Unlocking the Luminescent Potential of Fish-Scale-Derived Carbon Nanoparticles for Multicolor Conversion
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of Synthesis Parameters Employing the Box–Behnken Design and Response Surface Methodology Analysis
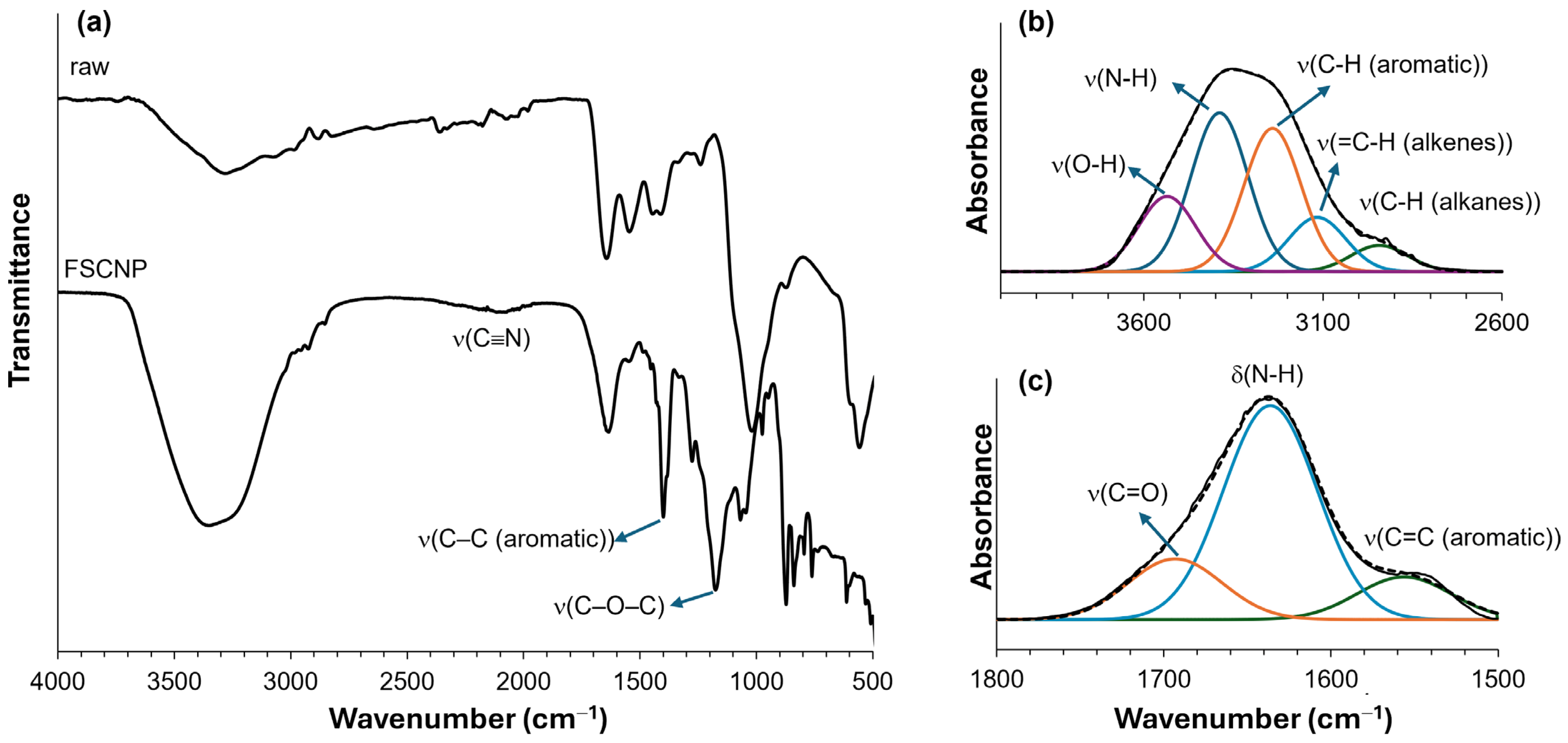
2.2. Characterization of FSCNP
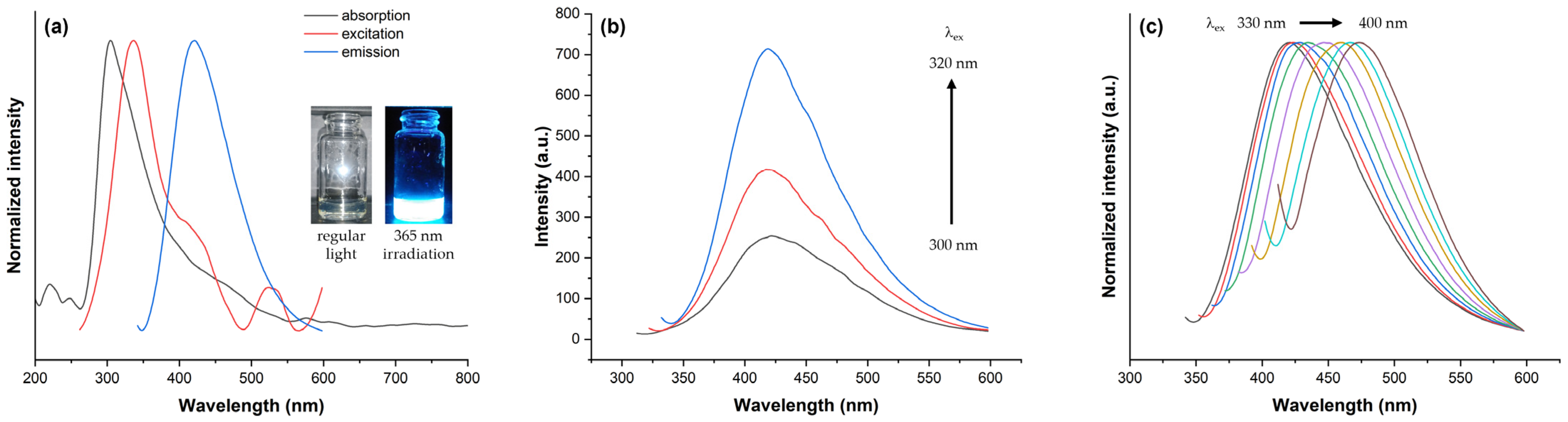
2.3. Optical Properties of FSCNPs
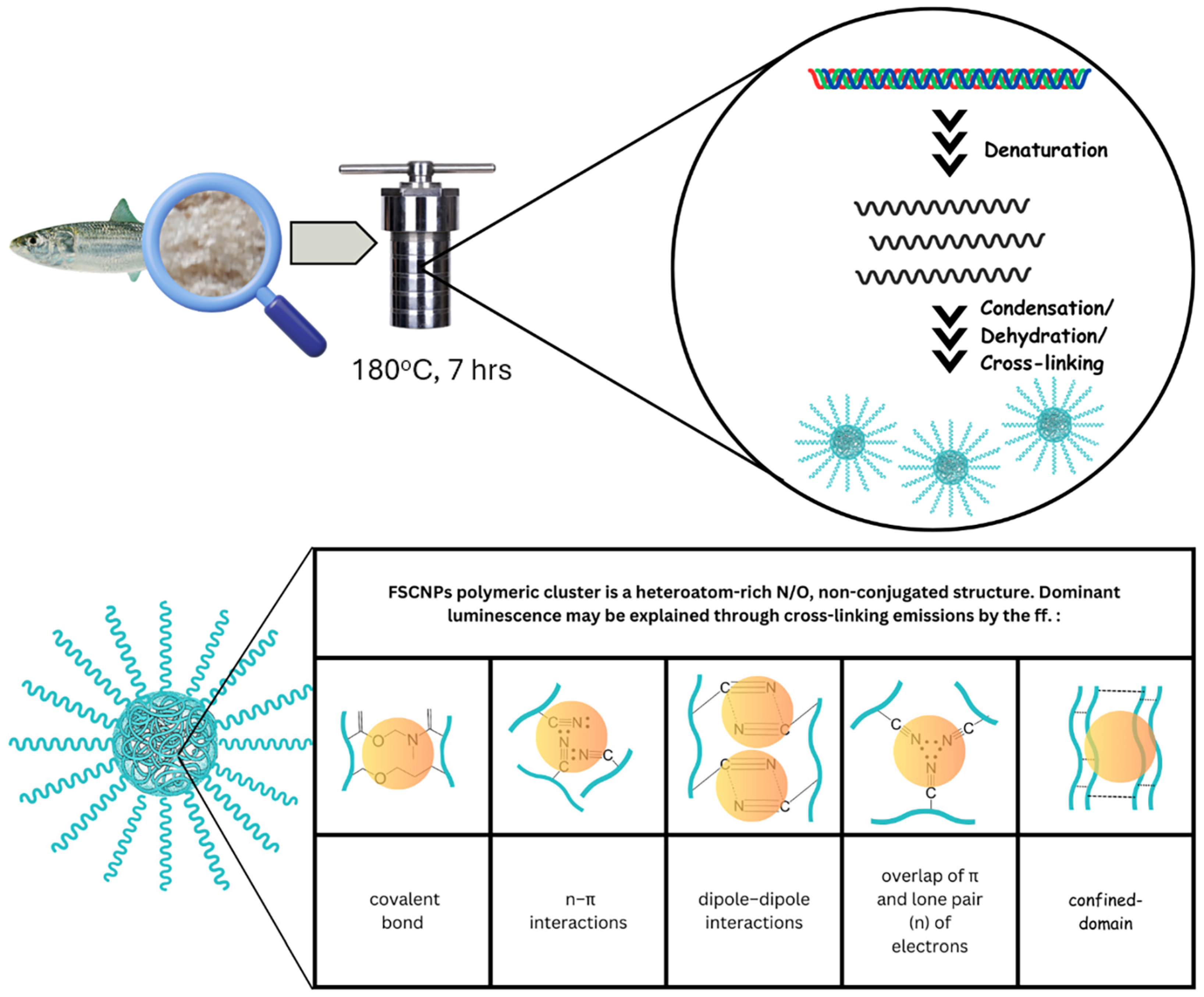
2.4. Luminescence Mechanism of FSCNPs
2.4.1. The Factors, Variables, and Parameters That Contributed to the FSCNPs’ Luminescence
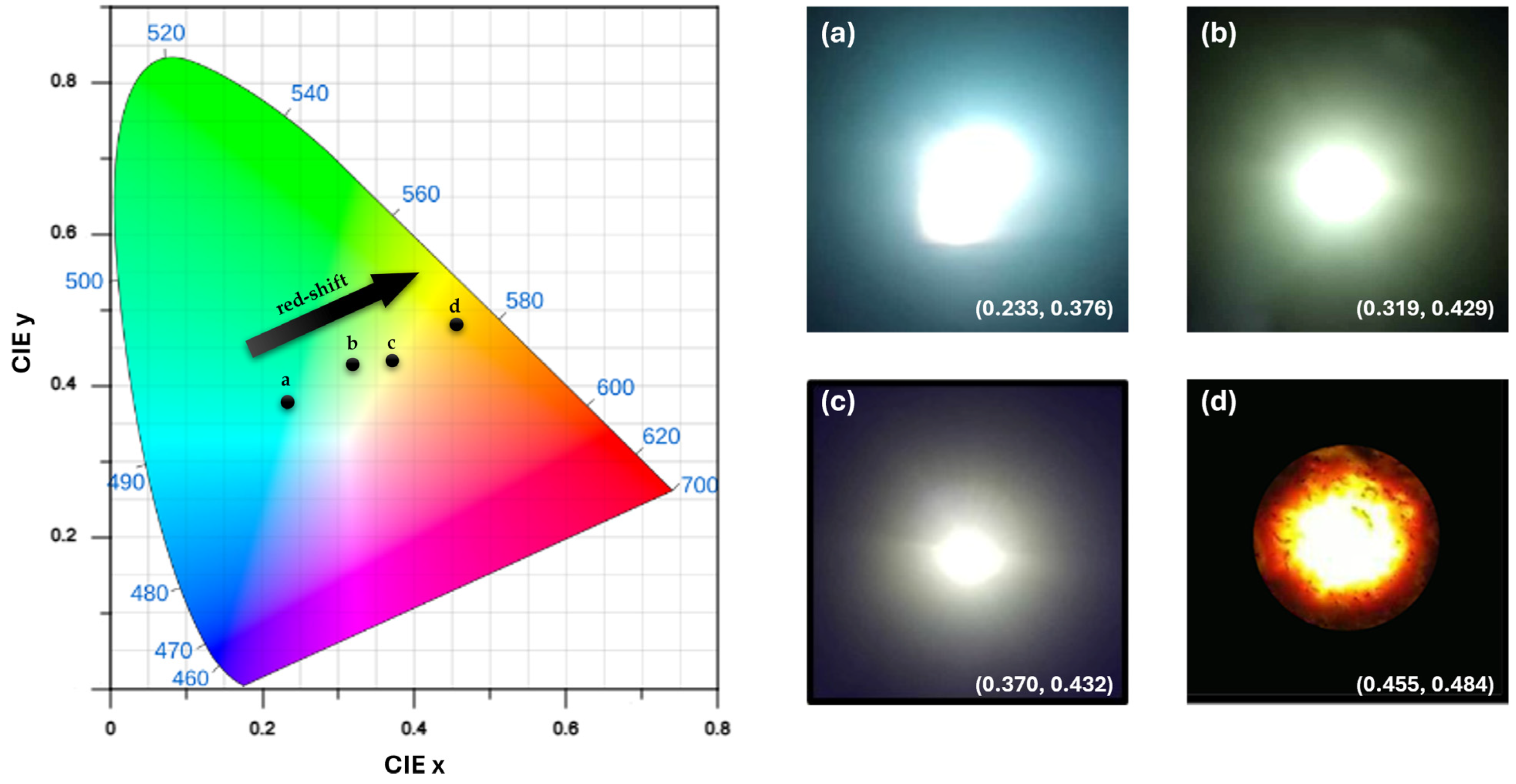
2.4.2. Origin FSCNPs’ Multicolor Luminescence
3. Materials and Methods
3.1. Materials
3.2. Sample Pre-Treatment
3.3. Sample Preparation
3.4. Preparation of FSCNP Phosphors
3.5. Characterization
3.5.1. Transmission Electron Microscopy
3.5.2. X-ray Photoelectron Spectroscopy
3.5.3. Fourier Transform Infrared Spectroscopy
3.5.4. X-ray Diffraction
3.5.5. Raman Spectroscopy
3.5.6. UV–Visible and Fluorescence Spectroscopy
3.5.7. Absolute Quantum Yield Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmad, F.; Saeed, Q.; Shah, S.M.U.; Gondal, M.A.; Mumtaz, S. Chapter 11—Environmental sustainability: Challenges and Approaches. In Natural Resources Conservation and Advances for Sustainability; Jhariya, M.K., Meena, R.S., Banerjee, A., Meena, S.N., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 243–270. [Google Scholar] [CrossRef]
- Oladzadabbasabadi, N.; Dheyab, M.A.; Nafchi, A.M.; Ghasemlou, M.; Ivanova, E.P.; Adhikari, B. Turning food waste into value-added carbon dots for sustainable food packaging application: A review. Adv. Colloid Interface Sci. 2023, 321, 103020. [Google Scholar] [CrossRef] [PubMed]
