The Anti-Inflammatory Effect of Lactococcus lactis-Ling-Zhi 8 on Ameliorating Atherosclerosis and Nonalcoholic Fatty Liver in High-Fat Diet Rabbits
Abstract
1. Introduction
2. Results
2.1. Effects of L. lactis-LZ8 Supplementation on Body Weight, Hematological Parameters, and Serum Lipid Profiles in HFD Rabbits
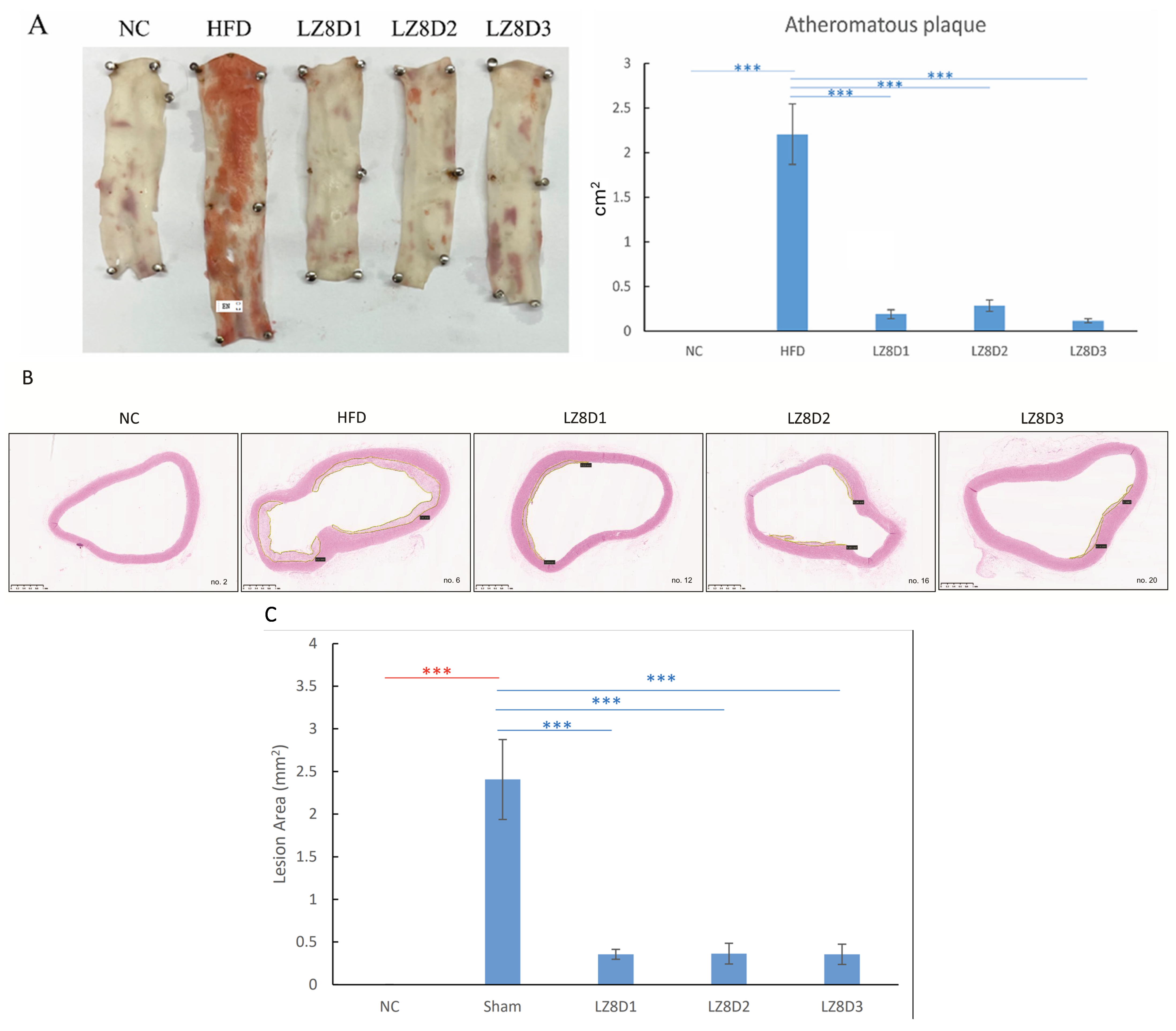
2.2. L. lactis-LZ8 Supplementation Reduces Lipid Plaques of the Aortas in HFD Rabbits
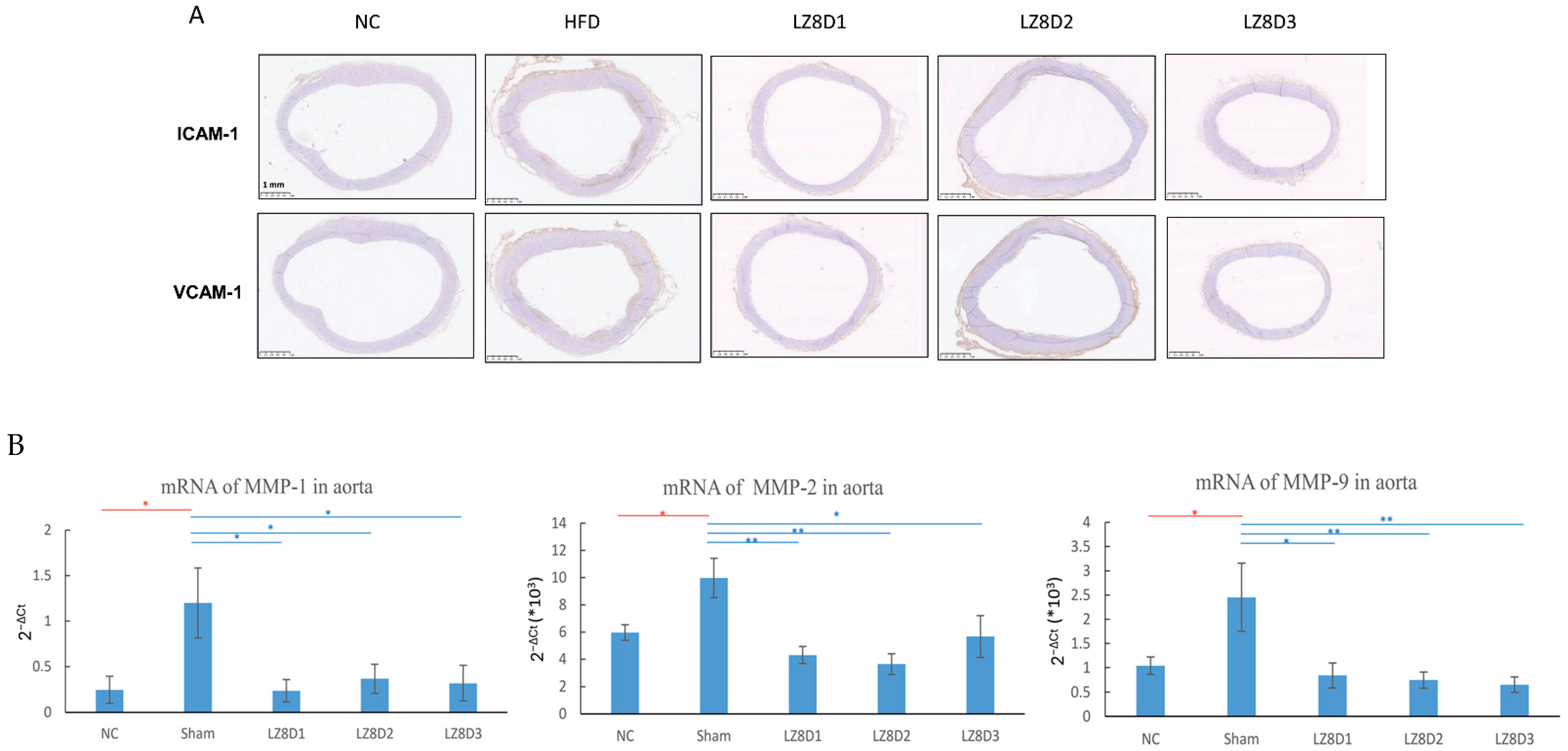
2.3. L. lactis-LZ8 Supplementation Downregulates Protein and Gene Expression Related to Inflammation in the Aortas of HFD Rabbits
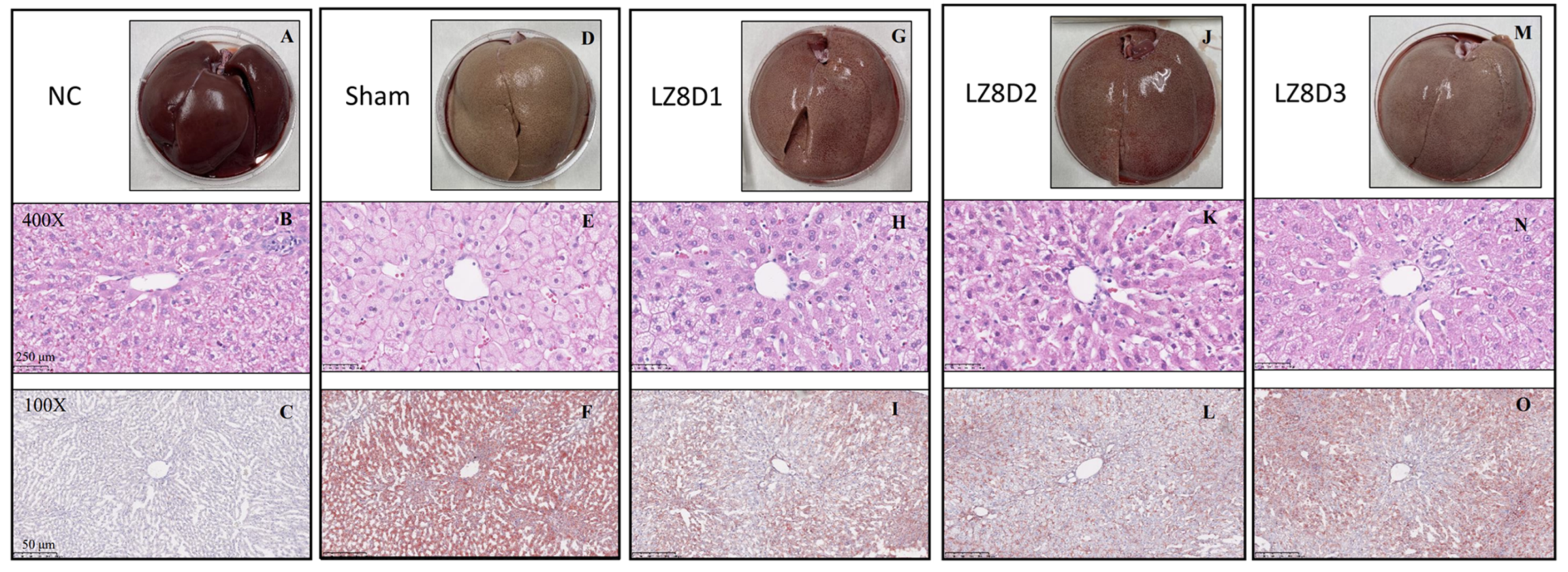
2.4. L. lactis-LZ8 Supplementation Reduces Hepatic Lipid Accumulation in HFD Rabbits
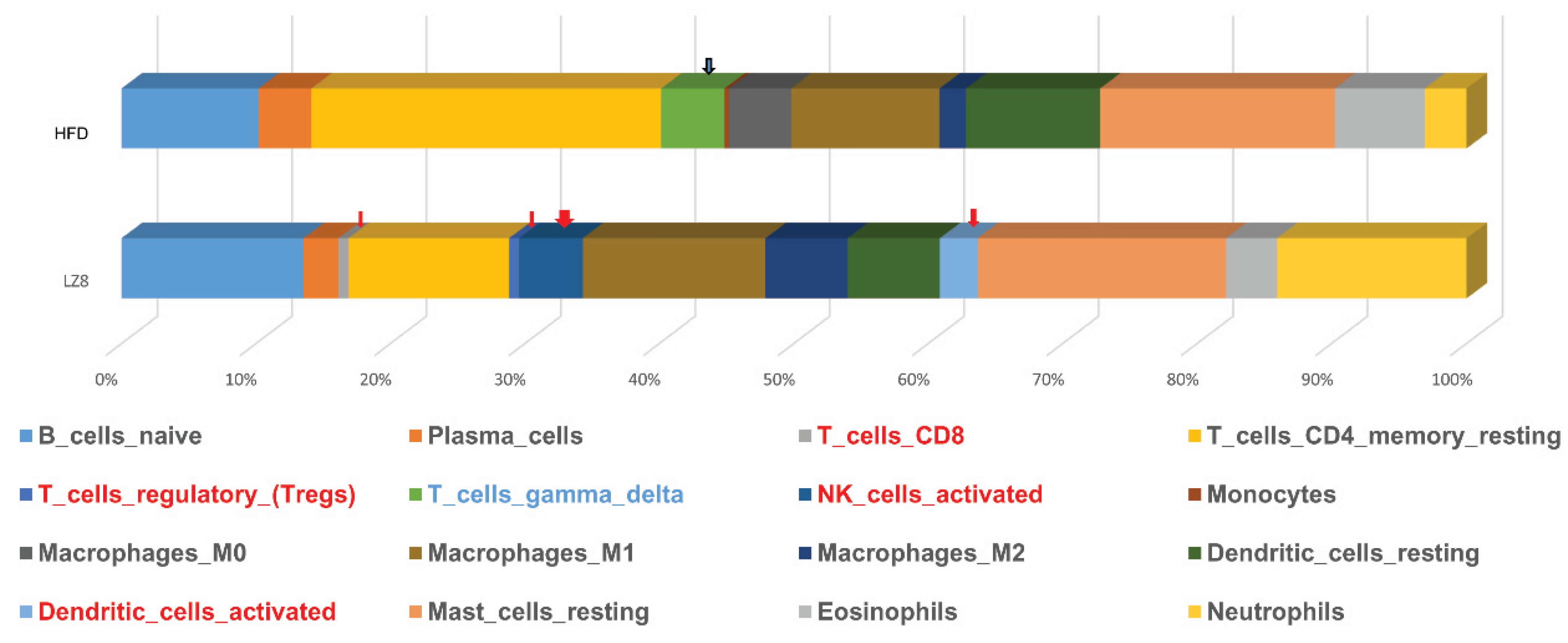
2.5. RNA Sequence Data of Livers from Rabbits of HFD and LZ8D1 Groups
3. Discussion
4. Materials and Methods
4.1. Preparation of Biotherapeutic L. lactis-LZ8
4.2. L. lactis-LZ8 Supplementation Treatment in an Established HFD Rabbit Model
4.3. Hematology and Serum Lipids Determination
4.4. Sudan Red Staining of Rabbit Aortas
4.5. H&E Staining of Rabbit Livers and Aorta Arches
4.6. IHC Analysis of Rabbit Aorta Arches
4.7. Oil Red O Staining of Rabbit Livers
4.8. Real-Time Polymerase Chain Reaction of Rabbit Aortas
4.9. RNA Sequencing of Rabbit Livers and Bioinformatics Analysis
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Khushalani, J.S.; Song, S.; Calhoun, B.H.; Puddy, R.W.; Kucik, J.E. Preventing Leading Causes of Death: Systematic Review of Cost-Utility Literature. Am. J. Prev. Med. 2022, 62, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Gaidai, O.; Cao, Y.; Loginov, S. Global Cardiovascular Diseases Death Rate Prediction. Curr. Probl. Cardiol. 2023, 48, 101622. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.H.; Ramji, D.P. Atherosclerosis: Pathogenesis and Key Cellular Processes, Current and Emerging Therapies, Key Challenges, and Future Research Directions. Methods Mol. Biol. 2022, 2419, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Athyros, V.G.; Alexandrides, T.K.; Bilianou, H.; Cholongitas, E.; Doumas, M.; Ganotakis, E.S.; Goudevenos, J.; Elisaf, M.S.; Germanidis, G.; Giouleme, O.; et al. The use of statins alone, or in combination with pioglitazone and other drugs, for the treatment of non-alcoholic fatty liver disease/non-alcoholic steatohepatitis and related cardiovascular risk. An Expert Panel Statement. Metabolism 2017, 71, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Pais, R.; Redheuil, A.; Cluzel, P.; Ratziu, V.; Giral, P. Relationship Among Fatty Liver, Specific and Multiple-Site Atherosclerosis, and 10-Year Framingham Score. Hepatology 2019, 69, 1453–1463. