Stress-Responsive Gene Expression, Metabolic, Physiological, and Agronomic Responses by Consortium Nano-Silica with Trichoderma against Drought Stress in Bread Wheat
Abstract
1. Introduction
2. Results
2.1. Plant Growth-Promoting Traits of T. harzianum
2.2. Synthesis and Characterization of Green Synthesized Silica Nanoparticles (SiO2NPs)
2.3. Drought Responses of T. aestivum at Different Stages
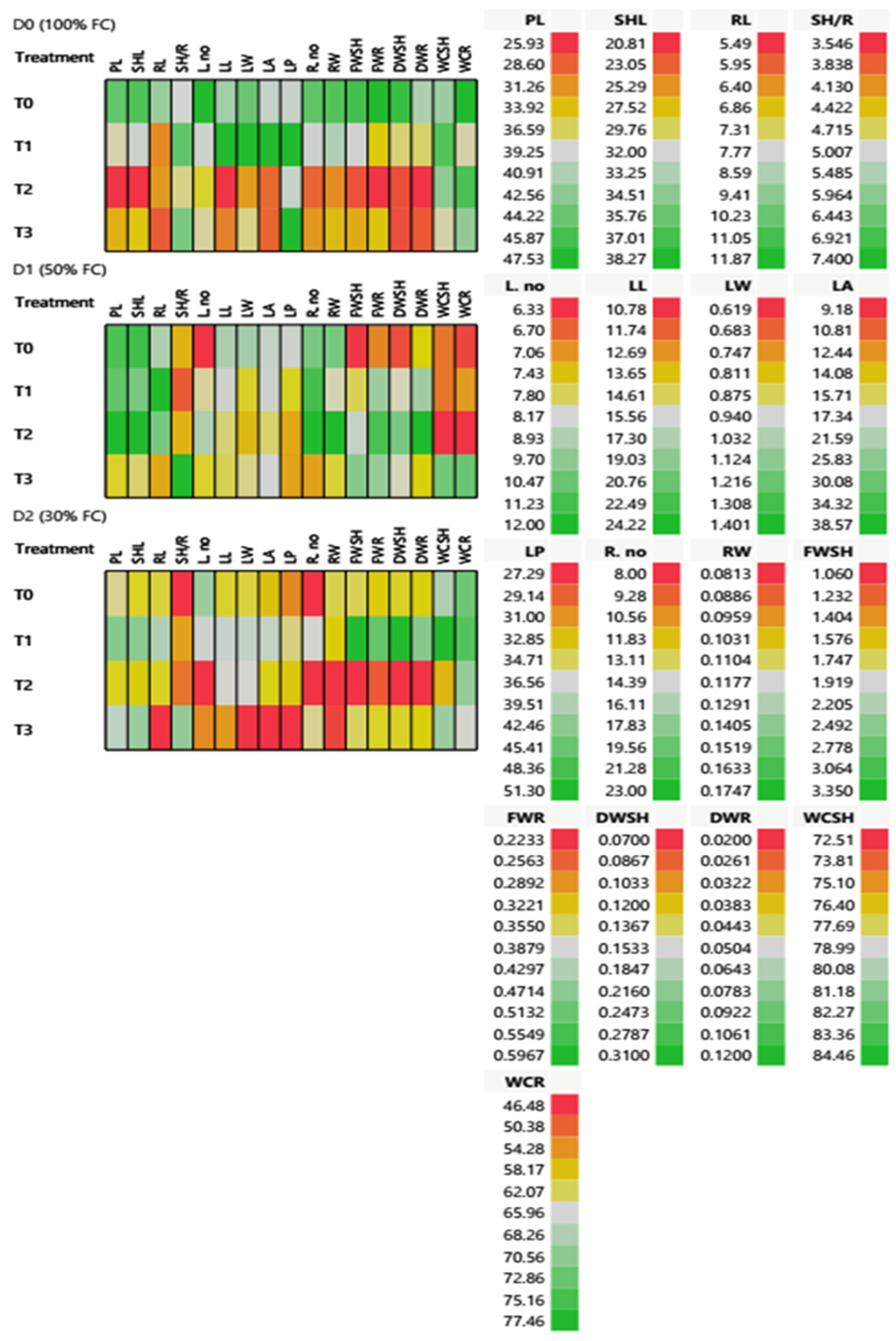
2.3.1. Morphological and Phenotypic Responses of T. aestivum at the Heading Stage under Different Water Regimes
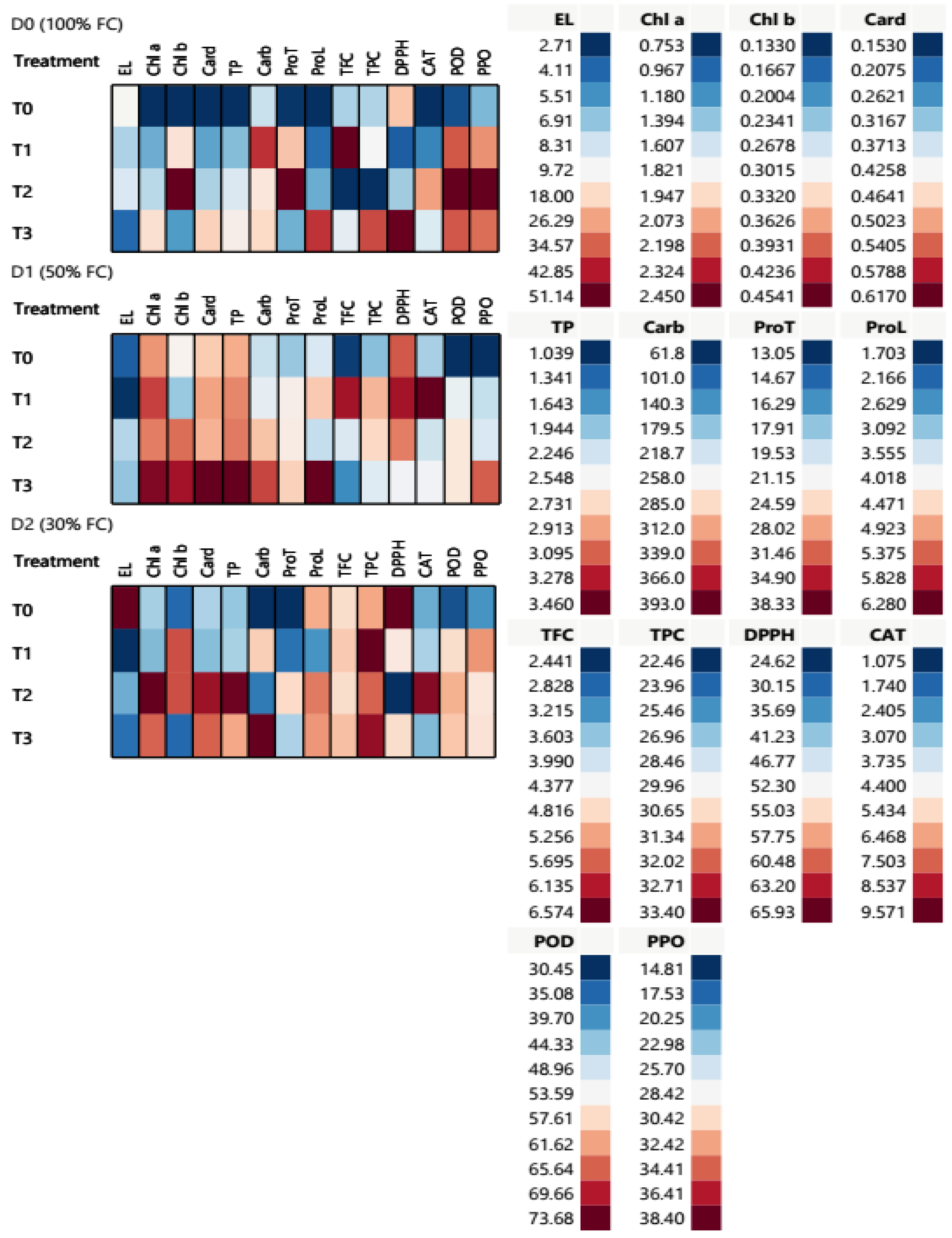
2.3.2. Physiological and Biochemical Parameter Response T. aestivum at the Heading Stage under Different Water Conditions
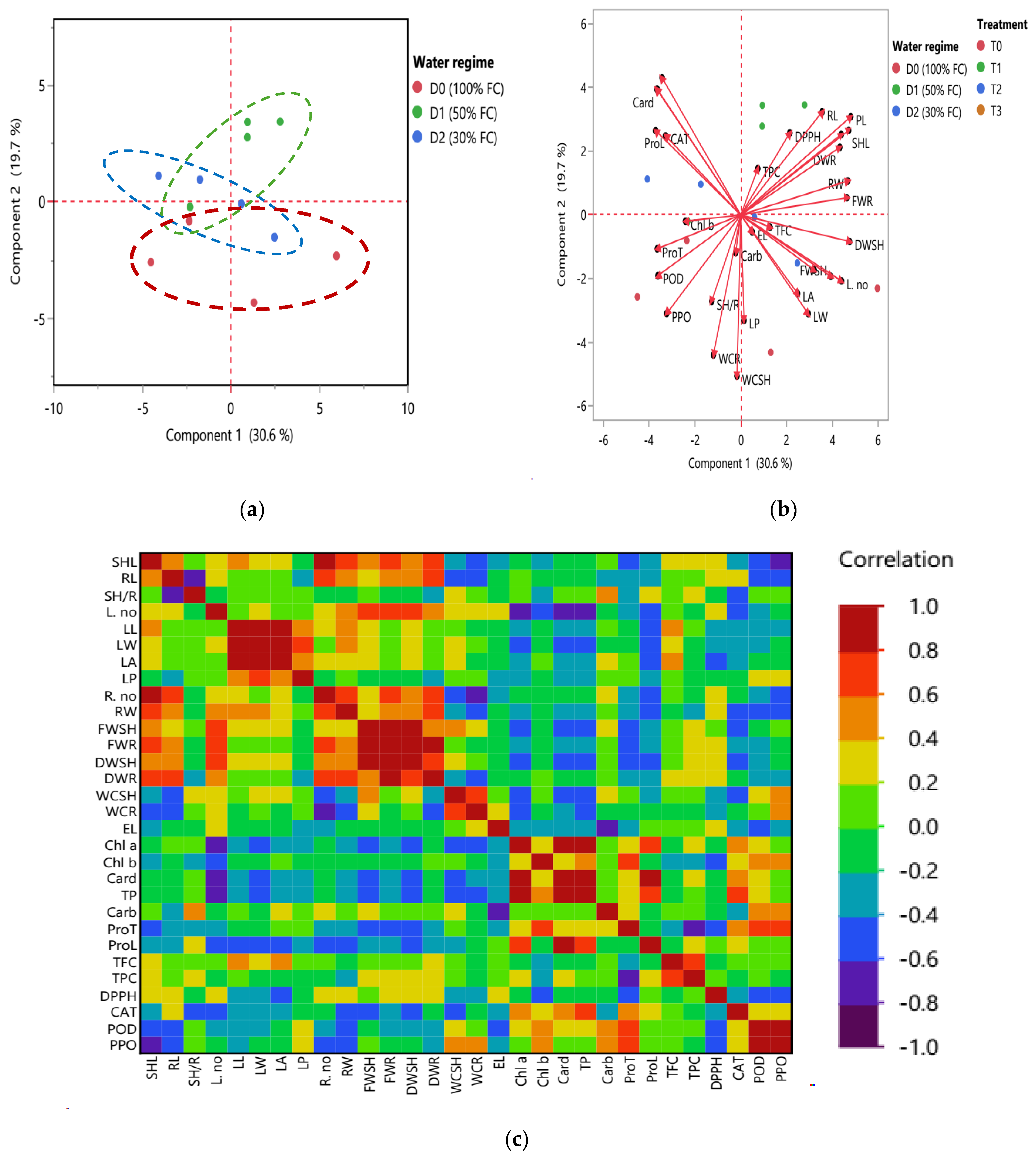
2.3.3. Multivariate Analysis and Inter-Correlation Based on Morpho-Physiological and Biochemical Traits at the Heading Stage: Principal Component Analysis (PCA) Biplot and Heatmap Partial Correlation
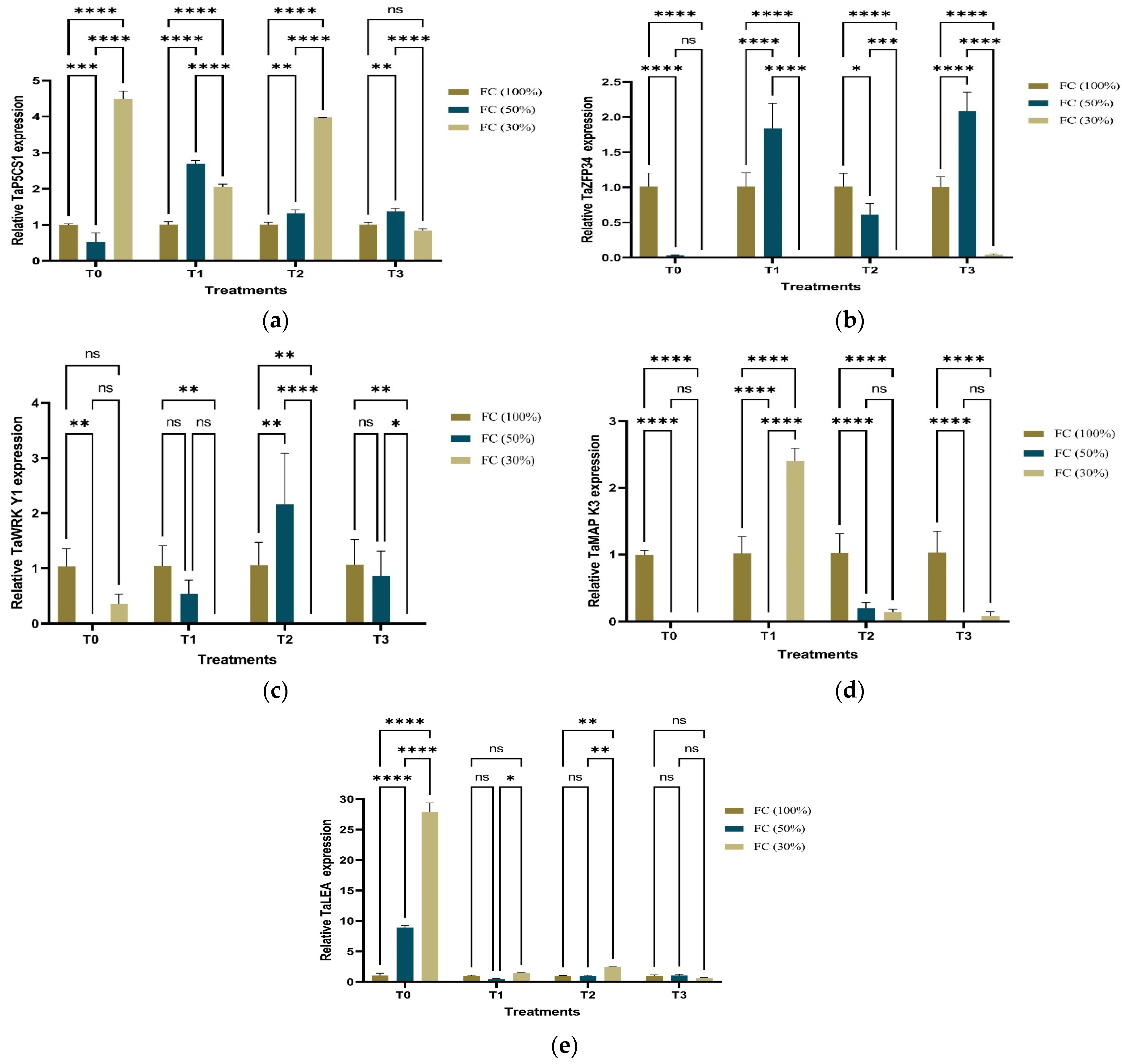
2.4. Expression Analysis: Quantitative RT-PCR of Five Drought-Responsive Genes
2.5. Yield-Related Agronomic Attributes of T. aestivum
2.5.1. Yield Traits with Multivariate Analysis
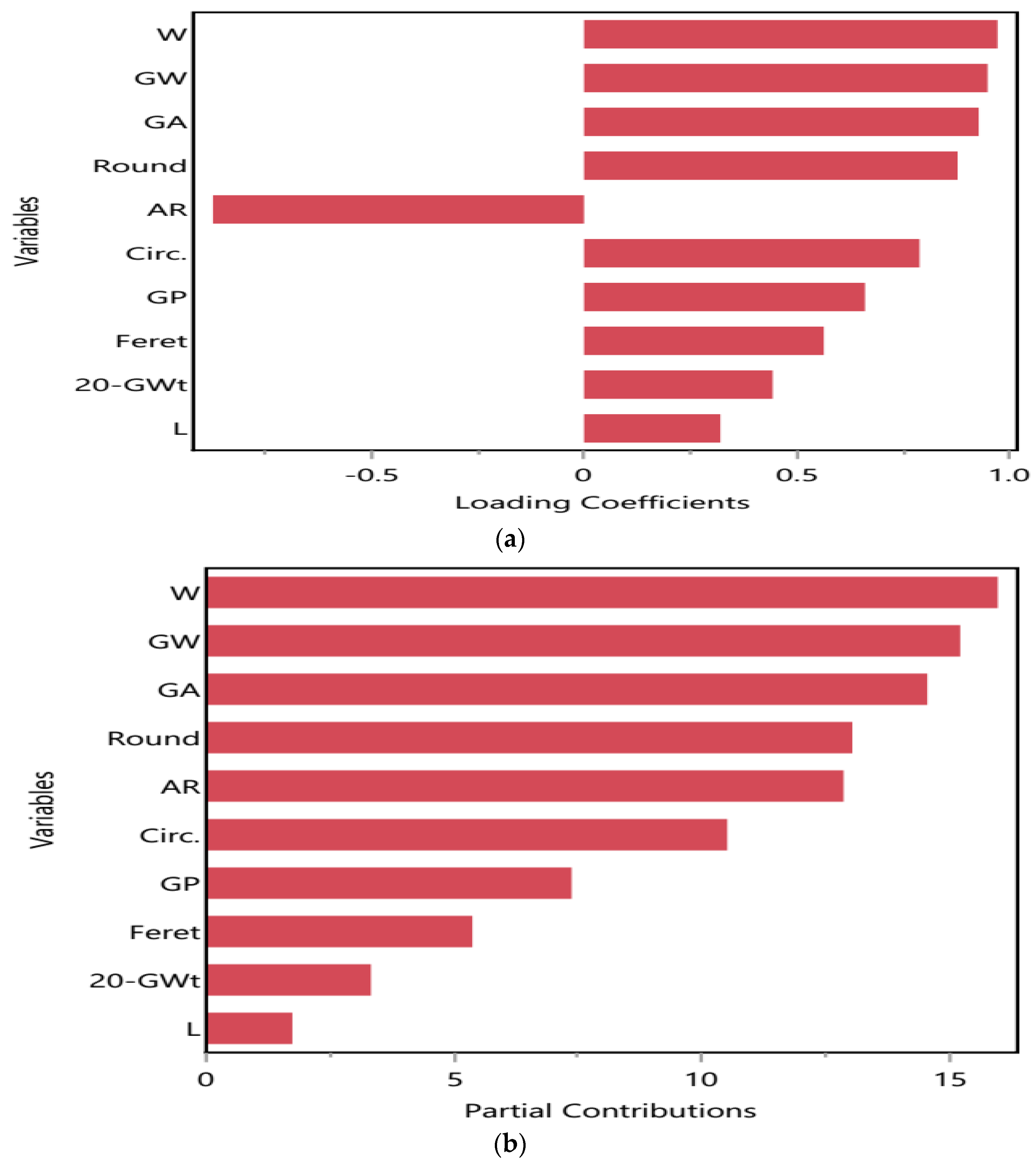
2.5.2. Kernel or Grain Attributes and Their Contribution to Drought Stress Tolerance
2.5.3. Drought Tolerance Indices and Their Intercorrelations with and Contributions to Drought Stress
2.6. Bioinformatic Analysis
2.6.1. Function Assessment of the qRT-PCR-Associated Genes
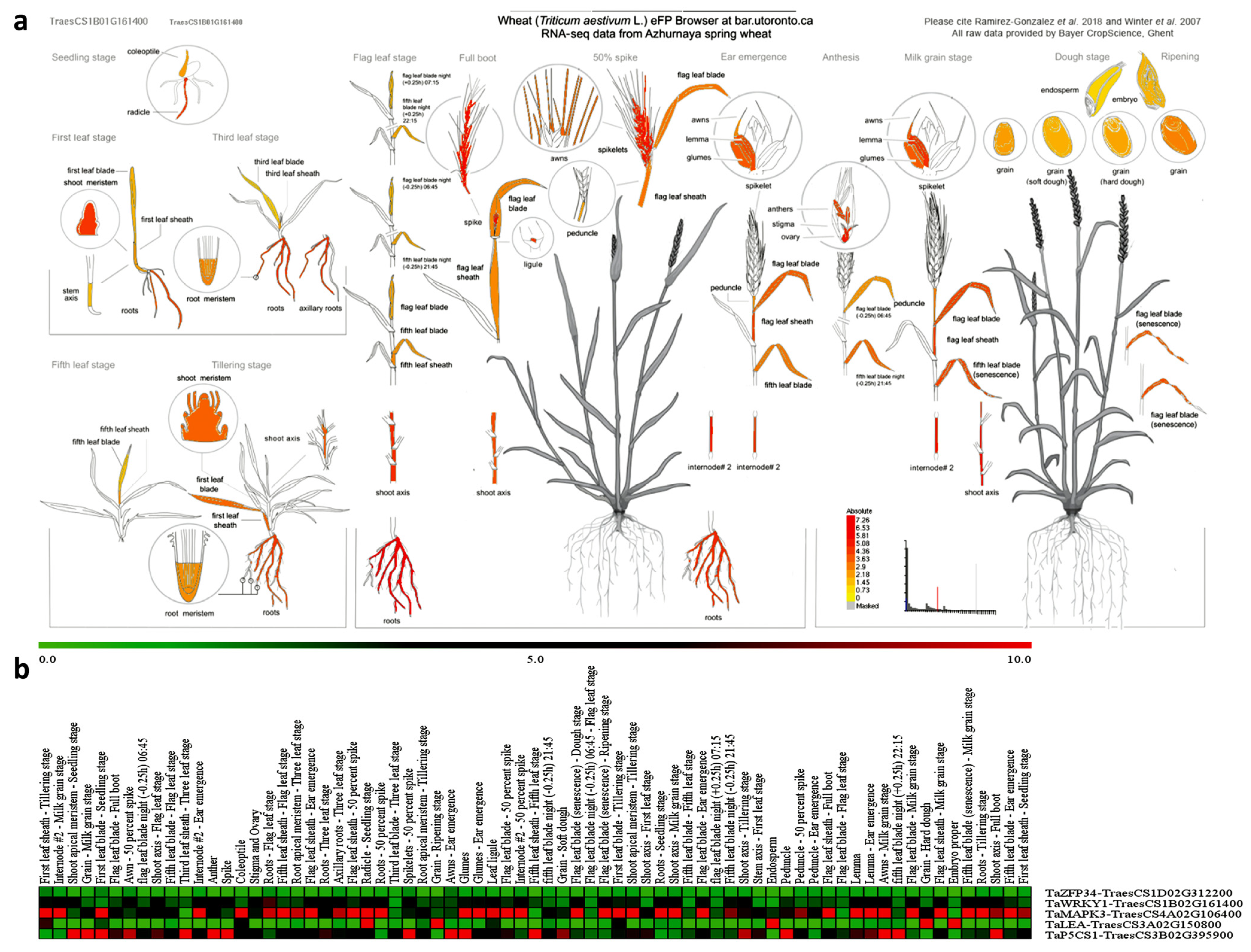
2.6.2. Putative Tissue Expression Patterns of Drought-Responsive Genes in T. aestivum
3. Discussion
4. Materials and Methods
4.1. Experimental Materials
4.2. Screening of Trichoderma Drought Tolerance
4.3. Testing the Effectiveness of T. harzianum as a Biostimulant: Plant Growth-Promoting Trait Characterization
Determination of Phytohormone-like Compounds
4.4. Determination of Nitrogen Fixation and Ammonia Production
4.5. Determination of Phosphate Solubilization Potential
4.6. Determination of Extracellular Enzymatic Activity
4.7. Eco-Friendly Rice Husk Ash Waste Silica Nanoparticle Synthesis: Preparation of Silica Oxide
4.8. Optical, Structural, and Morphological Characterizations of Nano-Silica
4.8.1. UV–Visible Spectroscopy Analysis and Zeta Potential
4.8.2. SEM-EDX Analysis: Scanning Electron Microscopy (SEM) and Energy-Dispersive X-ray (EDX)
4.8.3. Fourier Transform Infrared (FTIR) Spectroscopy, X-ray Diffraction (XRD), and Thermogravimetric Analysis (TGA) of Silica Nanoparticles
4.9. Field Experiment
4.9.1. Experimentation: Experimental Sit and Climate
4.9.2. Experimental Design and Treatments
4.9.3. Crop Husbandry
4.10. Morphological and Physiological Parameters of the Heading Stage
4.11. Biochemical Analyses
4.11.1. Determination of Total Proteins and Total Carbohydrates
4.11.2. Determination of Pigments and Total Proline
4.11.3. Determination of Antioxidant Enzymatic Activities
4.11.4. Determination of Non-Enzymatic Antioxidants
4.12. Molecular Analysis: Expression Analysis Using Quantitative RT-PCR
4.13. Yield-Related Agronomic Traits of Wheat
4.13.1. Yield Attributes
4.13.2. Grain Phenotypic Traits
4.13.3. Drought Tolerance Indices
4.14. Bioinformatics Approaches
4.14.1. Function Predictions of Wheat Genes Associated with Drought Responses
4.14.2. Potential Tissue Expression Patterns of the Target Genes
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramadas, S.; Kumari, B.; Khandoker, S.; Jalali, S.; Acharya, K.K.; Krishnappa, G.; Singh, G.; Joshi, A. Wheat in Asia: Trends, Challenges and Research Priorities; Springer: Berlin/Heidelberg, Germany, 2022; pp. 33–61. [Google Scholar]
- Erenstein, O.; Poole, N.; Donovan, J. Role of staple cereals in human nutrition: Separating the wheat from the chaff in the infodemics age. Trends Food Sci. Technol. 2022, 119, 508–513. [Google Scholar] [CrossRef]
- Asseng, S.; Foster, I.; Turner, N.C. The impact of temperature variability on wheat yields. Glob. Chang. Biol. 2011, 17, 997–1012. [Google Scholar] [CrossRef]
- Ortiz, R.; Ban, T.; Bandyopadhyay, R.; Bänziger, M.; Bergvinson, D.; Hell, K.; James, B.; Jeffers, D.; Kumar, L.; Menkir, A. CGIAR Research for Development Program on Mycotoxins; CABI: Wallingford, UK, 2008. [Google Scholar]
- El Sabagh, A.; Islam, M.S.; Skalicky, M.; Raza, M.; Singh, K.; Hossain, M.A.; Hossain, A.; Mahboob, W.; Iqbal, M.A.; Ratnasekera, D.; et al. Salinity Stress in Wheat (Triticum aestivum L.) in the Changing Climate: Adaptation and Management Strategies. Front. Agron. 2021, 3, 661932. [Google Scholar] [CrossRef]
- Karim, M.A.; Hamid, A.; Rahman, M.S. Grain growth and yield performance of wheat under subtropical conditions: I. Effect of sowing dates. Cereal Res. Commun. 1999, 27, 439–446. [Google Scholar] [CrossRef]
- Samad, N.; Bruno, V. The urgency of preserving water resources. Environ. News 2013, 21, 3–6. [Google Scholar]
- Luo, T.; Young, R.; Reig, P. Aqueduct projected water stress country rankings. Tech. Note 2015, 16, 1–16. [Google Scholar]
- Sowers, J.; Vengosh, A.; Weinthal, E. Climate change, water resources, and the politics of adaptation in the Middle East and North Africa. Clim. Chang. 2011, 104, 599–627. [Google Scholar] [CrossRef]
- Haque, M.I.; Khan, M.R. Impact of climate change on food security in Saudi Arabia: A roadmap to agriculture-water sustainability. J. Agribus. Dev. Emerg. Econ. 2022, 12, 1–18. [Google Scholar] [CrossRef]
- Sayed, H.I. Wheat land varieties collected from Saudi Arabia. I. Purification and classification. J. Coll. Agric. King Saud Univ. 1979, 1, 53–70. [Google Scholar]
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food Security: The Challenge of Feeding 9 Billion People. Science 2010, 327, 812–818. [Google Scholar] [CrossRef]
- Bhardwaj, D.; Ansari, M.; Sahoo, R.; Tuteja, N. Biofertilizers function as key player in sustainable agriculture by improving soil fertility, plant tolerance and crop productivity. Microb. Cell Factories 2014, 13, 66. [Google Scholar] [CrossRef] [PubMed]
- Salim, B.; Abou El-Yazied, A.; Salama, Y.; Raza, A.; Osman, H.S. Impact of silicon foliar application in enhancing antioxidants, growth, flowering and yield of squash plants under deficit irrigation condition. Ann. Agric. Sci. 2021, 66, 176–183. [Google Scholar] [CrossRef]
- Ghanem, H.E.; Al-Farouk, M. Wheat Drought Tolerance: Morpho-Physiological Criteria, Stress Indexes, and Yield Responses in Newly Sand Soils. J. Plant Growth Regul. 2024, 43, 2234–2250. [Google Scholar] [CrossRef]
- Daryanto, S.; Wang, L.; Jacinthe, P.-A. Global synthesis of drought effects on maize and wheat production. PLoS ONE 2016, 11, e0156362. [Google Scholar] [CrossRef]
