The Evolution of NLR Inflammasome and Its Mediated Pyroptosis in Metazoa
Abstract
1. Introduction
2. Results
2.1. The Existence of NLRs in Metazoa and Their Structural Domain Composition
2.2. The Evolutionary Relationship of NLRs in Metazoa
2.3. The Existence of ASCs and PYD in Metazoa
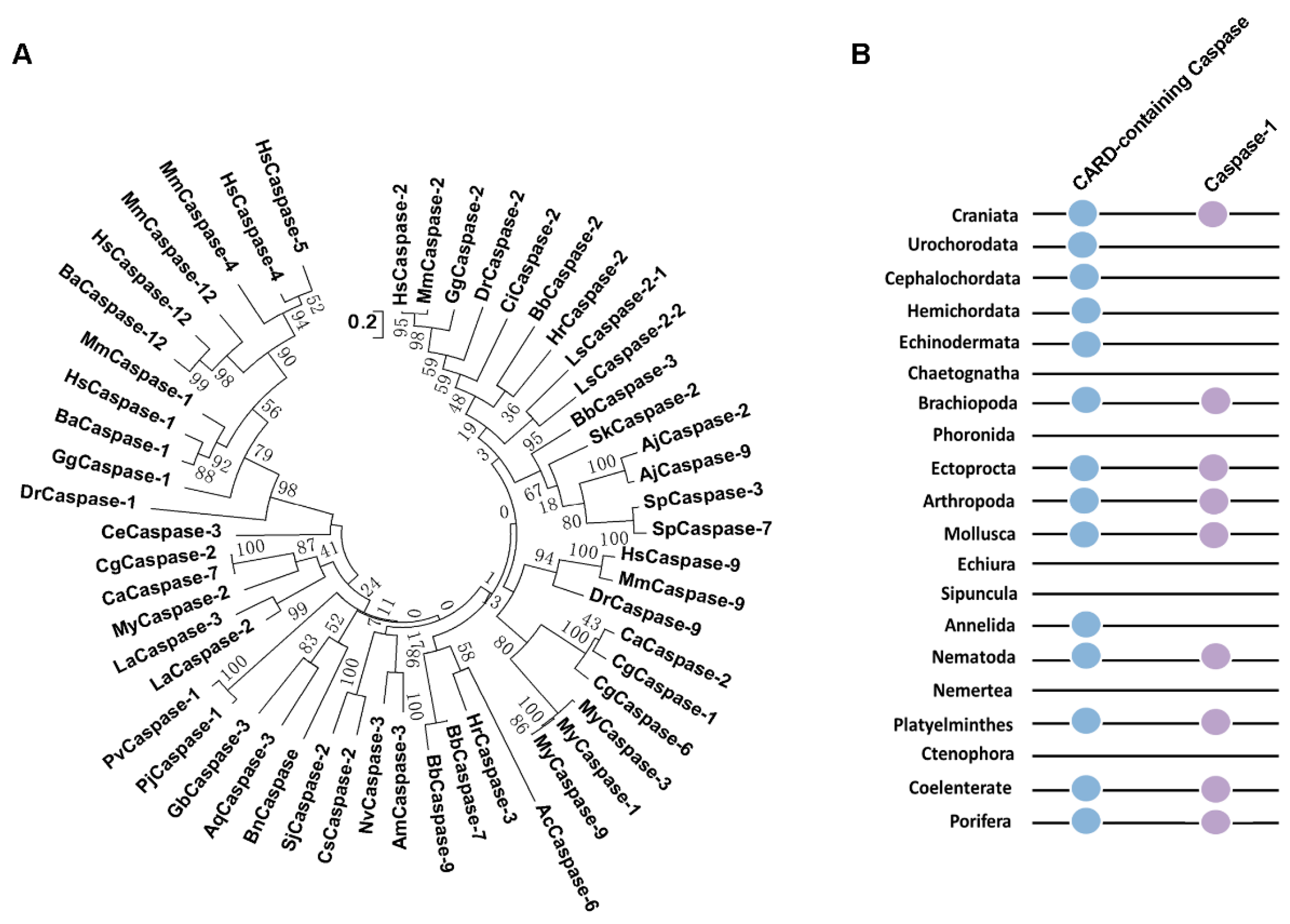
2.4. The Evolutionary Relationship of CARD-Containing Caspases in Metazoa
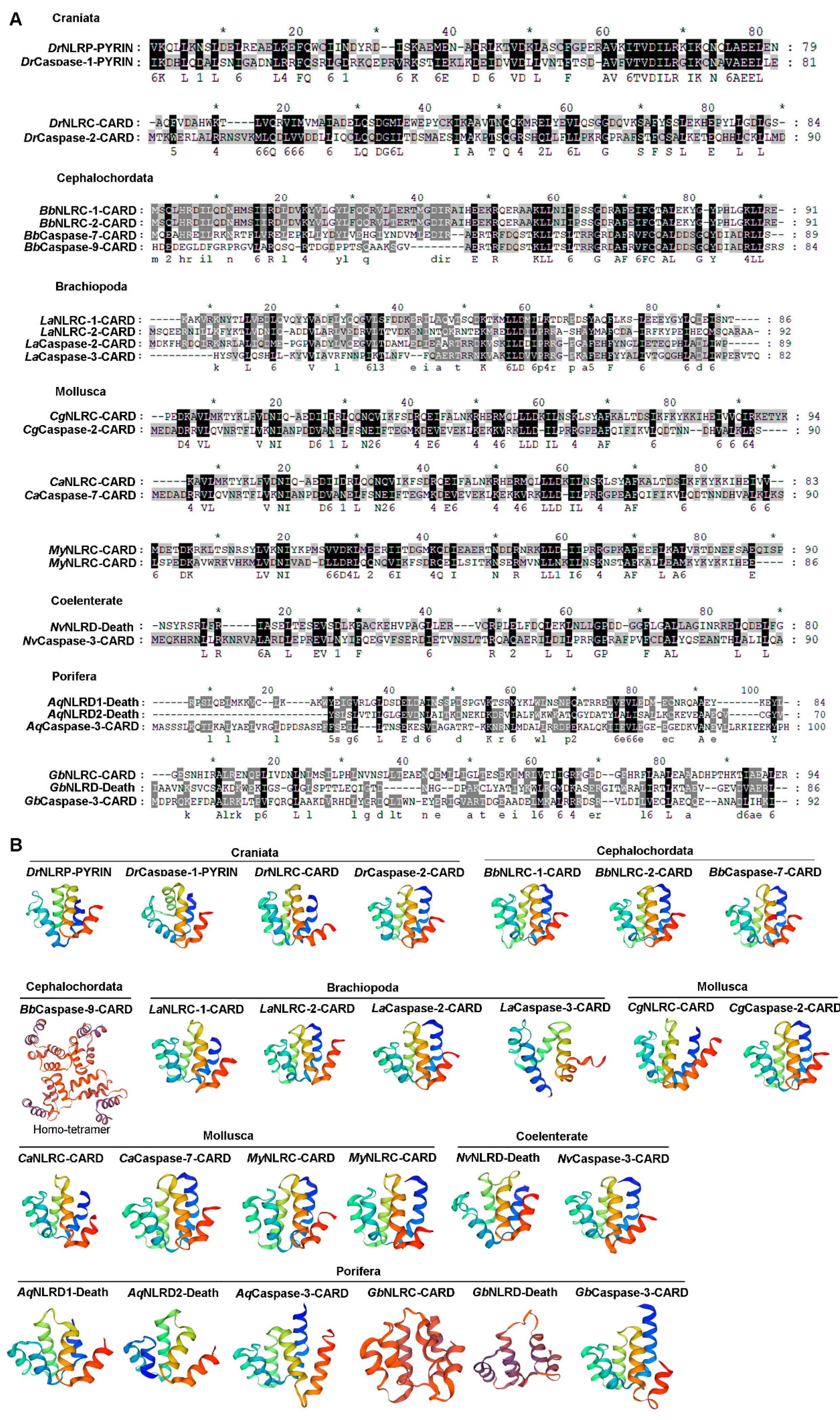
2.5. The Sequence Alignment and Three-Dimensional Structure of the CARD/PYD/Death Domain from NLRs and Caspase-1s
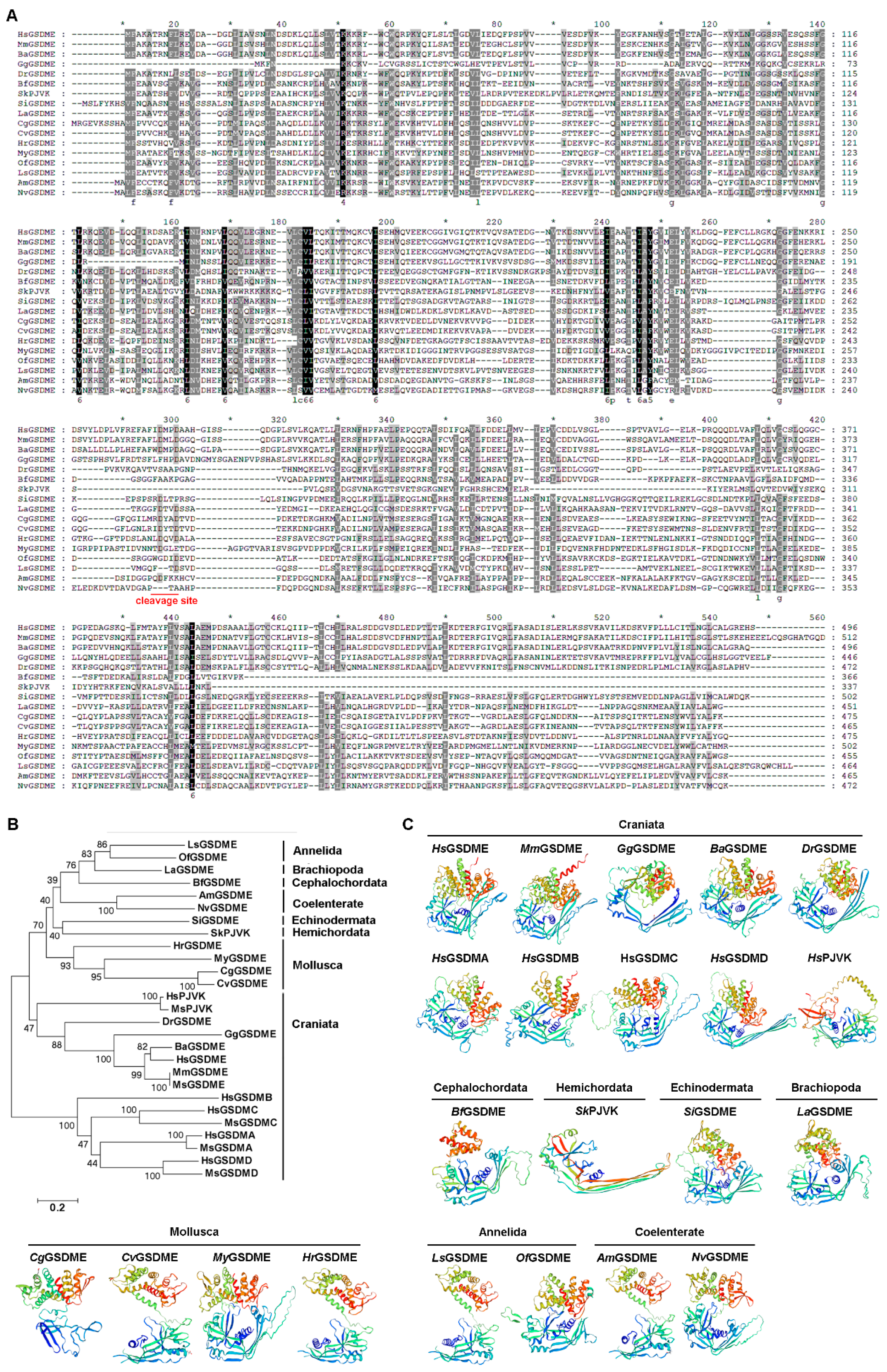
2.6. The Sequence Alignment, Evolutionary Relationship, and Three-Dimensional Structure of GSDMEs
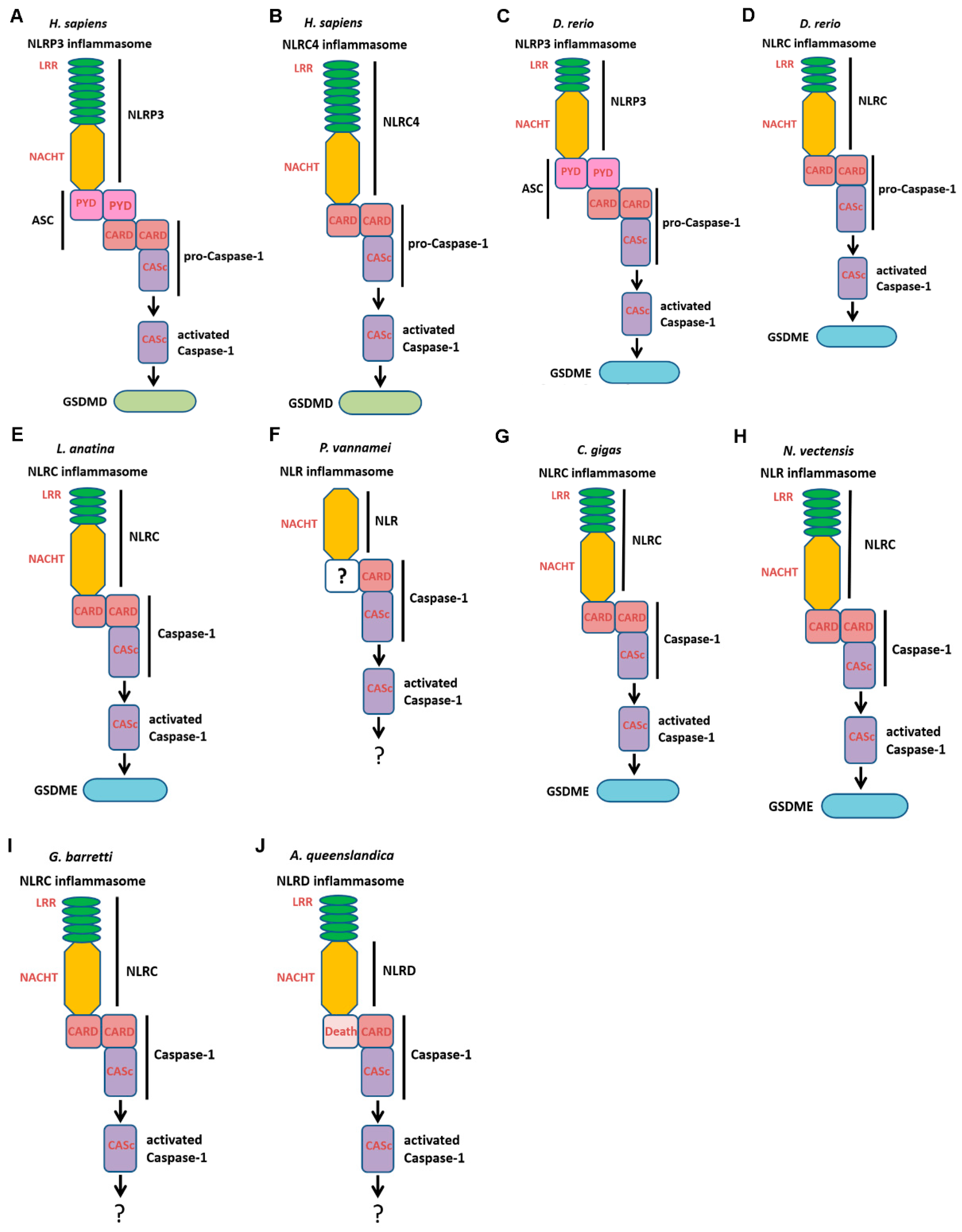
2.7. The NLR Inflammasome Composition and Its Mediated Pyroptosis in Different Metazoan Phyla
3. Discussion
4. Materials and Methods
4.1. The Amino Acid Sequences and BLASTp Analysis of NLRs, ASCs, CARD-Containing Caspases, and GSDMs
4.2. The Evolutionary Analysis of NLRs, CARD-Containing Caspases, and GSDMs
4.3. Multiple Sequence Alignment of NLRs, ASCs, Caspase-1s, and GSDMs
4.4. The Structural Domain and Three-Dimensional Structure Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Broz, P.; Dixit, V.M. Inflammasomes: Mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 2016, 16, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Broz, P. Recognition of Intracellular Bacteria by Inflammasomes. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Rathinam, V.A.; Fitzgerald, K.A. Inflammasome Complexes: Emerging Mechanisms and Effector Functions. Cell 2016, 165, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Miao, E.A.; Leaf, I.A.; Treuting, P.M.; Mao, D.P.; Dors, M.; Sarkar, A.; Warren, S.E.; Wewers, M.D.; Aderem, A. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat. Immunol. 2010, 11, 1136–1142. [Google Scholar] [CrossRef]
