Interconnection of CD133 Stem Cell Marker with Autophagy and Apoptosis in Colorectal Cancer
Abstract
1. Introduction
2. Structure and Function of CD133
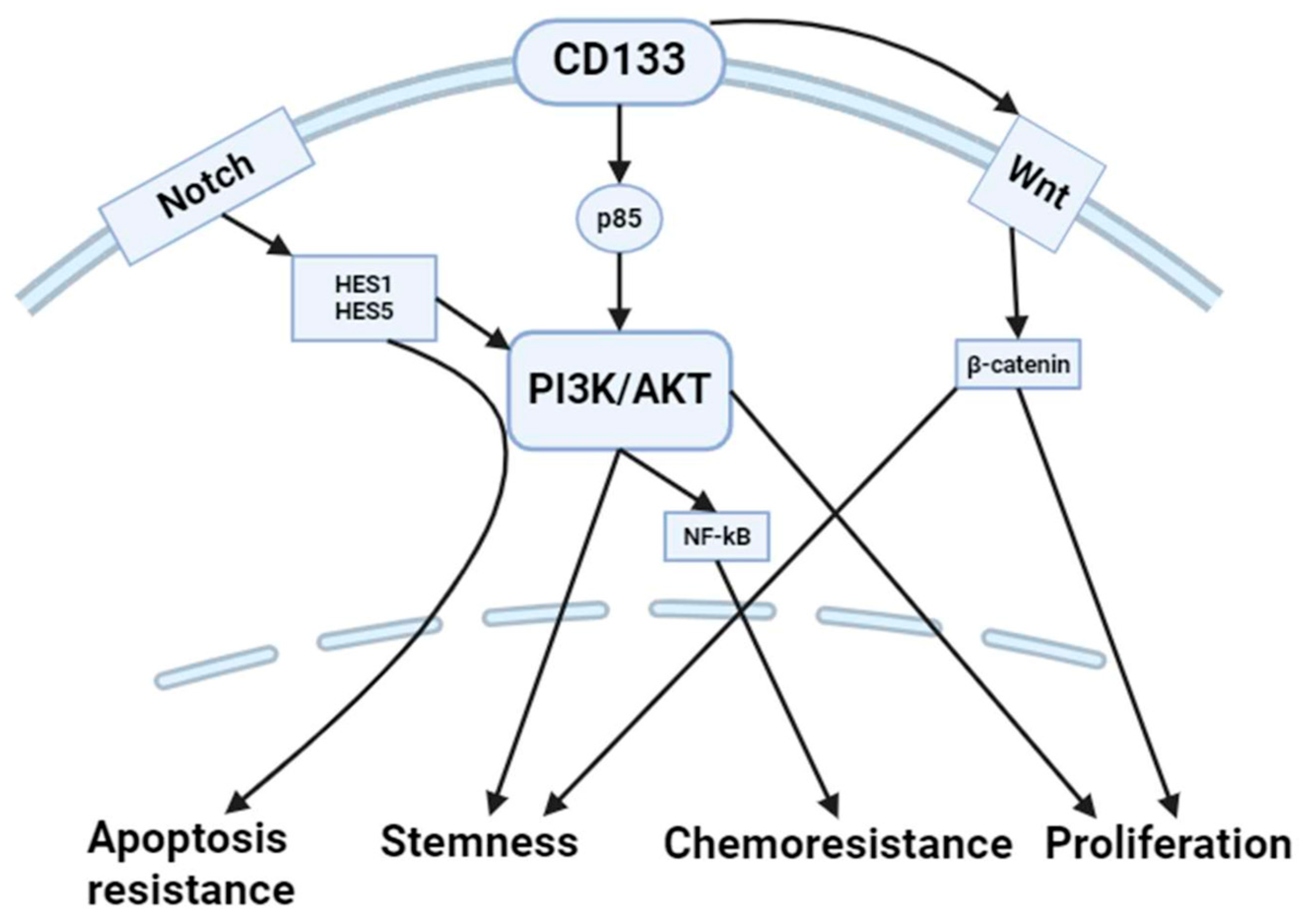
3. Molecular Links between CD133 and Apoptosis
3.1. PI3K/AKT Pathway
3.2. Notch Signaling
3.3. p53 Pathway
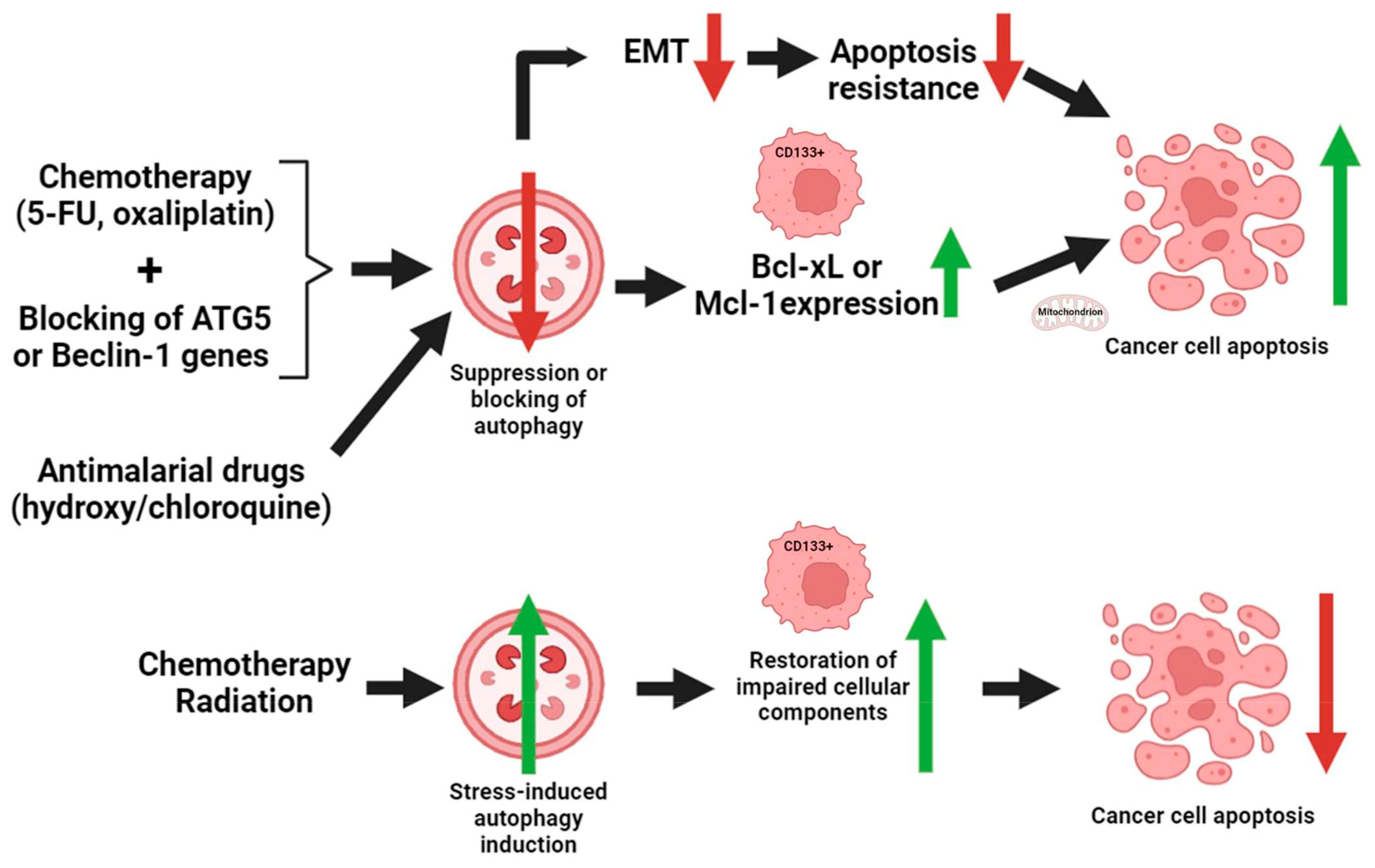
4. Interconnection of CD133 and Autophagy
4.1. PI3K/AKT/mTOR Pathway
4.2. AMPK Pathway
4.3. Hypoxia-Inducible Factors
4.4. Beclin-1 and Bcl-2 Interactions
4.5. Transcriptional Regulation
5. Interconnection of CD133-Mediated Autophagy and Apoptosis in CRC
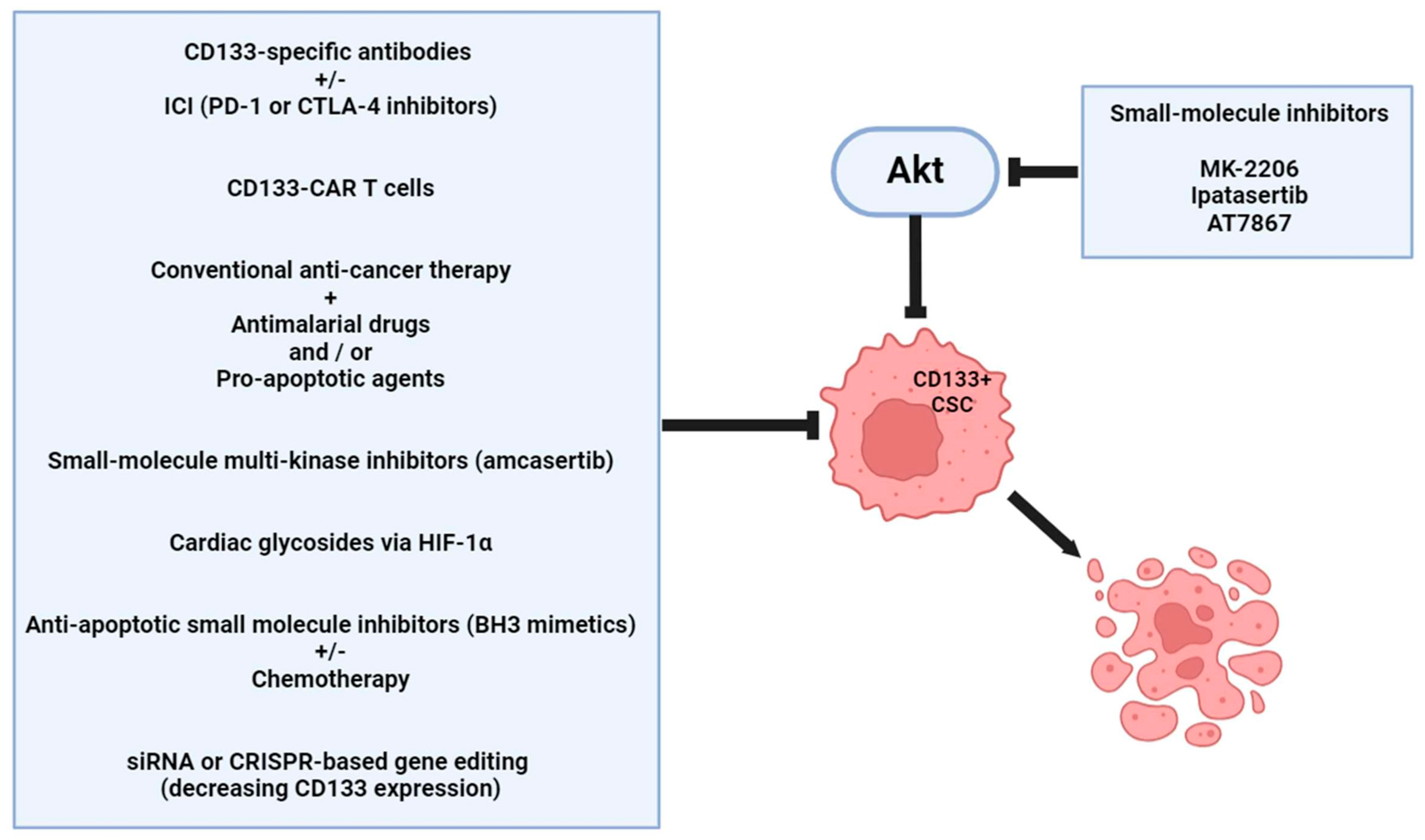
6. Therapeutic Considerations
7. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M. Colon Cancer: A Clinician’s Perspective in 2019. Gastroenterol. Res. 2020, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Brenner, H.; Kloor, M.; Pox, C.P. Colorectal cancer. Lancet 2014, 383, 1490–1502. [Google Scholar] [CrossRef] [PubMed]
- Jung, G.; Hernández-Illán, E.; Moreira, L.; Balaguer, F.; Goel, A. Epigenetics of colorectal cancer: Biomarker and therapeutic potential. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 111–130. [Google Scholar] [CrossRef]
- Schmitt, M.; Greten, F.R. The inflammatory pathogenesis of colorectal cancer. Nat. Rev. Immunol. 2021, 21, 653–667. [Google Scholar] [CrossRef]
- Cui, Z.; Cong, M.; Yin, S.; Li, Y.; Ye, Y.; Liu, X.; Tang, J. Role of protein degradation systems in colorectal cancer. Cell Death Discov. 2024, 10, 141. [Google Scholar] [CrossRef]
- Műzes, G.; Sipos, F. Inflammasomes Are Influenced by Epigenetic and Autophagy Mechanisms in Colorectal Cancer Signaling. Int. J. Mol. Sci. 2024, 25, 6167. [Google Scholar] [CrossRef]
- Xu, X.; Lai, Y.; Hua, Z.C. Apoptosis and apoptotic body: Disease message and therapeutic target potentials. Biosci. Rep. 2019, 39, BSR20180992. [Google Scholar] [CrossRef]
- Strasser, A.; Vaux, D.L. Cell Death in the Origin and Treatment of Cancer. Mol. Cell 2020, 78, 1045–1054. [Google Scholar] [CrossRef]
- Orlandi, G.; Roncucci, L.; Carnevale, G.; Sena, P. Different Roles of Apoptosis and Autophagy in the Development of Human Colorectal Cancer. Int. J. Mol. Sci. 2023, 24, 10201. [Google Scholar] [CrossRef]
- Kasprzak, A. Autophagy and the Insulin-like Growth Factor (IGF) System in Colonic Cells: Implications for Colorectal Neoplasia. Int. J. Mol. Sci. 2023, 24, 3665. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, S.; Muhammad, J.S.; Maghazachi, A.A.; Hamid, Q. Autophagy: A Versatile Player in the Progression of Colorectal Cancer and Drug Resistance. Front. Oncol. 2022, 12, 924290. [Google Scholar] [CrossRef] [PubMed]
- D’Arcy, M.S. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 2019, 43, 582–592. [Google Scholar] [CrossRef]
- Sell, S. On the stem cell origin of cancer. Am. J. Pathol. 2010, 176, 2584–2594. [Google Scholar] [CrossRef]
- Zhao, H.; Han, R.; Wang, Z.; Xian, J.; Bai, X. Colorectal Cancer Stem Cells and Targeted Agents. Pharmaceutics 2023, 15, 2763. [Google Scholar] [CrossRef] [PubMed]
- Marquardt, S.; Solanki, M.; Spitschak, A.; Vera, J.; Pützer, B.M. Emerging functional markers for cancer stem cell-based therapies: Understanding signaling networks for targeting metastasis. Semin. Cancer Biol. 2018, 53, 90–109. [Google Scholar] [CrossRef]
- Batlle, E.; Clevers, H. Cancer stem cells revisited. Nat. Med. 2017, 23, 1124–1134. [Google Scholar] [CrossRef]
- You, C.Z.; Xu, H.; Zhao, F.S.; Dou, J. A Validation Study of CD133 as a Reliable Marker for Identification of Colorectal Cancer Stem-Like Cells. Bull. Exp. Biol. Med. 2024, 176, 369–375. [Google Scholar] [CrossRef]
- Lim, S.H.; Jang, J.; Park, J.O.; Kim, K.M.; Kim, S.T.; Park, Y.S.; Lee, J.; Kim, H.C. CD133-positive tumor cell content is a predictor of early recurrence in colorectal cancer. J. Gastrointest. Oncol. 2014, 5, 447–456. [Google Scholar] [CrossRef]
- Abdou Hassan, W.; Muqresh, M.A.; Omer, M. The Potential Role of CD44 and CD133 in Colorectal Stem Cell Cancer. Cureus 2022, 14, e30509. [Google Scholar] [CrossRef]
- Bohusné Barta, B.; Simon, Á.; Nagy, L.; Dankó, T.; Raffay, R.E.; Petővári, G.; Zsiros, V.; Sebestyén, A.; Sipos, F.; Műzes, G. Survival of HT29 cancer cells is influenced by hepatocyte growth factor receptor inhibition through modulation of self-DNA-triggered TLR9-dependent autophagy response. PLoS ONE 2022, 17, e0268217. [Google Scholar] [CrossRef] [PubMed]
- Sipos, F.; Bohusné Barta, B.; Simon, Á.; Nagy, L.; Dankó, T.; Raffay, R.E.; Petővári, G.; Zsiros, V.; Wichmann, B.; Sebestyén, A.; et al. Survival of HT29 Cancer Cells Is Affected by IGF1R Inhibition via Modulation of Self-DNA-Triggered TLR9 Signaling and the Autophagy Response. Pathol. Oncol. Res. 2022, 28, 1610322. [Google Scholar] [CrossRef] [PubMed]