- Ahmad, Z.; Waraich, E.A.; Akhtar, S.; Anjum, S.; Ahmad, T.; Mahboob, W.; Hafeez, O.B.A.; Tapera, T.; Labuschagne, M.; Rizwan, M. Physiological responses of wheat to drought stress and its mitigation approaches. Acta Physiol. Plant. 2018, 40, 80. [Google Scholar] [CrossRef]
- Ali, S.; Hayat, K.; Iqbal, A.; Xie, L. Implications of abscisic acid in the drought stress tolerance of plants. Agronomy 2020, 10, 1323. [Google Scholar] [CrossRef]
- Dawood, M.G. Stimulating plant tolerance against abiotic stress through seed priming. In Advances in Seed Priming; Springer: Singapore, 2018; pp. 147–183. [Google Scholar]
- Dutta, P. Seed priming: New vistas and contemporary perspectives. In Advances in Seed Priming; Springer: Singapore, 2018; pp. 3–22. [Google Scholar]
- Rani, S.; Kumar, P.; Suneja, P. Biotechnological interventions for inducing abiotic stress tolerance in crops. Plant Gene 2021, 27, 100315. [Google Scholar] [CrossRef]
- Sarkar, D.; Singh, S.; Parihar, M.; Rakshit, A. Seed bio-priming with microbial inoculants: A tailored approach towards improved crop performance, nutritional security, and agricultural sustainability for smallholder farmers. Curr. Res. Environ. Sustain. 2021, 3, 100093. [Google Scholar] [CrossRef]
- Dimkpa, C.; Weinand, T.; Asch, F. Plant–rhizobacteria interactions alleviate abiotic stress conditions. Plant Cell Environ. 2009, 32, 1682–1694. [Google Scholar] [CrossRef]
- Ren, A.; Li, X.; Han, R.; Yin, L.; Wei, M.; Gao, Y. Benefits of a symbiotic association with endophytic fungi are subject to water and nutrient availability in Achnatherum sibiricum. Plant Soil 2011, 346, 363–373. [Google Scholar] [CrossRef]
- Xie, L.; He, X.; Wang, K.; Hou, L.; Sun, Q. Spatial dynamics of dark septate endophytes in the roots and rhizospheres of Hedysarum scoparium in northwest China and the influence of edaphic variables. Fungal Ecol. 2017, 26, 135–143. [Google Scholar] [CrossRef]
- Brotman, Y.; Landau, U.; Cuadros-Inostroza, Á.; Takayuki, T.; Fernie, A.R.; Chet, I.; Viterbo, A.; Willmitzer, L. Trichoderma-plant root colonization: Escaping early plant defense responses and activation of the antioxidant machinery for saline stress tolerance. PLoS Pathog. 2013, 9, e1003221. [Google Scholar] [CrossRef]
- Poveda, J.; Díaz-González, S.; Díaz-Urbano, M.; Velasco, P.; Sacristán, S. Fungal endophytes of Brassicaceae: Molecular interactions and crop benefits. Front. Plant Sci. 2022, 13, 932288. [Google Scholar] [CrossRef]
- Samolski, I.; Rincon, A.M.; Pinzon, L.M.; Viterbo, A.; Monte, E. The qid74 gene from Trichoderma harzianum has a role in root architecture and plant biofertilization. Microbiology 2012, 158, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Carrero-Carrón, I.; Rubio, M.; Niño-Sánchez, J.; Navas-Cortés, J.; Jiménez-Díaz, R.; Monte, E.; Hermosa, R. Interactions between Trichoderma harzianum and defoliating Verticillium dahliae in resistant and susceptible wild olive clones. Plant Pathol. 2018, 67, 1758–1767. [Google Scholar] [CrossRef]
- Irshad, K.; Siddiqui, Z.S.; Chen, J.; Rao, Y.; Ansari, H.; Wajid, D.; Nida, K.; Wei, X. Bio-priming with salt tolerant endophytes improved crop tolerance to salt stress via modulating photosystem II and antioxidant activities in a sub-optimal environment. Front. Plant Sci. 2023, 14, 1082480. [Google Scholar] [CrossRef]
- Estrada, B.; Aroca, R.; Barea, J.M.; Ruiz-Lozano, J.M. Native arbuscular mycorrhizal fungi isolated from a saline habitat improved maize antioxidant systems and plant tolerance to salinity. Plant Sci. 2013, 201, 42–51. [Google Scholar] [CrossRef]
- Illescas, M.; Pedrero-Méndez, A.; Pitorini-Bovolini, M.; Hermosa, R.; Monte, E. Phytohormone production profiles in Trichoderma species and their relationship to wheat plant responses to water stress. Pathogens 2021, 10, 991. [Google Scholar] [CrossRef]
- Snehal, S.; Lohani, P. Silica nanoparticles: Its green synthesis and importance in agriculture. J. Pharmacogn. Phytochem. 2018, 7, 3383–3393. [Google Scholar]
- Chandrasekhar, S.; Pramada, P.; Majeed, J. Effect of calcination temperature and heating rate on the optical properties and reactivity of rice husk ash. J. Mater. Sci. 2006, 41, 7926–7933. [Google Scholar] [CrossRef]
- Gautam, A.; Batra, R.; Singh, N. A Study on Use of Rice Husk Ash in Concrete. Eng. Herit. J. 2019, 3, 1–4. [Google Scholar] [CrossRef]
- Thiedeitz, M.; Schmidt, W.; Härder, M.; Kränkel, T. Performance of rice husk ash as supplementary cementitious material after production in the field and in the lab. Materials 2020, 13, 4319. [Google Scholar] [CrossRef] [PubMed]
- Liou, T.-H.; Wu, S.-J. Characteristics of microporous/mesoporous carbons prepared from rice husk under base-and acid-treated conditions. J. Hazard. Mater. 2009, 171, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Chen, B.; Wang, Q.; Shi, X.; Xiao, Z.; Lin, J.; Fang, X. Carbon nanotubes as molecular transporters for walled plant cells. Nano Lett. 2009, 9, 1007–1010. [Google Scholar] [CrossRef] [PubMed]
- Raza, M.A.S.; Zulfiqar, B.; Iqbal, R.; Muzamil, M.N.; Aslam, M.U.; Muhammad, F.; Amin, J.; Aslam, H.M.U.; Ibrahim, M.A.; Uzair, M. Morpho-physiological and biochemical response of wheat to various treatments of silicon nano-particles under drought stress conditions. Sci. Rep. 2023, 13, 2700. [Google Scholar] [CrossRef]
- Parveen, N.; Ashraf, M. Role of silicon in mitigating the adverse effects of salt stress on growth and photosynthetic attributes of two maize (Zea mays L.) cultivars grown hydroponically. Pak. J. Bot. 2010, 42, 1675–1684. [Google Scholar]
- Siddiqui, M.; Al-Whaibi, M.; Firoz, M.; Al-Khaishany, M. Role of Nanoparticles in Plants. In Nanotechnology and Plant Sciences; Springer: Cham, Switzerland, 2015; pp. 19–35. [Google Scholar]
- Abbasi Khalaki, M.; Ghorbani, A.; Dadjou, F. Influence of nano-priming on Festuca ovina seed germination and early seedling traits under drought stress, in laboratory condition. Ecopersia 2019, 7, 133–139. [Google Scholar]
- Kulkarni, M.; Soolanayakanahally, R.; Ogawa, S.; Uga, Y.; Selvaraj, M.G.; Kagale, S. Drought response in wheat: Key genes and regulatory mechanisms controlling root system architecture and transpiration efficiency. Front. Chem. 2017, 5, 106. [Google Scholar] [CrossRef]
- Paolacci, A.R.; Tanzarella, O.A.; Porceddu, E.; Ciaffi, M. Identification and validation of reference genes for quantitative RT-PCR normalization in wheat. BMC Mol. Biol. 2009, 10, 11. [Google Scholar] [CrossRef]
