Cartilage Laser Engraving for Fast-Track Tissue Engineering of Auricular Grafts
Abstract
:1. Introduction
2. Results
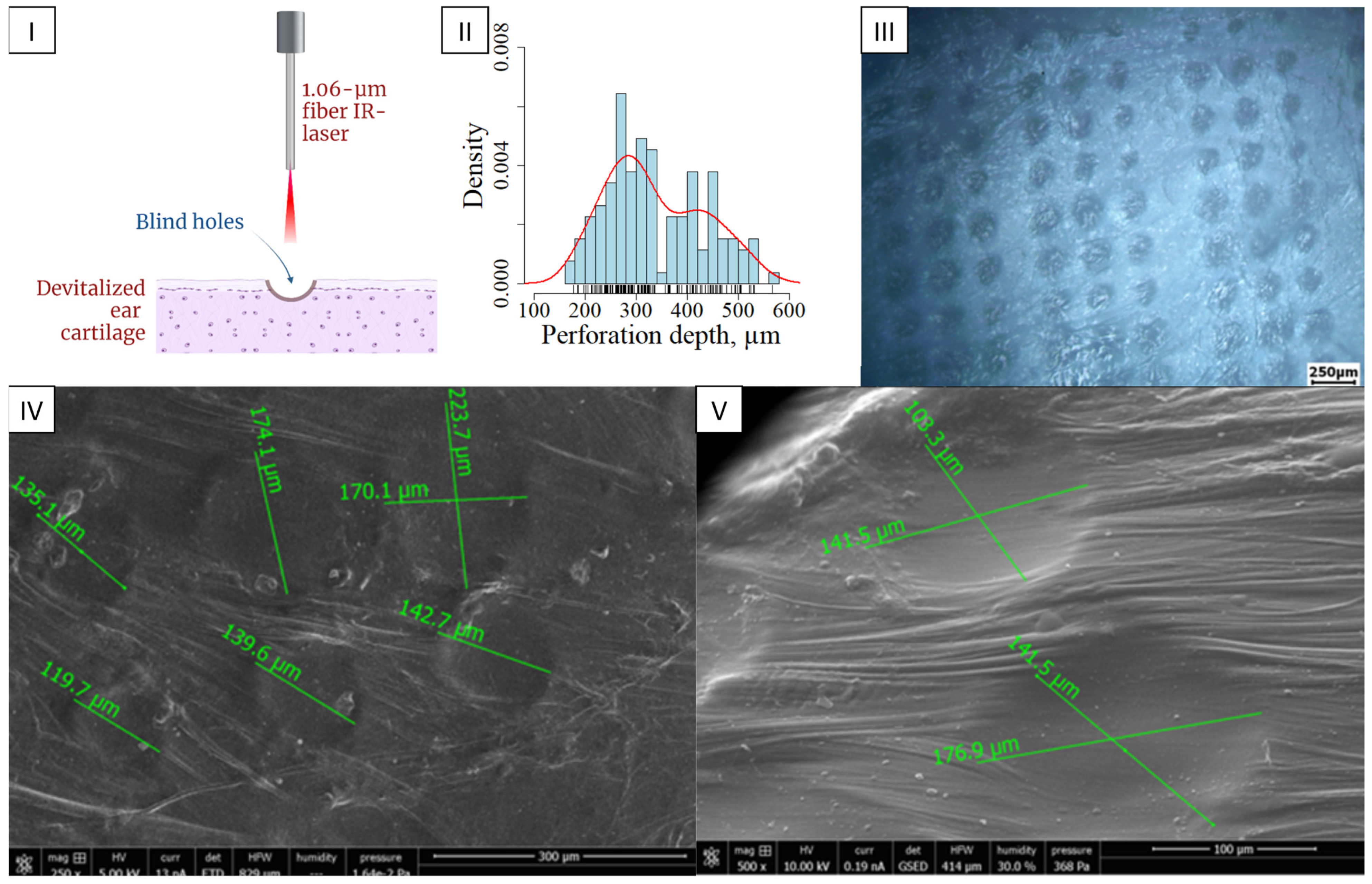
2.1. Laser Perforation (Engraving) of Cartilage Tissue
2.2. Isolation of Cells from Nasal Cartilages
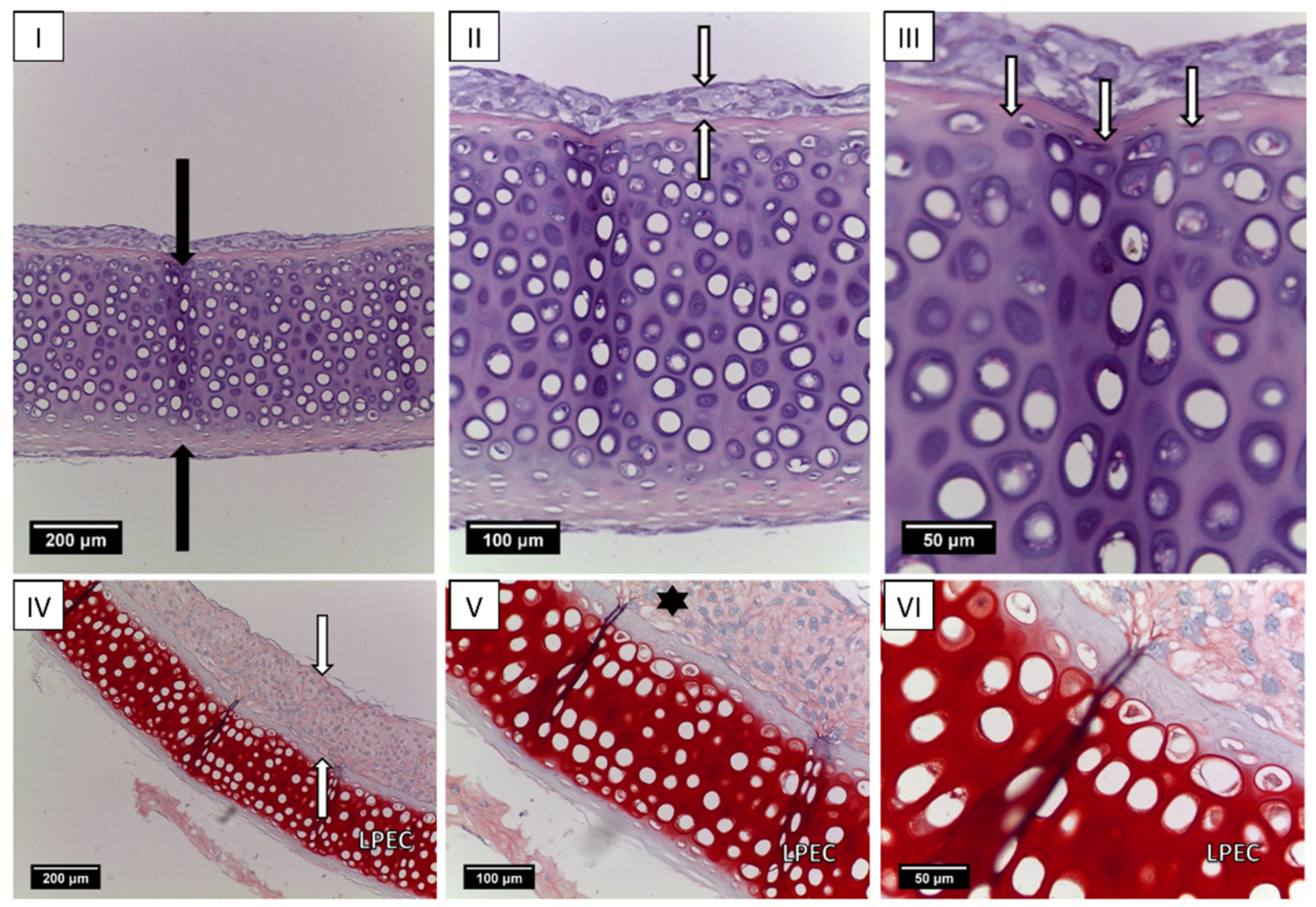
2.3. Creation of Tissue-Engineered Grafts
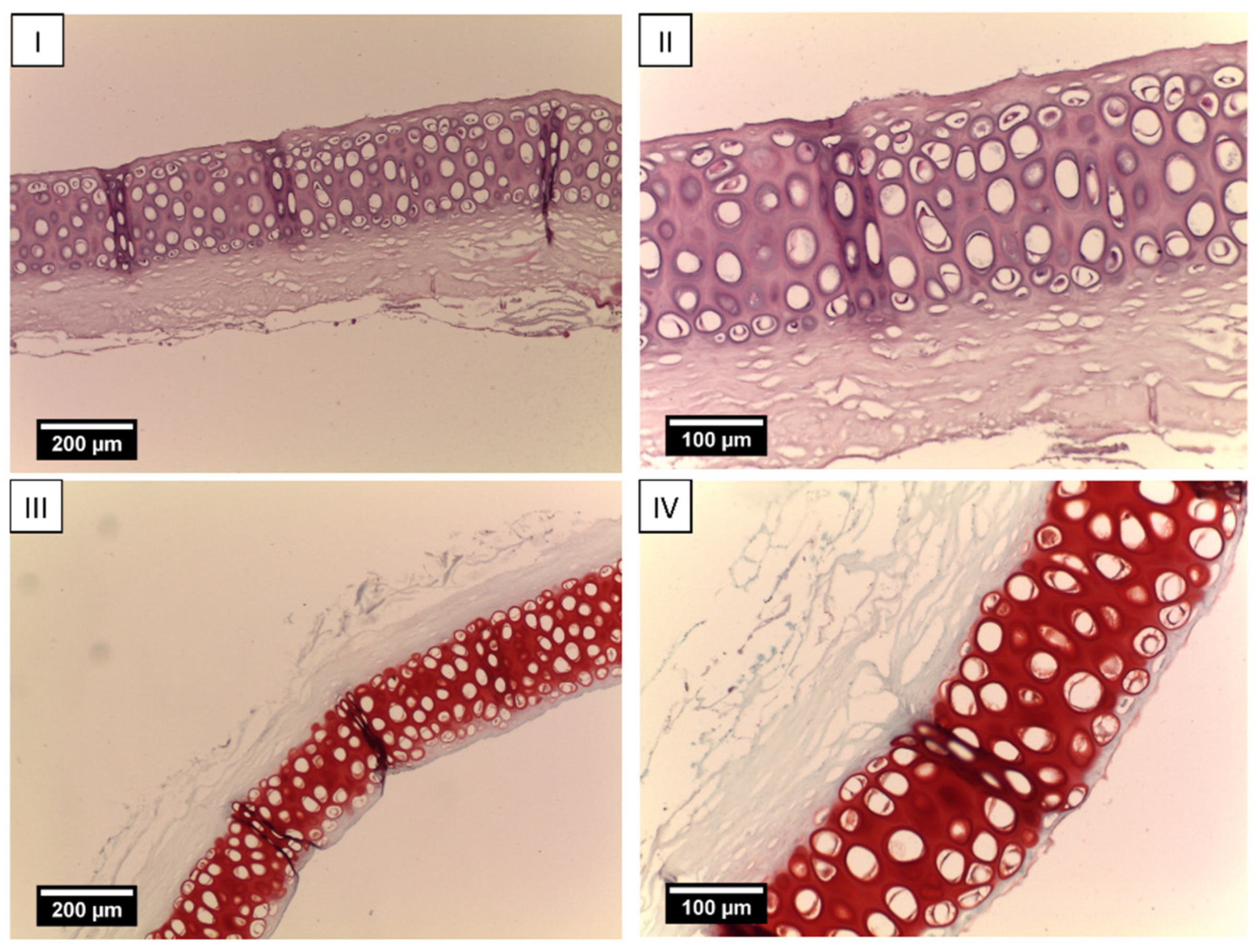
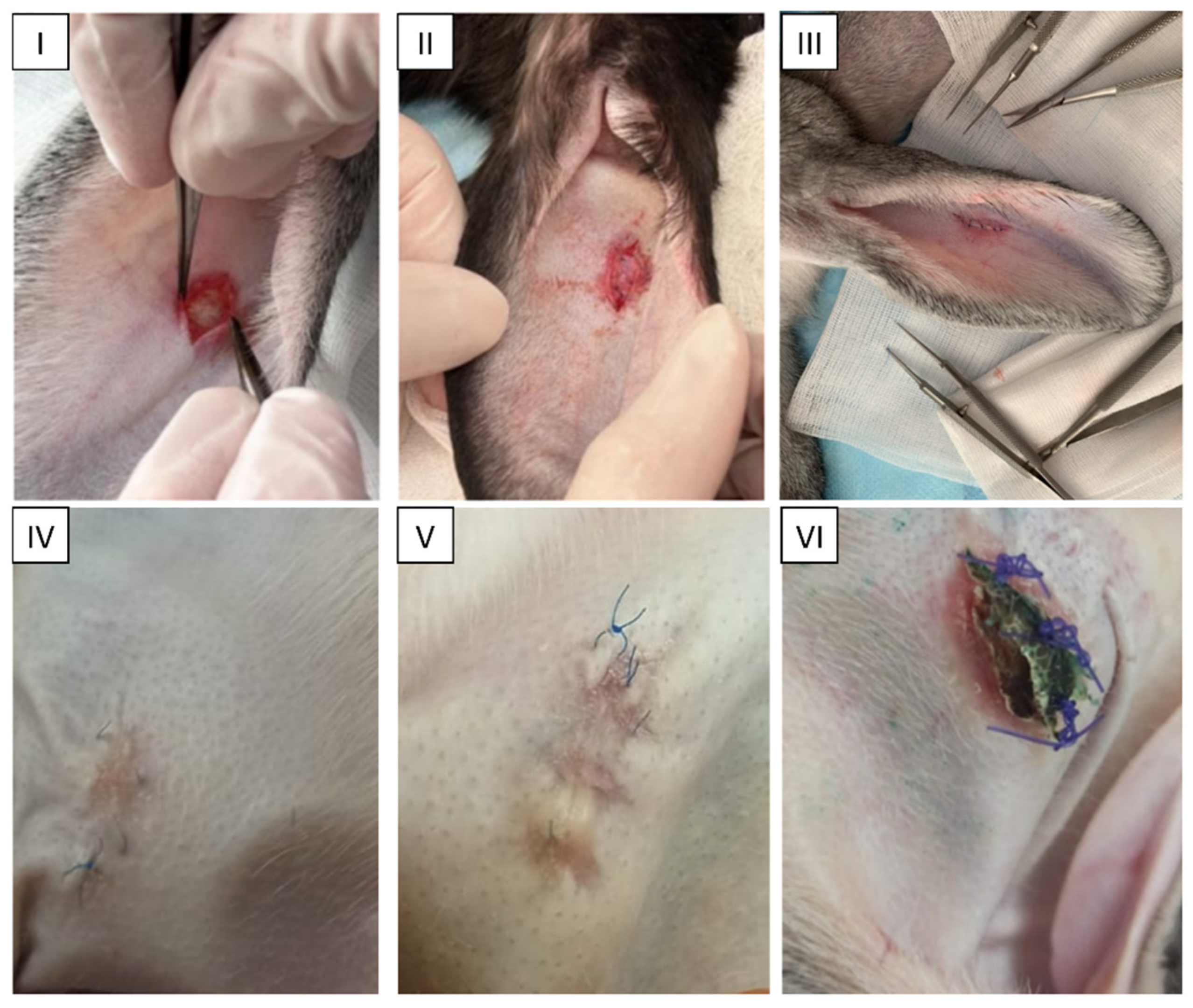
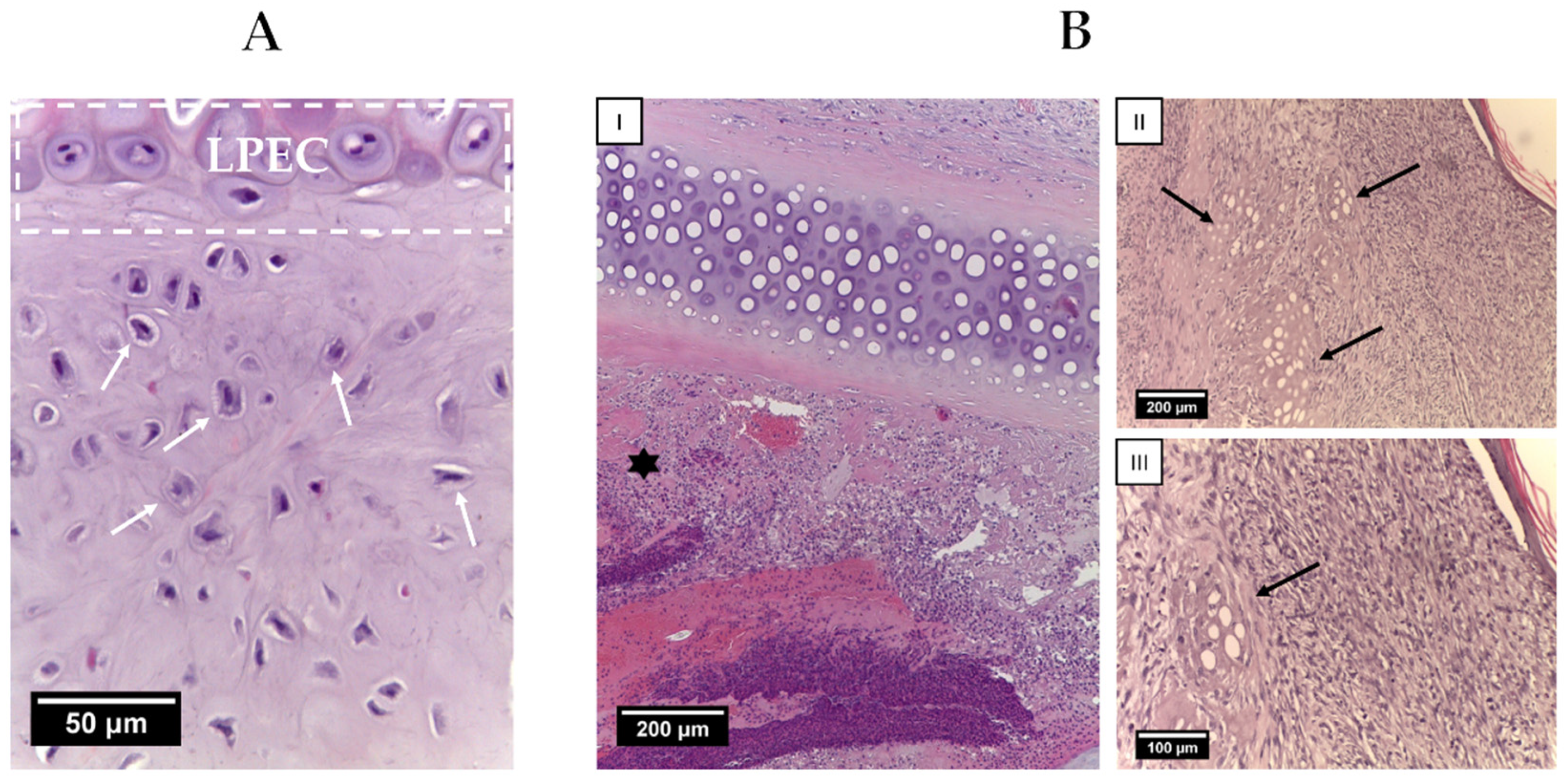
2.4. Animal Study
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Harvest and Devitalization of Rabbit EC (Ear Cartilage)
5.2. Laser Perforation (Engraving) of Decellularized Ear Cartilage (dEC)
5.3. Isolation and Culture of Rabbit Nasal Chondrocytes (rNCs)
5.4. Revitalization of Laser-Perforated Ear Cartilages (LPECs)
5.5. In Vivo Experiments: Orthotopic Study in Rabbits
5.6. Scanning Electron Microscopy
5.7. Histology
5.8. Immunohistochemistry
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sobol, E.; Shekhter, A.; Guller, A.; Baum, O.; Baskov, A. Laser-induced regeneration of cartilage. J. Biomed. Opt. 2011, 16, 080902. [Google Scholar] [CrossRef] [PubMed]
- Humphries, S.; Joshi, A.; Webb, W.R.; Kanegaonkar, R. Auricular reconstruction: Where are we now? A critical literature review. Eur. Arch. Oto-Rhino-Laryngol. 2022, 279, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Beketov, E.E.; Isaeva, E.V.; Yakovleva, N.D.; Demyashkin, G.A.; Arguchinskaya, N.V.; Kisel, A.A.; Lagoda, T.S.; Malakhov, E.P.; Kharlov, V.I.; Osidak, E.O.; et al. Bioprinting of Cartilage with Bioink Based on High-Concentration Collagen and Chondrocytes. Int. J. Mol. Sci. 2021, 22, 11351. [Google Scholar] [CrossRef] [PubMed]
- Pina, S.; Ribeiro, V.P.; Marques, C.F.; Maia, F.R.; Silva, T.H.; Reis, R.L.; Oliveira, J.M. Scaffolding strategies for tissue engineering and regenerative medicine applications. Materials 2019, 12, 1824. [Google Scholar] [CrossRef] [PubMed]