- Corbeil, D.; Karbanová, J.; Fargeas, C.A.; Jászai, J. Prominin-1 (CD133): Molecular and Cellular Features Across Species. Adv. Exp. Med. Biol. 2013, 777, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ren, S.; Xie, L.; Cui, C.; Xing, Y.; Liu, C.; Cao, B.; Yang, F.; Li, Y.; Chen, X.; et al. Mutation of N-linked glycosylation at Asn548 in CD133 decreases its ability to promote hepatoma cell growth. Oncotarget 2015, 6, 20650–20660. [Google Scholar] [CrossRef][Green Version]
- Thamm, K.; Graupner, S.; Werner, C.; Huttner, W.B.; Corbeil, D. Monoclonal Antibodies 13A4 and AC133 Do Not Recognize the Canine Ortholog of Mouse and Human Stem Cell Antigen Prominin-1 (CD133). PLoS ONE 2016, 11, e0164079. [Google Scholar] [CrossRef]
- Bidlingmaier, S.; Zhu, X.; Liu, B. The utility and limitations of glycosylated human CD133 epitopes in defining cancer stem cells. J. Mol. Med. 2008, 86, 1025–1032. [Google Scholar] [CrossRef]
- Kemper, K.; Sprick, M.R.; De Bree, M.; Scopelliti, A.; Vermeulen, L.; Hoek, M.; Zeilstra, J.; Pals, S.T.; Mehmet, H.; Stassi, G.; et al. The AC133 Epitope, but not the CD133 Protein, Is Lost upon Cancer Stem Cell Differentiation. Cancer Res. 2010, 70, 719–729. [Google Scholar] [CrossRef]
- Grosse-Gehling, P.; Fargeas, C.A.; Dittfeld, C.; Garbe, Y.; Alison, M.R.; Corbeil, D.; Kunz-Schughart, A.L. CD133 as a biomarker for putative cancer stem cells in solid tumours: Limitations, problems and challenges. J. Pathol. 2012, 229, 355–378. [Google Scholar] [CrossRef]
- Glumac, P.M.; LeBeau, A.M. The role of CD133 in cancer: A concise review. Clin. Transl. Med. 2018, 7, 18. [Google Scholar] [CrossRef]
- Corbeil, D.; Röper, K.; Fargeas, C.A.; Joester, A.; Huttner, W.B. Prominin: A story of cholesterol, plasma membrane protrusions and human pathology. Traffic 2001, 2, 82–91. [Google Scholar] [CrossRef]
- Su, Y.J.; Lin, W.H.; Chang, Y.W.; Wei, K.C.; Liang, C.L.; Chen, S.C.; Lee, J.L. Polarized cell migration induces cancer type-specific CD133/integrin/Src/Akt/GSK3β/β-catenin signaling required for maintenance of cancer stem cell properties. Oncotarget 2015, 6, 38029–38045. [Google Scholar] [CrossRef] [PubMed]
- Röper, K.; Corbeil, D.; Huttner, W.B. Retention of prominin in microvilli reveals distinct cholesterol-based lipid micro-domains in the apical plasma membrane. Nat. Cell Biol. 2000, 2, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Kosodo, Y.; Röper, K.; Haubensak, W.; Marzesco, A.-M.; Corbeil, D.; Huttner, W.B. Asymmetric distribution of the apical plasma membrane during neurogenic divisions of mammalian neuroepithelial cells. EMBO J. 2004, 23, 2314–2324. [Google Scholar] [CrossRef] [PubMed]
- Bauer, N.; Wilsch-Bräuninger, M.; Karbanová, J.; Fonseca, A.; Strauss, D.; Freund, D.; Thiele, C.; Huttner, W.B.; Bornhäuser, M.; Corbeil, D. Haematopoietic stem cell differentiation promotes the release of prominin-1/CD133-containing membrane vesicles—A role of the endocytic–exocytic pathway. EMBO Mol. Med. 2011, 3, 398–409. [Google Scholar] [CrossRef]
- Bauer, N.; Fonseca, A.V.; Florek, M.; Freund, D.; Jászai, J.; Bornhäuser, M.; Fargeas, C.A.; Corbeil, D. New insights into the cell biology of hematopoietic progenitors by studying prominin-1 (CD133). Cells Tissues Organs 2008, 188, 127–138. [Google Scholar] [CrossRef]
- Shmelkov, S.V.; Butler, J.M.; Hooper, A.T.; Hormigo, A.; Kushner, J.; Milde, T.; St Clair, R.; Baljevic, M.; White, I.; Jin, D.K.; et al. CD133 expression is not restricted to stem cells, and both CD133+ and CD133- metastatic colon cancer cells initiate tumors. J. Clin. Investig. 2008, 118, 2111–2120. [Google Scholar] [CrossRef]
- Jaksch, M.; Múnera, J.; Bajpai, R.; Terskikh, A.; Oshima, R.G. Cell cycle-dependent variation of a CD133 epitope in human embryonic stem cell, colon cancer, and melanoma cell lines. Cancer Res. 2008, 68, 7882–7886. [Google Scholar] [CrossRef]
- Bussolati, B.; Moggio, A.; Collino, F.; Aghemo, G.; D’Armento, G.; Grange, C.; Camussi, G. Hypoxia modulates the undifferentiated phenotype of human renal inner medullary CD133+ progenitors through Oct4/miR-145 balance. Am. J. Physiol. Physiol. 2012, 302, F116–F128. [Google Scholar] [CrossRef]
- Maeda, K.; Ding, Q.; Yoshimitsu, M.; Kuwahata, T.; Miyazaki, Y.; Tsukasa, K.; Hayashi, T.; Shinchi, H.; Natsugoe, S.; Takao, S. CD133 Modulate HIF-1α Expression under Hypoxia in EMT Phenotype Pancreatic Cancer Stem-Like Cells. Int. J. Mol. Sci. 2016, 17, 1025. [Google Scholar] [CrossRef]
- Pleskač, P.; Fargeas, C.A.; Veselska, R.; Corbeil, D.; Skoda, J. Emerging roles of prominin-1 (CD133) in the dynamics of plasma membrane architecture and cell signaling pathways in health and disease. Cell. Mol. Biol. Lett. 2024, 29, 41. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Z.; Ruan, S.; Yan, Q.; Chen, Y.; Cui, J.; Wang, X.; Huang, S.; Hou, B. Regulation of iron metabolism and ferroptosis in cancer stem cells. Front. Oncol. 2023, 13, 1251561. [Google Scholar] [CrossRef] [PubMed]