- Ramírez-González, R.; Borrill, P.; Lang, D.; Harrington, S.; Brinton, J.; Venturini, L.; Davey, M.; Jacobs, J.; Van Ex, F.; Pasha, A.; et al. The transcriptional landscape of polyploid wheat. Science 2018, 361, eaar6089. [Google Scholar] [CrossRef]
- Winter, D.; Vinegar, B.; Nahal, H.; Ammar, R.; Wilson, G.V.; Provart, N.J. An “Electronic Fluorescent Pictograph” browser for exploring and analyzing large-scale biological data sets. PLoS ONE 2007, 2, e718. [Google Scholar] [CrossRef] [PubMed]
- Akbari, S.I.; Prismantoro, D.; Permadi, N.; Rossiana, N.; Miranti, M.; Mispan, M.S.; Mohamed, Z.; Doni, F. Bioprospecting the roles of Trichoderma in alleviating plants’ drought tolerance: Principles, mechanisms of action, and prospects. Microbiol. Res. 2024, 283, 127665. [Google Scholar] [CrossRef] [PubMed]
- Esmail, S.M.; Omar, G.E.; Mourad, A.M. In-depth understanding of the genetic control of stripe rust resistance (Puccinia striiformis f. sp. tritici) induced in wheat (Triticum aestivum) by Trichoderma asperellum T34. Plant Dis. 2023, 107, 457–472. [Google Scholar] [CrossRef] [PubMed]
- Abdenaceur, R.; Farida, B.-t.; Mourad, D.; Rima, H.; Zahia, O.; Fatma, S.-H. Effective biofertilizer Trichoderma spp. isolates with enzymatic activity and metabolites enhancing plant growth. Int. Microbiol. 2022, 25, 817–829. [Google Scholar] [CrossRef]
- Zhang, S.; Gan, Y.; Xu, B. Mechanisms of the IAA and ACC-deaminase producing strain of Trichoderma longibrachiatum T6 in enhancing wheat seedling tolerance to NaCl stress. BMC Plant Biol. 2019, 19, 22. [Google Scholar] [CrossRef]
- Pedrero-Méndez, A.; Insuasti, H.C.; Neagu, T.; Illescas, M.; Rubio, M.B.; Monte, E.; Hermosa, R. Why is the correct selection of Trichoderma strains important? The case of wheat endophytic strains of T. harzianum and T. simmonsii. J. Fungi 2021, 7, 1087. [Google Scholar] [CrossRef]
- Bach, E.; dos Santos Seger, G.D.; de Carvalho Fernandes, G.; Lisboa, B.B.; Passaglia, L.M.P. Evaluation of biological control and rhizosphere competence of plant growth promoting bacteria. Appl. Soil Ecol. 2016, 99, 141–149. [Google Scholar] [CrossRef]
- Vaccaro, F.; Passeri, I.; Ajijah, N.; Bettini, P.; Courty, P.E.; Dębiec-Andrzejewska, K.; Joshi, N.; Kowalewska, Ł.; Stasiuk, R.; Musiałowski, M. Genotype-by-genotype interkingdom cross-talk between symbiotic nitrogen fixing Sinorhizobium meliloti strains and Trichoderma species. Microbiol. Res. 2024, 285, 127768. [Google Scholar] [CrossRef]
- de Oliveira, H.P.; de Melo, R.O.; Cavalcante, V.S.; Monteiro, T.S.A.; de Freitas, L.G.; Lambers, H.; Valadares, S.V. Phosphate fertilizers coated with phosphate-solubilising Trichoderma harzianum increase phosphorus uptake and growth of Zea mays. In Plant and Soil; Springer: Berlin/Heidelberg, Germany, 2024; pp. 1–12. [Google Scholar]
- Suárez, M.B.; Sanz, L.; Chamorro, M.I.; Rey, M.; González, F.J.; Llobell, A.; Monte, E. Proteomic analysis of secreted proteins from Trichoderma harzianum: Identification of a fungal cell wall-induced aspartic protease. Fungal Genet. Biol. 2005, 42, 924–934. [Google Scholar] [CrossRef]
- Rahman, M.; Borah, S.M.; Borah, P.K.; Bora, P.; Sarmah, B.K.; Lal, M.K.; Tiwari, R.K.; Kumar, R. Deciphering the antimicrobial activity of multifaceted rhizospheric biocontrol agents of solanaceous crops viz., Trichoderma harzianum MC2, and Trichoderma harzianum NBG. Front. Plant Sci. 2023, 14, 1141506. [Google Scholar] [CrossRef]
- Ali, M.; Drea, A.-A. Green synthesis and characterization of antibiotic amorphous nano silicon oxide powder extracted from rice husk ash. Int. J. Curr. Res. Rev. 2021, 13, 94–99. [Google Scholar] [CrossRef]
- A Ajeel, S.; A Sukkar, K.; K Zedin, N. Chemical extraction process for producing high purity nanosilica from Iraqi rice husk. Eng. Technol. J. 2021, 39, 56–63. [Google Scholar] [CrossRef]
- Imoisili, P.E.; Ukoba, K.O.; Jen, T.-C. Green technology extraction and characterization of silica nanoparticles from palm kernel shell ash via sol–gel. J. Mater. Res. Technol. 2020, 9, 307–313. [Google Scholar] [CrossRef]
- Gul, S.; Abideen, Z.; Adnan, M.Y.; Hanif, M.; Nawaz, M.F.; El-Keblawy, A. Seaweed-derived bio-stimulant improves growth and salt tolerance of radish varieties under saline conditions. Biocatal. Agric. Biotechnol. 2023, 52, 102822. [Google Scholar] [CrossRef]
- George, T.S.; Bulgarelli, D.; Carminati, A.; Chen, Y.; Jones, D.; Kuzyakov, Y.; Schnepf, A.; Wissuwa, M.; Roose, T. Bottom-up perspective–The role of roots and rhizosphere in climate change adaptation and mitigation in agroecosystems. Plant Soil 2024, 500, 297–323. [Google Scholar] [CrossRef]
- Anwar, A.; Kim, J.-K. Transgenic breeding approaches for improving abiotic stress tolerance: Recent progress and future perspectives. Int. J. Mol. Sci. 2020, 21, 2695. [Google Scholar] [CrossRef]
- Verma, N.; Sao, P.; Srivastava, A.; Singh, S. Physiological and molecular responses to drought, submergence and excessive watering in plants. In Harsh Environment and Plant Resilience; Springer: Cham, Switzerland, 2021; pp. 305–321. [Google Scholar]
- Xiong, S.; Wang, Y.; Chen, Y.; Gao, M.; Zhao, Y.; Wu, L. Effects of drought stress and rehydration on physiological and biochemical properties of four oak species in China. Plants 2022, 11, 679. [Google Scholar] [CrossRef]
- Estévez-Geffriaud, V.; Vicente, R.; Vergara-Díaz, O.; Narváez Reinaldo, J.J.; Trillas, M.I. Application of Trichoderma asperellum T34 on maize (Zea mays) seeds protects against drought stress. Planta 2020, 252, 8. [Google Scholar] [CrossRef]
- Flexas, J.; Medrano, H. Drought-inhibition of photosynthesis in C3 plants: Stomatal and non-stomatal limitations revisited. Ann. Bot. 2002, 89, 183–189. [Google Scholar] [CrossRef]
- Alharbi, K.; Rashwan, E.; Mohamed, H.H.; Awadalla, A.; Omara, A.E.-D.; Hafez, E.M.; Alshaal, T. Application of silica nanoparticles in combination with two bacterial strains improves the growth, antioxidant capacity and production of barley irrigated with saline water in salt-affected soil. Plants 2022, 11, 2026. [Google Scholar] [CrossRef]
- Yang, Y.; Song, H.; Yao, P.; Zhang, S.; Jia, H.; Ye, X. NtLTPI. 38, a plasma membrane-localized protein, mediates lipid metabolism and salt tolerance in Nicotiana tabacum. Int. J. Biol. Macromol. 2023, 242, 125007. [Google Scholar] [CrossRef] [PubMed]
- Ologundudu, F. Antioxidant enzymes and non-enzymatic antioxidants as defense mechanism of salinity stress in cowpea (Vigna unguiculata L. Walp)—Ife brown and Ife bpc. Bull. Natl. Res. Cent. 2021, 45, 152. [Google Scholar] [CrossRef]
- Sah, S.K.; Reddy, K.R.; Li, J. Abscisic acid and abiotic stress tolerance in crop plants. Front. Plant Sci. 2016, 7, 571. [Google Scholar] [CrossRef]
