Amino Acid Compound 2 (AAC2) Treatment Counteracts Insulin-Induced Synaptic Gene Expression and Seizure-Related Mortality in a Mouse Model of Alzheimer’s Disease
Abstract
:1. Introduction
2. Results
2.1. Normal Peripheral Glycemic Control but Altered Mortality in APP/PS1 Mice
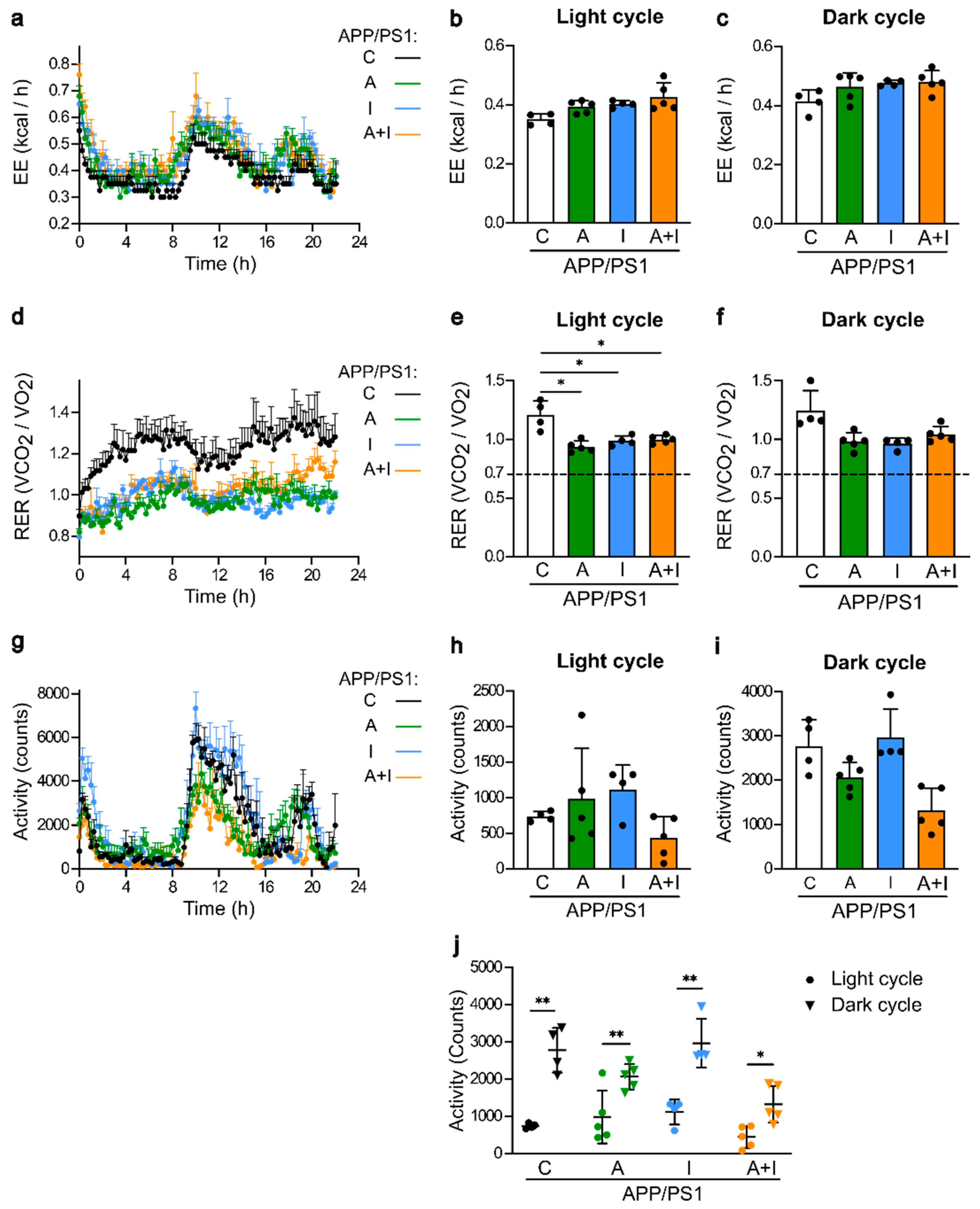
2.2. Similar Metabolic Parameters and Activity within Treated APP/PS1 Mice
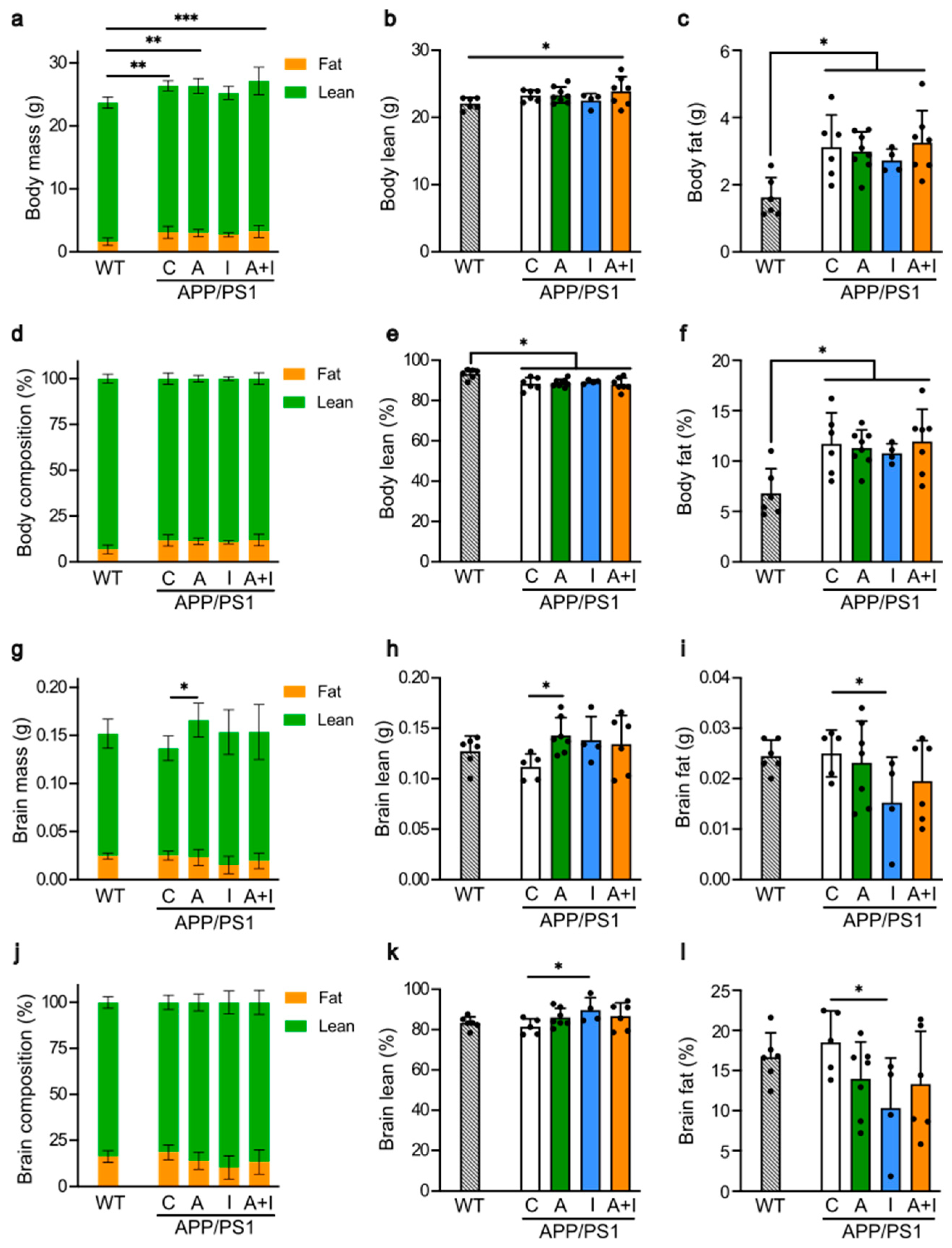
2.3. Similar Body and Different Brain Composition in Treated APP/PS1 Mice
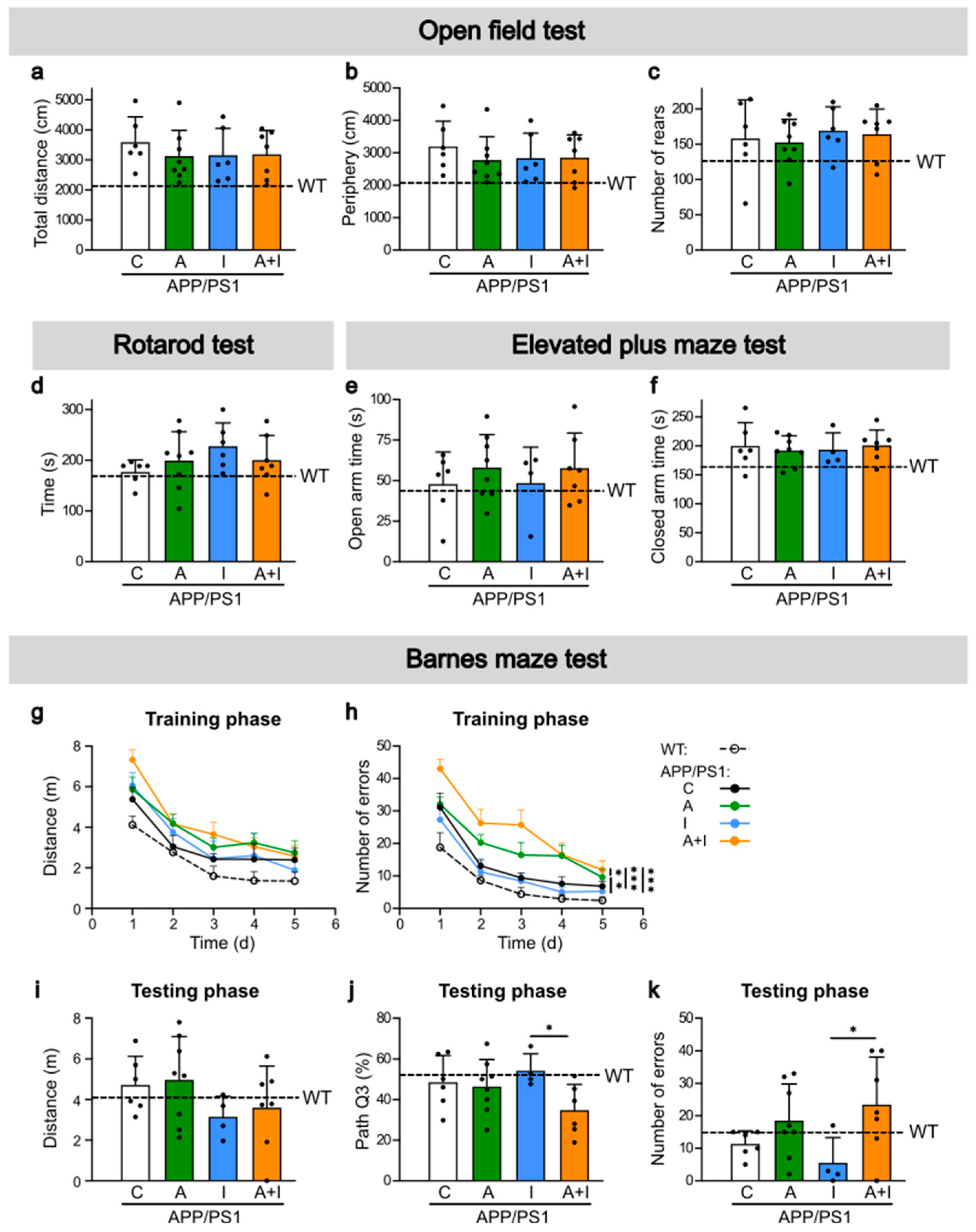
2.4. Similar Cognitive Performance in WT and APP/PS1 Mice but Improved Performance in INS Compared to AAC2–INS-Treated APP/PS1 Mice
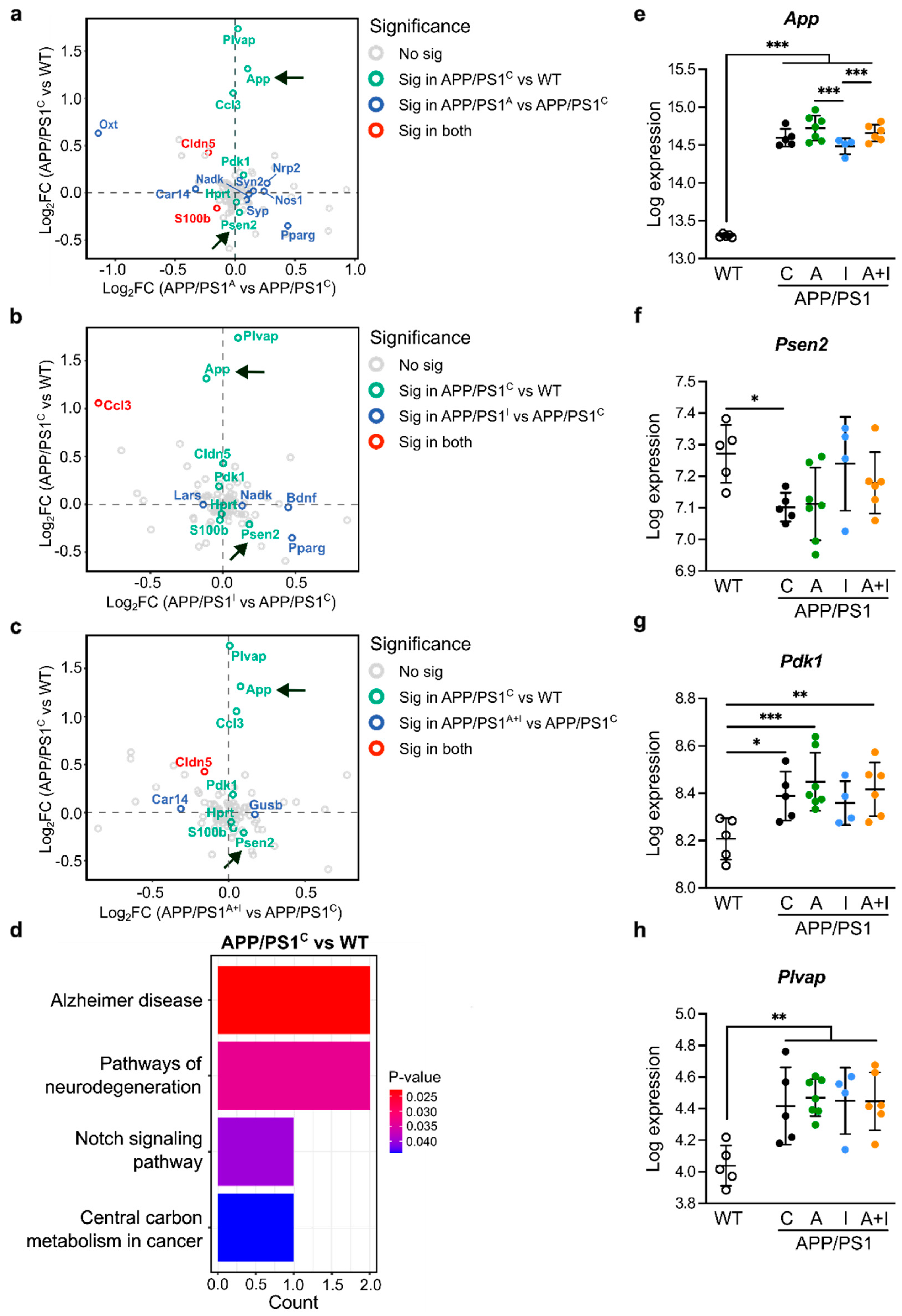
2.5. Increased Expression of Canonical AD Genes in APP/PS1 Mice with and Without Treatment Compared to WT Mice
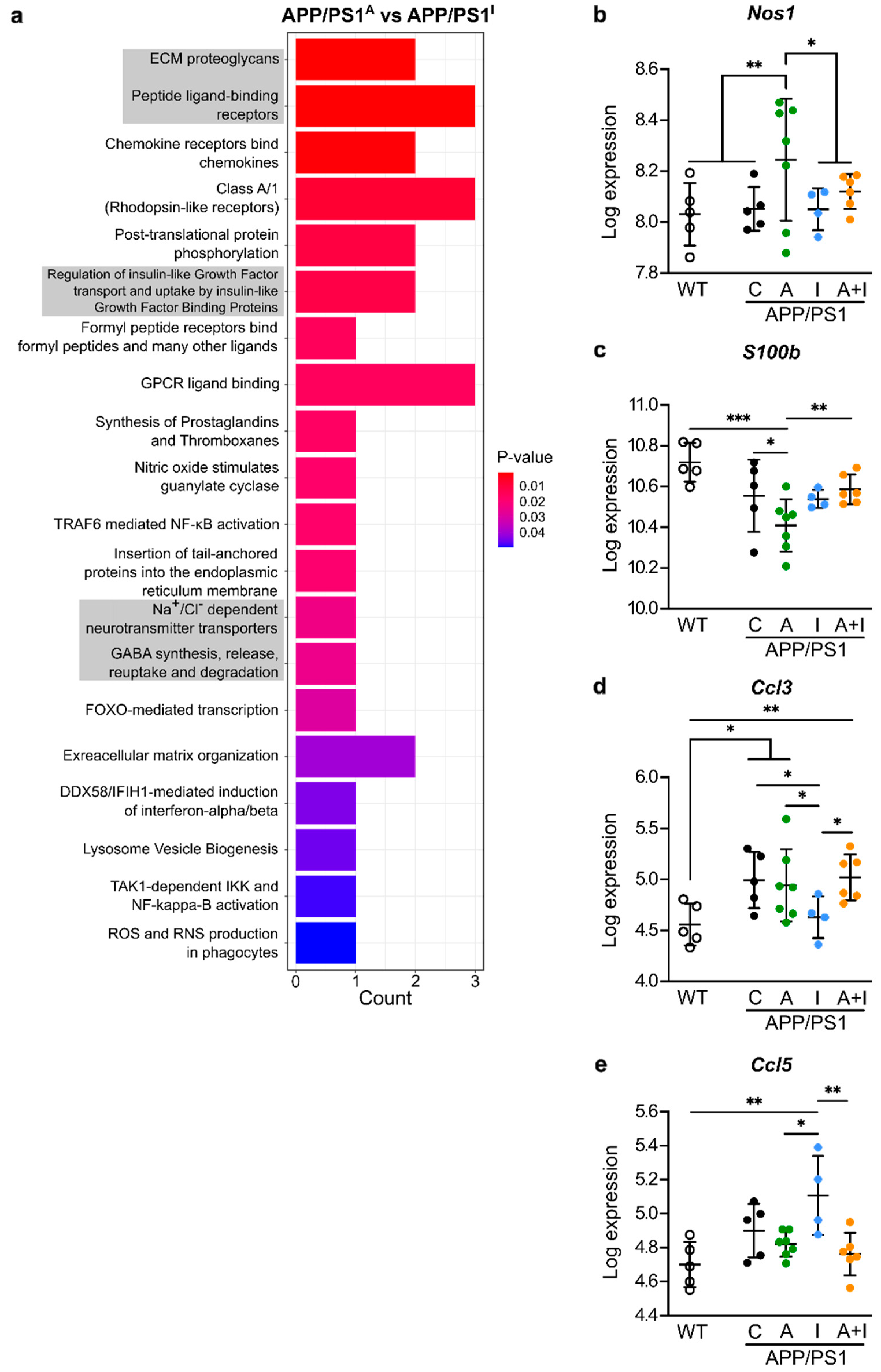
2.6. Distinct Regulation of Neuroinflammatory Genes by INS vs. AAC2 or AAC2–INS Nanofibers in APP/PS1 Mice
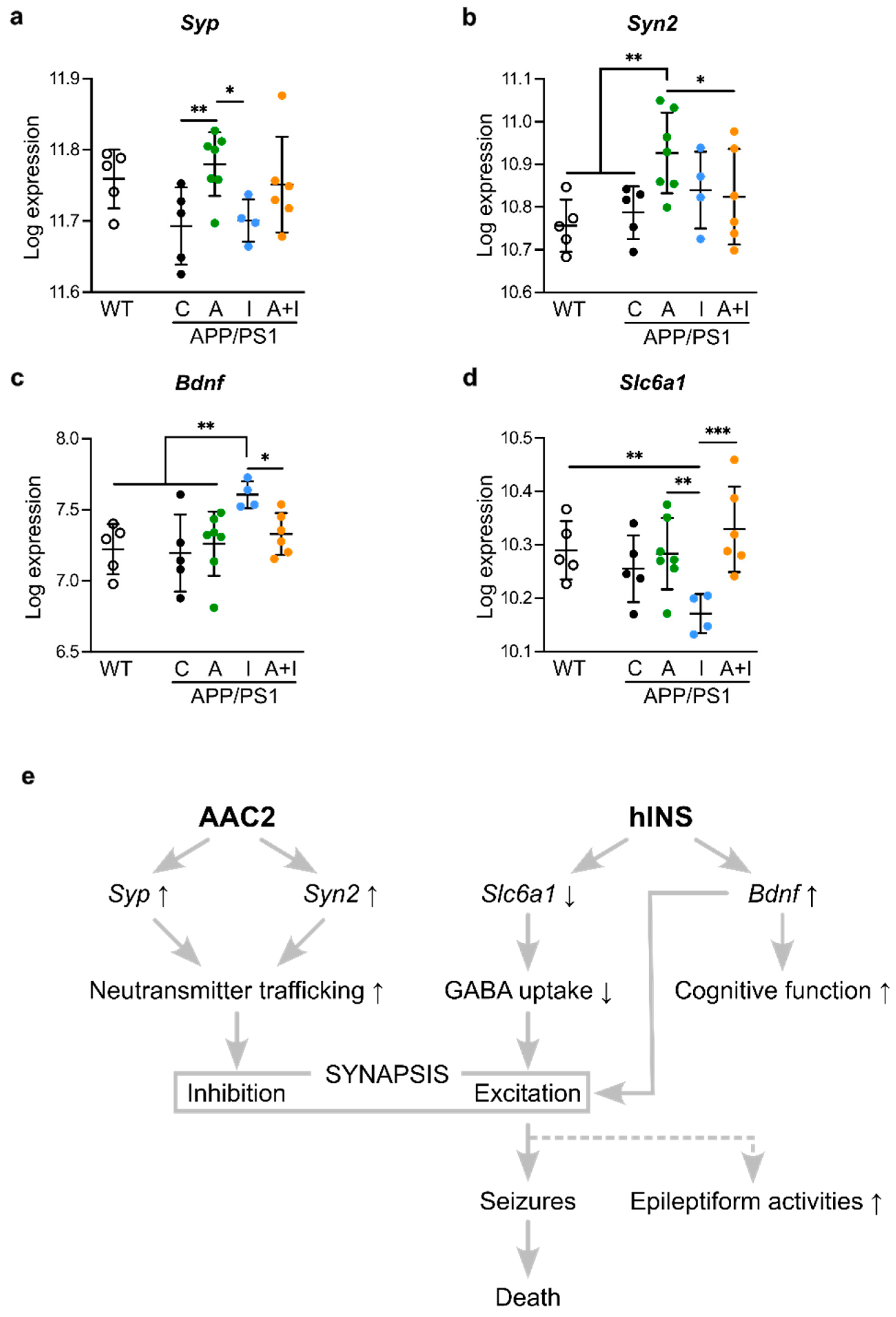
2.7. Opposite Regulation of Genes Determining Synaptic Excitation/Inhibition Balance by INS vs. AAC2 or AAC2–INS Nanofibers
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animal Study
4.3. Animal Study Design
- (1)
- WT, n = 6;
- (2)
- APP/PS1_control (C), n = 8;
- (3)
- APP/PS1_AAC2 (A), n = 9;
- (4)
- APP/PS1_hINS (I), n = 7;
- (5)
- APP/PS1_AAC2–hINS (A+I), n = 9.
4.4. Metabolic Parameters: Fasting Glucose, Body and Brain Composition, and Activity Measurements
4.5. RNA Isolation and NanoString mRNA Profiling
4.6. NanoString Data Analysis
4.7. Behavioral Tests
4.8. Statistics
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Swinburn, B.A.; Sacks, G.; Hall, K.D.; McPherson, K.; Finegood, D.T.; Moodie, M.L.; Gortmaker, S.L. The global obesity pandemic: Shaped by global drivers and local environments. Lancet 2011, 378, 804–814. [Google Scholar] [CrossRef] [PubMed]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in diabetes since 1980: A pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 2016, 387, 1513–1530. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Dementia Forecasting Collaborators. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022, 7, e105–e125. [Google Scholar] [CrossRef]
- Knopman, D.S.; Amieva, H.; Petersen, R.C.; Chetelat, G.; Holtzman, D.M.; Hyman, B.T.; Nixon, R.A.; Jones, D.T. Alzheimer disease. Nat. Rev. Dis. Primers 2021, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Janson, J.; Laedtke, T.; Parisi, J.E.; O’Brien, P.; Petersen, R.C.; Butler, P.C. Increased risk of type 2 diabetes in Alzheimer disease. Diabetes 2004, 53, 474–481. [Google Scholar] [CrossRef]
- Biessels, G.J.; Staekenborg, S.; Brunner, E.; Brayne, C.; Scheltens, P. Risk of dementia in diabetes mellitus: A systematic review. Lancet Neurol. 2006, 5, 64–74. [Google Scholar] [CrossRef]
- de la Monte, S.M. The Full Spectrum of Alzheimer’s Disease Is Rooted in Metabolic Derangements That Drive Type 3 Diabetes. Adv. Exp. Med. Biol. 2019, 1128, 45–83. [Google Scholar] [CrossRef]
- Kumar, V.; Kim, S.H.; Bishayee, K. Dysfunctional Glucose Metabolism in Alzheimer’s Disease Onset and Potential Pharmacological Interventions. Int. J. Mol. Sci. 2022, 23, 9540. [Google Scholar] [CrossRef]
- Biessels, G.J.; Despa, F. Cognitive decline and dementia in diabetes mellitus: Mechanisms and clinical implications. Nat. Rev. Endocrinol. 2018, 14, 591–604. [Google Scholar] [CrossRef]
