Poly-D,L-Lactic Acid Filler Attenuates Ultraviolet B-Induced Skin Pigmentation by Reducing Destruction of the Basement Membrane
Abstract
:1. Introduction
2. Results
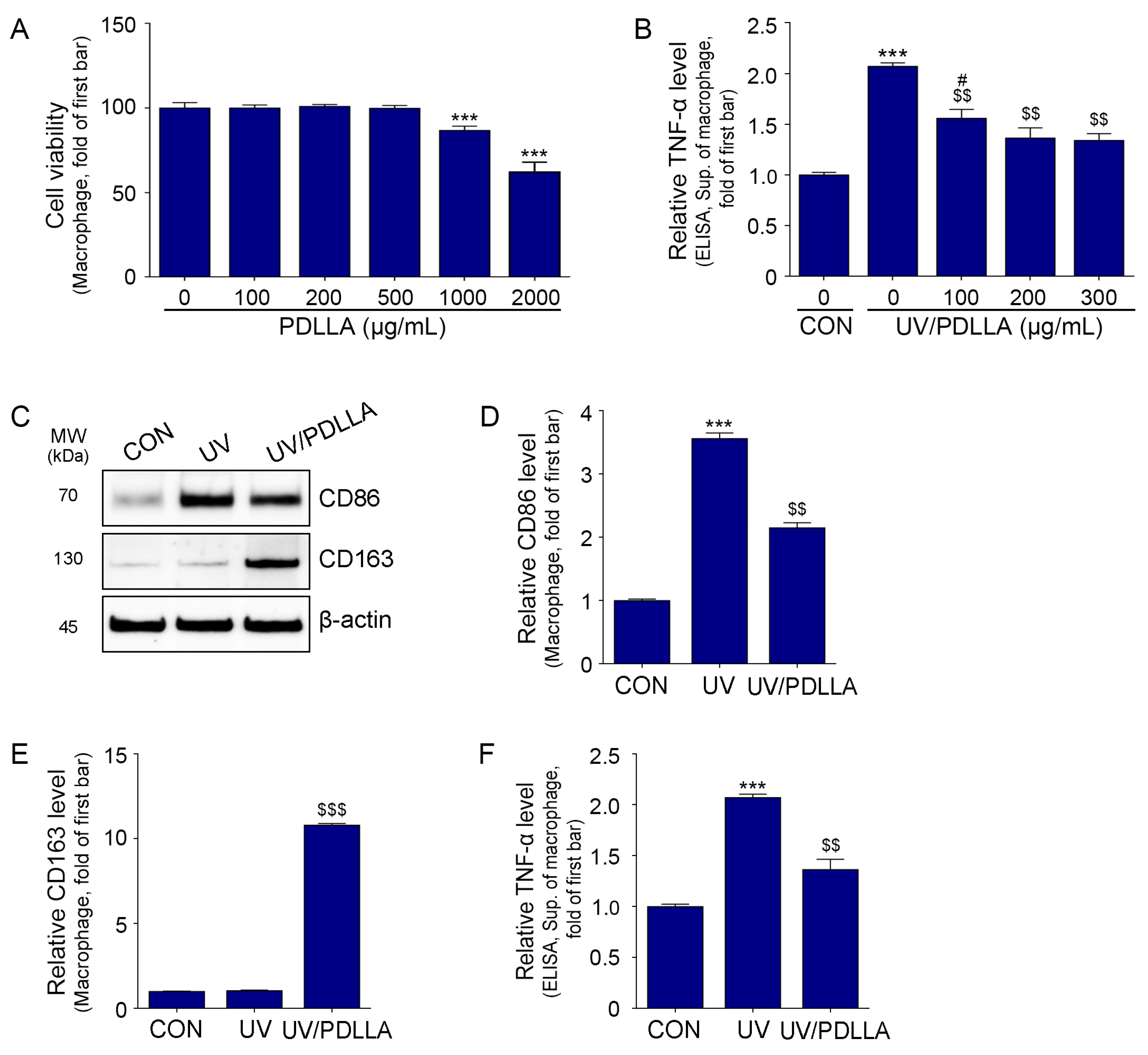
2.1. PDLLA Decreased Expression of CD86 and TNF-α in UV-Irradiated Macrophages
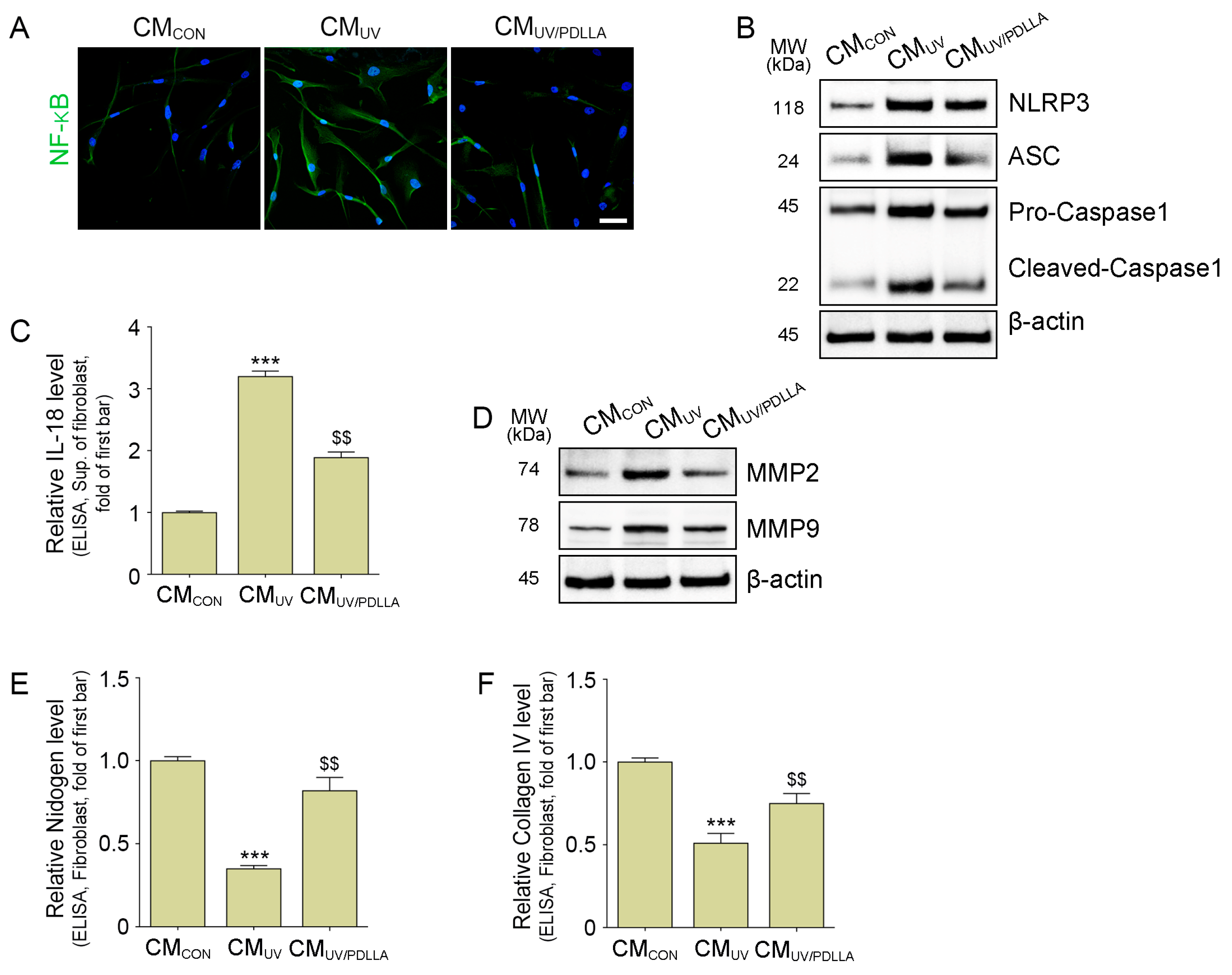
2.2. PDLLA Decreased the Translocation of NF-κB, Formation of NLRP3 Inflammasome, and Expression of MMP2 and MMP9 in Fibroblasts
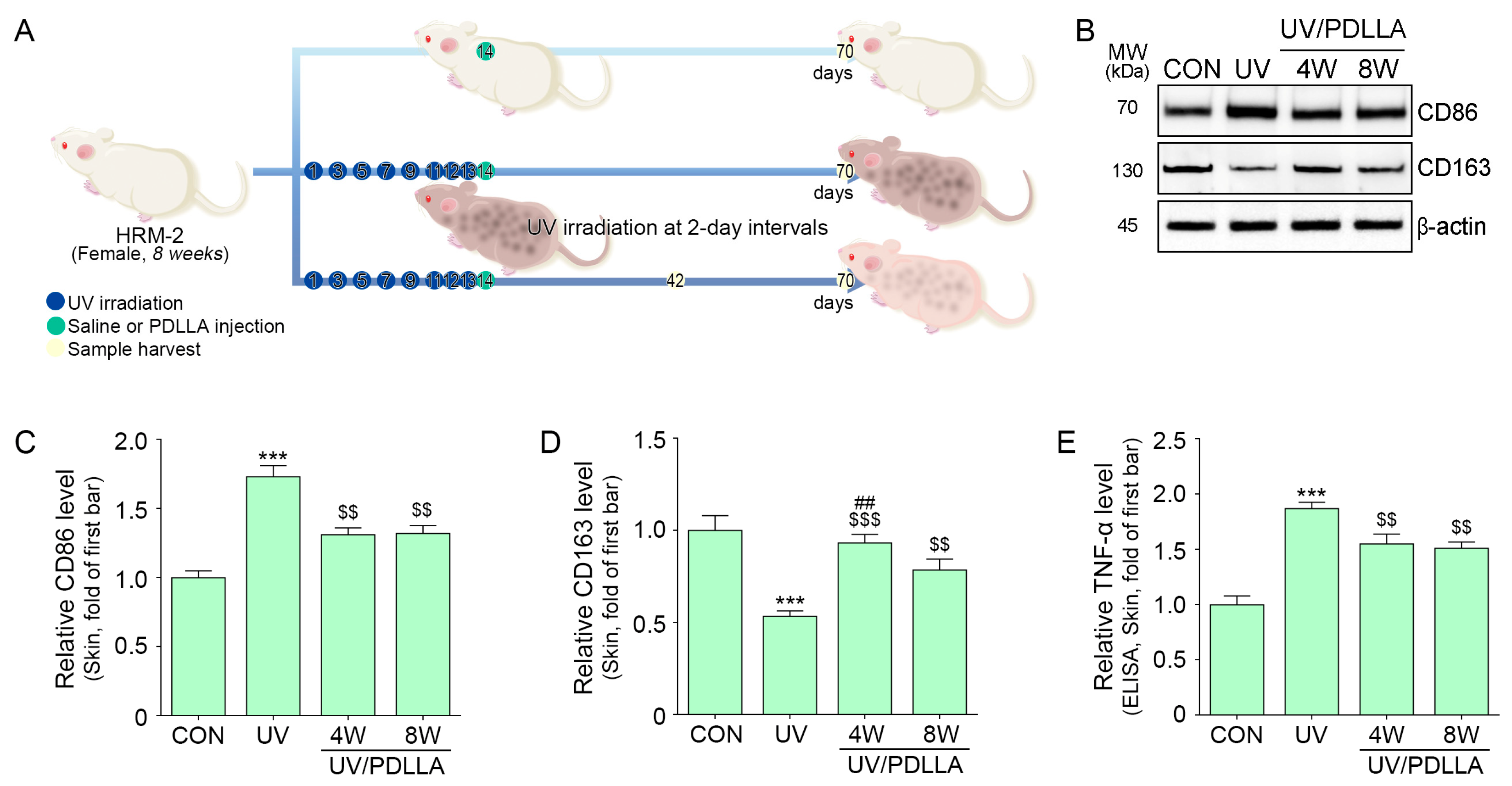
2.3. PDLLA Decreased Expression of CD86 and TNF-α in UV-Irradiated Mouse Skin
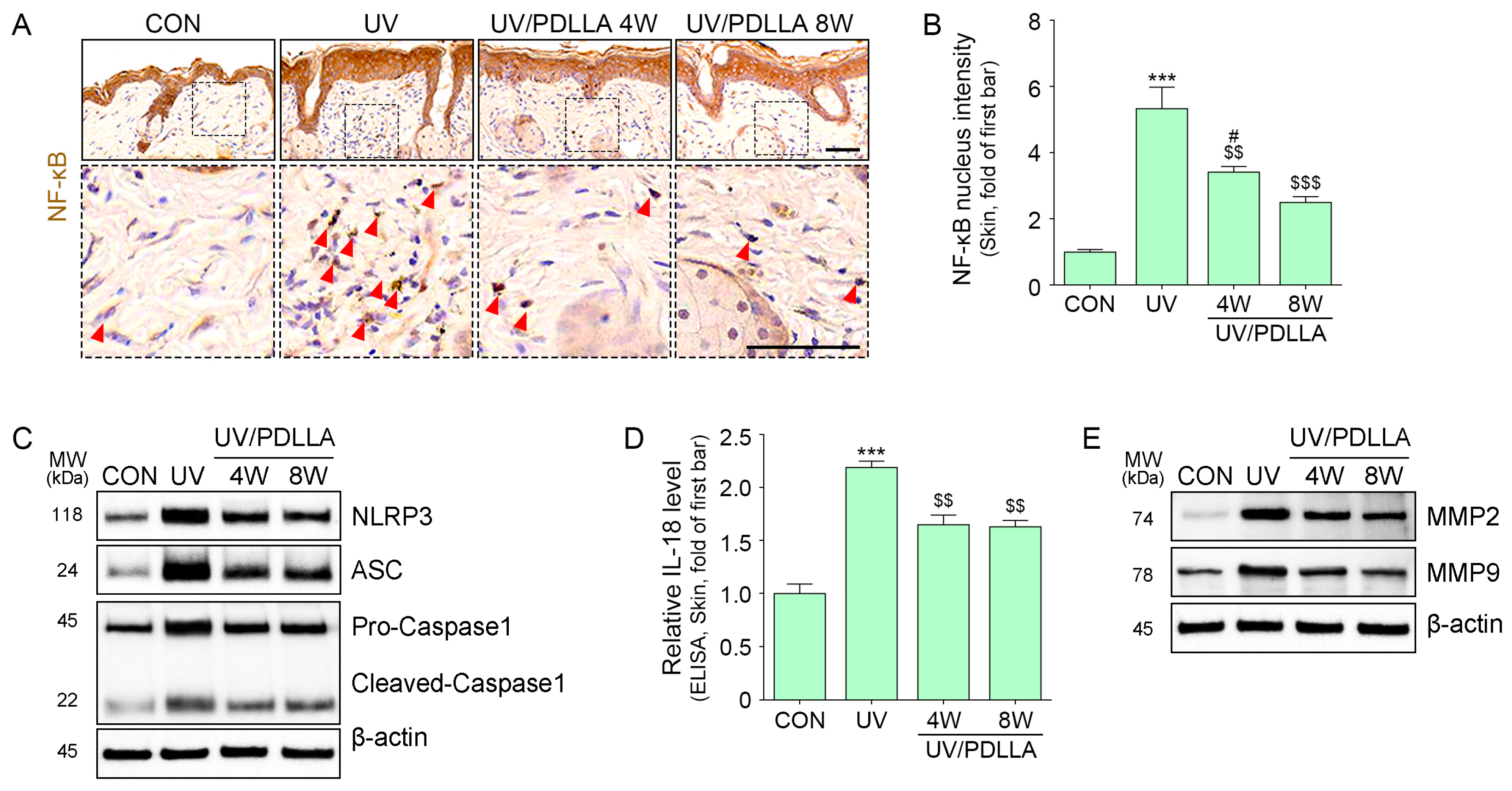
2.4. PDLLA Decreased Translocation of NF-κB, Formation of the NLRP3 Inflammasome, and Expression of MMP2 and MMP9 in UVB-Irradiated Mouse Skin
2.5. PDLLA Decreased BM Destruction and Melanin Accumulation in UVB-Irradiated Skin
3. Discussion
4. Materials and Methods
4.1. PDLLA Preparation
4.2. In Vitro Experiments
4.2.1. Cell Culture
4.2.2. Experimental Design of In Vitro
4.3. In Vivo Experiments
4.3.1. Mouse Model and Maintenance
4.3.2. Experimental Design of In Vivo
4.3.3. Skin Color
4.4. Sample Preparation
4.4.1. Protein Isolation
4.4.2. Paraffin-Embedded Block
4.5. Cell Viability
4.6. Enzyme-Linked Immunosorbent Assay (ELISA)
4.7. Western Blot
4.8. Staining
4.8.1. Immunocytochemistry (ICC)
4.8.2. Immunohistochemistry (IHC)
4.8.3. Fontana-Masson Staining
4.9. Transmission Electron Microscopy (TEM) Imaging
4.10. Tyrosinase Activity
4.11. Quantitative and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hruza, L.L.; Pentland, A.P. Mechanisms of UV-induced inflammation. J. Investig. Dermatol. 1993, 100, S35–S41. [Google Scholar] [CrossRef]
- Young, A.R. Acute effects of UVR on human eyes and skin. Prog. Biophys. Mol. Biol. 2006, 92, 80–85. [Google Scholar] [CrossRef] [PubMed]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV radiation and the skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef] [PubMed]
- Gaddameedhi, S.; Selby, C.P.; Kemp, M.G.; Ye, R.; Sancar, A. The circadian clock controls sunburn apoptosis and erythema in mouse skin. J. Investig. Dermatol. 2015, 135, 1119–1127. [Google Scholar] [CrossRef]
- Nikkola, V.; Miettinen, M.E.; Karisola, P.; Grönroos, M.; Ylianttila, L.; Alenius, H.; Snellman, E.; Partonen, T. Ultraviolet B radiation modifies circadian time in epidermal skin and in subcutaneous adipose tissue. Photodermatol. Photoimmunol. Photomed. 2019, 35, 157–163. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, P.; Li, H.; Dai, J. BMAL1 and CLOCK proteins in regulating UVB-induced apoptosis and DNA damage responses in human keratinocytes. J. Cell. Physiol. 2018, 233, 9563–9574. [Google Scholar] [CrossRef]
