Overexpression of Chromatin Remodeling Factor SRG3 Down-Regulates IL1β-Expressing M1 Macrophages and IL17-Producing T Cells in Adipose Tissues
Abstract
1. Introduction
2. Results
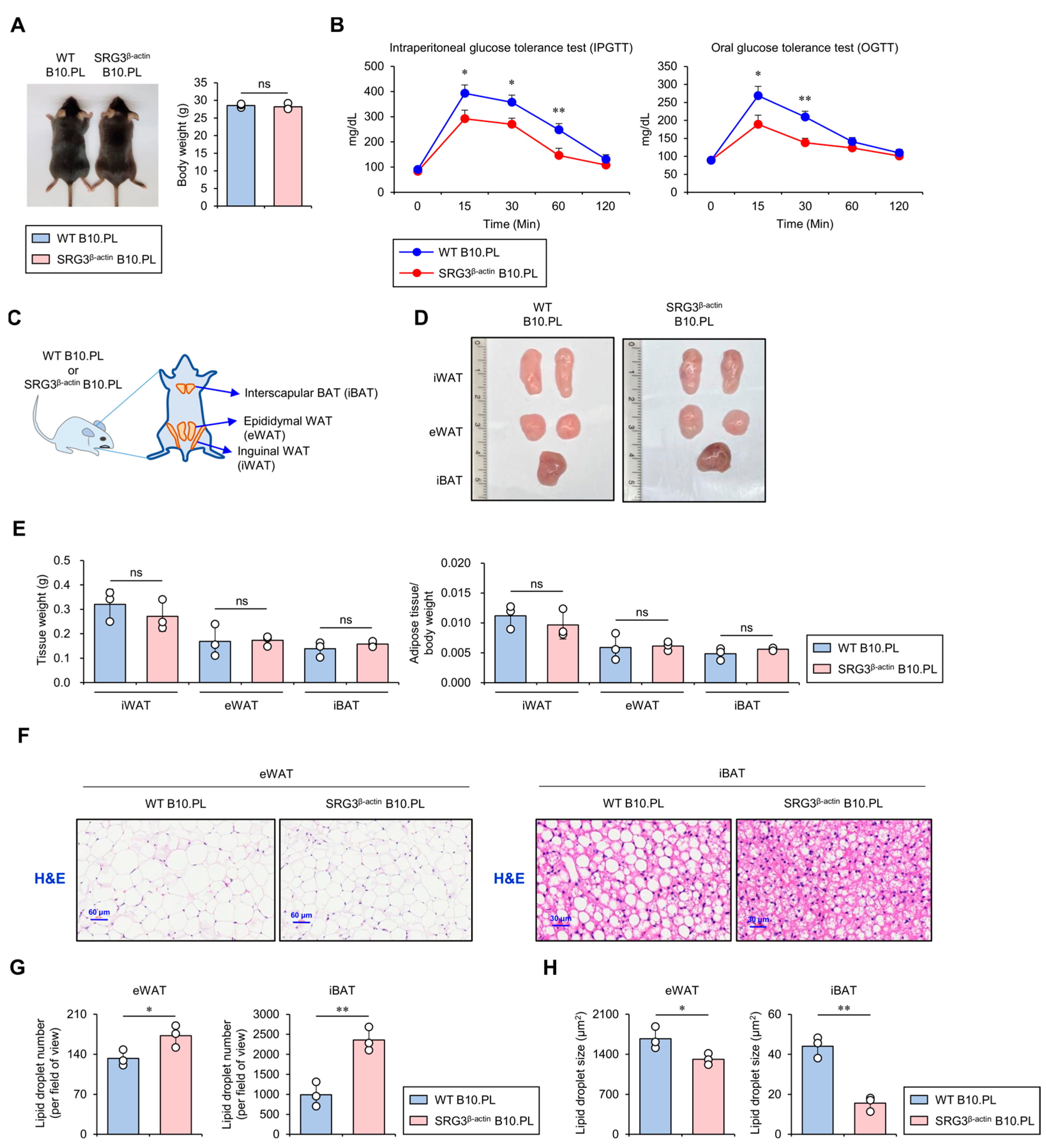
2.1. SRG3 Overexpression Improves Glucose Tolerance, Which Is Associated with a Reduction in Adipocyte Size in Adipose Tissues
2.2. SRG3 Overexpression Induces Selective Down-Regulation of the M1 Macrophage Population in the Adipose Tissues of SRG3β-actin B10.PL Mice
2.3. Correlation Analysis of SRG3 and Macrophage Subset Gene Expression in Human Adipose Tissues
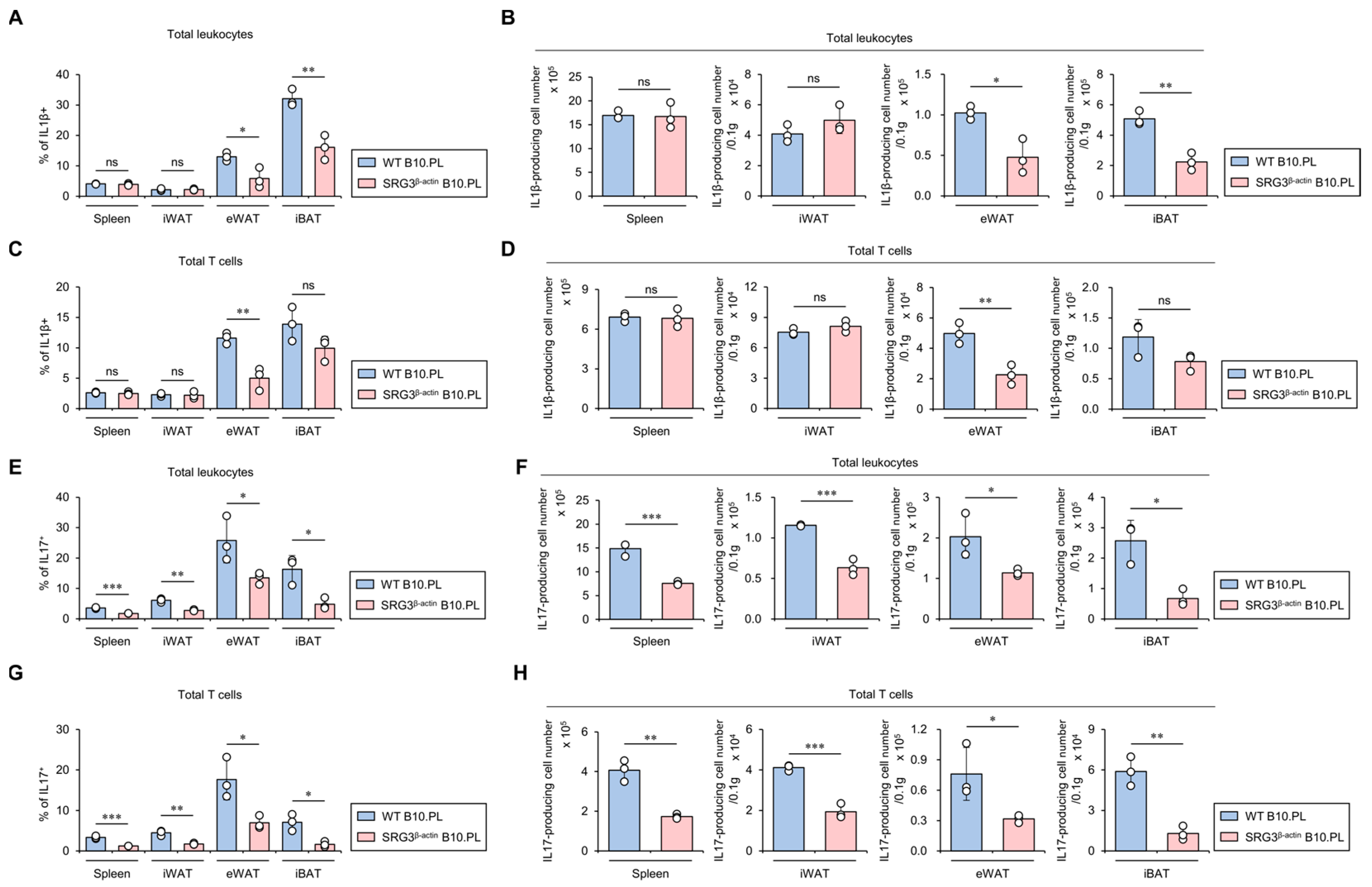
2.4. SRG3 Overexpression Down-Regulates the Accumulation of IL1β- and IL17-Producing T Cells in Adipose Tissues
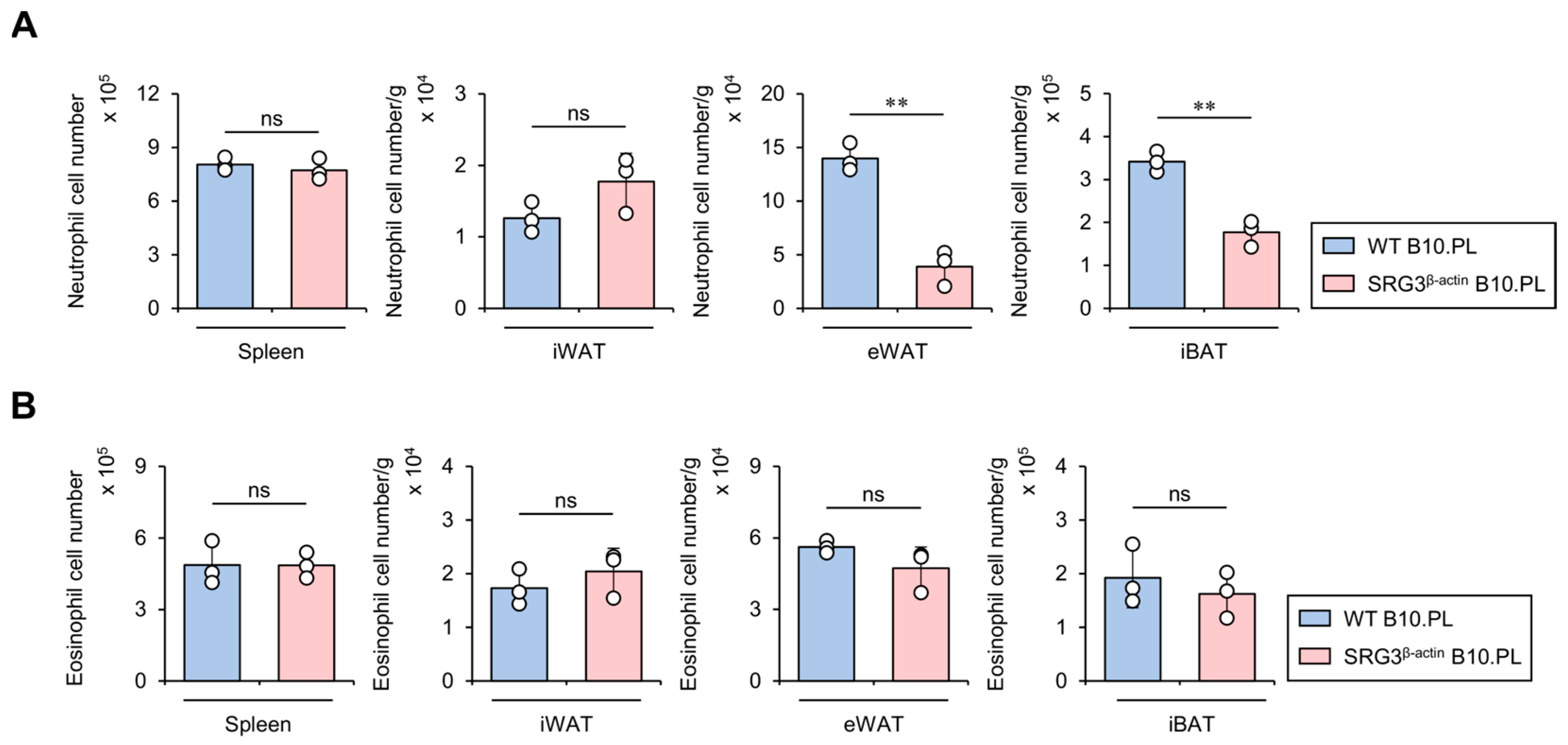
2.5. SRG3 Overexpression Limits the Accumulation of Neutrophils but Not Eosinophils in Adipose Tissues
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Mice
4.3. Genotyping of Mice
4.4. Glucose Tolerance Test (GTT)
4.5. Hematoxylin and Eosin (H&E) Staining and Analysis of Adipose Tissues
4.6. Isolation of Immune Cells from Adipose Tissues
4.7. Flow Cytometry
4.8. Intracellular Cytokine Staining
4.9. Data Collection in the GEPIA
4.10. Data Collection in the Human Protein Atlas
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jeon, S.H.; Kang, M.G.; Kim, Y.H.; Jin, Y.H.; Lee, C.; Chung, H.Y.; Kwon, H.; Park, S.D.; Seong, R.H. A new mouse gene, SRG3, related to the SWI3 of Saccharomyces cerevisiae, is required for apoptosis induced by glucocorticoids in a thymoma cell line. J. Exp. Med. 1997, 185, 1827–1836. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Huh, S.O.; Choi, H.; Lee, K.S.; Shin, D.; Lee, C.; Nam, J.S.; Kim, H.; Chung, H.; Lee, H.W.; et al. Srg3, a mouse homolog of yeast SWI3, is essential for early embryogenesis and involved in brain development. Mol. Cell Biol. 2001, 21, 7787–7795. