AtC3H3, an Arabidopsis Non-TZF Gene, Enhances Salt Tolerance by Increasing the Expression of Both ABA-Dependent and -Independent Stress-Responsive Genes
Abstract
:1. Introduction
2. Results
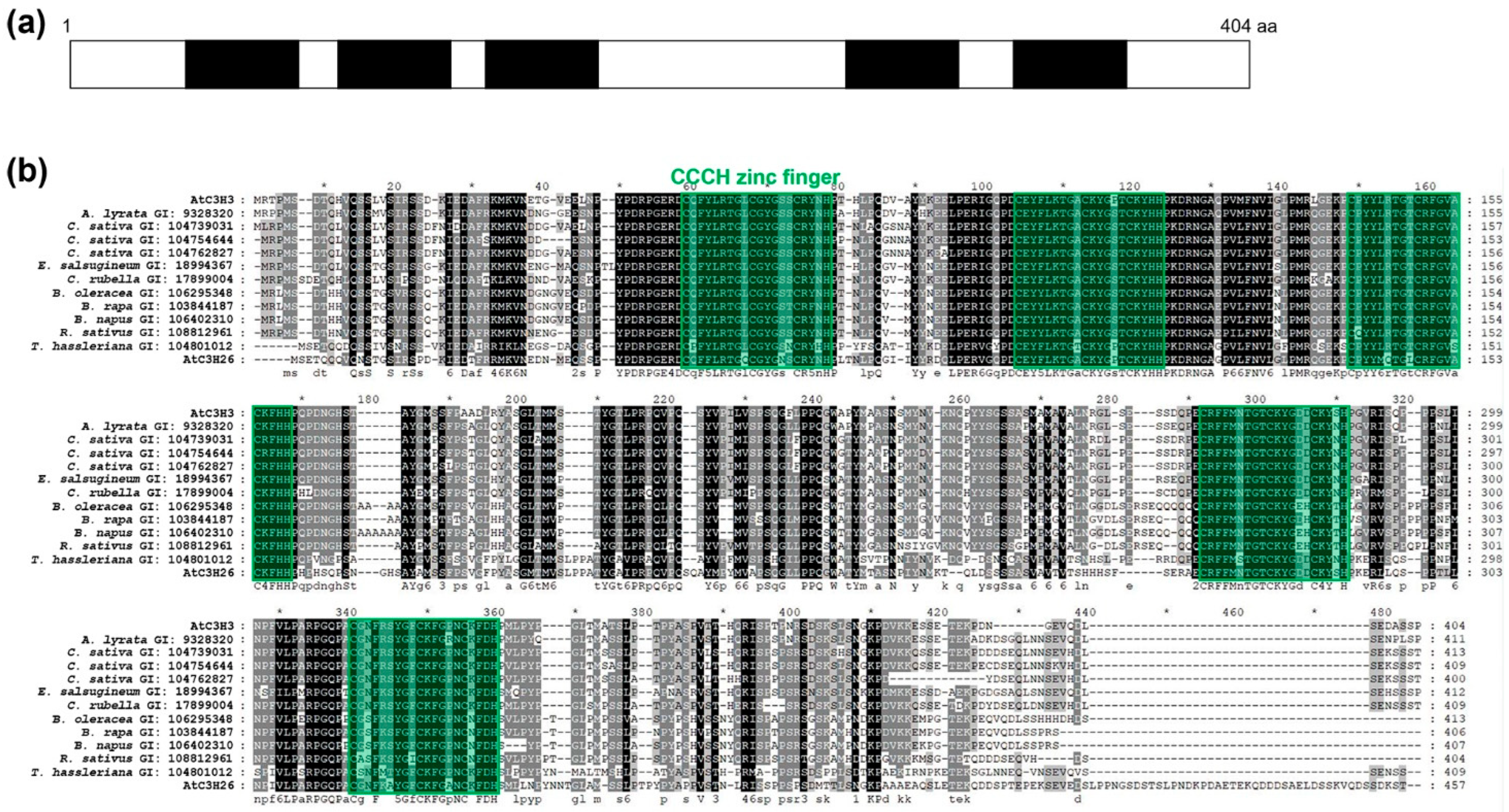
2.1. AtC3H3 Possesses Five CCCH Zinc-Finger Motifs
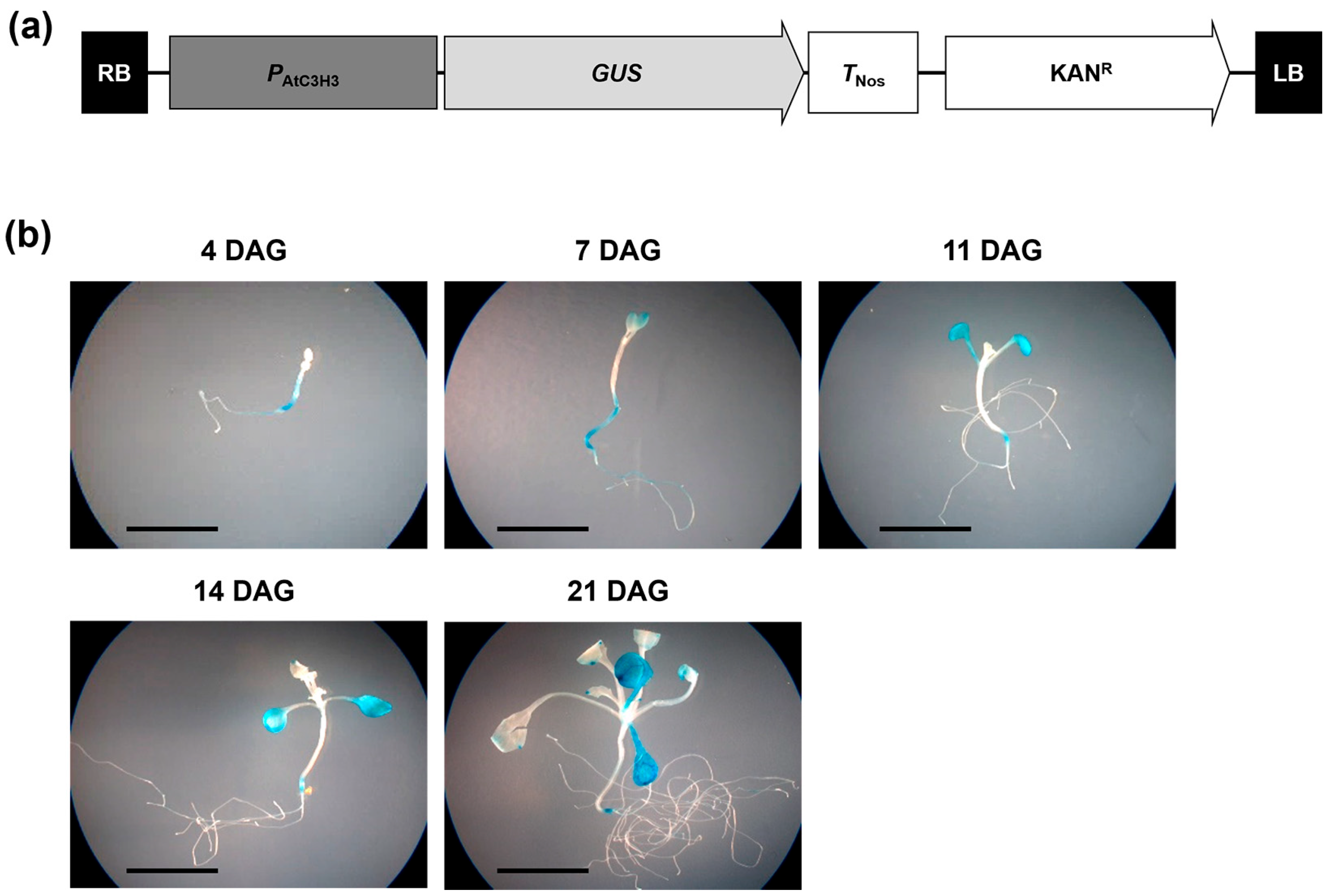
2.2. AtC3H3 Expression during Development and in Organs in Arabidopsis
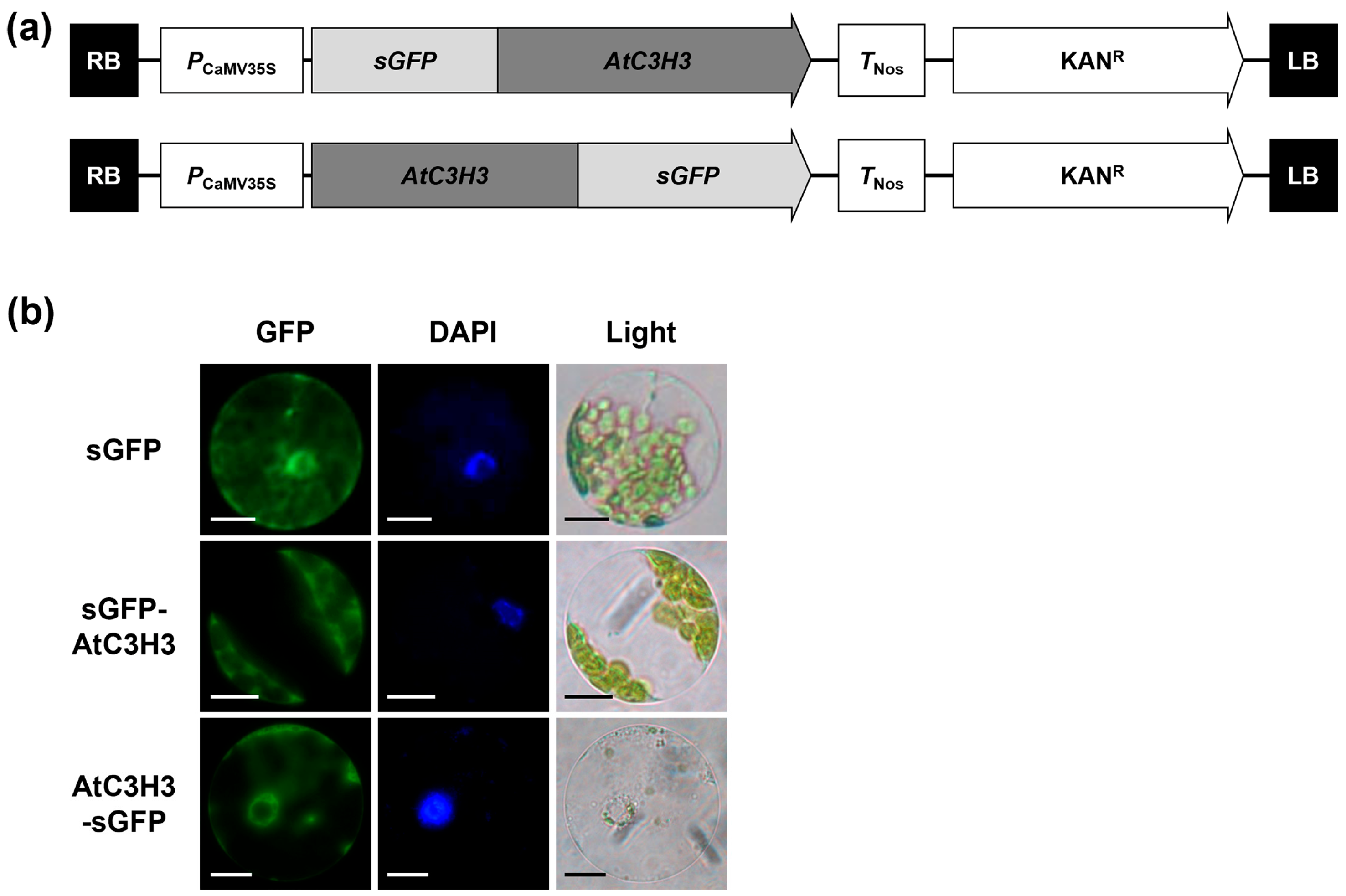
2.3. Subcellular Localization of AtC3H3 Protein
2.4. AtC3H3 Transcription Increases under Osmotic Stress Conditions
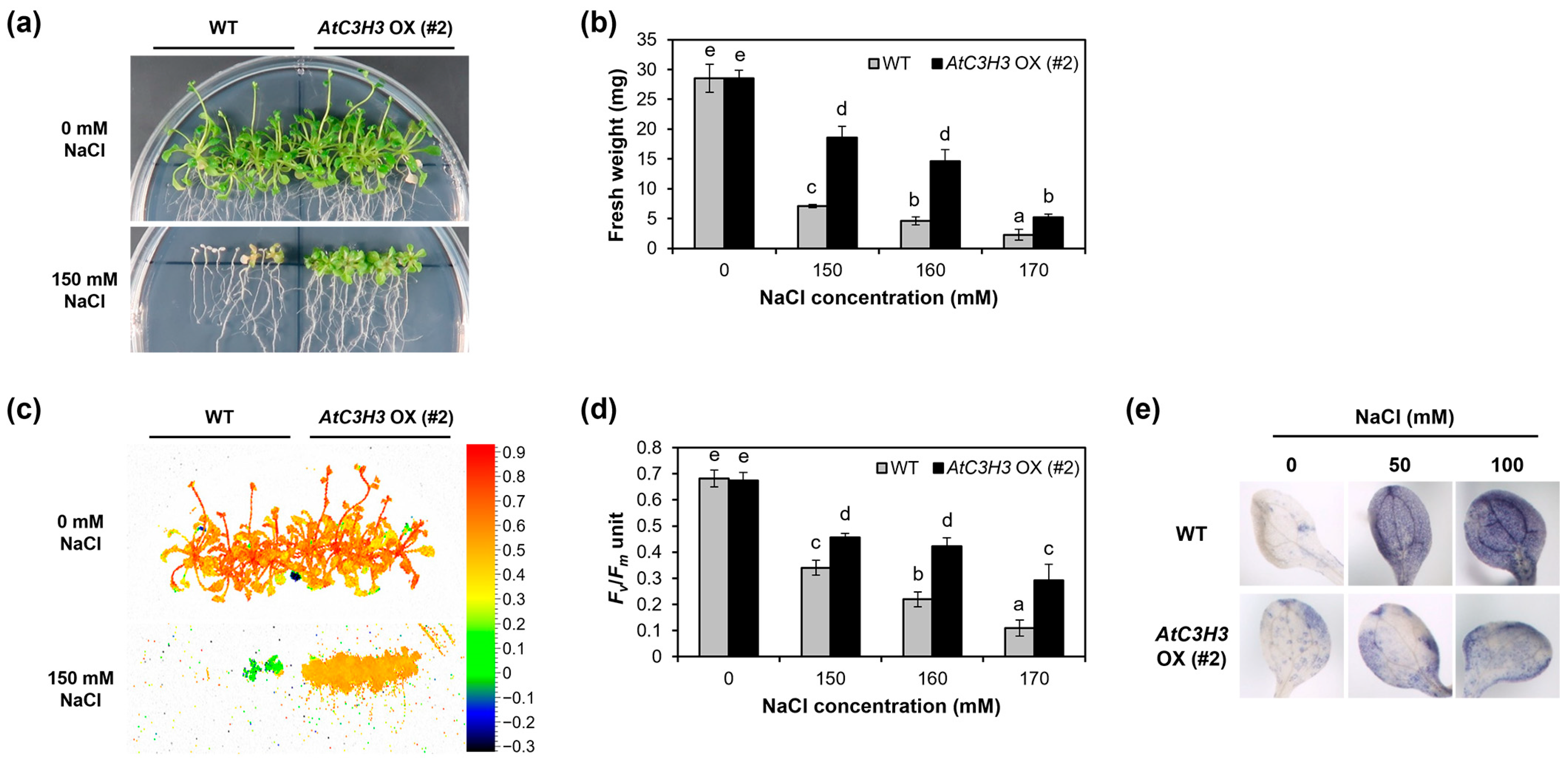
2.5. AtC3H3-Overexpressing Transgenic Plants Show Tolerance to Salt Stress
2.6. AtC3H3 OXs Show the Elevated Expression of Both ABA-Dependent and -Independent Salt Stress-Responsive Genes
2.7. Analysis of Target mRNAs of the RNase Function of AtC3H3 Using mRNA-Sequencing
3. Discussion
3.1. AtC3H3 OXs Show Salt Tolerance but Not Drought Tolerance
3.2. AtC3H3 Enhances Salt Tolerance by Increasing the Expression of Both ABA-Dependent and -Independent Salt Stress-Responsive Genes
3.3. Targets of the RNase Function of AtC3H3
4. Materials and Methods
4.1. Arabidopsis Growth
4.2. Multiple Sequence Alignment
4.3. Vector Construction
4.4. Transgenic Plants Generation
4.5. Stress Treatment
4.6. Histochemical Staining for Detection of Superoxide Production
4.7. Fv/Fm and SPAD Value Measurement
4.8. RNA Isolation and RT-PCR
4.9. Histochemical GUS Assay
4.10. Protoplast Transformation
4.11. Statistical Analysis
