Apoptosis, Mitochondrial Autophagy, Fission, and Fusion Maintain Mitochondrial Homeostasis in Mouse Liver Under Tail Suspension Conditions
Abstract
1. Introduction
2. Results
2.1. Influence of TS on Body Weight (BW), Body Length (BL), Carcass Weight (CW), Liver Weight (LW), LW-to-BW Ratio (LW/BW), and LW-to-CW Ratio (LW/CW) in Mice
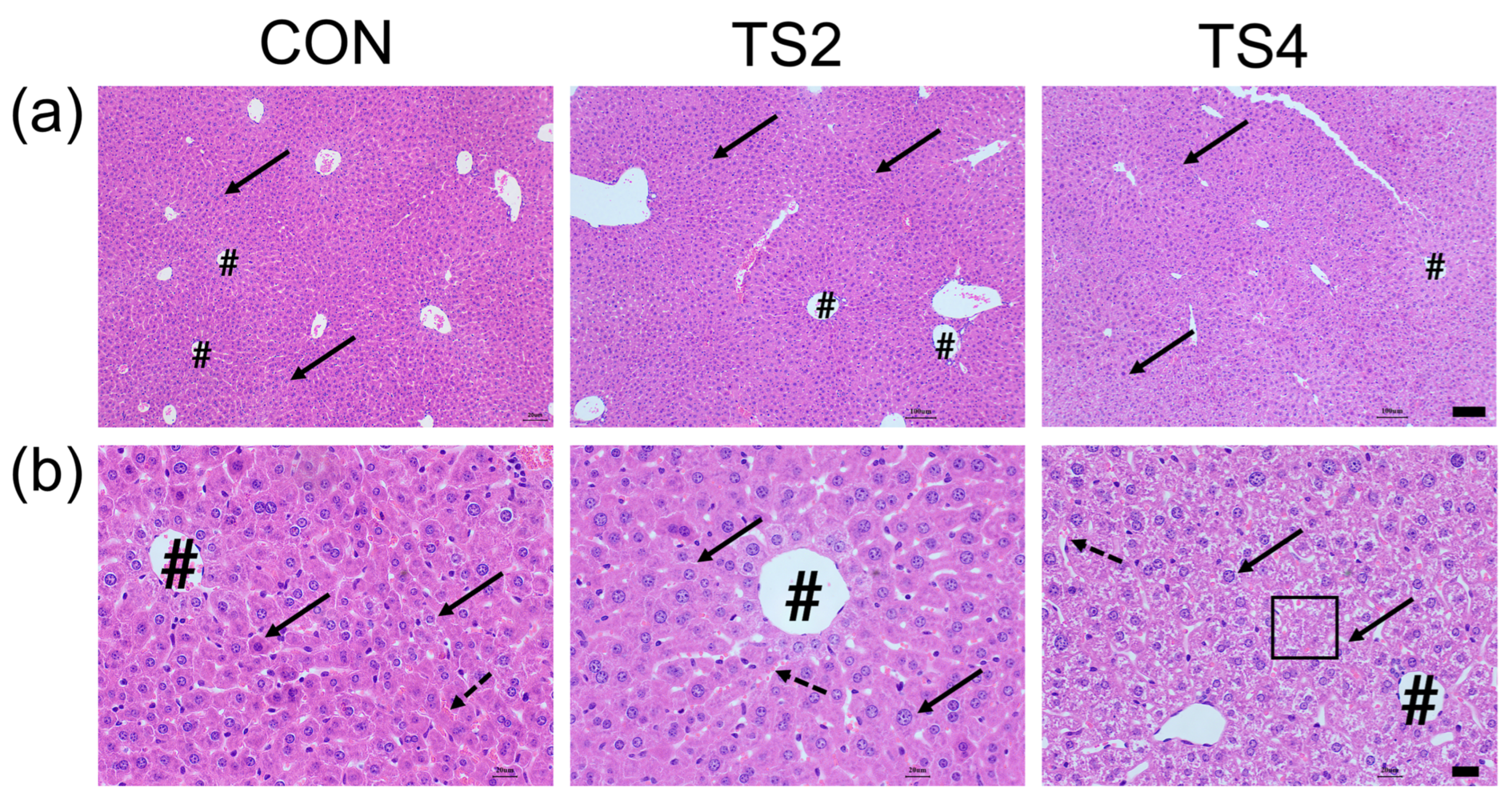
2.2. Hematoxylin and Eosin (H&E) Staining of Liver Tissue
2.3. Ultrastructural Changes in Liver Mitochondrial and Nuclei
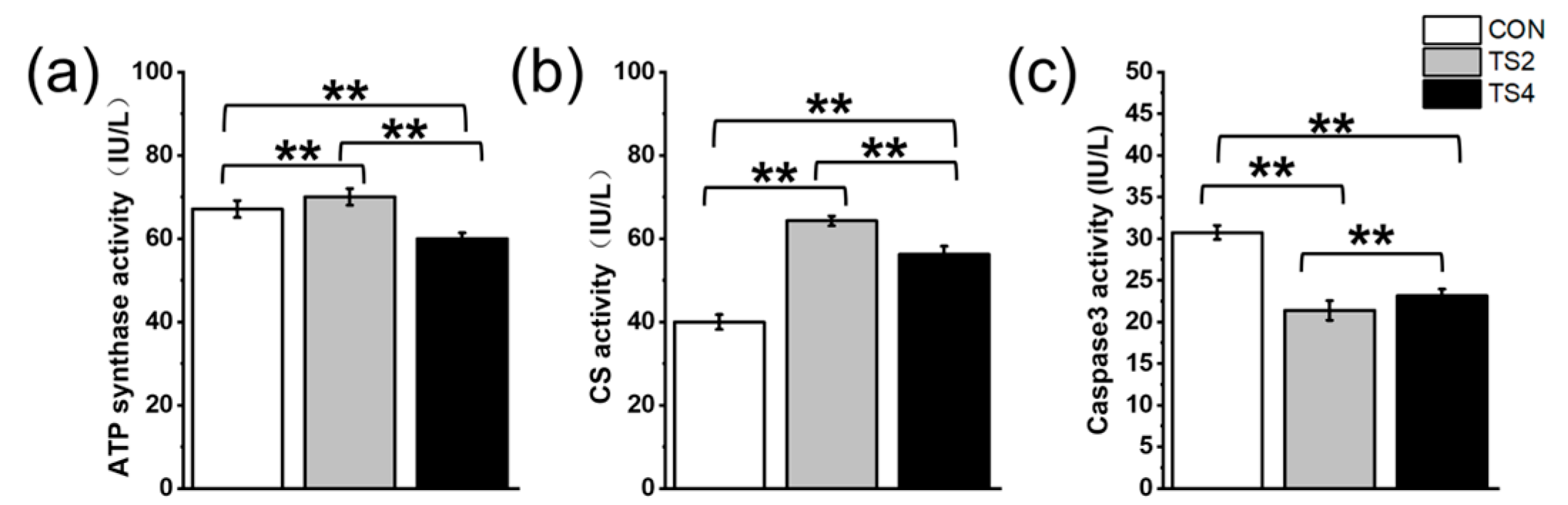
2.4. Variations in ATP Synthase, CS, and Caspase3 Activity
2.5. DNA Fragmentation
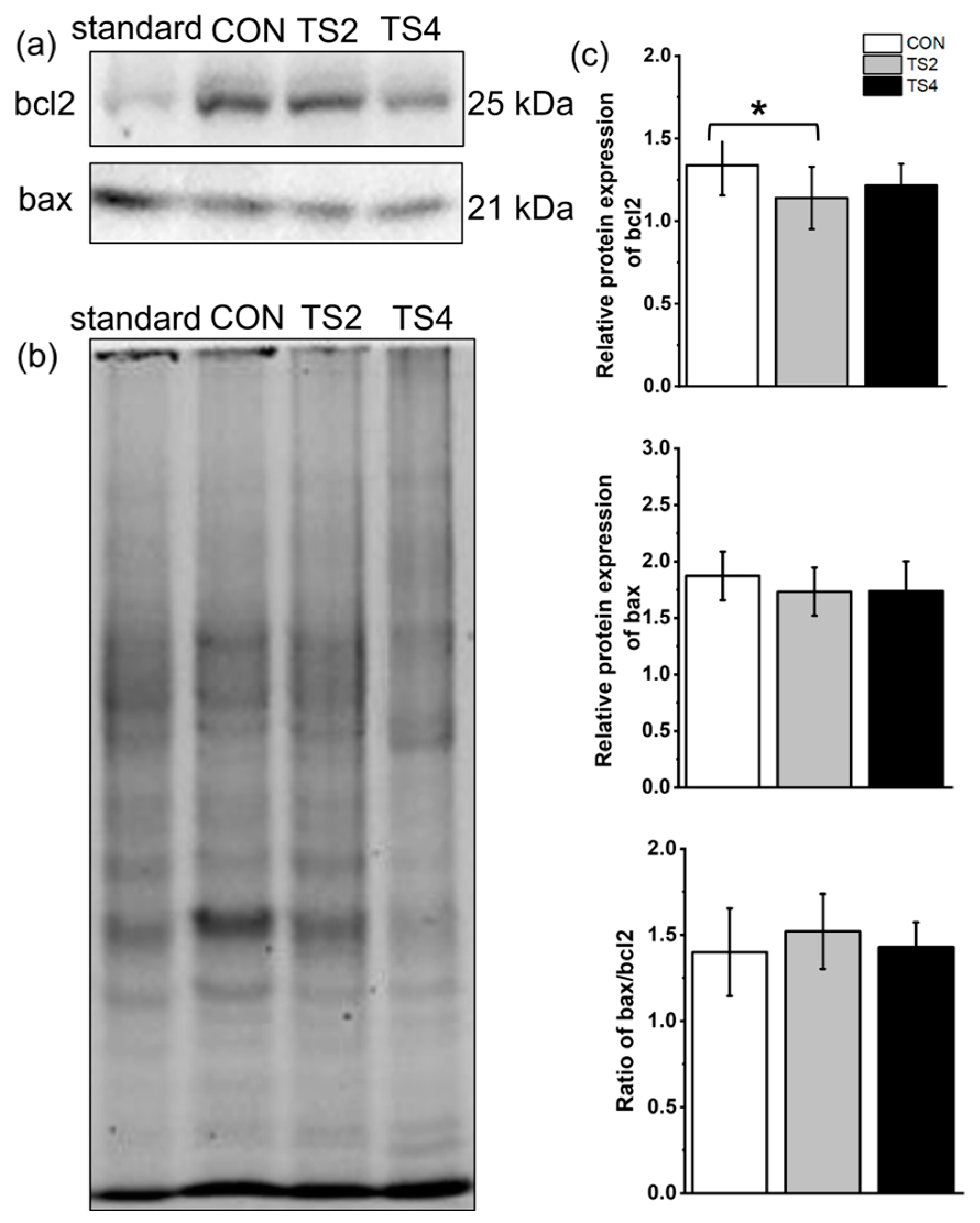
2.6. Variations in Expression of Apoptosis-Associated Proteins
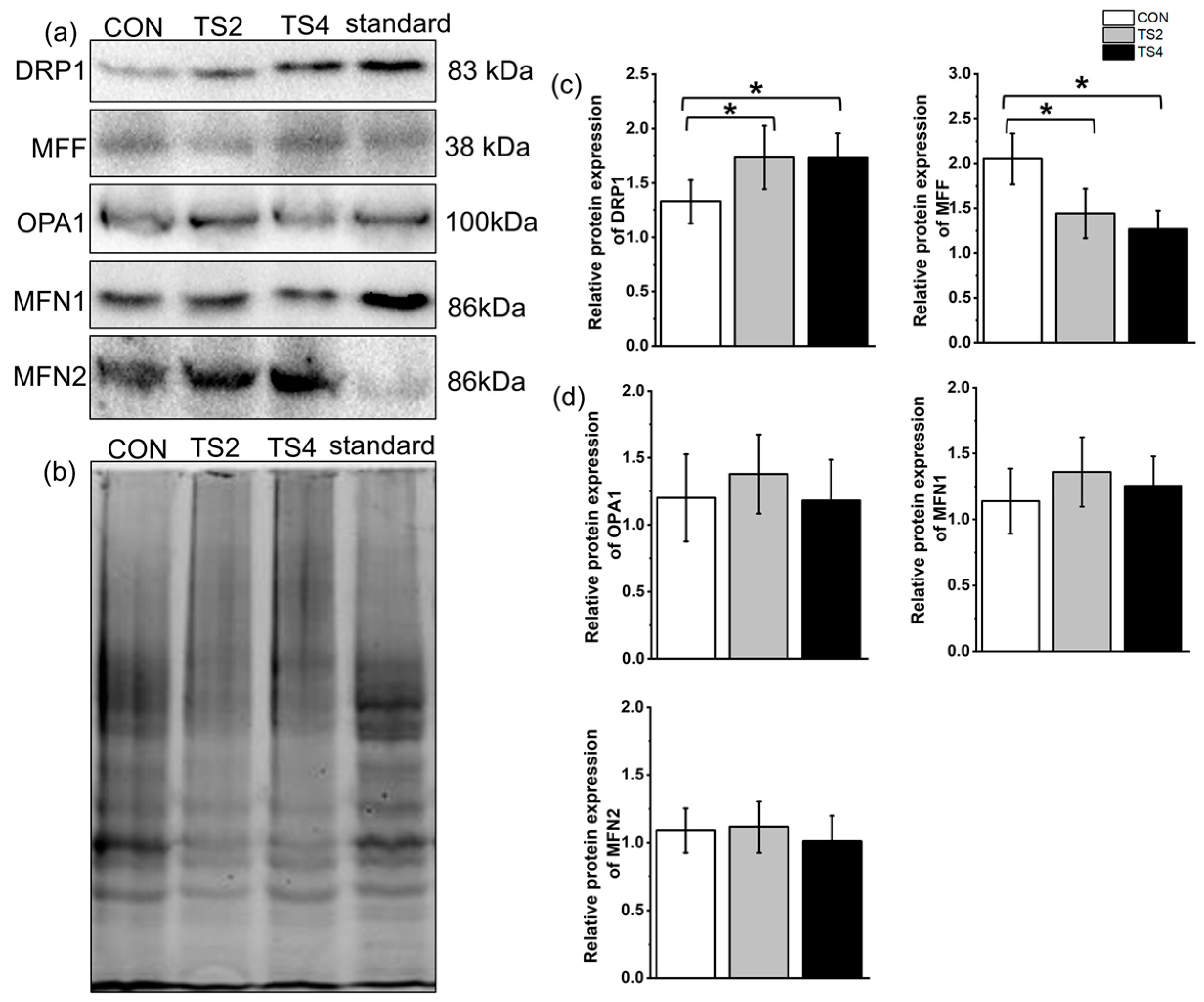
2.7. Variations in Expression of Mitochondrial Fission- and Fusion-Associated Proteins
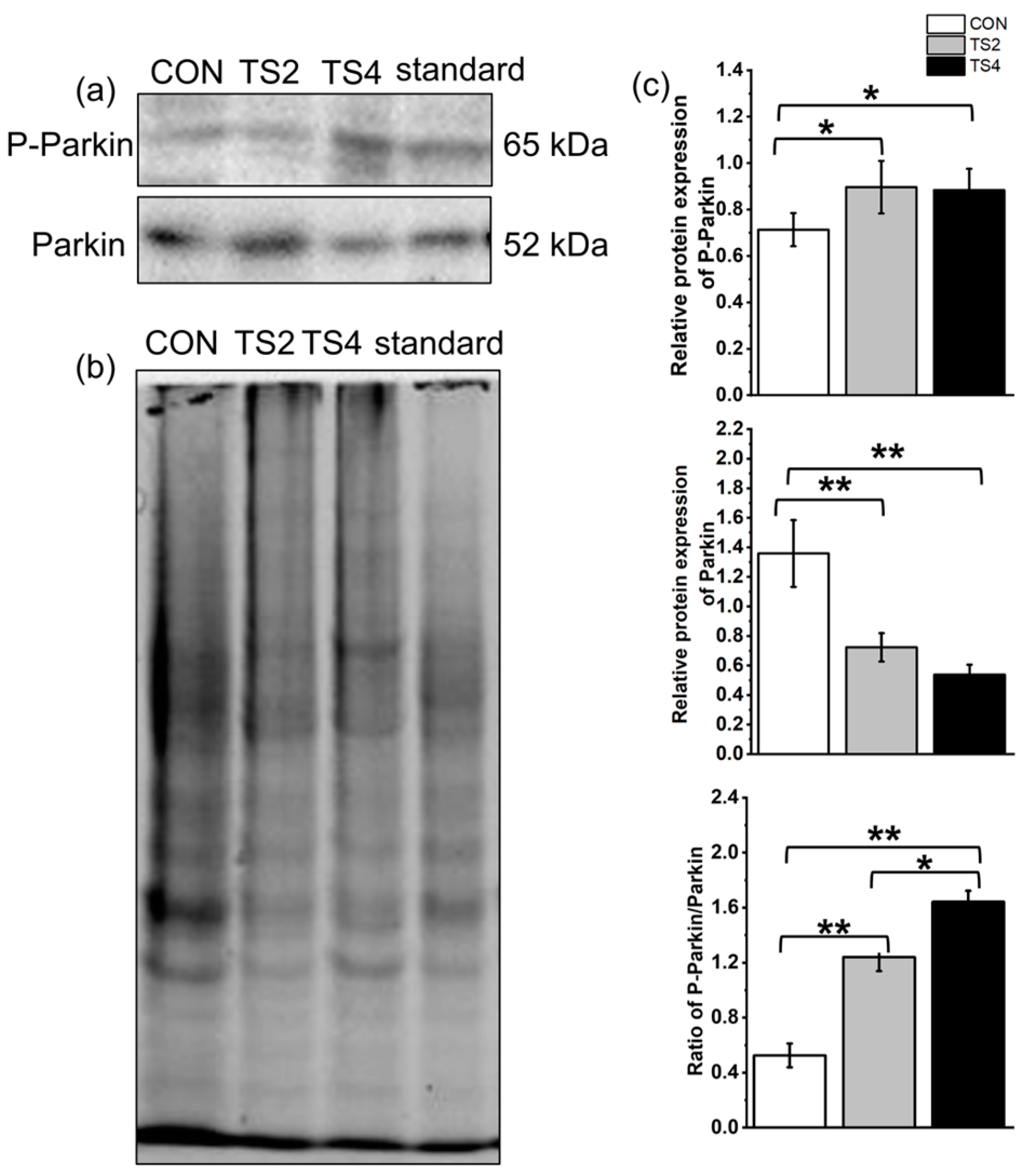
2.8. Variations in Expression of Mitochondrial Autophagy-Associated Proteins
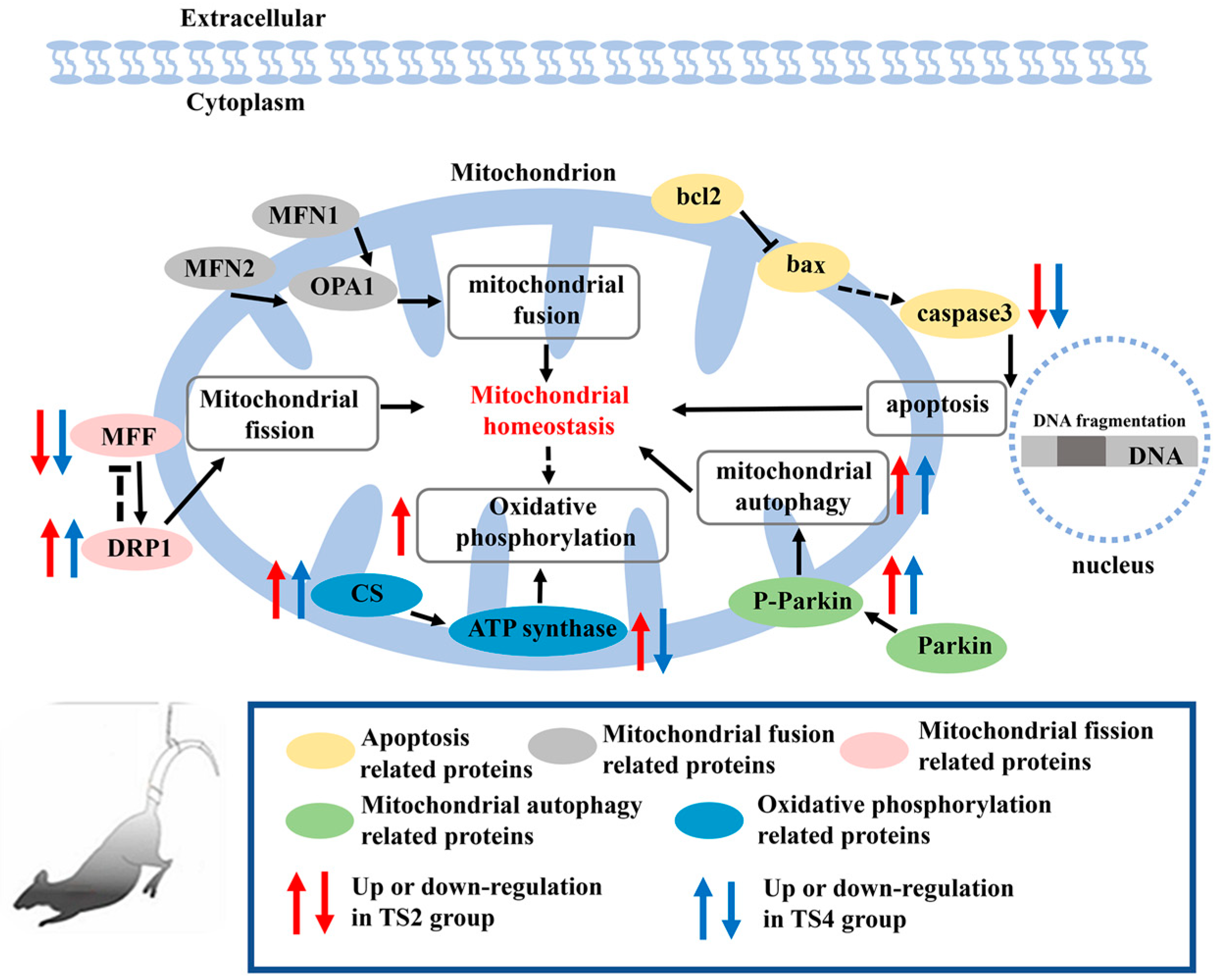
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Animals and Groups
4.3. Sample Preparation
4.4. Hematoxylin and Eosin (H&E) Staining
4.5. Transmission Electron Microscopy (TEM)
4.6. ATP Synthase, CS, and Caspase3 Activity
4.7. Terminal Deoxynucleotidyl Transferase Biotin-dUTP Nick End Labeling (TUNEL) Staining
4.8. Western Blotting
4.9. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pettis, C.R.; Witten, M.L. Gender differences in organ density in a rat simulated microgravity model. Acta Astronaut. 2004, 54, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.M.; Cheng-Campbell, M.; Blaber, E.A.; Anand, S.; Bhattacharya, S.; Zwart, S.R.; Crucian, B.E.; Smith, S.M.; Meller, R.; Grabham, P.; et al. Beyond Low-Earth Orbit: Characterizing Immune and Microrna Differentials Following Simulated Deep Spaceflight Conditions in Mice. iScience 2020, 23, 101747. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.P.; Tran, P.H.; Kim, K.S.; Yang, S.G. The effects of real and simulated microgravity on cellular mitochondrial function. NPJ Microgravity 2021, 7, 44. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, J.; Yang, C.; Zhang, H.; Wu, F.; Chen, J.; Li, K.; Wang, H.; Li, Y.; Li, Y.; et al. Upregulation of miR-223 in the rat liver inhibits proliferation of hepatocytes under simulated microgravity. Exp. Mol. Med. 2017, 49, e348. [Google Scholar] [CrossRef]
- Atiakshin, D.A.; Il’in, E.A.; Pashkov, A.N. Morphofunctional state of hepatocytes nuclear apparatus in Mongolian herbils after the flight on space apparatus Foton-M3. Aviakosmicheskaia Ekol. Med. 2010, 44, 29–34. [Google Scholar]
