Current State and Challenges of Tissue and Organ Cryopreservation in Biobanking
Abstract
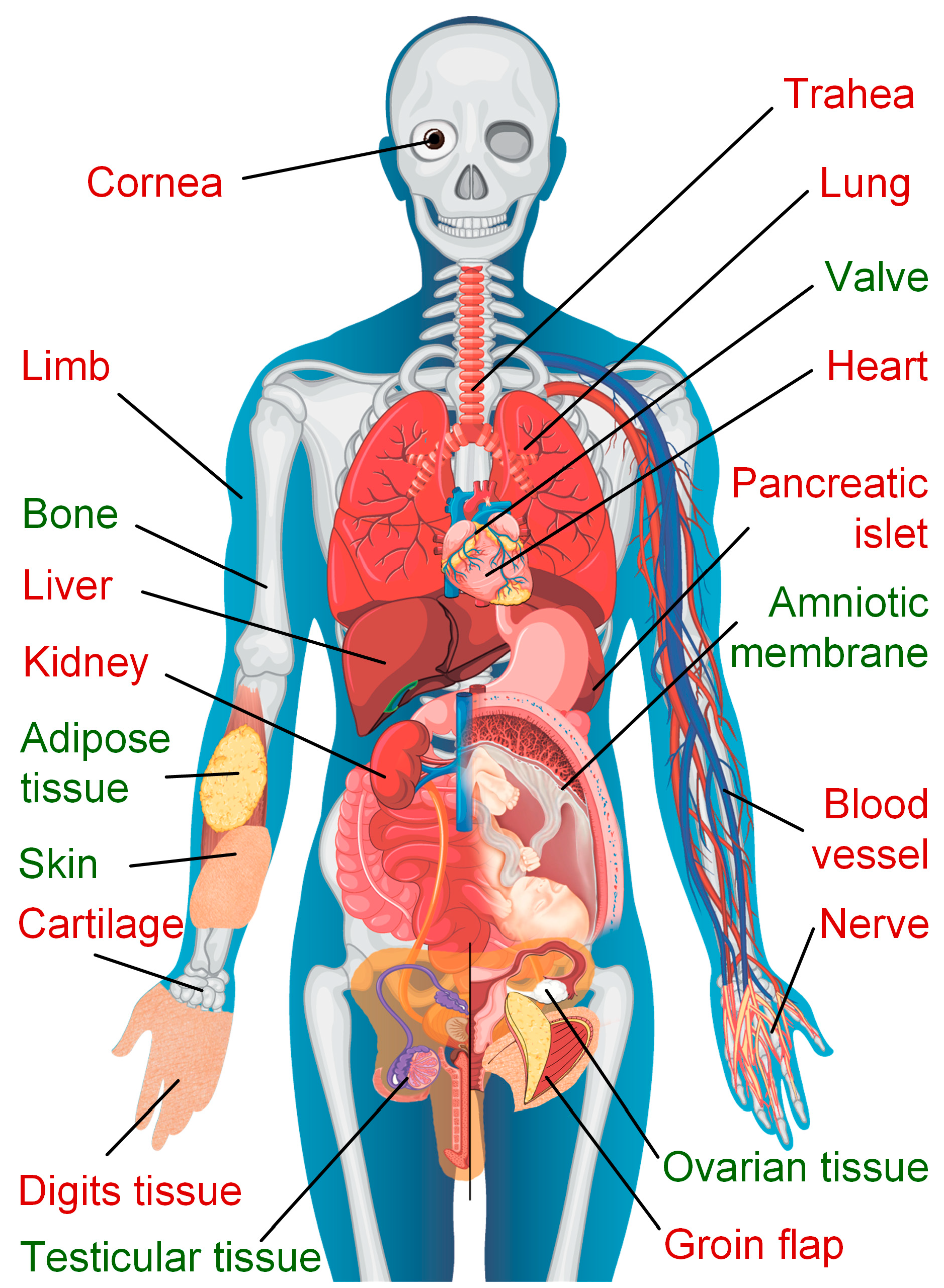
:1. Introduction
2. Current State of Tissue and Organ Cryopreservation
2.1. Ovarian Tissue
2.2. Testicular Tissue
2.3. Heart
2.4. Heart Valve
2.5. Vascular Tissue
2.6. Pancreatic Islet
2.7. Adipose Tissue
2.8. Amniotic Membrane
2.9. Cornea
2.10. Kidney
2.11. Liver
2.12. Cartilage
2.13. Bone
2.14. Trachea
2.15. Complex
2.16. Skin
2.17. Brain and Nerve
2.18. Muscle or Groin Flap
2.19. Other Tissue
3. Biobanking
4. Main Features and Parameters of Cryopreservation
4.1. Cryopreservation Methods
4.1.1. Slow Freezing
4.1.2. Vitrification
4.2. Cryoprotectants
- Permeating: dimethyl sulfoxide (DMSO), glycerol (GLY), 1,2-propanediol (PrOH), ethylene glycol (EG), methanol.
- Non-permeating: polymers (polyvinylpyrrolidone, hydroxyethyl starch, polyethylene glycol) and sugars (raffinose, sucrose, trehalose, glucose, mannitol, galactose).
5. Sperm Cryopreservation
6. Current Challenges of Cryopreservation
6.1. Optimization of Protocols
6.2. Storage and Transfer of Samples
6.3. Large Sample Size
6.4. Cryoinjury
- Controlled, Partial-Ice Freezing
- 2.
- Directional freezing
- 3.
- Machine perfusion
6.5. CPA Toxicity
- Liquidus Tracking
- 2.
- Neutralizing toxicity
- 3.
- Antifreeze proteins and (glyco)proteins as ice-binding proteins
- 4.
- Synthetic polymers
- 5.
- Natural low-molecular-weight CPAs
- 6.
- Permeation for npCPAs
- 7.
- Ice nucleators
- 8.
- Non-Newtonian and Rheomagnetic Fluids
- 9.
- Directional vitrification
- 10.
- Isochoric cryopreservation, thermodynamic equilibrium pressure-enabled cryopreservation
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yokoyama, W.M.; Thompson, M.L.; Ehrhardt, R.O. Cryopreservation and thawing of cells. Curr. Protoc. Immunol. 2012, 99, A.3G.1–A.3G.5. [Google Scholar] [CrossRef]
- Taylor, M.J.; Weegman, B.P.; Baicu, S.C.; Giwa, S.E. New Approaches to Cryopreservation of Cells, Tissues, and Organs. Transfus. Med. Hemother. 2019, 46, 197–215. [Google Scholar] [CrossRef]
- Qin, G.; Jernryd, V.; Sjöberg, T.; Steen, S.; Nilsson, J. Machine Perfusion for Human Heart Preservation: A Systematic Review. Transpl. Int. 2022, 35, 10258. [Google Scholar] [CrossRef]
- Radi, G.; Fallani, G.; Germinario, G.; Busutti, M.; La Manna, G.; Ravaioli, M. Hypothermic Perfusion of the Kidney: From Research to Clinical Practice. Eur. J. Transplant. 2023, 1, 79–91. [Google Scholar] [CrossRef]
- De Vries, R.J.; Tessier, S.N.; Banik, P.D.; Nagpal, S.; Cronin, S.E.J.; Ozer, S.; Hafiz, E.O.A.; van Gulik, T.M.; Yarmush, L.M.; Markmann, J.F.; et al. Supercooling extends preservation time of human livers. Nat. Biotechnol. 2019, 37, 1131–1136. [Google Scholar] [CrossRef]
- Bojic, S.; Murray, A.; Bentley, B.L.; Spindler, R.; Pawlik, P.; Cordeiro, J.L.; Bauer, R.; de Magalhães, J.P. Winter is coming: The future of cryopreservation. BMC Biol. 2021, 19, 56. [Google Scholar] [CrossRef]
- Giwa, S.; Lewis, J.; Alvarez, L.; Langer, R.; Roth, A.; Church, G.; Markmann, J.; Sachs, D.; Chandraker, A.; Wertheim, J.; et al. The promise of organ and tissue preservation to transform medicine. Nat. Biotechnol. 2017, 35, 530–542. [Google Scholar] [CrossRef]
- Meneghel, J.; Kilbride, P.; Morris, G.J. Cryopreservation as a Key Element in the Successful Delivery of Cell-Based Therapies—A Review. Front. Med. 2020, 7, 592242. [Google Scholar] [CrossRef]
- Bai, Y.; Krishnamoorthy, N.; Patel, K.R.; Rosas, I.; Sanderson, M.J.; Ai, X. Cryopreserved human precision-cut lung slices as a bioassay for live tissue banking a viability study of bronchodilation with bitter-taste receptor agonists. Am. J. Respir. Cell Mol. Biol. 2016, 54, 656–663. [Google Scholar] [CrossRef]
- El Cury-Silva, T.; Nunes, M.E.G.; Casalechi, M.; Comim, F.V.; Rodrigues, J.K.; Reis, F.M. Cryoprotectant agents for ovarian tissue vitrification: Systematic review. Cryobiology 2021, 103, 7–14. [Google Scholar] [CrossRef]
- Da Silva, A.M.; Pereira, A.F.; Comizzoli, P.; Silva, A.R. Cryopreservation and Culture of Testicular Tissues: An Essential Tool for Biodiversity Preservation. Biopreserv. Biobank 2020, 18, 235–243. [Google Scholar] [CrossRef]
- Panis, B.; Nagel, M.; Van den Houwe, I. Challenges and prospects for the conservation of crop genetic resources in field genebanks, in in vitro collections and/or in liquid nitrogen. Plants 2020, 9, 1634. [Google Scholar] [CrossRef]
- Sanou, I.; van Maaren, J.; Eliveld, J.; Lei, Q.; Meißner, A.; de Melker, A.A.; Hamer, G.; van Pelt, A.M.M.; Mulder, C.L. Spermatogonial Stem Cell-Based Therapies: Taking Preclinical Research to the Next Level. Front. Endocrinol. 2022, 13, 850219. [Google Scholar] [CrossRef]
- Kometas, M.; Christman, G.M.; Kramer, J.; Rhoton-Vlasak, A. Methods of Ovarian Tissue Cryopreservation: Is Vitrification Superior to Slow Freezing?—Ovarian Tissue Freezing Methods. Reprod. Sci. 2021, 28, 3291–3302. [Google Scholar] [CrossRef]
- Liu, Z.; Cao, H.; Fan, Y.; Wang, Y.; Wang, J. Strong Inhibition of Ice Growth by Biomimetic Crowding Coacervates. Angew. Chem. Int. Ed. 2023, 62, e202311047. [Google Scholar] [CrossRef]
- Argyle, C.E.; Harper, J.C.; Davies, M.C. Oocyte cryopreservation: Where are we now? Hum. Reprod. Update 2016, 22, 440–449. [Google Scholar] [CrossRef]
- Hezavehei, M.; Sharafi, M.; Kouchesfahani, H.M.; Henkel, R.; Agarwal, A.; Esmaeili, V.; Shahverdi, A. Sperm cryopreservation: A review on current molecular cryobiology and advanced approaches. Reprod. Biomed. Online 2018, 37, 327–339. [Google Scholar] [CrossRef]
- Bahsoun, S.; Coopman, K.; Akam, E.C. The impact of cryopreservation on bone marrow-derived mesenchymal stem cells: A systematic review. J. Transl. Med. 2019, 17, 397. [Google Scholar] [CrossRef]
- Fahy, G.M.; Wowk, B.; Pagotan, R.; Chang, A.; Phan, J.; Thomson, B.; Phan, L. Physical and biological aspects of renal vitrification. Organogenesis 2009, 5, 167–175. [Google Scholar] [CrossRef]
- Cole, M. Human Body Anatomy Vectors by Vecteezy. Available online: https://www.vecteezy.com/vector-art/7252402-anatomical-structure-human-bodies (accessed on 6 July 2024).