- Vossel, K.A.; Tartaglia, M.C.; Nygaard, H.B.; Zeman, A.Z.; Miller, B.L. Epileptic activity in Alzheimer’s disease: Causes and clinical relevance. Lancet Neurol. 2017, 16, 311–322. [Google Scholar] [CrossRef]
- Ramos-Rodriguez, J.J.; Ortiz, O.; Jimenez-Palomares, M.; Kay, K.R.; Berrocoso, E.; Murillo-Carretero, M.I.; Perdomo, G.; Spires-Jones, T.; Cozar-Castellano, I.; Lechuga-Sancho, A.M.; et al. Differential central pathology and cognitive impairment in pre-diabetic and diabetic mice. Psychoneuroendocrinology 2013, 38, 2462–2475. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.Q.; Wu, D.K.; Liu, J.K. mTOR and tau phosphorylated proteins in the hippocampal tissue of rats with type 2 diabetes and Alzheimer’s disease. Mol. Med. Rep. 2013, 7, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Mosconi, L. Glucose metabolism in normal aging and Alzheimer’s disease: Methodological and physiological considerations for PET studies. Clin. Transl. Imaging 2013, 1, 217–233. [Google Scholar] [CrossRef]
- Kim, B.; Feldman, E.L. Insulin resistance as a key link for the increased risk of cognitive impairment in the metabolic syndrome. Exp. Mol. Med. 2015, 47, e149. [Google Scholar] [CrossRef]
- Johnson, R.J.; Tolan, D.R.; Bredesen, D.; Nagel, M.; Sanchez-Lozada, L.G.; Fini, M.; Burtis, S.; Lanaspa, M.A.; Perlmutter, D. Could Alzheimer’s disease be a maladaptation of an evolutionary survival pathway mediated by intracerebral fructose and uric acid metabolism? Am. J. Clin. Nutr. 2023, 117, 455–466. [Google Scholar] [CrossRef]
- Sanabria-Diaz, G.; Martinez-Montes, E.; Melie-Garcia, L.; Alzheimer’s Disease Neuroimaging Initiative. Glucose metabolism during resting state reveals abnormal brain networks organization in the Alzheimer’s disease and mild cognitive impairment. PLoS ONE 2013, 8, e68860. [Google Scholar] [CrossRef]
- Riske, L.; Thomas, R.K.; Baker, G.B.; Dursun, S.M. Lactate in the brain: An update on its relevance to brain energy, neurons, glia and panic disorder. Ther. Adv. Psychopharmacol. 2017, 7, 85–89. [Google Scholar] [CrossRef]
- Chowdhury, G.M.I.; Wang, P.; Ciardi, A.; Mamillapalli, R.; Johnson, J.; Zhu, W.; Eid, T.; Behar, K.; Chan, O. Impaired Glutamatergic Neurotransmission in the Ventromedial Hypothalamus May Contribute to Defective Counterregulation in Recurrently Hypoglycemic Rats. Diabetes 2017, 66, 1979–1989. [Google Scholar] [CrossRef]
- Salvati, K.A.; Ritger, M.L.; Davoudian, P.A.; O’Dell, F.; Wyskiel, D.R.; Souza, G.; Lu, A.C.; Perez-Reyes, E.; Drake, J.C.; Yan, Z.; et al. AMPK-mediated potentiation of GABAergic signalling drives hypoglycaemia-provoked spike-wave seizures. Brain 2022, 145, 2332–2346. [Google Scholar] [CrossRef]
- Vossel, K.; Ranasinghe, K.G.; Beagle, A.J.; La, A.; Pook, K.A.; Castro, M.; Mizuiri, D.; Honma, S.M.; Venkateswaran, N.; Koestler, M.; et al. Effect of Levetiracetam on Cognition in Patients With Alzheimer Disease With and Without Epileptiform Activity: A Randomized Clinical Trial. JAMA Neurol. 2021, 78, 1345–1354. [Google Scholar] [CrossRef]
