Basal State Calibration of a Chemical Reaction Network Model for Autophagy
Abstract
:1. Introduction
Features of the Initial Model
2. Results
2.1. Initial Protein Concentrations
| Mechanism | R | Cell Type | Relevant Species | Source |
|---|---|---|---|---|
| Apoptosis | 23 | HeLa | procaspase | [15] |
| Apoptosis | 25 | HeLa | procaspase, BID, tBID | [16] |
| Apoptosis | 24 | E.coli | cyt c, BIT, Bax, procasp | [19] |
| Apoptosis | 20 | HeLa | p53, MDM2 | [17] |
| Apoptosis | 19 | MCF7 | PUMA, Bax, BCL2 and their complexes | [18] |
| Apoptosis | 5 | MCF7 | p53, MDM2 | [14] |
| signaling /Ras | 43 | not specified | Ca ions, SERCA, PIP, PLC, IP | [38] |
| signaling/Ras | 11 | eukaryotic | Ca ions, G-proteins, PLC, IP | [39] |
| JNK/p38 cas | 12 | not specified | JNK, MAPK | [45] |
| Autophagy and apoptosis | 13 | RTP | ATG5, autophagosomes, BCL2, BEC1, Ca ions, CALPAIN, caspase, DAPK, PI3R, JNK, mTOR | [46] |
| mTOR signaling | 25 | HeLa | AKT, mTOR, TSC1/2, PI3K | [44] |
| mTOR signaling | 18 | HeLa | AKT, mTOR | [47] |
| EGFR signaling | 129 | NSCLC | PIP2, AKT | [48] |
| Ras signaling | 6 | SB2 melanoma | PKC, cAMP, G-proteins, PIP2, PKA, MAPK, | [49] |
| mTOR signaling | 13 | Human | mTOR | [50] |
2.2. Revision of Incorrect Reactions
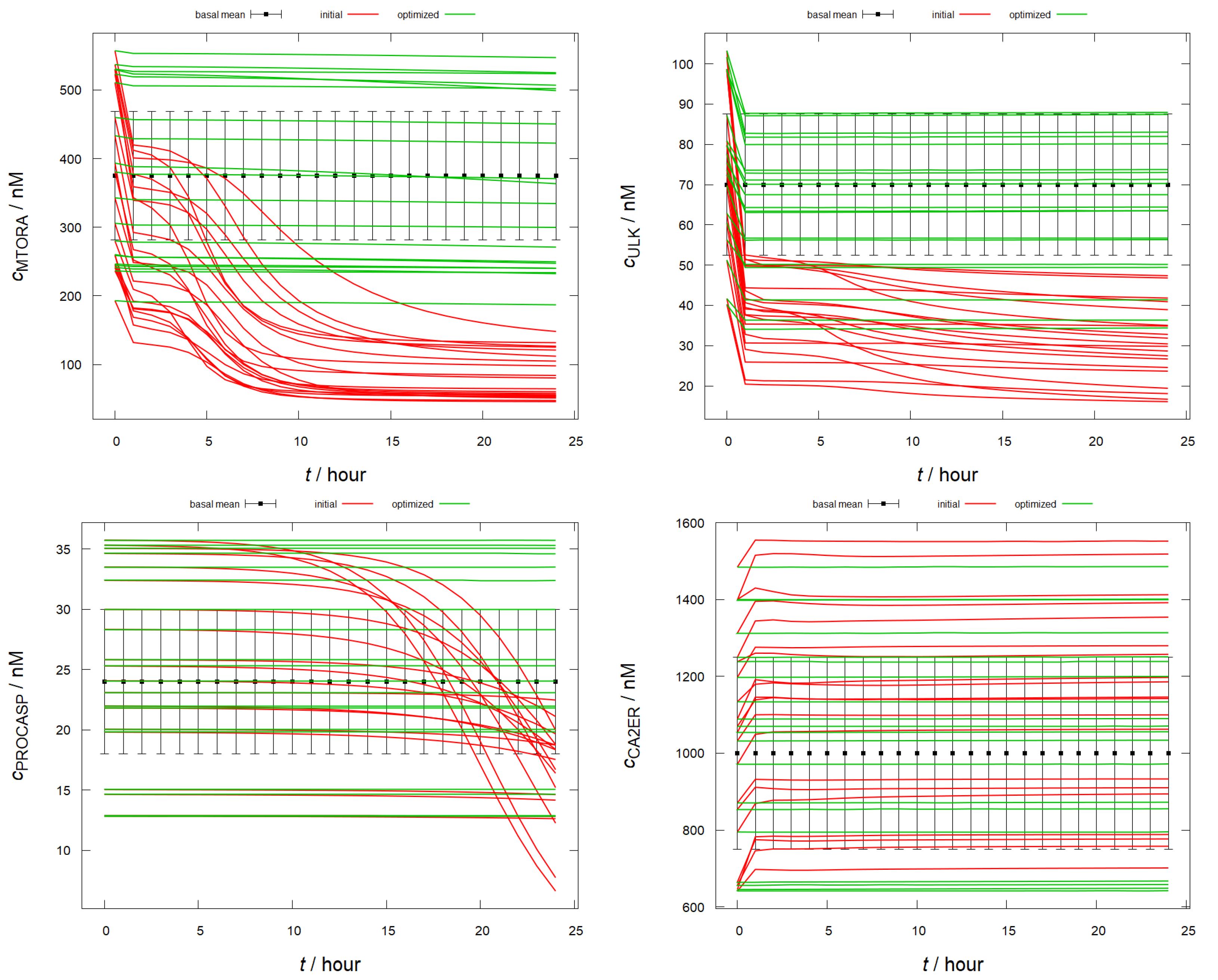
2.3. Simulating the Basal State
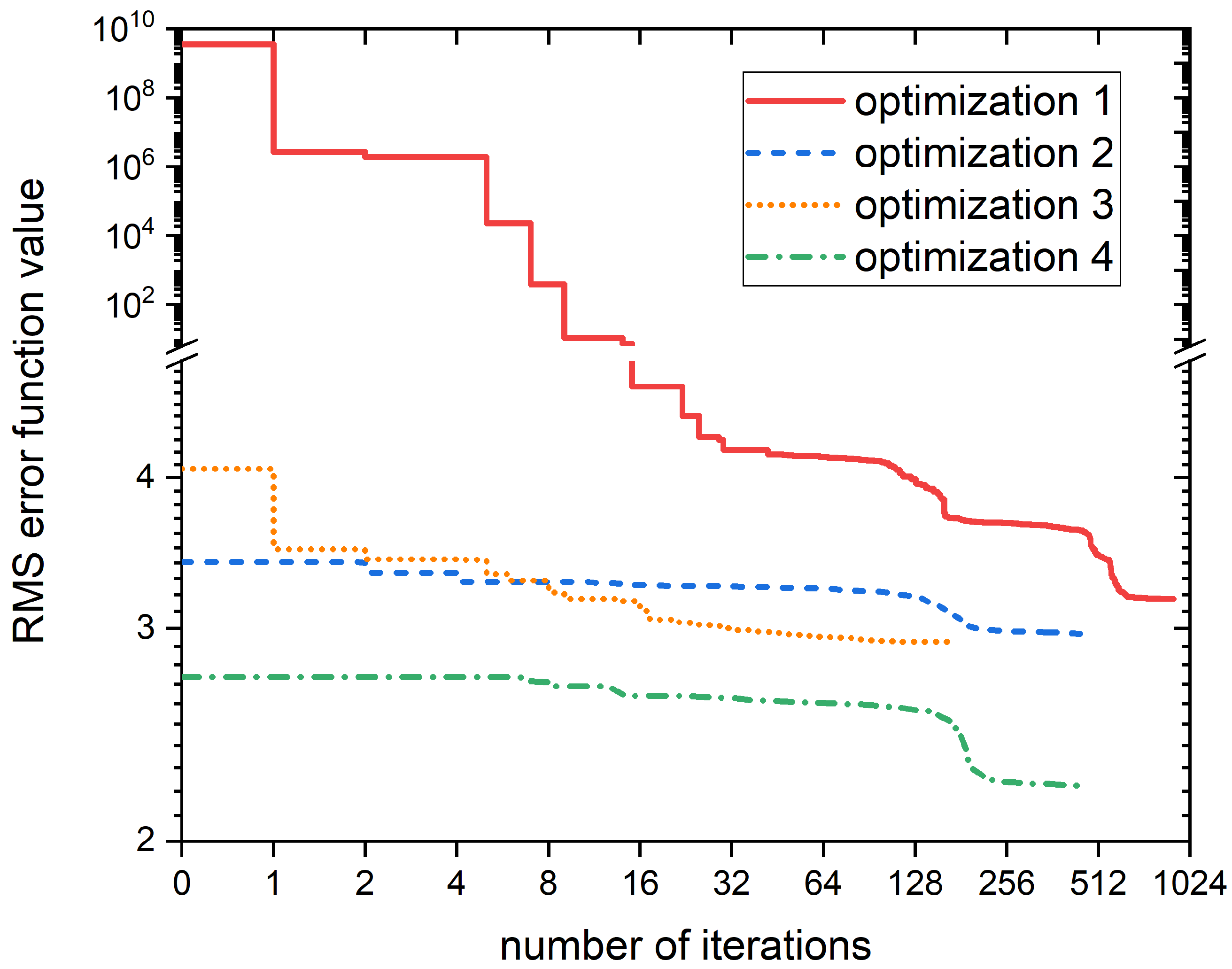
2.4. Identification of Influential Rate Coefficients
2.5. Optimization of Influential Rate Coefficients
3. Discussion
4. Materials and Methods
Mathematical Modeling
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CRN | Chemical reaction network |
| GFP-LC3B | Green Fluorescent Protein fused with LC3 |
| H2B-RFP | Histone H2B fused with Red Fluorescent Protein |
| ER | Endoplasmic Reticulum |
| Mdm2 | Mouse double minute 2 |
| mTOR | Mammalian target of rapamicyn |
| mTORC1 | Mammalian target of rapamicyn complex 1 |
| PERK | PKR-like endoplasmic reticulum kinase |
| JNK | Jun N-terminal kinase |
| UPR | unfolded protein response |
| ATF4 | Activating Transcription Factor 4 |
| BECN1 | Beclin 1 |
| BCL2 | Beclin 2 |
| eif2 | eukaryotic translational initiation factor 2 |
| ATG | autophagy-related gene |
| LC3B | Microtubule-associated proteins 1A/1B light chain 3B |
| ULK1 | Unc-51-like autophagy-activating kinases |
| CHOP | C/EBP homologous protein |
| cAMP | Cyclic adenosine monophosphate |
| MAPK15 | Mitogen-activated protein kinase 15 |
| AC | Adenylyl cyclase |
| AKTA | active form of AKT |
| AMPK | AMP-activated protein kinase |
| ATG5t | truncated ATG5 (ATG5T) |
| BCL2 | B-cell lymphoma 2 |
| BCL2_BAX | BCL2 and BAX complex |
| PUMA | p53 upregulated modulator of apoptosis |
| BCL2_PUMA | BCL2 and PUMA complex |
| BID | BH3 interacting-domain death agonist |
| tBID | truncated BH3 interacting-domain death agonist |
| CA2ER | Ca ion concentration in the ER |
| CA2IC | Ca ion in the cytoplasm |
| CAMKK | Calcium/calmodulin-dependent protein kinase kinase 2 |
| DAPK | Death associated protein kinase 1 |
| EPAC | exchange protein activated by cAMP |