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Jeon, S.; Choi, S.; Park, K.; Seong, R.H. The SWI/SNF chromatin remodeling complex regulates germinal center formation by repressing Blimp-1 expression. Proc. Natl. Acad. Sci. USA 2015, 112, E718–E727. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, J.; Min, H.; Seong, R.H. RORgammat-driven T(H)17 Cell Differentiation Requires Epigenetic Control by the Swi/Snf Chromatin Remodeling Complex. iScience 2020, 23, 101106. [Google Scholar] [CrossRef]
- Lee, S.W.; Park, H.J.; Jeon, J.; Park, Y.H.; Kim, T.C.; Jeon, S.H.; Seong, R.H.; Van Kaer, L.; Hong, S. Chromatin Regulator SRG3 Overexpression Protects against LPS/D-GalN-Induced Sepsis by Increasing IL10-Producing Macrophages and Decreasing IFNgamma-Producing NK Cells in the Liver. Int. J. Mol. Sci. 2021, 22, 3043. [Google Scholar] [CrossRef]
- Lee, S.W.; Park, H.J.; Jeon, S.H.; Lee, C.; Seong, R.H.; Park, S.H.; Hong, S. Ubiquitous Over-Expression of Chromatin Remodeling Factor SRG3 Ameliorates the T Cell-Mediated Exacerbation of EAE by Modulating the Phenotypes of both Dendritic Cells and Macrophages. PLoS ONE 2015, 10, e0132329. [Google Scholar] [CrossRef]
- Lee, Y.S.; Sohn, D.H.; Han, D.; Lee, H.W.; Seong, R.H.; Kim, J.B. Chromatin remodeling complex interacts with ADD1/SREBP1c to mediate insulin-dependent regulation of gene expression. Mol. Cell Biol. 2007, 27, 438–452. [Google Scholar] [CrossRef]
- Roder, P.V.; Wu, B.; Liu, Y.; Han, W. Pancreatic regulation of glucose homeostasis. Exp. Mol. Med. 2016, 48, e219. [Google Scholar] [CrossRef]
- Sinha, R.; Fisch, G.; Teague, B.; Tamborlane, W.V.; Banyas, B.; Allen, K.; Savoye, M.; Rieger, V.; Taksali, S.; Barbetta, G.; et al. Prevalence of impaired glucose tolerance among children and adolescents with marked obesity. N. Engl. J. Med. 2002, 346, 802–810. [Google Scholar] [CrossRef]
