New Insights on the Effects of Krill Oil Supplementation, a High-Fat Diet, and Aging on Hippocampal-Dependent Memory, Neuroinflammation, Synaptic Density, and Neurogenesis
Abstract
:1. Introduction
2. Results
2.1. Animals
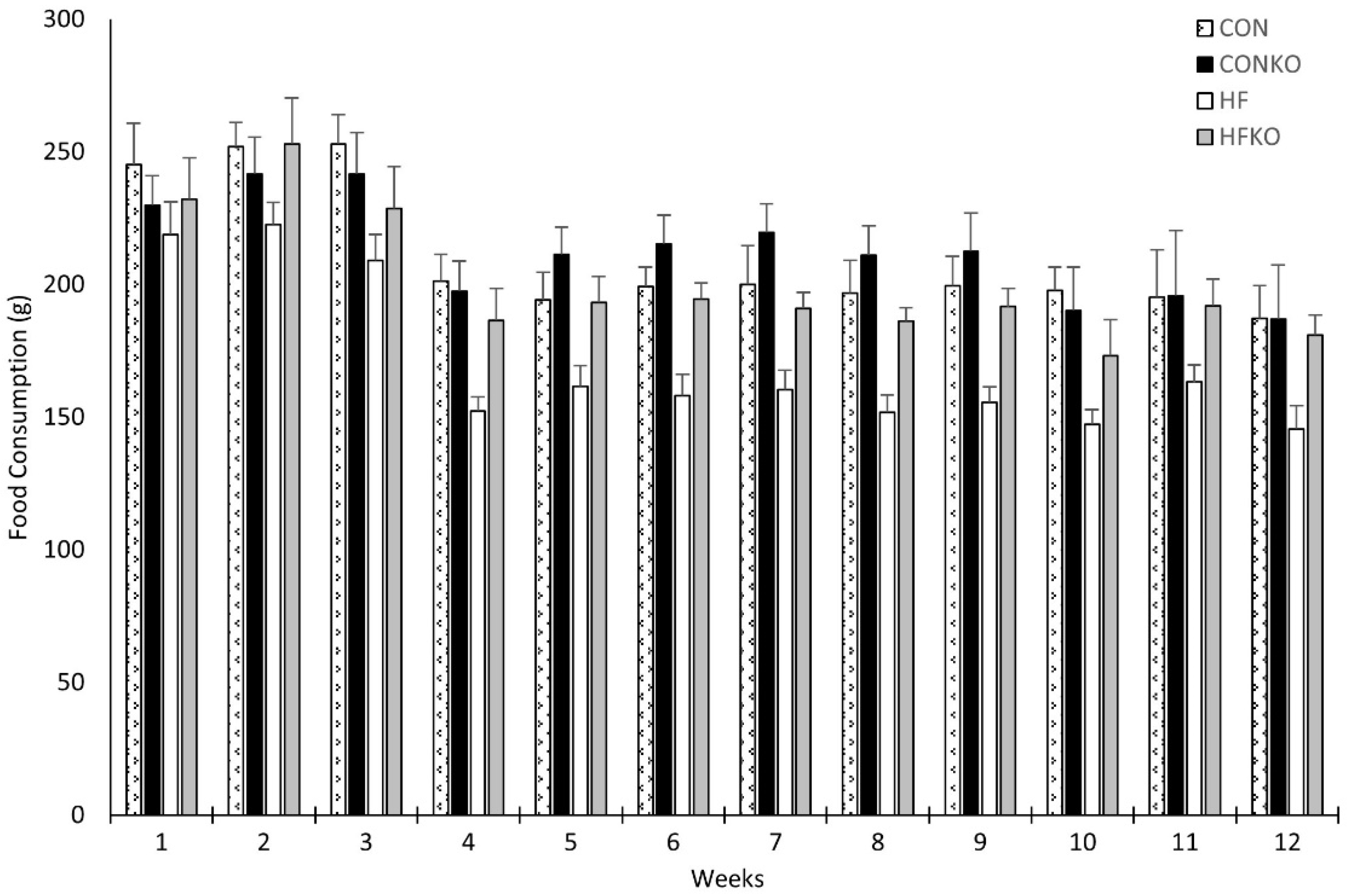
2.2. Food Consumption
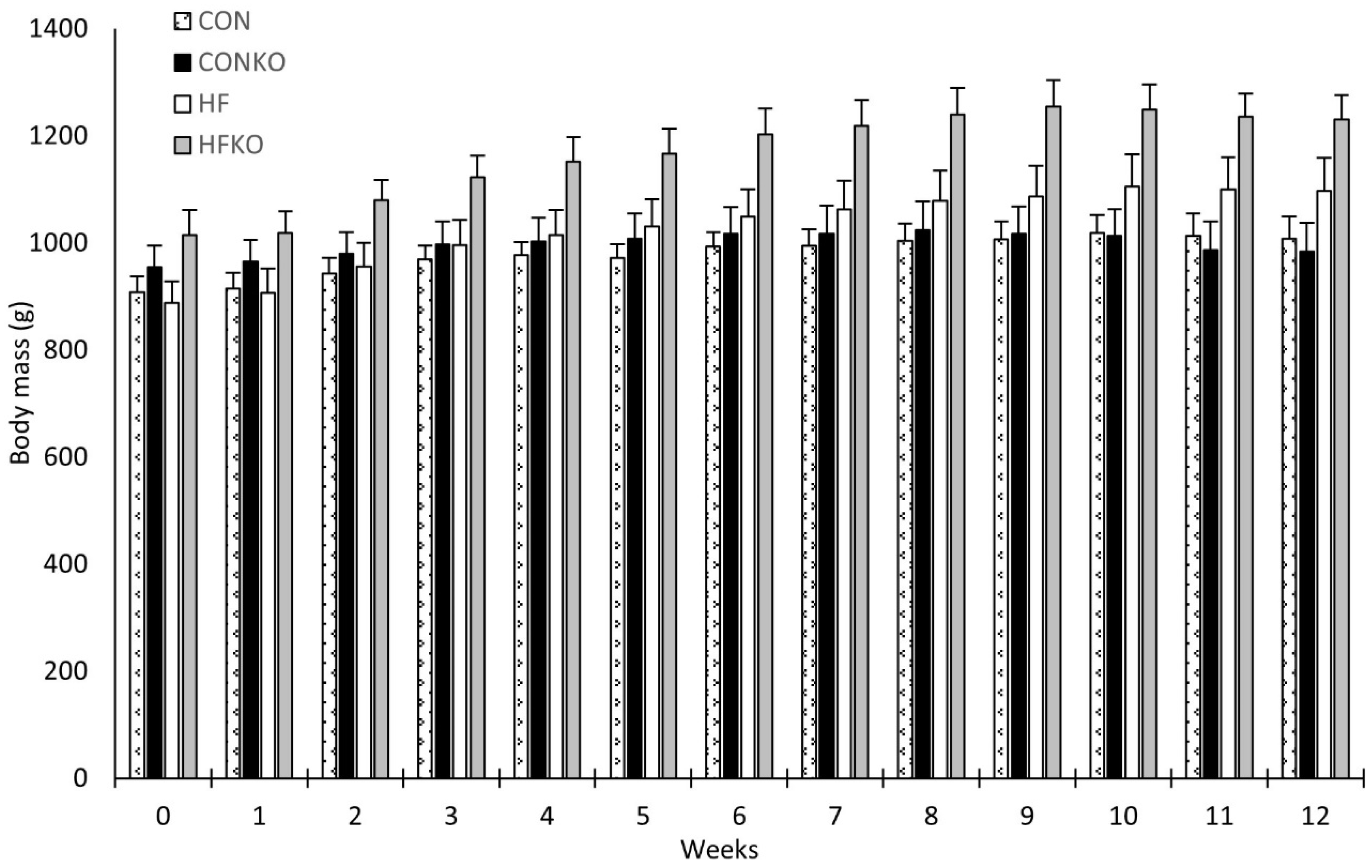
2.3. Anthropometric Data
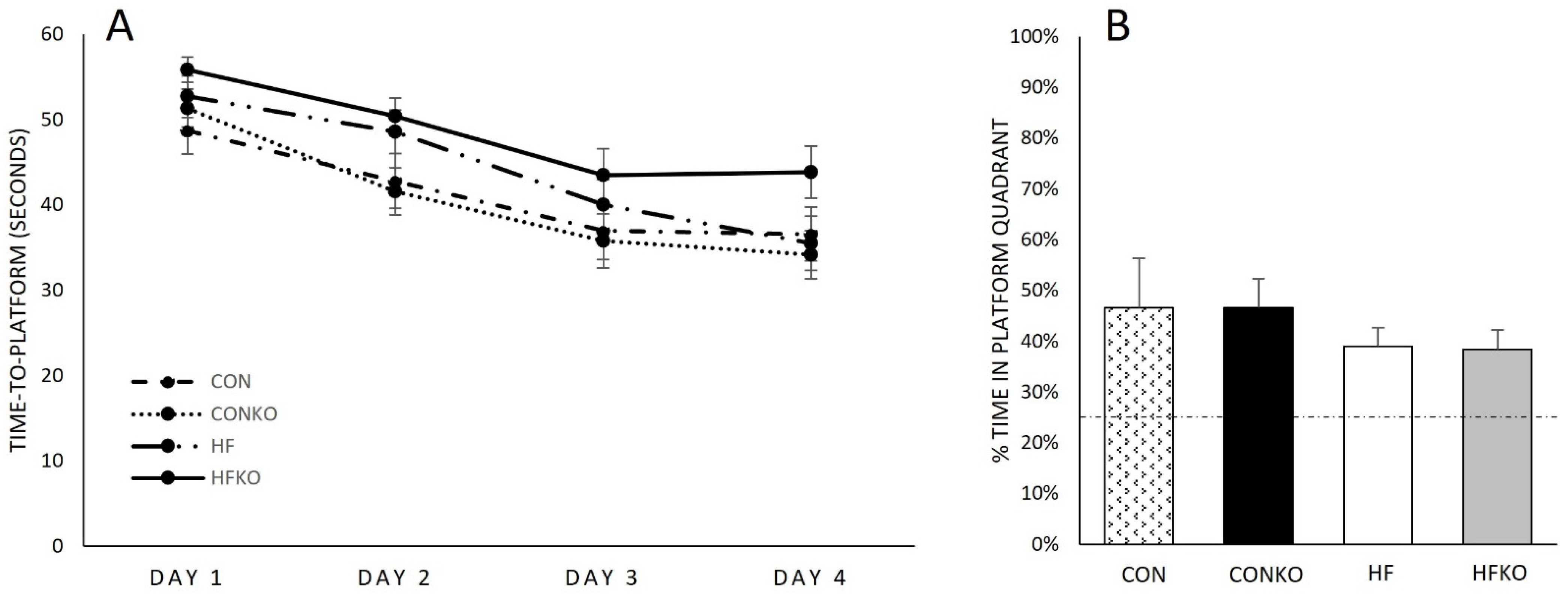
2.4. Behavioral Testing
2.5. Cytokine Concentrations
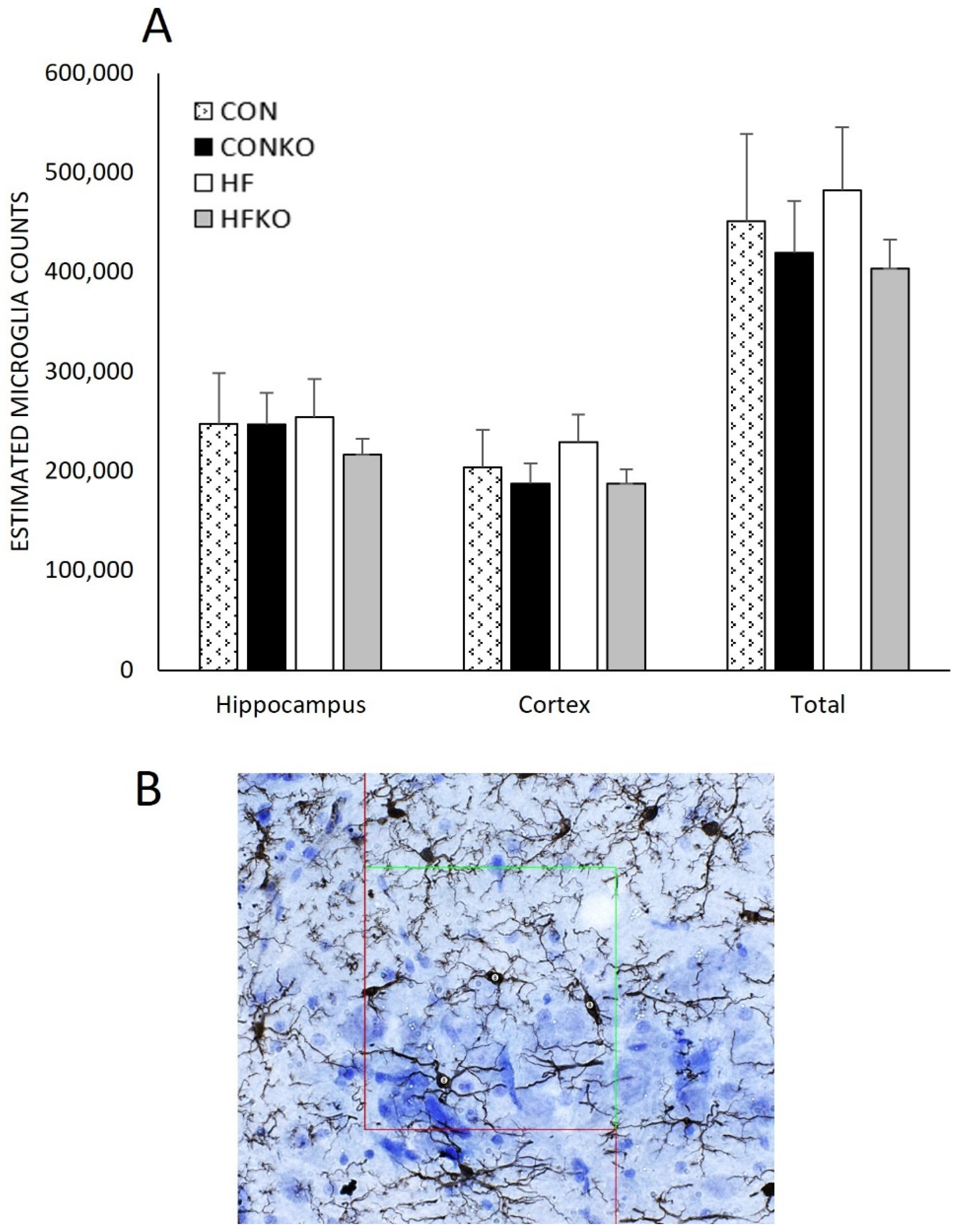
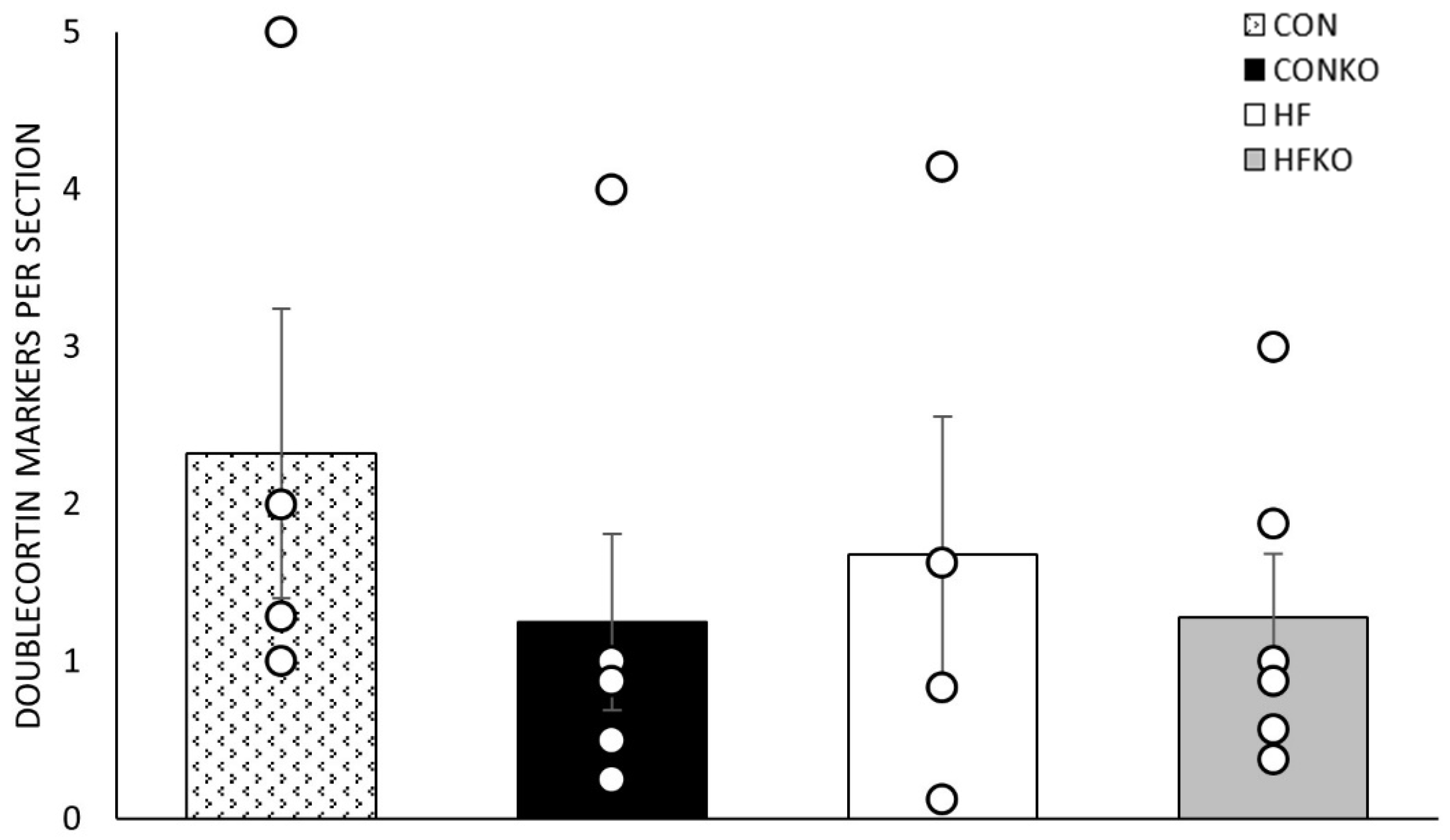
2.6. Microglia and Doublecortin Quantification
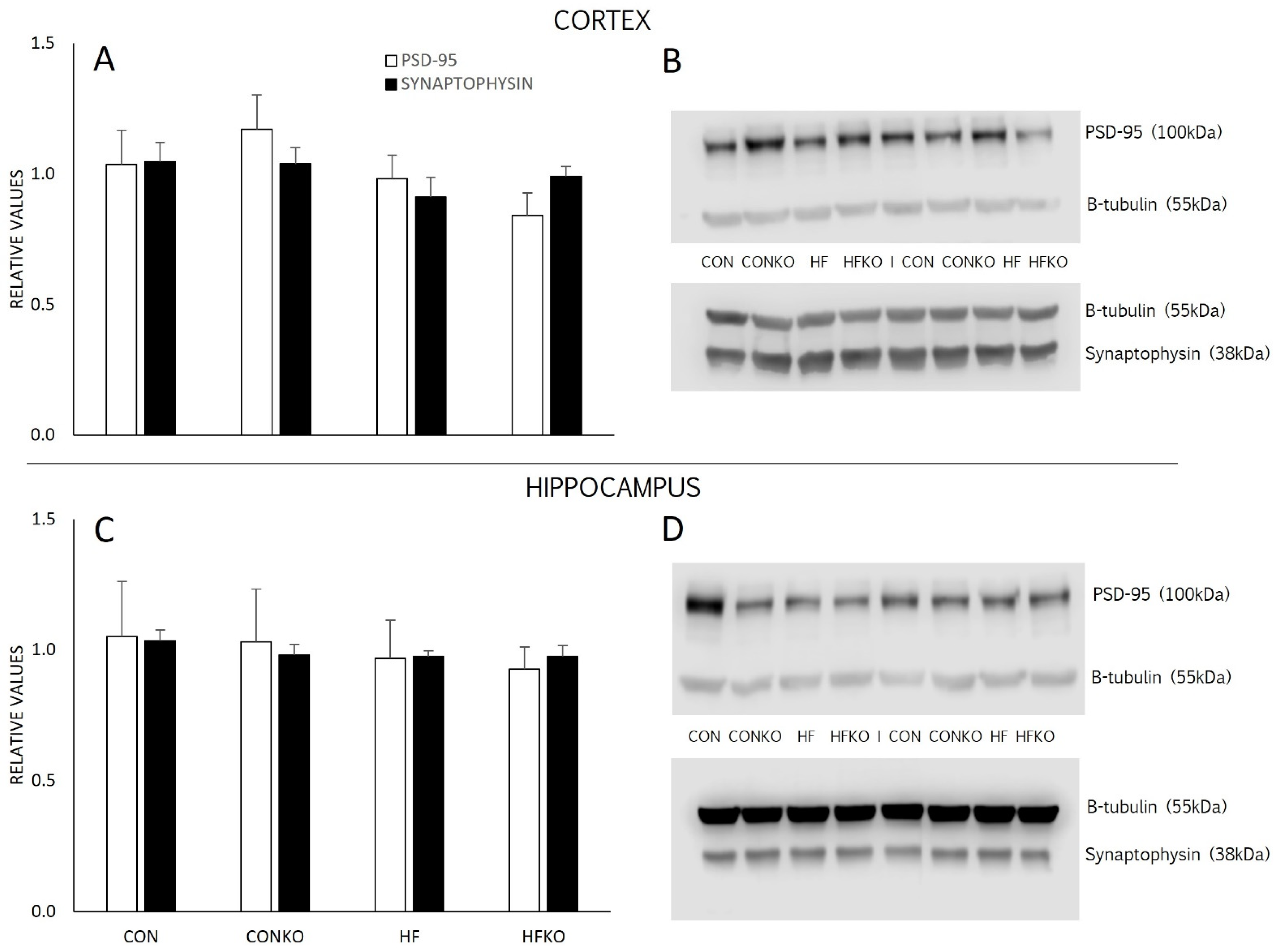
2.7. Synaptic Density
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animal Care and Dietary Groups
4.3. Behavioral Testing
4.4. Tissue Collection
4.5. Protein Analysis
4.6. Section Preparation and Stereology
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Babcock, K.R.; Page, J.S.; Fallon, J.R.; Webb, A.E. Adult Hippocampal Neurogenesis in Aging and Alzheimer’s Disease. Stem Cell Rep. 2021, 16, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Cameron, H.A.; Glover, L.R. Adult neurogenesis: Beyond learning and memory. Annu. Rev. Psychol. 2015, 66, 53–81. [Google Scholar] [CrossRef] [PubMed]
- Isaev, N.K.; Stelmashook, E.V.; Genrikhs, E.E. Neurogenesis and brain aging. Rev. Neurosci. 2019, 30, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, R.M.; Kitt, M.M.; Watkins, L.R.; Maier, S.F. Neuroinflammation in the normal aging hippocampus. Neuroscience 2015, 309, 84–99. [Google Scholar] [CrossRef]
- Dominguez-Gonzalez, M.; Puigpinos, M.; Jove, M.; Naudi, A.; Portero-Otin, M.; Pamplona, R.; Ferrer, I. Regional vulnerability to lipoxidative damage and inflammation in normal human brain aging. Exp. Gerontol. 2018, 111, 218–228. [Google Scholar] [CrossRef]
- Andraka, J.M.; Sharma, N.; Marchalant, Y. Can krill oil be of use for counteracting neuroinflammatory processes induced by high fat diet and aging? Neurosci. Res. 2020, 157, 1–14. [Google Scholar] [CrossRef]
- Derecki, N.C.; Cardani, A.N.; Yang, C.H.; Quinnies, K.M.; Crihfield, A.; Lynch, K.R.; Kipnis, J. Regulation of learning and memory by meningeal immunity: A key role for IL-4. J. Exp. Med. 2010, 207, 1067–1080. [Google Scholar] [CrossRef]
- Nakamura, Y. Regulating factors for microglial activation. Biol. Pharm. Bull. 2002, 25, 945–953. [Google Scholar] [CrossRef]
- Nimmerjahn, A.; Kirchhoff, F.; Helmchen, F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 2005, 308, 1314–1318. [Google Scholar] [CrossRef]
- Shadfar, S.; Hwang, C.J.; Lim, M.S.; Choi, D.Y.; Hong, J.T. Involvement of inflammation in Alzheimer’s disease pathogenesis and therapeutic potential of anti-inflammatory agents. Arch. Pharmacal Res. 2015, 38, 2106–2119. [Google Scholar] [CrossRef]
- Wang, W.-Y.; Tan, M.-S.; Yu, J.-T.; Tan, L. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann. Transl. Med. 2015, 3, 136. [Google Scholar] [PubMed]