- Saddalani, S.Y.; Pansacala, J.; Fernando, J.; Toledo, R. Chitin extraction from sardine fish scales. Int. J. Biosci. 2022, 21, 216–222. [Google Scholar] [CrossRef]
- Alcantara, F.S.; Francisco, J.T. Production and characterization of cellulose from the branch and leaves of Gigantochloa atter (Kawayang kayali). Int. J. Biosci. 2019, 14, 330–335. [Google Scholar]
- Escobar, B.; Martínez-Casillas, D.C.; Pérez-Salcedo, K.Y.; Rosas, D.; Morales, L.; Liao, S.J.; Huang, L.L.; Shi, X. Research progress on biomass-derived carbon electrode materials for electrochemical energy storage and conversion technologies. Int. J. Hydrogen Energy 2021, 46, 26053–26073. [Google Scholar] [CrossRef]
- Qin, D.; Bi, S.; You, X.; Wang, M.; Cong, X.; Yuan, C.; Yu, M.; Cheng, X.; Chen, X.-G. Development and application of fish scale wastes as versatile natural biomaterials. Chem. Eng. J. 2022, 428, 131102. [Google Scholar] [CrossRef]
- Aboudamia, F.Z.; Aatab, F.; Jaouad, A.; Bouchdoug, M.; Kharroubi, M. Sardine Scales: A Promising Source of Marine Biomaterials. Lett. Appl. NanoBioScience 2021, 11, 3954–3960. [Google Scholar] [CrossRef]
- Attia, S.; Hamdy, M.; Ezzeldin, S. Twenty-year tracking of lighting savings and power density in the residential sector. Energy Build. 2017, 154, 113–126. [Google Scholar] [CrossRef]
- Campalani, C.; Causin, V.; Selva, M.; Perosa, A. Fish-waste-derived gelatin and carbon dots for biobased UV-blocking films. ACS Appl. Mater. Interfaces 2022, 14, 35148–35156. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, Z.; Yang, X.; Chang, J.; Liu, Z.; Jiang, K. Fish-scale-derived carbon dots as efficient fluorescent nanoprobes for detection of ferric ions. RSC Adv. 2019, 9, 940–949. [Google Scholar] [CrossRef]
- Zhang, Y.; Lei, C.; Dong, P.; Fu, P.; Zhang, Y.; Hua, R. Green synthesis of carbon dots from fish scales for selective turn off–on detection of glutathione. RSC Adv. 2024, 14, 3578–3587. [Google Scholar] [CrossRef]