- Riley, D.A.; Ellis, S.; Slocum, G.R.; Satyanarayana, T.; Bain, J.L.; Sedlak, F.R. Hypogravity-induced atrophy of rat soleus and extensor digitorum longus muscles. Muscle Nerve 1987, 10, 560–568. [Google Scholar] [CrossRef]
- Nday, C.M.; Frantzidis, C.; Jackson, G.; Bamidis, P.; Kourtidou-Papadeli, C. Neurophysiological changes in simulated microgravity: An animal model. Neurol. India 2019, 67, 221–226. [Google Scholar] [CrossRef]
- Riley, D.A.; Slocum, G.R.; Bain, J.L.; Sedlak, F.R.; Sowa, T.E.; Mellender, J.W. Rat hindlimb unloading: Soleus histochemistry, ultrastructure, and electromyography. J. Appl. Physiol. 1990, 69, 58–66. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.-C.; Chen, Y.-F.; Wang, C.-L.; Chen, L.; Jiang, M.-Y.; Liu, X.-W.; Zhang, X.-X.; Feng, Y.-Z.; Xu, J.-H. Loss and recovery of myocardial mitochondria in mice under different tail suspension time: Apoptosis and mitochondrial fission, fusion and autophagy. Exp. Physiol. 2023, 108, 1189–1202. [Google Scholar] [CrossRef]
- Ma, K.; Chen, G.; Li, W.; Kepp, O.; Zhu, Y.; Chen, Q. Mitophagy, Mitochondrial Homeostasis, and Cell Fate. Front. Cell Dev. Biol. 2020, 8, 467. [Google Scholar] [CrossRef]
- Xie, L.-l.; Shi, F.; Tan, Z.; Li, Y.; Bode, A.M.; Cao, Y. Mitochondrial network structure homeostasis and cell death. Cancer Sci. 2018, 109, 3686–3694. [Google Scholar] [CrossRef] [PubMed]
- Belushkina, N.N.; Severin, S.E. Molecular mechanisms of apoptosis pathology. Arkhiv Patol. 2001, 63, 51–60. [Google Scholar]
- Wang, X.-C.; Wang, Z.; Chen, Y.-F.; Chen, L.; Zhang, B.-M.; Li, R.; Feng, Y.-Z.; Jiang, L.-N.; Xu, J.-H. Stimulated Microgravity Affects Mitochondrial Homeostasis in the Harderian Glands of Mice. J. Evol. Biochem. Physiol. 2023, 59, 1167–1181. [Google Scholar] [CrossRef]
- Ju, J.-S. Regulation of Mitochondrial Homeostasis in Response to Endurance Exercise Training in Skeletal Muscle. J. Life Sci. 2017, 27, 361–369. [Google Scholar] [CrossRef][Green Version]
- Bonet-Ponce, L.; Saez-Atienzar, S.; da Casa, C.; Flores-Bellver, M.; Barcia, J.M.; Sancho-Pelluz, J.; Romero, F.J.; Jordan, J.; Galindo, M.F. On the mechanism underlying ethanol-induced mitochondrial dynamic disruption and autophagy response. Biochim. Biophys. Acta-Mol. Basis Dis. 2015, 1852, 1400–1409. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, N.; Li, L.; Chen, S.; Wang, T. PINK1-dependent phosphorylation of PINK1 and Parkin is essential for mitochondrial quality control. Cell Death Dis. 2016, 7, e2501. [Google Scholar] [CrossRef]
- Lacombe, A.; Scorrano, L. The interplay between mitochondrial dynamics and autophagy: From a key homeostatic mechanism to a driver of pathology. In Seminars in Cell & Developmental Biology; Academic Press: Cambridge, MA, USA, 2024; Volume 161, pp. 1–19. [Google Scholar] [CrossRef]