4.12. Library Preparation and mRNA-Seq
4.13. mRNA-Seq
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Borkotoky, S.; Saravanan, V.; Jaiswal, A.; Das, B.; Selvaraj, S.; Murali, A.; Lakshmi, P.T.V. The Arabidopsis stress responsive gene database. Int. J. Plant Genom. 2013, 2013, 949564. [Google Scholar] [CrossRef]
- Hussain, Q.; Asim, M.; Zhang, R.; Khan, R.; Farooq, S.; Wu, J. Transcription factors interact with ABA through gene expression and signaling pathways to mitigate drought and salinity stress. Biomolecules 2021, 11, 1159. [Google Scholar] [CrossRef]
- Soma, F.; Takahashi, F.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Cellular phosphorylation signaling and gene expression in drought stress responses: ABA-dependent and ABA-independent regulatory systems. Plants 2021, 10, 759. [Google Scholar] [CrossRef]
- Wang, D.; Guo, Y.; Wu, C.; Yang, G.; Li, Y.; Zheng, C. Genome-wide analysis of CCCH zinc finger family in Arabidopsis and rice. BMC Genom. 2008, 9, 44. [Google Scholar] [CrossRef]
- Seok, H.Y.; Woo, D.H.; Park, H.Y.; Lee, S.Y.; Tran, H.T.; Lee, E.H.; Nguyen, L.V.; Moon, Y.H. AtC3H17, a non-tandem CCCH zinc finger protein, functions as a nuclear transcriptional activator and has pleiotropic effects on vegetative development, flowering and seed development in Arabidopsis. Plant Cell Physiol. 2016, 57, 603–615. [Google Scholar] [CrossRef]
- Han, G.; Qiao, Z.; Li, Y.; Wang, C.; Wang, B. The Roles of CCCH zinc-finger proteins in plant abiotic stress tolerance. Int. J. Mol. Sci. 2021, 22, 8327. [Google Scholar] [CrossRef]