- Chitnis, T.; Weiner, H.L. CNS inflammation and neurodegeneration. J. Clin. Investig. 2017, 127, 3577–3587. [Google Scholar] [CrossRef] [PubMed]
- Ransohoff, R.M. How neuroinflammation contributes to neurodegeneration. Science 2016, 353, 777–783. [Google Scholar] [CrossRef]
- McCarthy, M.M. Location, Location, Location: Microglia Are Where They Live. Neuron 2017, 95, 233–235. [Google Scholar] [CrossRef]
- Kongsui, R.; Beynon, S.B.; Johnson, S.J.; Walker, F.R. Quantitative assessment of microglial morphology and density reveals remarkable consistency in the distribution and morphology of cells within the healthy prefrontal cortex of the rat. J. Neuroinflamm. 2014, 11, 182. [Google Scholar] [CrossRef]
- Andoh, M.; Koyama, R. Assessing Microglial Dynamics by Live Imaging. Front. Immunol. 2021, 12, 617564. [Google Scholar] [CrossRef]
- Eyo, U.B.; Wu, L.-J. Microglia: Lifelong patrolling immune cells of the brain. Prog. Neurobiol. 2019, 179, 101614. [Google Scholar] [CrossRef]
- Cornell, J.; Salinas, S.; Huang, H.Y.; Zhou, M. Microglia regulation of synaptic plasticity and learning and memory. Neural Regen. Res. 2022, 17, 705–716. [Google Scholar]
- Kalamakis, G.; Brune, D.; Ravichandran, S.; Bolz, J.; Fan, W.; Ziebell, F.; Stiehl, T.; Catala-Martinez, F.; Kupke, J.; Zhao, S.; et al. Quiescence Modulates Stem Cell Maintenance and Regenerative Capacity in the Aging Brain. Cell 2019, 176, 1407–1419.e14. [Google Scholar] [CrossRef]
- Zaccard, C.R.; Gippo, I.; Song, A.; Geula, C.; Penzes, P. Dendritic spinule-mediated structural synaptic plasticity: Implications for development, aging, and psychiatric disease. Front. Mol. Neurosci. 2023, 16, 1059730. [Google Scholar] [CrossRef]
- Kozareva, D.A.; Cryan, J.F.; Nolan, Y.M. Born this way: Hippocampal neurogenesis across the lifespan. Aging Cell 2019, 18, e13007. [Google Scholar] [CrossRef] [PubMed]
- Marchalant, Y.; Brothers, H.M.; Norman, G.J.; Karelina, K.; DeVries, A.C.; Wenk, G.L. Cannabinoids attenuate the effects of aging upon neuroinflammation and neurogenesis. Neurobiol. Dis. 2009, 34, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Yao, X.; Zhang, H.; Wang, C.; Zhao, J.; Xu, D.; Xiao, Y.; Li, Q.; Zhuang, H.; Kang, X.; et al. Effects of Prolonged High-Fat Diet Consumption Starting at Different Ages on Behavioral Parameters and Hippocampal Neuroplasticity in Male Mice. J. Integr. Neurosci. 2023, 22, 16. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Yang, C.; Wang, C.; Li, H.; Zhao, J.; Kang, X.; Liu, Z.; Chen, L.; Chen, X.; Pu, T.; et al. High-Fat Diet Consumption in Adolescence Induces Emotional Behavior Alterations and Hippocampal Neurogenesis Deficits Accompanied by Excessive Microglial Activation. Int. J. Mol. Sci. 2022, 23, 8316. [Google Scholar] [CrossRef]
- Lindqvist, A.; Mohapel, P.; Bouter, B.; Frielingsdorf, H.; Pizzo, D.; Brundin, P.; Erlanson-Albertsson, C. High-fat diet impairs hippocampal neurogenesis in male rats. Eur. J. Neurol. 2006, 13, 1385–1388. [Google Scholar] [CrossRef]