- Campalani, C.; Cattaruzza, E.; Zorzi, S.; Vomiero, A.; You, S.; Matthews, L.; Capron, M.; Mondelli, C.; Selva, M.; Perosa, A. Biobased carbon dots: From fish scales to photocatalysis. Nanomaterials 2021, 11, 524. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Odachi, K.; Shirai, T. Fabrication of ultra-bright carbon nano-onions via a one-step microwave pyrolysis of fish scale waste in seconds. Green Chem. 2022, 24, 3969–3976. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Dela Cruz, M.I.S.; Thongsai, N.; de Luna, M.D.G.; In, I.; Paoprasert, P. Preparation of highly photoluminescent carbon dots from polyurethane: Optimization using response surface methodology and selective detection of silver (I) ion. Colloids Surf. A Physicochem. Eng. Asp. 2019, 568, 184–194. [Google Scholar] [CrossRef]
- Wyantuti, S.; Fadhilatunnisa, B.; Fauzia, R.P.; Jia, Q.; Rahmani, A.A.; Irkham; Bahti, H.H. Response surface methodology box-behnken design to optimise the hydrothermal synthesis of gadolinium nanoparticles. Chin. J. Anal. Chem. 2023, 51, 100316. [Google Scholar] [CrossRef]
- Barati, A.; Shamsipur, M.; Arkan, E.; Hosseinzadeh, L.; Abdollahi, H. Synthesis of biocompatible and highly photoluminescent nitrogen doped carbon dots from lime: Analytical applications and optimization using response surface methodology. Mater. Sci. Eng. C 2015, 47, 325–332. [Google Scholar] [CrossRef]
- Liu, B.; Chen, Z.; Chu, B.; Wang, Y.-L.; Li, N.; Zhang, H.; Yang, Y.; Hu, S.; Zhang, X.-H. Clustering-induced white light emission from carbonized polymer dots. Adv. Photonics Res. 2021, 2, 2000161. [Google Scholar] [CrossRef]