- Liu, J.; Peng, Y.; Cui, Z.; Wu, Z.; Qian, A.; Shang, P.; Qu, L.; Li, Y.; Liu, J.; Long, J. Depressed mitochondrial biogenesis and dynamic remodeling in mouse tibialis anterior and gastrocnemius induced by 4-week hindlimb unloading. Iubmb Life 2012, 64, 901–910. [Google Scholar] [CrossRef]
- Chen, Y.-F.; Wang, X.-C.; Wang, Z.; Chen, L.; Liu, X.-W.; Song, X.-Y.; Zhang, J.-W.; Wang, C.-L.; Guo, Y.-Y.; Xu, J.-H. Simulated Weightlessness Led to the Transformation of Glycolipid Metabolism in the Livers of Mice. Rev. Bras. Med. Esporte 2023, 29, e2022_0115. [Google Scholar] [CrossRef]
- Ramirez, J.; Periyakaruppan, A.; Sarkar, S.; Ramesh, G.T.; Sharma, S.C. Effect of Simulated Microgravity on the Activity of Regulatory Enzymes of Glycolysis and Gluconeogenesis in Mice Liver. Microgravity Sci. Technol. 2014, 25, 303–309. [Google Scholar] [CrossRef][Green Version]
- Ranade, A.; Khan, A.A.; Gul, M.T.; Suresh, S.; Qaisar, R.; Ahmad, F.; Karim, A. Suppression of endoplasmic reticulum stress reverses hindlimb unloading-induced hepatic cellular processes in mice. Biochim. Biophys. Acta. Gen. Subj. 2023, 1867, 130422. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Ye, B.Y.; Wei, J.J.; Wang, Q.W.; Xu, C.S.; Yu, G.Y. MiR-199a-5p regulates rat liver regeneration and hepatocyte proliferation by targeting TNF-? TNFR1/TRADD/CASPASE8/CASPASE3 signalling pathway. Artif. Cells Nanomed. Biotechnol. 2019, 47, 4110–4118. [Google Scholar] [CrossRef]
- Teijido, O.; Dejean, L. Upregulation of Bcl2 inhibits apoptosis-driven BAX insertion but favors BAX relocalization in mitochondria. FEBS Lett. 2010, 584, 3305–3310. [Google Scholar] [CrossRef] [PubMed]
- Grosse, J.; Wehland, M.; Pietsch, J.; Ma, X.; Ulbrich, C.; Schulz, H.; Saar, K.; Hübner, N.; Hauslage, J.; Hemmersbach, R.; et al. Short-term weightlessness produced by parabolic flight maneuvers altered gene expression patterns in human endothelial cells. FASEB J. 2012, 26, 639–655. [Google Scholar] [CrossRef] [PubMed]
- Wehland, M.; Ma, X.; Braun, M.; Hauslage, J.; Hemmersbach, R.; Bauer, J.; Grosse, J.; Infanger, M.; Grimm, D. The impact of altered gravity and vibration on endothelial cells during a parabolic flight. Cell. Physiol. Biochem. 2013, 31, 432–451. [Google Scholar] [CrossRef] [PubMed]
- Talbot, N.C.; Caperna, T.J.; Blomberg, L.; Graninger, P.G.; Stodieck, L.S. The effects of space flight and microgravity on the growth and differentiation of PICM-19 pig liver stem cells. In Vitro Cell. Dev. Biol. Anim. 2010, 46, 502–515. [Google Scholar] [CrossRef] [PubMed]