| GPCRA | active from of G protein-coupled receptor |
| GA | G protein subunit |
| GBC | G protein subunit |
| IP3 | Inositol trisphosphate |
| PIP2 | Phosphatidylinositol 4,5-bisphosphate |
| PKA | protein kinase A |
| PKC | protein kinase C |
| PLC | Inactive Phospholipase C epsilon 1 |
| RHEBA | Active Ras Homolog Enriched In Brain |
| SERCA | Sarco/endoplasmic reticulum -ATPase |
| TSC 1/2 | Inactive tuberous sclerosis proteins 1 and 2 |
| UVRAG | UV radiation resistance-associated gene protein |
| CYTCM | cytochrome c in the mitochondria |
| MTORA | active mammalian target of rapamicyn |
| STS | staurosporine |
References
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Galluzzi, L.; Kepp, O.; Zitvogel, L. Immunogenic cell death in cancer therapy. Annu. Rev. Immunol. 2013, 31, 51–72. [Google Scholar] [CrossRef]
- Green, D.R.; Llambi, F. Cell death signaling. Cold Spring Harb. Perspect. Biol. 2015, 7, a006080. [Google Scholar] [CrossRef]
- Li, J.; Yuan, J. Caspases in apoptosis and beyond. Oncogene 2008, 27, 6194–6206. [Google Scholar] [CrossRef]
- Kerr, J.F.; Wyllie, A.H.; Currie, A.R. Apoptosis: A basic biological phenomenon with wideranging implications in tissue kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef]
- Dutreix, M. Safety control for apoptotic irreversibility. Proc. Natl. Acad. Sci. USA 2012, 109, 12844–12845. [Google Scholar] [CrossRef]
- Kapuy, O. Mechanism of Decision Making between Autophagy and Apoptosis Induction upon Endoplasmic Reticulum Stress. Int. J. Mol. Sci. 2024, 25, 4368. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N. Autophagy: Process and function. Genes Dev. 2007, 21, 2861–2873. [Google Scholar] [CrossRef]
- Aita, V.M.; Liang, X.H.; Murty, V.; Pincus, D.L.; Yu, W.; Cayanis, E.; Kalachikov, S.; Gilliam, T.; Levine, B. Cloning and Genomic Organization of Beclin 1, a Candidate Tumor Suppressor Gene on Chromosome 17q21. Genomics 1999, 59, 59–65. [Google Scholar] [CrossRef]
- Mizushima, N.; Yoshimori, T.; Levine, B. Methods in Mammalian Autophagy Research. Cell 2010, 140, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Mariño, G.; Niso-Santano, M.; Baehrecke, E.H.; Kroemer, G. Self-consumption: The interplay of autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2014, 15, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.W.; Jeon, J.; Go, G.; Lee, J.H.; Lee, S.H. The Dual Role of Autophagy in Cancer Development and a Therapeutic Strategy for Cancer by Targeting Autophagy. Int. J. Mol. Sci. 2020, 22, 179. [Google Scholar] [CrossRef] [PubMed]
- Geva-Zatorsky, N.; Rosenfeld, N.; Itzkovitz, S.; Milo, R.; Sigal, A.; Dekel, E.; Yarnitzky, T.; Liron, Y.; Polak, P.; Lahav, G.; et al. Oscillations and variability in the p53 system. Mol. Syst. Biol. 2006, 2. [Google Scholar] [CrossRef]
- Neumann, L.; Pforr, C.; Beaudouin, J.; Pappa, A.; Fricker, N.; Krammer, P.H.; Lavrik, I.N.; Eils, R. Dynamics within the CD95 death-inducing signaling complex decide life and death of cells. Mol. Syst. Biol. 2010, 6, 352. [Google Scholar] [CrossRef]
