The Therapeutic Potential of Exosomes vs. Matrix-Bound Nanovesicles from Human Umbilical Cord Mesenchymal Stromal Cells in Osteoarthritis Treatment
Abstract
1. Introduction
2. Results
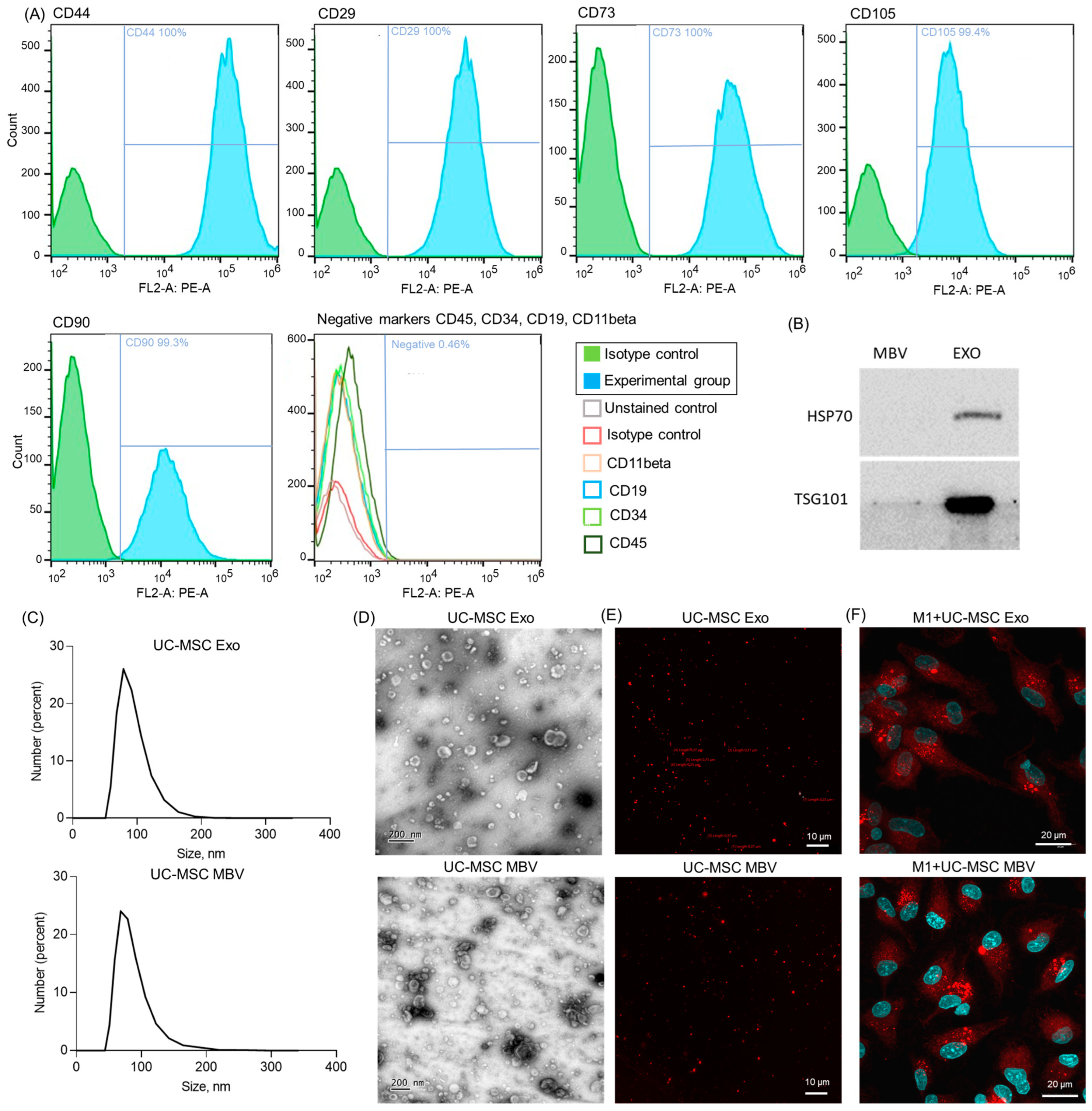
2.1. Immunophenotype Analysis of UC-MSC and Visualization of MBV and Exosomes from UC-MSC
2.2. Phagocytosis of Extracellular Vesicles (Exosomes and MBV from UC-MSC) by Human Macrophages Leads to Modulation of Pro-Inflammatory Cytokine Secretion
2.3. Impact of UC-MSC Exosomes and MBV on the Restoration of Articular Cartilage in Rats with OA
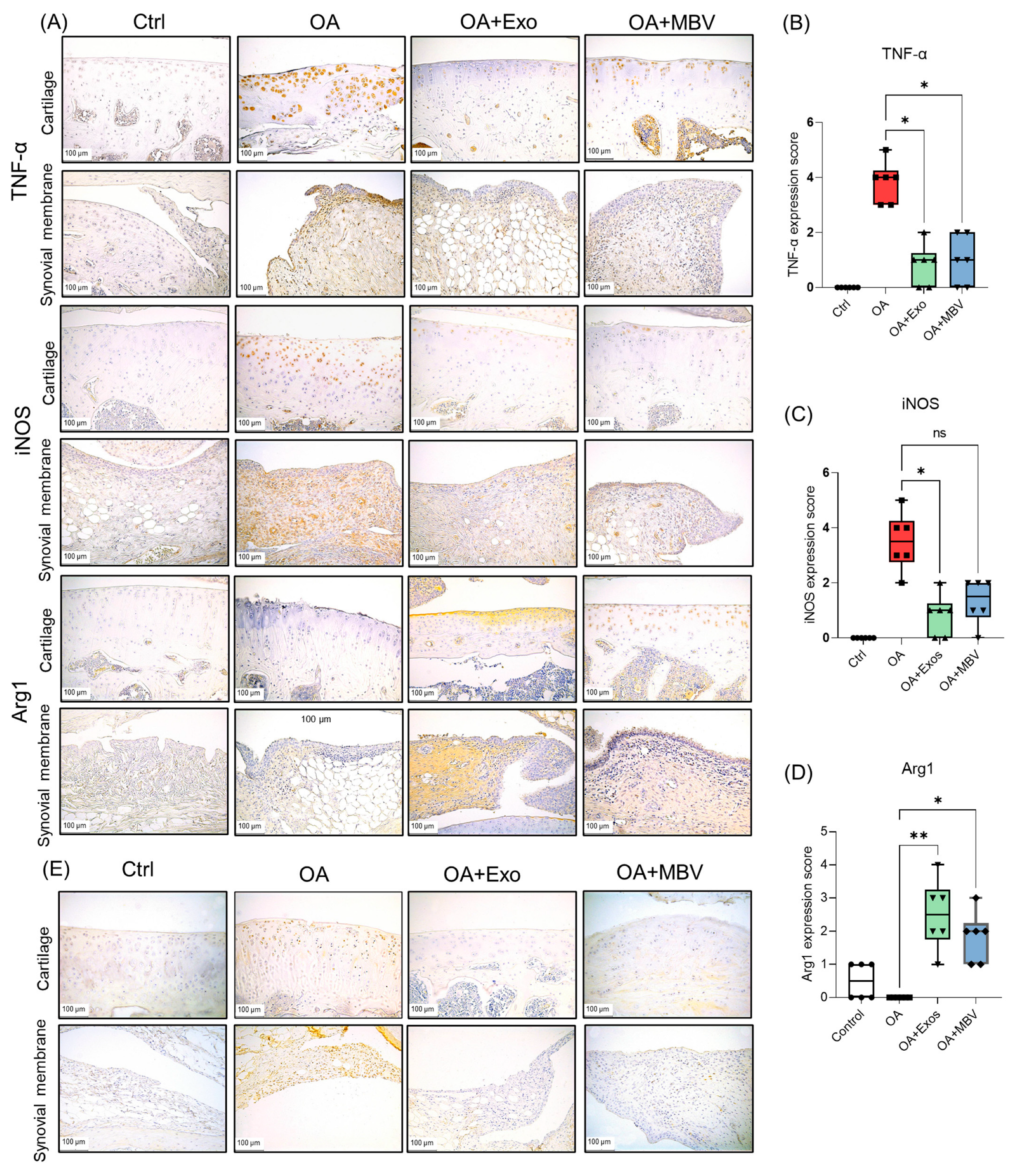
2.4. UC-MSC Exosomes More Effectively Reduce Inflammation in Synovial and Cartilage Tissue in Rats with OA Compared to MBV from UC-MSC
3. Discussion
4. Materials and Methods
4.1. Culturing of UC-MSC
4.2. Immunophenotype Analysis of UC-MSC
4.3. Isolation of MBV from ECM and Exosomes from the Conditioned Medium of UC-MSC
4.4. Evaluation of the Morphology and Size of UC-MSC Extracellular Vesicles
4.5. Analysis of Exosomal Markers in MBV and EV from UC-MSC by Western Blot
4.6. Staining of MBV and Exosomes from UC-MSC with the PKH26 Membrane Dye
4.7. Culturing Macrophages Derived from Peripheral Blood Mononuclear Cells (PBMC)
4.8. Phagocytosis of MDM of MBV or UC-MSC Exosomes Labeled with Membrane Dye PKH26
4.9. Quantitative Polymerase Chain Reaction with Reverse Transcription and ELISA
4.10. Induction of an OA Animal Model and Administration of EV
4.11. Histological Staining of Samples
4.12. Immunohistochemistry
4.13. In Situ Polymerase Chain Reaction
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Perruccio, A.V.; Young, J.J.; Wilfong, J.M.; Power, J.D.; Canizares, M.; Badley, E.M. Osteoarthritis Year in Review 2023: Epidemiology & Therapy. Osteoarthr. Cartil. 2024, 32, 159–165. [Google Scholar] [CrossRef]
- Hunter, D.J.; March, L.; Chew, M. Osteoarthritis in 2020 and beyond: A Lancet Commission. Lancet 2020, 396, 1711–1712. [Google Scholar] [CrossRef] [PubMed]
- Bo, K.; Xie, X.; Liu, X.; Ou, J.; Zhang, Y.; Wang, X.; Yang, S.; Zhang, W.; Zhang, L.; Chang, J. Predicting Incident Radiographic Knee Osteoarthritis through Quantitative Meniscal Lesion Parameters: Data from the Osteoarthritis Initiative. BMC Musculoskelet. Disord. 2024, 25, 626. [Google Scholar] [CrossRef] [PubMed]
- Pettenuzzo, S.; Arduino, A.; Belluzzi, E.; Pozzuoli, A.; Fontanella, C.G.; Ruggieri, P.; Salomoni, V.; Majorana, C.; Berardo, A. Biomechanics of Chondrocytes and Chondrons in Healthy Conditions and Osteoarthritis: A Review of the Mechanical Characterisations at the Microscale. Biomedicines 2023, 11, 1942. [Google Scholar] [CrossRef] [PubMed]
- Fontanella, C.G.; Belluzzi, E.; Pozzuoli, A.; Favero, M.; Ruggieri, P.; Macchi, V.; Carniel, E.L. Mechanical Behavior of Infrapatellar Fat Pad of Patients Affected by Osteoarthritis. J. Biomech. 2022, 131, 110931. [Google Scholar] [CrossRef]
- van den Bosch, M.H.J. Inflammation in Osteoarthritis: Is It Time to Dampen the Alarm(in) in This Debilitating Disease? Clin. Exp. Immunol. 2019, 195, 153–166. [Google Scholar] [CrossRef]