- Poulose, S.M.; Miller, M.G.; Scott, T.; Shukitt-Hale, B. Nutritional Factors Affecting Adult Neurogenesis and Cognitive Function. Adv. Nutrition. 2017, 8, 804–811. [Google Scholar] [CrossRef]
- Park, H.R.; Park, M.; Choi, J.; Park, K.Y.; Chung, H.Y.; Lee, J. A high-fat diet impairs neurogenesis: Involvement of lipid peroxidation and brain-derived neurotrophic factor. Neurosci. Lett. 2010, 482, 235–239. [Google Scholar] [CrossRef]
- Paulo, S.L.; Miranda-Lourenço, C.; Belo, R.F.; Rodrigues, R.S.; Fonseca-Gomes, J.; Tanqueiro, S.R.; Geraldes, V.; Rocha, I.; Sebastião, A.M.; Xapelli, S.; et al. High Caloric Diet Induces Memory Impairment and Disrupts Synaptic Plasticity in Aged Rats. Curr. Issues Mol. Biol. 2021, 43, 2305–2319. [Google Scholar] [CrossRef]
- Ilina, A.; Linkova, N. A Transgenic 5xFAD-M Line of Mice for Dendritic Spine Morphology Analysis in Alzheimer’s Disease. Brain Sci. 2023, 13, 307. [Google Scholar] [CrossRef]
- Schuster, T.; Krug, M.; Wenzel, J. Spinules in axospinous synapses of the rat dentate gyrus: Changes in density following long-term potentiation. Brain Res. 1990, 523, 171–174. [Google Scholar] [CrossRef]
- Glantz, L.A.; Gilmore, J.H.; Hamer, R.M.; Lieberman, J.A.; Jarskog, L.F. Synaptophysin and postsynaptic density protein 95 in the human prefrontal cortex from mid-gestation into early adulthood. Neuroscience 2007, 149, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Trépanier, M.-O.; Hopperton, K.E.; Orr, S.K.; Bazinet, R.P. N-3 polyunsaturated fatty acids in animal models with neuroinflammation: An update. Eur. J. Pharmacol. 2016, 785, 187–206. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, A.M.; Fernandes, M.D.C.; de Fraga, L.S.; Porawski, M.; Giovenardi, M.; Guedes, R.P. Omega-3 fatty acids revert high-fat diet-induced neuroinflammation but not recognition memory impairment in rats. Metab. Brain Dis. 2017, 32, 1871–1881. [Google Scholar] [CrossRef] [PubMed]
- Barros, M.P.; Poppe, S.C.; Bondan, E.F. Neuroprotective properties of the marine carotenoid astaxanthin and omega-3 fatty acids, and perspectives for the natural combination of both in krill oil. Nutrients 2014, 6, 1293–1317. [Google Scholar] [CrossRef] [PubMed]
- Blusztajn, J.K.; Slack, B.E.; Mellott, T.J. Neuroprotective Actions of Dietary Choline. Nutrients 2017, 9, 815. [Google Scholar] [CrossRef]
- Fakhri, S.; Aneva, I.Y.; Farzaei, M.H.; Sobarzo-Sanchez, E. The Neuroprotective Effects of Astaxanthin: Therapeutic Targets and Clinical Perspective. Molecules 2019, 24, 2640. [Google Scholar] [CrossRef]
- Joffre, C.; Dinel, A.L.; Chataigner, M.; Pallet, V.; Laye, S. n-3 Polyunsaturated Fatty Acids and Their Derivates Reduce Neuroinflammation during Aging. Nutrients 2020, 12, 647. [Google Scholar] [CrossRef]
- Maruszak, A.; Pilarski, A.; Murphy, T.; Branch, N.; Thuret, S. Hippocampal neurogenesis in Alzheimer’s disease: Is there a role for dietary modulation? J. Alzheimer’s Dis. 2014, 38, 11–38. [Google Scholar] [CrossRef]
