Melatonin Maintains Postharvest Quality in Fresh Gastrodia elata Tuber by Regulating Antioxidant Ability and Phenylpropanoid and Energy Metabolism During Storage
Abstract
1. Introduction
2. Results
2.1. Influence of Melatonin on Spoilage, Respiratory Rate, Malondialdehyde (MDA), Soluble Solid Content (SSC), and Weight Loss in G. elata Tubers
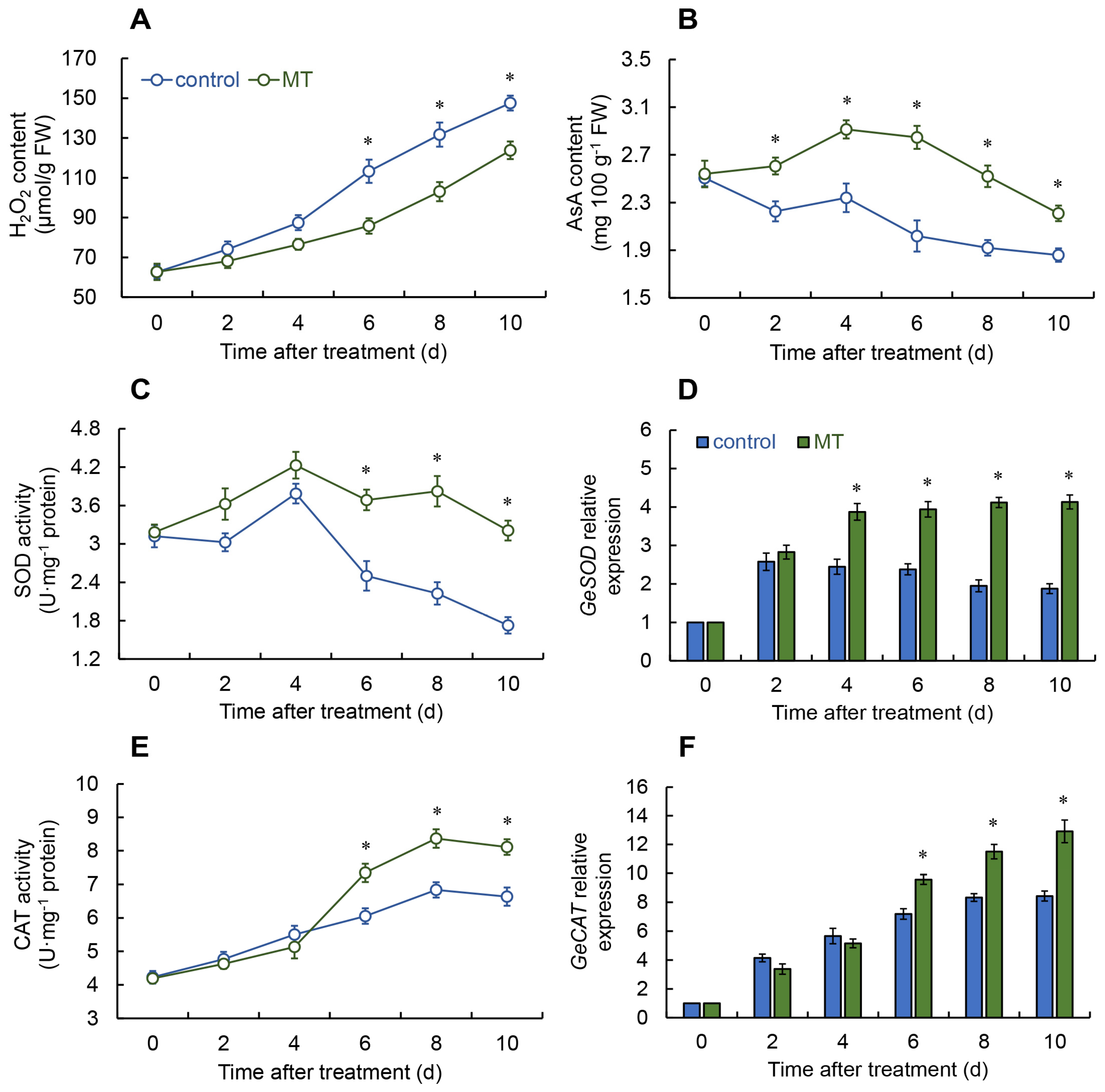
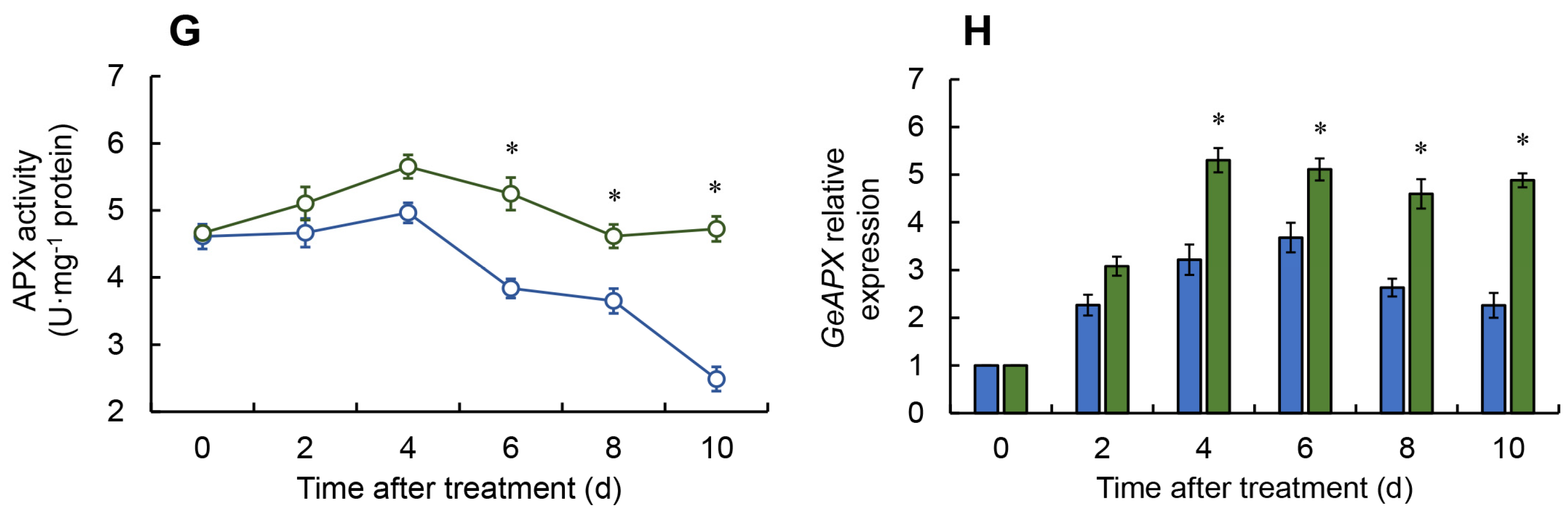
2.2. Effect of Melatonin on ROS Metabolism
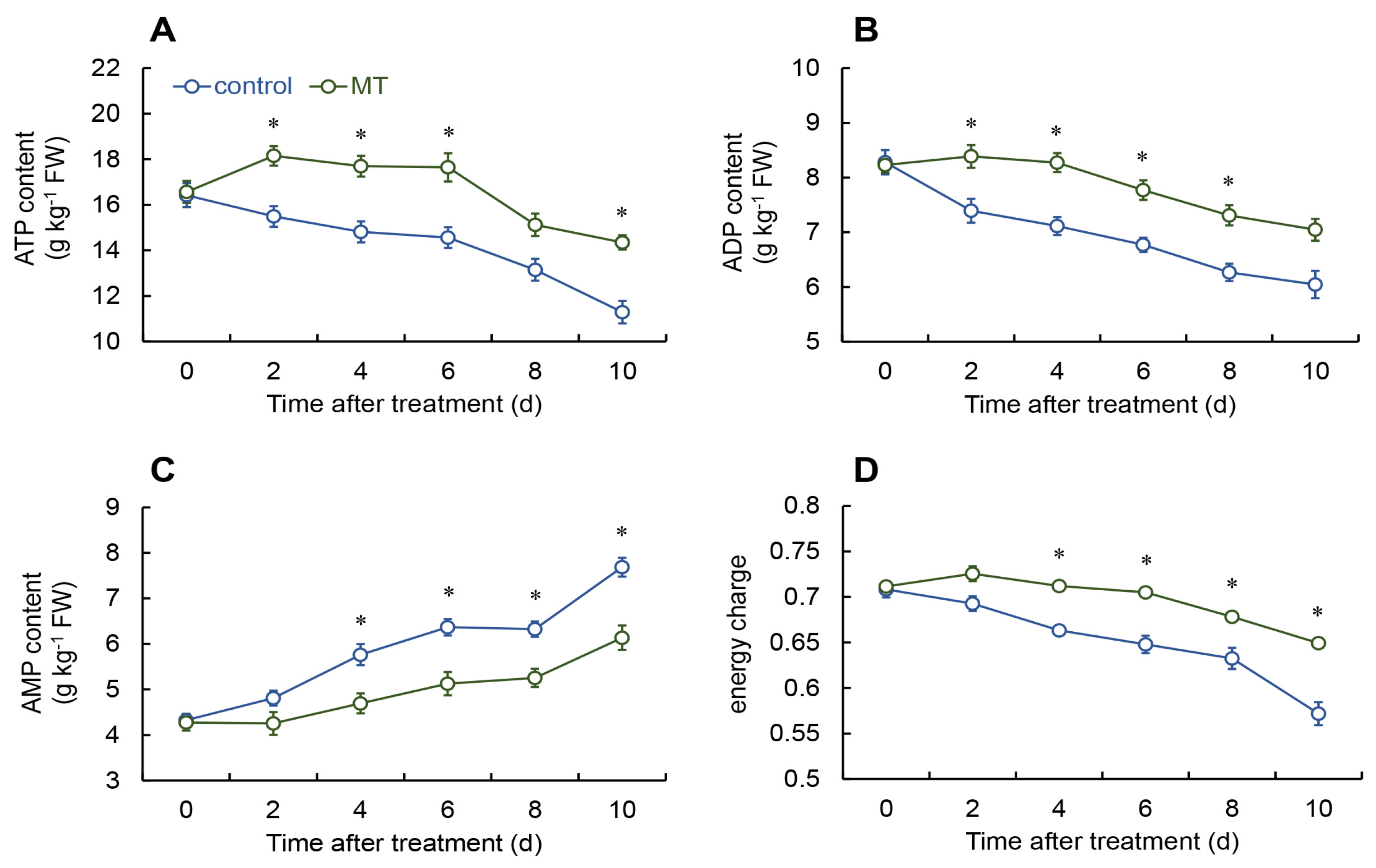
2.3. Measure the Levels of Adenosine Triphosphate (ATP), Adenosine Diphosphate (ADP), Adenosine Monophosphate (AMP), and Energy Charge
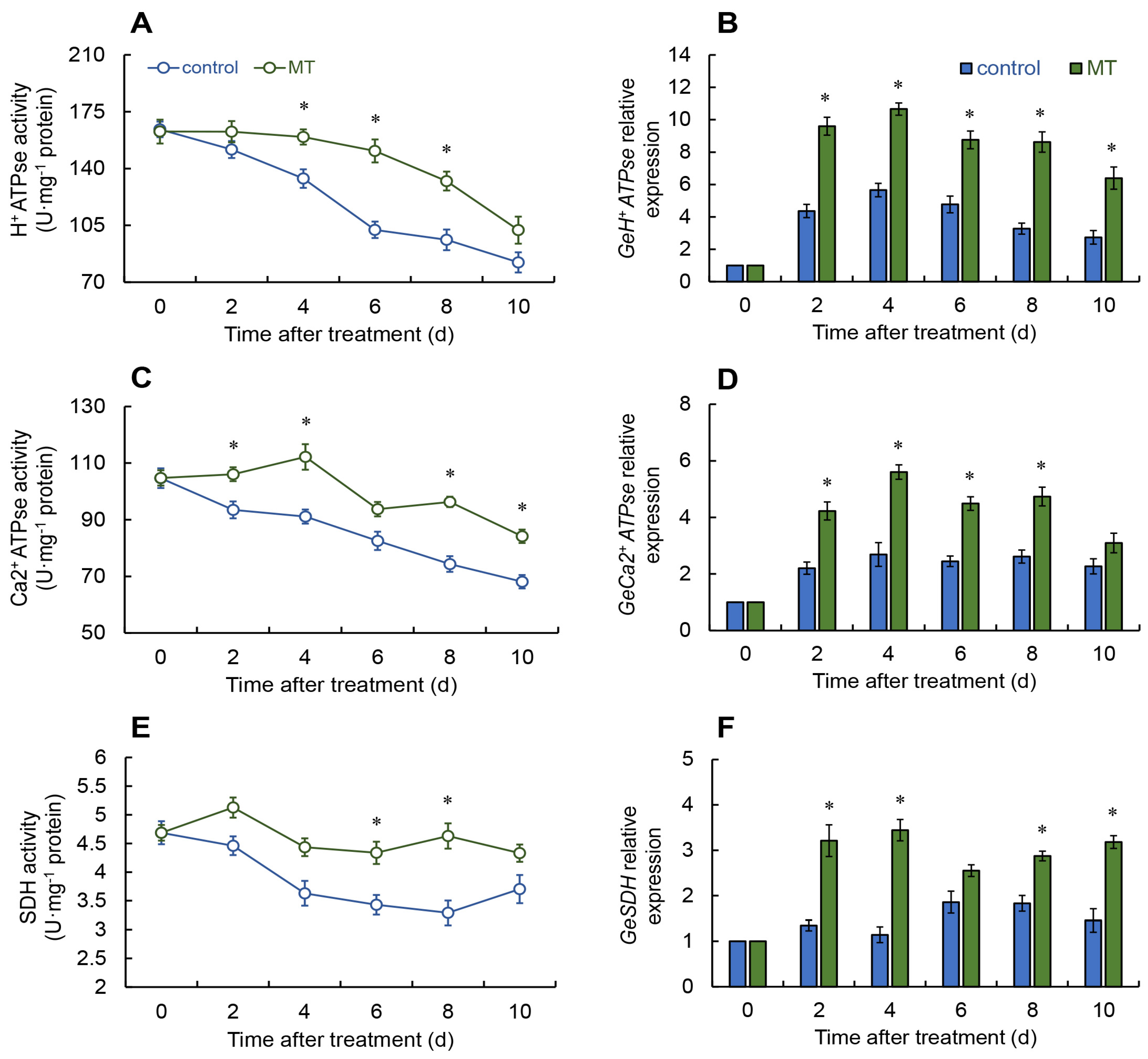
2.4. Assessment of Energy Metabolism Enzyme Activities
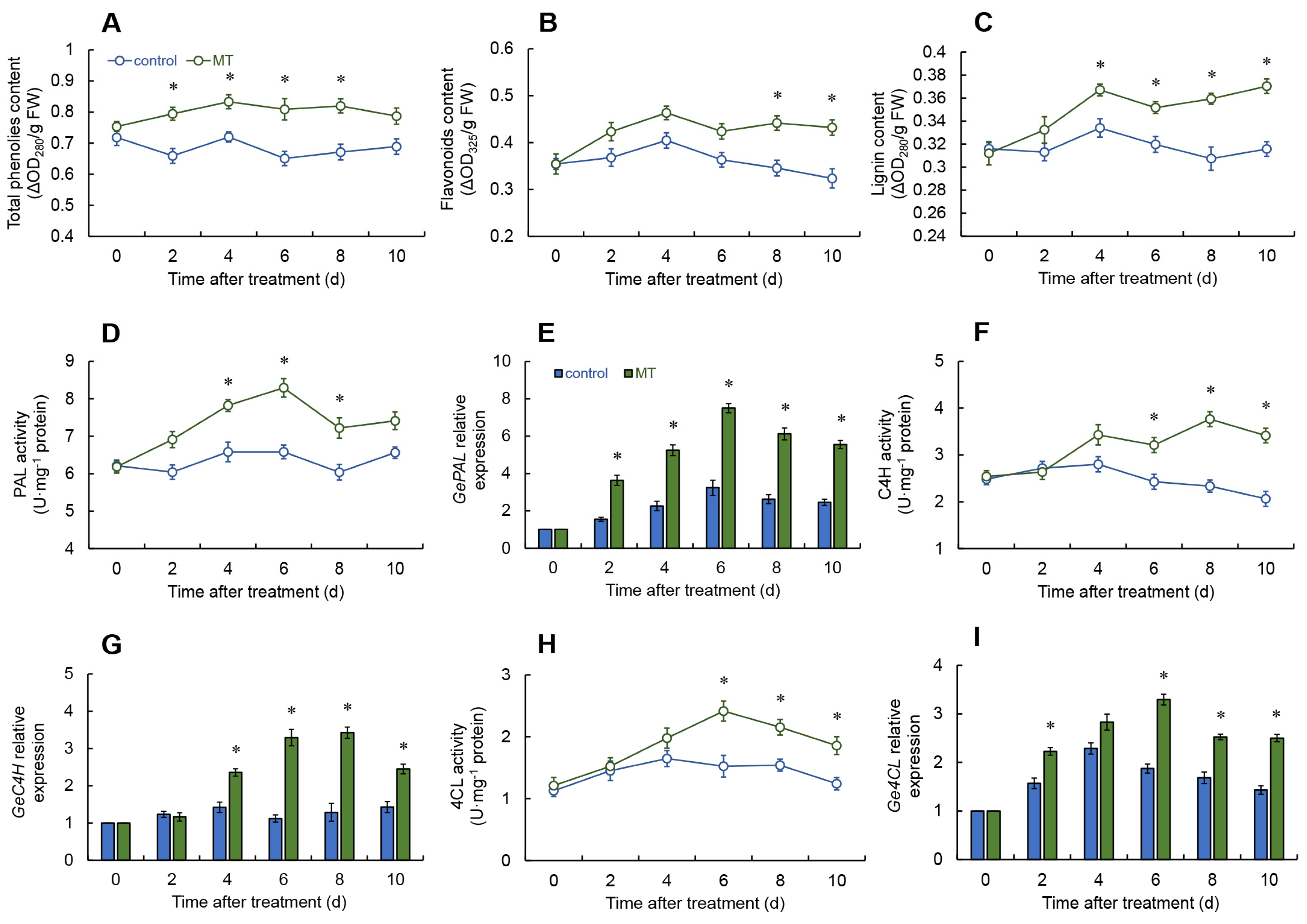
2.5. Effects of Melatonin Treatment on Total Phenolic, Flavonoid, and Lignin Contents
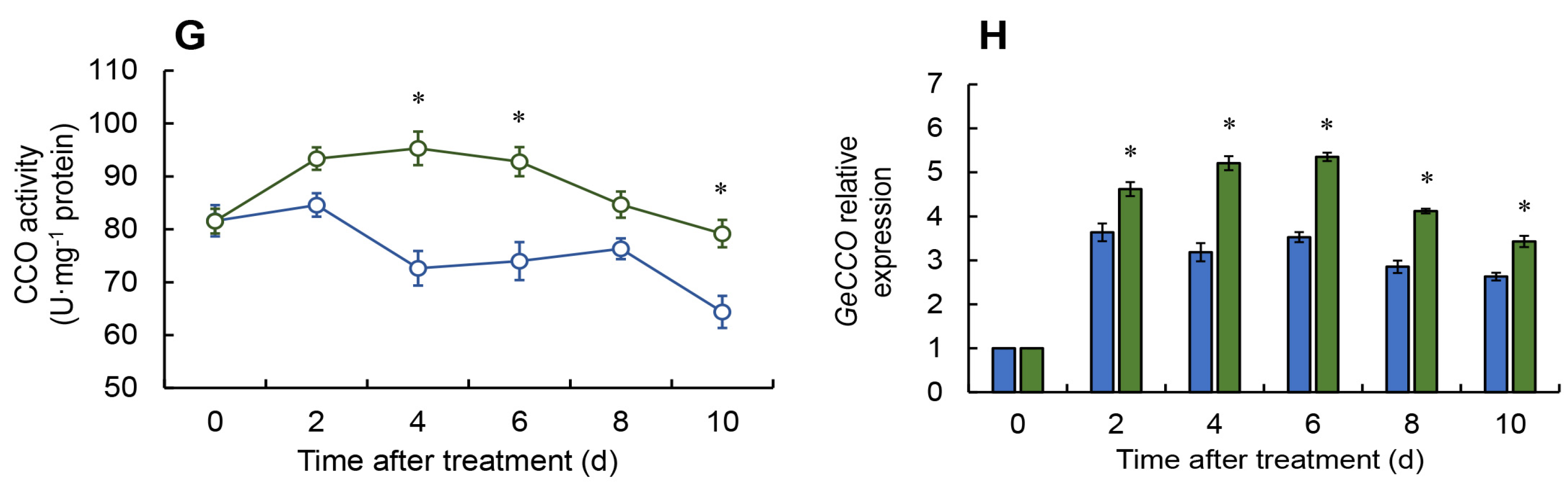
2.6. Effect of Melatonin on Phenylpropanoid Pathway
3. Discussion
4. Materials and Methods
4.1. G. elata and Chemicals
4.2. Treatment
4.3. G. elata Tubers Collection
4.4. Determination of Respiratory Rate, MDA, Weight Loss, Deterioration Rate, and SSC
4.5. Determination of H2O2 and AsA Content and Antioxidant-Related Enzyme Activity
4.6. Determine the Content of AMP, ADP, and ATP, Energy Charge, and Energy Metabolism-Related Enzyme Activity Determination
4.7. Analysis of Enzyme Activities and Metabolite Contents Related to the Phenylpropanoid Pathway
4.8. RNA Extraction and First-Strand cDNA Synthesis
4.9. Real-Time Quantitative (RT-PCR)
4.10. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fan, S.; Tian, W.; Wang, Q.; Shangguan, C.; Liu, X.; Zhang, X.; Chen, C. Evaluation of the changes in active substances and their effects on intestinal microflora during simulated digestion of Gastrodia elata. LWT-Food Sci. Technol. 2022, 169, 113924. [Google Scholar] [CrossRef]