- Peshkova, M.; Kosheleva, N.; Shpichka, A.; Radenska-Lopovok, S.; Telyshev, D.; Lychagin, A.; Li, F.; Timashev, P.; Liang, X.-J. Targeting Inflammation and Regeneration: Scaffolds, Extracellular Vesicles, and Nanotechnologies as Cell-Free Dual-Target Therapeutic Strategies. Int. J. Mol. Sci. 2022, 23, 13796. [Google Scholar] [CrossRef]
- Braun, S.; Zaucke, F.; Brenneis, M.; Rapp, A.E.; Pollinger, P.; Sohn, R.; Jenei-Lanzl, Z.; Meurer, A. The Corpus Adiposum Infrapatellare (Hoffa’s Fat Pad)—The Role of the Infrapatellar Fat Pad in Osteoarthritis Pathogenesis. Biomedicines 2022, 10, 1071. [Google Scholar] [CrossRef]
- Ioan-Facsinay, A.; Kloppenburg, M. An Emerging Player in Knee Osteoarthritis: The Infrapatellar Fat Pad. Arthritis Res. Ther. 2013, 15, 225. [Google Scholar] [CrossRef]
- Lambert, C.; Zappia, J.; Sanchez, C.; Florin, A.; Dubuc, J.-E.; Henrotin, Y. The Damage-Associated Molecular Patterns (DAMPs) as Potential Targets to Treat Osteoarthritis: Perspectives From a Review of the Literature. Front. Med. 2020, 7, 607186. [Google Scholar] [CrossRef]
- Chung, H.Y.; Kim, D.H.; Lee, E.K.; Chung, K.W.; Chung, S.; Lee, B.; Seo, A.Y.; Chung, J.H.; Jung, Y.S.; Im, E.; et al. Redefining Chronic Inflammation in Aging and Age-Related Diseases: Proposal of the Senoinflammation Concept. Aging Dis. 2019, 10, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.E.; Miller, R.J.; Malfait, A.-M. Osteoarthritis Joint Pain: The Cytokine Connection. Cytokine 2014, 70, 185–193. [Google Scholar] [CrossRef]
- Neogi, T. Clinical Significance of Bone Changes in Osteoarthritis. Ther. Adv. Musculoskelet. Dis. 2012, 4, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Bannuru, R.R.; Osani, M.C.; Vaysbrot, E.E.; Arden, N.K.; Bennell, K.; Bierma-Zeinstra, S.M.A.; Kraus, V.B.; Lohmander, L.S.; Abbott, J.H.; Bhandari, M.; et al. OARSI Guidelines for the Non-Surgical Management of Knee, Hip, and Polyarticular Osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1578–1589. [Google Scholar] [CrossRef]
- Kloppenburg, M.; Berenbaum, F. Osteoarthritis Year in Review 2019: Epidemiology and Therapy. Osteoarthr. Cartil. 2020, 28, 242–248. [Google Scholar] [CrossRef]
- Tschopp, M.; Pfirrmann, C.W.A.; Fucentese, S.F.; Brunner, F.; Catanzaro, S.; Kühne, N.; Zwyssig, I.; Sutter, R.; Götschi, T.; Tanadini, M.; et al. A Randomized Trial of Intra-Articular Injection Therapy for Knee Osteoarthritis. Investig. Radiol. 2023, 58, 355–362. [Google Scholar] [CrossRef]
- Bannuru, R.R.; Schmid, C.H.; Kent, D.M.; Vaysbrot, E.E.; Wong, J.B.; McAlindon, T.E. Comparative Effectiveness of Pharmacologic Interventions for Knee Osteoarthritis: A Systematic Review and Network Meta-Analysis. Ann. Intern. Med. 2015, 162, 46–54. [Google Scholar] [CrossRef]
- Rahmani Del Bakhshayesh, A.; Babaie, S.; Tayefi Nasrabadi, H.; Asadi, N.; Akbarzadeh, A.; Abedelahi, A. An Overview of Various Treatment Strategies, Especially Tissue Engineering for Damaged Articular Cartilage. Artif. Cells Nanomed. Biotechnol. 2020, 48, 1089–1104. [Google Scholar] [CrossRef] [PubMed]
- Velot, É.; Madry, H.; Venkatesan, J.K.; Bianchi, A.; Cucchiarini, M. Is Extracellular Vesicle-Based Therapy the Next Answer for Cartilage Regeneration? Front. Bioeng. Biotechnol. 2021, 9, 645039. [Google Scholar] [CrossRef]