- Miao, S.; Liang, K.; Zhu, J.; Yang, B.; Zhao, D.; Kong, B. Hetero-atom-doped carbon dots: Doping strategies, properties and applications. Nano Today 2020, 33, 100879. [Google Scholar] [CrossRef]
- Chen, T.-H.; Tseng, W.-L. Self-assembly of monodisperse carbon dots into high-brightness nanoaggregates for cellular uptake imaging and iron(III) sensing. Anal. Chem. 2017, 89, 11348–11356. [Google Scholar] [CrossRef]
- Gao, Y.; Han, H.; Lu, W.; Jiao, Y.; Liu, Y.; Gong, X.; Xian, M.; Shuang, S.; Dong, C. Matrix-free and highly efficient room-temperature phosphorescence of nitrogen-doped carbon dots. Langmuir 2018, 34, 12845–12852. [Google Scholar] [CrossRef]
- Tammina, S.K.; Yang, D.; Li, X.; Koppala, S.; Yang, Y. High photoluminescent nitrogen and zinc doped carbon dots for sensing Fe3+ ions and temperature. Spectrochim. Acta Part A 2019, 222, 117141. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Li, M.; Li, Q.; Liang, S.; Tan, Y.; Sheng, L.; Shi, W.; Zhang, S.X.-A. Carbon Dots with Continuously Tunable Full-Color Emission and Their Application in Ratiometric pH Sensing. Chem. Mater. 2014, 26, 3104–3112. [Google Scholar] [CrossRef]
- Shang, Y.; Yao, M.; Liu, Z.; Fu, R.; Yan, L.; Yang, L.; Zhang, Z.; Dong, J.; Zhai, C.; Hou, X.; et al. Enhancement of short/medium-range order and thermal conductivity in ultrahard sp3 amorphous carbon by C70 precursor. Nat. Commun. 2023, 14, 7860. [Google Scholar] [CrossRef] [PubMed]
- Moseenkov, S.I.; Kuznetsov, V.L.; Zolotarev, N.A.; Kolesov, B.A.; Prosvirin, I.P.; Ishchenko, A.V.; Zavorin, A.V. Investigation of amorphous carbon in nanostructured carbon materials (a comparative study by TEM, XPS, Raman spectroscopy and XRD). Materials 2023, 16, 1112. [Google Scholar] [CrossRef]