- Budden, T.; Bowden, N.A. The role of altered nucleotide excision repair and UVB-induced DNA damage in melanomagenesis. Int. J. Mol. Sci. 2013, 14, 11321151. [Google Scholar] [CrossRef]
- Nakajima, H.; Ezaki, Y.; Nagai, T.; Yoshioka, R.; Imokawa, G. Epithelial-mesenchymal interaction during UVB-induced up-regulation of neutral endopeptidase. Biochem J 2012, 443, 297–305. [Google Scholar] [CrossRef]
- O’Dea, E.L.; Kearns, J.D.; Hoffmann, A. UV as an amplifier rather than inducer of NF-κB activity. Mol. Cell 2008, 30, 632–641. [Google Scholar] [CrossRef]
- Tanaka, K.; Asamitsu, K.; Uranishi, H.; Iddamalgoda, A.; Ito, K.; Kojima, H.; Okamoto, T. Protecting skin photoaging by NF-κB inhibitor. Curr. Drug Metab. 2010, 11, 431–435. [Google Scholar] [CrossRef]
- Bond, M.; Fabunmi, R.P.; Baker, A.H.; Newby, A.C. Synergistic upregulation of metalloproteinase-9 by growth factors and inflammatory cytokines: An absolute requirement for transcription factor NF-κB. FEBS Lett. 1998, 435, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.P.; Tuan, T.L.; Wu, H.; Hughes, M.; Garner, W.L. TNF-α stimulates activation of pro-MMP2 in human skin through NF-κB mediated induction of MT1-MMP. J. Cell Sci. 2001, 114, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yan, C.; Gieling, R.G.; Kida, Y.; Garner, W.; Li, W.; Han, Y.P. Tumor necrosis factor-alpha induced expression of matrix metalloproteinase-9 through p21-activated kinase-1. BMC Immunol. 2009, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Hasegawa, J.; Asamitsu, K.; Okamoto, T. Prevention of the ultraviolet B-mediated skin photoaging by a nuclear factor κB inhibitor, parthenolide. J. Pharmacol. Exp. Ther. 2005, 315, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Jung, Y.R.; An, H.J.; Kim, D.H.; Jang, E.J.; Choi, Y.J.; Moon, K.M.; Park, M.H.; Park, C.H.; Chung, K.W.; et al. Anti-wrinkle and anti-inflammatory effects of active garlic components and the inhibition of MMPs via NF-κB signaling. PLoS ONE 2013, 8, e73877. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.; Qin, Z.; Xia, W.; Shao, Y.; Voorhees, J.J.; Fisher, G.J. Matrix-degrading metalloproteinases in photoaging. J. Investig. Dermatol. Symp. Proc. 2009, 14, 20–24. [Google Scholar] [CrossRef]
- Mauviel, A. Cytokine regulation of metalloproteinase gene expression. J. Cell. Biochem. 1993, 53, 288–295. [Google Scholar] [CrossRef]
- Kwon, S.H.; Hwang, Y.J.; Lee, S.K.; Park, K.C. Heterogeneous pathology of melasma and its clinical implications. Int. J. Mol. Sci. 2016, 17, 824. [Google Scholar] [CrossRef]
- Costell, M.; Gustafsson, E.; Aszódi, A.; Mörgelin, M.; Bloch, W.; Hunziker, E.; Addicks, K.; Timpl, R.; Fässler, R. Perlecan maintains the integrity of cartilage and some basement membranes. J. Cell Biol. 1999, 147, 1109–1122. [Google Scholar] [CrossRef]