- Klimak, M.; Nims, R.J.; Pferdehirt, L.; Collins, K.H.; Harasymowicz, N.S.; Oswald, S.J.; Setton, L.A.; Guilak, F. Immunoengineering the next Generation of Arthritis Therapies. Acta Biomater. 2021, 133, 74–86. [Google Scholar] [CrossRef]
- Colombini, A.; Libonati, F.; Lopa, S.; Peretti, G.M.; Moretti, M.; de Girolamo, L. Autologous Chondrocyte Implantation Provides Good Long-Term Clinical Results in the Treatment of Knee Osteoarthritis: A Systematic Review. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 2023, 31, 2338–2348. [Google Scholar] [CrossRef] [PubMed]
- Steinert, A.F.; Ghivizzani, S.C.; Rethwilm, A.; Tuan, R.S.; Evans, C.H.; Nöth, U. Major Biological Obstacles for Persistent Cell-Based Regeneration of Articular Cartilage. Arthritis Res. Ther. 2007, 9, 213. [Google Scholar] [CrossRef]
- Naji, A.; Eitoku, M.; Favier, B.; Deschaseaux, F.; Rouas-Freiss, N.; Suganuma, N. Biological Functions of Mesenchymal Stem Cells and Clinical Implications. Cell. Mol. Life Sci. CMLS 2019, 76, 3323–3348. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, L. Stem Cell Senescence: The Obstacle of the Treatment of Degenerative Disk Disease. Curr. Stem Cell Res. Ther. 2019, 14, 654–668. [Google Scholar] [CrossRef] [PubMed]
- Moradi, L.; Vasei, M.; Dehghan, M.M.; Majidi, M.; Farzad Mohajeri, S.; Bonakdar, S. Regeneration of Meniscus Tissue Using Adipose Mesenchymal Stem Cells-Chondrocytes Co-Culture on a Hybrid Scaffold: In Vivo Study. Biomaterials 2017, 126, 18–30. [Google Scholar] [CrossRef]
- Hamdalla, H.M.; Ahmed, R.R.; Galaly, S.R.; Ahmed, O.M.; Naguib, I.A.; Alghamdi, B.S.; Abdul-Hamid, M. Assessment of the Efficacy of Bone Marrow-Derived Mesenchymal Stem Cells against a Monoiodoacetate-Induced Osteoarthritis Model in Wistar Rats. Stem Cells Int. 2022, 2022, 1900403. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, R.-J.; Wu, Y.; Huang, D.; Li, Y.; Liu, Y. Advances in Stem Cell-Based Therapies in the Treatment of Osteoarthritis. Int. J. Mol. Sci. 2023, 25, 394. [Google Scholar] [CrossRef] [PubMed]
- Volarevic, V.; Markovic, B.S.; Gazdic, M.; Volarevic, A.; Jovicic, N.; Arsenijevic, N.; Armstrong, L.; Djonov, V.; Lako, M.; Stojkovic, M. Ethical and Safety Issues of Stem Cell-Based Therapy. Int. J. Med. Sci. 2018, 15, 36–45. [Google Scholar] [CrossRef]
- Gazdic, M.; Volarevic, V.; Arsenijevic, N.; Stojkovic, M. Mesenchymal Stem Cells: A Friend or Foe in Immune-Mediated Diseases. Stem Cell Rev. Rep. 2015, 11, 280–287. [Google Scholar] [CrossRef]
- Abreu, H.; Canciani, E.; Raineri, D.; Cappellano, G.; Rimondini, L.; Chiocchetti, A. Extracellular Vesicles in Musculoskeletal Regeneration: Modulating the Therapy of the Future. Cells 2021, 11, 43. [Google Scholar] [CrossRef]
- Kim, Y.G.; Choi, J.; Kim, K. Mesenchymal Stem Cell-Derived Exosomes for Effective Cartilage Tissue Repair and Treatment of Osteoarthritis. Biotechnol. J. 2020, 15, e2000082. [Google Scholar] [CrossRef] [PubMed]