- Arapaki, A.; Christopoulos, P.; Kalampokas, E.; Triantafyllidou, O.; Matsas, A.; Vlahos, N.F. Ovarian Tissue Cryopreservation in Children and Adolescents. Children 2022, 9, 1256. [Google Scholar] [CrossRef]
- Kolibianaki, E.E.; Goulis, D.G.; Kolibianakis, E.M. Ovarian tissue cryopreservation and transplantation to delay menopause: Facts and fiction. Maturitas 2020, 142, 64–67. [Google Scholar] [CrossRef]
- Silber, S. Chapter 13 Human Ovarian Tissue Vitrification. In Cryopreservation of Mammalian Gametes and Embryos: Methods and Protocols; Nagy, Z.P., Varghese, A.C., Agarwal, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 177–194. [Google Scholar] [CrossRef]
- Silber, S.J.; Derosa, M.; Goldsmith, S.; Fan, Y.; Castleman, L.; Melnick, J. Cryopreservation and transplantation of ovarian tissue: Results from one center in the USA. J. Assist. Reprod. Genet. 2018, 35, 2205–2213. [Google Scholar] [CrossRef]
- Arav, A.; Patrizio, P. Techniques of Cryopreservation for Ovarian Tissue and Whole Ovary. Clin. Med. Insights Reprod. Health 2019, 13, 117955811988494. [Google Scholar] [CrossRef]
- Peng, L.F. Ovarian tissue freezing and activation after thawing: An update. Middle East Fertil. Soc. J. 2021, 26, 9. [Google Scholar] [CrossRef]
- Donnez, J.; Dolmans, M.M.; Pellicer, A.; Diaz-Garcia, C.; Serrano, M.; Schmidt, K.; Ernst, E.; Luyckx, V.; Andersen, C. Restoration of ovarian activity and pregnancy after transplantation of cryopreserved ovarian tissue: A review of 60 cases of reimplantation. Fertil. Steril. 2013, 99, 1503–1513. [Google Scholar] [CrossRef]
- Bahroudi, Z.; Zarnaghi, M.R.; Izadpanah, M.; Abedelahi, A.; Niknafs, B.; Nasrabadi, H.T.; Seghinsara, A.M. Review of ovarian tissue cryopreservation techniques for fertility preservation. J. Gynecol. Obstet. Hum. Reprod. 2022, 51, 102290. [Google Scholar] [CrossRef]
- Xiao, Z.; Wang, Y.; Li, L.; Luo, S.; Li, S.W. Needle immersed vitrification can lower the concentration of cryoprotectant in human ovarian tissue cryopreservation. Fertil. Steril. 2010, 94, 2323–2328. [Google Scholar] [CrossRef]
- Maffei, S.; Hanenberg, M.; Pennarossa, G.; Silva, J.; Brevini, T.; Arav, A.; Gandolfi, F. Direct comparative analysis of conventional and directional freezing for the cryopreservation of whole ovaries. Fertil. Steril. 2013, 100, 1122–1131. [Google Scholar] [CrossRef]
- Kanbar, M.; de Michele, F.; Wyns, C. Cryostorage of testicular tissue and retransplantation of spermatogonial stem cells in the infertile male. Best Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 103–115. [Google Scholar] [CrossRef]
- Future Fertility Programme Oxford. Testicular Tissue. Available online: https://www.ouh.nhs.uk/future-fertility/patients/testicular.aspx (accessed on 4 July 2024).
- Testicular Tissue Cryopreservation. Available online: https://www.cuh.nhs.uk/patient-information/testicular-tissue-cryopreservation/ (accessed on 4 July 2024).
- Kaneko, H.; Kikuchi, K.; Nakai, M.; Somfai, T.; Noguchi, J.; Tanihara, F.; Ito, J.; Kashiwazaki, N. Generation of Live Piglets for the First Time Using Sperm Retrieved from Immature Testicular Tissue Cryopreserved and Grafted into Nude Mice. PLoS ONE 2013, 8, e70989. [Google Scholar] [CrossRef]
- Baert, Y.; Goossens, E.; Van Saen, D.; Ning, L.; Veld, P.I.; Tournaye, H. Orthotopic grafting of cryopreserved prepubertal testicular tissue: In search of a simple yet effective cryopreservation protocol. Fertil. Steril. 2012, 97, 1152–1157.e2. [Google Scholar] [CrossRef]
- Dumont, L.; Arkoun, B.; Jumeau, F.; Milazzo, J.-P.; Bironneau, A.; Liot, D.; Wils, J.; Rondanino, C.; Rives, N. Assessment of the optimal vitrification protocol for pre-pubertal mice testes leading to successful in vitro production of flagellated spermatozoa. Andrology 2015, 3, 611–625. [Google Scholar] [CrossRef]
- Lechiancole, A.; Sponga, S.; Benedetti, G.; Semeraro, A.; Guzzi, G.; Daffarra, C.; Meneguzzi, M.; Nalli, C.; Piani, D.; Bressan, M.; et al. Graft preservation in heart transplantation: Current approaches. Front. Cardiovasc. Med. 2023, 10, 1253579. [Google Scholar] [CrossRef]
- Offerijns, F.G.J.; Krijnen, H.W. The preservation of the rat heart in the frozen state. Cryobiology 1972, 9, 289–295. [Google Scholar] [CrossRef]
- Joshi, P.; Rabin, Y. Thermomechanical stress analyses of nanowarming-assisted recovery from cryopreservation by vitrification in human heart and rat heart models. PLoS ONE 2023, 18, e0290063. [Google Scholar] [CrossRef]
- Montagner, G.; Barbazza, A.; Lugas, A.T.; Terzini, M.; Serino, G.; Bignardi, C.; Cacciatore, M.; Vida, V.L.; Padalino, M.A.; Trojan, D. Decellularized cryopreserved human pericardium: A validation study towards tissue bank practice. Cell Tissue Bank. 2023, 25, 401–410. [Google Scholar] [CrossRef]
- Ma, W.; Lee, K.H.; Delligatti, C.E.; Davis, M.T.; Zheng, Y.; Gong, H.; Kirk, J.A.; Craig, R.; Irving, T. The structural and functional integrities of porcine myocardium are mostly preserved by cryopreservation. J. Gen. Physiol. 2023, 155, e202313345. [Google Scholar] [CrossRef]
- Skific, M.; Golemovic, M.; Safradin, I.; Duric, Z.; Biocina, B.; Cepulic, B.G. Cryopreserved human heart valve allografts: A ten-year single centre experience. Cell Tissue Bank. 2023, 24, 401–416. [Google Scholar] [CrossRef]
- Jashari, R. Transplantation of cryopreserved human heart valves in Europe: 30 years of banking in Brussels and future perspectives. Cell Tissue Bank. 2021, 22, 519–537. [Google Scholar] [CrossRef]
- Burkert, J.; Kochová, P.; Tonar, Z.; Cimrman, R.; Blassová, T.; Jashari, R.; Fiala, R.; Špatenka, J. The time has come to extend the expiration limit of cryopreserved allograft heart valves. Cell Tissue Bank. 2021, 22, 161–184. [Google Scholar] [CrossRef]
- Da Costa, F.D.A. Commentary: We should not forget the aortic valve allograft. J. Thorac. Cardiovasc. Surg. 2023, 165, 1316–1317. [Google Scholar] [CrossRef]
- Peters, M.C.; Kruithof, B.P.T.; Bouten, C.V.C.; Voets, I.K.; van den Bogaerdt, A.; Goumans, M.J.; van Wijk, A. Preservation of human heart valves for replacement in children with heart valve disease: Past, present and future. Cell Tissue Bank. 2023, 25, 67–85. [Google Scholar] [CrossRef]
- European Committee on Organ Transplantation. Cardiovascular tissue. In Guide to the Quality and Safety of Tissues and Cells for Human Application, 4th ed.; EDQM: Strasbourg, France, 2019; Chapter 20; pp. 249–254. [Google Scholar]
- Witten, J.C.; Houghtaling, P.L.; Shrestha, N.K.; Gordon, S.M.; Jaber, W.; Blackstone, E.H.; Pettersson, G.B. Aortic allograft infection risk. J. Thorac. Cardiovasc. Surg. 2023, 165, 1303–1315.e9. [Google Scholar] [CrossRef]
- Fiala, R.; Kochová, P.; Kubíková, T.; Cimrman, R.; Tonar, Z.; Špatenka, J.; Fabián, O.; Burkert, J. Mechanical and structural properties of human aortic and pulmonary allografts do not deteriorate in the first 10 years of cryopreservation and storage in nitrogen. Cell Tissue Bank. 2019, 20, 221–241. [Google Scholar] [CrossRef]
- Jashari, R.; Bouzet, V.; Blanco, M.J.A.; Oleffe, A.; Lecocq, E.; Mastrobuoni, S. Vascular allografts for clinical application in Europe: Assessment of 30 years of experience with vascular tissue banking in Brussels. Cell Tissue Bank. 2023, 24, 613–625. [Google Scholar] [CrossRef]
- Georges, G.; Dakkak, M.; Febrer, G.; Nourissat, G.; Allard, B. Tissue Banking in Canada: A Review of the First 5 Years in Activity of the Largest Public Canadian Vascular Graft Bank. J. Vasc. Surg. 2020, 72, e119–e120. [Google Scholar] [CrossRef]
- Hwang, S.; Bae, J.H.; Kim, I.-O.; Hong, J.-J. Current vascular allograft procurement, cryopreservation and transplantation techniques in the Asan Medical Center Tissue Bank. Ann. Liver Transplant. 2021, 1, 79–85. [Google Scholar] [CrossRef]
- Galeone, A.; Gardellini, J.; Trojan, D.; Nicola, V.D.; Gaetano, R.D.; Faggian, G.; Luciani, G.B. Three Decades of Experience with Aortic Prosthetic Valve Endocarditis. J. Cardiovasc. Dev. Dis. 2023, 10, 338. [Google Scholar] [CrossRef]
- Galeone, A.; Trojan, D.; Gardellini, J.; Di Gaetano, R.; Faggian, G.; Luciani, G.B. Cryopreserved aortic homografts for complex aortic valve or root endocarditis: A 28-year experience. Eur. J. Cardio-Thorac. Surg. 2022, 62, 13–16. [Google Scholar] [CrossRef]
- Fallani, G.; Maroni, L.; Bonatti, C.; Comai, G.; Buzzi, M.; Cuna, V.; Vasuri, F.; Caputo, F.; Prosperi, E.; Pisani, F.; et al. Renal Vessel Extension with Cryopreserved Vascular Grafts: Overcoming Surgical Pitfalls in Living Donor Kidney Transplant. Transpl. Int. 2023, 36, 11060. [Google Scholar] [CrossRef]
- Ahmed, S.B.; Louvancourt, A.; Daniel, G.; Combe, P.; Duprey, A.; Albertini, J.-N.; Favre, J.-P.; Rosset, E. Cryopreserved arterial allografts for in situ reconstruction of abdominal aortic native or secondary graft infection. J. Vasc. Surg. 2018, 67, 468–477. [Google Scholar] [CrossRef]
- Lehalle, B.; Geschier, C.; Fiévé, G.; Stoltz, J.F. Early rupture and degeneration of cryopreserved arterial allografts. J. Vasc. Surg. 1997, 25, 751–752. [Google Scholar] [CrossRef]