- Zaykov, A.N.; Mayer, J.P.; DiMarchi, R.D. Pursuit of a perfect insulin. Nat. Rev. Drug Discov. 2016, 15, 425–439. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.K.; Burns, J.M. Insulin: An emerging treatment for Alzheimer’s disease dementia? Curr. Neurol. Neurosci. Rep. 2012, 12, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Yang, J.; Wang, Y.; Fan, Y.; Tang, F.; Hou, C.; Yu, J.; Wang, X.; Jiang, G. Low-dose intranasal insulin improves cognitive function and suppresses the development of epilepsy. Brain Res. 2020, 1726, 146474. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Sun, Y.; Lin, T.; Song, N.J.; Mason, M.L.; Leung, J.H.; Kowdley, D.; Wall, J.; Brunetti, A.; Fitzgerald, J.; et al. Amino acid-based compound activates atypical PKC and leptin receptor pathways to improve glycemia and anxiety like behavior in diabetic mice. Biomaterials 2020, 239, 119839. [Google Scholar] [CrossRef]
- Lee, A.; Mason, M.L.; Lin, T.; Kumar, S.B.; Kowdley, D.; Leung, J.H.; Muhanna, D.; Sun, Y.; Ortega-Anaya, J.; Yu, L.; et al. Amino Acid Nanofibers Improve Glycemia and Confer Cognitive Therapeutic Efficacy to Bound Insulin. Pharmaceutics 2021, 14, 81. [Google Scholar] [CrossRef]
- Kumar, S.B.; Arnipalli, S.; Abushukur, A.; Carrau, S.; Mehta, P.; Ziouzenkova, O. Extracellular Glucose Depletion as an Indirect Measure of Glucose Uptake in Cells and Tissues Ex Vivo. J. Vis. Exp. 2022, 182, e63681. [Google Scholar] [CrossRef]
- Chiti, F.; Dobson, C.M. Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem. 2006, 75, 333–366. [Google Scholar] [CrossRef]
- de Ceballos, M.L.; Kofalvi, A. Boosting brain glucose metabolism to fight neurodegeneration? Oncotarget 2017, 8, 14273–14274. [Google Scholar] [CrossRef]
- Dodd, G.T.; Tiganis, T. Insulin action in the brain: Roles in energy and glucose homeostasis. J. Neuroendocrinol. 2017, 29, e12513. [Google Scholar] [CrossRef]
- Macklin, L.; Griffith, C.M.; Cai, Y.; Rose, G.M.; Yan, X.X.; Patrylo, P.R. Glucose tolerance and insulin sensitivity are impaired in APP/PS1 transgenic mice prior to amyloid plaque pathogenesis and cognitive decline. Exp. Gerontol. 2017, 88, 9–18. [Google Scholar] [CrossRef]
- Webster, S.J.; Bachstetter, A.D.; Van Eldik, L.J. Comprehensive behavioral characterization of an APP/PS-1 double knock-in mouse model of Alzheimer’s disease. Alzheimers Res. Ther. 2013, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Holcomb, L.A.; Gordon, M.N.; Jantzen, P.; Hsiao, K.; Duff, K.; Morgan, D. Behavioral changes in transgenic mice expressing both amyloid precursor protein and presenilin-1 mutations: Lack of association with amyloid deposits. Behav. Genet. 1999, 29, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.N.; King, D.L.; Diamond, D.M.; Jantzen, P.T.; Boyett, K.V.; Hope, C.E.; Hatcher, J.M.; DiCarlo, G.; Gottschall, W.P.; Morgan, D.; et al. Correlation between cognitive deficits and Abeta deposits in transgenic APP+PS1 mice. Neurobiol. Aging 2001, 22, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Mina, A.I.; LeClair, R.A.; LeClair, K.B.; Cohen, D.E.; Lantier, L.; Banks, A.S. CalR: A Web-Based Analysis Tool for Indirect Calorimetry Experiments. Cell Metab. 2018, 28, 656–666.e1. [Google Scholar] [CrossRef] [PubMed]
- McClave, S.A.; Snider, H.L. Use of indirect calorimetry in clinical nutrition. Nutr. Clin. Pract. 1992, 7, 207–221. [Google Scholar] [CrossRef]
- Reiserer, R.S.; Harrison, F.E.; Syverud, D.C.; McDonald, M.P. Impaired spatial learning in the APPSwe + PSEN1DeltaE9 bigenic mouse model of Alzheimer’s disease. Genes. Brain Behav. 2007, 6, 54–65. [Google Scholar] [CrossRef]