- Ocansey, D.K.W.; Zhang, L.; Wang, Y.; Yan, Y.; Qian, H.; Zhang, X.; Xu, W.; Mao, F. Exosome-Mediated Effects and Applications in Inflammatory Bowel Disease. Biol. Rev. Camb. Philos. Soc. 2020, 95, 1287–1307. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The Biology, Function, and Biomedical Applications of Exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Siljander, P.R.-M.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological Properties of Extracellular Vesicles and Their Physiological Functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed]
- Woo, C.H.; Kim, H.K.; Jung, G.Y.; Jung, Y.J.; Lee, K.S.; Yun, Y.E.; Han, J.; Lee, J.; Kim, W.S.; Choi, J.S.; et al. Small Extracellular Vesicles from Human Adipose-Derived Stem Cells Attenuate Cartilage Degeneration. J. Extracell. Vesicles 2020, 9, 1735249. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Cai, Z.; Zhou, Q.; Li, L.; Fu, P. Exosomes from Human Umbilical Cord Mesenchymal Stem Cells Inhibit ROS Production and Cell Apoptosis in Human Articular Chondrocytes via the miR-100-5p/NOX4 Axis. Cell Biol. Int. 2021, 45, 2096–2106. [Google Scholar] [CrossRef]
- Yan, L.; Wu, X. Exosomes Produced from 3D Cultures of Umbilical Cord Mesenchymal Stem Cells in a Hollow-Fiber Bioreactor Show Improved Osteochondral Regeneration Activity. Cell Biol. Toxicol. 2020, 36, 165. [Google Scholar] [CrossRef]
- Huleihel, L.; Hussey, G.S.; Naranjo, J.D.; Zhang, L.; Dziki, J.L.; Turner, N.J.; Stolz, D.B.; Badylak, S.F. Matrix-Bound Nanovesicles within ECM Bioscaffolds. Sci. Adv. 2016, 2, e1600502. [Google Scholar] [CrossRef]
- van Niel, G.; Charrin, S.; Simoes, S.; Romao, M.; Rochin, L.; Saftig, P.; Marks, M.S.; Rubinstein, E.; Raposo, G. The Tetraspanin CD63 Regulates ESCRT-Independent and -Dependent Endosomal Sorting during Melanogenesis. Dev. Cell 2011, 21, 708–721. [Google Scholar] [CrossRef]
- Piening, L.M.; Wachs, R.A. Matrix-Bound Nanovesicles: What Are They and What Do They Do? Cells Tissues Organs 2023, 212, 111–123. [Google Scholar] [CrossRef]
- Peshkova, M.; Korneev, A.; Revokatova, D.; Smirnova, O.; Klyucherev, T.; Shender, V.; Arapidi, G.; Kosheleva, N.; Timashev, P. Four Sides to the Story: A Proteomic Comparison of Liquid-Phase and Matrix-Bound Extracellular Vesicles in 2D and 3D Cell Cultures. Proteomics 2024, 24, e2300375. [Google Scholar] [CrossRef] [PubMed]
- Hussey, G.S.; Pineda Molina, C.; Cramer, M.C.; Tyurina, Y.Y.; Tyurin, V.A.; Lee, Y.C.; El-Mossier, S.O.; Murdock, M.H.; Timashev, P.S.; Kagan, V.E.; et al. Lipidomics and RNA Sequencing Reveal a Novel Subpopulation of Nanovesicle within Extracellular Matrix Biomaterials. Sci. Adv. 2020, 6, eaay4361. [Google Scholar] [CrossRef] [PubMed]
- Hussey, G.S.; Dziki, J.L.; Lee, Y.C.; Bartolacci, J.G.; Behun, M.; Turnquist, H.R.; Badylak, S.F. Matrix Bound Nanovesicle-Associated IL-33 Activates a pro-Remodeling Macrophage Phenotype via a Non-Canonical, ST2-Independent Pathway. J. Immunol. Regen. Med. 2019, 3, 26–35. [Google Scholar] [CrossRef]
- Huleihel, L.; Bartolacci, J.G.; Dziki, J.L.; Vorobyov, T.; Arnold, B.; Scarritt, M.E.; Pineda Molina, C.; LoPresti, S.T.; Brown, B.N.; Naranjo, J.D.; et al. Matrix-Bound Nanovesicles Recapitulate Extracellular Matrix Effects on Macrophage Phenotype. Tissue Eng. Part A 2017, 23, 1283–1294. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, W.; Yong, H.; He, M.; Yang, Y.; Deng, Z.; Li, Y. Macrophages in Osteoarthritis: Pathophysiology and Therapeutics. Am. J. Transl. Res. 2020, 12, 261–268. [Google Scholar] [PubMed]