- Ovsianikov, A.; Khademhosseini, A.; Mironov, V. The Synergy of Scaffold-Based and Scaffold-Free Tissue Engineering Strategies. Trends Biotechnol. 2018, 36, 348–357. [Google Scholar] [CrossRef]
- Beketov, E.E.; Isaeva, E.V.; Shegay, P.V.; Ivanov, S.A.; Kaprin, A.D. Current state of tissue engineering for cartilage regeneration. Genes Cells 2019, 14, 12–20. (In Russia) [Google Scholar] [CrossRef]
- Zhu, W.; Cao, L.; Song, C.; Pang, Z.; Jiang, H.; Guo, C. Cell-derived decellularized extracellular matrix scaffolds for articular cartilage repair. Int. J. Artif. Organs 2021, 44, 269–281. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, X.; Hong, H.; Hu, R.; Liu, J.; Liu, C. Decellularized extracellular matrix scaffolds: Recent trends and emerging strategies in tissue engineering. Bioact. Mater. 2022, 10, 15–31. [Google Scholar] [CrossRef]
- Lu, H.; Hoshiba, T.; Kawazoe, N.; Koda, I.; Song, M.; Chen, G. Cultured cell-derived extracellular matrix scaffolds for tissue engineering. Biomaterials 2011, 32, 9658–9666. [Google Scholar] [CrossRef]
- Meng, H.; Liu, X.; Liu, R.; Zheng, Y.; Hou, A.; Liu, S.; He, W.; Wang, Y.; Wang, A.; Guo, Q.; et al. Decellularized laser micro-patterned osteochondral implants exhibit zonal recellularization and self-fixing for osteochondral regeneration in a goat model. Journal of Orthopaedic Translation. 2024, 46, 18–32. [Google Scholar] [CrossRef]
- Isaeva, E.V.; Beketov, E.E.; Arguchinskaya, N.V.; Ivanov, S.A.; Shegay, P.V.; Kaprin, A.D. Decellularized Extracellular Matrix for Tissue Engineering (Review). Sovrem Tekhnologii Med. 2022, 14, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Pei, M.; Li, Q.; Zhang, Y. Decellularized extracellular matrix mediates tissue construction and regeneration. Front. Med. 2022, 16, 56–82. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Yang, B.; Xu, W.; Li, D.; Wang, S.; Ren, Z.; Zhang, J.; Zhang, T.; Liu, X.; Li, J.; et al. Cell-Derived Extracellular Matrix Materials for Tissue Engineering. Tissue Eng. Part B Rev. 2022, 28, 1007–1021. [Google Scholar] [CrossRef] [PubMed]
