Effects of Environmental Noise Stress on Mouse Metabolism
Abstract
1. Introduction
2. Results
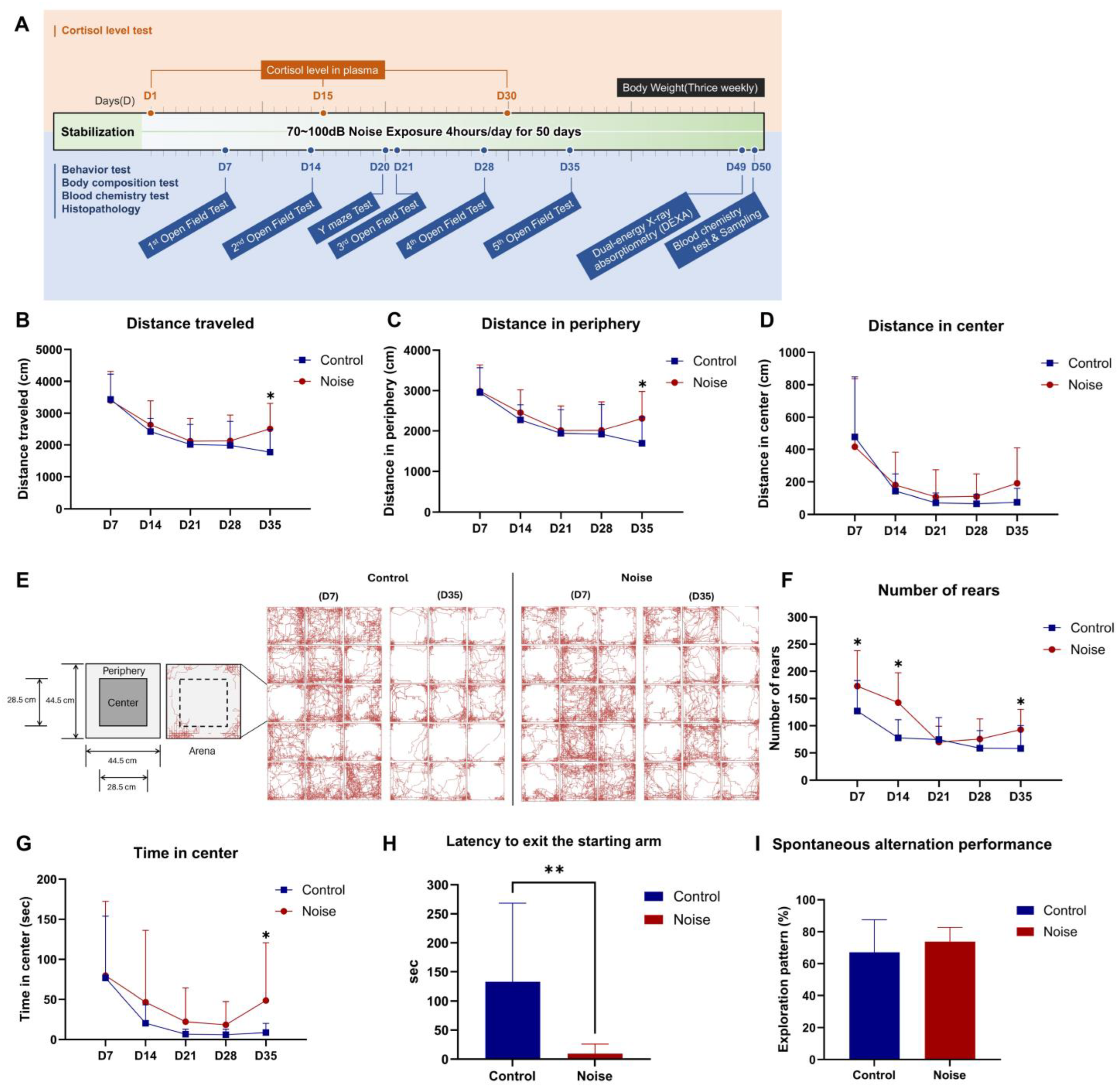
2.1. Noise Exposure Increases Locomotor Activity and Reduces Anxiety
2.2. Noise Exposure Reduces Body Weight
2.3. Noise Exposure Increases Plasma Cortisol Levels
2.4. Noise Exposure Significantly Reduced the Serum Levels of HDL Cholesterol and Triglyceride and Promoted the Expression of SR-B1 and Leptin in the Liver
2.5. Noise Exposure Positively Correlates with Increased Fat Mass and Adipocyte Hypertrophy
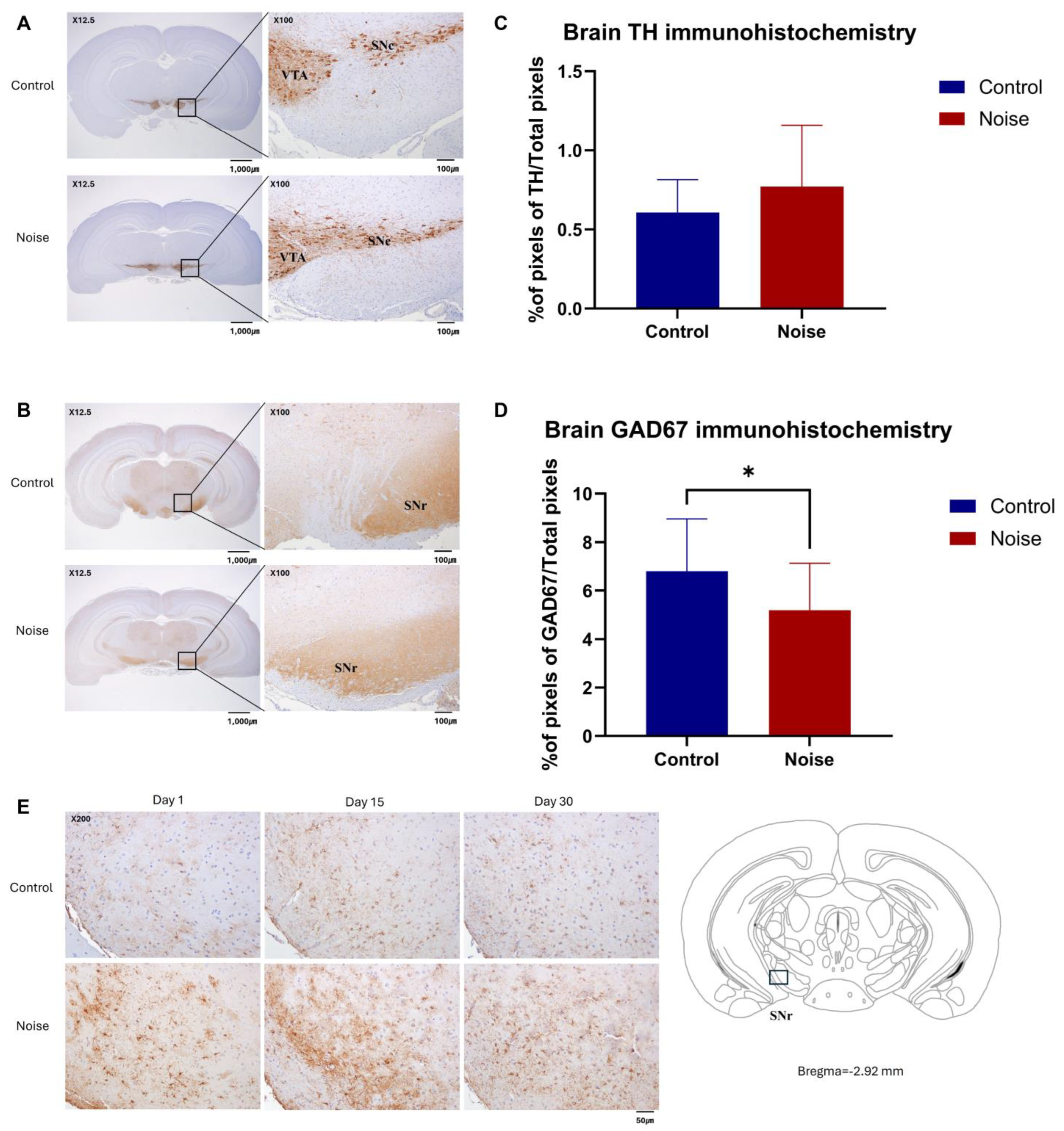
2.6. Noise Exposure Decreases GAD 67 Expression in Mice Brain
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Design
4.3. Cortisol Measurement
4.4. Open Field Test
4.5. Y-Maze Test
4.6. DEXA
4.7. Serum Chemistry
4.8. Histology and Immunohistochemistry
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Daiber, A.; Kröller-Schön, S.; Frenis, K.; Oelze, M.; Kalinovic, S.; Vujacic-Mirski, K.; Kuntic, M.; Bayo Jimenez, M.T.; Helmstädter, J.; Steven, S.; et al. Environmental noise induces the release of stress hormones and inflammatory signaling molecules leading to oxidative stress and vascular dysfunction-Signatures of the internal exposome. BioFactors 2019, 45, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Hahad, O.; Lelieveld, J.; Birklein, F.; Lieb, K.; Daiber, A.; Münzel, T. Ambient air pollution increases the risk of cerebrovascular and neuropsychiatric disorders through induction of inflammation and oxidative stress. Int. J. Mol. Sci. 2020, 21, 4306. [Google Scholar] [CrossRef] [PubMed]
- Münzel, T.; Sørensen, M.; Daiber, A. Transportation noise pollution and cardiovascular disease. Nat. Rev. Cardiol. 2021, 18, 619–636. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.; Sbihi, H.; Tamburic, L.; Brauer, M.; Frank, L.D.; Davies, H.W. Association of long-term exposure to transportation noise and traffic-related air pollution with the incidence of diabetes: A prospective cohort study. Environ. Health Perspect. 2017, 125, 087025. [Google Scholar] [CrossRef]
- He, S.; Smargiassi, A.; Low, N.; Bilodeau-Bertrand, M.; Ayoub, A.; Auger, N. Residential noise exposure and the longitudinal risk of hospitalization for depression after pregnancy: Postpartum and beyond. Environ. Res. 2019, 170, 26–32. [Google Scholar] [CrossRef]