- Sun, J.; Jiang, H.; Xu, Y.; Li, H.; Wu, X.; Xie, Q.; Li, C. The CCCH-type zinc finger proteins AtSZF1 and AtSZF2 regulate salt stress responses in Arabidopsis. Plant Cell Physiol. 2007, 48, 1148–1158. [Google Scholar] [CrossRef]
- Bogamuwa, S.; Jang, J.C. The Arabidopsis tandem CCCH zinc finger proteins AtTZF4, 5 and 6 are involved in light-, abscisic acid- and gibberellic acid mediated regulation of seed germination. Plant Cell Environ. 2013, 36, 1507–1519. [Google Scholar] [CrossRef]
- Lee, S.J.; Jung, H.J.; Kang, H.; Kim, S.Y. Arabidopsis zinc finger proteins AtC3H49/AtTZF3 and AtC3H20/AtTZF2 are involved in ABA and JA responses. Plant Cell Physiol. 2012, 53, 673–686. [Google Scholar] [CrossRef]
- Seok, H.Y.; Nguyen, L.V.; Park, H.Y.; Tarte, V.N.; Ha, J.; Lee, S.Y.; Moon, Y.H. Arabidopsis non-TZF gene AtC3H17 functions as a positive regulator in salt stress response. Biochem. Biophys. Res. Commun. 2018, 498, 954–959. [Google Scholar] [CrossRef]
- Jan, A.; Maruyama, K.; Todaka, D.; Kidokoro, S.; Abo, M.; Yoshimura, E.; Shinozaki, K.; Nakashima, K.; Yamaguchi-Shinozaki, K. OsTZF1, a CCCH-tandem zinc finger protein, confers delayed senescence and stress tolerance in rice by regulating stress-related genes. Plant Physiol. 2013, 161, 1202–1216. [Google Scholar] [CrossRef]
- Guo, Y.H.; Yu, Y.P.; Wang, D.; Wu, C.A.; Yang, G.D.; Huang, J.G.; Zheng, C.C. GhZFP1, a novel CCCH-type zinc finger protein from cotton, enhances salt stress tolerance and fungal disease resistance in transgenic tobacco by interacting with GZIRD21A and GZIPR5. New Phytol. 2009, 183, 62–75. [Google Scholar] [CrossRef]