- Lin, Y.; Berg, A.H.; Iyengar, P.; Lam, T.K.; Giacca, A.; Combs, T.P.; Rajala, M.W.; Du, X.; Rollman, B.; Li, W.; et al. The hyperglycemia-induced inflammatory response in adipocytes: The role of reactive oxygen species. J. Biol. Chem. 2005, 280, 4617–4626. [Google Scholar] [CrossRef]
- Qiu, Y.; Shan, B.; Yang, L.; Liu, Y. Adipose tissue macrophage in immune regulation of metabolism. Sci. China Life Sci. 2016, 59, 1232–1240. [Google Scholar] [CrossRef] [PubMed]
- da Peres Valgas Silva, C.; Shettigar, V.K.; Baer, L.A.; Abay, E.; Madaris, K.L.; Mehling, M.R.; Hernandez-Saavedra, D.; Pinckard, K.M.; Seculov, N.P.; Ziolo, M.T.; et al. Brown adipose tissue prevents glucose intolerance and cardiac remodeling in high-fat-fed mice after a mild myocardial infarction. Int. J. Obes. 2022, 46, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Babar, M.Z.M.; Akhtar, L.; Hussain, M.S. Neutrophil lymphocyte ratio (NLR): A well assessment tool of glycemic control in type 2 diabetic patients. Pak. J. Med. Sci. 2017, 33, 1366–1370. [Google Scholar] [CrossRef] [PubMed]
- Ou, R.; Liu, J.; Lv, M.; Wang, J.; Wang, J.; Zhu, L.; Zhao, L.; Xu, Y. Neutrophil depletion improves diet-induced non-alcoholic fatty liver disease in mice. Endocrine 2017, 57, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Ehses, J.A.; Lacraz, G.; Giroix, M.H.; Schmidlin, F.; Coulaud, J.; Kassis, N.; Irminger, J.C.; Kergoat, M.; Portha, B.; Homo-Delarche, F.; et al. IL-1 antagonism reduces hyperglycemia and tissue inflammation in the type 2 diabetic GK rat. Proc. Natl. Acad. Sci. USA 2009, 106, 13998–14003. [Google Scholar] [CrossRef]