- Mega, R.; Abe, F.; Kim, J.-S.; Tsuboi, Y.; Tanaka, K.; Kobayashi, H.; Sakata, Y.; Hanada, K.; Tsujimoto, H.; Kikuchi, J. Tuning water-use efficiency and drought tolerance in wheat using abscisic acid receptors. Nat. Plants 2019, 5, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Strizhov, N.; Ábrahám, E.; Ökrész, L.; Blickling, S.; Zilberstein, A.; Schell, J.; Koncz, C.; Szabados, L. Differential expression of two P5CS genes controlling proline accumulation during salt-stress requires ABA and is regulated by ABA1, ABI1 and AXR2 in Arabidopsis. Plant J. 1997, 12, 557–569. [Google Scholar] [CrossRef]
- Rodziewicz, P.; Swarcewicz, B.; Chmielewska, K.; Wojakowska, A.; Stobiecki, M. Influence of abiotic stresses on plant proteome and metabolome changes. Acta Physiol. Plant. 2014, 36, 1–19. [Google Scholar] [CrossRef]
- Itam, M.; Mega, R.; Tadano, S.; Abdelrahman, M.; Matsunaga, S.; Yamasaki, Y.; Akashi, K.; Tsujimoto, H. Metabolic and physiological responses to progressive drought stress in bread wheat. Sci. Rep. 2020, 10, 17189. [Google Scholar] [CrossRef]
- Kam, J.; Gresshoff, P.M.; Shorter, R.; Xue, G.-P. The Q-type C2H2 zinc finger subfamily of transcription factors in Triticum aestivum is predominantly expressed in roots and enriched with members containing an EAR repressor motif and responsive to drought stress. Plant Mol. Biol. 2008, 67, 305–322. [Google Scholar] [CrossRef]
- Sharp, R.E.; Poroyko, V.; Hejlek, L.G.; Spollen, W.G.; Springer, G.K.; Bohnert, H.J.; Nguyen, H.T. Root growth maintenance during water deficits: Physiology to functional genomics. J. Exp. Bot. 2004, 55, 2343–2351. [Google Scholar] [CrossRef]
- Rauf, M.; Munir, M.; ul Hassan, M.; Ahmad, M.; Afzal, M. Performance of wheat genotypes under osmotic stress at germination and early seedling growth stage. Afr. J. Biotechnol. 2007, 6, 8. [Google Scholar]
- Xu, W.; Jia, L.; Shi, W.; Liang, J.; Zhou, F.; Li, Q.; Zhang, J. Abscisic acid accumulation modulates auxin transport in the root tip to enhance proton secretion for maintaining root growth under moderate water stress. New Phytol. 2013, 197, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Meister, R.; Rajani, M.; Ruzicka, D.; Schachtman, D.P. Challenges of modifying root traits in crops for agriculture. Trends Plant Sci. 2014, 19, 779–788. [Google Scholar] [CrossRef]
- Chang, H.; Chen, D.; Kam, J.; Richardson, T.; Drenth, J.; Guo, X.; McIntyre, C.L.; Chai, S.; Rae, A.L.; Xue, G.-P. Abiotic stress upregulated TaZFP34 represses the expression of type-B response regulator and SHY2 genes and enhances root to shoot ratio in wheat. Plant Sci. 2016, 252, 88–102. [Google Scholar] [CrossRef]
- Yu, Y.; Song, T.; Wang, Y.; Zhang, M.; Li, N.; Yu, M.; Zhang, S.; Zhou, H.; Guo, S.; Bu, Y. The wheat WRKY transcription factor TaWRKY1-2D confers drought resistance in transgenic Arabidopsis and wheat (Triticum aestivum L.). Int. J. Biol. Macromol. 2023, 226, 1203–1217. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, L. Overexpression of TaWRKY46 enhances drought tolerance in transgenic wheat. Cereal Res. Commun. 2022, 50, 679–688. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, T.F.; Li, Y.T.; Zheng, L.; Lu, Z.W.; Zhou, Y.B.; Chen, J.; Chen, M.; Zhang, J.P.; Sun, G.Z. Mitogen-activated protein kinase TaMPK3 suppresses ABA response by destabilising TaPYL4 receptor in wheat. New Phytol. 2022, 236, 114–131. [Google Scholar] [CrossRef] [PubMed]
- Zan, T.; Li, L.; Li, J.; Zhang, L.; Li, X. Genome-wide identification and characterization of late embryogenesis abundant protein-encoding gene family in wheat: Evolution and expression profiles during development and stress. Gene 2020, 736, 144422. [Google Scholar] [CrossRef]
- Silletti, S.; Di Stasio, E.; Van Oosten, M.J.; Ventorino, V.; Pepe, O.; Napolitano, M.; Marra, R.; Woo, S.L.; Cirillo, V.; Maggio, A. Biostimulant activity of Azotobacter chroococcum and Trichoderma harzianum in durum wheat under water and nitrogen deficiency. Agronomy 2021, 11, 380. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Wang, F.; Wang, G.; Wang, C.; Wang, W.; Chen, K.; Gu, C.; Yu, Q.; Jiang, J. Growth adaptability and foreign gene stability of TaLEA transgenic Populus simonii × nigra. Ann. For. Sci. 2021, 78, 42. [Google Scholar] [CrossRef]
- Lei, S.; Yin, J.; Li, C.; Xu, Q.; Tian, B.; Cheng, X.; Cao, J.; Lu, J.; Ma, C.; Chang, C. Impacts of natural variations in the TaLEA-1A gene on seed dormancy and germination in wheat and transgenic Arabidopsis and rice. Environ. Exp. Bot. 2024, 220, 105715. [Google Scholar] [CrossRef]
- Zhao, P.; Liu, F.; Ma, M.; Gong, J.; Wang, Q.; Jia, P.; Zheng, G.; Liu, H. Overexpression of AtLEA3-3 confers resistance to cold stress in Escherichia coli and provides enhanced osmotic stress tolerance and ABA sensitivity in Arabidopsis thaliana. Mol. Biol. 2011, 45, 785–796. [Google Scholar] [CrossRef]
- Galau, G.A.; Hughes, D.W.; Dure, L. Abscisic acid induction of cloned cotton late embryogenesis-abundant (Lea) mRNAs. Plant Mol. Biol. 1986, 7, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Bai, S.; Li, Q.; Gao, C.; Liu, G.; Li, G.; Tan, F. Overexpression of TaLEA gene from Tamarix androssowii improves salt and drought tolerance in transgenic poplar (Populus simonii× P. nigra). PLoS ONE 2013, 8, e67462. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Hu, Y.; Zhang, W.; Xie, R.; Guan, H.; Xiong, H.; Jia, L.; Zhang, X.; Zhou, H.; Zheng, D. Revisiting the role of delta-1-pyrroline-5-carboxylate synthetase in drought-tolerant crop breeding. Crop J. 2022, 10, 1213–1218. [Google Scholar] [CrossRef]
- Zhang, P.; Chao, R.; Qiu, L.; Ge, W.; Liang, J.; Wen, P. ChaWRKY40 Enhances Drought Tolerance of ‘Dawei’ Hazelnuts by Positively Regulating Proline Synthesis. Forests 2024, 15, 407. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, Y.; Li, P.; Jian, J.; Zhao, C.; Wen, G. Mitogen-activated protein kinase and substrate identification in plant growth and development. Int. J. Mol. Sci. 2022, 23, 2744. [Google Scholar] [CrossRef]
- Abdelhameed, A.A.; Ali, M.; Darwish, D.B.E.; AlShaqhaa, M.A.; Selim, D.A.-F.H.; Nagah, A.; Zayed, M. Induced genetic diversity through mutagenesis in wheat gene pool and significant use of SCoT markers to underpin key agronomic traits. BMC Plant Biol. 2024, 24, 673. [Google Scholar] [CrossRef]
- Ali, M.; Ahmed, I.; Bibi, H.; Saeed, M.; Khalil, I.A.; Bari, A. Impact of irrigation schedules on yield-related traits of wheat under semi-arid region. Gesunde Pflanz. 2023, 75, 2413–2422. [Google Scholar] [CrossRef]
- Sallam, A.; Alqudah, A.M.; Dawood, M.F.; Baenziger, P.S.; Börner, A. Drought stress tolerance in wheat and barley: Advances in physiology, breeding and genetics research. Int. J. Mol. Sci. 2019, 20, 3137. [Google Scholar] [CrossRef]