- Walma, D.A.C.; Yamada, K.M. The extracellular matrix in development. Development 2020, 147, dev175596. [Google Scholar] [CrossRef]
- Cramer, M.C.; Badylak, S.F. Extracellular matrix-based biomaterials and their influence upon cell behavior. Ann. Biomed. Eng. 2020, 48, 2132–2153. [Google Scholar] [CrossRef]
- Chimerad, M.; Barazesh, A.; Zandi, M.; Zarkesh, I.; Moghaddam, A.; Borjian, P.; Chimehrad, R.; Asghari, A.; Akbarnejad, Z.; Khonakdar, H.A.; et al. Tissue engineered scaffold fabrication methods for medical applications. Int. J. Polym. Mater. Polym. Biomater. 2022, 72, 1455–1479. [Google Scholar] [CrossRef]
- Wong, B.J.; Milner, T.E.; Kim, H.K.; Chao, K.; Sun, C.H.; Sobol, E.N.; Nelson, J.S. Proteoglycan synthesis in porcine nasal cartilage grafts following Nd:YAG (lambda = 1.32 microns) laser-mediated reshaping. Photochem. Photobiol. 2000, 71, 218–224. [Google Scholar] [CrossRef]
- Kolarovszki, B.; Ficsor, S.; Frank, D.; Katona, K.; Soos, B.; Turzo, K. Unlocking the potential: Laser surface modifications for titanium dental implants. Lasers Med. Sci. 2024, 39, 162. [Google Scholar] [CrossRef]
- Khandaker, M.; Ait Moussa, A.; Sama, D.N.; Safavinia, F.; Hazra, S.; Kalay, O.C.; Karpat, F.; Clary, E.; Haleem, A. Laser-Induced Microgrooves Improve the Mechanical Responses of Cemented Implant Systems. Micromachines 2020, 11, 466. [Google Scholar] [CrossRef]
- Daskalova, A.; Sezanova, K.; Angelova, L.; Paunova-Krasteva, T.; Gergulova, R.; Kovacheva, D.; Rabadjieva, D. Ultra-Short Laser-Assisted Micro-Structure Formations on Mg/Zn Double-Doped Calcium Phosphate Ceramics for Enhanced Antimicrobial Activity. Materials 2023, 16, 6626. [Google Scholar] [CrossRef]
- Al-Kattan, A.; Nirwan, V.P.; Popov, A.; Ryabchikov, Y.V.; Tselikov, G.; Sentis, M.; Sentis, M.; Fahmi, A.; Kabashin, A.V. Recent advances in laser-ablative synthesis of bare Au and Si nanoparticles and assessment of their prospects for tissue engineering applications. Int. J. Mol. Sci. 2018, 19, 1563. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.S.; Kuan, T.Y.; Chen, Y.C.; Chu, Y.J.; Chen, J.S.; Chen, C.C.; Liu, T.Y. Simultaneous detection of SARS-CoV-2 S1 protein by using flexible electrochemical and Raman enhancing biochip. Biosens. Bioelectron. 2024, 249, 116021. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, Z.; Xie, C.; Wang, X.; Luo, F.; Hong, M.; Zhou, R.; Ma, C.; Lin, N.; Zhang, J.; et al. Scaffold with micro/macro-architecture for myocardial alignment engineering into complex 3D cell patterns. Adv. Healthc. Mater. 2019, 8, 1901015. [Google Scholar] [CrossRef] [PubMed]
- Luke, A.M.; Mathew, S.; Altawash, M.M.; Madan, B.M. Lasers: A review with their applications in oral medicine. J. Lasers Med. Sci. 2019, 10, 324. [Google Scholar] [CrossRef]
- Baranovsky, D.S.; Lyundup, A.V.; Balyasin, M.V.; Klabukov, I.D.; Krasilnikova, O.A.; Krasheninnikov, M.E.; Parshin, V.D. Interleukin IL-1β stimulates revitalization of cartilage matrix in vitro with human nasal chondrocytes. Russ. J. Transplantol. Artif. Organs 2019, 21, 88–95. [Google Scholar] [CrossRef]
- Xu, Y.; Li, D.; Yin, Z.; He, A.; Lin, M.; Jiang, G.; Song, X.; Hu, X.; Liu, Y.; Wang, J.; et al. Tissue-engineered trachea regeneration using decellularized trachea matrix treated with laser micropore technique. Acta Biomater. 2017, 58, 113–121. [Google Scholar] [CrossRef]
- Kasyanov, V.A.; Hodde, J.; Hiles, M.C.; Eisenberg, C.; Eisenberg, L.; De Castro, L.E.; Ozolanta, I.; Murovska, M.; Draughn, R.A.; Prestwich, G.D.; et al. Rapid biofabrication of tubular tissue constructs by centrifugal casting in a decellularized natural scaffold with laser-machined micropores. J. Mater. Sci. Mater. Med. 2009, 20, 329–337. [Google Scholar] [CrossRef]
- Goldberg-Bockhorn, E.; Schwarz, S.; Subedi, R.; Elsässer, A.; Riepl, R.; Walther, P.; Körber, L.; Breiter, R.; Stock, K.; Rotter, N. Laser surface modification of decellularized extracellular cartilage matrix for cartilage tissue engineering. Lasers Med. Sci. 2018, 33, 375–384. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Y.; Liu, Y.; Dan, L.; Yin, Z.; Huo, Y.; Jiang, G.; Yang, Y.; Wang, Z.; Li, Y.; et al. Porous decellularized trachea scaffold prepared by a laser micropore technique. J. Mech. Behav. Biomed. Mater. 2019, 90, 96–103. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Y.; Liu, Y.; Li, H.; Jia, Z.; Tang, Y.; Jiang, G.; Zhang, X.; Duan, L. Surface modification of decellularized trachea matrix with collagen and laser micropore technique to promote cartilage regeneration. Am. J. Transl. Res. 2019, 11, 5390–5403. [Google Scholar]
- Li, Y.; Xu, Y.; Liu, Y.; Wang, Z.; Chen, W.; Duan, L.; Gu, D. Decellularized cartilage matrix scaffolds with laser-machined micropores for cartilage regeneration and articular cartilage repair. Mater. Sci. Eng. C 2019, 105, 110139. [Google Scholar] [CrossRef] [PubMed]
- Juran, C.M.; Dolwick, M.F.; McFetridge, P.S. Engineered microporosity: Enhancing the early regenerative potential of decellularized temporomandibular joint discs. Tissue Eng. Part A 2015, 21, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Shakh, A.S.; Isaev, E.I.; Baranovsky, D.S.; Demyashkin, G.A.; Klabukov, I.D. The use of pulsed laser infrared radiation with a wavelength of 1.06 μm for the formation of a special microstructure in cartilaginous tissue. Russ. J. Gen. Chem. 2021, 65, 62–66. [Google Scholar]
- Baranovskii, D.; Demner, J.; Nürnberger, S.; Lyundup, A.; Redl, H.; Hilpert, M.; Pigeot, S.; Krasheninnikov, M.; Krasilnikova, O.; Klabukov, I.; et al. Engineering of tracheal grafts based on recellularization of laser-engraved human airway cartilage substrates. Cartilage 2022, 13, 19476035221075951. [Google Scholar] [CrossRef] [PubMed]
- Klabukov, I.; Atiakshin, D.; Kogan, E.; Ignatyuk, M.; Krasheninnikov, M.; Zharkov, N.; Yakimova, A.; Grinevich, V.; Pryanikov, P.; Parshin, V.; et al. Post-Implantation Inflammatory Responses to Xenogeneic Tissue-Engineered Cartilage Implanted in Rabbit Trachea: The Role of Cultured Chondrocytes in the Modification of Inflammation. Int. J. Mol. Sci. 2023, 24, 16783. [Google Scholar] [CrossRef]