- Zuniga, L.A.; Shen, W.J.; Joyce-Shaikh, B.; Pyatnova, E.A.; Richards, A.G.; Thom, C.; Andrade, S.M.; Cua, D.J.; Kraemer, F.B.; Butcher, E.C. IL-17 regulates adipogenesis, glucose homeostasis, and obesity. J. Immunol. 2010, 185, 6947–6959. [Google Scholar] [CrossRef]
- Stenkula, K.G.; Erlanson-Albertsson, C. Adipose cell size: Importance in health and disease. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R284–R295. [Google Scholar] [CrossRef]
- Itoh, M.; Suganami, T.; Hachiya, R.; Ogawa, Y. Adipose tissue remodeling as homeostatic inflammation. Int. J. Inflam. 2011, 2011, 720926. [Google Scholar] [CrossRef]
- Schulze, F.; Wehner, J.; Kratschmar, D.V.; Makshana, V.; Meier, D.T.; Hauselmann, S.P.; Dalmas, E.; Thienel, C.; Dror, E.; Wiedemann, S.J.; et al. Inhibition of IL-1beta improves Glycaemia in a Mouse Model for Gestational Diabetes. Sci. Rep. 2020, 10, 3035. [Google Scholar] [CrossRef]
- Ikumi, K.; Odanaka, M.; Shime, H.; Imai, M.; Osaga, S.; Taguchi, O.; Nishida, E.; Hemmi, H.; Kaisho, T.; Morita, A.; et al. Hyperglycemia Is Associated with Psoriatic Inflammation in Both Humans and Mice. J. Investig. Dermatol. 2019, 139, 1329–1338.e7. [Google Scholar] [CrossRef]
- Talukdar, S.; Oh, D.Y.; Bandyopadhyay, G.; Li, D.; Xu, J.; McNelis, J.; Lu, M.; Li, P.; Yan, Q.; Zhu, Y.; et al. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat. Med. 2012, 18, 1407–1412. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.H.; Itan, M.; Jang, J.; Gu, H.J.; Rozenberg, P.; Mingler, M.K.; Wen, T.; Yoon, J.; Park, S.Y.; Roh, J.Y.; et al. Eosinophils support adipocyte maturation and promote glucose tolerance in obesity. Sci. Rep. 2018, 8, 9894. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.M.; Lee, C.; Lee, S.K.; Kim, J.; Seong, R.H. The SWI/SNF chromatin-remodeling complex modulates peripheral T cell activation and proliferation by controlling AP-1 expression. J. Biol. Chem. 2010, 285, 2340–2350. [Google Scholar] [CrossRef]