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.L.; Brown, M.S. A century of cholesterol and coronaries: From plaques to genes to statins. Cell 2015, 161, 161–172. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Brody, M.; Bohm, I.; Bauer, R. Mechanism of action of methotrexate: Experimental evidence that methotrexate blocks the binding of interleukin 1 beta to the interleukin 1 receptor on target cells. Eur. J. Clin. Chem. Clin. Biochem. 1993, 31, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M. From C-Reactive Protein to Interleukin-6 to Interleukin-1: Moving Upstream To Identify Novel Targets for Atheroprotection. Circ. Res. 2016, 118, 145–156. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Leiva, O.; AbdelHameid, D.; Connors, J.M.; Cannon, C.P.; Bhatt, D.L. Common Pathophysiology in Cancer, Atrial Fibrillation, Atherosclerosis, and Thrombosis: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncol 2021, 3, 619–634. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kino, K.; Yamashita, A.; Yamaoka, K.; Watanabe, J.; Tanaka, S.; Ko, K.; Tsunoo, H. Isolation and characterization of a new immunomodulatory protein, ling zhi-8 (LZ-8), from Ganoderma lucidium. J. Biol. Chem. 1989, 264, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Yu, Y.-L.; Shih, C.-C.; Liu, K.-J.; Ou, K.-L.; Hong, L.-Z.; Chen, J.D.C.; Chu, C.-L. A novel adjuvant Ling Zhi-8 enhances the efficacy of DNA cancer vaccine by activating dendritic cells. Cancer Immunol. Immunother. 2011, 60, 1019–1027. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jan, J.-T.; Cheng, T.-J.R.; Juang, Y.-P.; Ma, H.-H.; Wu, Y.-T.; Yang, W.-B.; Cheng, C.-W.; Chen, X.; Chou, T.-H.; Shie, J.-J.; et al. Identification of existing pharmaceuticals and herbal medicines as inhibitors of SARS-CoV-2 infection. Proc. Natl. Acad. Sci. USA 2021, 118, e2021579118. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, L.; Sun, F.; Liang, C.; He, Y.; Bao, R.; Liu, L.; Zhou, C. Crystal structure of LZ-8 from the medicinal fungus Ganoderma lucidium. Proteins 2009, 75, 524–527. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-L.; Liang, Y.-C.; Tseng, Y.-S.; Huang, H.-Y.; Chou, S.-Y.; Hseu, R.-S.; Huang, C.-T.; Chiang, B.-L. An immunomodulatory protein, Ling Zhi-8, induced activation and maturation of human monocyte-derived dendritic cells by the NF-kappaB and MAPK pathways. J. Leukoc. Biol. 2009, 86, 877–889. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Z.; Chang, Y.Z.; He, Z.M.; Chen, L.; Zhou, X.W. Immunomodulatory activity of Ganoderma lucidum immunomodulatory protein via PI3K/Akt and MAPK signaling pathways in RAW264.7 cells. J. Cell Physiol. 2019, 234, 23337–23348. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-F.; Chiang, C.-H.; Lin, S.-J.; Song, P.-P.; Liu, H.-C.; Wu, T.-J.; Lin, W.-W. Recombinant Lactococcus lactis Expressing Ling Zhi 8 Protein Ameliorates Nonalcoholic Fatty Liver and Early Atherogenesis in Cholesterol-Fed Rabbits. Biomed. Res. Int. 2020, 2020, 3495682. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vestweber, D.; Blanks, J.E. Mechanisms that regulate the function of the selectins and their ligands. Physiol. Rev. 1999, 79, 181–213. [Google Scholar] [CrossRef] [PubMed]
- Ao, C.; Qi, L.; Xiong, Z.; Kang, A.; Guo, H.; Xue, L.; Huo, Y. Circulating secretory type IIA phospholipase A2, lipoprotein (a) and soluble cellular adhesion molecule levels in patients with angina pectoris. Clin. Biochem. 2008, 41, 1423–1428. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.L. Metalloproteinases in atherosclerosis. Eur. J. Pharmacol. 2017, 816, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Olejarz, W.; Lacheta, D.; Kubiak-Tomaszewska, G. Matrix Metalloproteinases as Biomarkers of Atherosclerotic Plaque Instability. Int. J. Mol. Sci. 2020, 21, 3946. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schoenfeld, S.R.; Kasturi, S.; Costenbader, K.H. The epidemiology of atherosclerotic cardiovascular disease among patients with SLE: A systematic review. Semin. Arthritis Rheum. 2013, 43, 77–95. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Kim, B.-H.; Seo, H.S.; Lee, Y.J.; Kim, H.H.; Son, H.-H.; Choi, M.H. Cholesterol-induced non-alcoholic fatty liver disease and atherosclerosis aggravated by systemic inflammation. PLoS ONE 2014, 9, e97841. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zheng, L.; Zhang, L.; Tan, F.; Wang, C.; Lv, X.; Bai, R.; Huo, N.; Zheng, M. Prevention and control of chicken coccidiosis construction of recombinant Lactococcus lactis expressing chicken IL-4 and IL-2 fusion protein and its immune synergistic effect on chicken coccidia live vaccine. Poult. Sci. 2023, 102, 102530. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lyu, C.; Yao, L.; Zhu, Q.; Mei, J.; Cao, Y.; Hu, S.; Zhao, W.; Huang, J.; Mei, L.; Yao, S.; et al. Reconstruction of the glutamate decarboxylase system in Lactococcus lactis for biosynthesis of food-grade γ-aminobutyric acid. Appl. Microbiol. Biotechnol. 2021, 105, 4127–4140. [Google Scholar] [CrossRef] [PubMed]
- How, Y.H.; Teo, M.Y.M.; In, L.L.A.; Yeo, S.K.; Bhandari, B.; Yusof, Y.A.; Pui, L.P. Stability and expression of K-ras mimotopes in freeze-dried recombinant Lactococcus lactis NZ3900-fermented milk powder during storage in vacuum packaging. J. Appl. Microbiol. 2024, 135, lxae162. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.Y.; Dong, M.; Hu, Z.Y.; Wu, J.; Li, Y.C.; Xu, H.D. Recombinant Lactococcus lactis NZ3900 expressing bioactive human FGF21 reduced body weight of Db/Db mice through the activity of brown adipose tissue. Benef. Microbes 2020, 11, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Mierau, I.; Kleerebezem, M. 10 years of the nisin-controlled gene expression system (NICE) in Lactococcus lactis. Appl. Microbiol. Biotechnol. 2005, 68, 705–717. [Google Scholar] [CrossRef] [PubMed]
- Frelet-Barrand, A. Lactococcus lactis, an Attractive Cell Factory for the Expression of Functional Membrane Proteins. Biomolecules 2022, 12, 180. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chan, S.W.; Tomlinson, B.; Chan, P.; Lam, C.W.K. The beneficial effects of Ganoderma lucidum on cardiovascular and metabolic disease risk. Pharm. Biol. 2021, 59, 1161–1171. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- van der Hem, L.G.; van der Vliet, J.A.; Bocken, C.F.; Kino, K.; Hoitsma, A.J.; Tax, W.J. Ling Zhi-8: Studies of a new immunomodulating agent. Transplantation 1995, 60, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Wachtel-Galor, S.; Tomlinson, B.; Benzie, I.F. Ganoderma lucidum (“Lingzhi”), a Chinese medicinal mushroom: Biomarker responses in a controlled human supplementation study. Br. J. Nutr. 2004, 91, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Matsui, Y.; Uhara, J.; Satoi, S.; Kaibori, M.; Yamada, H.; Kitade, H.; Imamura, A.; Takai, S.; Kawaguchi, Y.; Kwon, A.H.; et al. Improved prognosis of postoperative hepatocellular carcinoma patients when treated with functional foods: A prospective cohort study. J. Hepatol. 2002, 37, 78–86. [Google Scholar] [CrossRef]