- Min, D.H.; Zhao, Y.; Huo, D.Y.; Li, L.C.; Chen, M.; Xu, Z.S.; Ma, Y.Z. Isolation and identification of a wheat gene encoding a zinc finger protein (TaZnFP) responsive to abiotic stresses. Acta Physiol. Plant. 2013, 35, 1597–1604. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, X.; Zhi, Y.; Li, X.; Zhang, Q.; Niu, J.; Wang, J.; Zhai, H.; Zhao, N.; Li, J.; et al. A non-tandem CCCH-type zinc-finger protein, IbC3H18, functions as a nuclear transcriptional activator and enhances abiotic stress tolerance in sweet potato. New Phytol. 2019, 223, 1918–1936. [Google Scholar] [CrossRef]
- Bai, H.; Lin, P.; Li, X.; Liao, X.; Wan, L.; Yang, X.; Luo, Y.; Zhang, L.; Zhang, F.; Liu, S.; et al. DgC3H1, a CCCH zinc finger protein gene, confers cold tolerance in transgenic chrysanthemum. Sci. Hortic. 2021, 281, 109901. [Google Scholar] [CrossRef]
- Pi, B.; He, X.; Ruan, Y.; Jang, J.C.; Huang, Y. Genome-wide analysis and stress-responsive expression of CCCH zinc finger family genes in Brassica rapa. BMC Plant Biol. 2018, 18, 373. [Google Scholar] [CrossRef]
- Pomeranz, M.C.; Hah, C.; Lin, P.C.; Kang, S.G.; Finer, J.J.; Blackshear, P.J.; Jang, J.C. The Arabidopsis tandem zinc finger protein AtTZF1 traffics between the nucleus and cytoplasmic foci and binds both DNA and RNA. Plant Physiol. 2010, 152, 151–165. [Google Scholar] [CrossRef]