- Hulshof, L.A.; Frajmund, L.A.; van Nuijs, D.; van der Heijden, D.C.N.; Middeldorp, J.; Hol, E.M. Both male and female APPswe/PSEN1dE9 mice are impaired in spatial memory and cognitive flexibility at 9 months of age. Neurobiol. Aging 2022, 113, 28–38. [Google Scholar] [CrossRef]
- Shen, Q.; Yasmeen, R.; Marbourg, J.; Xu, L.; Yu, L.; Fadda, P.; Flechtner, A.; Lee, L.J.; Popovich, P.G.; Ziouzenkova, O. Induction of innervation by encapsulated adipocytes with engineered vitamin A metabolism. Transl. Res. 2018, 192, 1–14. [Google Scholar] [CrossRef]
- Lefterov, I.; Wolfe, C.M.; Fitz, N.F.; Nam, K.N.; Letronne, F.; Biedrzycki, R.J.; Kofler, J.; Han, X.; Wang, J.; Schug, J.; et al. APOE2 orchestrated differences in transcriptomic and lipidomic profiles of postmortem AD brain. Alzheimers Res. Ther. 2019, 11, 113. [Google Scholar] [CrossRef]
- Gandolfo, L.C.; Speed, T.P. RLE plots: Visualizing unwanted variation in high dimensional data. PLoS ONE 2018, 13, e0191629. [Google Scholar] [CrossRef]
- Pietri, M.; Dakowski, C.; Hannaoui, S.; Alleaume-Butaux, A.; Hernandez-Rapp, J.; Ragagnin, A.; Mouillet-Richard, S.; Haik, S.; Bailly, Y.; Peyrin, J.M.; et al. PDK1 decreases TACE-mediated alpha-secretase activity and promotes disease progression in prion and Alzheimer’s diseases. Nat. Med. 2013, 19, 1124–1131. [Google Scholar] [CrossRef] [PubMed]
- Mozer, A.B.; Whittemore, S.R.; Benton, R.L. Spinal microvascular expression of PV-1 is associated with inflammation, perivascular astrocyte loss, and diminished EC glucose transport potential in acute SCI. Curr. Neurovasc Res. 2010, 7, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Wei, M.; Yuan, L.; He, X.; Chen, C.; Ji, A.; Zhang, G. Claudin-5 relieves cognitive decline in Alzheimer’s disease mice through suppression of inhibitory GABAergic neurotransmission. Aging 2022, 14, 3554–3568. [Google Scholar] [CrossRef] [PubMed]
- Del Pozo, A.; Knox, K.M.; Lehmann, L.; Davidson, S.; Rho, S.; Jayadev, S.; Barker-Haliski, M. Chronic evoked seizures in young pre-symptomatic APP/PS1 mice induce serotonin changes and accelerate onset on Alzheimer’s disease-related neurpathology. bioRxiv 2023. [Google Scholar] [CrossRef]
- Li, T.; Zhu, J. Entanglement of CCR5 and Alzheimer’s Disease. Front. Aging Neurosci. 2019, 11, 209. [Google Scholar] [CrossRef]
- Cristovao, J.S.; Gomes, C.M. S100 Proteins in Alzheimer’s Disease. Front. Neurosci. 2019, 13, 463. [Google Scholar] [CrossRef]
- Leclerc, E.; Sturchler, E.; Vetter, S.W. The S100B/RAGE Axis in Alzheimer’s Disease. Cardiovasc. Psychiatry Neurol. 2010, 2010, 539581. [Google Scholar] [CrossRef]
- Aronica, E.; Bauer, S.; Bozzi, Y.; Caleo, M.; Dingledine, R.; Gorter, J.A.; Henshall, D.C.; Kaufer, D.; Koh, S.; Loscher, W.; et al. Neuroinflammatory targets and treatments for epilepsy validated in experimental models. Epilepsia 2017, 58 (Suppl. 3), 27–38. [Google Scholar] [CrossRef]
- Liao, W.; Luo, H.; Ruan, Y.; Mai, Y.; Liu, C.; Chen, J.; Yang, S.; Xuan, A.; Liu, J. Identification of candidate genes associated with clinical onset of Alzheimer’s disease. Front. Neurosci. 2022, 16, 1060111. [Google Scholar] [CrossRef]
- Tampellini, D.; Capetillo-Zarate, E.; Dumont, M.; Huang, Z.; Yu, F.; Lin, M.T.; Gouras, G.K. Effects of synaptic modulation on beta-amyloid, synaptophysin, and memory performance in Alzheimer’s disease transgenic mice. J. Neurosci. 2010, 30, 14299–14304. [Google Scholar] [CrossRef]
