The Impact of Different Anesthetics on the Distribution and Cytotoxic Function of NK Cell Subpopulations: An In Vitro Study
Abstract
1. Introduction
2. Results
2.1. Unaltered NK Cell Percentages and Cytotoxicity upon Anesthetic Exposure
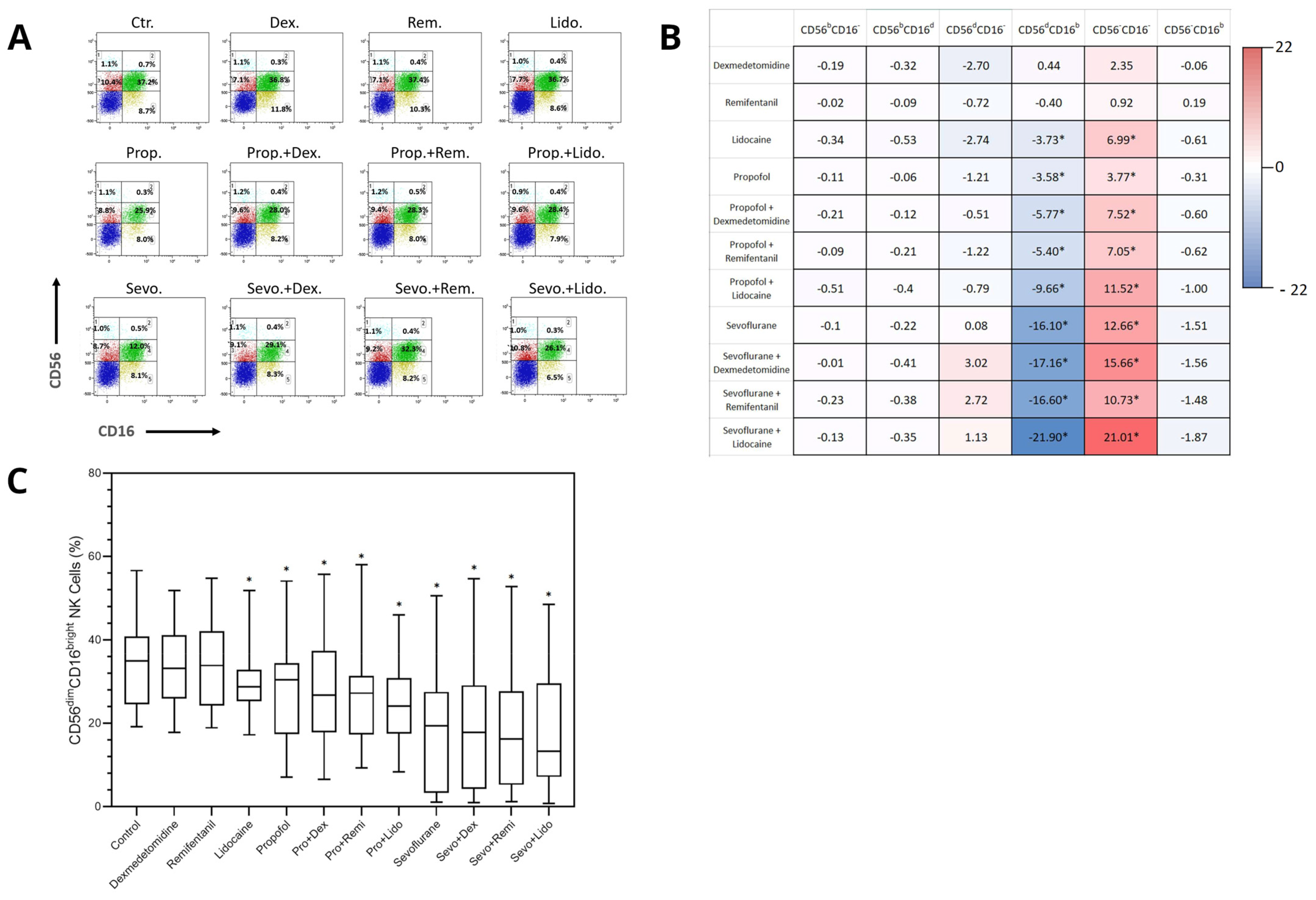
2.2. Differential Effects of Anesthetics on the Distribution of NK Cell Subpopulations
2.3. Anaesthesia Exposure Does Not Affect the Cytotoxicity of NK Cell Subpopulations
3. Discussion
4. Materials and Methods
4.1. Peripheral Blood Mononuclear Cells Isolation
4.2. Exposure of Cells to Anesthetic Agents
4.3. Flow Cytometry Analysis of NK Cells
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cassinello, F.; Prieto, I.; del Olmo, M.; Rivas, S.; Strichartz, G.R. Cancer surgery: How may anesthesia influence outcome? J. Clin. Anesth. 2015, 27, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Yap, A.; Lopez-Olivo, M.A.; Dubowitz, J.; Hiller, J.; Riedel, B. Global Onco-Anesthesia Research Collaboration Group. Anesthetic technique and cancer outcomes: A meta-analysis of total intravenous versus volatile anesthesia. Can. J. Anaesth. 2019, 66, 546–561, Erratum in: Can. J. Anaesth. 2019, 66, 1007–1008. (In English) [Google Scholar] [CrossRef] [PubMed]
- Chang, C.Y.; Wu, M.Y.; Chien, Y.J.; Su, I.M.; Wang, S.C.; Kao, M.C. Anesthesia and Long-term Oncological Outcomes: A Systematic Review and Meta-analysis. Anesth. Analg. 2021, 132, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Mukherjee, M.B.; Jin, Z.; Liu, H.; Lin, K.; Liu, Q.; Dilger, J.P.; Lin, J. The Potential Effect of General Anesthetics in Cancer Surgery: Meta-Analysis of Postoperative Metastasis and Inflammatory Cytokines. Cancers 2023, 15, 2759. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mitsuhata, H.; Shimizu, R.; Yokoyama, M.M. Suppressive effects of volatile anesthetics on cytokine release in human peripheral blood mononuclear cells. Int. J. Immunopharmacol. 1995, 17, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Tazawa, K.; Koutsogiannaki, S.; Chamberlain, M.; Yuki, K. The effect of different anesthetics on tumor cytotoxicity by natural killer cells. Toxicol. Lett. 2017, 266, 23–31. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Melamed, R.; Bar-Yosef, S.; Shakhar, G.; Shakhar, K.; Ben-Eliyahu, S. Suppression of natural killer cell activity and promotion of tumor metastasis by ketamine, thiopental, and halothane, but not by propofol: Mediating mechanisms and prophylactic measures. Anesth. Analg. 2003, 97, 1331–1339. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.A.; Oh, C.S.; Yoon, T.G.; Lee, J.Y.; Lee, S.H.; Yoo, Y.B.; Yang, J.H.; Kim, S.H. The effect of propofol and sevoflurane on cancer cell, natural killer cell, and cytotoxic T lymphocyte function in patients undergoing breast cancer surgery: An in vitro analysis. BMC Cancer 2018, 18, 159. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Buckley, A.; McQuaid, S.; Johnson, P.; Buggy, D.J. Effect of anaesthetic technique on the natural killer cell anti-tumour activity of serum from women undergoing breast cancer surgery: A pilot study. Br. J. Anaesth 2014, 113 (Suppl. 1), i56–i62. [Google Scholar] [CrossRef] [PubMed]
- Salagianni, M.; Baxevanis, C.N.; Papamichail, M.; Perez, S.A. New insights into the role of NK cells in cancer immunotherapy. Oncoimmunology 2012, 1, 205–207. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chan, I.S.; Ewald, A.J. The changing role of natural killer cells in cancer metastasis. J. Clin. Investig. 2022, 132, e143762. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Montaldo, E.; Del Zotto, G.; Della Chiesa, M.; Mingari, M.C.; Moretta, A.; De Maria, A.; Moretta, L. Human NK cell receptors/markers: A tool to analyze NK cell development, subsets and function. Cytom. Part A 2013, 83, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, H.; Hagerling, C.; Werb, Z. Roles of the immune system in cancer: From tumor initiation to metastatic progression. Genes Dev. 2018, 32, 1267–1284. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schneemilch, C.E.; Hachenberg, T.; Ansorge, S.; Ittenson, A.; Bank, U. Effects of different anaesthetic agents on immune cell function in vitro. Eur. J. Anaesthesiol. 2005, 22, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Wall, T.; Sherwin, A.; Ma, D.; Buggy, D.J. Influence of perioperative anaesthetic and analgesic interventions on oncological outcomes: A narrative review. Br. J. Anaesth. 2019, 123, 135–150. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oh, C.S.; Park, H.J.; Piao, L.; Sohn, K.M.; Koh, S.E.; Hwang, D.Y.; Kim, S.H. Expression Profiles of Immune Cells after Propofol or Sevoflurane Anesthesia for Colorectal Cancer Surgery: A Prospective Double-blind Randomized Trial. Anesthesiology 2022, 136, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Sacerdote, P.; Gaspani, L.; Rossoni, G.; Panerai, A.E.; Bianchi, M. Effect of the opioid remifentanil on cellular immune response in the rat. Int. Immunopharmacol. 2001, 1, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Cronin, A.J.; Aucutt-Walter, N.M.; Budinetz, T.; Bonafide, C.P.; DiVittore, N.A.; Gordin, V.; Schuler, H.G.; Bonneau, R.H. Low-dose remifentanil infusion does not impair natural killer cell function in healthy volunteers. Br. J. Anaesth. 