- Sutton, C.E.; Lalor, S.J.; Sweeney, C.M.; Brereton, C.F.; Lavelle, E.C.; Mills, K.H. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity 2009, 31, 331–341. [Google Scholar] [CrossRef]
- McGinley, A.M.; Sutton, C.E.; Edwards, S.C.; Leane, C.M.; DeCourcey, J.; Teijeiro, A.; Hamilton, J.A.; Boon, L.; Djouder, N.; Mills, K.H.G. Interleukin-17A Serves a Priming Role in Autoimmunity by Recruiting IL-1beta-Producing Myeloid Cells that Promote Pathogenic T Cells. Immunity 2020, 52, 342–356.e6. [Google Scholar] [CrossRef]
- Merz, K.E.; Thurmond, D.C. Role of Skeletal Muscle in Insulin Resistance and Glucose Uptake. Compr. Physiol. 2020, 10, 785–809. [Google Scholar]
- de la Serna, I.L.; Carlson, K.A.; Imbalzano, A.N. Mammalian SWI/SNF complexes promote MyoD-mediated muscle differentiation. Nat. Genet. 2001, 27, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, M.; Fang, H.; El-Mounayri, O.; Rodenberg, J.M.; Imbalzano, A.N.; Herring, B.P. The SWI/SNF chromatin remodeling complex regulates myocardin-induced smooth muscle-specific gene expression. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 921–928. [Google Scholar] [CrossRef]
- Choi, K.M.; Kim, J.H.; Kong, X.; Isik, M.; Zhang, J.; Lim, H.W.; Yoon, J.C. Defective brown adipose tissue thermogenesis and impaired glucose metabolism in mice lacking Letmd1. Cell Rep. 2021, 37, 110104. [Google Scholar] [CrossRef]
- Park, Y.K.; Lee, J.E.; Yan, Z.; McKernan, K.; O’Haren, T.; Wang, W.; Peng, W.; Ge, K. Interplay of BAF and MLL4 promotes cell type-specific enhancer activation. Nat. Commun. 2021, 12, 1630. [Google Scholar] [CrossRef]