- Guan, J.; Chen, Z.; Guo, L.; Cui, X.; Xu, T.; Wan, F.; Yang, Y. Evaluate how steaming and sulfur fumigation modify the microstructure, physicochemical properties and in vitro digestibility of Gastrodia elata Bl. starch. Front. Nutr. 2023, 9, 3183. [Google Scholar] [CrossRef] [PubMed]
- Badiche-El Hilali, F.; Valverde, J.M.; García-Pastor, M.E.; Serrano, M.; Castillo, S.; Valero, D. Melatonin postharvest treatment in leafy ‘Fino’ lemon maintains quality and bioactive compounds. Foods 2023, 12, 2979. [Google Scholar] [CrossRef]
- Cai, S.; Zhang, Z.; Wang, J.; Fu, Y.; Zhang, Z.; Khan, M.R.; Cong, X. Effect of exogenous melatonin on postharvest storage quality of passion fruit through antioxidant metabolism. LWT-Food Sci. Technol. 2024, 194, 115835. [Google Scholar] [CrossRef]
- Huang, T.; Kou, X.; Qiao, L.; Li, J.; Luo, D.; Wang, X.; Cao, S. Maintaining quality of postharvest green pepper fruit using melatonin by regulating membrane lipid metabolism and enhancing antioxidant capacity. Food Chem. 2024, 460, 140671. [Google Scholar] [CrossRef]
- Li, L.; Kitazawa, H.; Zhang, X.; Zhang, L.; Sun, Y.; Wang, X.; Yu, S. Melatonin retards senescence via regulation of the electron leakage of postharvest white mushroom (Agaricus bisporus). Food Chem. 2021, 340, 127833. [Google Scholar] [CrossRef]
- Promyou, S.; Raruang, Y.; Chen, Z.Y. Melatonin treatment of strawberry fruit during storage extends its post-harvest quality and reduces infection caused by Botrytis cinerea. Foods 2023, 12, 1445. [Google Scholar] [CrossRef]
- Li, X.; Li, C.; Cheng, Y.; Hou, J.; Zhang, J.; Ge, Y. Postharvest application of acibenzolar-S-methyl delays the senescence of pear fruit by regulating reactive oxygen species and fatty acid metabolism. J. Agric. Food Chem. 2020, 68, 4991–4999. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Ge, Y.; Li, C.; Han, X.; Qin, S.; Chen, Y.; Li, J. G6PDH regulated NADPH production and reactive oxygen species metabolism to enhance disease resistance against blue mold in apple fruit by acibenzolar-S-methyl. Postharvest Biol. Technol. 2019, 148, 228–235. [Google Scholar] [CrossRef]