- Zhang, X.S.; Zhang, X.; Wu, Q.; Li, W.; Wang, C.X.; Xie, G.B.; Zhou, X.M.; Shi, J.X.; Zhou, M.L. Astaxanthin offers neuroprotection and reduces neuroinflammation in experimental subarachnoid hemorrhage. J. Surg. Res. 2014, 192, 206–213. [Google Scholar] [CrossRef]
- Galasso, C.; Orefice, I.; Pellone, P.; Cirino, P.; Miele, R.; Ianora, A.; Brunet, C.; Sansone, C. On the Neuroprotective Role of Astaxanthin: New Perspectives? Mar. Drugs 2018, 16, 247. [Google Scholar] [CrossRef]
- Jadavji, N.M.; Emmerson, J.T.; MacFarlane, A.J.; Willmore, W.G.; Smith, P.D. B-vitamin and choline supplementation increases neuroplasticity and recovery after stroke. Neurobiol. Dis. 2017, 103, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Poly, C.; Massaro, J.M.; Seshadri, S.; Wolf, P.A.; Cho, E.; Krall, E.; Jacques, P.F.; Au, R. The relation of dietary choline to cognitive performance and white-matter hyperintensity in the Framingham Offspring Cohort. Am. J. Clin. Nutr. 2011, 94, 1584–1591. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Chen, M.; Gandhy, S.U.; Strawderman, M.; Levitsky, D.A.; Maclean, K.N.; Strupp, B.J. Perinatal choline supplementation improves cognitive functioning and emotion regulation in the Ts65Dn mouse model of Down syndrome. Behav. Neurosci. 2010, 124, 346–361. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, C.E.; Winocur, G. Learning and memory impairment in rats fed a high saturated fat diet. Behav. Neural Biol. 1990, 53, 74–87. [Google Scholar] [CrossRef]
- Winocur, G.; Greenwood, C.E. Studies of the effects of high fat diets on cognitive function in a rat model. Neurobiol. Aging 2005, 26 (Suppl. S1), 46–49. [Google Scholar] [CrossRef]
- Morrison, C.D.; Pistell, P.J.; Ingram, D.K.; Johnson, W.D.; Liu, Y.; Fernandez-Kim, S.O.; White, C.L.; Purpera, M.N.; Uranga, R.M.; Bruce-Keller, A.J.; et al. High fat diet increases hippocampal oxidative stress and cognitive impairment in aged mice: Implications for decreased Nrf2 signaling. J. Neurochem. 2010, 114, 1581–1589. [Google Scholar] [CrossRef]
- Pistell, P.J.; Morrison, C.D.; Gupta, S.; Knight, A.G.; Keller, J.N.; Ingram, D.K.; Bruce-Keller, A.J. Cognitive impairment following high fat diet consumption is associated with brain inflammation. J. Neuroimmunol. 2010, 219, 25–32. [Google Scholar] [CrossRef]
- White, C.L.; Pistell, P.J.; Purpera, M.N.; Gupta, S.; Fernandez-Kim, S.O.; Hise, T.L.; Keller, J.N.; Ingram, D.K.; Morrison, C.D.; Bruce-Keller, A.J. Effects of high fat diet on Morris maze performance, oxidative stress, and inflammation in rats: Contributions of maternal diet. Neurobiol. Dis. 2009, 35, 3–13. [Google Scholar] [CrossRef]
- Kim, J.H.; Seo, H.J.; Pang, Q.Q.; Kwon, Y.R.; Kim, J.H.; Cho, E.J. Protective effects of krill oil on high fat diet-induced cognitive impairment by regulation of oxidative stress. Free Radic. Res. 2021, 55, 799–809. [Google Scholar] [CrossRef]