- Zhang, J.; Addepalli, B.; Yun, K.Y.; Hunt, A.; Xu, R.; Rao, S.; Li, Q.Q.; Falcon, D.L. A polyadenylation factor subunit implicated in regulating oxidative signaling in Arabidopsis thaliana. PLoS ONE 2008, 3, e2410. [Google Scholar] [CrossRef]
- Chai, G.; Kong, Y.; Zhu, M.; Yu, L.; Qi, G.; Tang, X.; Wang, Z.; Cao, Y.; Yu, C.; Zhou, G. Arabidopsis C3H14 and C3H15 have overlapping roles in the regulation of secondary wall thickening and anther development. J. Exp. Bot. 2015, 66, 2595–2609. [Google Scholar] [CrossRef]
- Wu, Z.; Liang, J.; Li, T.; Zhang, D.; Teng, N. A LlMYB305-LlC3H18-LlWRKY33 module regulates thermotolerance in lily. Mol. Hortic. 2023, 3, 15. [Google Scholar] [CrossRef]
- Addepalli, B.; Hunt, A.G. Ribonuclease activity is a common property of Arabidopsis CCCH-containing zinc-finger proteins. FEBS Lett. 2008, 582, 2577–2582. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Potential biochemical indicators of salinity tolerance in plants. Plant Sci. 2004, 166, 3–16. [Google Scholar] [CrossRef]
- Ashraf, M. Biotechnological approach of improving plant salt tolerance using antioxidants as markers. Biotechnol. Adv. 2009, 27, 84–93. [Google Scholar] [CrossRef]
- Seong, S.Y.; Shim, J.S.; Bang, S.W.; Kim, J.K. Overexpression of OsC3H10, a CCCH-zinc finger, improves drought tolerance in rice by regulating stress-related genes. Plants 2020, 9, 1298. [Google Scholar] [CrossRef]