- Li, C.; Wei, M.; Ge, Y.; Zhao, J.; Chen, Y.; Hou, J.; Li, J. The role of glucose-6-phosphate dehydrogenase in reactive oxygen species metabolism in apple exocarp induced by acibenzolar-S-methyl. Food Chem. 2020, 308, 125663. [Google Scholar] [CrossRef]
- Li, F.; Hu, Y.; Shan, Y.; Liu, J.; Ding, X.; Duan, X.; Jiang, Y. Hydrogen-rich water maintains the color quality of fresh-cut Chinese water chestnut. Postharvest Biol. Technol. 2022, 183, 111743. [Google Scholar] [CrossRef]
- Ge, Y.; Duan, B.; Li, C.; Tang, Q.; Li, X.; Wei, M.; Li, J. γ-Aminobutyric acid delays senescence of blueberry fruit by regulation of reactive oxygen species metabolism and phenylpropanoid pathway. Sci. Hortic. 2018, 240, 303–309. [Google Scholar] [CrossRef]
- Lu, D.; Ren, Y.; Yan, T.; Jia, X.; Xu, H.; Yang, B.; He, J. Melatonin improves the postharvest anthracnose resistance of mango fruit by regulating antioxidant activity, the phenylpropane pathway and cell wall metabolism. Eur. J. Plant Pathol. 2024, 1–20. [Google Scholar] [CrossRef]
- Yu, C.; Zeng, L.; Sheng, K.; Chen, F.; Zhou, T.; Zheng, X.; Yu, T. γ-Aminobutyric acid induces resistance against Penicillium expansum by priming of defence responses in pear fruit. Food Chem. 2014, 159, 29–37. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, D.; Gao, X.; Yue, Z.; Zhou, H. Exogenous caffeic acid and epicatechin enhance resistance against Botrytis cinerea through activation of the phenylpropanoid pathway in apples. Sci. Hortic. 2020, 268, 109348. [Google Scholar] [CrossRef]