- Liu, C.; Meng, M.; Xu, B.; Xu, Y.; Li, G.; Cao, Y.; Wang, D.; Qiu, J.; Yu, J.; Xu, L.; et al. Fibroblast Growth Factor 6 Promotes Adipocyte Progenitor Cell Proliferation for Adipose Tissue Homeostasis. Diabetes 2023, 72, 467–482. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhang, Z.; Qian, G.; Zhou, J. Omentin-1 attenuates adipose tissue inflammation via restoration of TXNIP/NLRP3 signaling in high-fat diet-induced obese mice. Fundam. Clin. Pharmacol. 2020, 34, 721–735. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, L. Omentin-1 attenuates lipopolysaccharide-induced inflammation and osteogenic differentiation in periodontal ligament stem cells and reduces M1 macrophages polarization through repressing endoplasmic reticulum stress. Prostaglandins Other Lipid Mediat. 2024, 174, 106882. [Google Scholar] [CrossRef]
- Sena, C.M. Omentin: A Key Player in Glucose Homeostasis, Atheroprotection, and Anti-Inflammatory Potential for Cardiovascular Health in Obesity and Diabetes. Biomedicines 2024, 12, 284. [Google Scholar] [CrossRef]
- Kong, Q.; Zou, J.; Zhang, Z.; Pan, R.; Zhang, Z.Y.; Han, S.; Xu, Y.; Gao, Y.; Meng, Z.X. BAF60a Deficiency in Macrophage Promotes Diet-Induced Obesity and Metabolic Inflammation. Diabetes 2022, 71, 2136–2152. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, H.; Shao, J.; Zhang, S.; Jin, J. Hyperglycemia-Suppressed SMARCA5 Disrupts Transcriptional Homeostasis to Facilitate Endothelial Dysfunction in Diabetes. Diabetes Metab. J. 2023, 47, 366–381. [Google Scholar] [CrossRef]
- Brown, N.M.; Setchell, K.D. Animal models impacted by phytoestrogens in commercial chow: Implications for pathways influenced by hormones. Lab. Investig. 2001, 81, 735–747. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Oh, S.Y.; Park, H.J.; Kim, T.C.; Park, Y.H.; Van Kaer, L.; Hong, S. Phosphorothioate-linked guanine/cytosine-based stem-loop oligonucleotides induce the extracellular release of mitochondrial DNA from peritoneal B1a cells. Int. J. Biol. Macromol. 2022, 223 Pt A, 252–262. [Google Scholar] [CrossRef]