- He, L.; He, T.; Xing, J.; Zhou, Q.; Fan, L.; Liu, C.; Chen, Y.; Wu, D.; Tian, Z.; Liu, B.; et al. Bone Marrow Mesenchymal Stem Cell-Derived Exosomes Protect Cartilage Damage and Relieve Knee Osteoarthritis Pain in a Rat Model of Osteoarthritis. Stem Cell Res. Ther. 2020, 11, 276. [Google Scholar] [CrossRef]
- Cosenza, S.; Ruiz, M.; Toupet, K.; Jorgensen, C.; Noël, D. Mesenchymal Stem Cells Derived Exosomes and Microparticles Protect Cartilage and Bone from Degradation in Osteoarthritis. Sci. Rep. 2017, 7, 16214. [Google Scholar] [CrossRef]
- Zavatti, M.; Beretti, F.; Casciaro, F.; Bertucci, E.; Maraldi, T. Comparison of the Therapeutic Effect of Amniotic Fluid Stem Cells and Their Exosomes on Monoiodoacetate-Induced Animal Model of Osteoarthritis. BioFactors Oxf. Engl. 2020, 46, 106–117. [Google Scholar] [CrossRef]
- Zhou, H.; Shen, X.; Yan, C.; Xiong, W.; Ma, Z.; Tan, Z.; Wang, J.; Li, Y.; Liu, J.; Duan, A.; et al. Extracellular Vesicles Derived from Human Umbilical Cord Mesenchymal Stem Cells Alleviate Osteoarthritis of the Knee in Mice Model by Interacting with METTL3 to Reduce m6A of NLRP3 in Macrophage. Stem Cell Res. Ther. 2022, 13, 322. [Google Scholar] [CrossRef]
- Domenis, R.; Cifù, A.; Quaglia, S.; Pistis, C.; Moretti, M.; Vicario, A.; Parodi, P.C.; Fabris, M.; Niazi, K.R.; Soon-Shiong, P.; et al. Pro Inflammatory Stimuli Enhance the Immunosuppressive Functions of Adipose Mesenchymal Stem Cells-Derived Exosomes. Sci. Rep. 2018, 8, 13325. [Google Scholar] [CrossRef]
- Rong, Y.; Zhang, J.; Jiang, D.; Ji, C.; Liu, W.; Wang, J.; Ge, X.; Tang, P.; Yu, S.; Cui, W.; et al. Hypoxic Pretreatment of Small Extracellular Vesicles Mediates Cartilage Repair in Osteoarthritis by Delivering miR-216a-5p. Acta Biomater. 2021, 122, 325–342. [Google Scholar] [CrossRef] [PubMed]
- Hanai, H.; Hart, D.A.; Jacob, G.; Shimomura, K.; Ando, W.; Yoshioka, Y.; Ochiya, T.; Nakagawa, S.; Nakamura, M.; Okada, S.; et al. Small Extracellular Vesicles Derived from Human Adipose-derived Mesenchymal Stromal Cells Cultured in a New Chemically-defined Contaminate-free Media Exhibit Enhanced Biological and Therapeutic Effects on Human Chondrocytes in Vitro and in a Mouse Osteoarthritis Model. J. Extracell. Vesicles 2023, 12, 12337. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lee, Y.; Johansson, H.J.; Mäger, I.; Vader, P.; Nordin, J.Z.; Wiklander, O.P.B.; Lehtiö, J.; Wood, M.J.A.; Andaloussi, S.E. Serum-Free Culture Alters the Quantity and Protein Composition of Neuroblastoma-Derived Extracellular Vesicles. J. Extracell. Vesicles 2015, 4, 26883. [Google Scholar] [CrossRef] [PubMed]
- Forteza-Genestra, M.A.; Antich-Rosselló, M.; Calvo, J.; Gayà, A.; Monjo, M.; Ramis, J.M. Purity Determines the Effect of Extracellular Vesicles Derived from Mesenchymal Stromal Cells. Cells 2020, 9, 422. [Google Scholar] [CrossRef]
- Jin, H.J.; Bae, Y.K.; Kim, M.; Kwon, S.-J.; Jeon, H.B.; Choi, S.J.; Kim, S.W.; Yang, Y.S.; Oh, W.; Chang, J.W. Comparative Analysis of Human Mesenchymal Stem Cells from Bone Marrow, Adipose Tissue, and Umbilical Cord Blood as Sources of Cell Therapy. Int. J. Mol. Sci. 2013, 14, 17986–18001. [Google Scholar] [CrossRef]