- Ren, Z.Y.; Wu, Q.; Pan, B.; Liu, J.; He, Q.; Lang, R.; Lyu, S. Effects of different preservation schemes on isolated rat artery. J. Cell. Mol. Med. 2023, 27, 2362–2371. [Google Scholar] [CrossRef]
- Shani, N.; Friedman, O.; Arav, A.; Natan, Y.; Gur, E. Cryopreservation and Transplantation of Vascularized Composite Transplants: Unique Challenges and Opportunities. Plast. Reconstr. Surg. 2019, 143, 1074e–1080e. [Google Scholar] [CrossRef]
- Janko, M.R.; Bose, S.; Lawrence, P.F. Current status of treatment for aortic graft infection: When should cryopreserved allografts be used? ” Semin. Vasc. Surg. 2019, 32, 81–87. [Google Scholar] [CrossRef]
- Pelisek, J.; Yundung, Y.; Reutersberg, B.; Meuli, L.; Rössler, F.; Rabin, L.; Kopp, R.; Zimmermann, A. Swiss Vascular Biobank: Evaluation of Optimal Extraction Method and Admission Solution for Preserving RNA from Human Vascular Tissue. J. Clin. Med. 2023, 12, 5109. [Google Scholar] [CrossRef]
- Laschke, M.W.; Später, T.; Menger, M.D. Microvascular Fragments: More Than Just Natural Vascularization Units. Trends Biotechnol. 2021, 39, 24–33. [Google Scholar] [CrossRef]
- Nappi, F.; Nenna, A.; Spadaccio, C.; Singh, S.S.A.; Almazil, A.; Acar, C. The Use of the Cryopreserved Aortic Homograft for Aortic Valve Replacement: Is It Still an Option? J. Cardiovasc. Dev. Dis. 2023, 10, 248. [Google Scholar] [CrossRef]
- Kowert, A.; Vogt, F.; Beiras-Fernandez, A.; Reichart, B.; Kilian, E. Outcome after homograft redo operation in aortic position. Eur. J. Cardio-Thorac. Surg. 2012, 41, 404–408. [Google Scholar] [CrossRef]
- Antonopoulos, C.N.; Papakonstantinou, N.A.; Hardy, D.; Lyden, S.P. Editor’s Choice—Cryopreserved Allografts for Arterial Reconstruction after Aorto-Iliac Infection: A Systematic Review and Meta-Analysis. Eur. J. Vasc. Endovasc. Surg. 2019, 58, 120–128. [Google Scholar] [CrossRef]
- Pushkarev, A.V.; Burkov, I.A.; Ivannikova, V.M.; Saakyan, N.Y.; Krotov, A.S.; Tsiganov, D.I. Experimental studies of semiautomatic cryoconservation process for biological tissue specimens. Biomed. Eng. 2024, 57, 387–390. [Google Scholar] [CrossRef]
- Zhan, L.; Rao, J.S.; Sethia, N.; Slama, M.Q.; Han, Z.; Tobolt, D.; Etheridge, M.; Peterson, Q.P.; Dutcher, C.S.; Bischof, J.C. Pancreatic islet cryopreservation by vitrification achieves high viability, function, recovery and clinical scalability for transplantation. Nat. Med. 2022, 28, 798–808. [Google Scholar] [CrossRef]
- Komatsu, H.; Barriga, A.; Medrano, L.; Omori, K.; Kandeel, F.; Mullen, Y. Oxygenated thawing and rewarming alleviate rewarming injury of cryopreserved pancreatic islets. Biochem. Biophys. Res. Commun. 2017, 486, 817–823. [Google Scholar] [CrossRef]
- Kojayan, G.; Whaley, D.; Alexander, M.; Rodriguez, S.; Lee, S.; Lakey, J.R. Improved cryopreservation yield of pancreatic islets using combination of lower dose permeable cryoprotective agents. Cryobiology 2019, 88, 23–28. [Google Scholar] [CrossRef]
- Nakayama-Iwatsuki, K.; Yamanaka, T.; Negishi, J.; Teshima, J.; Tamada, Y.; Hirabayashi, M.; Hochi, S. Transplantation of rat pancreatic islets vitrified-warmed on the nylon mesh device and the silk fibroin sponge disc. Islets 2020, 12, 145–155. [Google Scholar] [CrossRef]
- Nakayama-Iwatsuki, K.; Hirabayashi, M.; Hochi, S. Fabrication of functional rat pseudo-islets after cryopreservation of pancreatic islets or dispersed islet cells. J. Tissue Eng. Regen. Med. 2021, 15, 686–696. [Google Scholar] [CrossRef]
- Ohashi, M. Fat Grafting for Facial Rejuvenation with Cryopreserved Fat Grafts. Clin. Plast. Surg. 2020, 47, 63–71. [Google Scholar] [CrossRef]
- Ibrahiem, S.M.S.; Farouk, A.; Salem, I.L. Facial rejuvenation: Serial fat graft transfer. Alex. J. Med. 2016, 52, 371–376. [Google Scholar] [CrossRef]
- Choudhery, M.S.; Mahmood, R.; Harris, D.T. Stem Cell Banking of Adipose Tissue. Curr. Stem Cell Rep. 2022, 8, 174–183. [Google Scholar] [CrossRef]
- MacRae, J.W.; Tholpady, S.S.; Ogle, R.C.; Morgan, R.F.; Carraway, J.H.; Vasconez, H.C. Ex Vivo Fat Graft Preservation: Effects and Implications of Cryopreservation. Ann. Plast. Surg. 2004, 52, 281–283. [Google Scholar] [CrossRef]
- Crowley, C.A.; Smith, W.P.W.; Seah, K.T.M.; Lim, S.-K.; Khan, W.S. Cryopreservation of Human Adipose Tissues and Adipose-Derived Stem Cells with DMSO and/or Trehalose: A Systematic Review. Cells 2021, 10, 1837. [Google Scholar] [CrossRef]
- Gal, S.; Pu, L.L.Q. An Update on Cryopreservation of Adipose Tissue. Plast. Reconstr. Surg. 2020, 145, 1089–1097. [Google Scholar] [CrossRef]
- Quan, Y.; Wang, J.; Lu, F.; Yuan, Y.; Cai, J. Sequential Grafting of Fresh and Cryopreserved Fat After Mechanical Processing is a Safe and Effective Facial Rejuvenation Strategy. Aesthetic Plast. Surg. 2022, 46, 1432–1438. [Google Scholar] [CrossRef]
- Fénelon, M.; Catros, S.; Meyer, C.; Fricain, J.-C.; Obert, L.; Auber, F.; Louvrier, A.; Gindraux, F. Applications of human amniotic membrane for tissue engineering. Membranes 2021, 11, 387. [Google Scholar] [CrossRef]
- Leal-Marin, S.; Kern, T.; Hofmann, N.; Pogozhykh, O.; Framme, C.; Börgel, M.; Figueiredo, C.; Glasmacher, B.; Gryshkov, O. Human Amniotic Membrane: A review on tissue engineering, application, and storage. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 1198–1215. [Google Scholar] [CrossRef]
- Elkhenany, H.; El-Derby, A.; Elkodous, M.A.; Salah, R.A.; Lotfy, A.; El-Badri, N. Applications of the amniotic membrane in tissue engineering and regeneration: The hundred-year challenge. Stem Cell Res. Ther. 2022, 13, 8. [Google Scholar] [CrossRef]
- Ehredt, D.J.; Zulauf, E.E.; Kim, H.M.; Connors, J. Cryopreserved Amniotic Membrane and Autogenous Adipose Tissue as an Interpositional Spacer After Resection of a Cubonavicular Coalition: A Case Report and Review of the Literature. J. Foot Ankle Surg. 2020, 59, 173–177. [Google Scholar] [CrossRef]
- Ashkani-Esfahani, S.; Waryasz, G.R.; Nakagawa, H.; Panero, A.; Sussman, W.I. Application of Amniotic Membrane Allograft in the Treatment of Foot and Ankle Pathologies: A Review of the Basic Science and Clinical Evidence. J. Foot Ankle Surg. 2022, 10, 209–215. [Google Scholar] [CrossRef]
- Thia, Z.Z.; Tong, L. Update on the role of impression cytology in ocular surface disease. Taiwan J. Ophthalmol. 2019, 9, 141–149. [Google Scholar] [CrossRef]
- Wagner, M.; Walter, P.; Salla, S.; Johnen, S.; Plange, N.; Rütten, S.; Goecke, T.W.; Fuest, M. Cryopreservation of amniotic membrane with and without glycerol additive. Graefe’s Arch. Clin. Exp. Ophthalmol. 2018, 256, 1117–1126. [Google Scholar] [CrossRef]
- Fenelon, M.; Maurel, D.B.; Siadous, R.; Gremare, A.; Delmond, S.; Durand, M.; Brun, S.; Catros, S.; Gindraux, F.; L’Heureux, N.; et al. Comparison of the impact of preservation methods on amniotic membrane properties for tissue engineering applications. Mater. Sci. Eng. C 2019, 104, 109903. [Google Scholar] [CrossRef]
- Bertolin, M.; Dalla Gassa, S.; Trojan, D.; Spagnol, L.; Donisi, P.M.; Camposampiero, D.; Ponzin, D.; Ferrari, S. Cryopreservation of human amniotic membrane (HAM) for ocular surface reconstruction: A comparison beetween protocols. BMJ Open Ophthalmol. 2022, 7, A10. [Google Scholar] [CrossRef]
- Walkden, A. Amniotic membrane transplantation in ophthalmology: An updated perspective. Clin. Ophthalmol. 2020, 14, 2057–2072. [Google Scholar] [CrossRef]
- Gilgenkrantz, H.; de l’Hortet, A.C. Understanding Liver Regeneration: From Mechanisms to Regenerative Medicine. Am. J. Pathol. 2018, 188, 1316–1327. [Google Scholar] [CrossRef]
- Smeringaiova, I.; Utheim, T.P.; Jirsova, K. Ex vivo expansion and characterization of human corneal endothelium for transplantation: A review. Stem Cell Res. Ther. 2021, 12, 554. [Google Scholar] [CrossRef]
- Rodríguez-Fernández, S.; Álvarez-Portela, M.; Rendal-Vázquez, E.; Piñeiro-Ramil, M.; Sanjurjo-Rodríguez, C.; Castro-Viñuelas, R.; Sánchez-Ibáñez, J.; Fuentes-Boquete, I.; Díaz-Prado, S. Analysis of cryopreservation protocols and their harmful effects on the endothelial integrity of human corneas. Int. J. Mol. Sci. 2021, 22, 12564. [Google Scholar] [CrossRef]
- Burgos-Blasco, B.; Vidal-Villegas, B.; Collado-Vincueria, I.; Soria-García, A.M.; Cuiña-Sardiña, R.; Mendez-Fernandez, R.; Diaz-Valle, D.; Ariño-Gutierez, M. Clinical outcomes of long-term corneas preserved frozen in Eusol-C used in emergency tectonic grafts. Cell Tissue Bank. 2023, 24, 351–356. [Google Scholar] [CrossRef]
- European Committee on Organ Transplantation. Ocular tissue. In Guide to the Quality and Safety of Tissues and Cells for Human Application, 4th ed.; EDQM: Strasbourg, France, 2019; Chapter 17; pp. 213–224. [Google Scholar]
- Ganesh, S.; Brar, S.; Rao, P.A. Cryopreservation of extracted corneal lenticules after small incision lenticule extraction for potential use in human subjects. Cornea 2014, 33, 1355–1362. [Google Scholar] [CrossRef]