- García-Serrano, A.; Tomé-Carneiro, J.; Carmen Crespo, M.; Visitación Calvo, M.; Pereda-Pérez, I.; Baliyan, S.; Burgos-Ramos, E.; Montero, O.; Dávalos, A.; Venero, C.; et al. Concentrates of buttermilk and krill oil improve cognition in aged rats. Prostaglandins Leukot. Essent. Fat. Acids 2020, 155, 102077. [Google Scholar] [CrossRef]
- Li, Q.; Wu, F.; Wen, M.; Yanagita, T.; Xue, C.; Zhang, T.; Wang, Y. The Protective Effect of Antarctic Krill Oil on Cognitive Function by Inhibiting Oxidative Stress in the Brain of Senescence-Accelerated Prone Mouse Strain 8 (SAMP8) Mice. J. Food Sci. 2018, 83, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Jang, J.S.; Son, D.J.; Im, H.S.; Kim, J.Y.; Park, J.E.; Choi, W.R.; Han, S.B.; Hong, J.T. Antarctic Krill Oil Diet Protects against Lipopolysaccharide-Induced Oxidative Stress, Neuroinflammation and Cognitive Impairment. Int. J. Mol. Sci. 2017, 18, 2554. [Google Scholar] [CrossRef] [PubMed]
- Skorve, J.; Hilvo, M.; Vihervaara, T.; Burri, L.; Bohov, P.; Tillander, V.; Bjorndal, B.; Suoniemi, M.; Laaksonen, R.; Ekroos, K.; et al. Fish oil and krill oil differentially modify the liver and brain lipidome when fed to mice. Lipids Health Dis. 2015, 14, 88. [Google Scholar] [CrossRef] [PubMed]
- Adarme-Vega, T.C.; Thomas-Hall, S.R.; Schenk, P.M. Towards sustainable sources for omega-3 fatty acids production. Curr. Opin. Biotechnol. 2014, 26, 14–18. [Google Scholar] [CrossRef]
- Ulven, S.M.; Holven, K.B. Comparison of bioavailability of krill oil versus fish oil and health effect. Vasc. Health Risk Manag. 2015, 11, 511–524. [Google Scholar] [CrossRef]
- Hopperton, K.E.; Trepanier, M.O.; Giuliano, V.; Bazinet, R.P. Brain omega-3 polyunsaturated fatty acids modulate microglia cell number and morphology in response to intracerebroventricular amyloid-beta 1-40 in mice. J. Neuroinflamm. 2016, 13, 257. [Google Scholar] [CrossRef]
- Sun, G.Y.; Simonyi, A.; Fritsche, K.L.; Chuang, D.Y.; Hannink, M.; Gu, Z.; Greenlief, C.M.; Yao, J.K.; Lee, J.C.; Beversdorf, D.Q. Docosahexaenoic acid (DHA): An essential nutrient and a nutraceutical for brain health and diseases. Prostaglandins Leukot. Essent. Fat. Acids 2018, 136, 3–13. [Google Scholar] [CrossRef]
- Pan, J.P.; Zhang, H.Q.; Wei, W.; Guo, Y.F.; Na, X.; Cao, X.H.; Liu, L.J. Some subtypes of endocannabinoid/endovanilloid receptors mediate docosahexaenoic acid-induced enhanced spatial memory in rats. Brain Res. 2011, 1412, 18–27. [Google Scholar] [CrossRef]
- Badesso, S.; Cartas-Cejudo, P.; Espelosin, M.; Santamaria, E.; Cuadrado-Tejedor, M.; Garcia-Osta, A. Docosahexaenoic Acid Ameliorates Contextual Fear Memory Deficits in the Tg2576 Alzheimer’s Disease Mouse Model: Cellular and Molecular Correlates. Pharmaceutics 2023, 15, 82. [Google Scholar]
- Jiang, L.H.; Shi, Y.; Wang, L.S.; Yang, Z.R. The influence of orally administered docosahexaenoic acid on cognitive ability in aged mice. J. Nutr. Biochem. 2009, 20, 735–741. [Google Scholar] [CrossRef]