- Williams, J.B.; Cao, Q.; Yan, Z. Transcriptomic analysis of human brains with Alzheimer’s disease reveals the altered expression of synaptic genes linked to cognitive deficits. Brain Commun. 2021, 3, fcab123. [Google Scholar] [CrossRef] [PubMed]
- Rosahl, T.W.; Spillane, D.; Missler, M.; Herz, J.; Selig, D.K.; Wolff, J.R.; Hammer, R.E.; Malenka, R.C.; Sudhof, T.C. Essential functions of synapsins I and II in synaptic vesicle regulation. Nature 1995, 375, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.G.; Mu, R.Z.; Liu, Y.; Jiang, D.; Jia, T.T.; Huang, Y.J. Increased Serum S100B Levels in Patients With Epilepsy: A Systematic Review and Meta-Analysis Study. Front. Neurosci. 2019, 13, 456. [Google Scholar] [CrossRef] [PubMed]
- Schwark, R.; Andrade, R.; Bykhovskaia, M. Synapsin II Directly Suppresses Epileptic Seizures In Vivo. Brain Sci. 2022, 12, 325. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Saito, H.; Matsuki, N. Inhibition of GABAA synaptic responses by brain-derived neurotrophic factor (BDNF) in rat hippocampus. J. Neurosci. 1997, 17, 2959–2966. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.Q. Epileptic Mechanisms Shared by Alzheimer’s Disease: Viewed via the Unique Lens of Genetic Epilepsy. Int. J. Mol. Sci. 2021, 22, 7133. [Google Scholar] [CrossRef]
- Rajasekaran, K.; Ma, Q.; Good, L.B.; Kathote, G.; Jakkamsetti, V.; Liu, P.; Avila, A.; Primeaux, S.; Enciso Alva, J.; Markussen, K.H.; et al. Metabolic modulation of synaptic failure and thalamocortical hypersynchronization with preserved consciousness in Glut1 deficiency. Sci. Transl. Med. 2022, 14, eabn2956. [Google Scholar] [CrossRef]
- Gimbel, D.A.; Nygaard, H.B.; Coffey, E.E.; Gunther, E.C.; Lauren, J.; Gimbel, Z.A.; Strittmatter, S.M. Memory impairment in transgenic Alzheimer mice requires cellular prion protein. J. Neurosci. 2010, 30, 6367–6374. [Google Scholar] [CrossRef]
- Kamondi, A.; Grigg-Damberger, M.; Loscher, W.; Tanila, H.; Horvath, A.A. Epilepsy and epileptiform activity in late-onset Alzheimer disease: Clinical and pathophysiological advances, gaps and conundrums. Nat. Rev. Neurol. 2024, 20, 162–182. [Google Scholar] [CrossRef]
- Gureviciene, I.; Ishchenko, I.; Ziyatdinova, S.; Jin, N.; Lipponen, A.; Gurevicius, K.; Tanila, H. Characterization of Epileptic Spiking Associated With Brain Amyloidosis in APP/PS1 Mice. Front. Neurol. 2019, 10, 1151. [Google Scholar] [CrossRef]
- Fruehwald-Schultes, B.; Kern, W.; Born, J.; Fehm, H.L.; Peters, A. Hyperinsulinemia causes activation of the hypothalamus-pituitary-adrenal axis in humans. Int. J. Obes. Relat. Metab. Disord. 2001, 25 (Suppl. 1), S38–S40. [Google Scholar] [CrossRef] [PubMed]
- Arieff, A.I.; Doerner, T.; Zelig, H.; Massry, S.G. Mechanisms of seizures and coma in hypoglycemia. Evidence for a direct effect of insulin on electrolyte transport in brain. J. Clin. Investig. 1974, 54, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Hallschmid, M. Intranasal Insulin for Alzheimer’s Disease. CNS Drugs 2021, 35, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Craft, S.; Raman, R.; Chow, T.W.; Rafii, M.S.; Sun, C.K.; Rissman, R.A.; Donohue, M.C.; Brewer, J.B.; Jenkins, C.; Harless, K.; et al. Safety, Efficacy, and Feasibility of Intranasal Insulin for the Treatment of Mild Cognitive Impairment and Alzheimer Disease Dementia: A Randomized Clinical Trial. JAMA Neurol. 2020, 77, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Palop, J.J.; Chin, J.; Roberson, E.D.; Wang, J.; Thwin, M.T.; Bien-Ly, N.; Yoo, J.; Ho, K.O.; Yu, G.Q.; Kreitzer, A.; et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron 2007, 55, 697–711. [Google Scholar] [CrossRef]
