Structural Features of DNA in tRNA Genes and Their Upstream Sequences
Abstract
1. Introduction
2. Results
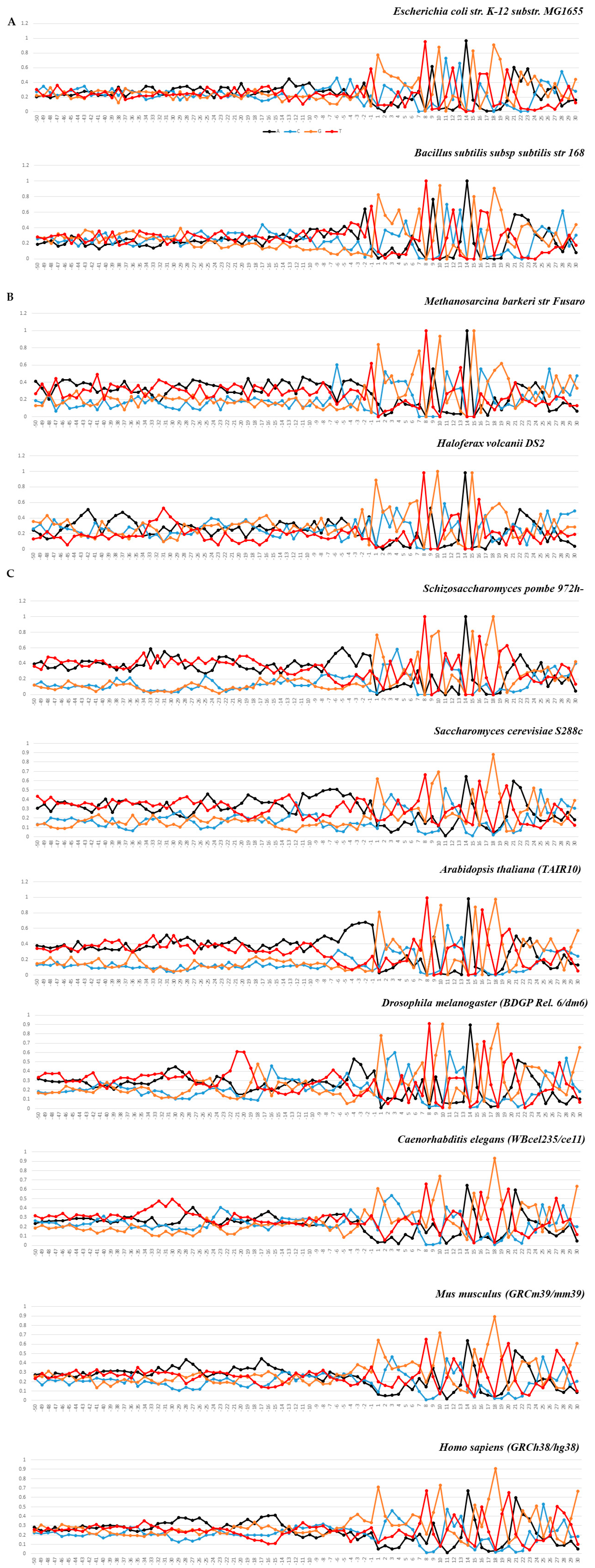