- Man, S.M.; Hopkins, L.J.; Nugent, E.; Cox, S.; Gluck, I.M.; Tourlomousis, P.; Wright, J.A.; Cicuta, P.; Monie, T.P.; Bryant, C.E. Inflammasome activation causes dual recruitment of NLRC4 and NLRP3 to the same macromolecular complex. Proc. Natl. Acad. Sci. USA 2014, 111, 7403–7408. [Google Scholar] [CrossRef]
- Broz, P.; von Moltke, J.; Jones, J.W.; Vance, R.E.; Monack, D.M. Differential requirement for Caspase-1 autoproteolysis in pathogen-induced cell death and cytokine processing. Cell Host Microbe 2010, 8, 471–483. [Google Scholar] [CrossRef]
- Meunier, E.; Broz, P. Evolutionary Convergence and Divergence in NLR Function and Structure. Trends Immunol. 2017, 38, 744–757. [Google Scholar] [CrossRef]
- Lange, C.; Hemmrich, G.; Klostermeier, U.C.; Lopez-Quintero, J.A.; Miller, D.J.; Rahn, T.; Weiss, Y.; Bosch, T.C.; Rosenstiel, P. Defining the origins of the NOD-like receptor system at the base of animal evolution. Mol. Biol. Evol. 2011, 28, 1687–1702. [Google Scholar] [CrossRef]
- Wang, J.; Chai, J. Molecular actions of NLR immune receptors in plants and animals. Sci. China. Life Sci. 2020, 63, 1303–1316. [Google Scholar] [CrossRef]
- Ting, J.P.; Lovering, R.C.; Alnemri, E.S.; Bertin, J.; Boss, J.M.; Davis, B.K.; Flavell, R.A.; Girardin, S.E.; Godzik, A.; Harton, J.A.; et al. The NLR gene family: A standard nomenclature. Immunity 2008, 28, 285–287. [Google Scholar] [CrossRef]