- Boland, M. Human digestion—A processing perspective. J. Sci. Food Agric. 2016, 96, 2275–2283. [Google Scholar] [CrossRef]
- Lloyd-Jones, D.M.; Lewis, C.E.; Schreiner, P.J.; Shikany, J.M.; Sidney, S.; Reis, J.P. The Coronary Artery Risk Development In Young Adults (CARDIA) Study: JACC Focus Seminar 8/8. J. Am. Coll. Cardiol. 2021, 78, 260–277. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Devesa, A.; Ibanez, B.; Malick, W.A.; Tinuoye, E.O.; Bustamante, J.; Peyra, C.; Rosenson, R.S.; Bhatt, D.L.; Stone, G.W.; Fuster, V. Primary Prevention of Subclinical Atherosclerosis in Young Adults: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2023, 82, 2152–2162. [Google Scholar] [CrossRef] [PubMed]
- Chou, R.; Cantor, A.; Dana, T.; Wagner, J.; Ahmed, A.Y.; Fu, R.; Ferencik, M. Statin Use for the Primary Prevention of Cardiovascular Disease in Adults: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2022, 328, 754–771. [Google Scholar] [CrossRef] [PubMed]
- Gera, P.; Wasserstein, D.H.; Frishman, W.H.; Aronow, W.S. Low-Dose Colchicine for the Prevention of Cardiovascular Events After Acute Coronary Syndrome. Cardiol. Rev. 2024. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.F.; Chu, Y.W.; Chiang, C.H.; Jiang, R.S.; Lin, W.W.; Wang, N.M. Preclinical Safety Evaluation of Engineered Lactococcus lactis NZ3900 Biotherapeutic Using a 13-week Repeated Dose Oral Toxicity Study in Rabbits. Food Biotechnol. 2023, 37, 219–234. [Google Scholar] [CrossRef]
- Singh, V.; Kaur, R.; Kumari, P.; Pasricha, C.; Singh, R. ICAM-1 and VCAM-1: Gatekeepers in various inflammatory and cardiovascular disorders. Clin. Chim. Acta 2023, 548, 117487. [Google Scholar] [CrossRef] [PubMed]
- Westerterp, M.; Fotakis, P.; Ouimet, M.; Bochem, A.E.; Zhang, H.; Molusky, M.M.; Wang, W.; Abramowicz, S.; la Bastide-van Gemert, S.; Wang, N.; et al. Cholesterol Efflux Pathways Suppress Inflammasome Activation, NETosis, and Atherogenesis. Circulation 2018, 138, 898–912. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tall, A.R.; Bornfeldt, K.E. Inflammasomes and Atherosclerosis: A Mixed Picture. Circ. Res. 2023, 132, 1505–1520. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lin, W.