- Kallenberger, S.M.; Beaudouin, J.; Claus, J.; Fischer, C.; Sorger, P.K.; Legewie, S.; Eils, R. Intra- and Interdimeric Caspase-8 Self-Cleavage Controls Strength and Timing of CD95-Induced Apoptosis. Sci. Signal. 2014, 7, ra23. [Google Scholar] [CrossRef]
- Chong, K.H.; Samarasinghe, S.; Kulasiri, D. Mathematical modelling of p53 basal dynamics and DNA damage response. Math. Biosci. 2015, 259, 27–42. [Google Scholar] [CrossRef]
- Chong, K.H.; Samarasinghe, S.; Kulasiri, D.; Zheng, J. Mathematical modelling of core regulatory mechanism in p53 protein that activates apoptotic switch. J. Theor. Biol. 2019, 462, 134–147. [Google Scholar] [CrossRef]
- Bagci, E.; Vodovotz, Y.; Billiar, T.; Ermentrout, G.; Bahar, I. Bistability in Apoptosis: Roles of Bax, Bcl-2, and Mitochondrial Permeability Transition Pores. Biophys. J. 2006, 90, 1546–1559. [Google Scholar] [CrossRef] [PubMed]
- Tiwary, B.K. Computational medicine: Quantitative modeling of complex diseases. Briefings Bioinform. 2019, 21, 429–440. [Google Scholar] [CrossRef]
- Aghamiri, S.S.; Amin, R.; Helikar, T. Recent applications of quantitative systems pharmacology and machine learning models across diseases. J. Pharmacokinet. Pharmacodyn. 2022, 49, 19–37. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Oltvai, Z.N.; Bayır, H.; Silverman, G.A.; Pak, S.C.; Perlmutter, D.H.; Bahar, I. Quantitative assessment of cell fate decision between autophagy and apoptosis. Sci. Rep. 2017, 7, 17605. [Google Scholar] [CrossRef]
- Wen, M.; Spotte-Smith, E.W.C.; Blau, S.M.; McDermott, M.J.; Krishnapriyan, A.S.; Persson, K.A. Chemical reaction networks and opportunities for machine learning. Nat. Comput. Sci. 2023, 3, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Müller, S.; Flamm, C.; Stadler, P.F. What makes a reaction network “chemical”? J. Cheminform. 2022, 14, 63. [Google Scholar] [CrossRef] [PubMed]
- Tyson, J.J.; Novák, B. Functional Motifs in Biochemical Reaction Networks. Annu. Rev. Phys. Chem. 2010, 61, 219–240. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Bhatt, D.; Oltvai, Z.N.; Greenberger, J.S.; Bahar, I. Significance of p53 dynamics in regulating apoptosis in response to ionizing radiation and polypharmacological strategies. Sci. Rep. 2014, 4, 6245. [Google Scholar] [CrossRef]
- Alers, S.; Löffler, A.S.; Wesselborg, S.; Stork, B. Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: Cross talk, shortcuts, and feedbacks. Mol. Cell. Biol. 2012, 32, 2–11. [Google Scholar] [CrossRef]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef]
- Lee, J.W.; Park, S.; Takahashi, Y.; Wang, H.G. The association of AMPK with ULK1 regulates autophagy. PLoS ONE 2010, 5, e15394. [Google Scholar] [CrossRef]
- Yin, X.; Cao, L.; Peng, Y.; Tan, Y.; Xie, M.; Kang, R.; Livesey, K.M.; Tang, D. A critical role for UVRAG in apoptosis. Autophagy 2011, 7, 1242–1244. [Google Scholar] [CrossRef]