- Erickson, A.C.; Couchman, J.R. Still more complexity in mammalian basement membranes. J. Histochem. Cytochem. 2000, 48, 1291–1306. [Google Scholar] [CrossRef]
- Yurchenco, P.D.; Patton, B.L. Developmental and pathogenic mechanisms of basement membrane assembly. Curr. Pharm. Des. 2009, 15, 1277–1294. [Google Scholar] [CrossRef] [PubMed]
- Stern, R.S. Prevalence of a history of skin cancer in 2007: Results of an incidence-based model. Arch. Dermatol. 2010, 146, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Cochran, A.J. The incidence of melanocytes in normal human skin. J. Investig. Dermatol. 1970, 55, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Tarnowski, W.M. Ultrastructure of the epidermal melanocyte dense plate. J. Investig. Dermatol. 1970, 55, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Panthier, J.J.; Arnheiter, H. Signaling and transcriptional regulation in the neural crest-derived melanocyte lineage: Interactions between KIT and MITF. Development 2000, 127, 5379–5389. [Google Scholar] [CrossRef]
- Cruickshank, C.N.; Harcourt, S.A. Pigment donation in vitro. J. Investig. Dermatol. 1964, 42, 183–184. [Google Scholar] [CrossRef]
- Torres-Álvarez, B.; Mesa-Garza, I.G.; Castanedo-Cázares, J.P.; Fuentes-Ahumada, C.; Oros-Ovalle, C.; Navarrete-Solis, J.; Moncada, B. Histochemical and immunohistochemical study in melasma: Evidence of damage in the basal membrane. Am. J. Dermatopathol. 2011, 33, 291–295. [Google Scholar] [CrossRef]
- Gilchrest, B.A.; Fitzpatrick, T.B.; Anderson, R.; Parrish, J.A. Localization of melanin pigmentation in the skin with Wood’s lamp. Br. J. Dermatol. 1977, 96, 245–248. [Google Scholar] [CrossRef]
- Thawabteh, A.M.; Jibreen, A.; Karaman, D.; Thawabteh, A.; Karaman, R. Skin pigmentation types, causes and treatment—A review. Molecules 2023, 28, 4839. [Google Scholar] [CrossRef]
- Rajanala, S.; Maymone, M.B.; Vashi, N.A. Melasma pathogenesis: A review of the latest research, pathological findings, and investigational therapies. Dermatol. Online J. 2019, 25, 1. [Google Scholar] [CrossRef]
- Horiba, S.; Kawamoto, M.; Tobita, R.; Kami, R.; Ogura, Y.; Hosoi, J. M1/M2 Macrophage Skewing is Related to Reduction in Types I, V, and VI Collagens with Aging in Sun-Exposed Human Skin. JID Innov. 2023, 3, 100222. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, L.; Nowroozi, M.R.; Amini, E.; Arami, M.K.; Ayati, M.; Mohsenzadegan, M. A review on the role of M2 macrophages in bladder cancer; pathophysiology and targeting. Int. Immunopharmacol. 2019, 76, 105880. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Smith, W.; Hao, D.; He, B.; Kong, L. M1 and M2 macrophage polarization and potentially therapeutic naturally occurring compounds. Int. Immunopharmacol. 2019, 70, 459–466. [Google Scholar] [CrossRef]