- Dobashi, S.; Naito, H.; Yoshihara, T. Cage restriction-induced physical inactivity promotes subsequent hepatic apoptosis during tail suspension in young male rats. Physiol. Rep. 2023, 11, e15695. [Google Scholar] [CrossRef]
- Du, F.; Ding, Y.; Zou, J.; Li, Z.; Tian, J.; She, R.; Wang, D.; Wang, H.; Lv, D.; Chang, L. Morphology and Molecular Mechanisms of Hepatic Injury in Rats under Simulated Weightlessness and the Protective Effects of Resistance Training. PLoS ONE 2015, 10, e0127047. [Google Scholar] [CrossRef]
- Bursch, W.; Wastl, U.; Hufnagl, K.; Schulte-Hermann, R. No increase of apoptosis in regressing mouse liver after withdrawal of growth stimuli or food restriction. Toxicol. Sci. Off. J. Soc. Toxicol. 2005, 85, 507–514. [Google Scholar] [CrossRef][Green Version]
- Tanaka, A.; Youle, R.J. A chemical inhibitor of DRP1 uncouples mitochondrial fission and apoptosis. Mol. Cell 2008, 29, 409–410. [Google Scholar] [CrossRef]
- Aguirre, J.I.; Plotkin, L.I.; Stewart, S.A.; Weinstein, R.S.; Parfitt, A.M.; Manolagas, S.C.; Bellido, T. Osteocyte apoptosis is induced by weightlessness in mice and precedes osteoclast recruitment and bone loss. J. Bone Miner. Res. 2006, 21, 605–615. [Google Scholar] [CrossRef]
- Labrousse, A.M.; Zappaterra, M.D.; Rube, D.A.; van der Bliek, A.M.C. elegans dynamin-related protein DRP-1 controls severing of the mitochondrial outer membrane. Mol. Cell 1999, 4, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Lendahl, U.; Nister, M.; Zhao, J. Regulation of Mammalian Mitochondrial Dynamics: Opportunities and Challenges. Front. Endocrinol. 2020, 11, 374. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Kage, F.; Higgs, H.N. Mff oligomerization is required for Drp1 activation and synergy with actin filaments during mitochondrial division. Mol. Biol. Cell 2021, 32, ar5. [Google Scholar] [CrossRef] [PubMed]
- Otera, H.; Mihara, K. Discovery of the membrane receptor for mitochondrial fission GTPase Drp1. Small GTPases 2011, 2, 167–172. [Google Scholar] [CrossRef]
- Otera, H.; Wang, C.X.; Cleland, M.M.; Setoguchi, K.; Yokota, S.; Youle, R.J.; Mihara, K. Mff is an essential factor for mitochondrial recruitment of Drp1 during mitochondrial fission in mammalian cells. J. Cell Biol. 2010, 191, 1141–1158. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ni, H.; Li, M.; Sanzari, J.K.; Diffenderfer, E.S.; Lin, L.; Kennedy, A.R.; Weissman, D. Effect of solar particle event radiation and hindlimb suspension on gastrointestinal tract bacterial translocation and immune activation. PLoS ONE 2012, 7, e44329. [Google Scholar] [CrossRef]