- National Research Council (U.S.). Guide for the care and use of laboratory animals. In Committee for the Update of the Guide for the Care and Use of Laboratory Animals, 8th ed.; Institute for Laboratory Animal Research; National Academies Press: Washington, DC, USA, 2011; Volume xxv, 220p. [Google Scholar]
- Peterson, E.A.; Augenstein, J.S.; Tanis, D.C.; Augenstein, D.G. Noise raises blood pressure without impairing auditory sensitivity. Science 1981, 211, 1450–1452. [Google Scholar] [CrossRef]
- Wu, C.C.; Chen, S.J.; Yen, M.H. Effects of noise on blood pressure and vascular reactivities. Clin. Exp. Pharmacol. Physiol. 1992, 19, 833–838. [Google Scholar] [CrossRef]
- Turner, J.G.; Parrish, J.L.; Hughes, L.F.; Toth, L.A.; Caspary, D.M. Hearing in laboratory animals: Strain differences and nonauditory effects of noise. Comp. Med. 2005, 55, 12–23. [Google Scholar] [PubMed]
- Kazi, A.I.; Oommen, A. Chronic noise stress-induced alterations of glutamate and gamma-aminobutyric acid and their metabolism in the rat brain. Noise Health 2014, 16, 343–349. [Google Scholar] [CrossRef]
- Smith, K.M. Hyperactivity in mice lacking one allele of the glutamic acid decarboxylase 67 gene. Atten. Defic. Hyperact. Disord. 2018, 10, 267–271. [Google Scholar] [CrossRef]
- Mahmoud, S.; Gharagozloo, M.; Simard, C.; Gris, D. Astrocytes maintain glutamate homeostasis in the CNS by controlling the balance between glutamate uptake and release. Cells 2019, 8, 184. [Google Scholar] [CrossRef] [PubMed]
- Dolotov, O.V.; Inozemtseva, L.S.; Myasoedov, N.F.; Grivennikov, I.A. Stress-induced depression and Alzheimer’s disease: Focus on astrocytes. Int. J. Mol. Sci. 2022, 23, 4999. [Google Scholar] [CrossRef] [PubMed]
- Eraslan, E.; Akyazi, I.; Erg L-Ekiz, E.; Matur, E. Noise stress changes mRNA expressions of corticotropin-releasing hormone, its receptors in amygdala, and anxiety-related behaviors. Noise Health 2015, 17, 141–147. [Google Scholar] [CrossRef]
- Koelsch, S.; Boehlig, A.; Hohenadel, M.; Nitsche, I.; Bauer, K.; Sack, U. The impact of acute stress on hormones and cytokines, and how their recovery is affected by music-evoked positive mood. Sci. Rep. 2016, 6, 23008. [Google Scholar] [CrossRef]
- Campos, A.C.; Fogaça, M.V.; Aguiar, D.C.; Guimarães, F.S. Animal models of anxiety disorders and stress. Braz. J. Psychiatry 2013, 35 (Suppl. 2), S101–S111. [Google Scholar] [CrossRef]
- Scott, K.A.; Melhorn, S.J.; Sakai, R.R. Effects of chronic social stress on obesity. Curr. Obes. Rep. 2012, 1, 16–25. [Google Scholar] [CrossRef]