- Wang, G.; Yuan, J.; Cai, X.; Xu, Z.; Wang, J.; Ocansey, D.K.W.; Yan, Y.; Qian, H.; Zhang, X.; Xu, W.; et al. HucMSC-Exosomes Carrying miR-326 Inhibit Neddylation to Relieve Inflammatory Bowel Disease in Mice. Clin. Transl. Med. 2020, 10, e113. [Google Scholar] [CrossRef]
- Suleimanov, S.K.; Efremov, Y.M.; Klyucherev, T.O.; Salimov, E.L.; Ragimov, A.A.; Timashev, P.S.; Vlasova, I.I. Radical-Generating Activity, Phagocytosis, and Mechanical Properties of Four Phenotypes of Human Macrophages. Int. J. Mol. Sci. 2024, 25, 1860. [Google Scholar] [CrossRef]
- Olivotto, E.; Minguzzi, M.; D’Adamo, S.; Astolfi, A.; Santi, S.; Uguccioni, M.; Marcu, K.B.; Borzì, R.M. Basal and IL-1β Enhanced Chondrocyte Chemotactic Activity on Monocytes Are Co-Dependent on Both IKKα and IKKβ NF-κB Activating Kinases. Sci. Rep. 2021, 11, 21697. [Google Scholar] [CrossRef]
- Goldring, M.B.; Otero, M.; Tsuchimochi, K.; Ijiri, K.; Li, Y. Defining the Roles of Inflammatory and Anabolic Cytokines in Cartilage Metabolism. Ann. Rheum. Dis. 2008, 67 (Suppl. S3), iii75–iii82. [Google Scholar] [CrossRef]
- Zhou, S.; Maleitzke, T.; Geissler, S.; Hildebrandt, A.; Fleckenstein, F.N.; Niemann, M.; Fischer, H.; Perka, C.; Duda, G.N.; Winkler, T. Source and Hub of Inflammation: The Infrapatellar Fat Pad and Its Interactions with Articular Tissues during Knee Osteoarthritis. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2022, 40, 1492–1504. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Duong, C.M.; Nguyen, X.-H.; Than, U.T.T. Mesenchymal Stem Cell-Derived Extracellular Vesicles for Osteoarthritis Treatment: Extracellular Matrix Protection, Chondrocyte and Osteocyte Physiology, Pain and Inflammation Management. Cells 2021, 10, 2887. [Google Scholar] [CrossRef] [PubMed]
- Burrello, J.; Monticone, S.; Gai, C.; Gomez, Y.; Kholia, S.; Camussi, G. Stem Cell-Derived Extracellular Vesicles and Immune-Modulation. Front. Cell Dev. Biol. 2016, 4, 83. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.D. Anatomy of a Discovery: M1 and M2 Macrophages. Front. Immunol. 2015, 6, 212. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liu, A.; Li, X.; Sun, Z.; Sun, Z.; Liu, Y.; Wang, G.; Huang, D.; Xiong, H.; Yu, S.; et al. Dual-Engineered Cartilage-Targeting Extracellular Vesicles Derived from Mesenchymal Stem Cells Enhance Osteoarthritis Treatment via miR-223/NLRP3/Pyroptosis Axis: Toward a Precision Therapy. Bioact. Mater. 2023, 30, 169–183. [Google Scholar] [CrossRef]
- Lo Sicco, C.; Reverberi, D.; Balbi, C.; Ulivi, V.; Principi, E.; Pascucci, L.; Becherini, P.; Bosco, M.C.; Varesio, L.; Franzin, C.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles as Mediators of Anti-Inflammatory Effects: Endorsement of Macrophage Polarization. Stem Cells Transl. Med. 2017, 6, 1018–1028. [Google Scholar] [CrossRef]
- Ragni, E.; Papait, A.; Perucca Orfei, C.; Silini, A.R.; Colombini, A.; Viganò, M.; Libonati, F.; Parolini, O.; de Girolamo, L. Amniotic Membrane-Mesenchymal Stromal Cells Secreted Factors and Extracellular Vesicle-miRNAs: Anti-Inflammatory and Regenerative Features for Musculoskeletal Tissues. Stem Cells Transl. Med. 2021, 10, 1044–1062. [Google Scholar] [CrossRef]