-W.; Lin, Y.-C.; Chang, T.-Y.; Tsai, S.-H.; Ho, H.-C.; Chen, Y.-T.; Yang, V.C. Caveolin-1 expression is associated with plaque formation in hypercholesterolemic rabbits. J. Histochem. Cytochem. 2006, 54, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Gomes, F.L.T.; Maranhão, R.C.; Tavares, E.R.; Carvalho, P.O.; Higuchi, M.L.; Mattos, F.R.; Pitta, F.G.; Hatab, S.A.; Kalil-Filho, R.; Serrano, C.V. Regression of Atherosclerotic Plaques of Cholesterol-Fed Rabbits by Combined Chemotherapy with Paclitaxel and Methotrexate Carried in Lipid Core Nanoparticles. J. Cardiovasc. Pharmacol. Ther. 2018, 23, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Karami, Z.; Mehrzad, J.; Akrami, M.; Hosseinkhani, S. Anti-inflammation-based treatment of atherosclerosis using Gliclazide-loaded biomimetic nanoghosts. Sci. Rep. 2023, 13, 13880. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhong, B.; Li, F.L.; Zhao, J.Y.; Fu, Y.; Peng, C. Sporoderm-broken spore powder of Ganoderma lucidum ameliorate obesity and inflammation process in high-fat diet-induced obese mice. Food Nutr. Res. 2022, 66, 8745. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, J.; Lee, D.H.; Hu, J.; Tabung, F.K.; Li, Y.; Bhupathiraju, S.N.; Rimm, E.B.; Rexrode, K.M.; Manson, J.E.; Willett, W.C.; et al. Dietary Inflammatory Potential and Risk of Cardiovascular Disease Among Men and Women in the U.S. J. Am. Coll. Cardiol. 2020, 76, 2181–2193. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shah, B.; Newman, J.D.; Woolf, K.; Ganguzza, L.; Guo, Y.; Allen, N.; Zhong, J.; Fisher, E.A.; Slater, J. Anti-Inflammatory Effects of a Vegan Diet Versus the American Heart Association-Recommended Diet in Coronary Artery Disease Trial. J. Am. Heart Assoc. 2018, 7, e011367. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Burmeister, Y.; Weyer, K.; Dorre, A.; Seilheimer, B. The Multicomponent Medicinal Product Hepar Compositum Reduces Hepatic Inflammation and Fibrosis in a Streptozotocin- and High-Fat Diet-Induced Model of Metabolic Dysfunction-Associated Steatotic Liver Disease/Metabolic Dysfunction-Associated Steatohepatitis. Biomedicines 2023, 11, 3216. [Google Scholar] [CrossRef]
- Dongiovanni, P.; Paolini, E.; Corsini, A.; Sirtori, C.R.; Ruscica, M. Nonalcoholic fatty liver disease or metabolic dysfunction-associated fatty liver disease diagnoses and cardiovascular diseases: From epidemiology to drug approaches. Eur. J. Clin. Investig. 2021, 51, e13519. [Google Scholar] [CrossRef]