- Nguyen, D.; Hägg, D.A.; Forsman, A.; Ekholm, J.; Nimkingratana, P.; Brantsing, C.; Kalogeropoulos, T.; Zaunz, S.; Concaro, S.; Brittberg, M.; et al. Cartilage tissue engineering by the 3D bioprinting of iPS cells in a nanocellulose/alginate bioink. Sci. Rep. 2017, 7, 658. [Google Scholar] [CrossRef]
- Nürnberger, S.; Schneider, C.; Keibl, C.; Schädl, B.; Heimel, P.; Monforte, X.; Teuschl, A.H.; Nalbach, M.; Thurner, P.J.; Grillari, J.; et al. Repopulation of decellularised articular cartilage by laser-based matrix engraving. EBioMedicine 2021, 64, 103196. [Google Scholar] [CrossRef]
- Lakes, E.H.; Matuska, A.M.; McFetridge, P.S.; Allen, K.D. Mechanical Integrity of a Decellularized and Laser Drilled Medial Meniscus. J. Biomech. Eng. 2016, 138, 4032381. [Google Scholar] [CrossRef]
- Okubo, R.; Asawa, Y.; Watanabe, M.; Nagata, S.; Nio, M.; Takato, T.; Hikita, A.; Hoshi, K. Proliferation medium in three-dimensional culture of auricular chondrocytes promotes effective cartilage regeneration in vivo. Regen. Ther. 2019, 11, 306–315. [Google Scholar] [CrossRef]
- Nayyer, L.; Patel, K.H.; Esmaeili, A.; Rippel, R.A.; Birchall, M.; O’toole, G.; Butler, P.E.; Seifalian, A.M. Tissue engineering: Revolution and challenge in auricular cartilage reconstruction. Plast. Reconstr. Surg. 2012, 129, 1123–1137. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, Y. Generation of Ear Cartilage for Auricular Reconstruction. Organ Tissue Eng. 2020, 70, 1507–1515. [Google Scholar] [CrossRef]
- Ingavle, G.C.; Frei, A.W.; Gehrke, S.H.; Detamore, M.S. Incorporation of aggrecan in interpenetrating network hydrogels to improve cellular performance for cartilage tissue engineering. Tissue Eng. Part A 2013, 19, 1349–1359. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, A.J.; Converse, G.L.; Hopkins, R.A.; Detamore, M.S. The bioactivity of cartilage extracellular matrix in articular cartilage regeneration. Adv. Healthc. Mater. 