- Dong, J.; Li, L.; Fang, X.; Zang, M. Exosome-Encapsulated microRNA-127-3p Released from Bone Marrow-Derived Mesenchymal Stem Cells Alleviates Osteoarthritis Through Regulating CDH11-Mediated Wnt/β-Catenin Pathway. J. Pain Res. 2021, 14, 297–310. [Google Scholar] [CrossRef]
- Li, S.; Stöckl, S.; Lukas, C.; Götz, J.; Herrmann, M.; Federlin, M.; Grässel, S. hBMSC-Derived Extracellular Vesicles Attenuate IL-1β-Induced Catabolic Effects on OA-Chondrocytes by Regulating Pro-Inflammatory Signaling Pathways. Front. Bioeng. Biotechnol. 2020, 8, 603598. [Google Scholar] [CrossRef]
- Cavallo, C.; Merli, G.; Borzì, R.M.; Zini, N.; D’Adamo, S.; Guescini, M.; Grigolo, B.; Di Martino, A.; Santi, S.; Filardo, G. Small Extracellular Vesicles from Adipose Derived Stromal Cells Significantly Attenuate in Vitro the NF-κB Dependent Inflammatory/Catabolic Environment of Osteoarthritis. Sci. Rep. 2021, 11, 1053. [Google Scholar] [CrossRef]
- Vergadi, E.; Vaporidi, K.; Theodorakis, E.E.; Doxaki, C.; Lagoudaki, E.; Ieronymaki, E.; Alexaki, V.I.; Helms, M.; Kondili, E.; Soennichsen, B.; et al. Akt2 Deficiency Protects from Acute Lung Injury via Alternative Macrophage Activation and miR-146a Induction in Mice. J. Immunol. Baltim. Md 1950 2014, 192, 394–406. [Google Scholar] [CrossRef]
- Liacini, A.; Sylvester, J.; Li, W.Q.; Zafarullah, M. Inhibition of Interleukin-1-Stimulated MAP Kinases, Activating Protein-1 (AP-1) and Nuclear Factor Kappa B (NF-Kappa B) Transcription Factors down-Regulates Matrix Metalloproteinase Gene Expression in Articular Chondrocytes. Matrix Biol. J. Int. Soc. Matrix Biol. 2002, 21, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Capelli, C.; Gotti, E.; Morigi, M.; Rota, C.; Weng, L.; Dazzi, F.; Spinelli, O.; Cazzaniga, G.; Trezzi, R.; Gianatti, A.; et al. Minimally Manipulated Whole Human Umbilical Cord Is a Rich Source of Clinical-Grade Human Mesenchymal Stromal Cells Expanded in Human Platelet Lysate. Cytotherapy 2011, 13, 786–801. [Google Scholar] [CrossRef] [PubMed]
- You, D.G.; Lim, G.T.; Kwon, S.; Um, W.; Oh, B.H.; Song, S.H.; Lee, J.; Jo, D.-G.; Cho, Y.W.; Park, J.H. Metabolically Engineered Stem Cell-Derived Exosomes to Regulate Macrophage Heterogeneity in Rheumatoid Arthritis. Sci. Adv. 2021, 7, eabe0083. [Google Scholar] [CrossRef]
- Wang, R.; Xu, B. TGF-Β1-Modified MSC-Derived Exosomal miR-135b Attenuates Cartilage Injury via Promoting M2 Synovial Macrophage Polarization by Targeting MAPK6. Cell Tissue Res. 2021, 384, 113–127. [Google Scholar] [CrossRef]
- Bartolacci, J.G.; Behun, M.N.; Warunek, J.P.; Li, T.; Sahu, A.; Dwyer, G.K.; Lucas, A.; Rong, J.; Ambrosio, F.; Turnquist, H.R.; et al. Matrix-Bound Nanovesicle-Associated IL-33 Supports Functional Recovery after Skeletal Muscle Injury by Initiating a pro-Regenerative Macrophage Phenotypic Transition. NPJ Regen. Med. 2024, 9, 7. [Google Scholar] [CrossRef]
- Cramer, M.; Pineda Molina, C.; Hussey, G.; Turnquist, H.R.; Badylak, S.F. Transcriptomic Regulation of Macrophages by Matrix-Bound Nanovesicle-Associated Interleukin-33. Tissue Eng. Part A 2022, 28, 867–878. [Google Scholar] [CrossRef]