- Rudolf, A.M.; Hood, W.R. Mitochondrial stress in the spaceflight environment. Mitochondrion 2024, 76, 101855. [Google Scholar] [CrossRef]
- Liu, X.; Hussain, R.; Mehmood, K.; Tang, Z.; Zhang, H.; Li, Y. Mitochondrial-Endoplasmic Reticulum Communication-Mediated Oxidative Stress and Autophagy. BioMed Res. Int. 2022, 2022, 6459585. [Google Scholar] [CrossRef]
- Xu, Y.; Shen, J.; Ran, Z. Emerging views of mitophagy in immunity and autoimmune diseases. Autophagy 2020, 16, 3–17. [Google Scholar] [CrossRef]
- Handwerk, L.; Schreier, H.K.; Kraft, D.; Shreder, K.; Hemmersbach, R.; Hauslage, J.; Bonig, H.; Wiesmüller, L.; Fournier, C.; Rall-Scharpf, M. Simulating Space Conditions Evokes Different DNA Damage Responses in Immature and Mature Cells of the Human Hematopoietic System. Int. J. Mol. Sci. 2023, 24, 13761. [Google Scholar] [CrossRef]
- Sidorenko, L.A.; Krasnov, I.B.; Gulevskaja, T.S.; Morgunov, V.A. Ultrastructure of ependyma in brain third ventricle of the rats exposed to repeated tail-suspension. Scanning electron microscopical study. J. Gravit. Physiol 2007, 14, P77–P78. [Google Scholar] [PubMed]
- Naeem, K.; Zulfaqar, H.; Gulzar, H.; Sehar, R.; Iqbal, J.; Rafiq, M.; Rehman, A.; Ashfaq, M.; Hasan, M. Simulated Microgravity Mediated Hypothalamus Response and Differential Expression of Key Proteins: A Review on Current Knowledge. Curr. Proteom. 2018, 15, 201–207. [Google Scholar] [CrossRef]
- Baek, S.H.; Cho, Y.; Lee, J.; Choi, B.Y.; Choi, Y.; Park, J.S.; Kim, H.; Sul, J.; Kim, E.; Park, J.H.; et al. Intracellular and Mitochondrial Reactive Oxygen Species Measurement in Primary Cultured Neurons. Bio-Protocol 2018, 8, e2871. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Quan, X.-B.; Zeng, W.-J.; Yang, X.-O.; Wang, M.-J. Mechanism of Hepatocyte Apoptosis. J. Cell Death 2016, 9, 19–29. [Google Scholar] [CrossRef]
- Grohm, J.; Plesnila, N.; Culmsee, C. Bid mediates fission, membrane permeabilization and peri-nuclear accumulation of mitochondria as a prerequisite for oxidative neuronal cell death. Brain Behav. Immun. 2010, 24, 831–838. [Google Scholar] [CrossRef]
- Su, L.J.; Zhang, J.H.; Gomez, H.; Kellum, J.A.; Peng, Z.Y. Mitochondria ROS and mitophagy in acute kidney injury. Autophagy 2023, 19, 401–414. [Google Scholar] [CrossRef]
- Lei, L.; Yang, S.; Lu, X.; Zhang, Y.; Li, T. Research Progress on the Mechanism of Mitochondrial Autophagy in Cerebral Stroke. Front. Aging Neurosci. 2021, 13, 698601. [Google Scholar] [CrossRef]
- Macho, L.; Nemeth, S.; Kvetnansky, R.; Fickova, M.; Tigranian, R.A.; Serova, L. Metabolic changes in the animals subjected to space flight. Acta Astronaut. 1982, 9, 385–389. [Google Scholar] [CrossRef]