- Brossa, A.; Papadimitriou, E.; Collino, F.; Incarnato, D.; Oliviero, S.; Camussi, G.; Bussolati, B. Role of CD133 Molecule in Wnt Response and Renal Repair. Stem Cells Transl. Med. 2018, 7, 283–294. [Google Scholar] [CrossRef]
- Rappa, G.; Mercapide, J.; Anzanello, F.; Le, T.T.; Johlfs, M.G.; Fiscus, R.R.; Wilsch-Bräuninger, M.; Corbeil, D.; Lorico, A. Wnt interaction and extracellular release of prominin-1/CD133 in human malignant melanoma cells. Exp. Cell Res. 2013, 319, 810–819. [Google Scholar] [CrossRef]
- Mak, A.B.; Nixon, A.M.; Kittanakom, S.; Stewart, J.M.; Chen, G.I.; Curak, J.; Gingras, A.C.; Mazitschek, R.; Neel, B.G.; Stagljar, I.; et al. Regulation of CD133 by HDAC6 promotes β-catenin signaling to suppress cancer cell differentiation. Cell Rep. 2012, 2, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Dubrovska, A.; Kim, S.; Salamone, R.J.; Walker, J.R.; Maira, S.M.; García-Echeverría, C.; Schultz, P.G.; Reddy, V.A. The role of PTEN/Akt/PI3K signaling in the maintenance and viability of prostate cancer stem-like cell populations. Proc. Natl. Acad. Sci. USA 2009, 106, 268–273. [Google Scholar] [CrossRef]
- Liu, R.; Chen, Y.; Liu, G.; Li, C.; Song, Y.; Cao, Z.; Li, W.; Hu, J.; Lu, C.; Liu, Y. PI3K/AKT pathway as a key link modulates the multidrug resistance of cancers. Cell Death Dis. 2020, 11, 797. [Google Scholar] [CrossRef]
- Wei, Y.; Jiang, Y.; Zou, F.; Liu, Y.; Wang, S.; Xu, N.; Xu, W.; Cui, C.; Xing, Y.; Liu, Y.; et al. Activation of PI3K/Akt pathway by CD133-p85 interaction promotes tumorigenic capacity of glioma stem cells. Proc. Natl. Acad. Sci. USA 2013, 110, 6829–6834. [Google Scholar] [CrossRef] [PubMed]
- Gisina, A.; Kim, Y.; Yarygin, K.; Lupatov, A. Can CD133 Be Regarded as a Prognostic Biomarker in Oncology: Pros and Cons. Int. J. Mol. Sci. 2023, 24, 17398. [Google Scholar] [CrossRef]
- Mori, Y.; Takeuchi, A.; Miyagawa, K.; Yoda, H.; Soda, H.; Nabeya, Y.; Watanabe, N.; Ozaki, T.; Shimozato, O. CD133 prevents colon cancer cell death induced by serum deprivation through activation of Akt-mediated protein synthesis and inhibition of apoptosis. FEBS Open Bio 2021, 11, 1382–1394. [Google Scholar] [CrossRef]
- Goto, S.; Kawabata, T.; Li, T.S. Enhanced Expression of ABCB1 and Nrf2 in CD133-Positive Cancer Stem Cells Associates with Doxorubicin Resistance. Stem Cells Int. 2020, 2020, 8868849. [Google Scholar] [CrossRef]
- Liu, Y.P.; Yang, C.J.; Huang, M.S.; Yeh, C.T.; Wu, A.T.; Lee, Y.C.; Lai, T.C.; Lee, C.H.; Hsiao, Y.W.; Lu, J.; et al. Cisplatin selects for multidrug-resistant CD133+ cells in lung adenocarcinoma by activating Notch signaling. Cancer Res. 2013, 73, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Liang, X.; Zhan, Y.; Wang, Z.; Xu, J.; Qiu, Y.; Wang, J.; Cao, Y.; Le, V.M.; Ly, H.T.; et al. Targeting CD133 reverses drug-resistance via the AKT/NF-κB/MDR1 pathway in colorectal cancer. Br. J. Cancer 2020, 122, 1342–1353. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, R.; Deng, L.; Shuai, Z.; Chen, M. The Effect of Metformin on the Proliferation, Apoptosis and CD133 mRNA Expression of Colon Cancer Stem Cells by Upregulation of miR 342-3p. Drug Des. Dev. Ther. 2021, 15, 4633–4648. [Google Scholar] [CrossRef] [PubMed]
- Pospieszna, J.; Dams-Kozlowska, H.; Udomsak, W.; Murias, M.; Kucinska, M. Unmasking the Deceptive Nature of Cancer Stem Cells: The Role of CD133 in Revealing Their Secrets. Int. J. Mol. Sci. 2023, 24, 10910. [Google Scholar] [CrossRef]
- Gao, F.; Zhang, Y.; Wang, S.; Liu, Y.; Zheng, L.; Yang, J.; Huang, W.; Ye, Y.; Luo, W.; Xiao, D. Hes1 is involved in the self-renewal and tumourigenicity of stem-like cancer cells in colon cancer. Sci. Rep. 2014, 4, 3963. [Google Scholar] [CrossRef]
- Liu, Z.H.; Dai, X.M.; Du, B. Hes1: A key role in stemness, metastasis and multidrug resistance. Cancer Biol. Ther. 2015, 16, 353–359. [Google Scholar] [CrossRef]
- Katoh, M.; Katoh, M. Notch signaling in gastrointestinal tract (review). Int. J. Oncol. 2007, 30, 247–251. [Google Scholar] [CrossRef]
- Roy, S.; Majumdar, A.P. Signaling in colon cancer stem cells. J. Mol. Signal. 2012, 7, 11. [Google Scholar] [CrossRef]
- Fre, S.; Pallavi, S.K.; Huyghe, M.; Laé, M.; Janssen, K.P.; Robine, S.; Artavanis-Tsakonas, S.; Louvard, D. Notch and Wnt signals cooperatively control cell proliferation and tumorigenesis in the intestine. Proc. Natl. Acad. Sci. USA 2009, 106, 6309–6314. [Google Scholar] [CrossRef]
- Srinivasan, T.; Walters, J.; Bu, P.; Than, E.B.; Tung, K.L.; Chen, K.Y.; Panarelli, N.; Milsom, J.; Augenlicht, L.; Lipkin, S.M.; et al. NOTCH Signaling Regulates Asymmetric Cell Fate of Fast- and Slow-Cycling Colon Cancer-Initiating Cells. Cancer Res. 2016, 76, 3411–3421. [Google Scholar] [CrossRef]