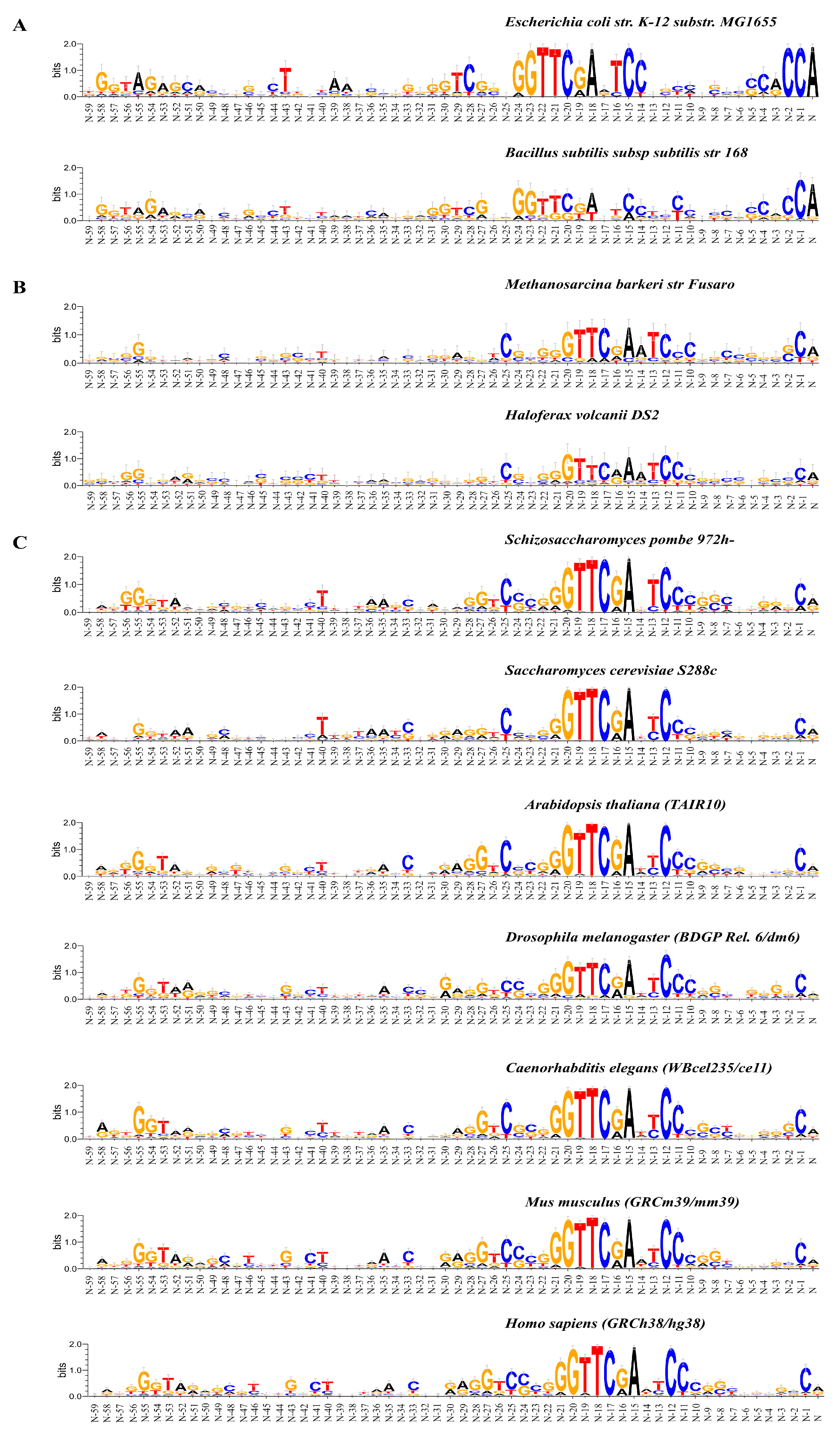
2.1. Statistical Characteristics of the Nucleotide Sequences of the tRNA Genes and Their Upstream Regions in