- Tang, Z.; Li, C.; Kang, B.; Gao, G.; Li, C.; Zhang, Z. GEPIA: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017, 45, W98–W102. [Google Scholar] [CrossRef]
- Uhlen, M.; Oksvold, P.; Fagerberg, L.; Lundberg, E.; Jonasson, K.; Forsberg, M.; Zwahlen, M.; Kampf, C.; Wester, K.; Hober, S.; et al. Towards a knowledge-based Human Protein Atlas. Nat. Biotechnol. 2010, 28, 1248–1250. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, J.; Lee, S.W.; Park, H.J.; Park, Y.H.; Kim, T.-C.; Lee, S.; Lee, S.; Van Kaer, L.; Hong, S. Overexpression of Chromatin Remodeling Factor SRG3 Down-Regulates IL1β-Expressing M1 Macrophages and IL17-Producing T Cells in Adipose Tissues. Int. J. Mol. Sci. 2024, 25, 11681. https://doi.org/10.3390/ijms252111681
Jeon J, Lee SW, Park HJ, Park YH, Kim T-C, Lee S, Lee S, Van Kaer L, Hong S. Overexpression of Chromatin Remodeling Factor SRG3 Down-Regulates IL1β-Expressing M1 Macrophages and IL17-Producing T Cells in Adipose Tissues. International Journal of Molecular Sciences. 2024; 25(21):11681. https://doi.org/10.3390/ijms252111681
Chicago/Turabian StyleJeon, Jungmin, Sung Won Lee, Hyun Jung Park, Yun Hoo Park, Tae-Cheol Kim, Sujin Lee, Seyeong Lee, Luc Van Kaer, and Seokmann Hong. 2024. "Overexpression of Chromatin Remodeling Factor SRG3 Down-Regulates IL1β-Expressing M1 Macrophages and IL17-Producing T Cells in Adipose Tissues" International Journal of Molecular Sciences 25, no. 21: 11681. https://doi.org/10.3390/ijms252111681
APA StyleJeon, J., Lee, S. W., Park, H. J., Park, Y. H., Kim, T.-C., Lee, S., Lee, S., Van Kaer, L., & Hong, S. (2024). Overexpression of Chromatin Remodeling Factor SRG3 Down-Regulates IL1β-Expressing M1 Macrophages and IL17-Producing T Cells in Adipose Tissues. International Journal of Molecular Sciences, 25(21), 11681. https://doi.org/10.3390/ijms252111681