- Sipos, F.; Constantinovits, M.; Műzes, G. Intratumoral functional heterogeneity and chemotherapy. World J. Gastroenterol. 2014, 20, 2429–2432. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Guan, H.; Liu, X.D.; Xie, D.F.; Wang, Y.; Ma, T.; Huang, B.; Zhou, P.K. p53 positively regulates the expression of cancer stem cell marker CD133 in HCT116 colon cancer cells. Oncol. Lett. 2018, 16, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Park, E.K.; Lee, J.C.; Park, J.W.; Bang, S.Y.; Yi, S.A.; Kim, B.K.; Park, J.H.; Kwon, S.H.; You, J.S.; Nam, S.W.; et al. Transcriptional repression of cancer stem cell marker CD133 by tumor suppressor p53. Cell Death Dis. 2015, 6, e1964. [Google Scholar] [CrossRef]
- Atashpour, S.; Fouladdel, S.; Movahhed, T.K.; Barzegar, E.; Ghahremani, M.H.; Ostad, S.N.; Azizi, E. Quercetin induces cell cycle arrest and apoptosis in CD133(+) cancer stem cells of human colorectal HT29 cancer cell line and enhances anticancer effects of doxorubicin. Iran. J. Basic Med. Sci. 2015, 18, 635–643. [Google Scholar] [PubMed]
- Therachiyil, L.; Haroon, J.; Sahir, F.; Siveen, K.S.; Uddin, S.; Kulinski, M.; Buddenkotte, J.; Steinhoff, M.; Krishnankutty, R. Dysregulated Phosphorylation of p53, Autophagy and Stemness Attributes the Mutant p53 Harboring Colon Cancer Cells Impaired Sensitivity to Oxaliplatin. Front. Oncol. 2020, 10, 1744. [Google Scholar] [CrossRef]
- Xia, P.; Xu, X.Y. PI3K/Akt/mTOR signaling pathway in cancer stem cells: From basic research to clinical application. Am. J. Cancer Res. 2015, 5, 1602–1609. [Google Scholar]
- Jung, C.H.; Ro, S.H.; Cao, J.; Otto, N.M.; Kim, D.H. mTOR regulation of autophagy. FEBS Lett. 2010, 584, 1287–1295. [Google Scholar] [CrossRef]
- Kharouf, N.; Flanagan, T.W.; Alamodi, A.A.; Al Hmada, Y.; Hassan, S.Y.; Shalaby, H.; Santourlidis, S.; Hassan, S.L.; Haikel, Y.; Megahed, M.; et al. CD133-Dependent Activation of Phosphoinositide 3-Kinase /AKT/Mammalian Target of Rapamycin Signaling in Melanoma Progression and Drug Resistance. Cells 2024, 13, 240. [Google Scholar] [CrossRef]
- Moreno-Londoño, A.P.; Robles-Flores, M. Functional Roles of CD133: More than Stemness Associated Factor Regulated by the Microenvironment. Stem Cell Rev. Rep. 2024, 20, 25–51. [Google Scholar] [CrossRef]
- Jang, J.W.; Song, Y.; Kim, S.H.; Kim, J.S.; Kim, K.M.; Choi, E.K.; Kim, J.; Seo, H.R. CD133 confers cancer stem-like cell properties by stabilizing EGFR-AKT signaling in hepatocellular carcinoma. Cancer Lett. 2017, 389, 1–10. [Google Scholar] [CrossRef]
- Weng, C.C.; Kuo, K.K.; Su, H.T.; Hsiao, P.J.; Chen, Y.W.; Wu, D.C.; Hung, W.C.; Cheng, K.H. Pancreatic Tumor Progression Associated With CD133 Overexpression: Involvement of Increased TERT Expression and Epidermal Growth Factor Receptor-Dependent Akt Activation. Pancreas 2016, 45, 443–457. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Ju, J.H.; Son, S.; Shin, I. Silencing of CD133 inhibits GLUT1-mediated glucose transport through downregulation of the HER3/Akt/mTOR pathway in colon cancer. FEBS Lett. 2020, 594, 1021–1035. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.R.; Hendershott, M.C.; Terragni, J.; Cooper, G.M. mRNA degradation plays a significant role in the program of gene expression regulated by phosphatidylinositol 3-kinase signaling. Mol. Cell. Biol. 2010, 30, 5295–5305. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, C.; Lan, L.; Yan, L.; Li, W.; Evans, I.; Ruiz, E.J.; Su, Q.; Zhao, G.; Wu, W.; et al. METTL3 promotes oxaliplatin resistance of gastric cancer CD133+ stem cells by promoting PARP1 mRNA stability. Cell. Mol. Life Sci. 2022, 79, 135. [Google Scholar] [CrossRef]
- Papadatos-Pastos, D.; Rabbie, R.; Ross, P.; Sarker, D. The role of the PI3K pathway in colorectal cancer. Crit. Rev. Oncol./Hematol. 2015, 94, 18–30. [Google Scholar] [CrossRef]
- Maharati, A.; Moghbeli, M. PI3K/AKT signaling pathway as a critical regulator of epithelial-mesenchymal transition in colorectal tumor cells. Cell Commun. Signal. CCS 2023, 21, 201. [Google Scholar] [CrossRef]
- Zhu, Y.; Kong, F.; Zhang, C.; Ma, C.; Xia, H.; Quan, B.; Cui, H. CD133 mediates the TGF-β1-induced activation of the PI3K/ERK/P70S6K signaling pathway in gastric cancer cells. Oncol. Lett. 2017, 14, 7211–7216. [Google Scholar] [CrossRef][Green Version]
- Jang, J.W.; Song, Y.; Kim, S.H.; Kim, J.; Seo, H.R. Potential mechanisms of CD133 in cancer stem cells. Life Sci. 2017, 184, 25–29. [Google Scholar] [CrossRef]
- Karim, M.R.; Fisher, C.R.; Kapphahn, R.J.; Polanco, J.R.; Ferrington, D.A. Investigating AKT activation and autophagy in immunoproteasome-deficient retinal cells. PLoS ONE 2020, 15, e0231212. [Google Scholar] [CrossRef]