2003, 91, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wu, M.; Xu, J.; Wu, C.; Zhang, B.; Wang, G.; Ma, D. Effects of dexmedetomidine on perioperative stress, inflammation, and immune function: Systematic review and meta-analysis. Br. J. Anaesth. 2019, 123, 777–794. [Google Scholar] [CrossRef] [PubMed]
- Cata, J.P.; Ramirez, M.F.; Velasquez, J.F.; Di, A.I.; Popat, K.U.; Gottumukkala, V.; Black, D.M.; Lewis, V.O.; Vauthey, J.N. Lidocaine Stimulates the Function of Natural Killer Cells in Different Experimental Settings. Anticancer Res. 2017, 37, 4727–4732. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, M.F.; Tran, P.; Cata, J.P. The effect of clinically therapeutic plasma concentrations of lidocaine on natural killer cell cytotoxicity. Reg. Anesth. Pain Med. 2015, 40, 43–48. [Google Scholar] [CrossRef] [PubMed]
| Antibody-Mediated Immunity | Proliferation Ability | Cytokine Production | Cytolytic Molecule Production | |
|---|---|---|---|---|
| CD56brightCD16neg | − | ++ | ++ | − |
| CD56brightCD16dim | + | ++ | ++ | − |
| CD56dimCD16neg | − | + | + | ++ |
| CD56dimCD16bright | ++ | + | + | ++ |
| CD56negCD16bright | ++ | + | − | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vulcano, T.J.; Abdulahad, W.H.; van Meurs, M.; Jongman, R.M.; Struys, M.M.R.F.; Bosch, D.J. The Impact of Different Anesthetics on the Distribution and Cytotoxic Function of NK Cell Subpopulations: An In Vitro Study. Int. J. Mol. Sci. 2024, 25, 11045. https://doi.org/10.3390/ijms252011045
Vulcano TJ, Abdulahad WH, van Meurs M, Jongman RM, Struys MMRF, Bosch DJ. The Impact of Different Anesthetics on the Distribution and Cytotoxic Function of NK Cell Subpopulations: An In Vitro Study. International Journal of Molecular Sciences. 2024; 25(20):11045. https://doi.org/10.3390/ijms252011045
Chicago/Turabian StyleVulcano, Tristan J., Wayel H. Abdulahad, Matijs van Meurs, Rianne M. Jongman, Michel M. R. F. Struys, and Dirk J. Bosch. 2024. "The Impact of Different Anesthetics on the Distribution and Cytotoxic Function of NK Cell Subpopulations: An In Vitro Study" International Journal of Molecular Sciences 25, no. 20: 11045. https://doi.org/10.3390/ijms252011045
APA StyleVulcano, T. J., Abdulahad, W. H., van Meurs, M., Jongman, R. M., Struys, M. M. R. F., & Bosch, D. J. (2024). The Impact of Different Anesthetics on the Distribution and Cytotoxic Function of NK Cell Subpopulations: An In Vitro Study. International Journal of Molecular Sciences, 25(20), 11045. https://doi.org/10.3390/ijms252011045