- Hu, X.; Wei, R.; Liu, C.; Wang, Y.; Yang, D.; Sun, L.; Xia, F.; Liu, S.; Li, M.; Zhou, X. Recent advances in small incision lenticule extraction (SMILE)-derived refractive lenticule preservation and clinical reuse. Eng. Regen. 2023, 4, 103–121. [Google Scholar] [CrossRef]
- Nemcokova, M.; Dite, J.; Klimesova, Y.M.; Netukova, M.; Studeny, P. Preservation of corneal stromal lenticule: Review. Cell Tissue Bank. 2022, 23, 627–639. [Google Scholar] [CrossRef]
- Bandeira, F.; Yam, G.H.F.; Liu, Y.C.; Devarajan, K.; Mehta, J.S. Three-dimensional neurite characterization of small incision lenticule extraction derived lenticules. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4408–4415. [Google Scholar] [CrossRef]
- Liu, Y.; Williams, G.P.; George, B.L.; Soh, Y.Q.; Seah, X.Y.; Peh, G.S.L.; Yam, G.H.F.; Mehta, J.S. Corneal lenticule storage before reimplantation Yu-Chi. Mol. Vis. 2017, 23, 753–764. [Google Scholar]
- Sharma, A.; Rao, J.S.; Han, Z.; Gangwar, L.; Namsrai, B.; Gao, Z.; Ring, H.L.; Magnuson, E.; Etheridge, M.; Wowk, B.; et al. Vitrification and Nanowarming of Kidneys. Adv. Sci. 2021, 8, 2101691. [Google Scholar] [CrossRef]
- Zhan, T.; Liu, K.; Yang, J.; Dang, H.; Chen, L.; Xu, Y. Fe3O4 Nanoparticles with Carboxylic Acid Functionality for Improving the Structural Integrity of Whole Vitrified Rat Kidneys. ACS Appl. Nano Mater. 2021, 4, 13552–13561. [Google Scholar] [CrossRef]
- Zhan, L.; Han, Z.; Shao, Q.; Etheridge, M.L.; Hays, T.; Bischof, J.C. Rapid joule heating improves vitrification based cryopreservation. Nat. Commun. 2022, 13, 6017. [Google Scholar] [CrossRef]
- Han, Z.; Rao, J.S.; Ramesh, S.; Hergesell, J.; Namsrai, B.; Etheridge, M.L.; Finger, E.B.; Bischof, J.C. Model-Guided Design and Optimization of CPA Perfusion Protocols for Whole Organ Cryopreservation. Ann. Biomed. Eng. 2023, 51, 2216–2228. [Google Scholar] [CrossRef]
- Garcia-Dominguez, X.; Vicente, J.S.; Vera-Donoso, C.D.; Marco-Jiménez, F. Successful development of vitrified embryonic kidney after laparoscopy transplantation into non-immunosuppressed hosts. Trends Transplant. 2017, 10, 1–5. [Google Scholar] [CrossRef]
- Matsui, K.; Kinoshita, Y.; Inage, Y.; Matsumoto, N.; Morimoto, K.; Saito, Y.; Takamura, T.; Matsunari, H.; Yamanaka, S.; Nagashima, H. Cryopreservation of Fetal Porcine Kidneys for Xenogeneic Regenerative Medicine. J. Clin. Med. 2023, 12, 2293. [Google Scholar] [CrossRef]
- Marco-Jiménez, F.; Garcia-Dominguez, X.; Jimenez-Trigos, E.; Vera-Donoso, C.D.; Vicente, J.S. Vitrification of kidney precursors as a new source for organ transplantation. Cryobiology 2015, 70, 278–282. [Google Scholar] [CrossRef]
- Takamura, T.; Nagashima, H.; Matsunari, H.; Yamanaka, S.; Saito, Y.; Kinoshita, Y.; Fujimoto, T.; Matsumoto, K.; Nakano, K.; Okano, H. Development of a Cryopreservation Technique for Xenogeneic Kidney Grafts: Evaluation Using a Mouse Model. J. Clin. Med. 2022, 11, 7237. [Google Scholar] [CrossRef]
- Han, Z.; Rao, J.S.; Gangwar, L.; Namsrai, B.; Pasek-Allen, J.L.; Etheridge, M.L.; Wolf, S.M.; Pruett, T.L.; Bischof, J.C.; Finger, E.B. Vitrification and nanowarming enable long-term organ cryopreservation and life-sustaining kidney transplantation in a rat model. Nat. Commun. 2023, 14, 3407. [Google Scholar] [CrossRef]
- Sharma, A.; Bischof, J.C.; Finger, E.B. Liver Cryopreservation for Regenerative Medicine Applications. Regen. Eng. Transl. Med. 2021, 7, 57–65. [Google Scholar] [CrossRef]
- Sharma, A.; Lee, C.Y.; Namsrai, B.; Han, Z.; Tobolt, D.; Rao, J.S.; Gao, Z.; Etheridge, M.L.; Garwood, M.; Clemens, M.G.; et al. Cryopreservation of Whole Rat Livers by Vitrification and Nanowarming. Ann. Biomed. Eng. 2023, 51, 566–577. [Google Scholar] [CrossRef]
- Dou, M.; Lu, C.; Rao, W. Bioinspired materials and technology for advanced cryopreservation. Trends Biotechnol. 2022, 40, 93–106. [Google Scholar] [CrossRef]
- Berendsen, T.A.; Bruinsma, B.G.; Puts, C.F.; Saeidi, N.; Usta, O.B.; Uygun, B.E.; Izamis, M.; Toner, M.; Yarmush, M.L.; Uygun, K. Supercooling enables long-term transplantation survival following 4 days of liver preservation. Nat. Med. 2014, 20, 790–793. [Google Scholar] [CrossRef]
- Gavish, Z.; Ben-Haim, M.; Arav, A. Cryopreservation of whole murine and porcine livers. Rejuvenation Res. 2008, 11, 765–772. [Google Scholar] [CrossRef]
- Manuchehrabadi, N.; Gao, Z.; Zhang, J.; Ring, H.L.; Shao, Q.; Liu, F.; McDermott, M.; Fok, A.; Rabin, Y.; Brockbank, K.G.M. Improved tissue cryopreservation using inductive heating of magnetic nanoparticles. Sci. Transl. Med. 2017, 9, eaah4586. [Google Scholar] [CrossRef]
- He, J.; Wine, I.; Wu, K.; Sevick, J.; Laouar, L.; Jomha, N.M.; Westover, L. Effect of vitrification on mechanical properties of porcine articular cartilage. Proc. Inst. Mech. Eng. H 2022, 236, 1521–1527. [Google Scholar] [CrossRef]
- Ead, M.; Wu, K.; Jar, C.; Duke, K.; Jomha, N.; Westover, L. Mechanical Properties of Fresh, Frozen and Vitrified Articular Cartilage. Ann. Biomed. Eng. 2023, 51, 2001–2012. [Google Scholar] [CrossRef]
- Chen, P.; Wang, S.; Chen, Z.; Ren, P.; Hepfer, R.G.; Greene, E.D.; Campbell, L.H.; Helke, K.L.; Nie, X.; Jensen, J.H.; et al. Nanowarming and ice-free cryopreservation of large sized, intact porcine articular cartilage. Commun. Biol. 2023, 6, 220. [Google Scholar] [CrossRef]
- Williams, S.K.; Amiel, D.; Ball, S.T.; Allen, R.T.; Wong, V.W.; Chen, A.C.; Sah, R.L.; Bugbee, W.D. Prolonged Storage Effects on the Articular Cartilage of Fresh Human Osteochondral Allografts. J. Bone Jt. Surg.-Am. Vol. 2003, 85, 2111–2120. [Google Scholar] [CrossRef]
- Wu, K.; Yong, K.W.; Ead, M.; Sommerfeldt, M.; Skene-Arnold, T.D.; Westover, L.; Duke, K.; Laouar, L.; Elliott, J.A.W.; Jomha, N.M. Vitrified Particulated Articular Cartilage for Joint Resurfacing: A Swine Model. Am. J. Sports Med. 2022, 50, 3671–3680. [Google Scholar] [CrossRef]
- Johnston, D.T.; Lohmeier, S.J.; Langdell, H.C.; Pyfer, B.J.; Komisarow, J.; Powers, D.B.; Erdmann, D. Current Concepts in Cranial Reconstruction: Review of Alloplastic Materials. Plast. Reconstr. Surg. Glob. Open 2022, 10, E4466. [Google Scholar] [CrossRef]
- Younger, E.M.; Chapman, M.W. Morbidity at Bone Graft Donor Sites. J. Orthop. Trauma 1989, 3, 192–195. [Google Scholar] [CrossRef]
- Visser, N.; Rezaie, E.; Ducharme, A.; Shin, A.Y.; Bishop, A.T. The effect of surgical revascularization on the mechanical properties of cryopreserved bone allograft in a porcine tibia model. J. Orthop. Res. 2023, 41, 815–822. [Google Scholar] [CrossRef]
- Donnelly, B.M.; Smolar, D.E.; Baig, A.A.; Soliman, M.A.R.; Monteiro, A.; Gibbons, K.J.; Levy, E.I.; Snyder, K.V. Analysis of craniectomy bone flaps stored in a neurosurgical cryopreservation freezer: Microorganism culture results and reimplantation rates. Acta Neurochir. 2023, 165, 3187–3195. [Google Scholar] [CrossRef]
- Rezaie, E.S.; Visser, N.J.; van den Berg, C.; Shin, A.Y.; Bishop, A.T. Vasculogenic gene therapy: No role for revitalization of structural bone allografts. J. Orthop. Res. 2023, 41, 1014–1021. [Google Scholar] [CrossRef]
- Deluiz, D.; Delcroix, G.J.-R.; Fraga, S.R.G.; D’Ippolito, G.; Grau-Monge, C.; Bonnin-Marquez, A.; Reiner, T.; Amadeu, T.; Tinoco, E.M.B.; Schiller, P.C. Viable cryopreserved human bone graft exhibit superior osteogenic properties in mandibular lateral augmentation. Sci. Rep. 2023, 13, 1422. [Google Scholar] [CrossRef]
- Forcier, R.J.; Heussner, R.T.; Newsom, L.; Giers, M.B.; Al Rawashdeh, W.; Buchanan, K.A.; Woods, E.J.; Johnstone, B.H.; Higgins, A.Z. Accelerating cryoprotectant delivery using vacuum infiltration. Cryobiology 2023, 112, 104558. [Google Scholar] [CrossRef]
- Pilbauerová, N.; Suchánek, J. Cryopreservation of Dental Stem Cells. Acta Medica 2018, 61, 1–7. [Google Scholar] [CrossRef]
- Kaku, M.; Kamada, H.; Kawata, T.; Koseki, H.; Abedini, S.; Kojima, S.; Motokawa, M.; Fujita, T.; Ohtani, J.; Tsuka, N.; et al. Cryopreservation of periodontal ligament cells with magnetic field for tooth banking. Cryobiology 2010, 61, 73–78. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, L.; Zhou, M.; Yi, S.; Ran, J.; Long, Y.; Luo, J.; Tian, K. Can delayed grafting of frozen teeth achieve periodontal ligament healing? Med. Hypotheses 2022, 167, 110945. [Google Scholar] [CrossRef]
- Stocco, E.; Barbon, S.; Mammana, M.; Trojan, D.; Bianchin, A.; Favaretto, F.; Contran, M.; Zambello, G.; Vogliardi, A.; Confalonieri, M. Development and In Vitro/In Vivo Comparative Characterization of Cryopreserved and Decellularized Tracheal Grafts. Cells 2023, 12, 888. [Google Scholar] [CrossRef]
- Wei, S.; Yang, B.; Bi, T.; Zhang, W.; Sun, H.; Cui, Y.; Li, G.; Zhang, A. Tracheal replacement with aortic grafts: Bench to clinical practice. Regen. Ther. 2023, 24, 434–442. [Google Scholar] [CrossRef]
- Hysi, I.; Kipnis, E.; Fayoux, P.; Copin, M.; Zawadzki, C.; Jashari, R.; Hubert, T.; Ung, A.; Ramon, P.; Jude, B. Successful orthotopic transplantation of short tracheal segments without immunosuppressive therapy. Eur. J. Cardio-Thorac. Surg. 2014, 47, e54–e61. [Google Scholar] [CrossRef]