- Ahmad, A.; Aslam, Z.; Javed, T.; Hussain, S.; Raza, A.; Shabbir, R.; Mora-Poblete, F.; Saeed, T.; Zulfiqar, F.; Ali, M.M. Screening of wheat (Triticum aestivum L.) genotypes for drought tolerance through agronomic and physiological response. Agronomy 2022, 12, 287. [Google Scholar] [CrossRef]
- Fischer, R.; Edmeades, G.O. Breeding and cereal yield progress. Crop Sci. 2010, 50, S-85–S-98. [Google Scholar] [CrossRef]
- Goriewa-Duba, K.; Duba, A.; Wachowska, U.; Wiwart, M. An evaluation of the variation in the morphometric parameters of grain of six Triticum species with the use of digital image analysis. Agronomy 2018, 8, 296. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Elashtokhy, M.M.; Shamseldin, S.A.; El-Ballat, E.M.; Zayed, E.M.; Heikal, Y.M. Analysis of genetic diversity and phylogenetic relationships of wheat (Triticum aestivum L.) genotypes using phenological, molecular and DNA barcoding markers. Genes 2022, 14, 34. [Google Scholar] [CrossRef] [PubMed]
- Choukan, R.; Taherkhani, T.; Ghannadha, M.; Khodarahmi, M. Evaluation of drought tolerance maize lines by drought stress tolerance indices. Iran. J. Agric. Sci. 2006, 8, 2000–2010. [Google Scholar]
- Ladoui, K.K.; Mefti, M.; Benkherbache, N. Selection de genotypes d’orge (Hordeumvulgare L.) tolerants au stress hydrique par les indices de tolérance a la secheresse. Rev. Agrobiol. 2020, 10, 1805–1812. [Google Scholar]
- Aujla, I.S.; Paulitz, T.C. An improved method for establishing accurate water potential levels at different temperatures in growth media. Front. Microbiol. 2017, 8, 1497. [Google Scholar] [CrossRef] [PubMed]
- Bric, J.M.; Bostock, R.M.; Silverstone, S.E. Rapid in situ assay for indoleacetic acid production by bacteria immobilized on a nitrocellulose membrane. Appl. Environ. Microbiol. 1991, 57, 535–538. [Google Scholar] [CrossRef]
- Holbrook, A.A.; Edge, W.; Bailey, F. Spectrophotometric Method for Determination of Gibberellic Acid; ACS Publications: Washington, DC, USA, 1961. [Google Scholar]
- Sharma, S.; Sharma, A.; Kaur, M. Extraction and evaluation of gibberellic acid from Pseudomonas spp. Plant growth promoting rhizobacteria. J. Pharmacogn. Phytochem. 2018, 7, 2790–2795. [Google Scholar]
- Jensen, H. The azotobacteriaceae. Bacteriol. Rev. 1954, 18, 195–214. [Google Scholar] [CrossRef]
- Bakker, A.W.; Schippers, B. Microbial cyanide production in the rhizosphere in relation to potato yield reduction and Pseudomonas spp-mediated plant growth-stimulation. Soil Biol. Biochem. 1987, 19, 451–457. [Google Scholar] [CrossRef]
- Nautiyal, C.S. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol. Lett. 1999, 170, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Noori, M.S.; Saud, H.M. Potential plant growth-promoting activity of Pseudomonas sp. isolated from paddy soil in Malaysia as biocontrol agent. J. Plant Pathol. Microbiol. 2012, 3, 1000120. [Google Scholar]
- Lumi Abe, C.A.; Bertechini Faria, C.; Fernandes de Castro, F.; De Souza, S.R.; dos Santos, F.C.; Novais da Silva, C.; Tessmann, D.J.; Barbosa-Tessmann, I.P. Fungi isolated from maize (Zea mays L.) grains and production of associated enzyme activities. Int. J. Mol. Sci. 2015, 16, 15328–15346. [Google Scholar] [CrossRef]
- Samanta, R.; Pal, D.; Sen, S. Production of hydrolases by N2-fixing microorganisms. Biochem. Physiol. Pflanz. 1989, 185, 75–81. [Google Scholar] [CrossRef]
- Khianngam, S.; Pootaeng-on, Y.; Techakriengkrai, T.; Tanasupawat, S. Screening and identification of cellulase producing bacteria isolated from oil palm meal. J. Appl. Pharm. Sci. 2014, 4, 90. [Google Scholar]
- Sarath, G.; De la Motte, R.; Wagner, F. Protease assay methods. In Proteolytic Enzymes–A Practical Approach; Beynon, R.J., Bond, J.S., Eds.; Merck: Darmstadt, Germany, 1989. [Google Scholar]
- Jyoti, A.; Singh, R.K.; Kumar, N.; Aman, A.K.; Kar, M. Synthesis and properties of amorphous nanosilica from rice husk and its composites. Mater. Sci. Eng. B 2021, 263, 114871. [Google Scholar] [CrossRef]
- Nawaz, A.; Shahbaz, M.; Asadullah; Imran, A.; Marghoob, M.U.; Imtiaz, M.; Mubeen, F. Potential of salt tolerant PGPR in growth and yield augmentation of wheat (Triticum aestivum L.) under saline conditions. Front. Microbiol. 2020, 11, 2019. [Google Scholar] [CrossRef] [PubMed]
- Bajji, M.; Kinet, J.-M.; Lutts, S. The use of the electrolyte leakage method for assessing cell membrane stability as a water stress tolerance test in durum wheat. Plant Growth Regul. 2002, 36, 61–70. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Hedge, J.; Hofreiter, B.; Whistler, R. Carbohydrate Chemistry; Academic Press: New York, NY, USA, 1962; Volume 17. [Google Scholar]
- Barnes, J.D.; Balaguer, L.; Manrique, E.; Elvira, S.; Davison, A. A reappraisal of the use of DMSO for the extraction and determination of chlorophylls a and b in lichens and higher plants. Environ. Exp. Bot. 1992, 32, 85–100. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Khalil, R.R.; Moustafa, A.N.; Bassuony, F.M.; Haroun, S.A. Kinetin and/or calcium affect growth of Phaseolus vulgaris L. plant grown under heavy metals stress. J. Environ. Sci. 2017, 46, 103–120. [Google Scholar]
- Rahaman, M.M.; Zwart, R.S.; Thompson, J.P. Constitutive and induced expression of total phenol and phenol oxidases in wheat genotypes ranging in resistance/susceptibility to the root-lesion nematode Pratylenchus thornei. Plants 2020, 9, 485. [Google Scholar] [CrossRef]
- Kosem, N.; Han, Y.-H.; Moongkarndi, P. Antioxidant and cytoprotective activities of methanolic extract from Garcinia mangostana hulls. Sci. Asia 2007, 33, 283–292. [Google Scholar] [CrossRef]
- McDonald, S.; Prenzler, P.D.; Antolovich, M.; Robards, K. Phenolic content and antioxidant activity of olive extracts. Food Chem. 2001, 73, 73–84. [Google Scholar] [CrossRef]
- Gorinstein, S.; Vargas, O.J.M.; Jaramillo, N.O.; Salas, I.A.; Ayala, A.L.M.; Arancibia-Avila, P.; Toledo, F.; Katrich, E.; Trakhtenberg, S. The total polyphenols and the antioxidant potentials of some selected cereals and pseudocereals. Eur. Food Res. Technol. 2007, 225, 321–328. [Google Scholar] [CrossRef]
- Rosidah; Yam, M.; Sadikun, A.; Asmawi, M. Antioxidant potential of Gynura procumbens. Pharm. Biol. 2008, 46, 616–625. [Google Scholar] [CrossRef]
- Ding, W.; Fang, W.; Shi, S.; Zhao, Y.; Li, X.; Xiao, K. Wheat WRKY type transcription factor gene TaWRKY1 is essential in mediating drought tolerance associated with an ABA-dependent pathway. Plant Mol. Biol. Report. 2016, 34, 1111–1126. [Google Scholar] [CrossRef]