- Merrill, A.H., Jr.; Hoel, M.; Wang, E.; Mullins, R.E.; Hargrove, J.L.; Jones, D.P.; Popova, I.A. Altered carbohydrate, lipid, and xenobiotic metabolism by liver from rats flown on Cosmos 1887. FASEB J. 1990, 4, 95–100. [Google Scholar] [CrossRef]
- Michaletti, A.; Gioia, M.; Tarantino, U.; Zolla, L. Effects of microgravity on osteoblast mitochondria: A proteomic and metabolomics profile. Sci. Rep. 2017, 7, 15376. [Google Scholar] [CrossRef]
- Hauslage, J.; Cevik, V.; Hemmersbach, R. Pyrocystis noctiluca represents an excellent bioassay for shear forces induced in ground-based microgravity simulators (clinostat and random positioning machine). NPJ Microgravity 2017, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Belova, S.P.; Zaripova, K.; Sharlo, K.; Kostrominova, T.Y.; Shenkman, B.S.; Nemirovskaya, T.L. Metformin attenuates an increase of calcium-dependent and ubiquitin-proteasome markers in unloaded muscle. J. Appl. Physiol. 2022, 133, 1149–1163. [Google Scholar] [CrossRef] [PubMed]
- Zaripova, K.A.; Kalashnikova, E.P.; Belova, S.P.; Kostrominova, T.Y.; Shenkman, B.S.; Nemirovskaya, T.L. Role of Pannexin 1 ATP-Permeable Channels in the Regulation of Signaling Pathways during Skeletal Muscle Unloading. Int. J. Mol. Sci. 2021, 22, 10444. [Google Scholar] [CrossRef]
- Wagatsuma, A.; Kotake, N.; Kawachi, T.; Shiozuka, M.; Yamada, S.; Matsuda, R. Mitochondrial adaptations in skeletal muscle to hindlimb unloading. Mol. Cell. Biochem. 2011, 350, 1–11. [Google Scholar] [CrossRef]
- Bigard, A.X.; Lienhard, F.; Merino, D.; Serrurier, B.; Guezennec, C.Y. Effects of growth hormone on rat skeletal muscle after hindlimb suspension. Eur. J. Appl. Physiol. Occup. Physiol. 1994, 69, 337–343. [Google Scholar] [CrossRef]
- LeBlanc, A.; Marsh, C.; Evans, H.; Johnson, P.; Schneider, V.; Jhingran, S. Bone and muscle atrophy with suspension of the rat. J. Appl. Physiol. 1985, 58, 1669–1675. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Li, G.Q.; Li, Y.Z.; Cho, J.L.; Wang, J.P.; Gao, J.Y.; Deng, Y.L.; Li, Y.J. Label-free quantitative proteomics of rat liver exposed to simulated microgravity. Acta Astronaut. 2020, 170, 251–260. [Google Scholar] [CrossRef]
- Duarte, F.V.; Amorim, J.A.; Palmeira, C.M.; Rolo, A.P. Regulation of Mitochondrial Function and its Impact in Metabolic Stress. Curr. Med. Chem. 2015, 22, 2468–2479. [Google Scholar] [CrossRef]
- Ryter, S.W.; Choi, A.M.K. Regulation of Autophagy in Oxygen-Dependent Cellular Stress. Curr. Pharm. Des. 2013, 19, 2747–2756. [Google Scholar] [CrossRef]