- Hu, Z.; Yan, C.; Liu, P.; Huang, Z.; Ma, R.; Zhang, C.; Wang, R.; Zhang, Y.; Martinon, F.; Miao, D.; et al. Crystal structure of NLRC4 reveals its autoinhibition mechanism. Science 2013, 341, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.X. Emerging mechanisms and functions of inflammasome complexes in teleost fish. Front. Immunol. 2023, 14, 1065181. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Zou, J.; Wang, M.; Chen, Z.; Wang, Q. A Comparative Review of Pyroptosis in Mammals and Fish. J. Inflamm. Res. 2022, 15, 2323–2331. [Google Scholar] [CrossRef] [PubMed]
- Yuen, B.; Bayes, J.M.; Degnan, S.M. The characterization of sponge NLRs provides insight into the origin and evolution of this innate immune gene family in animals. Mol. Biol. Evol. 2014, 31, 106–120. [Google Scholar] [CrossRef]
- Lamkanfi, M.; Dixit, V.M. Mechanisms and functions of inflammasomes. Cell 2014, 157, 1013–1022. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, S.; Ruan, J.; Wu, J.; Tong, A.B.; Yin, Q.; Li, Y.; David, L.; Lu, A.; Wang, W.L.; et al. Cryo-EM structure of the activated NAIP2-NLRC4 inflammasome reveals nucleated polymerization. Science 2015, 350, 404–409. [Google Scholar] [CrossRef]
- Li, J.Y.; Wang, Y.Y.; Shao, T.; Fan, D.D.; Lin, A.F.; Xiang, L.X.; Shao, J.Z. The zebrafish NLRP3 inflammasome has functional roles in ASC-dependent interleukin-1beta maturation and gasdermin E-mediated pyroptosis. J. Biol. Chem. 2020, 295, 1120–1141. [Google Scholar] [CrossRef]
- Messier-Solek, C.; Buckley, K.M.; Rast, J.P. Highly diversified innate receptor systems and new forms of animal immunity. Semin. Immunol. 2010, 22, 39–47. [Google Scholar] [CrossRef]