- Zeng, N.; Chen, Y.; Wu, Y.; Zang, M.; Largo, R.; Chang, E.; Schaverien, M.; Yu, P.; Zhang, Q. Pre-epithelialized cryopreserved tracheal allograft for neo-trachea flap engineering. Front. Bioeng. Biotechnol. 2023, 11, 1196521. [Google Scholar] [CrossRef]
- Tsou, K.C.; Hung, W.T.; Ju, Y.T.; Liao, H.C.; Hsu, H.H.; Chen, J.S. Application of aortic allograft in trachea transplantation. J. Formos. Med. Assoc. 2023, 122, 940–946. [Google Scholar] [CrossRef]
- Dickey, R.M.; Hembd, A.S.; Fruge, S.; Haddock, N.T.; Papas, K.K.; Suszynski, T.M. Composite Tissue Preservation. Ann. Plast. Surg. 2020, 84, 711–716. [Google Scholar] [CrossRef]
- He, B.; Su, S.; Yuan, G.; Duan, J.; Zhu, Z.; Wang, Z. Clinical guideline for vascularized composite tissue cryopreservation. J. Tissue Eng. Regen. Med. 2021, 15, 527–533. [Google Scholar] [CrossRef]
- Wang, Z.; He, B.; Duan, Y.; Shen, Y.; Zhu, L.; Zhu, X.; Zhu, Z. Cryopreservation and replantation of amputated rat hind limbs. Eur. J. Med. Res. 2014, 19, 28. [Google Scholar] [CrossRef]
- Arav, A.; Friedman, O.; Natan, Y.; Gur, E.; Shani, N. Rat Hindlimb Cryopreservation and Transplantation: A Step Toward “Organ Banking”. Am. J. Transplant. 2017, 17, 2820–2828. [Google Scholar] [CrossRef]
- Bakhach, J. Xenotransplantation of cryopreserved composite organs on the rabbit. Organogenesis 2009, 5, 127–133. [Google Scholar] [CrossRef]
- Osada, A.; Matsumine, H.; Kamei, W.; Sakurai, H. Usefulness of avulsed fingertip skin for reconstruction after digital amputation. Case Rep. Plast. Surg. Hand Surg. 2020, 7, 23–29. [Google Scholar] [CrossRef]
- Antonioli, B.; Tosca, M.C.; Pintaudi, B.; Guidoni, F.; Galuzzi, M.; Pelizzoni, C.; Manasseri, B.; Grimaldi, M.C.; Sesana, G.; Bertuzzi, F. Human skin processing affects clinical outcome in allograft recipients. Burns 2023, 49, 797–805. [Google Scholar] [CrossRef]
- Schlottmann, F.; Strauß, S.; Ziesing, S.; Reineke, C.; Ipaktchi, R.; Weyand, B.; Krezdorn, N.; Vogt, P.M.; Bucan, V. Organization of Hannover Skin Bank: Sterile culture and procurement protocols for viable cryopreserved allogeneic skin grafts of living donors. Int. Wound, J. 2024, 21, e14374. [Google Scholar] [CrossRef]
- Staud, C.J.; Resch, A.; Christ, A.; Borger, A.; Zaussinger, M.; Teufelsbauer, M.; Worel, N.; Radtke, C. Skin Bank Establishment in Treatment of Severe Burn Injuries: Overview and Experience with Skin Allografts at the Vienna Burn Center. J. Clin. Med. 2023, 12, 4717. [Google Scholar] [CrossRef]
- Matera, D.; Jacob, L. The Novel Use of Cryopreserved Human Allograft in Extensive Hurley Stage III Hidradenitis Suppurativa. Wounds 2023, 35, E134–E138. [Google Scholar] [CrossRef]
- Iyun, A.; Ademola, S.A.; Olawoye, O.A.; Michael, A.I.; Aderibigbe, R.O.; Iyun, O.I.; Oluwatosin, O.M. Glycerolised skin allografts for extensive burns in low- and middle-income countries. J. West Afr. Coll. Surg. 2021, 11, 35. [Google Scholar] [CrossRef]
- Canatelli-Mallat, M.; Lascaray, F.; Entraigues-Abramson, M.; Portiansky, E.L.; Blamaceda, N.; Morel, G.R.; Goya, R.G. Cryopreservation of a Human Brain and Its Experimental Correlate in Rats. Rejuvenation Res. 2020, 23, 516–525. [Google Scholar] [CrossRef]
- Melnik, M.; Miyoshi, E.; Ma, R.; Corrada, M.; Kawas, C.; Bohannan, R.; Caraway, C.; Miller, C.A.; Hinman, J.D.; John, V.; et al. Simultaneous isolation of intact brain cells and cell-specific extracellular vesicles from cryopreserved Alzheimer’s disease cortex. J. Neurosci. Methods 2024, 406, 110137. [Google Scholar] [CrossRef]
- Xue, W.; Li, H.; Xu, J.; Yu, X.; Liu, L.; Liu, H.; Zhao, R.; Shao, Z. Effective cryopreservation of human brain tissue and neural organoids. Cell Rep. Methods 2024, 4, 100777. [Google Scholar] [CrossRef]
- Holzer, P.; Chang, E.J.; Rogers, K.; Tarlton, J.; Lu, D.; Gillespie, N.; Adkins, J.; Metea, M.; LaRochelle, A.; Wicks, J. Large-gap peripheral nerve repair using xenogeneic transplants in rhesus macaques. Xenotransplantation 2023, 30, e12792. [Google Scholar] [CrossRef]
- Arav, A.; Li, S.; Friedman, O.; Solodeev, I.; Aouizerate, J.; Kedar, D.; De Antonio, M.; Natan, D.; Gur, E.; Shani, N. Long-Term Survival and Functional Recovery of Cryopreserved Vascularized Groin Flap and Below-the-Knee Rat Limb Transplants. Rejuvenation Res. 2023, 26, 180–193. [Google Scholar] [CrossRef]
- Zhang, F.; Attkiss, K.J.; Walker, M.; Buncke, H.J. Effect of cryopreservation on survival of composite tissue grafts. J. Reconstr. Microsurg. 1998, 14, 559–564. [Google Scholar] [CrossRef]
- Lautner, L.J.; Freed, D.H.; Nagendran, J.; Acker, J.P. Current techniques and the future of lung preservation. Cryobiology 2020, 94, 1–8. [Google Scholar] [CrossRef]
- Gook, D.A. Human Ovarian Tissue Slow Freezing. In Cryopreservation of Mammalian Gametes and Embryos: Methods and Protocols; Nagy, Z.P., Varghese, A.C., Agarwal, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; Chapter 12; pp. 161–176. [Google Scholar] [CrossRef]
- Dhonnabháin, B.N.; Elfaki, N.; Fraser, K.; Petrie, A.; Jones, B.; Saso, S.; Hardiman, P.J.; Getreu, N. A comparison of fertility preservation outcomes in patients who froze oocytes, embryos, or ovarian tissue for medically indicated circumstances: A systematic review and meta-analysis. Fertil. Steril. 2022, 117, 1266–1276. [Google Scholar] [CrossRef]
- Nakano, M.S.; Simoes, R.S.; Baracat, M.C.P.; Lobel, A.; Shiroma, M.E.; Igami, D.Z.; Serafini, P.C.; Soares, J.M., Jr.; Baracat, E.C. Live birth rate after ovarian tissue cryopreservation followed by autotransplantation in cancer patients: A systematic review. Gynecological and Reproductive. Gynecol. Reprod. Endocrinol. Metab. 2020, 1, 89–94. Available online: https://gremjournal.com/wp-content/uploads/2020/06/04_Soares.pdf (accessed on 10 July 2024).
- Faizal, A.M.; Sugishita, Y.; Suzuki-Takahashi, Y.; Iwahata, H.; Takae, S.; Horage-Okutsu, Y.; Suzuki, N. Twenty-first century oocyte cryopreservation—In vitro maturation of immature oocytes from ovarian tissue cryopreservation in cancer patients: A systematic review. Women’s Health 2022, 18, 17455057221114269. [Google Scholar] [CrossRef]
- Onofre, J.; Kadam, P.; Baert, Y.; Goossens, E. Testicular tissue cryopreservation is the preferred method to preserve spermatogonial stem cells prior to transplantation. Reprod. Biomed. Online 2020, 40, 261–269. [Google Scholar] [CrossRef]
- Kabiri, D.; Safrai, M.; Gropp, M.; Hidas, G.; Mordechai-Daniel, T.; Meir, K.; Revel, A.; Imbar, T.; Reubinoff, B. Establishment of a controlled slow freezing-based approach for experimental clinical cryopreservation of human prepubertal testicular tissues. F S Rep. 2022, 3, 47–56. [Google Scholar] [CrossRef]
- Golemovic, M. Ten-year experience with cryopreserved vascular allografts in the Croatian Cardiovascular Tissue Bank. Cell Tissue Bank. 2022, 23, 807–824. [Google Scholar] [CrossRef]
- Brockbank, K.G.M.; Bischof, J.C.; Chen, Z.; Greene, E.D.; Gao, Z.; Campbell, L.H. Ice Control during Cryopreservation of Heart Valves and Maintenance of Post-Warming Cell Viability. Cells 2022, 11, 1856. [Google Scholar] [CrossRef]
- Mohkam, K.; Darnis, B.; Rode, A.; Hetsch, N.; Balbo, G.; Bourgeot, J.-P.; Mezoughi, S.; Demian, H.; Ducerf, C.; Mabrut, J.-Y. Rescue arterial revascularization using cryopreserved iliac artery allograft in liver transplant patients. Exp. Clin. Transplant. 2017, 15, 420–424. [Google Scholar] [CrossRef]
- Favaretto, F.; Compagnin, C.; Cogliati, E.; Montagner, G.; Dell’Antonia, F.; Berna, G.; Vettor, R.; Milan, G.; Trojan, D. Characterization of Human Subcutaneous Adipose Tissue and Validation of the Banking Procedure for Autologous Transplantation. Int. J. Mol. Sci. 2023, 24, 8190. [Google Scholar] [CrossRef]
- Rodríguez-Ares, M.T.; López-Valladares, M.J.; Touriño, R.; Vieites, B.; Gude, F.; Silva, M.T.; Couceiro, J. Effects of lyophilization on human amniotic membrane. Acta Ophthalmol. 2009, 87, 396–403. [Google Scholar] [CrossRef]
- Pogozhykh, O.; Prokopyuk, V.; Prokopyuk, O.; Kuleshova, L.; Goltsev, A.; Figueiredo, C.; Pogozhykh, D. Towards biobanking technologies for natural and bioengineered multicellular placental constructs. Biomaterials 2018, 185, 39–50. [Google Scholar] [CrossRef]
- European Committee on Organ Transplantation. Amniotic membrane. In Guide to the Quality and Safety of Tissues and Cells for Human Application; EDQM: Strasbourg, France, 2019; Chapter 18; pp. 227–233. [Google Scholar]
- Gousias, K.; Stricker, I.; Hoyer, A.; Theocharous, T.; Rompf, C.; Pranada, A.B.; Tannapfel, A.; Agrawal, R.; Tischoff, I. Explanted Skull Flaps after Decompressive Hemicraniectomy Demonstrate Relevant Bone Avitality-Is Their Reimplantation Worth the Risk? Brain Sci. 2023, 13, 1277. [Google Scholar] [CrossRef]
- Lin, M.T.Y.; Lee, I.X.Y.; Chen, W.-L.; Chen, M.-Y.; Mehta, J.S.; Yam, G.H.F.; Peh, G.S.L.; Liu, Y.-C. Culture of Primary Neurons from Dissociated and Cryopreserved Mouse Trigeminal Ganglion. Tissue Eng. Part C Methods 2023, 29, 381–393. [Google Scholar] [CrossRef]
- University of British Columbia Office of Research Ethics. Available online: https://ethics.research.ubc.ca (accessed on 1 May 2021).