- Williams, J.A.; Ni, H.M.; Ding, Y.F.; Ding, W.X. Parkin regulates mitophagy and mitochondrial function to protect against alcohol-induced liver injury and steatosis in mice. Am. J. Physiol.-Gastrointest. Liver Physiol. 2015, 309, G324–G340. [Google Scholar] [CrossRef]
- MacArthur Clark, J.A.; Sun, D. Guidelines for the ethical review of laboratory animal welfare People’s Republic of China National Standard GB/T 35892-2018 [Issued 6 February 2018 Effective from 1 September 2018]. Anim. Models Exp. Med. 2020, 3, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.F.; Ye, S.M.; Chen, X.P.; Jiang, T.; Huang, H.X.; Li, W.J.; Yu, H.Q.; Bao, J.H.; Chen, H. Rodent retinal microcirculation and visual electrophysiology following simulated microgravity. Exp. Eye Res. 2020, 194, 108023. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, C.M.; Liu, Q.S.; Guo, M.Z.; Yang, Y.S.; Mao, G.P.; Wang, P. Effects of Simulated Weightlessness on Tight Junction Protein Occludin and Zonula Occluden-1 Expression Levels in the Intestinal Mucosa of Rats. J. Huazhong Univ. Sci. Technol.-Med. Sci. 2011, 31, 26–32. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, J.-H.; Mou, J.-J.; Kong, X.-T.; Wu, M.; Xue, H.-L.; Xu, L.-X. Photoperiod Affects Harderian Gland Morphology and Secretion in Female Cricetulus barabensis: Autophagy, Apoptosis, and Mitochondria. Front. Physiol. 2020, 11, 408. [Google Scholar] [CrossRef]
- Zhou, Z.Y.; Vidales, J.; Gonzalez-Reyes, J.A.; Shibata, B.; Baar, K.; Rutkowsky, J.M.; Ramsey, J.J. A 1-Month Ketogenic Diet Increased Mitochondrial Mass in Red Gastrocnemius Muscle, but Not in the Brain or Liver of Middle-Aged Mice. Nutrients 2021, 13, 2533. [Google Scholar] [CrossRef]
- Xu, J.H.; Wang, Z.; Mou, J.J.; Wang, C.L.; Huang, W.M.; Xue, H.L.; Wu, M.; Chen, L.; Xu, L.X. Up-Regulation of Glycogen Synthesis and Degradation Enzyme Level Maintained Myocardial Glycogen in Huddling Brandt’s Voles Under Cool Environments. Front. Physiol. 2021, 12, 593129. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Qi, H.; Liu, J.; Zhang, G.; Liu, J.; Liu, N.; Zhu, M.; Zhao, X.; Song, C.; Zhou, Z.; et al. Deubiquitinase OTUD3 regulates metabolism homeostasis in response to nutritional stresses. Cell Metab. 2022, 34, 1023–1041.e1028. [Google Scholar] [CrossRef]
- Song, M.; Chen, F.F.; Li, Y.H.; Zhang, L.; Wang, F.; Qin, R.R.; Wang, Z.H.; Zhong, M.; Tang, M.X.; Zhang, W.; et al. Trimetazidine restores the positive adaptation to exercise training by mitigating statin-induced skeletal muscle injury. J. Cachexia Sarcopenia Muscle 2018, 9, 106–118. [Google Scholar] [CrossRef]