- Privitera, G.; Rana, N.; Armuzzi, A.; Pizarro, T.T. The gasdermin protein family: Emerging roles in gastrointestinal health and disease. Nat. Reviews. Gastroenterol. Hepatol. 2023, 20, 366–387. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, J.; Qiao, D.; Gao, F.; Gu, Y.; Jiang, X.; Zhu, L.; Kong, X. CcGSDMEa functions the pore-formation in cytomembrane and the regulation on the secretion of IL-lbeta in common carp (Cyprinus carpio haematopterus). Front. Immunol. 2022, 13, 1110322. [Google Scholar] [CrossRef]
- Chen, S.; Li, S.; Chen, H.; Gong, Y.; Yang, D.; Zhang, Y.; Liu, Q. Caspase-mediated LPS sensing and pyroptosis signaling in Hydra. Sci. Adv. 2023, 9, eadh4054. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Zhou, Z.; Sun, Y.; Zhang, T.; Sun, L. Coral gasdermin triggers pyroptosis. Sci. Immunol. 2020, 5, eabd2591. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yan, X.; Leng, J.; Wang, W.; Li, Y.; Yang, C.; Sun, J.; Wang, L.; Song, L. CgCaspase-3 activates the translocation of CgGSDME in haemocytes of Pacific oyster Crassostrea gigas. Fish Shellfish Immunol. 2022, 131, 757–765. [Google Scholar] [CrossRef]
- Chen, S.; Gong, Y.; Li, S.; Yang, D.; Zhang, Y.; Liu, Q. Hydra gasdermin-gated pyroptosis signalling regulates tissue regeneration. Dev. Comp. Immunol. 2023, 149, 104904. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Tang, D.; Zhao, W.; Wan, Z.; Yu, M.; Huang, Z.; Li, L.; Aweya, J.J.; Zhang, Y. NLRP3-like protein negatively regulates the expression of antimicrobial peptides in Penaeus vannamei hemocyates. Fish Shellfish Immunol. Rep. 2021, 2, 100039. [Google Scholar] [CrossRef]
- Malik, A.; Kanneganti, T.D. Inflammasome activation and assembly at a glance. J. Cell Sci. 2017, 130, 3955–3963. [Google Scholar] [CrossRef]