- Li, S.; Jiang, H.; Wang, Y.; Lyu, L.; Prusky, D.; Ji, Y.; Bi, Y. Effect of benzothiadiazole treatment on improving the mitochondrial energy metabolism involved in induced resistance of apple fruit during postharvest storage. Food Chem. 2020, 302, 125288. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, J.; Wei, M.; Ge, Y.; Hou, J.; Cheng, Y.; Chen, J. Methyl jasmonate maintained antioxidative ability of ginger rhizomes by regulating antioxidant enzymes and energy metabolism. Sci. Hortic. 2019, 256, 108578. [Google Scholar] [CrossRef]
- Zuo, X.; Cao, S.; Jia, W.; Zhao, Z.; Jin, P.; Zheng, Y. Near-saturated relative humidity alleviates chilling injury in zucchini fruit through its regulation of antioxidant response and energy metabolism. Food Chem. 2021, 351, 129336. [Google Scholar] [CrossRef]
- Shu, C.; Zhang, W.; Zhao, H.; Cao, J.; Jiang, W. Chlorogenic acid treatment alleviates the adverse physiological responses of vibration injury in apple fruit through the regulation of energy metabolism. Postharvest Biol. Technol. 2020, 159, 110997. [Google Scholar] [CrossRef]
- Ge, Y.; Li, X.; Li, C.; Tang, Q.; Duan, B.; Cheng, Y.; Li, J. Effect of sodium nitroprusside on antioxidative enzymes and the phenylpropanoid pathway in blueberry fruit. Food Chem. 2019, 295, 607–612. [Google Scholar] [CrossRef]
- Tan, X.L.; Fan, Z.Q.; Zeng, Z.X.; Shan, W.; Kuang, J.F.; Lu, W.J.; Zhao, Y.T. Exogenous melatonin maintains leaf quality of postharvest Chinese flowering cabbage by modulating respiratory metabolism and energy status. Postharvest Biol. Technol. 2021, 177, 111524. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Nawaz, G.; Zhao, C.; Li, Y.; Dong, T.; Xu, T. Exogenous melatonin attenuates post-harvest decay by increasing antioxidant activity in wax apple (Syzygium samarangense). Front. Plant Sci. 2020, 11, 569779. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhang, Y.; Yun, Z.; Hu, M.; Liu, J.; Jiang, Y.; Zhang, Z. Melatonin enhances cold tolerance by regulating energy and proline metabolism in litchi fruit. Foods 2020, 9, 454. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Li, C.; Fan, Y.; Qu, L.; Huang, R.; Liu, J.; Ge, Y. γ-Aminobutyric acid regulates mitochondrial energy metabolism and organic acids metabolism in apples during postharvest ripening. Postharvest Biol. Technol. 2022, 186, 111846. [Google Scholar] [CrossRef]
