Alkaline Mineral Complex Water Attenuates Transportation-Induced Hepatic Lipid Metabolism Dysregulation by AMPKα-SREBP-1c/PPARα Pathways
Abstract
:1. Introduction
2. Results
2.1. Effect of AMC on Blood Lipid Metabolism in Transported Rats
2.2. Effect of AMC on Hepatic Lipid Accumulation in Transported Rats
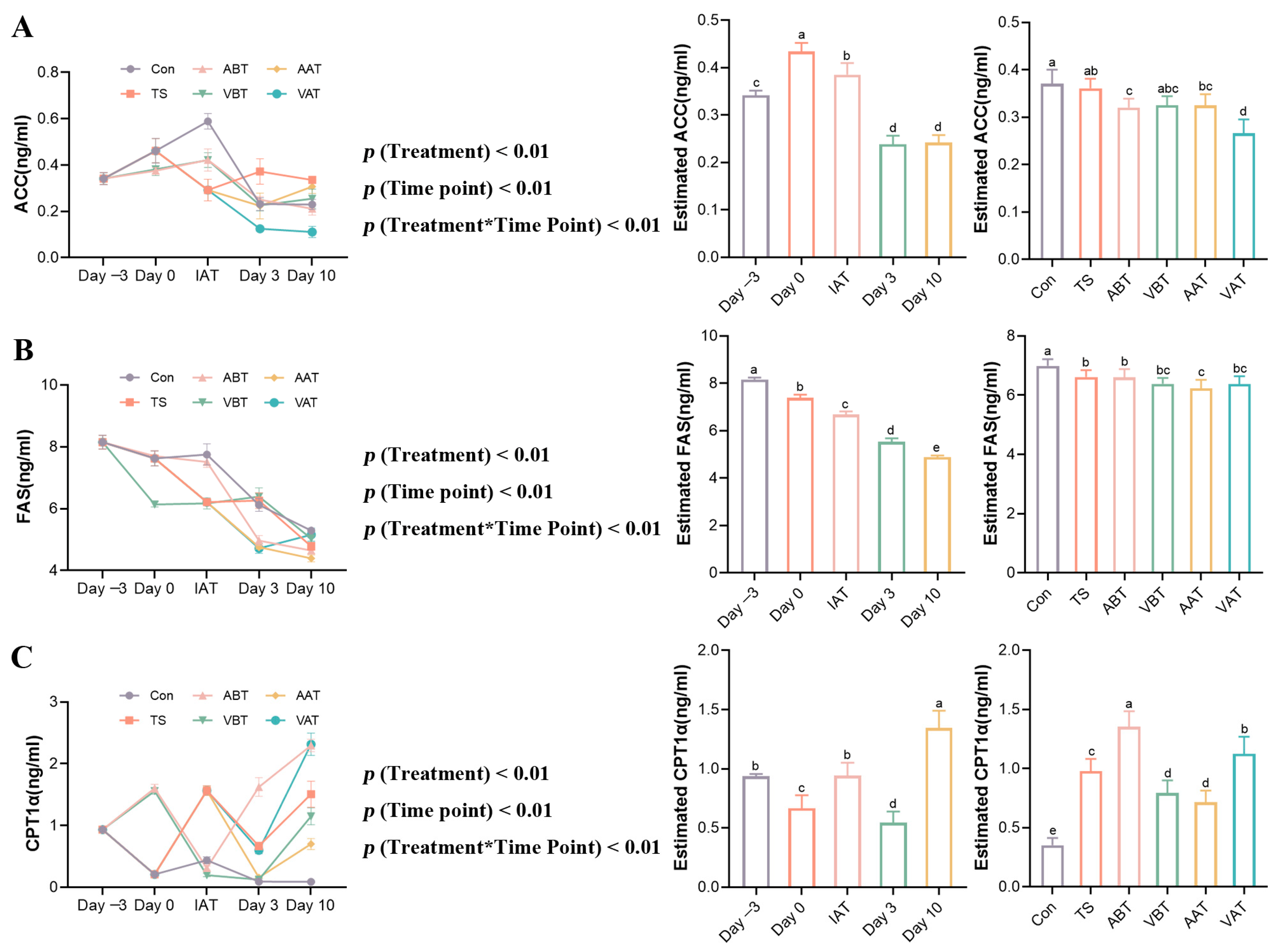
2.3. Effects of AMC on Lipid-Metabolizing Enzymes in Transported Rats
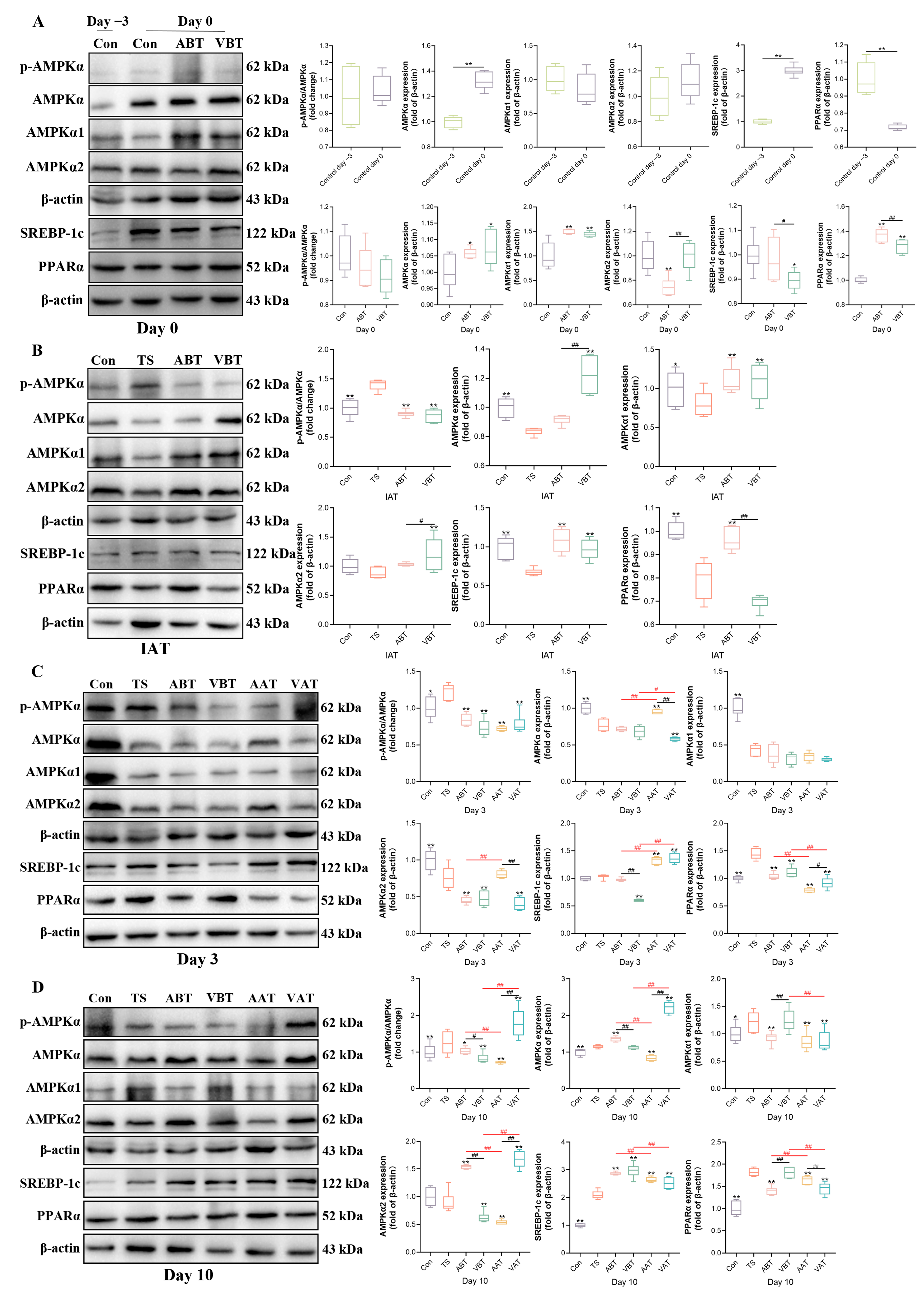
2.4. Effects of AMC on the Expression of AMPKα-SREBP-1c/PPARα-Regulating Hepatic Lipid Metabolism in Transported Rats
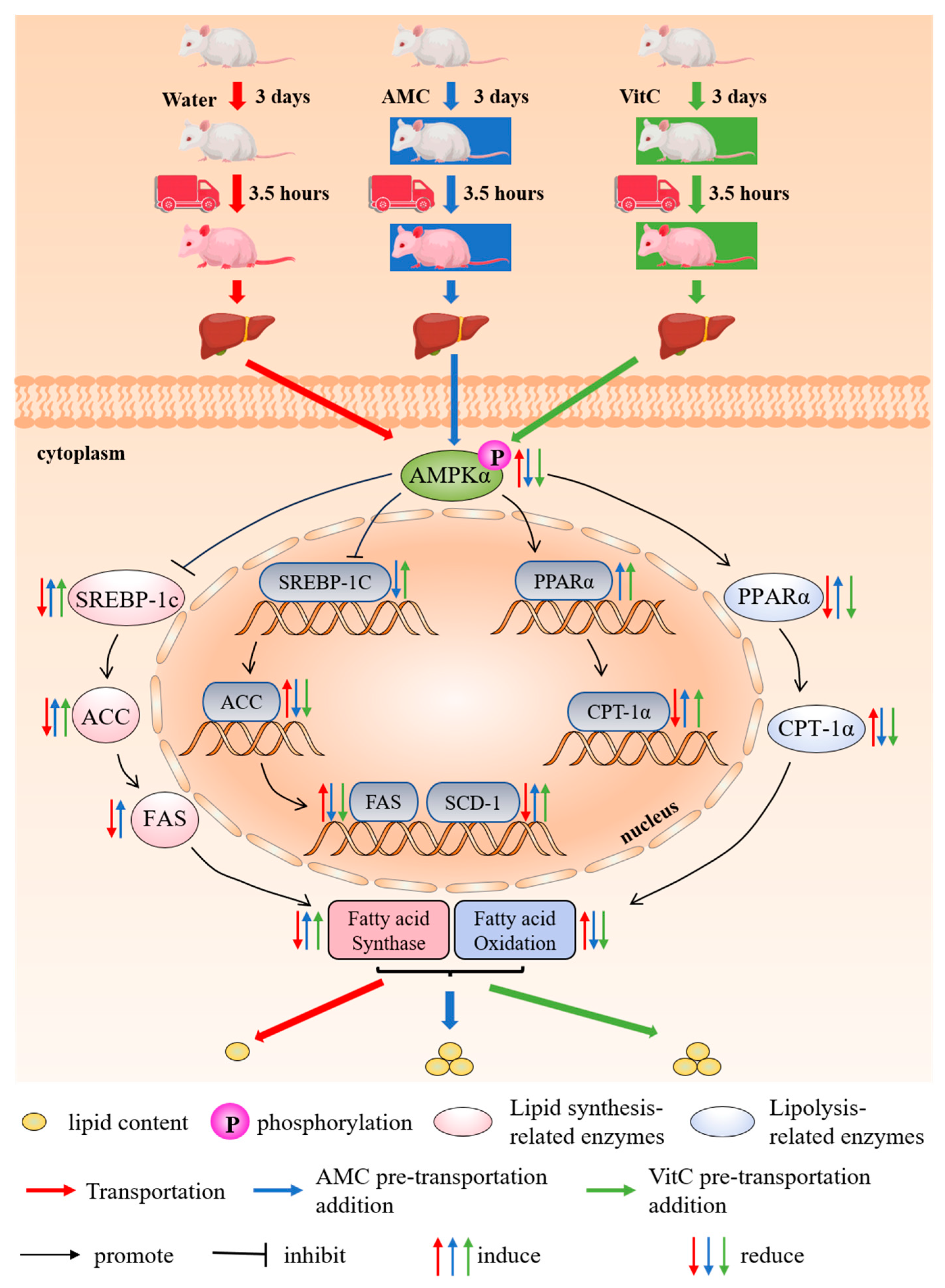
3. Discussion
4. Materials and Methods
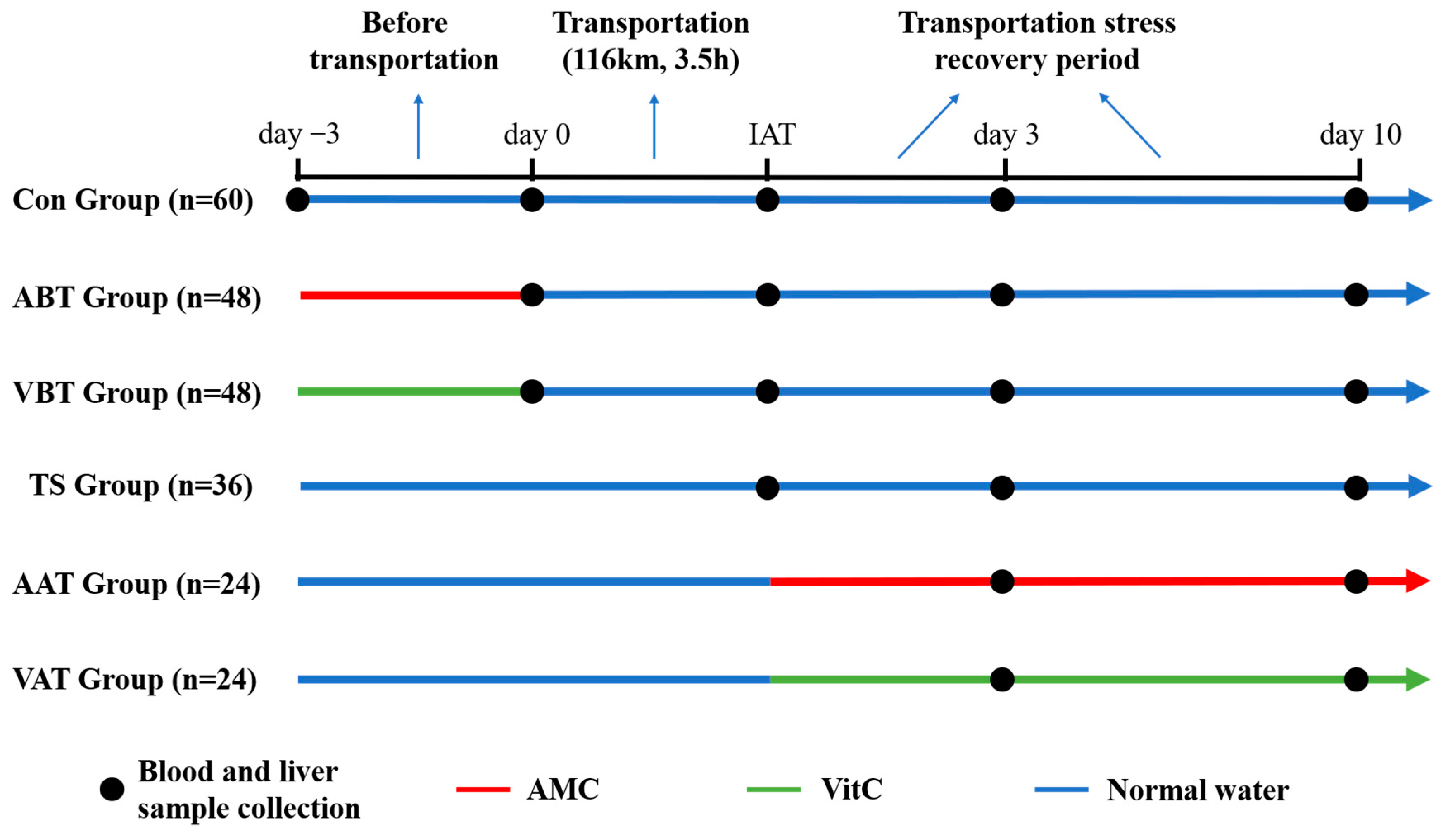
4.1. Animals and Treatments
4.2. Oil-Red O Staining
4.3. Lipid Metabolism Characteristic Assay
4.4. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.5. Western Blot Assays
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roadknight, N.; Mansell, P.; Jongman, E.; Courtman, N.; Fisher, A. Invited review: The welfare of young calves transported by road. J. Dairy Sci. 2021, 104, 6343–6357. [Google Scholar] [CrossRef]
- Hong, H.; Lee, E.; Lee, I.H.; Lee, S.R. Effects of transport stress on physiological responses and milk production in lactating dairy cows. Asian-Australas. J. Anim. Sci. 2019, 32, 442–451. [Google Scholar] [CrossRef]
- Buckham-Sporer, K.; Earley, B.; Marti, S. Current Knowledge on the Transportation by Road of Cattle, including Unweaned Calves. Animals 2023, 13, 3393. [Google Scholar] [CrossRef]
- He, Y.; Sang, Z.; Zhuo, Y.; Wang, X.; Guo, Z.; He, L.; Zeng, C.; Dai, H. Transport stress induces pig jejunum tissue oxidative damage and results in autophagy/mitophagy activation. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1521–1529. [Google Scholar] [CrossRef]