- Afshordel, S.; Hagl, S.; Werner, D.; Rohner, N.; Kogel, D.; Bazan, N.G.; Eckert, G.P. Omega-3 polyunsaturated fatty acids improve mitochondrial dysfunction in brain aging--impact of Bcl-2 and NPD-1 like metabolites. Prostaglandins Leukot. Essent. Fat. Acids 2015, 92, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Liu, Q.; Kalavagunta, P.K.; Huang, Q.; Lv, W.; An, X.; Chen, H.; Wang, T.; Heriniaina, R.M.; Qiao, T.; et al. Normal diet Vs High fat diet—A comparative study: Behavioral and neuroimmunological changes in adolescent male mice. Metab. Brain Dis. 2018, 33, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Cameron, T.P.; Lattuada, C.P.; Kornreich, M.R.; Tarone, R.E. Longevity and reproductive comparisons for male ACI and Sprague-Dawley rat aging colonies. Lab. Anim. Sci. 1982, 32, 495–499. [Google Scholar]
- Cavaliere, G.; Trinchese, G.; Penna, E.; Cimmino, F.; Pirozzi, C.; Lama, A.; Annunziata, C.; Catapano, A.; Mattace Raso, G.; Meli, R.; et al. High-Fat Diet Induces Neuroinflammation and Mitochondrial Impairment in Mice Cerebral Cortex and Synaptic Fraction. Front. Cell. Neurosci. 2019, 13, 509. [Google Scholar] [CrossRef] [PubMed]
- Sobesky, J.L.; Barrientos, R.M.; De May, H.S.; Thompson, B.M.; Weber, M.D.; Watkins, L.R.; Maier, S.F. High-fat diet consumption disrupts memory and primes elevations in hippocampal IL-1beta, an effect that can be prevented with dietary reversal or IL-1 receptor antagonism. Brain Behav. Immun. 2014, 42, 22–32. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes: From molecules to man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef]
- Patterson, E.; Wall, R.; Fitzgerald, G.F.; Ross, R.P.; Stanton, C. Health implications of high dietary omega-6 polyunsaturated Fatty acids. J. Nutr. Metab. 2012, 2012, 539426. [Google Scholar] [CrossRef]
- Labrousse, V.F.; Leyrolle, Q.; Amadieu, C.; Aubert, A.; Sere, A.; Coutureau, E.; Gregoire, S.; Bretillon, L.; Pallet, V.; Gressens, P.; et al. Dietary omega-3 deficiency exacerbates inflammation and reveals spatial memory deficits in mice exposed to lipopolysaccharide during gestation. Brain Behav. Immun. 2018, 73, 427–440. [Google Scholar] [CrossRef]
- Loef, M.; Walach, H. The omega-6/omega-3 ratio and dementia or cognitive decline: A systematic review on human studies and biological evidence. J. Nutr. Gerontol. Geriatr. 2013, 32, 1–23. [Google Scholar] [CrossRef]
- Andruchow, N.D.; Konishi, K.; Shatenstein, B.; Bohbot, V.D. A lower ratio of omega-6 to omega-3 fatty acids predicts better hippocampus-dependent spatial memory and cognitive status in older adults. Neuropsychology 2017, 31, 724–734. [Google Scholar] [CrossRef]
- Burdge, G.C.; Calder, P.C. Conversion of alpha-linolenic acid to longer-chain polyunsaturated fatty acids in human adults. Reprod. Nutr. Dev. 2005, 45, 581–597. [Google Scholar] [CrossRef] [PubMed]
- Nichols, P.D.; Petrie, J.; Singh, S. Long-chain omega-3 oils-an update on sustainable sources. Nutrients 2010, 2, 572–585. [Google Scholar] [CrossRef] [PubMed]