- Russo, R.; Berliocchi, L.; Adornetto, A.; Varano, G.; Cavaliere, F.; Nucci, C.; Rotiroti, D.; Morrone, L.A.; Bagetta, G.; Corasaniti, M. Calpain-mediated cleavage of Beclin-1 and autophagy deregulation following retinal ischemic injury in vivo. Cell Death Dis. 2011, 2, e144. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Saeki, E.; Otsu, K.; Morita, T.; Takeda, H.; Kuzuya, T.; Hori, M.; Kusuoka, H. Intracellular calcium level required for calpain activation in a single myocardial cell. J. Mol. Cell. Cardiol. 2001, 33, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Decuypere, J.P.; Bultynck, G.; Parys, J.B. A dual role for Ca2+ in autophagy regulation. Cell Calcium 2011, 50, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Racioppi, L.; Means, A.R. Calcium/calmodulin-dependent protein kinase kinase 2: Roles in signaling and pathophysiology. J. Biol. Chem. 2012, 287, 31658–31665. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, S.; Perozzo, R.; Schmid, I.; Ziemiecki, A.; Schaffner, T.; Scapozza, L.; Brunner, T.; Simon, H.U. Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis. Nat. Cell Biol. 2006, 8, 1124–1132. [Google Scholar] [CrossRef]
- Berridge, M.J.; Bootman, M.D.; Roderick, H.L. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. [Google Scholar] [CrossRef]
- Rubinsztein, D.C.; Codogno, P.; Levine, B. Autophagy modulation as a potential therapeutic target for diverse diseases. Nat. Rev. Drug Discov. 2012, 11, 709–730. [Google Scholar] [CrossRef]
- Eungdamrong, N.J.; Iyengar, R. Compartment-Specific Feedback Loop and Regulated Trafficking Can Result in Sustained Activation of Ras at the Golgi. Biophys. J. 2007, 92, 808–815. [Google Scholar] [CrossRef]
- Wang, J.; Huang, X.; Huang, W. A quantitative kinetic model for ATP-induced intracellular oscillations. J. Theor. Biol. 2007, 245, 510–519. [Google Scholar] [CrossRef]
- Xu, Y.; Yuan, J.; Lipinski, M.M. Live imaging and single-cell analysis reveal differential dynamics of autophagy and apoptosis. Autophagy 2013, 9, 1418–1430. [Google Scholar] [CrossRef]
- du Toit, A.; Hofmeyr, J.H.S.; Gniadek, T.J.; Loos, B. Measuring autophagosome flux. Autophagy 2018, 14, 1060–1071. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Guan, K.L. mTOR as a central hub of nutrient signalling and cell growth. Nat. Cell Biol. 2019, 21, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Tao, T.; Li, H.; Zhu, X. mTOR signaling pathway and mTOR inhibitors in cancer: Progress and challenges. Cell Biosci. 2020, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Dalle Pezze, P.; Sonntag, A.G.; Thien, A.; Prentzell, M.T.; Gödel, M.; Fischer, S.; Neumann-Haefelin, E.; Huber, T.B.; Baumeister, R.; Shanley, D.P.; et al. A Dynamic Network Model of mTOR Signaling Reveals TSC-Independent mTORC2 Regulation. Sci. Signal. 2012, 5, ra25. [Google Scholar] [CrossRef]
- Sundaramurthy, P.; Gakkhar, S.; Sowdhamini, R. Computational prediction and analysis of impact of the cross-talks between JNK and P38 kinase cascades. Bioinformation 2009, 3, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Tavassoly, I.; Parmar, J.; Shajahan-Haq, A.; Clarke, R.; Baumann, W.; Tyson, J. Dynamic Modeling of the Interaction Between Autophagy and Apoptosis in Mammalian Cells. CPT Pharmacometrics Syst. Pharmacol. 2015, 4, 263–272. [Google Scholar] [CrossRef]