- Hirata, Y.; Tabata, M.; Kurobe, H.; Motoki, T.; Akaike, M.; Nishio, C.; Higashida, M.; Mikasa, H.; Nakaya, Y.; Takanashi, S.; et al. Coronary atherosclerosis is associated with macrophage polarization in epicardial adipose tissue. J. Am. Coll. Cardiol. 2011, 58, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V. Targeting macrophage immunometabolism: Dawn in the darkness of sepsis. International Immunopharmacology 2018, 58, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Feldmeyer, L.; Keller, M.; Niklaus, G.; Hohl, D.; Werner, S.; Beer, H.D. The inflammasome mediates UVB-induced activation and secretion of interleukin-1β by keratinocytes. Curr. Biol. 2007, 17, 1140–1145. [Google Scholar] [CrossRef]
- Hasegawa, T.; Nakashima, M.; Suzuki, Y. Nuclear DNA damage-triggered NLRP3 inflammasome activation promotes UVB-induced inflammatory responses in human keratinocytes. Biochem. Biophys. Res. Commun. 2016, 477, 329–335. [Google Scholar] [CrossRef]
- Hasegawa, T.; Noguchi, S.; Nakashima, M.; Miyai, M.; Goto, M.; Matsumoto, Y.; Torii, S.; Honda, S.; Shimizu, S. Alternative autophagy dampens UVB-induced NLRP3 inflammasome activation in human keratinocytes. J. Biol. Chem. 2024, 300, 107173. [Google Scholar] [CrossRef]
- Bauernfeind, F.G.; Horvath, G.; Stutz, A.; Alnemri, E.S.; MacDonald, K.; Speert, D.; Fernandes-Alnemri, T.; Wu, J.; Monks, B.G.; Fitzgerald, K.A.; et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 2009, 183, 787–791. [Google Scholar] [CrossRef]
- He, Y.; Hara, H.; Núñez, G. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem. Sci. 2016, 41, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Jo, E.K.; Kim, J.K.; Shin, D.M.; Sasakawa, C. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell. Mol. Immunol. 2016, 13, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F.; Burns, K.; Tschopp, J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol. Cell 2002, 10, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Sutterwala, F.S.; Ogura, Y.; Szczepanik, M.; Lara-Tejero, M.; Lichtenberger, G.S.; Grant, E.P.; Flavell, R.A. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity 2006, 24, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Afonina, I.S.; Müller, C.; Martin, S.J.; Beyaert, R. Proteolytic processing of interleukin-1 family cytokines: Variations on a common theme. Immunity 2015, 42, 991–1004. [Google Scholar] [CrossRef]
- Bernard, J.J.; Gallo, R.L.; Krutmann, J. Photoimmunology: How ultraviolet radiation affects the immune system. Nat. Rev. Immunol. 2019, 19, 688–701. [Google Scholar] [CrossRef]