- Ma, Q.; Lin, X.; Wei, Q.; Yang, X.; Zhang, Y.N.; Chen, J. Melatonin treatment delays postharvest senescence and maintains the organoleptic quality of ‘Newhall’navel orange (Citrus sinensis (L.) Osbeck) by inhibiting respiration and enhancing antioxidant capacity. Sci. Hortic. 2021, 286, 110236. [Google Scholar] [CrossRef]
- Xia, H.; Shen, Y.; Shen, T.; Wang, X.; Zhang, X.; Hu, P.; Lv, X. Melatonin accumulation in sweet cherry and its influence on fruit quality and antioxidant properties. Molecules 2020, 25, 753. [Google Scholar] [CrossRef]
- Waszczak, C.; Carmody, M.; Kangasjärvi, J. Reactive oxygen species in plant signaling. Annu. Rev. Plant Biol. 2018, 69, 209–236. [Google Scholar] [CrossRef]
- Razzaq, K.; Khan, A.S.; Malik, A.U.; Shahid, M. Ripening period influences fruit softening and antioxidative system of ‘Samar Bahisht Chaunsa’ mango. Sci. Hortic. 2013, 160, 108–114. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, M.; Zhang, W.; Gao, Y.; Ma, X.; Cheng, S.; Chen, G. Exogenous melatonin activates the antioxidant system and maintains postharvest organoleptic quality in Hami melon (Cucumis. melo var. inodorus Jacq.). Front. Plant Sci. 2023, 14, 1274939. [Google Scholar] [CrossRef]
- Rastegar, S.; Khankahdani, H.H.; Rahimzadeh, M. Effects of melatonin treatment on the biochemical changes and antioxidant enzyme activity of mango fruit during storage. Sci. Hortic. 2020, 259, 108835. [Google Scholar] [CrossRef]
- Guo, Y.; Li, X.; Li, C.; Jinyue, R.; Xu, H.; Ge, Y. Acibenzolar-S-methyl activates phenylpropanoid pathway to enhance resistance against Alternaria alternata in pear fruit. J. Sci. Food Agric. 2023, 103, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Xu, D.; Liu, C.; Chen, C.; Tian, M.; Jiang, A. Ascorbic acid treatment inhibits wound healing of fresh-cut potato strips by controlling phenylpropanoid metabolism. Postharvest Biol. Technol. 2021, 181, 111644. [Google Scholar] [CrossRef]