- Wirthgen, E.; Goumon, S.; Kunze, M.; Walz, C.; Spitschak, M.; Tuchscherer, A.; Brown, J.; Höflich, C.; Faucitano, L.; Hoeflich, A. Effects of Transport Duration and Environmental Conditions in Winter or Summer on the Concentrations of Insulin-Like Growth Factors and Insulin-Like Growth Factor-Binding Proteins in the Plasma of Market-Weight Pigs. Front. Endocrinol. 2018, 9, 36. [Google Scholar] [CrossRef]
- Naldurtiker, A.; Batchu, P.; Kouakou, B.; Terrill, T.H.; McCommon, G.W.; Kannan, G. Differential gene expression analysis using RNA-seq in the blood of goats exposed to transportation stress. Sci. Rep. 2023, 13, 1984. [Google Scholar] [CrossRef]
- Ijiri, M.; Akioka, K.; Kitano, T.; Miura, H.; Ono, H.K.; Terashima, R.; Fujimoto, Y.; Matsuo, T.; Yamato, O.; Kawaguchi, H. Acupuncture Treatment Improves Transport Stress in Microminipigs Through the Acupoint in Ears. Vivo 2023, 37, 2100–2104. [Google Scholar] [CrossRef]
- Wan, C.; Yin, P.; Xu, X.; Liu, M.; He, S.; Song, S.; Liu, F.; Xu, J. Effect of simulated transport stress on the rat small intestine: A morphological and gene expression study. Res. Vet. Sci. 2014, 96, 355–364. [Google Scholar] [CrossRef]
- Zhao, H.; Tang, X.; Wu, M.; Li, Q.; Yi, X.; Liu, S.; Jiang, J.; Wang, S.; Sun, X. Transcriptome Characterization of Short Distance Transport Stress in Beef Cattle Blood. Front. Genet. 2021, 12, 616388. [Google Scholar] [CrossRef]
- Beenken, A.M.; Deters, E.L.; Hansen, S.L. The effect of injectable vitamin C and road transit duration on inflammation, muscle fatigue, and performance in pre-conditioned beef steer calves. J. Anim. Sci. 2021, 99, skab312. [Google Scholar] [CrossRef]
- Meléndez, D.M.; Marti, S.; Haley, D.B.; Schwinghamer, T.D.; Schwartzkopf-Genswein, K.S. Effect of transport and rest stop duration on the welfare of conditioned cattle transported by road. PLoS ONE 2020, 15, e0228492. [Google Scholar] [CrossRef]