- Yazid, M.D.; Hung-Chih, C. Perturbation of PI3K/Akt signaling affected autophagy modulation in dystrophin-deficient myoblasts. Cell Commun. Signal. CCS 2021, 19, 105. [Google Scholar] [CrossRef]
- Lu, R.; Zhao, G.; Yang, Y.; Jiang, Z.; Cai, J.; Hu, H. Inhibition of CD133 Overcomes Cisplatin Resistance Through Inhibiting PI3K/AKT/mTOR Signaling Pathway and Autophagy in CD133-Positive Gastric Cancer Cells. Technol. Cancer Res. Treat. 2019, 18, 1533033819864311. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yu, J.; Wang, S.; Lu, R.; Wu, J.; Jiang, B. Overexpression of CD133 enhances chemoresistance to 5-fluorouracil by activating the PI3K/Akt/p70S6K pathway in gastric cancer cells. Oncol. Rep. 2014, 32, 2437–2444. [Google Scholar] [CrossRef][Green Version]
- Yang, Y.; Jiang, C.; Yang, Y.; Guo, L.; Huang, J.; Liu, X.; Wu, C.; Zou, J. Silencing of LncRNA-HOTAIR decreases drug resistance of Non-Small Cell Lung Cancer cells by inactivating autophagy via suppressing the phosphorylation of ULK1. Biochem. Biophys. Res. Commun. 2018, 497, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Wang, J.; Ma, D.; Wang, P.; Zhang, Y.; Fang, Q. Heme oxygenase-1 contributes to imatinib resistance by promoting autophagy in chronic myeloid leukemia through disrupting the mTOR signaling pathway. Biomed. Pharmacother. 2016, 78, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Han, X.; Tkach, D.; Huang, S.G.; Zhang, D. AMPK promotes the survival of colorectal cancer stem cells. Anim. Model. Exp. Med. 2018, 1, 134–142. [Google Scholar] [CrossRef]
- Roach, P.J. AMPK -> ULK1 -> autophagy. Mol. Cell. Biol. 2011, 31, 3082–3084. [Google Scholar] [CrossRef]
- Lee, J.W.; Park, S.; Takahashi, Y.; Wang, H.G. The association of AMPK with ULK1 regulates autophagy. PLoS ONE 2010, 5, e15394. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Yin, J.; Huo, W.; Chaum, E. Loss of Prom1 impairs autophagy and promotes epithelial-mesenchymal transition in mouse retinal pigment epithelial cells. J. Cell. Physiol. 2023, 238, 2373–2389. [Google Scholar] [CrossRef]
- Feng, H.; Zhao, X.; Guo, Q.; Feng, Y.; Ma, M.; Guo, W.; Dong, X.; Deng, C.; Li, C.; Song, X.; et al. Autophagy resists EMT process to maintain retinal pigment epithelium homeostasis. Int. J. Biol. Sci. 2019, 15, 507–521. [Google Scholar] [CrossRef]
- Petrova, V.; Annicchiarico-Petruzzelli, M.; Melino, G.; Amelio, I. The hypoxic tumour microenvironment. Oncogenesis 2018, 7, 10. [Google Scholar] [CrossRef]
- Yun, Z.; Lin, Q. Hypoxia and regulation of cancer cell stemness. Adv. Exp. Med. Biol. 2014, 772, 41–53. [Google Scholar] [CrossRef]
- Zaarour, R.F.; Azakir, B.; Hajam, E.Y.; Nawafleh, H.; Zeinelabdin, N.A.; Engelsen, A.S.T.; Thiery, J.; Jamora, C.; Chouaib, S. Role of Hypoxia-Mediated Autophagy in Tumor Cell Death and Survival. Cancers 2021, 13, 533. [Google Scholar] [CrossRef]
- Fathi, A.; Mosaad, H.; Hussein, S.; Roshdy, M.; Ismail, E.I. Prognostic significance of CD133 and ezrin expression in colorectal carcinoma. IUBMB Life 2017, 69, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Jin, J.; Li, X.; Zhang, S.; Choi, Y.H.; Piao, Y.; Shen, X.; Lin, Z. Prognostic implications of ezrin and phosphorylated ezrin expression in non-small cell lung cancer. BMC Cancer 2014, 14, 191. [Google Scholar] [CrossRef] [PubMed]
- Qureshi-Baig, K.; Kuhn, D.; Viry, E.; Pozdeev, V.I.; Schmitz, M.; Rodriguez, F.; Ullmann, P.; Koncina, E.; Nurmik, M.; Frasquilho, S.; et al. Hypoxia-induced autophagy drives colorectal cancer initiation and progression by activating the PRKC/PKC-EZR (ezrin) pathway. Autophagy 2020, 16, 1436–1452. [Google Scholar] [CrossRef] [PubMed]
- Li, D.D.; Guo, J.F.; Huang, J.J.; Wang, L.L.; Deng, R.; Liu, J.N.; Feng, G.K.; Xiao, D.J.; Deng, S.Z.; Zhang, X.S.; et al. Rhabdastrellic acid-A induced autophagy-associated cell death through blocking Akt pathway in human cancer cells. PLoS ONE 2010, 5, e12176. [Google Scholar] [CrossRef]
- Li, X.; Wu, X.Q.; Deng, R.; Li, D.D.; Tang, J.; Chen, W.D.; Chen, J.H.; Ji, J.; Jiao, L.; Jiang, S.; et al. CaMKII-mediated Beclin 1 phosphorylation regulates autophagy that promotes degradation of Id and neuroblastoma cell differentiation. Nat. Commun. 2017, 8, 1159. [Google Scholar] [CrossRef]
- Jamal, S.M.E.; Alamodi, A.; Wahl, R.U.; Grada, Z.; Shareef, M.A.; Hassan, S.Y.; Murad, F.; Hassan, S.L.; Santourlidis, S.; Gomez, C.R.; et al. Melanoma stem cell maintenance and chemo-resistance are mediated by CD133 signal to PI3K-dependent pathways. Oncogene 2020, 39, 5468–5478. [Google Scholar] [CrossRef]
- Chiu, C.F.; Chang, Y.W.; Kuo, K.T.; Shen, Y.S.; Liu, C.Y.; Yu, Y.H.; Cheng, C.C.; Lee, K.Y.; Chen, F.C.; Hsu, M.K.; et al. NF-κB-driven suppression of FOXO3a contributes to EGFR mutation-independent gefitinib resistance. Proc. Natl. Acad. Sci. USA 2016, 113, E2526–E2535. [Google Scholar] [CrossRef]