- Xie, Z.; Lin, W.; Yu, G.; Cheng, Q.; Xu, B.; Huang, B. Improved cold tolerance in switchgrass by a novel CCCH-type zinc finger transcription factor gene, PvC3H72, associated with ICE1–CBF–COR regulon and ABA-responsive genes. Biotechnol. Biofuels 2019, 12, 224. [Google Scholar] [CrossRef]
- Barreau, C.; Paillard, L.; Osborne, H.B. AU-rich elements and associated factors: Are there unifying principles? Nucleic Acids Res. 2005, 33, 7138–7150. [Google Scholar] [CrossRef]
- Lai, W.S.; Kennington, E.A.; Blackshear, P.J. Tristetraprolin and its family members can promote the cell-free deadenylation of AU-rich element-containing mRNAs by poly(A) ribonuclease. Mol. Cell. Biol. 2003, 23, 3798–3812. [Google Scholar] [CrossRef]
- Bogamuwa, S.; Jang, J. Plant tandem CCCH zinc finger proteins interact with ABA, drought, and stress response regulators in processing-bodies and stress granules. PLoS ONE 2016, 11, e0151574. [Google Scholar] [CrossRef]
- Jang, J. Arginine-rich motif-tandem CCCH zinc finger proteins in plant stress responses and post-transcriptional regulation of gene expression. Plant Sci. 2016, 252, 118–124. [Google Scholar] [CrossRef]
- Srivastava, M.; Duan, G.; Kershaw, N.J.; Athanasopoulos, V.; Yeo, J.H.C.; Ose, T.; Hu, D.; Brown, S.H.J.; Jergic, S.; Patel, H.R.; et al. Roquin binds microRNA-146a and Argonaute2 to regulate microRNA homeostasis. Nat. Commun. 2015, 6, 6253. [Google Scholar] [CrossRef]
- Suzuki, H.I.; Arase, M.; Matsuyama, H.; Choi, Y.L.; Ueno, T.; Mano, H.; Sugimoto, K.; Miyazono, K. MCPIP1 ribonuclease antagonizes dicer and terminates microRNA biogenesis through precursor microRNA degradation. Mol. Cell 2011, 44, 424–436. [Google Scholar] [CrossRef]