- Varusai, T.M.; Nguyen, L.K. Dynamic modelling of the mTOR signalling network reveals complex emergent behaviours conferred by DEPTOR. Sci. Rep. 2018, 8, 643. [Google Scholar] [CrossRef]
- Bidkhori, G.; Moeini, A.; Masoudi-Nejad, A. Modeling of Tumor Progression in NSCLC and Intrinsic Resistance to TKI in Loss of PTEN Expression. PLoS ONE 2012, 7, e48004. [Google Scholar] [CrossRef]
- Muller, M.; Obeyesekere, M.; Mills, G.B.; Ram, P.T. Network topology determines dynamics of the mammalian MAPK1,2 signaling network: Bifan motif regulation of C-Raf and B-Raf isoforms by FGFR and MC1R. FASEB J. 2008, 22, 1393–1403. [Google Scholar] [CrossRef]
- Dorvash, M.; Farahmandnia, M.; Mosaddeghi, P.; Farahmandnejad, M.; Saber, H.; Khorraminejad-Shirazi, M.; Azadi, A.; Tavassoly, I. Dynamic modeling of signal transduction by mTOR complexes in cancer. J. Theor. Biol. 2019, 483, 109992. [Google Scholar] [CrossRef]
- Siwecka, N.; Galita, G.; Granek, Z.; Wiese, W.; Majsterek, I.; Rozpędek-Kamińska, W. IRE1/JNK Is the Leading UPR Pathway in 6-OHDA-Induced Degeneration of Differentiated SH-SY5Y Cells. Int. J. Mol. Sci. 2024, 25, 7679. [Google Scholar] [CrossRef] [PubMed]
- Luhr, M.; Torgersen, M.L.; Szalai, P.; Hashim, A.; Brech, A.; Staerk, J.; Engedal, N. The kinase PERK and the transcription factor ATF4 play distinct and essential roles in autophagy resulting from tunicamycin-induced ER stress. J. Biol. Chem. 2019, 294, 8197–8217. [Google Scholar] [CrossRef] [PubMed]
- Uehara, Y.; Hirose, J.; Yamabe, S.; Okamoto, N.; Okada, T.; Oyadomari, S.; Mizuta, H. Endoplasmic reticulum stress-induced apoptosis contributes to articular cartilage degeneration via C/EBP homologous protein. Osteoarthr. Cartil. 2014, 22, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Huang, Z.; Zhang, H.; Lu, J.; Wei, Y.; Yang, Y.; Bai, L. IRE1-mTOR-PERK Axis Coordinates Autophagy and ER Stress-Apoptosis Induced by P2X7-Mediated Ca2+ Influx in Osteoarthritis. Front. Cell Dev. Biol. 2021, 9, 695041. [Google Scholar] [CrossRef]
- Liu, Z.; Lv, Y.; Zhao, N.; Guan, G.; Wang, J. Protein kinase R-like ER kinase and its role in endoplasmic reticulum stress-decided cell fate. Cell Death Dis. 2015, 6, e1822. [Google Scholar] [CrossRef]
- Fan, P.; Jordan, V.C. PERK, Beyond an Unfolded Protein Response Sensor in Estrogen-Induced Apoptosis in Endocrine-Resistant Breast Cancer. Mol. Cancer Res. 2022, 20, 193–201. [Google Scholar] [CrossRef]
- Cao, J.; Dai, D.L.; Yao, L.; Yu, H.H.; Ning, B.; Zhang, Q.; Chen, J.; Cheng, W.H.; Shen, W.; Yang, Z.X. Saturated fatty acid induction of endoplasmic reticulum stress and apoptosis in human liver cells via the PERK/ATF4/CHOP signaling pathway. Mol. Cell. Biochem. 2012, 364, 115–129. [Google Scholar] [CrossRef]
- Ogata, M.; Hino, S.i.; Saito, A.; Morikawa, K.; Kondo, S.; Kanemoto, S.; Murakami, T.; Taniguchi, M.; Tanii, I.; Yoshinaga, K.; et al. Autophagy Is Activated for Cell Survival after Endoplasmic ReticulumStress. Mol. Cell. Biol. 2006, 26, 9220–9231. [Google Scholar] [CrossRef]