- Beasley, K.L.; Weiss, M.A.; Weiss, R.A. Hyaluronic acid fillers: A comprehensive review. Facial Plast. Surg. 2009, 25, 086–094. [Google Scholar] [CrossRef]
- Burgess, C.M. Principles of soft tissue augmentation for the aging face. Clin. Interv. Aging 2006, 1, 349–355. [Google Scholar] [CrossRef]
- Moyle, G.J.; Lysakova, L.; Brown, S.; Sibtain, N.; Healy, J.; Priest, C.; Mandalia, S.; Barton, S.E. A randomized open-label study of immediate versus delayed polylactic acid injections for the cosmetic management of facial lipoatrophy in persons with HIV infection. HIV Med. 2004, 5, 82–87. [Google Scholar] [CrossRef]
- Brady, J.M.; Cutright, D.E.; Miller, R.A.; Battistone, G.C.; Hunsuck, E.E. Resorption rate, route of elimination, and ultrastructure of the implant site of polylactic acid in the abdominal wall of the rat. J. Biomed. Mater. Res. 1973, 7, 155–166. [Google Scholar] [CrossRef]
- Alijotas-Reig, J.; Fernández-Figueras, M.T.; Puig, L. Inflammatory, immune-mediated adverse reactions related to soft tissue dermal fillers. Semin. Arthritis Rheum. 2013, 43, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Eppley, B.L.; Summerlin, D.J.; Prevel, C.D.; Sadove, A.M. Effects of a positively charged biomaterial for dermal and subcutaneous augmentation. Aesthetic Plast. Surg. 1994, 18, 413–416. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Wang, B.; Yang, G.; Gauthier, M. Biomedical Science, Engineering and Technology; Books on Demand: London, UK, 2012; pp. 247–282. [Google Scholar]
- Pretula, J.; Slomkowski, S.; Penczek, S. Polylactides-Methods of synthesis and characterization. Adv. Drug Deliv. Rev. 2016, 107, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.A.; Chan, L.K.W.; Lee, A.W.K.; Lee, C.H.; Wong, S.T.H.; Yi, K.H. Poly-d,l-lactic Acid (PDLLA) Application in Dermatology: A Literature Review. Polymers 2024, 16, 2583. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Lin, J.Y.; Yang, D.Y.; Lee, S.H.; Kim, J.Y.; Kang, M. Efficacy and safety of poly-D, L-lactic acid microspheres as subdermal fillers in animals. Plast. Aesthet. Res. 2019, 6, 16. [Google Scholar] [CrossRef]
- Ramot, Y.; Haim-Zada, M.; Domb, A.J.; Nyska, A. Biocompatibility and safety of PLA and its copolymers. Adv. Drug Deliv. Rev. 2016, 107, 153–162. [Google Scholar] [CrossRef]
- Oh, S.; Seo, S.B.; Kim, G.; Batsukh, S.; Son, K.H.; Byun, K. Poly-D, L-lactic acid stimulates angiogenesis and collagen synthesis in aged animal skin. Int. J. Mol. Sci. 2023, 24, 7986. [Google Scholar] [CrossRef]
- Oh, S.; Seo, S.B.; Kim, G.; Batsukh, S.; Park, C.H.; Son, K.H.; Byun, K. Poly-D, L-lactic acid filler increases extracellular matrix by modulating macrophages and adipose-derived stem cells in aged animal skin. Antioxidants 2023, 12, 1204. [Google Scholar] [CrossRef]