- Wang, B.; Wang, K.; Jin, T.L.; Xu, Q.L.; He, Y.Y.; Cui, B.Z.; Wang, Y.Z. NCK1-AS1 enhances glioma cell proliferation, radioresistance and chemoresistance via miR-22-3p/IGF1R ceRNA pathway. Biomed. Pharmacother. 2020, 129, 110395. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, M.D.; Wang, X.C.; Chen, L.; Li, L.F.; Jiang, L.N.; Xu, J.H.; Kai, D. High levels of mitochondrial dynamics, autophagy, and apoptosis contribute to stable testicular status in hibernating Daurian ground squirrels. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2024, 297, 111705. [Google Scholar] [CrossRef]
- Eaton, S.L.; Roche, S.L.; Llavero Hurtado, M.; Oldknow, K.J.; Farquharson, C.; Gillingwater, T.H.; Wishart, T.M. Total protein analysis as a reliable loading control for quantitative fluorescent Western blotting. PLoS ONE 2013, 8, e72457. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, R.E.; Carroll, H.P.; Harris, A.; Maher, E.R.; Selby, P.J.; Banks, R.E. Housekeeping proteins: A preliminary study illustrating some limitations as useful references in protein expression studies. Proteomics 2005, 5, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Bass, J.J.; Wilkinson, D.J.; Rankin, D.; Phillips, B.E.; Szewczyk, N.J.; Smith, K.; Atherton, P.J. An overview of technical considerations for Western blotting applications to physiological research. Scand. J. Med. Sci. Sports 2017, 27, 4–25. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.-F.; Yu, J.; Li, R.; Li, S.-S.; Huang, J.-Y.; Wang, M.-D.; Jiang, L.-N.; Xu, J.-H.; Wang, Z. Apoptosis, Mitochondrial Autophagy, Fission, and Fusion Maintain Mitochondrial Homeostasis in Mouse Liver Under Tail Suspension Conditions. Int. J. Mol. Sci. 2024, 25, 11196. https://doi.org/10.3390/ijms252011196
Li L-F, Yu J, Li R, Li S-S, Huang J-Y, Wang M-D, Jiang L-N, Xu J-H, Wang Z. Apoptosis, Mitochondrial Autophagy, Fission, and Fusion Maintain Mitochondrial Homeostasis in Mouse Liver Under Tail Suspension Conditions. International Journal of Molecular Sciences. 2024; 25(20):11196. https://doi.org/10.3390/ijms252011196
Chicago/Turabian StyleLi, Lu-Fan, Jiao Yu, Rui Li, Shan-Shan Li, Jun-Yao Huang, Ming-Di Wang, Li-Na Jiang, Jin-Hui Xu, and Zhe Wang. 2024. "Apoptosis, Mitochondrial Autophagy, Fission, and Fusion Maintain Mitochondrial Homeostasis in Mouse Liver Under Tail Suspension Conditions" International Journal of Molecular Sciences 25, no. 20: 11196. https://doi.org/10.3390/ijms252011196
APA StyleLi, L.-F., Yu, J., Li, R., Li, S.-S., Huang, J.-Y., Wang, M.-D., Jiang, L.-N., Xu, J.-H., & Wang, Z. (2024). Apoptosis, Mitochondrial Autophagy, Fission, and Fusion Maintain Mitochondrial Homeostasis in Mouse Liver Under Tail Suspension Conditions. International Journal of Molecular Sciences, 25(20), 11196. https://doi.org/10.3390/ijms252011196