- Dudziak, K.; Zapalska, M.; Börner, A.; Szczerba, H.; Kowalczyk, K.; Nowak, M. Analysis of wheat gene expression related to the oxidative stress response and signal transduction under short-term osmotic stress. Sci. Rep. 2019, 9, 2743. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Makhadmeh, I.M.; Thabet, S.G.; Ali, M.; Alabbadi, B.; Albalasmeh, A.; Alqudah, A.M. Exploring genetic variation among Jordanian Solanum lycopersicon L. landraces and their performance under salt stress using SSR markers. J. Genet. Eng. Biotechnol. 2022, 20, 45. [Google Scholar] [CrossRef] [PubMed]
- Makhadmeh, I.; Albalasmeh, A.A.; Ali, M.; Thabet, S.G.; Darabseh, W.A.; Jaradat, S.; Alqudah, A.M. Molecular characterization of tomato (Solanum lycopersicum L.) accessions under drought stress. Horticulturae 2022, 8, 600. [Google Scholar] [CrossRef]
- Duncan, D.B. Multiple range and multiple F tests. Biometrics 1955, 11, 1–42. [Google Scholar] [CrossRef]

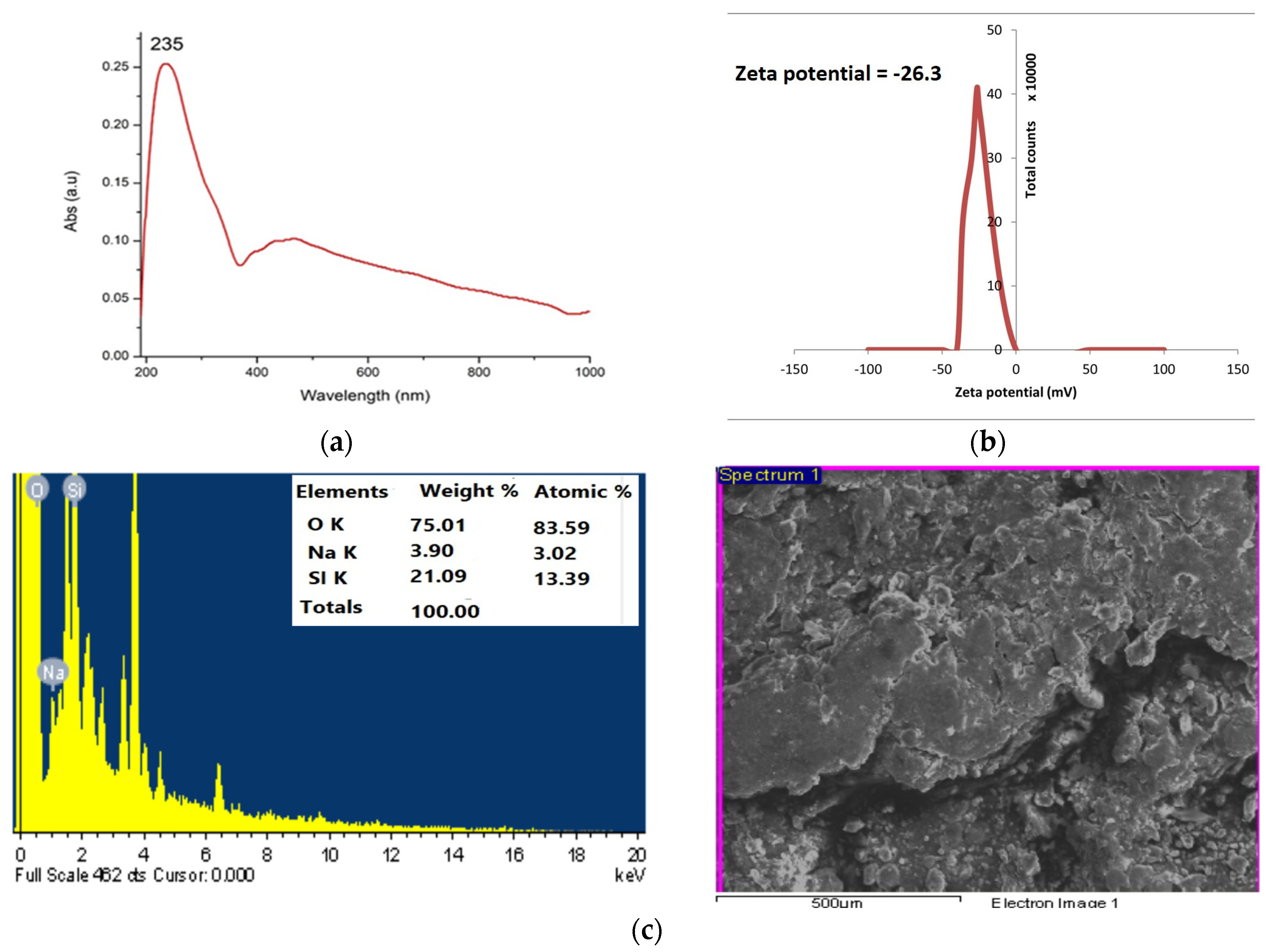
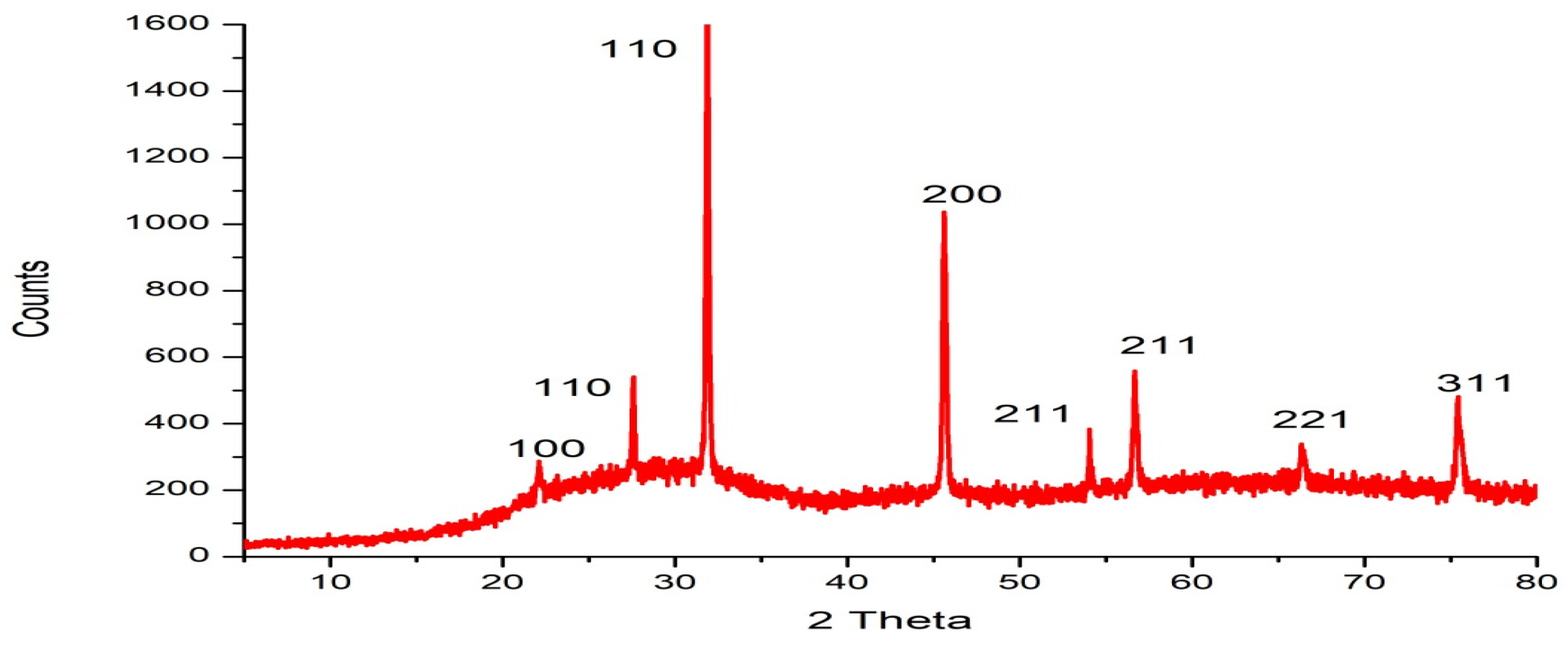
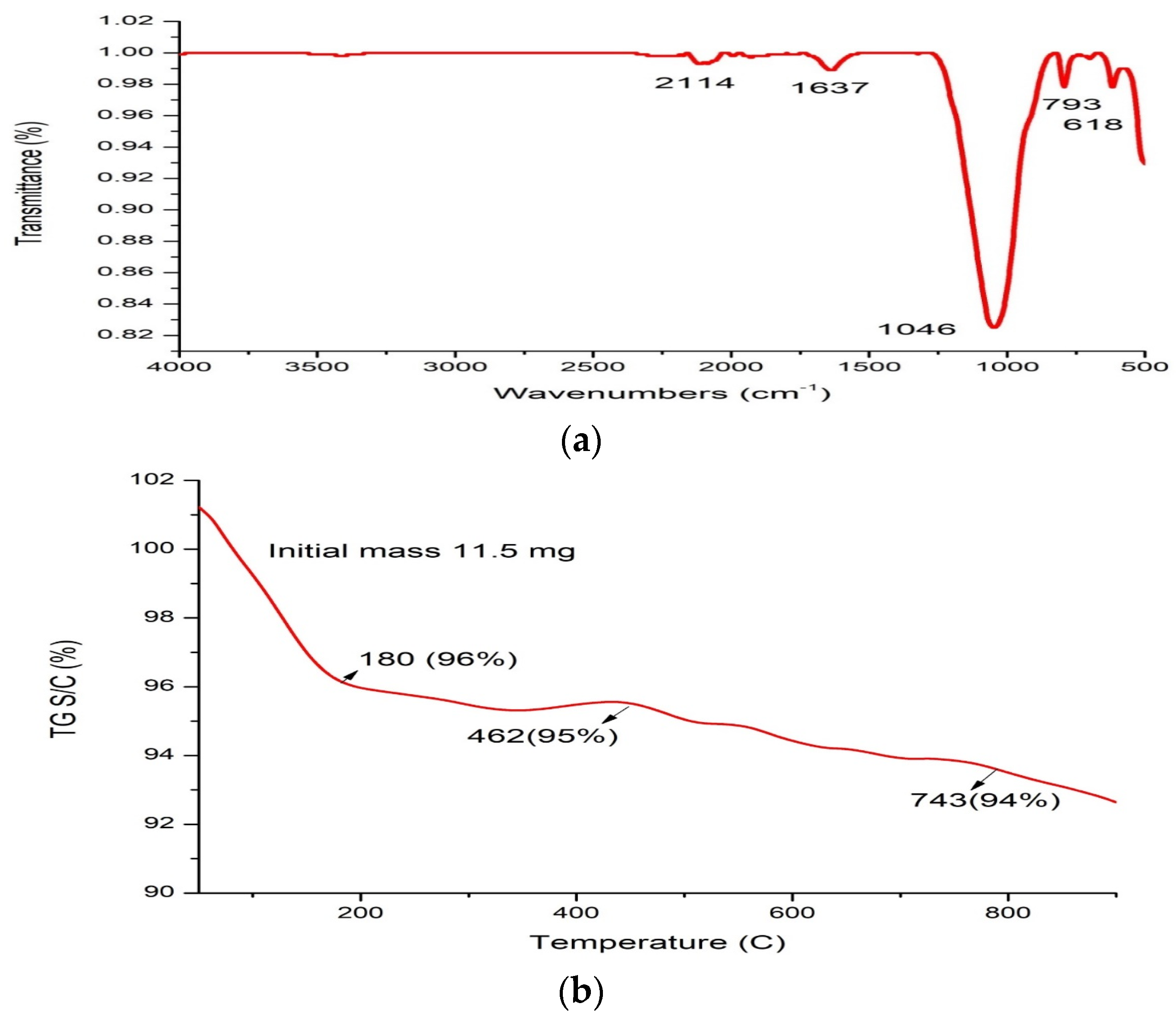



| Quantitative assay | |
| Indole acetic acid (IAA) (µg/mL) | 8.50 ± 0.59 |
| Gibberellic acid (GA) (µg/mL) | 28.62 ± 1.76 |
| Qualitative assay | |
| Cellulase | +ve |
| Amylase | +ve |
| Protease | −ve |
| Phosphate solubilization | −ve |
| Ammonia | −ve |
| Nitrogen fixation | +ve |
| Treatment | PL (cm) | SHL (cm) | RL (cm) | SH/R | L. No | Leaf Growth Parameter | R. No. | RW (cm) | FWSH (g) | FWR (g) | DWSH (g) | DWR (g) | WCSH (%) | WCR (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | LL (cm) | LW (cm) | LA (cm2) | LP (cm) | ||||||||||||||
| 100% FC (D0) | T0 | 44.38 ± 2.05 a | 36.67 ± 1.53 a | 9.10 ± 1.88 a | 5.05 ± 0.29 a | 12.00 ± 2.00 a | 17.84 ± 1.20 b | 1.21 ± 0.12 b | 18.98 ± 2.67 b | 37.60 ± 2.59 b | 20.0 ± 1.53 a | 0.16 ± 0.03 a | 3.08 ± 0.14 a | 0.29 ± 0.06 a | 0.60 ± 0.05 a | 0.07 ± 0.01 a | 80.68 ± 0.85 c | 77.46 ± 1.22 a |
| T1 | 38.42 ± 1.12 b | 32.39 ± 0.98 b | 6.35 ± 1.65 a | 6.56 ± 2.28 a | 8.33 ± 0.88 a | 24.22 ± 0.42 a | 1.40 ± 0.09 a | 38.57 ± 1.48 a | 51.30 ± 1.57 a | 14.6 ± 2.91 b | 0.13 ± 0.02 a | 1.96 ± 0.11 b | 0.14 ± 0.03 b | 0.33 ± 0.02 b | 0.05 ± 0.00 b | 82.97 ± 0.77 a | 64.56 ± 9.68 a | |
| T2 | 25.93 ± 2.21 c | 20.81 ± 1.23 d | 6.4 ± 1.67 a | 4.82 ± 1.45 a | 7.67 ± 1.76 a | 10.78 ± 0.62 c | 0.76 ± 0.06 d | 11.08 ± 0.56 c | 37.60 ± 0.89 c | 9.33 ± 1.76 d | 0.10 ± 0.01 a | 1.18 ± 0.14 d | 0.08 ± 0.02 b | 0.22 ± 0.03 b | 0.02 ± 0.01 c | 81.06 ± 0.68 b | 74.83 ± 1.92 a | |
| T3 | 33.02 ± 3.41 d | 27.79 ± 2.85 c | 5.87 ± 2.23 a | 6.20 ± 1.73 a | 8.00 ± 0.00 a | 12.35 ± 0.37 c | 0.90 ± 0.19 c | 11.01 ± 1.21 c | 51.30 ± 0.76 c | 10.67 ± 0.88 c | 0.10 ± 0.01 a | 1.50 ± 0.25 c | 0.08 ± 0.01 b | 0.33 ± 0.06 b | 0.03 ± 0.00 c | 78.53 ± 0.92 d | 70.06 ± 1.02 a | |
| 50% FC (D1) | T0 | 45.61 ± 2.21 a | 37.27 ± 1.91 a | 8.67 ± 1.25 b | 4.34 ± 0.04 a | 6.33 ± 0.88 a | 17.56 ± 2.60 a | 1.06 ± 0.04 a | 19.14 ± 1.84 a | 37.19 ± 5.21 a | 18.6 ± 1.20 a | 0.15 ± 0.04 a | 1.08 ± 0.19 d | 0.08 ± 0.00 b | 0.28 ± 0.11 d | 0.04 ± 0.01 b | 74.37 ± 0.82 b | 48.04 ± 9.03 b |
| T1 | 44.57 ± 2.45 a | 35.16 ± 2.31 a | 11.8 ± 1.24 a | 3.79 ± 0.41 a | 8.00 ± 0.58 a | 15.88 ± 5.00 a | 0.85 ± 0.20 a | 18.14 ± 7.92 a | 33.80 ± 9.69 a | 21.3 ± 6.36 a | 0.12 ± 0.00 a | 1.74 ± 0.19 c | 0.15 ± 0.02 a | 0.45 ± 0.33 a | 0.07 ± 0.02 b | 74.38 ± 3.19 b | 55.08 ± 7.60 b | |
| T2 | 47.53 ± 2.78 a | 38.27 ± 1.69 a | 9.84 ± 1.76 b | 4.34 ± 0.70 a | 9.00 ± 1.53 a | 14.81 ± 2.22 a | 0.80 ± 0.07 a | 15.95 ± 3.93 a | 31.96 ± 5.04 a | 23.0 ± 6.03 a | 0.17 ± 0.01 a | 2.05 ± 0.29 b | 0.23 ± 0.04 a | 0.55 ± 0.30 c | 0.12 ± 0.02 a | 72.51 ± 4.11 b | 46.48 ± 2.74 b | |
| T3 | 35.54 ± 2.33 b | 30.15 ± 3.40 a | 6.63 ± 1.16 c | 7.40 ± 2.92 a | 7.67 ± 1.67 a | 14.56 ± 1.86 a | 0.90 ± 0.14 a | 17.69 ± 3.85 a | 31.70 ± 3.93 a | 11.0 ± 0.58 a | 0.11 ± 0.03 a | 2.53 ± 0.22 a | 0.15 ± 0.03 a | 0.46 ± 0.22 b | 0.04 ± 0.01 b | 81.99 ± 0.54 a | 72.62 ± 3.48 a | |
| 30% FC (D2) | T0 | 37.79 ± 0.26 b | 28.77 ± 1.42 b | 7.20 ± 1.26 b | 3.55 ± 0.70 c | 9.33 ± 1.20 a | 14.24 ± 1.10 b | 0.86 ± 0.05 a | 14.08 ± 3.65 b | 30.64 ± 3.26 b | 8.00 ± 2.01 a | 0.11 ± 0.00 a | 1.74 ± 0.38 b | 0.13 ± 0.04 b | 0.33 ± 0.08 b | 0.04 ± 0.01 b | 80.17 ± 4.73 b | 72.42 ± 5.73 a |
| T1 | 42.81 ± 0.82 a | 34.57 ± 1.08 a | 8.58 ± 0.49 a | 4.22 ± 0.35 b | 8.33 ± 1.45 a | 16.15 ± 0.76 a | 0.98 ± 0.20 a | 19.45 ± 1.92 a | 35.31 ± 2.13 a | 14.33 ± 2.67 a | 0.11 ± 0.01 a | 3.35 ± 0.38 a | 0.31 ± 0.05 a | 0.52 ± 0.12 b | 0.08 ± 0.02 a | 84.46 ± 1.39 a | 74.21 ± 3.14 a | |
| T2 | 35.27 ± 0.51 c | 28.12 ± 0.21 b | 7.11 ± 0.85 b | 3.95 ± 0.31 c | 6.33 ± 0.33 a | 15.52 ± 1.10 b | 0.94 ± 0.04 a | 14.82 ± 1.16 a | 33.09 ± 3.31 b | 8.00 ± 2.63 a | 0.08 ± 0.02 a | 1.06 ± 0.12 b | 0.07 ± 0.01 b | 0.25 ± 1.66 a | 0.02 ± 0.00 b | 76.11 ± 2.61 b | 69.94 ± 13.16 a | |
| T3 | 40.13 ± 1.04 b | 34.02 ± 0.74 a | 5.49 ± 0.99 c | 5.86 ± 0.72 a | 7.00 ± 0.58 a | 12.86 ± 0.56 c | 0.62 ± 0.08 a | 9.18 ± 0.78 c | 27.29 ± 1. 46 c | 13.67 ± 3.07 a | 0.08 ± 0.03 a | 1.76 ± 0.15 b | 0.13 ± 0.04 b | 0.34 ± 0.09 b | 0.04 ± 0.01 b | 80.63 ± 0.69 b | 65.83 ± 2.90 a | |
| Two-Way ANOVA | ||||||||||||||||||
| Interaction | **** | *** | ns | ns | ns | * | ns | ** | * | * | ns | **** | * | **** | **** | *** | * | |
| Treatment | *** | ** | ns | ns | ns | ** | * | *** | ** | ns | ns | *** | ns | ns | ** | ns | ns | |
| Water regimes | **** | *** | ns | ** | ** | ns | * | ns | ns | ** | * | ns | **** | ns | **** | **** | **** | |
| Treatment | EL (%) | Chl a (mg/g) | Chl b (mg/g) | Carotenoids (mg/g) | Total Pigment (mg/g) | Carbohydrates (mg/g) | Protein (mg/g) | Proline (mg/g) | Flavonoids (mg/g) | Phenolic (mg/g) | DPPH (%) | CAT (U/g) | POD (U/g) | PPO (U/g) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | |||||||||||||||
| 100% FC (D0) | T0 | 10.31 ± 1.20 a | 0.75 ± 0.12 c | 0.13 ± 0.01 d | 0.15 ± 0.03 d | 1.04 ± 0.16 d | 215.17 ± 6.21 c | 13.23 ± 0.38 d | 1.70 ± 0.04 d | 3.76 ± 0.06 b | 27.70 ± 0.79 c | 56.00 ± 0.71 b | 1.07 ± 0.03 d | 33.11 ± 0.96 c | 22.28 ± 0.64 c |
| T1 | 7.53 ± 0.68 b | 1.29 ± 0.02 b | 0.32 ± 0.05 b | 0.28 ± 0.02 c | 1.90 ± 0.04 c | 357.14 ± 12.35 a | 25.97 ± 1.63 b | 2.25 ± 0.04 c | 6.57 ± 0.14 a | 29.91 ± 0.50 d | 29.43 ± 3.45 d | 2.18 ± 0.08 c | 66.22 ± 1.15 b | 32.97 ± 0.99 b | |
| T2 | 8.63 ± 1.26 b | 1.53 ± 0.44 b | 0.45 ± 0.06 a | 0.34 ± 0.07 b | 2.33 ± 0.56 b | 273.28 ± 10.00 b | 38.33 ± 1.47 a | 2.87 ± 0.07 b | 2.44 ± 0.09 c | 22.46 ± 0.98 b | 42.54 ± 2.72 c | 6.53 ± 1.11 a | 73.68 ± 2.86 a | 38.40 ± 1.03 a | |