- Vautard, F.; Ozcan, S.; Paulauskas, F.; Spruiell, J.E.; Meyer, H.; Lance, M.J. Influence of the carbon fiber surface microstructure on the surface chemistry generated by a thermo-chemical surface treatment. Appl. Surf. Sci. 2012, 261, 473–480. [Google Scholar] [CrossRef]
- Couzi, M.; Bruneel, J.-L.; Talaga, D.; Bokobza, L. A multi wavelength Raman scattering study of defective graphitic carbon materials: The first order Raman spectra revisited. Carbon 2016, 107, 388–394. [Google Scholar] [CrossRef]
- Siddique, A.B.; Hossain, S.M.; Pramanick, A.K.; Ray, M. Excitation dependence and independence of photoluminescence in carbon dots and graphene quantum dots: Insights into the mechanism of emission. Nanoscale 2021, 13, 16662–16671. [Google Scholar] [CrossRef]
- Xia, C.; Zhu, S.; Feng, T.; Yang, M.; Yang, B. Evolution and synthesis of carbon dots: From carbon dots to carbonized polymer dots. Adv. Sci. 2019, 6, 1901316. [Google Scholar] [CrossRef]
- Lagunay, R.A.E.; Akhetova, B.; O’Reilly, R.J.; Balanay, M.P. Tailoring the optoelectronic properties of soybean-derived nitrogen self-doped carbon dots through composite formation with KCl and zeolite, synthesized using autogenic atmosphere pyrolysis. Crystals 2024, 14, 348. [Google Scholar] [CrossRef]
- Xu, C.; Xiao, X.; Cai, C.; Cheng, Q.; Zhu, L.; Zhang, J.; Wei, B.; Wang, H. Insight into the differences in carbon dots prepared from fish scales using conventional hydrothermal and microwave methods. Environ. Sci. Pollut. Res. 2023, 30, 54616–54627. [Google Scholar] [CrossRef]
- Nazatul Akmal, N.; Mohammad Faiz, Z.; Che Azurahanim Che, A. Hydrothermal synthesis of carbon quantum dots: An updated review. J. Adv. Res. Fluid Mech. Therm. Sci. 2023, 101, 192–206. [Google Scholar] [CrossRef]