- Deters, E.L.; Hansen, S.L. Pre-transit vitamin C injection improves post-transit performance of beef steers. Anim. Int. J. Anim. Biosci. 2020, 14, 2083–2090. [Google Scholar] [CrossRef]
- Dai, Y.; Qiu, C.; Zhang, D.; Li, M.; Liu, W. Yam Gruel alone and in combination with metformin regulates hepatic lipid metabolism disorders in a diabetic rat model by activating the AMPK/ACC/CPT-1 pathway. Lipids Health Dis. 2024, 23, 28. [Google Scholar] [CrossRef]
- Li, L.; Lu, Z.; Wang, Y.; Yang, Y.; Wang, H.; Ma, H. Genistein alleviates chronic heat stress-induced lipid metabolism disorder and mitochondrial energetic dysfunction by activating the GPR30-AMPK-PGC-1α signaling pathways in the livers of broiler chickens. Poult. Sci. 2024, 103, 103251. [Google Scholar] [CrossRef]
- Lan, R.; Luo, H.; Wu, F.; Wang, Y.; Zhao, Z. Chitosan Oligosaccharides Alleviate Heat-Stress-Induced Lipid Metabolism Disorders by Suppressing the Oxidative Stress and Inflammatory Response in the Liver of Broilers. Antioxidants 2023, 12, 1497. [Google Scholar] [CrossRef]
- Qi, J.; Gan, L.; Huang, F.; Xie, Y.; Guo, H.; Cui, H.; Deng, J.; Gou, L.; Cai, D.; Pan, C.; et al. Multi-omics reveals that alkaline mineral water improves the respiratory health and growth performance of transported calves. Microbiome 2024, 12, 48. [Google Scholar] [CrossRef]
- Yang, M.; Hu, B.; Sun, D.; Zhao, C.; Wei, H.; Li, D.; Liao, Z.; Zhao, Y.; Liang, J.; Shi, M.; et al. Growth hormone receptor gene influences mitochondrial function and chicken lipid metabolism by AMPK-PGC1α-PPAR signaling pathway. BMC Genom. 2022, 23, 219. [Google Scholar] [CrossRef]
- Guo, S.; Gong, L.; Shen, Q.; Xing, D. Photobiomodulation reduces hepatic lipogenesis and enhances insulin sensitivity through activation of CaMKKβ/AMPK signaling pathway. J. Photochem. Photobiol. B Biol. 2020, 213, 112075. [Google Scholar] [CrossRef]