- Hao, X.D.; Liu, J.X.; Zhang, J.S. Longevity factor FOXO3a: A potential therapeutic target for age-related ocular diseases. Life Sci. 2024, 350, 122769. [Google Scholar] [CrossRef]
- Meng, F.; Zhang, Z.; Chen, C.; Liu, Y.; Yuan, D.; Hei, Z.; Luo, G. PI3K/AKT activation attenuates acute kidney injury following liver transplantation by inducing FoxO3a nuclear export and deacetylation. Life Sci. 2021, 272, 119119. [Google Scholar] [CrossRef] [PubMed]
- Diez, A.F.; Leroux, L.P.; Chagneau, S.; Plouffe, A.; Gold, M.; Chaparro, V.; Jaramillo, M. Toxoplasma gondii inhibits the expression of autophagy-related genes through AKT-dependent inactivation of the transcription factor FOXO3a. mBio 2023, 14, e0079523. [Google Scholar] [CrossRef] [PubMed]
- Behrooz, A.B.; Syahir, A. Could We Address the Interplay Between CD133, Wnt/β-Catenin, and TERT Signaling Pathways as a Potential Target for Glioblastoma Therapy? Front. Oncol. 2021, 11, 642719. [Google Scholar] [CrossRef] [PubMed]
- Mazo, C.E.; D’Lugos, A.C.; Sweeney, K.R.; Haus, J.M.; Angadi, S.S.; Carroll, C.C.; Dickinson, J.M. The effects of acute aerobic and resistance exercise on mTOR signaling and autophagy markers in untrained human skeletal muscle. Eur. J. Appl. Physiol. 2021, 121, 2913–2924. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, Y. AMPK and Autophagy. Adv. Exp. Med. Biol. 2019, 1206, 85–108. [Google Scholar] [CrossRef]
- Gong, Q.; Cao, X.; Cao, J.; Yang, X.; Zeng, W. Casticin suppresses the carcinogenesis of small cell lung cancer H446 cells through activation of AMPK/FoxO3a signaling. Oncol. Rep. 2018, 40, 1401–1410. [Google Scholar] [CrossRef] [PubMed]
- Smit, L.; Berns, K.; Spence, K.; Ryder, W.D.; Zeps, N.; Madiredjo, M.; Beijersbergen, R.; Bernards, R.; Clarke, R.B. An integrated genomic approach identifies that the PI3K/AKT/FOXO pathway is involved in breast cancer tumor initiation. Oncotarget 2016, 7, 2596–2610. [Google Scholar] [CrossRef]
- Kim, R.J.; Bae, E.; Hong, Y.K.; Hong, J.Y.; Kim, N.K.; Ahn, H.J.; Oh, J.J.; Park, D.S. PTEN loss-mediated Akt activation increases the properties of cancer stem-like cell populations in prostate cancer. Oncology 2014, 87, 270–279. [Google Scholar] [CrossRef]
- Liu, Q.Q.; Wu, G.H.; Wang, X.C.; Xiong, X.W.; Wang, R.; Yao, B.L. The role of Foxo3a in neuron-mediated cognitive impairment. Front. Mol. Neurosci. 2024, 17, 1424561. [Google Scholar] [CrossRef]
- Yun, H.R.; Jo, Y.H.; Kim, J.; Shin, Y.; Kim, S.S.; Choi, T.G. Roles of Autophagy in Oxidative Stress. Int. J. Mol. Sci. 2020, 21, 3289. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, D.; Liu, Y.; Su, Z.; Zhang, L.; Chen, F.; Zhou, Y.; Wu, Y.; Yu, M.; Zhang, Z.; et al. Role of the Hypoxia-inducible factor-1 alpha induced autophagy in the conversion of non-stem pancreatic cancer cells into CD133+ pancreatic cancer stem-like cells. Cancer Cell Int. 2013, 13, 119. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.J.; Zhang, S.S.; Guo, X.L.; Sun, K.; Han, Z.P.; Li, R.; Zhao, Q.D.; Deng, W.J.; Xie, X.Q.; Zhang, J.W.; et al. Autophagy contributes to the survival of CD133+ liver cancer stem cells in the hypoxic and nutrient-deprived tumor microenvironment. Cancer Lett. 2013, 339, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Khor, Y.S.; Wong, P.F. MicroRNAs-associated with FOXO3 in cellular senescence and other stress responses. Biogerontology 2024, 25, 23–51. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Lin, M.; Gu, W.; Su, Z.; Duan, Y.; Song, W.; Liu, H.; Zhang, F. The rules and regulatory mechanisms of FOXO3 on inflammation, metabolism, cell death and aging in hosts. Life Sci. 2023, 328, 121877. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, C.; Wang, P.; Gao, J.; Liu, X.; Li, Y.; Yan, S.; Shi, Y. HIF-1α affects trophoblastic apoptosis involved in the onset of preeclampsia by regulating FOXO3a under hypoxic conditions. Mol. Med. Rep. 2020, 21, 2484–2492. [Google Scholar] [CrossRef] [PubMed]
- Iida, H.; Suzuki, M.; Goitsuka, R.; Ueno, H. Hypoxia induces CD133 expression in human lung cancer cells by up-regulation of OCT3/4 and Fself2. Int. J. Oncol. 2012, 40, 71–79. [Google Scholar] [CrossRef]
- Yang, H.Z.; Ma, Y.; Zhou, Y.; Xu, L.M.; Chen, X.J.; Ding, W.B.; Zou, H.B. Autophagy contributes to the enrichment and survival of colorectal cancer stem cells under oxaliplatin treatment. Cancer Lett. 2015, 361, 128–136. [Google Scholar] [CrossRef]
- Mahgoub, E.; Taneera, J.; Sulaiman, N.; Saber-Ayad, M. The role of autophagy in colorectal cancer: Impact on pathogenesis and implications in therapy. Front. Med. 2022, 9, 959348. [Google Scholar] [CrossRef]
- Levy, J.M.; Thorburn, A. Targeting autophagy during cancer therapy to improve clinical outcomes. Pharmacol. Ther. 2011, 131, 130–141. [Google Scholar] [CrossRef]