- Go, D.H.; Lee, Y.G.; Lee, D.H.; Kim, J.A.; Jo, I.H.; Han, Y.S.; Jo, Y.H.; Kim, K.Y.; Seo, Y.K.; Moon, J.H.; et al. 3-Decylcatechol induces autophagy-mediated cell death through the IRE1α/JNK/p62 in hepatocellular carcinoma cells. Oncotarget 2017, 8, 58790–58800. [Google Scholar] [CrossRef]
- Maeyashiki, C.; Melhem, H.; Hering, L.; Baebler, K.; Cosin-Roger, J.; Schefer, F.; Weder, B.; Hausmann, M.; Scharl, M.; Rogler, G.; et al. Activation of pH-Sensing Receptor OGR1 (GPR68) Induces ER Stress Via the IRE1α/JNK Pathway in an Intestinal Epithelial Cell Model. Sci. Rep. 2020, 10, 1438. [Google Scholar] [CrossRef]
- Wang, L.; Fan, Y.; Gui, Y.; Yang, X.; Ye, X.; Cao, Y.; Zhang, Z. Endoplasmic reticulum stress triggered autophagy and regulated the phenotype transformation of rheumatoid arthritis synovial fibroblasts via the IRE1/JNK pathway. Ann. Transl. Med. 2022, 10, 725. [Google Scholar] [CrossRef] [PubMed]
- Cherra, S.J.; Kulich, S.M.; Uechi, G.; Balasubramani, M.; Mountzouris, J.; Day, B.W.; Chu, C.T. Regulation of the autophagy protein LC3 by phosphorylation. J. Cell Biol. 2010, 190, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, S.; Jiménez, C.; Carrera, A.; Diaz-Nido, J.; Avila, J.; Wandosell, F. A cAMP-activated pathway, including PKA and PI3K, regulates neuronal differentiation. Neurochem. Int. 2004, 44, 231–242. [Google Scholar] [CrossRef]
- Taylor, S.S.; Buechler, J.A.; Yonemoto, W. cAMP-dependent protein kinase: Framework for a diverse family of regulatory enzymes. Annu. Rev. Biochem. 1990, 59, 971–1005. [Google Scholar] [CrossRef]
- Taylor, S.S.; Zhang, P.; Steichen, J.M.; Keshwani, M.M.; Kornev, A.P. PKA: Lessons learned after twenty years. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2013, 1834, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Dolan, A.; Diamond, S. Systems Modeling of Ca2+ Homeostasis and Mobilization in Platelets Mediated by IP3 and Store-Operated Ca2+ Entry. Biophys. J. 2014, 106, 2049–2060. [Google Scholar] [CrossRef]
- Wani, W.Y.; Boyer-Guittaut, M.; Dodson, M.; Chatham, J.; Darley-Usmar, V.; Zhang, J. Regulation of autophagy by protein post-translational modification. Lab. Investig. 2015, 95, 14–25. [Google Scholar] [CrossRef]
- Jung, C.H.; Ro, S.H.; Cao, J.; Otto, N.M.; Kim, D.H. mTOR regulation of autophagy. FEBS Lett. 2010, 584, 1287–1295. [Google Scholar] [CrossRef]
- Egan, D.; Chun, M.; Vamos, M.; Zou, H.; Rong, J.; Miller, C.; Lou, H.; Raveendra-Panickar, D.; Yang, C.C.; Sheffler, D.; et al. Small Molecule Inhibition of the Autophagy Kinase ULK1 and Identification of ULK1 Substrates. Mol. Cell 2015, 59, 285–297. [Google Scholar] [CrossRef]
- Zsély, I.G. Determination of rate parameters based on both direct and indirect measurements. Int. J. Chem. Kinet. 2012, 44, 284–302. [Google Scholar]
- Papp, M.; Varga, T.; Busai, Á.; Zsély, I.G.; Nagy, T.; Turányi, T. Optima++ v2. 5: A General C++ Framework for Performing Combustion Simulations and Mechanism Optimization. Available online: http://respecth.hu/ (accessed on 30 August 2024).