- Minkeviciene, R.; Rheims, S.; Dobszay, M.B.; Zilberter, M.; Hartikainen, J.; Fulop, L.; Penke, B.; Zilberter, Y.; Harkany, T.; Pitkanen, A.; et al. Amyloid beta-induced neuronal hyperexcitability triggers progressive epilepsy. J. Neurosci. 2009, 29, 3453–3462. [Google Scholar] [CrossRef]
- Hoogmartens, J.; Hens, E.; Engelborghs, S.; De Deyn, P.P.; van der Zee, J.; Van Broeckhoven, C.; Cacace, R.; Consortium, B. Investigation of the role of matrix metalloproteinases in the genetic etiology of Alzheimer’s disease. Neurobiol. Aging 2021, 104, 105.e1–105.e6. [Google Scholar] [CrossRef]
- DiFrancesco, J.C.; Tremolizzo, L.; Polonia, V.; Giussani, G.; Bianchi, E.; Franchi, C.; Nobili, A.; Appollonio, I.; Beghi, E.; Ferrarese, C. Adult-Onset Epilepsy in Presymptomatic Alzheimer’s Disease: A Retrospective Study. J. Alzheimers Dis. 2017, 60, 1267–1274. [Google Scholar] [CrossRef]
- Alves, S.S.; de Oliveira, J.A.C.; Lazarini-Lopes, W.; Servilha-Menezes, G.; Grigorio-de-Sant, M.; Del Vecchio, F.; Mazzei, R.F.; Almeida, S.S.; da Silva Junior, R.M.P.; Garcia-Cairasco, N. Audiogenic Seizures in the Streptozotocin-Induced Rat Alzheimer’s Disease Model. J. Alzheimers Dis. 2023, 94, 1179–1196. [Google Scholar] [CrossRef]
- Geiss, G.K.; Bumgarner, R.E.; Birditt, B.; Dahl, T.; Dowidar, N.; Dunaway, D.L.; Fell, H.P.; Ferree, S.; George, R.D.; Grogan, T.; et al. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat. Biotechnol. 2008, 26, 317–325. [Google Scholar] [CrossRef]
- Elder, G.A.; Sosa, M.A.G.; De Gasperi, R. Transgenic mouse models of Alzheimer’s disease. Mt. Sinai J. Med. 2010, 77, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Du, Y.; Zhao, X.; Wu, C.; Yu, P. Reducing PDK1/Akt Activity: An Effective Therapeutic Target in the Treatment of Alzheimer’s Disease. Cells 2022, 11, 1735. [Google Scholar] [CrossRef] [PubMed]
- Thorsen, S.U.; Eising, S.; Mortensen, H.B.; Skogstrand, K.; Pociot, F.; Johannesen, J.; Svensson, J.; Danish Childhood Diabetes Registry. Systemic levels of CCL2, CCL3, CCL4 and CXCL8 differ according to age, time period and season among children newly diagnosed with type 1 diabetes and their healthy siblings. Scand. J. Immunol. 2014, 80, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Marciniak, E.; Faivre, E.; Dutar, P.; Pires, C.A.; Demeyer, D.; Caillierez, R.; Laloux, C.; Buee, L.; Blum, D.; Humez, S. The Chemokine MIP-1alpha/CCL3 impairs mouse hippocampal synaptic transmission, plasticity and memory. Sci. Rep. 2015, 5, 15862. [Google Scholar] [CrossRef] [PubMed]
- Wolinski, P.; Ksiazek-Winiarek, D.; Glabinski, A. Cytokines and Neurodegeneration in Epileptogenesis. Brain Sci. 2022, 12, 380. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Y.; Jiang, S.; Shi, F.D.; Shi, K.; Jin, W.N. Targeting CCL5 signaling attenuates neuroinflammation after seizure. CNS Neurosci. Ther. 2023, 29, 317–330. [Google Scholar] [CrossRef]