- Muthumari, K.; Anand, M.; Maruthupandy, M. Collagen extract from marine finfish scales as a potential mosquito larvicide. Protein J. 2016, 35, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Chinh, N.T.; Manh, V.Q.; Trung, V.Q.; Lam, T.D.; Huynh, M.D.; Tung, N.Q.; Trinh, N.D.; Hoang, T. Characterization of collagen derived from tropical freshwater carp fish scale wastes and its amino acid sequence. Nat. Prod. Commun. 2019, 14, 1934578X19866288. [Google Scholar] [CrossRef]
- Tao, S.; Zhu, S.; Feng, T.; Xia, C.; Song, Y.; Yang, B. The polymeric characteristics and photoluminescence mechanism in polymer carbon dots: A review. Mater. Today Chem. 2017, 6, 13–25. [Google Scholar] [CrossRef]
- Wang, B.; Yu, J.; Sui, L.; Zhu, S.; Tang, Z.; Yang, B.; Lu, S. Rational design of multi-color-emissive carbon dots in a single reaction system by hydrothermal. Adv. Sci. 2021, 8, 2001453. [Google Scholar] [CrossRef]
- Tepliakov, N.V.; Kundelev, E.V.; Khavlyuk, P.D.; Xiong, Y.; Leonov, M.Y.; Zhu, W.; Baranov, A.V.; Fedorov, A.V.; Rogach, A.L.; Rukhlenko, I.D. sp2-sp3-hybridized atomic domains determine optical features of carbon dots. ACS Nano 2019, 13, 10737–10744. [Google Scholar] [CrossRef]
- Kang, C.; Tao, S.; Yang, F.; Yang, B. Aggregation and luminescence in carbonized polymer dots. Aggregate 2022, 3, e169. [Google Scholar] [CrossRef]
- Zhou, Q.; Cao, B.; Zhu, C.; Xu, S.; Gong, Y.; Yuan, W.Z.; Zhang, Y. Clustering-triggered emission of nonconjugated polyacrylonitrile. Small 2016, 12, 6586–6592. [Google Scholar] [CrossRef]
- Tao, S.; Zhou, C.; Kang, C.; Zhu, S.; Feng, T.; Zhang, S.-T.; Ding, Z.; Zheng, C.; Xia, C.; Yang, B. Confined-domain crosslink-enhanced emission effect in carbonized polymer dots. Light Sci. Appl. 2022, 11, 56. [Google Scholar] [CrossRef]
- He, Q.; Ren, J.; Liu, Y. Dispersion-assisted tunable fluorescence from carbon dots. Nanotechnology 2022, 33, 175705. [Google Scholar] [CrossRef]