- Ardizzone, A.; Mannino, D.; Capra, A.P.; Repici, A.; Filippone, A.; Esposito, E.; Campolo, M. New Insights into the Mechanism of Ulva pertusa on Colitis in Mice: Modulation of the Pain and Immune System. Mar. Drugs 2023, 21, 298. [Google Scholar] [CrossRef]
- De Schutter, E.; Roelandt, R.; Riquet, F.B.; Van Camp, G.; Wullaert, A.; Vandenabeele, P. Punching Holes in Cellular Membranes: Biology and Evolution of Gasdermins. Trends Cell Biol. 2021, 31, 500–513. [Google Scholar] [CrossRef]
- Yuan, Z.; Jiang, S.; Qin, K.; Sun, L. New insights into the evolutionary dynamic and lineage divergence of gasdermin E in metazoa. Front. Cell Dev. Biol. 2022, 10, 952015. [Google Scholar] [CrossRef]
- Forn-Cuni, G.; Meijer, A.H.; Varela, M. Zebrafish in Inflammasome Research. Cells 2019, 8, 901. [Google Scholar] [CrossRef]
- Huang, S.; Yuan, S.; Guo, L.; Yu, Y.; Li, J.; Wu, T.; Liu, T.; Yang, M.; Wu, K.; Liu, H.; et al. Genomic analysis of the immune gene repertoire of amphioxus reveals extraordinary innate complexity and diversity. Genome Res. 2008, 18, 1112–1126. [Google Scholar] [CrossRef] [PubMed]
- Sodergren, E.; Weinstock, G.M.; Davidson, E.H.; Cameron, R.A.; Gibbs, R.A.; Angerer, R.C.; Angerer, L.M.; Arnone, M.I.; Burgess, D.R.; Burke, R.D.; et al. The genome of the sea urchin Strongylocentrotus purpuratus. Science 2006, 314, 941–952. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.A.; Kirkness, E.F.; Simakov, O.; Hampson, S.E.; Mitros, T.; Weinmaier, T.; Rattei, T.; Balasubramanian, P.G.; Borman, J.; Busam, D.; et al. The dynamic genome of Hydra. Nature 2010, 464, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Putnam, N.H.; Srivastava, M.; Hellsten, U.; Dirks, B.; Chapman, J.; Salamov, A.; Terry, A.; Shapiro, H.; Lindquist, E.; Kapitonov, V.V.; et al. Sea anemone genome reveals ancestral eumetazoan gene repertoire and genomic organization. Science 2007, 317, 86–94. [Google Scholar] [CrossRef]