- Deleye, Y.; Cotte, A.K.; Hannou, S.A.; Hennuyer, N.; Bernard, L.; Derudas, B.; Caron, S.; Legry, V.; Vallez, E.; Dorchies, E.; et al. CDKN2A/p16INK4a suppresses hepatic fatty acid oxidation through the AMPKα2-SIRT1-PPARα signaling pathway. J. Biol. Chem. 2020, 295, 17310–17322. [Google Scholar] [CrossRef]
- Zhu, X.; Bian, H.; Wang, L.; Sun, X.; Xu, X.; Yan, H.; Xia, M.; Chang, X.; Lu, Y.; Li, Y.; et al. Berberine attenuates nonalcoholic hepatic steatosis through the AMPK-SREBP-1c-SCD1 pathway. Free Radic. Biol. Med. 2019, 141, 192–204. [Google Scholar] [CrossRef]
- Wang, Y.G.; Han, X.G.; Yang, Y.; Qiao, H.; Dai, K.R.; Fan, Q.M.; Tang, T.T. Functional differences between AMPK α1 and α2 subunits in osteogenesis, osteoblast-associated induction of osteoclastogenesis, and adipogenesis. Sci. Rep. 2016, 6, 32771. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, T.; Ihato, M. Peroxisome Proliferator-Activated Receptor α Has a Protective Effect on Fatty Liver Caused by Excessive Sucrose Intake. Biomedicines 2022, 10, 2199. [Google Scholar] [CrossRef]
- Ding, R.B.; Bao, J.; Deng, C.X. Emerging roles of SIRT1 in fatty liver diseases. Int. J. Biol. Sci. 2017, 13, 852–867. [Google Scholar] [CrossRef] [PubMed]
- Ferré, P.; Phan, F.; Foufelle, F. SREBP-1c and lipogenesis in the liver: An update1. Biochem. J. 2021, 478, 3723–3739. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.W. Advances in Understanding of the Role of Lipid Metabolism in Aging. Cells 2021, 10, 880. [Google Scholar] [CrossRef]
- Janovick, N.A.; Dann, H.M.; Loor, J.J.; Drackley, J.K. Prepartum dietary energy intake alters hepatic expression of genes related to peroxisome proliferator-activated receptor and inflammation in peripartal dairy cows. J. Dairy Sci. 2022, 105, 8069–8086. [Google Scholar] [CrossRef]