- Brindley, D.N.; McCann, B.S.; Niaura, R.; Stoney, C.M.; Suarez, E.C. Stress and lipoprotein metabolism: Modulators and mechanisms. Metabolism 1993, 42 (Suppl. 1), 3–15. [Google Scholar] [CrossRef]
- Shen, W.J.; Azhar, S.; Kraemer, F.B.; Shen, W.J. SR-B1: A unique multifunctional receptor for cholesterol influx and efflux. Annu. Rev. Physiol. 2018, 80, 95–116. [Google Scholar] [CrossRef] [PubMed]
- Kozarsky, K.F.; Donahee, M.H.; Rigotti, A.; Iqbal, S.N.; Edelman, E.R.; Krieger, M. Overexpression of the HDL receptor SR-BI alters plasma HDL and bile cholesterol levels. Nature 1997, 387, 414–417. [Google Scholar] [CrossRef]
- Ito, M.; Ye, X.; Wang, Q.; Guo, L.; Hao, D.; Howatt, D.; Daugherty, A.; Cai, L.; Temel, R.; Li, X.A. SR-BI (scavenger receptor BI), not LDL (low-density lipoprotein) receptor, mediates adrenal stress response-brief report. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1830–1837. [Google Scholar] [CrossRef]
- Martínez-Uña, M.; López-Mancheño, Y.; Diéguez, C.; Fernández-Rojo, M.A.; Novelle, M.G. Unraveling the role of leptin in liver function and its relationship with liver diseases. Int. J. Mol. Sci. 2020, 21, 9368. [Google Scholar] [CrossRef] [PubMed]
- Bouillon-Minois, J.B.; Trousselard, M.; Thivel, D.; Benson, A.C.; Schmidt, J.; Moustafa, F.; Bouvier, D.; Dutheil, F. Leptin as a biomarker of stress: A systematic review and meta-analysis. Nutrients 2021, 13, 3350. [Google Scholar] [CrossRef]
- Morera, P.; Basiricò, L.; Hosoda, K.; Bernabucci, U. Chronic heat stress up-regulates leptin and adiponectin secretion and expression and improves leptin, adiponectin and insulin sensitivity in mice. J. Mol. Endocrinol. 2012, 48, 129–138. [Google Scholar] [CrossRef]
- Chandralekha, G.; Jeganathan, R.; Viswanathan, C.J.C.; Charan, J.C. Serum leptin and corticosterone levels after exposure to noise stress in rats. Malays. J. Med. Sci. 2005, 12, 51–56. [Google Scholar]
- Otsuka, R.; Yatsuya, H.; Tamakoshi, K.; Matsushita, K.; Wada, K.; Toyoshima, H. Perceived psychological stress and serum leptin concentrations in Japanese men. Obesity 2006, 14, 1832–1838. [Google Scholar] [CrossRef]
- Lundåsen, T.; Liao, W.; Angelin, B.; Rudling, M. Leptin induces the hepatic high density lipoprotein receptor scavenger receptor B type I (SR-BI) but not cholesterol 7alpha-hydroxylase (Cyp7a1) in leptin-deficient (ob/ob) mice. J. Biol. Chem. 2003, 278, 43224–43228. [Google Scholar] [CrossRef]