- Benson, E.E.; Betsou, F.; Fuller, B.J.; Harding, K.; Kofanova, O. Translating cryobiology principles into trans-disciplinary storage guidelines for biorepositories and biobanks: A concept paper. Cryo-Letters 2013, 34, 277–312. [Google Scholar]
- Fuller, B.J.; Petrenko, A.Y.; Rodriguez, J.V.; Somov, A.Y.; Balaban, C.L.; Guibert, E.E. Biopreservation of hepatocytes: Current concepts on hypothermic preservation, cryopreservation, and vitrification. Cryo-Letters 2016, 37, 432–452. [Google Scholar]
- Liu, D.; Pan, F. Advances in cryopreservation of organs. J. Huazhong Univ. Sci. Technol.-Med. Sci. 2016, 36, 153–161. [Google Scholar] [CrossRef]
- Jang, T.H.; Park, S.C.; Yang, J.H.; Kim, J.Y.; Seok, J.H.; Park, U.S.; Choi, C.W.; Lee, S.R.; Han, J. Cryopreservation and its clinical applications. Integr. Med. Res. 2017, 6, 12–18. [Google Scholar] [CrossRef]
- Parihar, A.; Kumar, A.; Panda, U.; Khan, R.; Parihar, D.S.; Khan, R. Cryopreservation: A Comprehensive Overview, Challenges, and Future Perspectives. Adv. Biol. 2023, 7, 2200285. [Google Scholar] [CrossRef]
- Raju, R.; Bryant, S.J.; Wilkinson, B.L.; Bryant, G. The need for novel cryoprotectants and cryopreservation protocols: Insights into the importance of biophysical investigation and cell permeability. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2021, 1865, 129749. [Google Scholar] [CrossRef]
- Liu, J.; Phy, J.; Yeomans, E. Theoretic considerations regarding slow cooling and vitrification during cryopreservation. Theriogenology 2012, 78, 1641–1652. [Google Scholar] [CrossRef]
- Onofre, J.; Baert, Y.; Faes, K.; Goossens, E. Cryopreservation of testicular tissue or testicular cell suspensions: A pivotal step in fertility preservation. Hum. Reprod. Update 2016, 22, 744–761. [Google Scholar] [CrossRef]
- Chang, T.; Zhao, G. Ice Inhibition for Cryopreservation: Materials, Strategies, and Challenges. Adv. Sci. 2021, 8, 2002425. [Google Scholar] [CrossRef]
- Roumiantsev, P.O.; Mudunov, A.M. Biobanking in oncology and radiology. Endocr. Surg. 2018, 11, 170–177. [Google Scholar] [CrossRef]
- Sieme, H.; Oldenhof, H.; Wolkers, W.F. Mode of action of cryoprotectants for sperm preservation. Anim. Reprod. Sci. 2016, 169, 2–5. [Google Scholar] [CrossRef]
- Ozimic, S.; Ban-Frangez, H.; Stimpfel, M. Sperm Cryopreservation Today: Approaches, Efficiency, and Pitfalls. Curr. Issues Mol. Biol. 2023, 45, 4716–4734. [Google Scholar] [CrossRef]
- Tharasanit, T.; Thuwanut, P. Oocyte Cryopreservation in Domestic Animals and Humans: Principles, Techniques and Updated Outcomes. Animals 2021, 11, 2949. [Google Scholar] [CrossRef]
- Benson, J.D.; Woods, E.J.; Walters, E.M.; Critser, J.K. The cryobiology of spermatozoa. Theriogenology 2012, 78, 1682–1699. [Google Scholar] [CrossRef]
- Peris, S.I.; Morrier, A.; Dufour, M.; Bailey, J.L. Cryopreservation of Ram Semen Facilitates Sperm DNA Damage: Relationship Between Sperm Andrological Parameters and the Sperm Chromatin Structure Assay. J. Androl. 2004, 25, 224–233. [Google Scholar] [CrossRef]
- Isachenko, E.; Isachenko, V.; Katkov, I.I.; Dessole, S.; Nawroth, F. Vitrification of mammalian spermatozoa in the absence of cryoprotectants: From past practical difficulties to present success. Reprod. BioMed. Online 2003, 6, 191–200. [Google Scholar] [CrossRef]
- Barbas, J.P.; Mascarenhas, R.D. Cryopreservation of domestic animal sperm cells. Cell Tissue Bank. 2009, 10, 49–62. [Google Scholar] [CrossRef]
- Desrosiers, P.; Légaré, C.; Leclerc, P.; Sullivan, R. Membranous and structural damage that occur during cryopreservation of human sperm may be time-related events. Fertil. Steril. 2006, 85, 1744–1752. [Google Scholar] [CrossRef]
- Tao, Y.; Sanger, E.; Saewu, A.; Leveille, M.-C. Human sperm vitrification: The state of the art. Reprod. Biol. Endocrinol. 2020, 18, 17. [Google Scholar] [CrossRef]
- Karimfar, M.; Niazvand, F.; Haghani, K.; Ghafourian, S.; Shirazi, R.; Bakhtiyari, S. The protective effects of melatonin against cryopreservation-induced oxidative stress in human sperm. Int. J. Immunopathol. Pharmacol. 2015, 28, 69–76. [Google Scholar] [CrossRef]
- Öztürk, A.E.; Bucak, M.N.; Bodu, M.; Başpınar, N.; Çelik, İ.; Shu, Z.; Keskin, N.; Gao, D. Cryobiology and Cryopreservation of Sperm. In Cryopreservation—Current Advances and Evaluations; Quain, M., Ed.; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Squires, E.L.; Keith, S.L.; Graham, J.K. Evaluation of alternative cryoprotectants for preserving stallion spermatozoa. Theriogenology 2004, 62, 1056–1065. [Google Scholar] [CrossRef]
- Best, B.P. Cryoprotectant Toxicity: Facts, Issues, and Questions. Rejuvenation Res. 2015, 18, 422–436. [Google Scholar] [CrossRef]
- Ahmad, E.; Aksoy, M. Trehalose as a Cryoprotective Agent for the Sperm Cells: A mini review Trehalose as a Cryoprotective Agent for the Sperm Cells: A Mini Review. Anim. Health Prod. Hyg. 2012, 1, 123–129. [Google Scholar]
- Hungerford, A.; Bakos, H.W.; Aitken, R.J. Sperm cryopreservation: Current status and future developments. Reprod. Fertil. Dev. 2022, 35, 265–281. [Google Scholar] [CrossRef]
- Murray, A.; Kilbride, P.; Gibson, M.I. Trehalose in cryopreservation. Applications, mechanisms and intracellular delivery opportunities. RSC Med. Chem. 2024, 15, 2980–2995. [Google Scholar] [CrossRef]
- Blommaert, D.; Franck, T.; Donnay, I.; Lejeune, J.-P.; Detilleux, J.; Serteyn, D. Substitution of egg yolk by a cyclodextrin-cholesterol complex allows a reduction of the glycerol concentration into the freezing medium of equine sperm. Cryobiology 2016, 72, 27–32. [Google Scholar] [CrossRef]
- Maulida, S.; Eriani, K.; Nur, F.M.; Fadli, N.; Batubara, A.S.; Muhammadar, A.A.; Siti-Azizah, M.N.; Wilkes, M.; Muchlisin, Z.A. Effect of cryoprotectant on the motility, viability, fertilization, and DNA integrity of naleh fish Barbonymus sp. (Cyprinidae) sperm. Braz. J. Vet. Res. Anim. Sci. 2021, 58, e168702. [Google Scholar] [CrossRef]
- Lewis, J.K.; Bischof, J.C.; Braslavsky, I.; Brockbank, K.G.M.; Fahy, G.M.; Fuller, B.J.; Rabin, Y.; Tocchio, A.; Woods, E.J.; Wowk, B.G.; et al. The Grand Challenges of Organ Banking: Proceedings from the first global summit on complex tissue cryopreservation. Cryobiology 2016, 72, 169–182. [Google Scholar] [CrossRef]
- Baust, J.G.; Snyder, K.K.; Van Buskirk, R.; Baust, J.M. Integrating Molecular Control to Improve Cryopreservation Outcome. Biopreserv. Biobank. 2017, 15, 134–141. [Google Scholar] [CrossRef]
- Strand, J.; homsen, H.; Jensen, J.B.; Marcussen, C.; Nicolajsen, T.B.; Skriver, M.B.; Søgaard, I.M.; Ezaz, T.; Purup, S.; Callesen, H.; et al. Biobanking in amphibian and reptilian conservation and management: Opportunities and challenges. Conserv. Genet. Resour. 2020, 12, 709–725. [Google Scholar] [CrossRef]
- Criswell, T.; Swart, C.; Stoudemire, J.; Brockbank, K.G.M.; Powell-Palm, M.; Stilwell, R.; Floren, M. Freezing Biological Time: A Modern Perspective on Organ Preservation. Stem Cells Transl. Med. 2023, 12, 17–25. [Google Scholar] [CrossRef]
- Fioretta, E.S.; Motta, S.E.; Lintas, V.; Loerakker, S.; Parker, K.K.; Baaijens, F.P.T.; Falk, V.; Hoerstrup, S.P.; Emmert, M.Y. Next-generation tissue-engineered heart valves with repair, remodelling and regeneration capacity. Nat. Rev. Cardiol. 2021, 18, 92–116. [Google Scholar] [CrossRef]
- Baker, M. Building better biobanks. Nature 2012, 486, 141–146. [Google Scholar] [CrossRef]
- Gurruchaga, H.; Saenz del Burgo, L.; Hernandez, R.M.; Orive, G.; Selden, C.; Fuller, B.; Ciriza, J.; Pedraz, J.L. Advances in the slow freezing cryopreservation of microencapsulated cells. J. Control. Release 2018, 281, 119–138. [Google Scholar] [CrossRef]
- Pan, J.; Shu, Z.; Ren, S.; Gao, D. Determination of Dielectric Properties of Cryoprotective Agent Solutions with a Resonant Cavity for the Electromagnetic Rewarming in Cryopreservation. Biopreserv. Biobank. 2017, 15, 404–409. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, G.; Zhang, Z.; Xu, X.; He, X. Magnetic induction heating of superparamagnetic nanoparticles during rewarming augments the recovery of hUCM-MSCs cryopreserved by vitrification. Acta Biomater. 2016, 33, 264–274. [Google Scholar] [CrossRef]
- Arav, A. Cryopreservation by Directional Freezing and Vitrification Focusing on Large Tissues and Organs. Cells 2022, 11, 1072. [Google Scholar] [CrossRef]
- Davidson, A.F.; Glasscock, C.; McClanahan, D.R.; Benson, J.D.; Higgins, A.Z. Toxicity Minimized Cryoprotectant Addition and Removal Procedures for Adherent Endothelial Cells. PLoS ONE 2015, 10, e0142828. [Google Scholar] [CrossRef]
- Wolkers, W.F.; Oldenhof, H. Cryopreservation and Freeze-Drying Protocols; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Hughes, Z.E.; Mancera, R.L. Molecular Mechanism of the Synergistic Effects of Vitrification Solutions on the Stability of Phospholipid Bilayers. Biophys. J. 2014, 106, 2617–2624. [Google Scholar] [CrossRef]
- Budke, C.; Dreyer, A.; Jaeger, J.; Gimpel, K.; Berkemeier, T.; Bonin, A.S.; Nagel, L.; Plattner, C.; DeVries, A.L.; Sewald, N.; et al. Quantitative efficacy classification of ice recrystallization inhibition agents. Cryst. Growth Des. 2014, 14, 4285–4294. [Google Scholar] [CrossRef]
- Naing, A.H.; Kim, C.K. A brief review of applications of antifreeze proteins in cryopreservation and metabolic genetic engineering. 3 Biotech 2019, 9, 329. [Google Scholar] [CrossRef]
- Murray, K.A.; Gibson, M.I. Chemical approaches to cryopreservation. Nat. Rev. Chem. 2022, 6, 579–593. [Google Scholar] [CrossRef]
- Dou, M.; Lu, C.; Sun, Z.; Rao, W. Natural cryoprotectants combinations of L-proline and trehalose for red blood cells cryopreservation. Cryobiology 2019, 91, 23–29. [Google Scholar] [CrossRef]
- Rao, W.; Huang, H.; Wang, H.; Zhao, S.; Dumbleton, J.; Zhao, G.; He, X. Nanoparticle-mediated intracellular delivery enables cryopreservation of human adipose-derived stem cells using trehalose as the sole cryoprotectant. ACS Appl. Mater. Interfaces 2015, 7, 5017–5028. [Google Scholar] [CrossRef]
- Kilbride, P.; Meneghel, J.; Lamb, S.; Morris, J.; Pouzet, J.; Jurgielewicz, M.; Leonforte, C.; Gibson, D.; Madrigal, A. Recovery and Post-Thaw Assessment of Human Umbilical Cord Blood Cryopreserved as Quality Control Segments and Bulk Samples. Biol. Blood Marrow Transpl. 2019, 25, 2447–2453. [Google Scholar] [CrossRef]
- Preciado, J.A.; Rubinsky, B. Isochoric preservation: A novel characterization method. Cryobiology 2010, 60, 23–29. [Google Scholar] [CrossRef]