- Qian, M.; Lyu, Q.; Liu, Y.; Hu, H.; Wang, S.; Pan, C.; Duan, X.; Gao, Y.; Qi, L.W.; Liu, W.; et al. Chitosan Oligosaccharide Ameliorates Nonalcoholic Fatty Liver Disease (NAFLD) in Diet-Induced Obese Mice. Mar. Drugs 2019, 17, 391. [Google Scholar] [CrossRef]
- Li, Y.; Xu, S.; Mihaylova, M.M.; Zheng, B.; Hou, X.; Jiang, B.; Park, O.; Luo, Z.; Lefai, E.; Shyy, J.Y.; et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011, 13, 376–388. [Google Scholar] [CrossRef]
- Barroso, E.; Rodríguez-Calvo, R.; Serrano-Marco, L.; Astudillo, A.M.; Balsinde, J.; Palomer, X.; Vázquez-Carrera, M. The PPARβ/δ activator GW501516 prevents the down-regulation of AMPK caused by a high-fat diet in liver and amplifies the PGC-1α-Lipin 1-PPARα pathway leading to increased fatty acid oxidation. Endocrinology 2011, 152, 1848–1859. [Google Scholar] [CrossRef]
- Ha, J.H.; Jang, J.; Chung, S.I.; Yoon, Y. AMPK and SREBP-1c mediate the anti-adipogenic effect of β-hydroxyisovalerylshikonin. Int. J. Mol. Med. 2016, 37, 816–824. [Google Scholar] [CrossRef]
- Cannon, C.P. Mixed dyslipidemia, metabolic syndrome, diabetes mellitus, and cardiovascular disease: Clinical implications. Am. J. Cardiol. 2008, 102, 5l–9l. [Google Scholar] [CrossRef] [PubMed]
- Patsouris, D.; Reddy, J.K.; Müller, M.; Kersten, S. Peroxisome proliferator-activated receptor alpha mediates the effects of high-fat diet on hepatic gene expression. Endocrinology 2006, 147, 1508–1516. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Dai, X.; Wang, L.; Cai, J.; Shen, J.; Shen, Y.; Li, X.; Zhao, Y. Iron overload accelerated lipid metabolism disorder and liver injury in rats with non-alcoholic fatty liver disease. Front. Nutr. 2022, 9, 961892. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Arya, J.K.; Rizvi, S.I. Chitosan reduces inflammation and protects against oxidative stress in a hyperlipidemic rat model: Relevance to nonalcoholic fatty liver disease. Mol. Biol. Rep. 2022, 49, 9465–9472. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zeng, Y.; Liu, S.; Wang, S. Acute stress show great influences on liver function and the expression of hepatic genes associated with lipid metabolism in rats. Lipids Health Dis. 2013, 12, 118. [Google Scholar] [CrossRef]