- Crum, R.J.; Hall, K.; Molina, C.P.; Hussey, G.S.; Graham, E.; Li, H.; Badylak, S.F. Immunomodulatory Matrix-Bound Nanovesicles Mitigate Acute and Chronic Pristane-Induced Rheumatoid Arthritis. NPJ Regen. Med. 2022, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Peshkova, M.; Korneev, A.; Suleimanov, S.; Vlasova, I.I.; Svistunov, A.; Kosheleva, N.; Timashev, P. MSCs’ Conditioned Media Cytokine and Growth Factor Profiles and Their Impact on Macrophage Polarization. Stem Cell Res. Ther. 2023, 14, 142. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, K.; Muneta, T.; Ozeki, N.; Nakagawa, Y.; Udo, M.; Saito, R.; Koga, H.; Tsuji, K.; Sekiya, I. Weekly Injections of Hylan G-F 20 Delay Cartilage Degeneration in Partial Meniscectomized Rat Knees. BMC Musculoskelet. Disord. 2016, 17, 188. [Google Scholar] [CrossRef]
- Leary, S.; Underwood, W.J.; Anthony, R.; Cartner, S.; Corey, D.; Grandin, T.; Greenacre, C.; Gwaltney-Bran, S.; Mccrackin, M.; Meyer, R.; et al. AVMA Guidelines for the Euthanasia of Animals 2013 ed. J. Am. Vet. Med. Assoc. 2013, 1–102. [Google Scholar]
- Moody, H.R.; Heard, B.J.; Frank, C.B.; Shrive, N.G.; Oloyede, A.O. Investigating the Potential Value of Individual Parameters of Histological Grading Systems in a Sheep Model of Cartilage Damage: The Modified Mankin Method. J. Anat. 2012, 221, 47–54. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klyucherev, T.O.; Peshkova, M.A.; Revokatova, D.P.; Serejnikova, N.B.; Fayzullina, N.M.; Fayzullin, A.L.; Ershov, B.P.; Khristidis, Y.I.; Vlasova, I.I.; Kosheleva, N.V.; et al. The Therapeutic Potential of Exosomes vs. Matrix-Bound Nanovesicles from Human Umbilical Cord Mesenchymal Stromal Cells in Osteoarthritis Treatment. Int. J. Mol. Sci. 2024, 25, 11564. https://doi.org/10.3390/ijms252111564
Klyucherev TO, Peshkova MA, Revokatova DP, Serejnikova NB, Fayzullina NM, Fayzullin AL, Ershov BP, Khristidis YI, Vlasova II, Kosheleva NV, et al. The Therapeutic Potential of Exosomes vs. Matrix-Bound Nanovesicles from Human Umbilical Cord Mesenchymal Stromal Cells in Osteoarthritis Treatment. International Journal of Molecular Sciences. 2024; 25(21):11564. https://doi.org/10.3390/ijms252111564
Chicago/Turabian StyleKlyucherev, Timofey O., Maria A. Peshkova, Daria P. Revokatova, Natalia B. Serejnikova, Nafisa M. Fayzullina, Alexey L. Fayzullin, Boris P. Ershov, Yana I. Khristidis, Irina I. Vlasova, Nastasia V. Kosheleva, and et al. 2024. "The Therapeutic Potential of Exosomes vs. Matrix-Bound Nanovesicles from Human Umbilical Cord Mesenchymal Stromal Cells in Osteoarthritis Treatment" International Journal of Molecular Sciences 25, no. 21: 11564. https://doi.org/10.3390/ijms252111564
APA StyleKlyucherev, T. O., Peshkova, M. A., Revokatova, D. P., Serejnikova, N. B., Fayzullina, N. M., Fayzullin, A. L., Ershov, B. P., Khristidis, Y. I., Vlasova, I. I., Kosheleva, N. V., Svistunov, A. A., & Timashev, P. S. (2024). The Therapeutic Potential of Exosomes vs. Matrix-Bound Nanovesicles from Human Umbilical Cord Mesenchymal Stromal Cells in Osteoarthritis Treatment. International Journal of Molecular Sciences, 25(21), 11564. https://doi.org/10.3390/ijms252111564