- Goitom, S.K.; Papp, M.; Kovács, M.; Nagy, T.; Zsély, I.G.; Turányi, T.; Pál, L. Efficient numerical methods for the optimisation of large kinetic reaction mechanisms. Combust. Theory Model. 2022, 26, 1071–1097. [Google Scholar] [CrossRef]
- Goodwin, D.G.; Speth, R.L.; Moffat, H.K.; Weber, B.W. Cantera: An Object-Oriented Software Toolkit for Chemical Kinetics, Thermodynamics, and Transport Processes. Version 2.5.1. Available online: https://www.cantera.org (accessed on 13 October 2024).
- Kovács, M.; Papp, M.; Turányi, T.; Nagy, T. A novel active parameter selection strategy for the efficient optimization of combustion mechanisms. Proc. Combust. Inst. 2023, 39, 5259–5267. [Google Scholar] [CrossRef]
- Pilling, J.T.; Walker, R.; Warnatz, J. Evaluated kinetic data for combustion modelling. J. Phys. Chem. Ref. Data 1992, 21. [Google Scholar]
- Dahal, S.; Yurkovich, J.T.; Xu, H.; Palsson, B.O.; Yang, L. Synthesizing systems biology knowledge from omics using genome-scale models. Proteomics 2020, 20, 1900282. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.D.; Kumbale, C.M.; Zhang, Q.; Voit, E.O. Dynamical systems approaches to personalized medicine. Curr. Opin. Biotechnol. 2019, 58, 168–174. [Google Scholar] [CrossRef]
- Nicholson, J.K. Global systems biology, personalized medicine and molecular epidemiology. Mol. Syst. Biol. 2006, 2, 52. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, Y.S.; Son, N.I.; Kang, C.K.; Kim, A.R. Recent omics technologies and their emerging applications for personalised medicine. IET Syst. Biol. 2017, 11, 87–98. [Google Scholar] [CrossRef]
- Iancu, R.V.; Jones, S.W.; Harvey, R.D. Compartmentation of camp signaling in cardiac myocytes: A computational study. Biophys. J. 2007, 92, 3317–3331. [Google Scholar] [CrossRef]
- Nakano, T.; Doi, T.; Yoshimoto, J.; Doya, K. A kinetic model of dopamine- and calcium-dependent striatal synaptic plasticity. PLoS Comput. Biol. 2010, 6, e1000670. [Google Scholar] [CrossRef]
- Shin, S.-Y.; Nguyen, L. Unveiling hidden dynamics of hippo signalling: A systems analysis. Genes 2016, 7, 44. [Google Scholar] [CrossRef]
- Hat, B.; Kochańczyk, M.; Bogdał, M.N.; Lipniacki, T. Feedbacks, bifurcations, and cell fate decision-making in the p53 system. PLoS Comput. Biol. 2016, 12, e1004787. [Google Scholar] [CrossRef] [PubMed]
- Broadbent, D.G.; Barnaba, C.; Perez, G.I.; Schmidt, J.C. Quantitative analysis of autophagy reveals the role of atg9 and atg2 in autophagosome formation. J. Cell Biol. 2023, 222, e202210078. [Google Scholar] [CrossRef] [PubMed]
- Raychaudhuri, S.; Das, S. Low probability activation of bax/bak can induce selective killing of cancer cells by generating heterogeneity in apoptosis. J. Healthc. Eng. 2013, 4, 47–66. [Google Scholar] [CrossRef] [PubMed]
- Lemon, G.; Gibson, W.G.; Bennett, M.R. Metabotropic receptor activation, desensitization and sequestration—i: Modelling calcium and inositol 1,4,5-trisphosphate dynamics following receptor activation. J. Theor. Biol. 2003, 223, 93–111. [Google Scholar] [CrossRef]
- Ashraf, J.; Ahmad, J.; Ul-Haq, Z. Deciphering the Role of PKC in Calpain-CAST System Through Formal Modeling Approach; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; pp. 60–71. [Google Scholar]