- Song, S.H.; Cho, E.J.; Park, M.S.; Lee, Y.R.; Joo, H.K.; Kang, G.; Jeon, B.H. Redox regulating protein APE1/Ref-1 expression is increased in abdominal aortic coarctation-induced hypertension rats. J. Korean Soc. Hypertens. 2012, 18, 126–135. [Google Scholar] [CrossRef]
- Lavker, R.M. Structural alterations in exposed and unexposed aged skin. J. Investig. Dermatol. 1979, 73, 59–66. [Google Scholar] [CrossRef]
- Amano, S. Possible involvement of basement membrane damage in skin photoaging. J. Investig. Dermatol. Symp. Proc. 2009, 14, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Moolla, S.; Miller-Monthrope, Y. Dermatology: How to manage facial hyperpigmentation in skin of colour. Drugs Context 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Miyachi, K.; Yamada, T.; Sanada, A.; Inoue, Y.; Hasebe, Y.; Arima, M.; Iwata, Y.; Hasegawa, S.; Sugiura, K.; Akamatsu, H. Melanin accumulation in dermal stem cells deteriorates their exosome-mediated skin basement membrane construction in solar lentigo. Exp. Dermatol. 2022, 31, 1881–1890. [Google Scholar] [CrossRef] [PubMed]
- Grimes, P.E. Management of hyperpigmentation in darker racial ethnic groups. Semin. Cutan. Med. Surg. 2009, 28, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Davis, E.C.; Callender, V.D. Postinflammatory hyperpigmentation: A review of the epidemiology, clinical features, and treatment options in skin of color. J. Clin. Aesthetic Dermatol. 2010, 3, 20. [Google Scholar]
- Vashi, N.A.; Kundu, R.V. Facial hyperpigmentation: Causes and treatment. Br. J. Dermatol. 2013, 169, 41–56. [Google Scholar] [CrossRef]
- Ando, H.; Niki, Y.; Ito, M.; Akiyama, K.; Matsui, M.S.; Yarosh, D.B.; Ichihashi, M. Melanosomes are transferred from melanocytes to keratinocytes through the processes of packaging, release, uptake, and dispersion. J. Investig. Dermatol. 2012, 132, 1222–1229. [Google Scholar] [CrossRef]
- Wu, X.S.; Masedunskas, A.; Weigert, R.; Copeland, N.G.; Jenkins, N.A.; Hammer, J.A. Melanoregulin regulates a shedding mechanism that drives melanosome transfer from melanocytes to keratinocytes. Proc. Natl. Acad. Sci. USA 2012, 109, E2101–E2109. [Google Scholar] [CrossRef]
- Watanabe, T.; Tahira, M.; Morino, S.; Horie, T.; Adachi, K.; Tsutsumi, R.; Yamada, N.; Yoshida, Y.; Yamamoto, O. Novel morphological study of solar lentigines by immunohistochemical and electron microscopic evaluation. J. Dermatol. 2013, 40, 528–532. [Google Scholar] [CrossRef]