- Damani, M.R.; Zhao, L.; Fontainhas, A.M.; Amaral, J.; Fariss, R.N.; Wong, W.T. Age-related alterations in the dynamic behavior of microglia. Aging Cell 2011, 10, 263–276. [Google Scholar] [CrossRef]
- Rodriguez-Callejas, J.D.; Fuchs, E.; Perez-Cruz, C. Evidence of Tau Hyperphosphorylation and Dystrophic Microglia in the Common Marmoset. Front. Aging Neurosci. 2016, 8, 315. [Google Scholar] [CrossRef]
- Galatro, T.F.; Holtman, I.R.; Lerario, A.M.; Vainchtein, I.D.; Brouwer, N.; Sola, P.R.; Veras, M.M.; Pereira, T.F.; Leite, R.E.P.; Moller, T.; et al. Transcriptomic analysis of purified human cortical microglia reveals age-associated changes. Nat. Neurosci. 2017, 20, 1162–1171. [Google Scholar] [CrossRef]
- Mahalakshmi, A.M.; Ray, B.; Tuladhar, S.; Hediyal, T.A.; Raj, P.; Rathipriya, A.G.; Qoronfleh, M.W.; Essa, M.M.; Chidambaram, S.B. Impact of Pharmacological and Non-Pharmacological Modulators on Dendritic Spines Structure and Functions in Brain. Cells 2021, 10, 3405. [Google Scholar] [CrossRef]
- Oh, W.C.; Hill, T.C.; Zito, K. Synapse-specific and size-dependent mechanisms of spine structural plasticity accompanying synaptic weakening. Proc. Natl. Acad. Sci. USA 2013, 110, E305–E312. [Google Scholar] [CrossRef]
- Bocarsly, M.E.; Fasolino, M.; Kane, G.A.; LaMarca, E.A.; Kirschen, G.W.; Karatsoreos, I.N.; McEwen, B.S.; Gould, E. Obesity diminishes synaptic markers, alters microglial morphology, and impairs cognitive function. Proc. Natl. Acad. Sci. USA 2015, 112, 15731–15736. [Google Scholar] [CrossRef]
- Kao, Y.C.; Wei, W.Y.; Tsai, K.J.; Wang, L.C. High Fat Diet Suppresses Peroxisome Proliferator-Activated Receptors and Reduces Dopaminergic Neurons in the Substantia Nigra. Int. J. Mol. Sci. 2019, 21, 207. [Google Scholar] [CrossRef]
- Petralia, R.S.; Mattson, M.P.; Yao, P.J. Communication breakdown: The impact of ageing on synapse structure. Ageing Res. Rev. 2014, 14, 31–42. [Google Scholar] [CrossRef]
- Alcantara-Gonzalez, F.; Juarez, I.; Solis, O.; Martinez-Tellez, I.; Camacho-Abrego, I.; Masliah, E.; Mena, R.; Flores, G. Enhanced dendritic spine number of neurons of the prefrontal cortex, hippocampus, and nucleus accumbens in old rats after chronic donepezil administration. Synapse 2010, 64, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Stranahan, A.M.; Lee, K.; Martin, B.; Maudsley, S.; Golden, E.; Cutler, R.G.; Mattson, M.P. Voluntary exercise and caloric restriction enhance hippocampal dendritic spine density and BDNF levels in diabetic mice. Hippocampus 2009, 19, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Haughey, N.J.; Liu, D.; Nath, A.; Borchard, A.C.; Mattson, M.P. Disruption of neurogenesis in the subventricular zone of adult mice, and in human cortical neuronal precursor cells in culture, by amyloid beta-peptide: Implications for the pathogenesis of Alzheimer’s disease. Neuromol. Med. 2002, 1, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Deng, W.; Gage, F.H. Mechanisms and functional implications of adult neurogenesis. Cell 2008, 132, 645–660. [Google Scholar] [CrossRef]