- Smith, B.; Hill, C.; Godfrey, E.L.; Rand, D.; van den Berg, H.; Thornton, S.; Hodgkin, M.; Davey, J.; Ladds, G. Dual positive and negative regulation of gpcr signaling by gtp hydrolysis. Cell. Signal. 2009, 21, 1151–1160. [Google Scholar] [CrossRef]
- Tiveci, S.; Akın, A.; Çakır, T.; Saybaşılı, H.; Ülgen, K. Modelling of calcium dynamics in brain energy metabolism and alzheimer’s disease. Comput. Biol. Chem. 2005, 29, 151–162. [Google Scholar] [CrossRef]
| Species | ini | opt | Species | ini | opt |
|---|---|---|---|---|---|
| BAX | 2.09 × | 3.37 | CAMKKB | 2.72 | 2.24 |
| BCL2_BAX | 39.0 | 2.24 | DAPK | 2.63 | 2.63 |
| UVG | 7.85 | 2.39 | PROCASP | 2.38 | 2.56 |
| BCL2 | 7.85 | 1.81 | PIP2 | 2.46 | 2.32 |
| BCL2_PUMA | 7.83 | 2.26 | AC | 2.35 | 2.36 |
| AKTA | 7.71 | 2.20 | CALPAIN | 2.36 | 2.23 |
| BEC1 | 7.22 | 2.27 | GPCRA | 2.34 | 2.34 |
| ATG5T | 7.01 | 1.67 | GA | 1.99 | 2.28 |
| RHEBA | 6.63 | 2.08 | PKA | 2.25 | 2.26 |
| CA2IC | 6.40 | 2.09 | P53 | 2.24 | 2.24 |
| ATG5 | 5.93 | 1.14 | CA2ER | 2.22 | 2.12 |
| TSC | 5.68 | 2.06 | GBC | 2.20 | 2.13 |
| MTORA | 5.44 | 2.60 | SERCA | 2.18 | 2.18 |
| ULK | 4.21 | 1.92 | AMPK | 2.17 | 2.12 |
| IP3 | 4.06 | 2.28 | CYTCM | 2.05 | 2.03 |
| BID | 3.61 | 1.74 | EPAC | 1.84 | 1.84 |
| PKC | 3.21 | 2.53 | PLCE | 1.80 | 1.80 |
| # | Reaction | |||||
|---|---|---|---|---|---|---|
| 73 | ATG5T+BCL2→ATG5_BCL2 | 6.50 | 4.00 | 4.79 | 0.13 | 1.35 |
| 43 | IP3→PIP2 | 4.00 | 0.14 | 1.37 | ||
| 104 | ATG5→REF | 4.00 | 0.19 | 1.55 | ||
| 102 | REF→ATG5 | 4.00 | 0.20 | 1.57 | ||
| 109 | PKC+CA2IC→PKC_CA2IC | 5.44 | 4.00 | 4.17 | 0.26 | 1.81 |
| 10 | BCL2_BAX→BCL2+BAX | 4.00 | 0.26 | 1.84 | ||
| 63 | MTORA→MTOR | 4.00 | 0.60 | 3.98 | ||
| 71 | AKTA→AKT | 4.00 | 0.63 | 4.25 | ||
| 54 | EPACA→EPAC | 4.00 | 0.63 | 4.26 | ||
| 18 | REF→BID | 4.00 | 0.65 | 4.49 | ||
| 30 | PUMA→REF | 4.00 | 0.73 | 5.36 | ||
| 29 | BCL2_PUMA→PUMA+BCL2 | 4.00 | 0.87 | 7.36 | ||
| 69 | RHEBA+MTOR→RHEBA+MTORA | 6.45 | 4.00 | 8.16 | 0.88 | 7.65 |
| 28 | PUMA+BCL2→BCL2_PUMA | 7.22 | 4.00 | 7.91 | 0.89 | 7.70 |
| 9 | BCL2+BAX→BCL2_BAX | 6.54 | 4.00 | 3.18 | 0.94 | 8.77 |
| 8 | P53A_BCL2→P53A+BCL2 | 4.00 | 0.97 | 9.32 | ||
| 44 | CA2IC+CAMKKB→CA2IC+CAMKKBA | 5.44 | 4.00 | 3.12 | 0.99 | 9.73 |
| 34 | CA2IC+SERCA→CA2ER+SERCA | 7.01 | 4.00 | 3.47 | 1.00 | 10.00 |
| 45 | K+CAMKKBA→AMPKA+CAMKKBA | 6.44 | 4.00 | 6.05 | 1.00 | 10.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hajdú, B.; Kapuy, O.; Nagy, T. Basal State Calibration of a Chemical Reaction Network Model for Autophagy. Int. J. Mol. Sci. 2024, 25, 11316. https://doi.org/10.3390/ijms252011316
Hajdú B, Kapuy O, Nagy T. Basal State Calibration of a Chemical Reaction Network Model for Autophagy. International Journal of Molecular Sciences. 2024; 25(20):11316. https://doi.org/10.3390/ijms252011316
Chicago/Turabian StyleHajdú, Bence, Orsolya Kapuy, and Tibor Nagy. 2024. "Basal State Calibration of a Chemical Reaction Network Model for Autophagy" International Journal of Molecular Sciences 25, no. 20: 11316. https://doi.org/10.3390/ijms252011316
APA StyleHajdú, B., Kapuy, O., & Nagy, T. (2024). Basal State Calibration of a Chemical Reaction Network Model for Autophagy. International Journal of Molecular Sciences, 25(20), 11316. https://doi.org/10.3390/ijms252011316