- Porcher, C.; Medina, I.; Gaiarsa, J.L. Mechanism of BDNF Modulation in GABAergic Synaptic Transmission in Healthy and Disease Brains. Front. Cell Neurosci. 2018, 12, 273. [Google Scholar] [CrossRef]
- Fischer, F.P.; Kasture, A.S.; Hummel, T.; Sucic, S. Molecular and Clinical Repercussions of GABA Transporter 1 Variants Gone Amiss: Links to Epilepsy and Developmental Spectrum Disorders. Front. Mol. Biosci. 2022, 9, 834498. [Google Scholar] [CrossRef]
- Gliwinska, A.; Czubilinska-Lada, J.; Wieckiewicz, G.; Swietochowska, E.; Badenski, A.; Dworak, M.; Szczepanska, M. The Role of Brain-Derived Neurotrophic Factor (BDNF) in Diagnosis and Treatment of Epilepsy, Depression, Schizophrenia, Anorexia Nervosa and Alzheimer’s Disease as Highly Drug-Resistant Diseases: A Narrative Review. Brain Sci. 2023, 13, 163. [Google Scholar] [CrossRef]
- Reibel, S.; Depaulis, A.; Larmet, Y. BDNF and epilepsy--the bad could turn out to be good. Trends Neurosci. 2001, 24, 318–319. [Google Scholar] [CrossRef]
- Scharfman, H.E.; Goodman, J.H.; Sollas, A.L.; Croll, S.D. Spontaneous limbic seizures after intrahippocampal infusion of brain-derived neurotrophic factor. Exp. Neurol. 2002, 174, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, Y.; Kitamura, A.; Wake, H.; Ishibashi, H.; Watanabe, M.; Nishimaki, T.; Nabekura, J. BDNF occludes GABA receptor-mediated inhibition of GABA release in rat hippocampal CA1 pyramidal neurons. Eur. J. Neurosci. 2006, 24, 2135–2144. [Google Scholar] [CrossRef] [PubMed]
- Johannesen, K.M.; Nielsen, J.; Sabers, A.; Isidor, B.; Kattentidt-Mouravieva, A.A.; Zieglgansberger, D.; Heidlebaugh, A.R.; Oetjens, K.F.; Vidal, A.A.; Christensen, J.; et al. The phenotypic presentation of adult individuals with SLC6A1-related neurodevelopmental disorders. Front. Neurosci. 2023, 17, 1216653. [Google Scholar] [CrossRef]
- Devries, S.; Mulder, M.; Charron, J.G.; Prokop, J.W.; Mark, P.R. SLC6A1 G443D associated with developmental delay and epilepsy. Cold Spring Harb. Mol. Case Stud. 2020, 6, a005371. [Google Scholar] [CrossRef]
- Cousin, M.A. Synaptophysin-dependent synaptobrevin-2 trafficking at the presynapse-Mechanism and function. J. Neurochem. 2021, 159, 78–89. [Google Scholar] [CrossRef]
- Harper, C.B.; Mancini, G.M.S.; van Slegtenhorst, M.; Cousin, M.A. Altered synaptobrevin-II trafficking in neurons expressing a synaptophysin mutation associated with a severe neurodevelopmental disorder. Neurobiol. Dis. 2017, 108, 298–306. [Google Scholar] [CrossRef]
- Xin, Y.; Lin, G.; Hua, T.; Liang, J.; Sun, T.; Wu, X. The altered expression of cytoskeletal and synaptic remodeling proteins during epilepsy. Open Life Sci. 2023, 18, 20220595. [Google Scholar] [CrossRef]
- Liu, J.J.; Bello, N.T.; Pang, Z.P. Presynaptic Regulation of Leptin in a Defined Lateral Hypothalamus-Ventral Tegmental Area Neurocircuitry Depends on Energy State. J. Neurosci. 2017, 37, 11854–11866. [Google Scholar] [CrossRef]
- Walker, C.D.; Long, H.; Williams, S.; Richard, D. Long-lasting effects of elevated neonatal leptin on rat hippocampal function, synaptic proteins and NMDA receptor subunits. J. Neurosci. Res. 2007, 85, 816–828. [Google Scholar] [CrossRef]
- Li, X.; Feng, Y.; Wu, W.; Zhao, J.; Fu, C.; Li, Y.; Ding, Y.; Wu, B.; Gong, Y.; Yang, G.; et al. Sex differences between APPswePS1dE9 mice in A-beta accumulation and pancreatic islet function during the development of Alzheimer’s disease. Lab. Anim. 2016, 50, 275–285. [Google Scholar] [CrossRef]