- Marchalant, Y.; Cerbai, F.; Brothers, H.M.; Wenk, G.L. Cannabinoid receptor stimulation is anti-inflammatory and improves memory in old rats. Neurobiol. Aging 2008, 29, 1894–1901. [Google Scholar] [CrossRef]

| (a) | |||||
| Dietary Groups | CHO | PRO | LARD | KO | SO |
| Control (CON) | 57.2 | 18.6 | 2.0 | 0 | 8.0 |
| Control with Krill oil (CONKO) | 57.2 | 18.6 | 2.0 | 8.0 | 0 |
| High fat (HF) | 27.6 | 23.5 | 26.0 | 0 | 8.0 |
| High fat with Krill oil (HFKO) | 27.6 | 23.5 | 26.0 | 8.0 | 0 |
| (b) | |||||
| Dietary Groups | CHO | PRO | FAT | kcal/g | |
| Control (CON)/ Control with krill oil (CONKO) | 57.9 | 18.8 | 23.3 | 3.9 | |
| High fat (HF)/ High fat with krill oil (HFKO) | 21.5 | 18.3 | 60.2 | 5.1 | |
| (c) | |||||
| CON | CONKO | HF | HFKO | ||
| Krill oil | 0 | 8 | 0 | 8 | |
| Soybean oil | 8 | 0 | 8 | 0 | |
| Lard | 2 | 2 | 26 | 26 | |
| Casein | 21 | 21 | 27 | 27 | |
| L-cystine | 0.3 | 0.3 | 0.4 | 0.4 | |
| Corn starch | 40.5 | 40.5 | 0 | 0 | |
| Malodextrin | 10 | 10 | 16.5 | 16.5 | |
| Sucrose | 9 | 9 | 9 | 9 | |
| Cellulose | 3.7 | 3.7 | 6.6 | 6.6 | |
| Mineral mix, AIN-93G-MX | 3.5 | 3.5 | 4.5 | 4.5 | |
| Calcium phosphate, dibasic | 0.2 | 0.2 | 0.3 | 0.3 | |
| Vitamin mix, AIN-93-VX | 1.5 | 1.5 | 1.9 | 1.9 | |
| Choline bitrartrae | 0.3 | 0.3 | 0.3 | 0.3 | |
| (d) | |||||
| Krill Oil | Soybean Oil | Lard | |||
| Eicosapentanoic acid (20:5) | 15 | ─ | ─ | ||
| Docosahexaenoic acid (22:6) | 7 | ─ | ─ | ||
| Palmitic acid (16:0) | 23 | 10 | 23 | ||
| Stearic acid (18:0) | 1 | 4 | 13 | ||
| Oleic acid (18:1) | 18 | 18 | 39 | ||
| Linoleic acid (18:2) | 3 | 55 | 18 | ||
| Linolenic acid (18:3) | 1 | 13 | 1 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andraka, J.M.; Sharma, N.; Marchalant, Y. New Insights on the Effects of Krill Oil Supplementation, a High-Fat Diet, and Aging on Hippocampal-Dependent Memory, Neuroinflammation, Synaptic Density, and Neurogenesis. Int. J. Mol. Sci. 2024, 25, 11554. https://doi.org/10.3390/ijms252111554
Andraka JM, Sharma N, Marchalant Y. New Insights on the Effects of Krill Oil Supplementation, a High-Fat Diet, and Aging on Hippocampal-Dependent Memory, Neuroinflammation, Synaptic Density, and Neurogenesis. International Journal of Molecular Sciences. 2024; 25(21):11554. https://doi.org/10.3390/ijms252111554
Chicago/Turabian StyleAndraka, John M., Naveen Sharma, and Yannick Marchalant. 2024. "New Insights on the Effects of Krill Oil Supplementation, a High-Fat Diet, and Aging on Hippocampal-Dependent Memory, Neuroinflammation, Synaptic Density, and Neurogenesis" International Journal of Molecular Sciences 25, no. 21: 11554. https://doi.org/10.3390/ijms252111554
APA StyleAndraka, J. M., Sharma, N., & Marchalant, Y. (2024). New Insights on the Effects of Krill Oil Supplementation, a High-Fat Diet, and Aging on Hippocampal-Dependent Memory, Neuroinflammation, Synaptic Density, and Neurogenesis. International Journal of Molecular Sciences, 25(21), 11554. https://doi.org/10.3390/ijms252111554