- Dong, B.; Tang, H.; Zhu, D.; Yao, Q.; Han, H.; He, K.; Ding, X. Benzothiazole treatment regulates the reactive oxygen species metabolism and phenylpropanoid pathway of Rosa roxburghii fruit to delay senescence during low temperature storage. Front. Plant Sci. 2021, 12, 753261. [Google Scholar] [CrossRef] [PubMed]
- Aghdam, M.S.; Luo, Z.; Li, L.; Jannatizadeh, A.; Fard, J.R.; Pirzad, F. Melatonin treatment maintains nutraceutical properties of pomegranate fruits during cold storage. Food Chem. 2020, 303, 125385. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Xiong, T.; Lei, Q.; Tan, Q.; Cai, J.; Song, Z.; Zhu, X. Melatonin treatment improves postharvest preservation and resistance of guava fruit (Psidium guajava L.). Foods 2022, 11, 262. [Google Scholar] [CrossRef]
- Fan, S.; Li, Q.; Feng, S.; Lei, Q.; Abbas, F.; Yao, Y.; Zhu, X. Melatonin maintains fruit quality and reduces anthracnose in postharvest papaya via enhancement of antioxidants and inhibition of pathogen development. Antioxidants 2022, 11, 804. [Google Scholar] [CrossRef]
- Carrión-Antolí, A.; Martínez-Romero, D.; Guillén, F.; Zapata, P.J.; Serrano, M.; Valero, D. Melatonin pre-harvest treatments leads to maintenance of sweet cherry quality during storage by increasing antioxidant systems. Front. Plant Sci. 2022, 13, 863467. [Google Scholar] [CrossRef]
- Li, D.; Wang, D.; Fang, Y.; Li, L.; Lin, X.; Xu, Y.; Luo, Z. A novel phase change coolant promoted quality attributes and glutamate accumulation in postharvest shiitake mushrooms involved in energy metabolism. Food Chem. 2021, 351, 129227. [Google Scholar] [CrossRef]