2015, 4, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.C.; Estes, B.T.; Awad, H.A.; Guilak, F. Chondrogenic differentiation of adipose-derived adult stem cells by a porous scaffold derived from native articular cartilage extracellular matrix. Tissue Eng. Part A 2009, 15, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Almeida, H.V.; Eswaramoorthy, R.; Cunniffe, G.M.; Buckley, C.T.; O’brien, F.; Kelly, D. Fibrin hydrogels functionalized with cartilage extracellular matrix and incorporating freshly isolated stromal cells as an injectable for cartilage regeneration. Acta Biomater. 2016, 36, 55–62. [Google Scholar] [CrossRef]
- Rutgers, M.; Saris, D.B.; Vonk, L.A.; van Rijen, M.H.; Akrum, V.; Langeveld, D.; Van Boxtel, A.; Dhert, W.J.; Creemers, L.B. Effect of collagen type I or type II on chondrogenesis by cultured human articular chondrocytes. Tissue Eng. Part A 2013, 19, 59–65. [Google Scholar] [CrossRef]
- Ragetly, G.; Griffon, D.J.; Chung, Y.S. The effect of type II collagen coating of chitosan fibrous scaffolds on mesenchymal stem cell adhesion and chondrogenesis. Acta Biomater. 2010, 6, 3988–3997. [Google Scholar] [CrossRef]
- Isaeva, E.V.; Beketov, E.E.; Demyashkin, G.A.; Yakovleva, N.D.; Arguchinskaya, N.V.; Kisel, A.A.; Lagoda, T.S.; Malakhov, E.P.; Smirnova, A.N.; Petriev, V.M.; et al. Cartilage formation in vivo using high concentration collagen-based bioink with MSC and decellularized ECM granules. Int. J. Mol. Sci. 2022, 23, 2703. [Google Scholar] [CrossRef]
- Cheng, J.; Shen, K.; Zuo, Q.; Yan, K.; Zhang, X.; Liang, W.; Fan, W. A xenogeneic decellularized multiphasic scaffold for the repair of osteochondral defects in a rabbit model. Mater. Des. 2023, 225, 111450. [Google Scholar] [CrossRef]
- Roth, S.P.; Glauche, S.M.; Plenge, A.; Erbe, I.; Heller, S.; Burk, J. Automated freeze-thaw cycles for decellularization of tendon tissue-a pilot study. BMC Biotechnol. 2017, 17, 13. [Google Scholar] [CrossRef]
- Chen, W.; Li, C.; Peng, M.; Xie, B.; Zhang, L.; Tang, X. Autologous nasal chondrocytes delivered by injectable hydrogel for In vivo articular cartilage regeneration. Cell Tissue Bank. 2018, 19, 35–46. [Google Scholar] [CrossRef] [PubMed]