- Mohammad, R.M.; Muqbil, I.; Lowe, L.; Yedjou, C.; Hsu, H.Y.; Lin, L.T.; Siegelin, M.D.; Fimognari, C.; Kumar, N.B.; Dou, Q.P.; et al. Broad targeting of resistance to apoptosis in cancer. Semin. Cancer Biol. 2015, 35, S78–S103. [Google Scholar] [CrossRef]
- Rainho, M.A.; Siqueira, P.B.; de Amorim, Í.S.S.; Mencalha, A.L.; Thole, A.A. Mitochondria in colorectal cancer stem cells–A target in drug resistance. Cancer Drug Resist. 2023, 6, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, X.; Zhu, W.; Sui, Z.; Wei, X.; Zhang, Y.; Qi, J.; Xing, Y.; Wang, W. TRPML1-induced autophagy inhibition triggers mitochondrial mediated apoptosis. Cancer Lett. 2022, 541, 215752. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Zhao, M.; Fan, L.; Cao, X.; Xia, Q.; Zhou, J.; Yin, H.; Zhao, L. Chitopentaose inhibits hepatocellular carcinoma by inducing mitochondrial mediated apoptosis and suppressing protective autophagy. Bioresour. Bioprocess. 2021, 8, 4. [Google Scholar] [CrossRef]
- Han, C.; Xing, G.; Zhang, M.; Zhong, M.; Han, Z.; He, C.; Liu, X. Wogonoside inhibits cell growth and induces mitochondrial-mediated autophagy-related apoptosis in human colon cancer cells through the PI3K/AKT/mTOR/p70S6K signaling pathway. Oncol. Lett. 2018, 15, 4463–4470. [Google Scholar] [CrossRef]
- Zhuang, Y.W.; Wu, C.E.; Zhou, J.Y.; Zhao, Z.M.; Liu, C.L.; Shen, J.Y.; Cai, H.; Liu, S.L. Solasodine reverses stemness and epithelial-mesenchymal transition in human colorectal cancer. Biochem. Biophys. Res. Commun. 2018, 505, 485–491. [Google Scholar] [CrossRef]
- Tessmann, J.W.; Rocha, M.R.; Morgado-Díaz, J.A. Mechanisms of radioresistance and the underlying signaling pathways in colorectal cancer cells. J. Cell. Biochem. 2023, 124, 31–45. [Google Scholar] [CrossRef]
- Strippoli, R.; Niayesh-Mehr, R.; Adelipour, M.; Khosravi, A.; Cordani, M.; Zarrabi, A.; Allameh, A. Contribution of Autophagy to Epithelial Mesenchymal Transition Induction during Cancer Progression. Cancers 2024, 16, 807. [Google Scholar] [CrossRef]
- Yan, C.; Luo, L.; Goto, S.; Urata, Y.; Guo, C.Y.; Doi, H.; Kitazato, K.; Li, T.S. Enhanced autophagy in colorectal cancer stem cells does not contribute to radio-resistance. Oncotarget 2016, 7, 45112–45121. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hu, Y.L.; Jahangiri, A.; Delay, M.; Aghi, M.K. Tumor cell autophagy as an adaptive response mediating resistance to treatments such as antiangiogenic therapy. Cancer Res. 2012, 72, 4294–4299. [Google Scholar] [CrossRef]
- Bristol, M.L.; Emery, S.M.; Maycotte, P.; Thorburn, A.; Chakradeo, S.; Gewirtz, D.A. Autophagy inhibition for chemosensitization and radiosensitization in cancer: Do the preclinical data support this therapeutic strategy? J. Pharmacol. Exp. Ther. 2013, 344, 544–552. [Google Scholar] [CrossRef]
- Wei, M.F.; Chen, M.W.; Chen, K.C.; Lou, P.J.; Lin, S.Y.; Hung, S.C.; Hsiao, M.; Yao, C.J.; Shieh, M.J. Autophagy promotes resistance to photodynamic therapy-induced apoptosis selectively in colorectal cancer stem-like cells. Autophagy 2014, 10, 1179–1192. [Google Scholar] [CrossRef] [PubMed]
- Schmohl, J.U.; Vallera, D.A. CD133, Selectively Targeting the Root of Cancer. Toxins 2016, 8, 165. [Google Scholar] [CrossRef] [PubMed]
- Ning, S.T.; Lee, S.Y.; Wei, M.F.; Peng, C.L.; Lin, S.Y.; Tsai, M.H.; Lee, P.C.; Shih, Y.H.; Lin, C.Y.; Luo, T.Y.; et al. Targeting Colorectal Cancer Stem-Like Cells with Anti-CD133 Antibody-Conjugated SN-38 Nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 17793–17804. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, M.; Wu, Z.; Tong, C.; Dai, H.; Guo, Y.; Liu, Y.; Huang, J.; Lv, H.; Luo, C.; et al. CD133-directed CAR T cells for advanced metastasis malignancies: A phase I trial. OncoImmunology 2018, 7, e1440169. [Google Scholar] [CrossRef]
- Mokarram, P.; Albokashy, M.; Zarghooni, M.; Moosavi, M.A.; Sepehri, Z.; Chen, Q.M.; Hudecki, A.; Sargazi, A.; Alizadeh, J.; Moghadam, A.R.; et al. New frontiers in the treatment of colorectal cancer: Autophagy and the unfolded protein response as promising targets. Autophagy 2017, 13, 781–819. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, J.; Li, K.; Deng, L.; Wang, H. Combination of an Autophagy Inducer and an Autophagy Inhibitor: A Smarter Strategy Emerging in Cancer Therapy. Front. Pharmacol. 2020, 11, 408. [Google Scholar] [CrossRef]
- Zhao, Q.; Guo, Y.; Feng, B.; Li, L.; Huang, C.; Jiao, B. Neriifolin from seeds of Cerbera manghas L. induces cell cycle arrest and apoptosis in human hepatocellular carcinoma HepG2 cells. Fitoterapia 2011, 82, 735–741. [Google Scholar] [CrossRef]
- Pan, L.; Zhang, Y.; Zhao, W.; Zhou, X.; Wang, C.; Deng, F. The cardiac glycoside oleandrin induces apoptosis in human colon cancer cells via the mitochondrial pathway. Cancer Chemother. Pharmacol. 2017, 80, 91–100. [Google Scholar] [CrossRef]