- Zhu, L.; Bao, E.; Zhao, R.; Hartung, J. Expression of heat shock protein 60 in the tissues of transported piglets. Cell Stress Chaperones 2009, 14, 61–69. [Google Scholar] [CrossRef]
- Costantino, M.; Conti, V.; Corbi, G.; Filippelli, A. Hydropinotherapy with Sulphurous Mineral Water as Complementary Treatment to Improve Glucose Metabolism, Oxidative Status, and Quality of Life. Antioxidants 2021, 10, 1773. [Google Scholar] [CrossRef]
- Li, R.; Li, Y.; Yang, X.; Hu, Y.; Yu, H.; Li, Y. Reducing VEGFB accelerates NAFLD and insulin resistance in mice via inhibiting AMPK signaling pathway. J. Transl. Med. 2022, 20, 341. [Google Scholar] [CrossRef]
- Kim, B.; Woo, M.J.; Park, C.S.; Lee, S.H.; Kim, J.S.; Kim, B.; An, S.; Kim, S.H. Hovenia Dulcis Extract Reduces Lipid Accumulation in Oleic Acid-Induced Steatosis of Hep G2 Cells via Activation of AMPK and PPARα/CPT-1 Pathway and in Acute Hyperlipidemia Mouse Model. Phytother. Res. PTR 2017, 31, 132–139. [Google Scholar] [CrossRef]
- Tang, C.C.; Huang, H.P.; Lee, Y.J.; Tang, Y.H.; Wang, C.J. Hepatoprotective effect of mulberry water extracts on ethanol-induced liver injury via anti-inflammation and inhibition of lipogenesis in C57BL/6J mice. Food Chem. Toxicol. 2013, 62, 786–796. [Google Scholar] [CrossRef]
- Teng, T.; Sun, G.; Ding, H.; Song, X.; Bai, G.; Shi, B.; Shang, T. Characteristics of glucose and lipid metabolism and the interaction between gut microbiota and colonic mucosal immunity in pigs during cold exposure. J. Anim. Sci. Biotechnol. 2023, 14, 84. [Google Scholar] [CrossRef] [PubMed]