- Webster, S.J.; Bachstetter, A.D.; Nelson, P.T.; Schmitt, F.A.; Van Eldik, L.J. Using mice to model Alzheimer’s dementia: An overview of the clinical disease and the preclinical behavioral changes in 10 mouse models. Front. Genet. 2014, 5, 88. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.; Hamilton, A.M.; Furberg, H.; Pietzak, E.; Purdue, M.P.; Troester, M.A.; Hoadley, K.A.; Love, M.I. An approach for normalization and quality control for NanoString RNA expression data. Brief. Bioinform. 2021, 22, bbaa163. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, research0034. [Google Scholar] [CrossRef] [PubMed]
- Risso, D.; Ngai, J.; Speed, T.P.; Dudoit, S. Normalization of RNA-seq data using factor analysis of control genes or samples. Nat. Biotechnol. 2014, 32, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef]
- Carrera, I.; Etcheverria, I.; Fernandez-Novoa, L.; Lombardi, V.R.; Lakshmana, M.K.; Cacabelos, R.; Vigo, C. A comparative evaluation of a novel vaccine in APP/PS1 mouse models of Alzheimer’s disease. Biomed. Res. Int. 2015, 2015, 807146. [Google Scholar] [CrossRef]
- O’Leary, T.P.; Brown, R.E. Visuo-spatial learning and memory deficits on the Barnes maze in the 16-month-old APPswe/PS1dE9 mouse model of Alzheimer’s disease. Behav. Brain Res. 2009, 201, 120–127. [Google Scholar] [CrossRef]
- Palop, J.J.; Chin, J.; Mucke, L. A network dysfunction perspective on neurodegenerative diseases. Nature 2006, 443, 768–773. [Google Scholar] [CrossRef]
- Kumar, A.; Chugani, H.T. The Role of Radionuclide Imaging in Epilepsy, Part 1: Sporadic Temporal and Extratemporal Lobe Epilepsy. J. Nucl. Med. Technol. 2017, 45, 14–21. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, Z.; Lee, A.; Lin, T.; Taneja, S.; Kowdley, D.; Leung, J.H.; Hill, M.; Tao, T.; Fitzgerald, J.; Yu, L.; et al. Amino Acid Compound 2 (AAC2) Treatment Counteracts Insulin-Induced Synaptic Gene Expression and Seizure-Related Mortality in a Mouse Model of Alzheimer’s Disease. Int. J. Mol. Sci. 2024, 25, 11689. https://doi.org/10.3390/ijms252111689
Deng Z, Lee A, Lin T, Taneja S, Kowdley D, Leung JH, Hill M, Tao T, Fitzgerald J, Yu L, et al. Amino Acid Compound 2 (AAC2) Treatment Counteracts Insulin-Induced Synaptic Gene Expression and Seizure-Related Mortality in a Mouse Model of Alzheimer’s Disease. International Journal of Molecular Sciences. 2024; 25(21):11689. https://doi.org/10.3390/ijms252111689
Chicago/Turabian StyleDeng, Zhijie, Aejin Lee, Tao Lin, Sagarika Taneja, Devan Kowdley, Jacob H. Leung, Marykate Hill, Tianyi Tao, Julie Fitzgerald, Lianbo Yu, and et al. 2024. "Amino Acid Compound 2 (AAC2) Treatment Counteracts Insulin-Induced Synaptic Gene Expression and Seizure-Related Mortality in a Mouse Model of Alzheimer’s Disease" International Journal of Molecular Sciences 25, no. 21: 11689. https://doi.org/10.3390/ijms252111689
APA StyleDeng, Z., Lee, A., Lin, T., Taneja, S., Kowdley, D., Leung, J. H., Hill, M., Tao, T., Fitzgerald, J., Yu, L., Blakeslee, J. J., Townsend, K., Weil, Z. M., Parquette, J. R., & Ziouzenkova, O. (2024). Amino Acid Compound 2 (AAC2) Treatment Counteracts Insulin-Induced Synaptic Gene Expression and Seizure-Related Mortality in a Mouse Model of Alzheimer’s Disease. International Journal of Molecular Sciences, 25(21), 11689. https://doi.org/10.3390/ijms252111689