- Kasper, P.; Martin, A.; Lang, S.; Kütting, F.; Goeser, T.; Demir, M.; Steffen, H.-M. NAFLD and cardiovascular diseases: A clinical review. Clin. Res. Cardiol. 2020, 110, 921–937. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.H.; Chen, H.C.; Yang, J.J.; Chuang, W.I.; Sheu, F. Polysaccharides PS-G and protein LZ-8 from Reishi (Ganoderma lucidum) exhibit diverse functions in regulating murine macrophages and T lymphocytes. J. Agric. Food Chem. 2010, 58, 8535–8544. [Google Scholar] [CrossRef] [PubMed]
- Schriml, L.M.; Mitraka, E.; Munro, J.; Tauber, B.; Schor, M.; Nickle, L.; Felix, V.; Jeng, L.; Bearer, C.; Lichenstein, R.; et al. Human Disease Ontology 2018 update: Classification, content and workflow expansion. Nucleic Acids Res. 2019, 47, D955–D962. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liberzon, A.; Birger, C.; Thorvaldsdottir, H.; Ghandi, M.; Mesirov, J.P.; Tamayo, P. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 2015, 1, 417–425. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]



| Groups | NC | HFD | LZ8D1 | LZ8D2 | LZ8D3 |
|---|---|---|---|---|---|
| Cholesterol (mg/dL) | 52.67 ± 31.47 | 2152.5 ± 596.09 * | 2313.5 ± 653.25 ** | 2586.75 ± 998 ** | 2813.25 ± 646.19 ** |
| γ-Glutamyltransferase (IU/L) | 11.33 ± 1.15 | 9.75 ± 3.59 | 7.75 ± 2.75 | 12.25 ± 0.96 | 8.75 ± 1.5 |
| 4.17 ± 0.12 | 4.13 ± 0.26 | 4.2 ± 0.27 | 4.05 ± 0.19 | 4.18 ± 0.15 | |
| Globulin (g/dL) | 1.5 ± 0.1 | 1.83 ± 0.41 | 2 ± 0.52 | 2.23 ± 0.55 | 1.98 ± 0.63 |
| Total protein (g/dL) | 5.67 ± 0.21 | 5.95 ± 0.24 | 6.2 ± 0.6 | 6.28 ± 0.69 | 6.15 ± 0.52 |
| Blood Urea Nitrogen (mg/dL) | 20.67 ± 1.53 | 20 ± 2.58 | 19.75 ± 2.87 | 17.5 ± 1.29 | 21.25 ± 4.11 |
| Alkaline Phosphatase (U/L) | 215.5 ± 97.5 | 199.25 ± 123.79 | 227.38 ± 129.58 | 153 ± 113.86 | 131.75 ± 59.66 |
| Creatinine (mg/dL) | 1.27 ± 0.06 | 1.3 ± 0.16 | 1.28 ± 0.13 | 1.18 ± 0.19 | 1.23 ± 0.17 |
| AST (U/L) | 31.33 ± 10.21 | 36.75 ± 4.57 | 32.75 ± 10.81 | 40.5 ± 11.47 | 58.67 ± 4.03 |
| ALT (U/L) | 40 ± 3 | 25.5 ± 4.2 | 17.5 ± 5.92 ** | 22.5 ± 9.95 * | 20.33 ± 2.87 ** |
| ID | Description | GeneRatio | BgRatio | p Value | p Adjust | q Value | Gene ID | Count |
|---|---|---|---|---|---|---|---|---|
| DOID:1936 | atherosclerosis | 17/131 | 364/10,312 | 3.3491 × 10−6 | 0.00083924 | 0.00066311 | PLIN2/APOA1/MMP12/CLU/APCS/S100A9/CD163/LPL/SPP1/ALOX15/SAMD9/CPE/RSAD2/TNFRSF12A/SERPINE1/FGF21/CPT1A | 17 |