| T3 | 4.22 ± 1.97 c | 1.92 ± 0.29 a | 0.21 ± 0.02 c | 0.47 ± 0.06 a | 2.60 ± 0.34 a | 284.46 ± 10.31 b | 17.23 ± 0.22 c | 5.66 ± 0.50 a | 4.22 ± 0.53 b | 32.24 ± 0.62 a | 65.90 ± 0.85 a | 3.94 ± 0.11 b | 66.10 ± 2.81 b | 34.05 ± 1.86 b | |
| 50% FC (D1) | T0 | 3.91 ± 0.90 b | 2.10 ± 0.15 a | 0.31 ± 0.07 a | 0.475 ± 0.02 a | 2.88 ± 0.24 a | 215.23 ± 10.08 d | 18.09 ± 0.59 c | 3.67 ± 0.15 b | 2.54 ± 0.12 b | 26.78 ± 0.41 c | 60.74 ± 1.27 a | 3.30 ± 0.11 d | 30.45 ± 0.88 b | 14.81 ± 0.43 c |
| T1 | 2.77 ± 0.53 c | 2.25 ± 0.17 a | 0.24 ± 0.02 a | 0.502 ± 0.04 a | 2.99 ± 0.22 a | 241.20 ± 5.20 c | 22.14 ± 0.53 b | 4.61 ± 0.81 b | 6.22 ± 0.58 a | 31.11 ± 0.58 a | 63.67 ± 1.61 a | 9.57 ± 0.33 a | 51.93 ± 1.72 a | 25.25 ± 0.87 b | |
| T2 | 7.69 ± 0.72 a | 2.14 ± 0.28 a | 0.39 ± 0.02 a | 0.492 ± 0.08 a | 3.02 ± 0.36 a | 296.13 ± 28.35 b | 22.46 ± 0.81 b | 3.47 ± 0.06 b | 4.10 ± 0.32 b | 30.68 ± 0.43 a | 59.27 ± 2.04 a | 3.71 ± 0.30 c | 55.76 ± 1.61 a | 26.34 ± 0.98 b | |
| T3 | 6.95 ± 1.78 b | 2.41 ± 0.43 a | 0.43 ± 0.02 a | 0.617 ± 0.12 a | 3.46 ± 0.54 a | 349.31 ± 25.61 a | 25.21 ± 1.19 a | 6.28 ± 0.38 a | 3.16 ± 0.67 b | 28.97 ± 0.52 b | 51.26 ± 1.45 b | 4.28 ± 0.22 b | 55.79 ± 1.16 a | 34.44 ± 2.49 a | |
| 30% FC (D2) | T0 | 51.14 ± 6.75 a | 1.46 ± 0.03 b | 0.17 ± 0.02 a | 0.34 ± 0.02 b | 1.97 ± 0.03 d | 61.80 ± 9.24 d | 13.05 ± 0.50 c | 4.86 ± 0.20 a | 4.77 ± 0.14 a | 31.29 ± 1.09 a | 65.93 ± 1.11 a | 2.75 ± 0.08 b | 33.28 ± 0.96 b | 20.36 ± 2.23 b |
| T1 | 2.71 ± 0.01 b | 1.35 ± 0.09 b | 0.40 ± 0.14 a | 0.31 ± 0.04 b | 2.06 ± 0.02 c | 290.57 ± 6.12 b | 15.09 ± 0.43 c | 2.65 ± 0.13 b | 4.94 ± 0.15 a | 33.40 ± 0.94 a | 53.63 ± 4.32 b | 3.35 ± 0.12 b | 57.01 ± 1.85 a | 32.85 ± 0.75 a | |
| T2 | 6.26 ± 0.07 b | 2.45 ± 0.33 a | 0.40 ± 0.12 a | 0.59 ± 0.06 a | 3.44 ± 0.50 a | 118.10 ± 15.64 c | 24.44 ± 0.60 a | 5.20 ± 0.06 a | 4.75 ± 0.06 a | 31.98 ± 1.83 a | 24.62 ± 1.46 c | 9.17 ± 1.66 a | 60.63 ± 1.18 a | 29.60 ± 1.14 a | |
| T3 | 4.46 ± 0.10 b | 2.19 ± 0.10 a | 0.17 ± 0.07 a | 0.54 ± 0.02 a | 2.90 ± 0.18 b | 393.01 ± 18.22 a | 18.62 ± 1.14 b | 5.01 ± 0.66 a | 5.05 ± 0.85 a | 32.98 ± 1.22 a | 54.67 ± 1.27 b | 2.93 ± 0.09 b | 59.10 ± 1.25 a | 29.75 ± 0.52 a | |
| Two-Way ANOVA | |||||||||||||||
| Interaction | **** | ns | ns | * | ns | **** | **** | **** | *** | **** | **** | **** | ** | *** | |
| Treatment | **** | ** | ** | **** | *** | **** | **** | **** | **** | *** | **** | **** | **** | **** | |
| Drought regimes | **** | **** | ns | **** | *** | **** | **** | **** | * | **** | **** | ** | **** | **** | |
| Treatment | PL (cm) | SHL (cm) | RL (cm) | SH/R | L. No | Flag Leaf Growth Parameter | RW (cm) | R. No | FWSH (g) | DWSH (g) | FWR (g) | DWR (g) | WCSH (%) | WCR (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | FLL (cm) | FLW (cm) | FLL/FLW | FLA (cm2) | FLP (cm) | FLAn (°) | ||||||||||||||
| 100% FC (D0) | T0 | 46.13 ± 0.64 b | 37.65 ± 0.27 b | 9.24 ± 1.24 b | 4.52 ± 0.54 a | 8.33 ± 0.33 a | 9.38 ± 0.24 b | 0.67 ± 0.07 b | 14.23 ± 1.55 c | 6.84 ± 0.19 b | 20.49 ± 0.61 b | 17.08 ± 1.27 c | 0.12 ± 0.03 b | 15.33 ± 1.76 b | 1.40 ± 0.10 a | 1.24 ± 0.09 a | 0.09 ± 0.02 a | 0.09 ± 0.02 a | 11.36 ± 0.86 b | 7.63 ± 2.89 d |
| T1 | 51.77 ± 1.02 a | 42.55 ± 0.62 a | 11.03 ± 0.27 a | 4.57 ± 0.56 a | 9.33 ± 0.88 a | 15.22 ± 1.22 a | 0.84 ± 0.05 a | 18.21 ± 1.27 a | 17.30 ± 1.39 a | 32.97 ± 2.13 a | 17.41 ± 1.51 c | 0.18 ± 0.00 a | 20.33 ± 0.88 a | 1.37 ± 0.06 b | 1.15 ± 0.07 a | 0.07 ± 0.00 a | 0.07 ± 0.00 a | 16.12 ± 2.93 a | 13.80 ± 4.54 c | |
| T2 | 42.10 ± 1.27 b | 34.82 ± 1.06 b | 7.40 ± 0.21 c | 4.65 ± 0.10 a | 8.67 ± 0.33 a | 9.45 ± 0.27 b | 0.56 ± 0.03 b | 16.79 ± 0.49 b | 6.05 ± 0.55 c | 20.05 ± 0.49 b | 22.38 ± 0.96 b | 0.08 ± 0.02 c | 9.67 ± 1.20 d | 0.71 ± 0.10 c | 0.68 ± 0.10 b | 0.03 ± 0.01 a | 0.03 ± 0.01 b | 4.20 ± 0.40 c | 22.62 ± 4.29 b | |
| T3 | 33.99 ± 2.33 c | 27.88 ± 1.35 c | 9.13 ± 0.84 b | 4.64 ± 0.72 a | 8.00 ± 0.58 a | 7.15 ± 0.40 c | 0.54 ± 0.04 b | 13.20 ± 0.27 d | 3.92 ± 0.56 d | 15.40 ± 0.97 c | 32.11 ± 0.93 a | 0.11 ± 0.03 b | 10.33 ± 2.03 c | 0.54 ± 0.11 c | 0.47 ± 0.08 b | 0.12 ± 0.09 a | 0.03 ± 0.00 b | 13.12 ± 3.32 a | 37.78 ± 2.22 a | |
| 50% FC (D1) | T0 | 47.91 ± 0.90 b | 38.83 ± 0.71 b | 11.33 ± 0.78 b | 4.42 ± 0.52 a | 7.67 ± 1.76 c | 12.44 ± 0.71 b | 0.81 ± 0.09 a | 16.01 ± 2.86 a | 10.71 ± 0.54 b | 26.58 ± 1.33 a | 19.84 ± 0.57 b | 0.15 ± 0.02 b | 20.67 ± 3.18 b | 0.76 ± 0.08 b | 0.67 ± 0.08 c | 0.10 ± 0.01 b | 0.06 ± 0.01 b | 12.48 ± 0.94 a | 5.16 ± 2.60 a |
| T1 | 59.72 ± 0.53 a | 49.29 ± 0.49 a | 12.48 ± 1.13 a | 4.66 ± 0.25 a | 7.33 ± 0.88 c | 13.08 ± 0.38 a | 0.88 ± 0.07 a | 15.04 ± 1.19 a | 12.22 ± 0.55 a | 28.01 ± 0.93 a | 26.19 ± 0.42 a | 0.21 ± 0.02 a | 18.33 ± 3.18 b | 0.67 ± 0.13 b | 0.59 ± 0.13 c | 0.08 ± 0.00 a | 0.04 ± 0.01 b | 13.30 ± 3.47 a | 18.01 ± 2.63 a | |
| T2 | 43.77 ± 1.82 b | 36.08 ± 1.47 b | 9.27 ± 1.08 c | 4.68 ± 0.20 a | 9.00 ± 1.53 b | 11.22 ± 0.52 b | 0.67 ± 0.05 a | 16.81 ± 1.44 a | 8.58 ± 0.76 c | 24.15 ± 1.08 b | 15.39 ± 0.81 c | 0.12 ± 0.03 c | 14.33 ± 1.86 c | 0.86 ± 0.02 b | 0.76 ± 0.06 b | 0.04 ± 0.01 b | 0.07 ± 0.01 b | 11.45 ± 4.65 a | 3.33 ± 3.33 a | |
| T3 | 46.2 ± 1.35 b | 38.82 ± 0.86 b | 8.52 ± 0.5 c | 5.05 ± 0.24 a | 12.67 ± 0.88 a | 10.35 ± 0.79 c | 0.68 ± 0.06 a | 15.32 ± 1.02 a | 7.14 ± 1.10 d | 22.16 ± 1.53 b | 16.26 ± 0.47 c | 0.18 ± 0.02 b | 24.33 ± 2.85 a | 1.41 ± 0.28 a | 1.20 ± 0.21 a | 0.05 ± 0.00 b | 0.10 ± 0.01 a | 13.87 ± 2.14 a | 17.48 ± 7.20 a | |
| 30% FC (D2) | T0 | 43.50 ± 0.29 c | 36.77 ± 1.34 a | 7.04 ± 1.69 a | 5.82 ± 1.13 a | 8.67 ± 0.88 a | 10.31 ± 0.31 a | 0.51 ± 0.01 d | 20.19 ± 0.60 a | 6.62 ± 0.26 c | 21.92 ± 0.50 a | 40.89 ± 6.31 b | 0.13 ± 0.03 b | 16.67 ± 0.88 a | 0.93 ± 0.02 c | 0.81 ± 0.04 c | 0.09 ± 0.01 a | 0.08 ± 0.01 a | 13.34 ± 2.51 a | 9.44 ± 0.56 b |
| T1 | 49.57 ± 1.43 a | 40.65 ± 1.76 a | 9.41 ± 0.60 a | 4.63 ± 0.51 a | 6.00 ± 1.15 a | 12.62 ± 1.20 a | 0.61 ± 0.01 c | 20.55 ± 1.96 a | 10.09 ± 0.96 b | 26.49 ± 2.26 a | 37.47 ± 4.32 a | 0.20 ± 0.03 a | 19.00 ± 0.58 a | 1.56 ± 0.21 a | 1.27 ± 0.15 a | 0.16 ± 0.05 a | 0.09 ± 0.01 a | 18.54 ± 1.65 a | 40.95 ± 8.38 a | |
| T2 | 46.94 ± 1.12 b | 38.04 ± 0.61 a | 8.99 ± 1.33 a | 4.22 ± 0.69 a | 6.67 ± 0.33 a | 12.61 ± 1.74 a | 0.70 ± 0.10 b | 18.07 ± 1.64 a | 11.95 ± 2.10 a | 27.12 ± 3.54 a | 9.08 ± 0.93 c | 0.11 ± 0.01 b | 10.33 ± 1.33 b | 0.76 ± 0.11 d | 0.67 ± 0.10 d | 0.08 ± 0.00 a | 0.05 ± 0.00 b | 11.67 ± 1.33 a | 38.80 ± 0.72 a | |
| T3 | 46.20 ± 0.95 b | 40.19 ± 0.72 a | 6.71 ± 0.20 a | 6.85 ± 0.66 a | 8.00 ± 0.58 a | 11.88 ± 0.88 a | 0.92 ± 0.05 a | 12.95 ± 0.34 b | 11.97 ± 1.96 a | 25.59 ± 2.04 a | 55.94 ± 6.41 d | 0.12 ± 0.01 b | 15.67 ± 1.86 a | 1.37 ± 0.17 b | 1.11 ± 0.13 b | 0.15 ± 0.00 a | 0.09 ± 0.00 a | 17.97 ± 4.26 a | 38.52 ± 1.70 a | |