- Cui, B.; Gai, Z.; She, X.; Wang, R.; Xi, Z. Effects of chronic noise on glucose metabolism and gut microbiota-host inflammatory homeostasis in rats. Sci. Rep. 2016, 6, 36693. [Google Scholar] [CrossRef]
- Sturman, O.; Germain, P.L.; Bohacek, J. Exploratory rearing: A context- and stress-sensitive behavior recorded in the open-field test. Stress 2018, 21, 443–452. [Google Scholar] [CrossRef]
- Burow, A.; Day, H.E.W.; Campeau, S. A detailed characterization of loud noise stress: Intensity analysis of hypothalamo-pituitary-adrenocortical axis and brain activation. Brain Res. 2005, 1062, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Sung, J.H.; Bang, J.H.; Cho, S.W.; Lee, J.; Sim, C.S. Effects of self-reported sensitivity and road-traffic noise levels on the immune system. PLoS ONE 2017, 12, e0187084. [Google Scholar] [CrossRef] [PubMed]
- Petroff, O.A.C. GABA and glutamate in the human brain. Neuroscientist 2002, 8, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Baik, J.H. Stress and the dopaminergic reward system. Exp. Mol. Med. 2020, 52, 1879–1890. [Google Scholar] [CrossRef]
- Ding, S.; Wei, W.; Zhou, F.M. Molecular and functional differences in voltage-activated sodium currents between GABA projection neurons and dopamine neurons in the substantia nigra. J. Neurophysiol. 2011, 106, 3019–3034. [Google Scholar] [CrossRef]
- Tan, T.; Wang, W.; Williams, J.; Ma, K.; Cao, Q.; Yan, Z. Stress exposure in dopamine d4 receptor knockout mice induces schizophrenia-like behaviors via disruption of GABAergic Transmission. Schizophr. Bull. 2019, 45, 1012–1023. [Google Scholar] [CrossRef]
- Fogaça, M.V.; Duman, R.S. Cortical GABAergic dysfunction in stress and depression: New insights for therapeutic interventions. Front. Cell. Neurosci. 2019, 13, 87. [Google Scholar] [CrossRef]
- Harris, R.B.S. Chronic and acute effects of stress on energy balance: Are there appropriate animal models? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, R250–R265. [Google Scholar] [CrossRef]
- Wysocki, A.B. The effect of intermittent noise exposure on wound healing. Adv. Wound Care 1996, 9, 35–39. [Google Scholar] [PubMed]
- Pyko, A.; Eriksson, C.; Lind, T.; Mitkovskaya, N.; Wallas, A.; Ögren, M.; Östenson, C.G.; Pershagen, G. Long-term exposure to transportation noise in relation to development of obesity-a cohort study. Environ. Health Perspect. 2017, 125, 117005. [Google Scholar] [CrossRef]