- Nakano, S.; Abe, Y.; Nakajima, K.; Sano, S.; Yamamoto, O.; Wakamatsu, K.; Ito, S.; Hayashi, M.; Suzuki, T. Establishment of a mouse model for post-inflammatory hyperpigmentation. Pigment. Cell Melanoma Res. 2021, 34, 101–110. [Google Scholar] [CrossRef]
- Tang, L.; Zhou, F. Inflammasomes in common immune-related skin diseases. Front. Immunol. 2020, 11, 882. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Ouyang, M.; Qu, Y.; Wang, M.; Huang, X.; Lan, J.; Lai, W.; Xu, Q. Advanced Glycation End Products Promote Melanogenesis by Activating NLRP3 Inflammasome in Human Dermal Fibroblasts. J. Investig. Dermatol. 2022, 142, 2591–2602.e8. [Google Scholar] [CrossRef] [PubMed]
- Sutterwala, F.S.; Haasken, S.; Cassel, S.L. Mechanism of NLRP3 inflammasome activation. Ann. N. Y. Acad. Sci. 2014, 1319, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.; Xiong, J.; Goeddel, D.V. The TNF receptor 1-associated protein TRADD signals cell death and NF-κB activation. Cell 1995, 81, 495–504. [Google Scholar] [CrossRef]
- Al-Roub, A.; Akhter, N.; Al-Rashed, F.; Wilson, A.; Alzaid, F.; Al-Mulla, F.; Sindhu, S.; Ahmad, R. TNFα induces matrix metalloproteinase-9 expression in monocytic cells through ACSL1/JNK/ERK/NF-kB signaling pathways. Sci. Rep. 2023, 13, 14351. [Google Scholar] [CrossRef]
- Iriyama, S.; Tsunenaga, M.; Amano, S.; Adachi, E. Key role of heparan sulfate chains in assembly of anchoring complex at the dermal–epidermal junction. Exp. Dermatol. 2011, 20, 953–955. [Google Scholar] [CrossRef]
- Amano, S.; Nishiyama, T.; Burgeson, R.E. A specific and sensitive ELISA for laminin 5. J. Immunol. Methods 1999, 224, 161–169. [Google Scholar] [CrossRef]
- Aberdam, D.; Galliano, M.F.; Vailly, J.; Pulkkinen, L.; Bonifas, J.; Christiano, A.M.; Tryggvason, K.; Uitto, J.; Epstein, E.H.; Ortonne, J.P., Jr. Herlitz’s junctional epidermolysis bullosa is linked to mutations in the gene (LAMC2) for the gamma 2 subunit of nicein/kalinin (LAMININ-5). Nat. Genet. 1994, 6, 299–304. [Google Scholar] [CrossRef]
- Lawrence, E.; Al Aboud, K.M. Postinflammatory Hyperpigmentation. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Huerth, K.A.; Hassan, S.; Callender, V.D. Therapeutic insights in melasma and hyperpigmentation management. J. Drugs Dermatol. JDD 2019, 18, 718–729. [Google Scholar]
- Taylor, S.; Grimes, P.; Lim, J.; Im, S.; Lui, H. Postinflammatory hyperpigmentation. J Cutan Med. Surg. 2009, 13, 183–191. [Google Scholar] [CrossRef]
- Passeron, T.; Genedy, R.; Salah, L.; Fusade, T.; Kositratna, G.; Laubach, H.J.; Marini, L.; Badawi, A. Laser treatment of hyperpigmented lesions: Position statement of the European Society of Laser in Dermatology. J. Eur. Acad. Dermatol. Venereol. JEADV 2019, 33, 987–1005. [Google Scholar] [CrossRef] [PubMed]