- Shi, Z.H.; Zhang, C.; Xu, X.F.; Zhu, J.; Zhou, Q.; Ma, L.J.; Niu, J.; Yang, Z.N. Overexpression of AtTTP affects ARF17 expression and leads to male sterility in Arabidopsis. PLoS ONE 2015, 10, e0117317. [Google Scholar] [CrossRef]
- Seok, H.Y.; Kim, T.; Lee, S.Y.; Moon, Y.H. Non-TZF transcriptional activator AtC3H12 negatively affects seed germination and seedling development in Arabidopsis. Int. J. Mol. Sci. 2022, 23, 1572. [Google Scholar] [CrossRef]
- Hofgen, R.; Willmitzer, L. Storage of competent cells for Agrobacterium transformation. Nucleic Acids Res. 1988, 16, 9877. [Google Scholar] [CrossRef] [PubMed]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.D.; Cho, Y.H.; Sheen, J. Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nat. Protoc. 2007, 2, 1565–1572. [Google Scholar] [CrossRef]
- Baek, H.S.; Kwon, T.U.; Shin, S.; Kwon, Y.J.; Chun, Y.J. Steroid sulfatase deficiency causes cellular senescence and abnormal differentiation by inducing Yippee-like 3 expression in human keratinocytes. Sci. Rep. 2021, 11, 20867. [Google Scholar] [CrossRef]
- FastQC. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 29 April 2022).
- FASTX-Toolkit. Available online: https://github.com/agordon/fastx_toolkit/ (accessed on 29 April 2022).
- BBMap. Available online: https://sourceforge.net/projects/bbmap/ (accessed on 29 April 2022).
- Trapnell, C.; Pachter, L.; Salzberg, S.L. TopHat: Discovering splice junctions with RNA-Seq. Bioinformatics 2009, 25, 1105–1111. [Google Scholar] [CrossRef]
