.K.; Roupa, Z.; Giannaki, C.D. The Effects of Zinc and Selenium Co-Supplementation on Resting Metabolic Rate, Thyroid Function, Physical Fitness, and Functional Capacity in Overweight and Obese People under a Hypocaloric Diet: A Randomized, Double-Blind, and Placebo-Controlled Trial. Nutrients 2023, 15, 3133. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, Y.; Yuan, Z.; Li, Y.; Liu, S.; Zeng, X.; Qiu, X.; Ye, L.; Huang, D. The association between iron status and thyroid hormone levels during pregnancy. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. 2022, 74, 127047. [Google Scholar] [CrossRef]
- Shimizu, Y.; Matsuyama, M.; Noguchi, Y.; Takada, M.; Kawashiri, S.Y.; Fukui, S.; Nakamichi, S.; Nagata, Y.; Maeda, T.; Hayashida, N. Association between anti-thyroid peroxidase antibody and thyroid stimulating hormone: A cross-sectional study. Sci. Rep. 2023, 13, 14358. [Google Scholar] [CrossRef] [PubMed]
- McKay, A.K.A.; Peeling, P.; Pyne, D.B.; Tee, N.; Whitfield, J.; Sharma, A.P.; Heikura, I.A.; Burke, L.M. Six Days of Low Carbohydrate, Not Energy Availability, Alters the Iron and Immune Response to Exercise in Elite Athletes. Med. Sci. Sports Exerc. 2022, 54, 377–387. [Google Scholar] [CrossRef]
- Rabbani, E.; Golgiri, F.; Janani, L.; Moradi, N.; Fallah, S.; Abiri, B.; Vafa, M. Randomized Study of the Effects of Zinc, Vitamin A, and Magnesium Co-supplementation on Thyroid Function, Oxidative Stress, and hs-CRP in Patients with Hypothyroidism. Biol. Trace Elem. Res. 2021, 199, 4074–4083. [Google Scholar] [CrossRef]
- Lu, L.; Huang, Z.; Wang, X.; Chen, J. Interaction between Dietary Selenium and Zinc Intakes on Hypothyroidism. Biol. Trace Elem. Res. 2023, 201, 4667–4676. [Google Scholar] [CrossRef]
- Kijima, K.; Ono, G.; Kobayakawa, K.; Saiwai, H.; Hara, M.; Yoshizaki, S.; Yokota, K.; Saito, T.; Tamaru, T.; Iura, H.; et al. Zinc deficiency impairs axonal regeneration and functional recovery after spinal cord injury by modulating macrophage polarization via NF-κB pathway. Front. Immunol. 2023, 14, 1290100. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, S.; Hu, L.; Dong, L.; Liu, Q.; Liu, Y.; Cheng, W.; Liu, D.; Yang, G.; Li, K. Vitamin D categories and postpartum thyroid function in women with hypothyroidism. Front. Nutr. 2022, 9, 953745. [Google Scholar] [CrossRef] [PubMed]
- Català-Moll, F.; Ferreté-Bonastre, A.G.; Godoy-Tena, G.; Morante-Palacios, O.; Ciudad, L.; Barberà, L.; Fondelli, F.; Martínez-Cáceres, E.M.; Rodríguez-Ubreva, J.; Li, T.; et al. Vitamin D receptor, STAT3, and TET2 cooperate to establish tolerogenesis. Cell Rep. 2022, 38, 110244. [Google Scholar] [PubMed]
- Maciejewska-Markiewicz, D.; Kochman, J.; Jakubczyk, K.; Bargiel, P.; Szlosser, Z.; Stachowska, E.; Markowska, M.; Bucka, A.; Czapla, N.; Petriczko, J.; et al. Vitamin D Status in Patients before Thyroidectomy. Int. J. Mol. Sci. 2023, 24, 3228. [Google Scholar] [CrossRef]


| Phyla Level | Genus/Species Level | References | |
|---|---|---|---|
| Augmented: | Reduced: | ||
| Firmicutes | Anaerostipes, Faecalibacterium | [3] | |
| Clostridiaceae 02d06, Clostridium estertheticum | [47] | ||
| Megamonas, Veillonella | Butyricimonas, Anaerostipes | [37] | |
| Ruminococcus | E.ventriosum, Flavonifractor, Romboutsia, Oscillospiaceae UCG 002 | [38] | |
| Enterococcus | Roseburia, Dialister | [28] | |
| Anaerofilum, Intestinimonas, Peptococcus, Ruminococcaceae UCG 005 | [43] | ||
| Phascolarctobacterium | [44] | ||
| Pediococcus, Streptococcus parasanguinis, Streptococcus salivarius, Veillonella parvula | Faecalibacterium, prausnitzii, Butyricimonas faecalis | [33] | |
| Ruminococcus sp., Dorea sp., Eubacterium ventriosum | [30] | ||
| Bacteroidota | Bacteroides, Prevotella 9 | [9] | |
| Prevotella | [12] | ||
| Odoribacter, Rikenellaceae | [28] | ||
| Parabacteroides | [34] | ||
| Acfinobacteria | Actinobacillus | [3] | |
| Rothia mucilaginosa | [47] | ||
| Eggerthella lenta, Collinsella | Bifdobacterium, Corynebacterium | [33] | |
| Phyla Level | Genus/Species Level | References | |
|---|---|---|---|
| Augmented: | Reduced: | ||
| Firmicutes | Ruminococcus, Roseburia, Dorea, [Eubacterium] hallii group | Lachnoclostridium, Fecalibacterium | [52] |
| Megamonas, Clostridia, Holdemania, oscillospirales, Lachnospiraceae NC2004 group | Faecalibacterium | [49] | |
| Enterococcus | [41] | ||
| Ruminococcus 2 | [31] | ||
| Lachnospiraceae incertae sedis, Subdoligranulum, Subdoligranulum | [37] | ||
| Granulicatella | [54] | ||
| Bacteroidota | Flavobacteriaceae | [53] | |
| Escherichia Shigella, Parasutterella Bacteroides, Blautia | Prevotella 9 | [29] | |
| Paraprevotella | [32] | ||
| Proteobacteria | Ralstonia, Acetitomaculum | [53] | |
| Alistipes | [41] | ||
| Veillonella | Neisseria, Rheinheimera | [32] | |
| Haemophilus | [52] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, K.; Sun, X.; Fan, C.; Wang, X.; Yu, H. Unveiling the Role of Gut Microbiota and Metabolites in Autoimmune Thyroid Diseases: Emerging Perspectives. Int. J. Mol. Sci. 2024, 25, 10918. https://doi.org/10.3390/ijms252010918
Yan K, Sun X, Fan C, Wang X, Yu H. Unveiling the Role of Gut Microbiota and Metabolites in Autoimmune Thyroid Diseases: Emerging Perspectives. International Journal of Molecular Sciences. 2024; 25(20):10918. https://doi.org/10.3390/ijms252010918
Chicago/Turabian StyleYan, Kai, Xin Sun, Chenxi Fan, Xin Wang, and Hongsong Yu. 2024. "Unveiling the Role of Gut Microbiota and Metabolites in Autoimmune Thyroid Diseases: Emerging Perspectives" International Journal of Molecular Sciences 25, no. 20: 10918. https://doi.org/10.3390/ijms252010918