- Park, H.H.; Lo, Y.C.; Lin, S.C.; Wang, L.; Yang, J.K.; Wu, H. The death domain superfamily in intracellular signaling of apoptosis and inflammation. Annu. Rev. Immunol. 2007, 25, 561–586. [Google Scholar] [CrossRef]
- Dai, Y.; Zhou, J.; Shi, C. Inflammasome: Structure, biological functions, and therapeutic targets. MedComm 2023, 4, e391. [Google Scholar] [CrossRef]
- Poyet, J.L.; Srinivasula, S.M.; Tnani, M.; Razmara, M.; Fernandes-Alnemri, T.; Alnemri, E.S. Identification of Ipaf, a human caspase-1-activating protein related to Apaf-1. J. Biol. Chem. 2001, 276, 28309–28313. [Google Scholar] [CrossRef]
- Proell, M.; Gerlic, M.; Mace, P.D.; Reed, J.C.; Riedl, S.J. The CARD plays a critical role in ASC foci formation and inflammasome signalling. Biochem. J. 2013, 449, 613–621. [Google Scholar] [CrossRef]
- Zaki, M.H.; Boyd, K.L.; Vogel, P.; Kastan, M.B.; Lamkanfi, M.; Kanneganti, T.D. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity 2010, 32, 379–391. [Google Scholar] [CrossRef]
- Vance, R.E. The NAIP/NLRC4 inflammasomes. Curr. Opin. Immunol. 2015, 32, 84–89. [Google Scholar] [CrossRef]
- Pandey, A.; Li, Z.; Gautam, M.; Ghosh, A.; Man, S.M. Molecular mechanisms of emerging inflammasome complexes and their activation and signaling in inflammation and pyroptosis. Immunol. Rev. 2024, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Qin, K.; Jiang, S.; Xu, H.; Yuan, Z.; Sun, L. Pyroptotic gasdermin exists in Mollusca and is vital to eliminating bacterial infection. Cell Rep. 2023, 42, 112414. [Google Scholar] [CrossRef] [PubMed]