| Tissue/Cryopreservation Method | Object | CPA | Sample Size | Results | Reference |
|---|---|---|---|---|---|
| Ovarian Tissue | |||||
| SF | Human | PrOH/SUC/ALB | 1 mm | 85% of intact oocytes and 74% of intact pre-granulosa cells. Preservation of function assessed by xenografting and longevity—in 1 human patient over 5 years. | [152] |
| V | Human | EG/DMSO/SSS + SUC | 10 × 10 × 1 mm3 | Hormonal return by 150 days and menstruation by 130 days. 92% viability of oocytes and 5 live births. | [23,24] |
| Mostly SF | Human | - | - | 43.8% clinical pregnancy rate, 32.3% live birth rate, 7.5% miscarriage rate. | [153] |
| Mostly SF | Human | - | - | 57% live birth rate. | [154] |
| DF | Sheep | FBS/DMSO | Whole organ | Results similar to fresh organ and higher than slow freezing: 89% intact follicles; 17,041 nuclei/mm2 stromal cell density; intact vessels. | [30] |
| Not specified | Human | - | Slices | 33.84% successful in vitro maturation of immature oocytes. 50–76.9% fertilization rate. | [155] |
| Testicular Tissue | |||||
| CSF | Mouse | DMSO/SUC | <1.8 mL | Similar to control: 47.6 ± 19.2 donor-derived colony numbers and 13.4 ± 7.2 × 104 cell recovery. Worse than control: 83 ± 2% post-thaw cell viability and 6.2 ± 0.2 pups per litter. | [156] |
| USF | Human | DMSO/SUC/HSA | 3 mm3 | Similar to control: structural integrity/morphology; intact seminiferous tubules; SSCs and Sertoli cells. | [157] |
| USF | Mice | DMSO/SUC | - | Similar to control: cellular integrity; 92% graft survival. Lower than control: 77 ± 12% intact tubules. | [35] |
| CSF | Human | DMSO/SUC/HSA | 3 mm3 | Similar to control: structural integrity/morphology; Sertoli cells; intact seminiferous tubules. Higher than control: SSCs with number of OCT4-stained cells. | [157] |
| V | Human | DMSO/EG/HSA | 3 mm3 | Similar to control: structural integrity/morphology; SSCs and Sertoli cells; intact seminiferous tubules. | [157] |
| Solid-surface V | Mice | DMSO/EG | 3 mm3 | Similar to control: 75% graft survival; 157 ± 109% of tubules completed spermatogenesis. Worse than control: 24 ± 22% damaged SSCs; 75 ± 9% intact tubules. | [35] |
| Heart | |||||
| CSF and USF | Wistar Rats | DMSO, O2, CO2 | Whole organ | 10–15% successful reanimation. Always could be reanimated, if supercooling occurred. Below freezing point, resuscitation impossible. 10–18-days-old animals survived after −30 °C. | [38] |
| USF | Minipigs | Skinning solution | ~0.5 cm3 | Similar to control: myofilament lattice arrangements; structural integrity; contractility. | [41] |
| Heart Valve | |||||
| CSF | Human | DMSO | - | Infective endocarditis: 47% 3-year survival rates; 76% freedom from re-operation. Non-infectious heart disease: 1 case of hemothorax; no graft-related complications. | [42,158] |
| CSF and V | Pig | VS55, VS49, or DP6/DIS/SUC/TRE | - | Muscle and leaflet viability were the same after vitrification and nanowarming. Increasing the CPA loading duration increased aorta viability after vitrification. | [159] |
| CSF | Human | DMSO | - | Similar to control: Young’s modulus and thickness. For AAHV, Young’s modulus is higher if stored <5 years; for PAHV, lower. | [49] |
| Not specified | Human | - | - | Mortality: 2.8% (nonendocarditis group) and 7.2% (endocarditis group). Probability of infection: 5.6%/14% (20 years) in the nonendocarditis/endocarditis groups. | [48] |
| Vascular Tissue | |||||
| CSF | Human | DMSO | 5 cm | Used for reconstruction of the middle hepatic vein branches, hepatobiliary and vascular surgeries. | [52] |
| Not specified | Human | DMSO | - | Commercial protocol. No available data. | [60] |
| CSF | Human | HA/DMSO | - | Similar to control: 1.9% vascular complications. 1-year survival rate: 100%. Higher than control: 5.6% intraoperative complications. Lower: 37% postoperative. | [60] |
| CSF | Human | DMSO/HA | - | Early postoperative complications: 50.7%; survival rates at 5 years: 54%. Early postoperative reintervention: 15.5%; late reinterventions: 18.3%. | [56,160] |
| Pancreatic Islet | |||||
| V | Mouse, Pig, Human | EG/DMSO | - | Similar to control: 87.4% islet viability in humans at 9 months. 96.7 ± 1.5% islet recovery in humans; 92% restored normoglycemia within 24–48 h in mice. | [67] |
| CSF | Mouse, Pig, Human | DMSO | - | 59.1–62.2% islet viability (lower than vitrification). | [67] |
| Not specified | Human | Islet cryopreservation solution | 0.075 mm2 | Volume change was only reduced by oxygenation in the long-term recovery rate. The oxygenated method suppressed ischemia- and inflammation-related gene upregulation. | [68] |
| CSF | Human, Mice, Rat | DMSO | - | Better with EG: 80.4 ± 2.6% yield, 80 ± 1.5% viability, and 3.4 ± 0.4 glucose-stimulated insulin release after 48-h culture in humans; euglycemia achieved 12 days sooner. | [69] |
| V | Rat | EG/DMSO/FBS + SUC | 0.03 mm2 | Lower than control: viability. Similar to control: stimulation index and response to glucose uptake. 80.0–83.3% euglycemia in 3 weeks; 93.8–97.1% recovery rate. | [70] |
| Adipose tissue | |||||
| CSF | Human | DMSO/TRE or TRE | - | Less functional (G3PDH), even with no obvious differences. SVF = 90% of control. Similar to control: cell viability at 2 weeks. | [77] |
| CSF | Human | Not precise | 4 mL | Similar to control: histology. No complications for all patients. Higher than control: 14.8 × 105/mL SVF. | [72] |
| USF | Human | 10 mL | 43.1 ± 7.2% of initial volume preserved at 6 months. Complications: 9 infections; 7 formations of nodules; 3 hematomas; 3 traumatic fat necroses. | [78] | |
| USF | Human | 10 mL | 91% of initial volume preserved at 12 months. The least favorable results were for leg and lip. Complications: 7 formations of nodules; 3 hematomas; 3 traumatic fat necroses. | [73] | |
| CSF | Human | DMSO/HA | 50 mL | Similar to control: 65.7–86.7% cell viability (not affected by subsequent temporary storage at −80 °C); expression of specific membrane markers; adipogenic abilities. | [161] |
| Amniotic Membrane | |||||
| USF | Human | GLY | 7.6 cm × 1.9 cm | Similar to control: % of degenerated cells without CPA, integrity ± CPA, tensile strength, and Young’s modulus. Worse than control: % of degenerated cells with CPA; cell viability. | [85] |
| USF | Human | GLY/RPMI or GLY/DMEM | 3.8 × 0.7 cm2 [86] 4 × 4 cm2 [162] | Similar to control: strain at break, resorption behavior [86], protein and growth factor levels [162]. Worse than control: maximum force [86]. Epithelial cells slightly damaged by cryopreservation. | [86,162] |
| CSF | Human | DMSO/FBS | 25 mm2 | Similar to control: levels of pluripotency gene expression. Worse than control: metabolic activity, significantly. | [163] |
| CSF | Human | GLY or DMSO | - | - | [164] |
| Cornea | |||||
| CSF | Human | HSA/DMSO (#1); DMSO (#2); VS55 (#3); EC/PrG/DMSO (#4). | 1 cm2 | Worse than control: all cell monolayers disrupted; cell density decreased; disruption in the collagen matrix of stroma (#1, 3, 4 CPA); epithelia flattened (#1, 2); separation between endothelia and Bowman’s layers (2); endothelia not visible in central part (#3, 4). | [91] |
| Not specified | Human | Eusol-C | 1 cm2 | 76% of corneas achieved epithelization; 50% were clear; 38% developed neovascularization. Complications: 34% reintervention; 8% positive microbial cultures; 4% corneal abscess. | [92] |
| Kidney | |||||
| V | Rat | EC/DMSO/FA/EG/X-1000/Z-1000 | Whole organ | After transplantation, kidneys reperfused immediately and uniformly, making urine in 2 min. | [102] |
| V, Nano | Rat | EC + VS55 + mNPs | 5.5 × 4.5 × 1.5 cm | Cooling faster than CCR at −45 to −90 °C and rewarming faster than CWR; intact morphology. Good viability; increased red cell death in the glomeruli; vascular endothelial lining intact. | [99] |
| V, Nano | Rat | VS55 | <5 mL | Better than bath thawing: maximum thermal stress, flow resistance. Rewarming faster than CWR. Internal renal cell structure slightly damaged; vascular network slightly injured; 38 ± 8% renal cell apoptosis. | [100] |
| V | Rabbit (Embryonic Kidneys) | EG/DMSO/N-MET/MP/POLY/X-1000 or Z-1000/FBS | - | 35% metanephroi from 16-day-old embryos were successfully grown, underwent differentiation, and developed histologically mature glomeruli. Histomorphometry similar to nonfrozen. | [103] |
| V | Microminiature Pig (Embryonic Kidneys) | FBS/EG/DMSO | 1.37 × 0.90 mm2 | Kidneys increased in size and reddish at 14 days. 4 glomeruli per slide and sparse medulla, similar to native neonatal. Presence of glomeruli, proximal and distal tubules, and collecting ducts. | [104] |
| Liver | |||||
| V, Nano | Rat | EG/sucrose/EC + mNPs | <8 × 13 cm2 | Complete removal of the mNPs and CPA. Temperature gradient below cracking. Intact cellular and sinusoidal architecture, preserved endothelium and hepatocyte function, and homogeneous perfusion. ALT levels slightly higher than control; LDH levels similar to control. | [109] |
| DF | Rat, Pig | UW/EG | Whole organ | Pig: 75–90% viability; satisfactory reperfusion, no blood flow obstructions. Warming up with homogeneous color within a few minutes; slow bilious-stained secretion. | [112] |
| CSF | Murine | EDTA+ UW/EG | Whole liver | Less than control: 60 ± 15% bile production (1) and 84 ± 17% (2); 63 ± 11% integrity of hepatocyte cell (1) and 85 ± 6% (2). Similar to control: tissue architecture. Normal presence of the epitope. | [112] |
| CSF | Pig | UW/EG | Whole liver | Viability ranging from 75% to 90%. Transplanted liver was functional. | [112] |
| Cartilage | |||||
| V | Pig | VS83,VS70 or VS55 | 30 × 20 × 14 mm | Better 1 than 2: no crack formation; cell metabolic activity; cell viability; live cells. Similar among 1, 2, control: conductivities; tissue porosity; permeability. | [116] |
| CSF and V | Pig | DMSO (CSF); EG/DMSO/CS/PrOH/SUC(V) | Ø7 mm | Similar among CSF, V, control: peak modulus. Higher in V and control than CSF: equilibrium modulus and relaxation time constants. | [115] |
| USF and V | Pig | -(USF) DMSO/EG/PrOH(V) | Ø10 mm | Similar among USF, V, control: peak stress; secant modulus; equilibrium stress values and Young’s modulus. Different from control: USF and V in stress relaxation time constants. | [114] |
| Bone | |||||
| USF | Mini-Pig | - | 3.5 cm | Worse than control: 616 ± 67 bone mineral density; TID, IDI, AvgCID, AvgED higher; compressive modulus. 50–87% healed tibiae. | [121] |