- Huan, C.; Li, H.; Jiang, Z.; Shen, S.; Zheng, X. Effect of hypobaric treatment on off-flavour development and energy metabolism in ‘Bruno’ kiwi fruit. LWT-Food Sci. Technol. 2021, 136, 110349. [Google Scholar] [CrossRef]
- Jin, P.; Zhu, H.; Wang, L.; Shan, T.; Zheng, Y. Oxalic acid alleviates chilling injury in peach fruit by regulating energy metabolism and fatty acid contents. Food Chem. 2014, 161, 87–93. [Google Scholar] [CrossRef]
- Duan, B.; Ge, Y.; Li, C.; Gao, X.; Tang, Q.; Li, X.; Chen, Y. Effect of exogenous ATP treatment on sucrose metabolism and quality of Nanguo pear fruit. Sci. Hortic. 2019, 249, 71–76. [Google Scholar] [CrossRef]
- Tao, D.; Wang, J.; Zhang, L.; Jiang, Y.; Lv, M. 1-Methylcyclopropene alleviates peel browning of ‘Nanguo’pears by regulating energy, antioxidant and lipid metabolisms after long term refrigeration. Sci. Hortic. 2019, 247, 254–263. [Google Scholar] [CrossRef]
- Wang, T.; Hu, M.; Yuan, D.; Yun, Z.; Gao, Z.; Su, Z.; Zhang, Z. Melatonin alleviates pericarp browning in litchi fruit by regulating membrane lipid and energy metabolisms. Postharvest Biol. Technol. 2020, 160, 111066. [Google Scholar] [CrossRef]
- Luo, S.; Hu, H.; Wang, Y.; Zhou, H.; Zhang, Y.; Zhang, L.; Li, P. The role of melatonin in alleviating the postharvest browning of lotus seeds through energy metabolism and membrane lipid metabolism. Postharvest Biol. Technol. 2020, 167, 111243. [Google Scholar] [CrossRef]
- Jannatizadeh, A.; Aghdam, M.S.; Luo, Z.; Razavi, F. Impact of exogenous melatonin application on chilling injury in tomato fruits during cold storage. Food Bioprocess Technol. 2019, 12, 741–750. [Google Scholar] [CrossRef]
- Jincy, M.; Djanaguiraman, M.; Jeyakumar, P.; Subramanian, K.S.; Jayasankar, S.; Paliyath, G. Inhibition of phospholipase D enzyme activity through hexanal leads to delayed mango (Mangifera indica L.) fruit ripening through changes in oxidants and antioxidant enzymes activity. Sci. Hortic. 2017, 218, 316–325. [Google Scholar] [CrossRef]