- Defourny, J.; Aghaie, A.; Perfettini, I.; Avan, P.; Delmaghani, S.; Petit, C. Pejvakin-mediated pexophagy protects auditory hair cells against noise-induced damage. Proc. Natl. Acad. Sci. USA 2019, 116, 8010–8017. [Google Scholar] [CrossRef] [PubMed]
- Angosto-Bazarra, D.; Alarcon-Vila, C.; Hurtado-Navarro, L.; Banos, M.C.; Rivers-Auty, J.; Pelegrin, P. Evolutionary analyses of the gasdermin family suggest conserved roles in infection response despite loss of pore-forming functionality. BMC Biol. 2022, 20, 9. [Google Scholar] [CrossRef] [PubMed]
- Jiejie Sun, L.S. Inflammation and its mechanism in molluscs: A review. J. Dalian Ocean Univ. 2023, 3, 369–379. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Leng, J.; Song, L. The Evolution of NLR Inflammasome and Its Mediated Pyroptosis in Metazoa. Int. J. Mol. Sci. 2024, 25, 11167. https://doi.org/10.3390/ijms252011167
Sun J, Leng J, Song L. The Evolution of NLR Inflammasome and Its Mediated Pyroptosis in Metazoa. International Journal of Molecular Sciences. 2024; 25(20):11167. https://doi.org/10.3390/ijms252011167
Chicago/Turabian StyleSun, Jiejie, Jinyuan Leng, and Linsheng Song. 2024. "The Evolution of NLR Inflammasome and Its Mediated Pyroptosis in Metazoa" International Journal of Molecular Sciences 25, no. 20: 11167. https://doi.org/10.3390/ijms252011167
APA StyleSun, J., Leng, J., & Song, L. (2024). The Evolution of NLR Inflammasome and Its Mediated Pyroptosis in Metazoa. International Journal of Molecular Sciences, 25(20), 11167. https://doi.org/10.3390/ijms252011167