| USF | Mini-Pig | - | 3.5 cm | Higher in group with growth factors: healing, periosteal bridging, callus remodeling and union, intramedullary and cortical vascular volumes. 31% cutaneous vascular tumors. | [123] |
| USF | Human | - | - | Postcranioplasty infections in 12% of patients with reimplantation. 1-year mortality rate in reimplantation group: 16%. | [122] |
| USF | Human | DMSO | 250–1000 µm | Better than control: 35.18 ± 4.98 mm3 volume gain, collagen and osteopontin expression. Worse than control: 0.63 ± 0.03 mg/cm3 bone mineral density, 46.25 ± 4.36 percent bone volume. | [124] |
| USF | Human | 0.5 × 0.5 cm | Similar for 1 and 2: 1.61% vs. 2.34% PTH1 positive cells. Better 1 than 2: 6.91% OPG-positive cells. Avital/total tissue surface: 2.51% (#1) vs. 0.03% (#2). | [165] | |
| Trachea | |||||
| USF | Pig | DMSO | 2.5–3.0 cm | The shortest/longest survival period was 1 day/147 days. Average 61.8 days. Fused tissue was formed between the allograft aorta and tracheal cartilage of the original trachea. | [133] |
| CSF | Pig | DMSO, HSA | 5 cm | Similar to control: shape, compression, structure, cytoplasmic granules, GAGs, collagen. Less than control: DNA content, immunoreactivity, elastic fiber pattern and content. Cartilagineous compartment intact and cellular elements at the epithelium side at implantation. | [129] |
| CSF | Rabbit | DMSO | ~6 cm | 19.6 ± 16.7 survival time. Satisfactory stiffness. Varying levels of neoangiogenesis and inflammatory infiltration. | [131] |
| CSF | Rat | FBS/DMSO/SUC | - | Similar to control: chondrocyte viability; at implantation: obliteration, cartilage necrosis, epithelium loss, abundant fibroblasts, macrophage infiltration, and significant immunorejection. | [132] |
| Complex | |||||
| DF | Rat | FBS/DMSO/sucrose | Whole limb | Greatest cell viability in 10% DMSO (88.8 ± 0.9%). Cell necrosis and debris increased without cryoprotectant (88.0 ± 6.2%). | [136] |
| DF | Rat | DMSO/FBS/TRE | Whole limb | Limbs had viable muscle, skin, and blood vessels. Histological structure, nuclei were preserved. Cell size was uniform, and there was no evidence of extreme inflammation. | [137] |
| V | Rat | DMSO/EG/TRE | Whole limb | Limbs had viable muscle, skin, and blood vessels. Histological structure, nuclei were preserved. Cell size was uniform, and there was no evidence of extreme inflammation. | [137] |
| Skin | |||||
| CSF | Human | DMSO | 4 × 15 cm | No dead cells or tissue observed over the period of 21 days. | [141] |
| Not specified | Human | DMSO | 1 mm × 3–8 cm | Tissue viability decreased significantly after freezing. | [140] |
| Not specified | Human | DMSO | 25 × 5 cm × 0.5 mm | Intact layers of epidermal tissue. | [142] |
| Not specified | Human | - | - | 99% of graft over the wound at 1 week. Wound contracture, granulation tissue and improved contour after 2nd allograft. Grafts well incorporated with no disease recurrence at 1 year. | [143] |
| Brain and nerve | |||||
| USF | Human | DMSO/GLY | Whole brain | Tissue appeared significantly softened | [145] |
| USF | Rat | DMSO/GLY | Whole brain | No significant differences were detected among groups in brain weight and dimensions, thickness of the cortex and hippocampus, and cell diameter of NeuNir neurons. Mature neuron numbers were comparable; neuron diameter revealed a significant shrinkage. Well-preserved density of synaptic connections. DCX neurons showed a significant shrinkage. No significant changes in TH cell diameter. | [145] |
| CSF | Mice | FBS/DMSO | 0.6–0.7 cm in length | Neurite outgrowth and morphology were comparable to control. Successful isolation of TG neuron cells. No apparent difference in the length of neurite outgrowth; slightly thinner neurites in cryopreserved group. Comparable length and morphology of typical long thread-like axon and short branched dendrite to control. Comparable results in the following parameters: number of neuronal bodies, total neurite length, number of neurite nodes, and number of neurites. | [166] |
| USF | Human | Methylcellulose, EG, DMSO, Y27632 | - | Apoptotic cells reduced in methylcellulose-, EG-, and PVP- treated organoids. TRE-, SUC-, glucose-, and proline-treated organoids showed a larger number of dead cells. Almost all cells in DMSO apoptotic. Functional cytoarchitectures of cortical organoids well preserved. Cell populations, gene expression, or cell spatial distribution of cortical organoids not disrupted. Glutamatergic synapse connections maintained, survival and neural function of organoids maintained. Cell diversity and structure in multiple brain-region-specific organoids preserved. | [147] |
| CSF | Pig | - | 4 cm | No adverse events. Functional recovery equivalent between xenogeneic and autologous limbs for fresh and frozen. Partial remyelination of fast-conducting fibers (autologous and xenogeneic). For xenogeneic transplants, little to no myelination was observed. Median sensory nerve conduction velocity did not return to preoperative baseline levels. Sensory nerve conduction velocity reduced after operation. A nearly complete loss of action potential was observed in all limbs. Maximum recovery was observed 8 months postoperatively. No major qualitative differences were observed in xenogeneic transplant vs. autograft electrophysiology. White blood cell counts and individual component percentages remained within normal ranges following nerve reconstruction with xenogeneic nerve transplant for the entirety of the study (12 months). Xenogeneic transplantation sites had a greater degree of inflammation. No residual porcine tissue in the xenogeneic nerve tissue. | [148] |
| Muscle or groin flap | |||||
| CSF | Rat | DMSO/GLY/glucose | 4.5 × 4.5 cm | All groin cutaneous flaps, gracilis muscle flaps were necrotic at 3 to 5 days postoperatively. Microscopic evidence of edema, red cell extravasation, and fibrin clot formation in the vascular vessels of groin cutaneous flaps. | [150] |
| V and CSF | Rat | DMSO/EG/Trehalose/HSA(V) | ~10 × 4 × 3 cm | Blood vessel damage after >10 s in LN slush and 2 min in LN vapor. Reduced survival of cryopreserved for 8–9 days compared to flaps cryopreserved for 24–48 h (protocol: two-step vitrification, cryopreservation in −80 °C for 24–48 h or 8–9 days, then transplantation). All surviving below-the-knee limbs demonstrated normal skin, fat muscle, and bone histology. «Safe cooling». Long-term survival of below-the-knee limbs cryopreserved by vitrification >Tg was significantly superior to that of limbs cryopreserved by DF or cooled to temperatures below the vitrification solution Tg. Below-the-knee limb was viable and able to support the rat’s weight, also demonstrating the regrowth of both total neuronal fibers and motor neurons. | [149] |
| Parameter | Slow Freezing | Vitrification |
|---|---|---|
| working time [171] | more than 3 h | fast, less than 10 min |
| cost [171] | expensive, freezing machine needed | inexpensive, no special machine needed |
| sample volume (µL) [171] | 100–250, wide range of sample volumes [6] | 1–2 |
| concentration of CPA [171] | low, more homogeneous distribution of CPAs, which need to diffuse through and permeate the extracellular space to take full effect [6] | high |
| risk of cryoinjury including ice crystal formation [171] | high due to the formation of extracellular ice [171] | low |
| post-thaw viability [171] | high | high |
| risk of toxicity due to CPA [171] | low | high |
| status of system [171] | closed system only | open or closed system |
| potential contamination with pathogenic agents [8,171] | low | high |
| manipulation skill [171] | easy | difficult |
| costly components [6] | equipment: variable-speed freezer [171] | operation costs |
| controllability/reproducibility [6] | high | low |
| main risks [6] | extracellular ice formation, osmotic damage | intracellular ice formation, CPA toxicity, fracturing, osmotic stress [6] risk of contamination with pathogenic agents [171] |
| Cryoprotectant | Advantages | Disadvantages |
|---|---|---|
| Glycerol [178,188,189] | The most widely used penetrating cryoprotectant, preserves sperm viability and motility at relatively low concentrations | Toxic at high concentrations [190] |
| DMSO [181,184] | Offers better protection of motility compared to glycerol [13] | Toxic at high concentrations [190] |
| Trehalose [191,192] | Effective in maintaining high sperm motility and mitochondrial activity, allows reducing the concentration of penetrating protectants and increases sperm survival [193] | No data on its effect on sperm preservation for some animal species [193] |
| Cyclodextrin–cholesterol [178,194] | Maintains membrane stability during cryopreservation, allows reducing the concentration of penetrating cryoprotectants | Only used in combination with penetrating cryoprotectants |
| Egg yolk [178,189,194,195] | Maintains membrane stability during cryopreservation | Only used in combination with penetrating cryoprotectants, undefined composition |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khaydukova, I.V.; Ivannikova, V.M.; Zhidkov, D.A.; Belikov, N.V.; Peshkova, M.A.; Timashev, P.S.; Tsiganov, D.I.; Pushkarev, A.V. Current State and Challenges of Tissue and Organ Cryopreservation in Biobanking. Int. J. Mol. Sci. 2024, 25, 11124. https://doi.org/10.3390/ijms252011124
Khaydukova IV, Ivannikova VM, Zhidkov DA, Belikov NV, Peshkova MA, Timashev PS, Tsiganov DI, Pushkarev AV. Current State and Challenges of Tissue and Organ Cryopreservation in Biobanking. International Journal of Molecular Sciences. 2024; 25(20):11124. https://doi.org/10.3390/ijms252011124
Chicago/Turabian StyleKhaydukova, Irina V., Valeria M. Ivannikova, Dmitry A. Zhidkov, Nikita V. Belikov, Maria A. Peshkova, Peter S. Timashev, Dmitry I. Tsiganov, and Aleksandr V. Pushkarev. 2024. "Current State and Challenges of Tissue and Organ Cryopreservation in Biobanking" International Journal of Molecular Sciences 25, no. 20: 11124. https://doi.org/10.3390/ijms252011124
APA StyleKhaydukova, I. V., Ivannikova, V. M., Zhidkov, D. A., Belikov, N. V., Peshkova, M. A., Timashev, P. S., Tsiganov, D. I., & Pushkarev, A. V. (2024). Current State and Challenges of Tissue and Organ Cryopreservation in Biobanking. International Journal of Molecular Sciences, 25(20), 11124. https://doi.org/10.3390/ijms252011124