| DOID:2348 | arteriosclerotic cardiovascular disease | 17/131 | 365/10,312 | 3.4753 × 10−6 | 0.00083924 | 0.00066311 | PLIN2/APOA1/MMP12/CLU/APCS/S100A9/CD163/LPL/SPP1/ALOX15/SAMD9/CPE/RSAD2/TNFRSF12A/SERPINE1/FGF21/CPT1A | 17 |
| DOID:2349 | arteriosclerosis | 18/131 | 411/10,312 | 4.165 × 10−6 | 0.00083924 | 0.00066311 | PLIN2/APOA1/MMP12/CLU/APCS/S100A9/CD163/LPL/SPP1/ALOX15/SAMD9/CDH13/CPE/RSAD2/TNFRSF12A/SERPINE1/FGF21/CPT1A | 18 |
| Group | N | Diets during Days 1–56 (50 g/kg/day) | 3 mL/oral during Days 1–56 |
|---|---|---|---|
| NC | 4 | Normal chow (NC) | - |
| HFD | 4 | 94% NC +1% cholesterol + 5% peanut oil | 15% fructose syrup |
| LZ8D1 | 4 | 94% NC +1% cholesterol + 5% peanut oil | L. lactis-LZ8 in 15% fructose syrup (5 × 1010 CFU/oral) |
| LZ8D2 | 4 | 94% NC +1% cholesterol + 5% peanut oil | L. lactis-LZ8 in 15% fructose syrup (2.5 × 1011 CFU/oral) |
| LZ8D3 | 4 | 94% NC +1% cholesterol + 5% peanut oil | L. lactis-LZ8 in 15% fructose syrup (5 × 1011 CFU/oral) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.-F.; Wang, N.M.; Chu, Y.-W.; Wu, C.-S.; Lin, W.-W. The Anti-Inflammatory Effect of Lactococcus lactis-Ling-Zhi 8 on Ameliorating Atherosclerosis and Nonalcoholic Fatty Liver in High-Fat Diet Rabbits. Int. J. Mol. Sci. 2024, 25, 11278. https://doi.org/10.3390/ijms252011278
Lee M-F, Wang NM, Chu Y-W, Wu C-S, Lin W-W. The Anti-Inflammatory Effect of Lactococcus lactis-Ling-Zhi 8 on Ameliorating Atherosclerosis and Nonalcoholic Fatty Liver in High-Fat Diet Rabbits. International Journal of Molecular Sciences. 2024; 25(20):11278. https://doi.org/10.3390/ijms252011278
Chicago/Turabian StyleLee, Mey-Fann, Nancy M. Wang, Yu-Wen Chu, Chi-Sheng Wu, and Wei-Wen Lin. 2024. "The Anti-Inflammatory Effect of Lactococcus lactis-Ling-Zhi 8 on Ameliorating Atherosclerosis and Nonalcoholic Fatty Liver in High-Fat Diet Rabbits" International Journal of Molecular Sciences 25, no. 20: 11278. https://doi.org/10.3390/ijms252011278
APA StyleLee, M.-F., Wang, N. M., Chu, Y.-W., Wu, C.-S., & Lin, W.-W. (2024). The Anti-Inflammatory Effect of Lactococcus lactis-Ling-Zhi 8 on Ameliorating Atherosclerosis and Nonalcoholic Fatty Liver in High-Fat Diet Rabbits. International Journal of Molecular Sciences, 25(20), 11278. https://doi.org/10.3390/ijms252011278