- Roberts, A.; Trapnell, C.; Donaghey, J.; Rinn, J.L.; Pachter, L. Improving RNA-Seq expression estimates by correcting for fragment bias. Genome Biol. 2011, 12, R22. [Google Scholar] [CrossRef]
- The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 29 April 2022).




| Experiment | Up-Regulated Gene Number | Down-Regulated Gene Number |
|---|---|---|
| AtC3H3 OX NaCl vs WT NaCl | 10 | 11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seok, H.-Y.; Lee, S.-Y.; Nguyen, L.V.; Bayzid, M.; Jang, Y.; Moon, Y.-H. AtC3H3, an Arabidopsis Non-TZF Gene, Enhances Salt Tolerance by Increasing the Expression of Both ABA-Dependent and -Independent Stress-Responsive Genes. Int. J. Mol. Sci. 2024, 25, 10943. https://doi.org/10.3390/ijms252010943
Seok H-Y, Lee S-Y, Nguyen LV, Bayzid M, Jang Y, Moon Y-H. AtC3H3, an Arabidopsis Non-TZF Gene, Enhances Salt Tolerance by Increasing the Expression of Both ABA-Dependent and -Independent Stress-Responsive Genes. International Journal of Molecular Sciences. 2024; 25(20):10943. https://doi.org/10.3390/ijms252010943
Chicago/Turabian StyleSeok, Hye-Yeon, Sun-Young Lee, Linh Vu Nguyen, Md Bayzid, Yunseong Jang, and Yong-Hwan Moon. 2024. "AtC3H3, an Arabidopsis Non-TZF Gene, Enhances Salt Tolerance by Increasing the Expression of Both ABA-Dependent and -Independent Stress-Responsive Genes" International Journal of Molecular Sciences 25, no. 20: 10943. https://doi.org/10.3390/ijms252010943