- Zou, D.; Liu, R.; Shi, S.; Du, J.; Tian, M.; Wang, X.; Hou, M.; Duan, Z.; Ma, Y. BHBA regulates the expressions of lipid synthesis and oxidation genes in sheep hepatocytes through the AMPK pathway. Res. Vet. Sci. 2021, 140, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Feng, Q. Pharmacological Effects and Molecular Protective Mechanisms of Astragalus Polysaccharides on Nonalcoholic Fatty Liver Disease. Front. Pharmacol. 2022, 13, 854674. [Google Scholar] [CrossRef]
- Entezari, M.; Hashemi, D.; Taheriazam, A.; Zabolian, A.; Mohammadi, S.; Fakhri, F.; Hashemi, M.; Hushmandi, K.; Ashrafizadeh, M.; Zarrabi, A.; et al. AMPK signaling in diabetes mellitus, insulin resistance and diabetic complications: A pre-clinical and clinical investigation. Biomed. Pharmacother. Biomed. Pharmacother. 2022, 146, 112563. [Google Scholar] [CrossRef] [PubMed]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef]
- Gongol, B.; Sari, I.; Bryant, T.; Rosete, G.; Marin, T. AMPK: An Epigenetic Landscape Modulator. Int. J. Mol. Sci. 2018, 19, 3238. [Google Scholar] [CrossRef]


| Gene | Gene Bank ID | Primer Sequences (5′ → 3′) |
|---|---|---|
| AMPKα1 | NM_019142.3 | Forward: TTCGGGAAAGTGAAGGTGGG |
| Reverse: GGTTCTGGATCTCTCTGCGG | ||
| AMPKα2 | NM_023991.1 | Forward: TCGGCAAAGTGAAGATTGGAGA |
| Reverse: TCCAACAACATCTAAACTGCGA | ||
| SREBP-1c | NM_001276708.1 | Forward: AGCTGATGGAGACAGGGAGT |
| Reverse: GTGGTAGCCATGCTGGAACT | ||
| ACC1 | NM_022193.2 | Forward: GGCGGCTCTGGAGGTATATG |
| Reverse: ATGTGGGCAGCATGAACTGA | ||
| FAS | NM_017332.2 | Forward: CCCCTCACATCAAGTGGGAC |
| Reverse: CTCGGAACTGGCGTCAATGT | ||
| SCD-1 | NM_139192.2 | Forward: ACACCTTGCTCTGGGGGATA |
| Reverse: TCAGAGAACTTGTGGTGGGC | ||
| PPARα | NM_013196.2 | Forward: TCGTGGAGTCCTGGAACTGA |
| Reverse: CTTCAGTCTTGGCTCGCCTC | ||
| CPT-1α | NM_031559.2 | Forward: CTGGGGAAGAGACAGACACC |
| Reverse: CCATCGTGGTAGAGCCAGAC | ||