- Zheng, C.; Tao, S.; Yang, B. The current progress and challenges of carbonized polymer dot-based room-temperature phosphorescent materials. CCS Chem. 2024, 6, 604–622. [Google Scholar] [CrossRef]
- Haque, A.; Alenezi, K.M.; Khan, M.S.; Wong, W.-Y.; Raithby, P.R. Non-covalent interactions (NCIs) in π-conjugated functional materials: Advances and perspectives. Chem. Soc. Rev. 2023, 52, 454–472. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, S.; Yin, P.; Li, J.; Tang, Y.; Yang, M. Response surface methodology optimization for the synthesis of N, S-codoped carbon dots and its application for tetracyclines detection. Chemosphere 2022, 303, 135145. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdulla II, N.S.; Fernandez, M.J.F.; Baptayev, B.; Balanay, M.P. Unlocking the Luminescent Potential of Fish-Scale-Derived Carbon Nanoparticles for Multicolor Conversion. Int. J. Mol. Sci. 2024, 25, 10929. https://doi.org/10.3390/ijms252010929
Abdulla II NS, Fernandez MJF, Baptayev B, Balanay MP. Unlocking the Luminescent Potential of Fish-Scale-Derived Carbon Nanoparticles for Multicolor Conversion. International Journal of Molecular Sciences. 2024; 25(20):10929. https://doi.org/10.3390/ijms252010929
Chicago/Turabian StyleAbdulla II, Najeeb S., Marvin Jose F. Fernandez, Bakhytzhan Baptayev, and Mannix P. Balanay. 2024. "Unlocking the Luminescent Potential of Fish-Scale-Derived Carbon Nanoparticles for Multicolor Conversion" International Journal of Molecular Sciences 25, no. 20: 10929. https://doi.org/10.3390/ijms252010929
APA StyleAbdulla II, N. S., Fernandez, M. J. F., Baptayev, B., & Balanay, M. P. (2024). Unlocking the Luminescent Potential of Fish-Scale-Derived Carbon Nanoparticles for Multicolor Conversion. International Journal of Molecular Sciences, 25(20), 10929. https://doi.org/10.3390/ijms252010929