- No, Y.A.; Seok, J.; Hyun, M.Y.; Kwon, T.R.; Oh, C.T.; Choi, E.J.; Kim, B.J. Long-term (24-month) safety evaluation of poly-DL-lactic acid filler injection for the nasolabial fold: A multicenter, open, randomized, evaluator-blind, active-controlled design. Plast. Reconstr. Surg. 2015, 135, 1074e–1075e. [Google Scholar] [CrossRef] [PubMed]
- Sofen, B.; Prado, G.; Emer, J. Melasma and post inflammatory hyperpigmentation: Management update and expert opinion. Ski. Ther. Lett. 2016, 21, 1–7. [Google Scholar]
- Chung, K.W.; Jeong, H.O.; Jang, E.J.; Choi, Y.J.; Kim, D.H.; Kim, S.R.; Lee, K.J.; Lee, H.J.; Chun, P.; Byun, Y.; et al. Characterization of a small molecule inhibitor of melanogenesis that inhibits tyrosinase activity and scavenges nitric oxide (NO). Biochim. Biophys. Acta 2013, 1830, 4752–4761. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, J.; Cho, S.H.; Roh, G.; Park, H.J.; Lee, Y.J.; Jeon, H.E.; Lee, Y.S.; Bae, S.H.; Youn, S.B.; et al. Assessing the impact of mRNA vaccination in chronic inflammatory murine model. NPJ Vaccines 2024, 9, 34. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kim, Y.M.; Hong, S. DNAJB9 suppresses the metastasis of triple-negative breast cancer by promoting FBXO45-mediated degradation of ZEB1. Cell Death Dis. 2021, 12, 461. [Google Scholar] [CrossRef]
- Umair, Z.; Baek, M.O.; Song, J.; An, S.; Chon, S.J.; Yoon, M.S. MicroRNA-4516 in urinary exosomes as a biomarker of premature ovarian insufficiency. Cells 2022, 11, 2797. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Byun, K.-A.; Seo, S.B.; Oh, S.; Jang, J.-W.; Son, K.H.; Byun, K. Poly-D,L-Lactic Acid Filler Attenuates Ultraviolet B-Induced Skin Pigmentation by Reducing Destruction of the Basement Membrane. Int. J. Mol. Sci. 2024, 25, 11568. https://doi.org/10.3390/ijms252111568
Byun K-A, Seo SB, Oh S, Jang J-W, Son KH, Byun K. Poly-D,L-Lactic Acid Filler Attenuates Ultraviolet B-Induced Skin Pigmentation by Reducing Destruction of the Basement Membrane. International Journal of Molecular Sciences. 2024; 25(21):11568. https://doi.org/10.3390/ijms252111568
Chicago/Turabian StyleByun, Kyung-A, Suk Bae Seo, Seyeon Oh, Jong-Won Jang, Kuk Hui Son, and Kyunghee Byun. 2024. "Poly-D,L-Lactic Acid Filler Attenuates Ultraviolet B-Induced Skin Pigmentation by Reducing Destruction of the Basement Membrane" International Journal of Molecular Sciences 25, no. 21: 11568. https://doi.org/10.3390/ijms252111568
APA StyleByun, K.-A., Seo, S. B., Oh, S., Jang, J.-W., Son, K. H., & Byun, K. (2024). Poly-D,L-Lactic Acid Filler Attenuates Ultraviolet B-Induced Skin Pigmentation by Reducing Destruction of the Basement Membrane. International Journal of Molecular Sciences, 25(21), 11568. https://doi.org/10.3390/ijms252111568