- Liu, Y.; Ge, Y.; Bi, Y.; Li, C.; Deng, H.; Hu, L.; Dong, B. Effect of postharvest acibenzolar-S-methyl dipping on phenylpropanoid pathway metabolism in muskmelon (Cucumis melo L.) fruits. Sci. Hortic. 2014, 168, 113–119. [Google Scholar] [CrossRef]
- Li, Z.; Li, B.; Li, M.; Fu, X.; Zhao, X.; Min, D.; Zhang, X. Hot air pretreatment alleviates browning of fresh-cut pitaya fruit by regulating phenylpropanoid pathway and ascorbate-glutathione cycle. Postharvest Biol. Technol. 2022, 190, 111954. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, B.; Kuang, C.; Chen, Y.; Da, F.; Yao, Q.; Zhu, D.; Ding, X. Melatonin Maintains Postharvest Quality in Fresh Gastrodia elata Tuber by Regulating Antioxidant Ability and Phenylpropanoid and Energy Metabolism During Storage. Int. J. Mol. Sci. 2024, 25, 11752. https://doi.org/10.3390/ijms252111752
Dong B, Kuang C, Chen Y, Da F, Yao Q, Zhu D, Ding X. Melatonin Maintains Postharvest Quality in Fresh Gastrodia elata Tuber by Regulating Antioxidant Ability and Phenylpropanoid and Energy Metabolism During Storage. International Journal of Molecular Sciences. 2024; 25(21):11752. https://doi.org/10.3390/ijms252111752
Chicago/Turabian StyleDong, Boyu, Chengyue Kuang, Yulong Chen, Fangfang Da, Qiuping Yao, Dequan Zhu, and Xiaochun Ding. 2024. "Melatonin Maintains Postharvest Quality in Fresh Gastrodia elata Tuber by Regulating Antioxidant Ability and Phenylpropanoid and Energy Metabolism During Storage" International Journal of Molecular Sciences 25, no. 21: 11752. https://doi.org/10.3390/ijms252111752
APA StyleDong, B., Kuang, C., Chen, Y., Da, F., Yao, Q., Zhu, D., & Ding, X. (2024). Melatonin Maintains Postharvest Quality in Fresh Gastrodia elata Tuber by Regulating Antioxidant Ability and Phenylpropanoid and Energy Metabolism During Storage. International Journal of Molecular Sciences, 25(21), 11752. https://doi.org/10.3390/ijms252111752