- Foraster, M.; Eze, I.C.; Vienneau, D.; Schaffner, E.; Jeong, A.; Héritier, H.; Rudzik, F.; Thiesse, L.; Pieren, R.; Brink, M.; et al. Long-term exposure to transportation noise and its association with adiposity markers and development of obesity. Environ. Int. 2018, 121, 879–889. [Google Scholar] [CrossRef]
- Gamber, K.M.; Huo, L.; Ha, S.; Hairston, J.E.; Greeley, S.; Bjørbæk, C. Over-expression of leptin receptors in hypothalamic POMC neurons increases susceptibility to diet-induced obesity. PLoS ONE 2012, 7, e30485. [Google Scholar] [CrossRef]
- Mc Auley, M.T. Effects of obesity on cholesterol metabolism and its implications for healthy ageing. Nutr. Res. Rev. 2020, 33, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Foraster, M.; Eze, I.C.; Vienneau, D.; Brink, M.; Cajochen, C.; Caviezel, S.; Héritier, H.; Schaffner, E.; Schindler, C.; Wanner, M.; et al. Long-term transportation noise annoyance is associated with subsequent lower levels of physical activity. Environ. Int. 2016, 91, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Markicevic, M.; Savvateev, I.; Grimm, C.; Zerbi, V. Emerging imaging methods to study whole-brain function in rodent models. Transl. Psychiatry 2021, 11, 457. [Google Scholar]
- Hu, Y.; Yu, J.; Cui, X.; Zhang, Z.; Li, Q.; Guo, W.; Zhao, C.; Chen, X.; Meng, M.; Li, Y.; et al. Combination usage of AdipoCount and Image-Pro Plus/ImageJ software for quantification of adipocyte sizes. Front. Endocrinol. 2021, 12, 642000. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Yang, J.; Kim, J.; Jang, Y.; Lee, J.; Han, D.; Kim, H.; Jeong, B.C.; Seong, J.K. Effects of Environmental Noise Stress on Mouse Metabolism. Int. J. Mol. Sci. 2024, 25, 10985. https://doi.org/10.3390/ijms252010985
Lee J, Yang J, Kim J, Jang Y, Lee J, Han D, Kim H, Jeong BC, Seong JK. Effects of Environmental Noise Stress on Mouse Metabolism. International Journal of Molecular Sciences. 2024; 25(20):10985. https://doi.org/10.3390/ijms252010985
Chicago/Turabian StyleLee, Jungmin, Jehoon Yang, Jeyun Kim, Yoonjung Jang, Jisun Lee, Daehyun Han, Hunnyun Kim, Byong Chang Jeong, and Je Kyung Seong. 2024. "Effects of Environmental Noise Stress on Mouse Metabolism" International Journal of Molecular Sciences 25, no. 20: 10985. https://doi.org/10.3390/ijms252010985
APA StyleLee, J., Yang, J., Kim, J., Jang, Y., Lee, J., Han, D., Kim, H., Jeong, B. C., & Seong, J. K. (2024). Effects of Environmental Noise Stress on Mouse Metabolism. International Journal of Molecular Sciences, 25(20), 10985. https://doi.org/10.3390/ijms252010985