| β-actin | NM_031144.3 | Forward: GGAGATTACTGCCCTGGCTCCTAGC |
| Reverse: GGCCGGACTCATCGTACTCCTGCTT |
| Antibody | Company | Cat Number | Dilution |
|---|---|---|---|
| AMPKα | Cell Signaling Technology | 5831 | 1:1000 |
| p-AMPKα | Cell Signaling Technology | 2535 | 1:1000 |
| AMPKα1 | Cell Signaling Technology | 5832 | 1:1000 |
| AMPKα2 | Abclonal | A14052 | 1:1000 |
| PPARα | Boster | A00600-2 | 1:1000 |
| SREBP-1c | Affinity | AF4728 | 1:1000 |
| β-actin | TransGen Biotech | HC201 | 1:1000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gan, L.; Guo, H.; Yang, Q.; Zhou, X.; Xie, Y.; Ma, X.; Gou, L.; Fang, J.; Zuo, Z. Alkaline Mineral Complex Water Attenuates Transportation-Induced Hepatic Lipid Metabolism Dysregulation by AMPKα-SREBP-1c/PPARα Pathways. Int. J. Mol. Sci. 2024, 25, 11373. https://doi.org/10.3390/ijms252111373
Gan L, Guo H, Yang Q, Zhou X, Xie Y, Ma X, Gou L, Fang J, Zuo Z. Alkaline Mineral Complex Water Attenuates Transportation-Induced Hepatic Lipid Metabolism Dysregulation by AMPKα-SREBP-1c/PPARα Pathways. International Journal of Molecular Sciences. 2024; 25(21):11373. https://doi.org/10.3390/ijms252111373
Chicago/Turabian StyleGan, Linli, Hongrui Guo, Qiyuan Yang, Xueke Zhou, Yue Xie, Xiaoping Ma, Liping Gou, Jing Fang, and Zhicai Zuo. 2024. "Alkaline Mineral Complex Water Attenuates Transportation-Induced Hepatic Lipid Metabolism Dysregulation by AMPKα-SREBP-1c/PPARα Pathways" International Journal of Molecular Sciences 25, no. 21: 11373. https://doi.org/10.3390/ijms252111373
APA StyleGan, L., Guo, H., Yang, Q., Zhou, X., Xie, Y., Ma, X., Gou, L., Fang, J., & Zuo, Z. (2024). Alkaline Mineral Complex Water Attenuates Transportation-Induced Hepatic Lipid Metabolism Dysregulation by AMPKα-SREBP-1c/PPARα Pathways. International Journal of Molecular Sciences, 25(21), 11373. https://doi.org/10.3390/ijms252111373