| Two-Way ANOVA | ||||||||||||||||||||
| Interaction | **** | **** | ns | * | * | ** | *** | ns | **** | *** | **** | ns | * | **** | **** | **** | **** | * | * | |
| Treatment | **** | **** | ** | ns | ns | **** | ns | ** | **** | **** | **** | *** | *** | *** | ns | * | ns | * | ns | |
| Drought regimes | **** | **** | ** | ** | * | * | ns | ns | ns | * | **** | * | ** | ns | ns | ns | ** | *** | **** | |
| Treatment | Weight of 20 Grains | Grain Width (cm) | Grain Perimeter (cm) | Grain Area (cm2) | L (cm) | W (cm) | Aspect Ratio (AR) | Circ. | Round | Feret | Fertile Spikelets (FS) | Sterile Spikelets (SS) | No. of Spikelets/Spike (S. No.) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | ||||||||||||||
| 100% FC (D0) | T0 | 0.17 ± 0.01 b | 0.20 ± 0.00 a | 1.50 ± 0.09 a | 0.11 ± 0.01 a | 0.65 ± 0.05 a | 0.22 ± 0.01 a | 2.30 ± 0.31 a | 0.59 ± 0.03 a | 0.34 ± 0.04 a | 0.63 ± 0.02 a | 16.33 ± 2.85 b | 21.00 ± 2.65 a | 38.00 ± 5.00 a |
| T1 | 0.31 ± 0.01 a | 0.21 ± 0.01 a | 1.46 ± 0.04 a | 0.13 ± 0.02 a | 0.65 ± 0.03 a | 0.26 ± 0.03 a | 2.59 ± 0.26 a | 0.65 ± 0.02 a | 0.39 ± 0.04 a | 0.66 ± 0.02 a | 32.00 ± 5.29 a | 11.00 ± 3.79 c | 43.00 ± 2.08 a | |
| T2 | 0.02 ± 0.00 d | 0.20 ± 0.00 a | 1.40 ± 0.03 a | 0.13 ± 0.01 a | 0.64 ± 0.01 a | 0.25 ± 0.01 a | 2.49 ± 0.24 a | 0.64 ± 0.03 a | 0.41 ± 0.04 a | 0.62 ± 0.00 a | 1.00 ± 0.58 c | 21.00 ± 2.65 a | 21.00 ± 2.65 b | |
| T3 | 0.13 ± 0.00 c | 0.18 ± 0.02 a | 1.40 ± 0.01 a | 0.10 ± 0.00 a | 0.62 ± 0.02 a | 0.20 ± 0.01 a | 3.17 ± 0.25 a | 0.58 ± 0.02 a | 0.32 ± 0.02 a | 0.62 ± 0.00 a | 8.00 ± 3.21 d | 17.00 ± 1.73 b | 25.00 ± 1.53 b | |
| 50% FC (D1) | T0 | 0.31 ± 0.01 a | 0.22 ± 0.04 a | 1.43 ± 0.10 a | 0.13 ± 0.01 b | 0.61 ± 0.03 a | 0.26 ± 0.01 b | 2.34 ± 0.04 a | 0.70 ± 0.03 a | 0.43 ± 0.01 b | 0.65 ± 0.06 a | 15.00 ± 4.51 a | 19.00 ± 4.00 a | 34.00 ± 0.58 a |
| T1 | 0.24 ± 0.01 b | 0.22 ± 0.00 a | 1.43 ± 0.03 a | 0.11 ± 0.00 b | 0.59 ± 0.02 a | 0.23 ± 0.00 b | 2.61 ± 0.13 a | 0.67 ± 0.01 a | 0.39 ± 0.02 b | 0.61 ± 0.02 a | 26.00 ± 2.31 a | 14.33 ± 1.86 a | 37.00 ± 3.61 a | |
| T2 | 0.23 ± 0.01 b | 0.26 ± 0.00 a | 1.49 ± 0.02 a | 0.16 ± 0.01 a | 0.61 ± 0.03 a | 0.34 ± 0.02 a | 1.82 ± 0.18 b | 0.72 ± 0.02 a | 0.56 ± 0.06 a | 0.62 ± 0.02 a | 18.67 ± 1.20 a | 13.00 ± 1.15 a | 31.67 ± 2.19 a | |
| T3 | 0.16 ± 0.00 c | 0.20 ± 0.00 a | 1.45 ± 0.09 a | 0.11 ± 0.01 b | 0.61 ± 0.04 a | 0.23 ± 0.01 b | 2.64 ± 0.16 a | 0.65 ± 0.03 a | 0.38 ± 0.02 b | 0.58 ± 0.03 a | 20.00 ± 3.61 a | 15.00 ± 1.15 a | 35.00 ± 2.52 a | |
| 30% FC (D2) | T0 | 0.21 ± 0.01 d | 0.19 ± 0.01 b | 1.24 ± 0.02 c | 0.08 ± 0.00 d | 0.51 ± 0.02 b | 0.20 ± 0.01 c | 2.63 ± 0.17 a | 0.67 ± 0.02 b | 0.38 ± 0.02 b | 0.52 ± 0.00 b | 19.33 ± 3.38 b | 14.67 ± 2.73 a | 34.00 ± 4.04 c |
| T1 | 0.25 ± 0.01 b | 0.21 ± 0.02 b | 1.38 ± 0.02 b | 0.11 ± 0.02 c | 0.60 ± 0.03 a | 0.23 ± 0.03 c | 2.66 ± 0.19 a | 0.65 ± 0.01 c | 0.38 ± 0.03 b | 0.61 ± 0.02 a | 31.67 ± 0.88 a | 11.67 ± 1.20 a | 43.33 ± 1.45 a | |
| T2 | 0.27 ± 0.01 a | 0.28 ± 0.10 a | 1.57 ± 0.01 a | 0.14 ± 0.00 a | 0.63 ± 0.02 a | 0.29 ± 0.01 b | 2.16 ± 0.15 a | 0.76 ± 0.03 a | 0.47 ± 0.03 a | 0.64 ± 0.02 a | 12.00 ± 6.24 c | 16.67 ± 7.06 a | 28.67 ± 0.88 d | |
| T3 | 0.25 ± 0.01 c | 0.22 ± 0.00 b | 1.54 ± 0.08 a | 0.13 ± 0.00 b | 0.66 ± 0.02 a | 0.24 ± 0.01 a | 2.71 ± 0.17 a | 0.64 ± 0.04 b | 0.37 ± 0.02 c | 0.63 ± 0.01 a | 27.00 ± 2.52 a | 11.67 ± 1.33 a | 39.33 ± 2.60 b | |
| Two-Way ANOVA | ||||||||||||||
| Interaction | **** | ns | * | ** | ns | * | ns | ns | ns | * | * | ns | * | |
| Treatment | **** | ** | ns | *** | ns | **** | ** | ** | *** | ns | **** | ns | **** | |
| Drought regimes | **** | * | ns | ns | ns | * | * | *** | * | ns | ** | ns | ns | |
| Treatment | MP | GMP | TOL | SI | SSI | STI | HARM | YSI | RDI | DRI | YRR | YI | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | |||||||||||||
| FC (50%) (D1) | T0 | 20.00 | 19.38 | 2.00 | 0.12 | −4.98 | 1.24 | 18.80 | 0.98 | 0.76 | 0.92 | 0.02 | 0.84 |
| T1 | 12.67 | 12.34 | −3.33 | −5.60 | 0.55 | 12.04 | 1.60 | 1.24 | 0.98 | −0.60 | 0.64 | ||
| T2 | 17.00 | 16.40 | 8.00 | −4.64 | 0.88 | 15.82 | 0.64 | 0.50 | 0.38 | 0.36 | 0.58 | ||
| T3 | 16.00 | 15.87 | 2.00 | −4.91 | 0.83 | 15.75 | 0.91 | 0.71 | 0.62 | 0.09 | 0.67 | ||
| FC (30%) (D2) | T0 | 20.17 | 19.85 | 1.67 | −0.29 | −4.96 | 1.31 | 19.54 | 0.96 | 0.75 | 0.88 | 0.04 | 0.86 |
| T1 | 21.33 | 18.14 | −20.67 | −7.69 | 1.15 | 15.65 | 3.69 | 2.87 | 5.12 | −2.69 | 1.41 | ||
| T2 | 16.50 | 12.88 | 9.00 | −4.60 | 0.81 | 12.60 | 0.60 | 0.47 | 0.51 | 0.40 | 0.53 | ||
| T3 | 22.00 | 21.31 | −10.00 | −5.63 | 1.50 | 20.65 | 1.52 | 1.27 | 2.00 | −0.63 | 1.31 |
| Primer | Primer (5′→3′) Forward Sequence | Primer (5′→3′) Reverse Sequence | Reference |
|---|---|---|---|
| TaZFP34 | ACGGCGATCAGTGGGTGT | GACGAACAGCTCGAGCAAGA | Chang, et al. [80] |
| TaWRKY1 | ATGTGGGAAAATGGTAAAA | CTATCTTTCCTTTCTTTGC | Ding et al. [128] |
| TaMAPK3 | CTTTAACCCGCTGCAGAGGA | GTCAAAGGAGAAGGGGTCCG | Dudziak et al. [129] |
| TaLEA | GACAACACCATCACCACCAAGGACA | TAATACAGAACCGGACACGAGGAGT | Itam et al. [74] |
| TaP5CS1 | TGGCCTTGTGAAAAGCAAAGA | GCCTGTTACTGCCTCTTGGA | Itam et al. [74] |
| TaActin | CCTCTCTGCGCCAATCGT | TCAGCCGAGCGGGAAATTGT | Itam et al. [74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aljeddani, G.S.; Hamouda, R.A.; Abdelsattar, A.M.; Heikal, Y.M. Stress-Responsive Gene Expression, Metabolic, Physiological, and Agronomic Responses by Consortium Nano-Silica with Trichoderma against Drought Stress in Bread Wheat. Int. J. Mol. Sci. 2024, 25, 10954. https://doi.org/10.3390/ijms252010954
Aljeddani GS, Hamouda RA, Abdelsattar AM, Heikal YM. Stress-Responsive Gene Expression, Metabolic, Physiological, and Agronomic Responses by Consortium Nano-Silica with Trichoderma against Drought Stress in Bread Wheat. International Journal of Molecular Sciences. 2024; 25(20):10954. https://doi.org/10.3390/ijms252010954
Chicago/Turabian StyleAljeddani, Ghalia S., Ragaa A. Hamouda, Amal M. Abdelsattar, and Yasmin M. Heikal. 2024. "Stress-Responsive Gene Expression, Metabolic, Physiological, and Agronomic Responses by Consortium Nano-Silica with Trichoderma against Drought Stress in Bread Wheat" International Journal of Molecular Sciences 25, no. 20: 10954. https://doi.org/10.3390/ijms252010954
APA StyleAljeddani, G. S., Hamouda, R. A., Abdelsattar, A. M., & Heikal, Y. M. (2024). Stress-Responsive Gene Expression, Metabolic, Physiological, and Agronomic Responses by Consortium Nano-Silica with Trichoderma against Drought Stress in Bread Wheat. International Journal of Molecular Sciences, 25(20), 10954. https://doi.org/10.3390/ijms252010954