| Laser source type | ytterbium fiber laser source |
| Laser source power | 20 W |
| Pulse frequency range | 20–50 kHz |
| Laser source wavelength | 1064 nm |
| Positioning accuracy | 3 µm |
| Pulse energy | 0.33–1 mJ |
| Contact spot | 50 µm |
| Laser Source Cooling | Air |
| Type of lifting mechanism (Z-axis) | Manual drive on the scanning stand |
| Software | EZCAD 2.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kisel, A.A.; Stepanov, V.A.; Isaeva, E.V.; Demyashkin, G.A.; Isaev, E.I.; Smirnova, E.I.; Yatsenko, E.M.; Afonin, G.V.; Ivanov, S.A.; Atiakshin, D.A.; et al. Cartilage Laser Engraving for Fast-Track Tissue Engineering of Auricular Grafts. Int. J. Mol. Sci. 2024, 25, 11538. https://doi.org/10.3390/ijms252111538
Kisel AA, Stepanov VA, Isaeva EV, Demyashkin GA, Isaev EI, Smirnova EI, Yatsenko EM, Afonin GV, Ivanov SA, Atiakshin DA, et al. Cartilage Laser Engraving for Fast-Track Tissue Engineering of Auricular Grafts. International Journal of Molecular Sciences. 2024; 25(21):11538. https://doi.org/10.3390/ijms252111538
Chicago/Turabian StyleKisel, Anastas A., Vladimir A. Stepanov, Elena V. Isaeva, Grigory A. Demyashkin, Evgeny I. Isaev, Ekaterina I. Smirnova, Elena M. Yatsenko, Grigoriy V. Afonin, Sergey A. Ivanov, Dmitrii A. Atiakshin, and et al. 2024. "Cartilage Laser Engraving for Fast-Track Tissue Engineering of Auricular Grafts" International Journal of Molecular Sciences 25, no. 21: 11538. https://doi.org/10.3390/ijms252111538
APA StyleKisel, A. A., Stepanov, V. A., Isaeva, E. V., Demyashkin, G. A., Isaev, E. I., Smirnova, E. I., Yatsenko, E. M., Afonin, G. V., Ivanov, S. A., Atiakshin, D. A., Shegay, P. V., Kaprin, A. D., Klabukov, I. D., & Baranovskii, D. S. (2024). Cartilage Laser Engraving for Fast-Track Tissue Engineering of Auricular Grafts. International Journal of Molecular Sciences, 25(21), 11538. https://doi.org/10.3390/ijms252111538