- Škubník, J.; Svobodová Pavlíčková, V.; Psotová, J.; Rimpelová, S. Cardiac Glycosides as Autophagy Modulators. Cells 2021, 10, 3341. [Google Scholar] [CrossRef]
- Lee, D.H.; Cheul Oh, S.; Giles, A.J.; Jung, J.; Gilbert, M.R.; Park, D.M. Cardiac glycosides suppress the maintenance of stemness and malignancy via inhibiting HIF-1α in human glioma stem cells. Oncotarget 2017, 8, 40233–40245. [Google Scholar] [CrossRef]
- Klose, J.; Eissele, J.; Volz, C.; Schmitt, S.; Ritter, A.; Ying, S.; Schmidt, T.; Heger, U.; Schneider, M.; Ulrich, A. Salinomycin inhibits metastatic colorectal cancer growth and interferes with Wnt/β-catenin signaling in CD133+ human colorectal cancer cells. BMC Cancer 2016, 16, 896. [Google Scholar] [CrossRef] [PubMed]
- Asadzadeh, Z.; Mansoori, B.; Mohammadi, A.; Kazemi, T.; Mokhtarzadeh, A.; Shanehbandi, D.; Hemmat, N.; Derakhshani, A.; Brunetti, O.; Safaei, S.; et al. The combination effect of Prominin1 (CD133) suppression and Oxaliplatin treatment in colorectal cancer therapy. Biomed. Pharmacother. 2021, 137, 111364. [Google Scholar] [CrossRef] [PubMed]
- Szaryńska, M.; Olejniczak, A.; Kobiela, J.; Spychalski, P.; Kmieć, Z. Therapeutic strategies against cancer stem cells in human colorectal cancer. Oncol. Lett. 2017, 14, 7653–7668. [Google Scholar] [CrossRef]
- Mattoo, A.R.; Zhang, J.; Espinoza, L.A.; Jessup, J.M. Inhibition of NANOG/NANOGP8 downregulates MCL-1 in colorectal cancer cells and enhances the therapeutic efficacy of BH3 mimetics. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2014, 20, 5446–5455. [Google Scholar] [CrossRef]
- Dou, J.; Ni, Y.; He, X.; Wu, D.; Li, M.; Wu, S.; Zhang, R.; Guo, M.; Zhao, F. Decreasing lncRNA HOTAIR expression inhibits human colorectal cancer stem cells. Am. J. Transl. Res. 2016, 8, 98–108. [Google Scholar]
- Li, W.; Cho, M.Y.; Lee, S.; Jang, M.; Park, J.; Park, R. CRISPR-Cas9 mediated CD133 knockout inhibits colon cancer invasion through reduced epithelial-mesenchymal transition. PLoS ONE 2019, 14, e0220860. [Google Scholar] [CrossRef]
- Merhi, M.; Ahmad, F.; Taib, N.; Inchakalody, V.; Uddin, S.; Shablak, A.; Dermime, S. The complex network of transcription factors, immune checkpoint inhibitors and stemness features in colorectal cancer: A recent update. Semin. Cancer Biol. 2023, 89, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Do, A.S.S.; Amano, T.; Edwards, L.A.; Zhang, L.; De Peralta-Venturina, M.; Yu, J.S. CD133 mRNA-Loaded Dendritic Cell Vaccination Abrogates Glioma Stem Cell Propagation in Humanized Glioblastoma Mouse Model. Mol. Ther. Oncolytics 2020, 18, 295–303. [Google Scholar] [CrossRef]
- Guler Kara, H.; Ozates, N.P.; Asik, A.; Gunduz, C. Cancer stemness kinase inhibitor amcasertib: A promising therapeutic agent in ovarian cancer stem and cancer cell models with different genetic profiles. Med. Oncol. 2023, 40, 342. [Google Scholar] [CrossRef]
- Malkomes, P.; Lunger, I.; Luetticke, A.; Oppermann, E.; Haetscher, N.; Serve, H.; Holzer, K.; Bechstein, W.O.; Rieger, M.A. Selective AKT Inhibition by MK-2206 Represses Colorectal Cancer-Initiating Stem Cells. Ann. Surg. Oncol. 2016, 23, 2849–2857. [Google Scholar] [CrossRef]
- Sun, L.; Huang, Y.; Liu, Y.; Zhao, Y.; He, X.; Zhang, L.; Wang, F.; Zhang, Y. Ipatasertib, a novel Akt inhibitor, induces transcription factor FoxO3a and NF-κB directly regulates PUMA-dependent apoptosis. Cell Death Dis. 2018, 9, 911. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yuan, Y.; Yang, L.; Chen, H.; Zhang, X.; Wen, T.; Liao, W.; Zhao, M.; Zhao, Z.; Hu, Q. AT7867 Inhibits the Growth of Colorectal Cancer Stem-Like Cells and Stemness by Regulating the Stem Cell Maintenance Factor Ascl2 and Akt Signaling. Stem Cells Int. 2023, 2023, 4199052. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sipos, F.; Műzes, G. Interconnection of CD133 Stem Cell Marker with Autophagy and Apoptosis in Colorectal Cancer. Int. J. Mol. Sci. 2024, 25, 11201. https://doi.org/10.3390/ijms252011201
Sipos F, Műzes G. Interconnection of CD133 Stem Cell Marker with Autophagy and Apoptosis in Colorectal Cancer. International Journal of Molecular Sciences. 2024; 25(20):11201. https://doi.org/10.3390/ijms252011201
Chicago/Turabian StyleSipos, Ferenc, and Györgyi Műzes. 2024. "Interconnection of CD133 Stem Cell Marker with Autophagy and Apoptosis in Colorectal Cancer" International Journal of Molecular Sciences 25, no. 20: 11201. https://doi.org/10.3390/ijms252011201
APA StyleSipos, F., & Műzes, G. (2024). Interconnection of CD133 Stem Cell Marker with Autophagy and Apoptosis in Colorectal Cancer. International Journal of Molecular Sciences, 25(20), 11201. https://doi.org/10.3390/ijms252011201