Bacteria, Archaea, and Eukarya
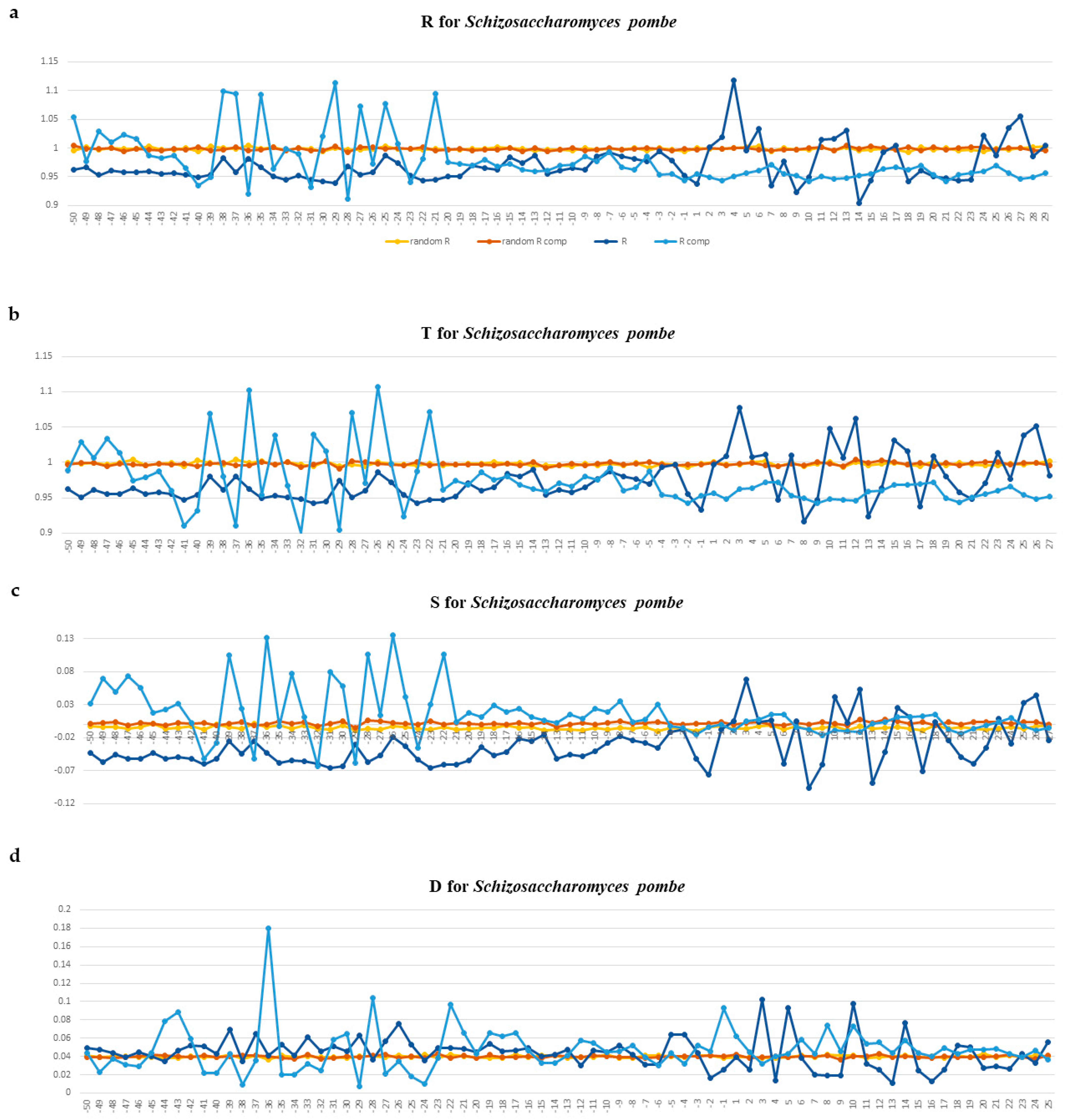
2.1.1. Characteristics of Nucleotide Texts
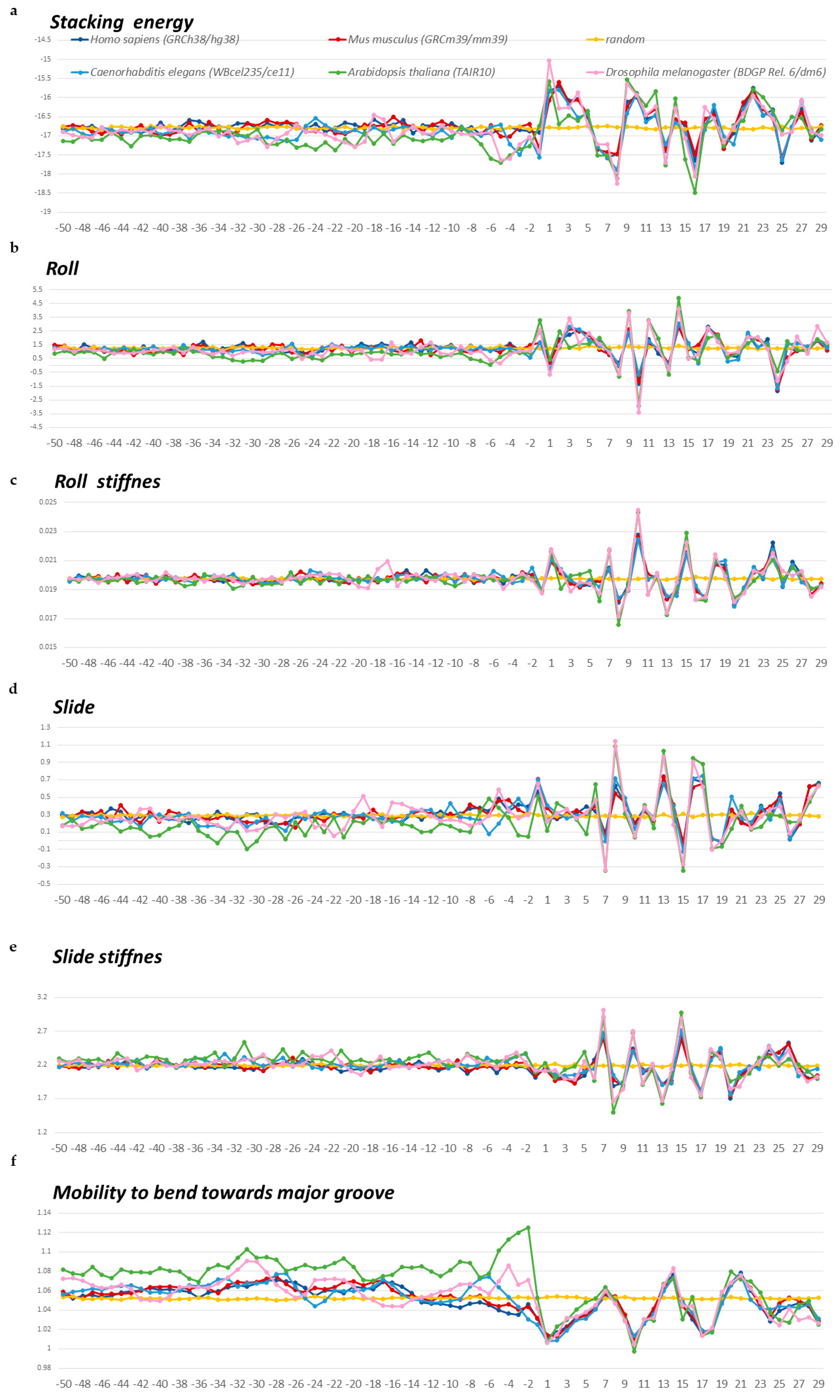
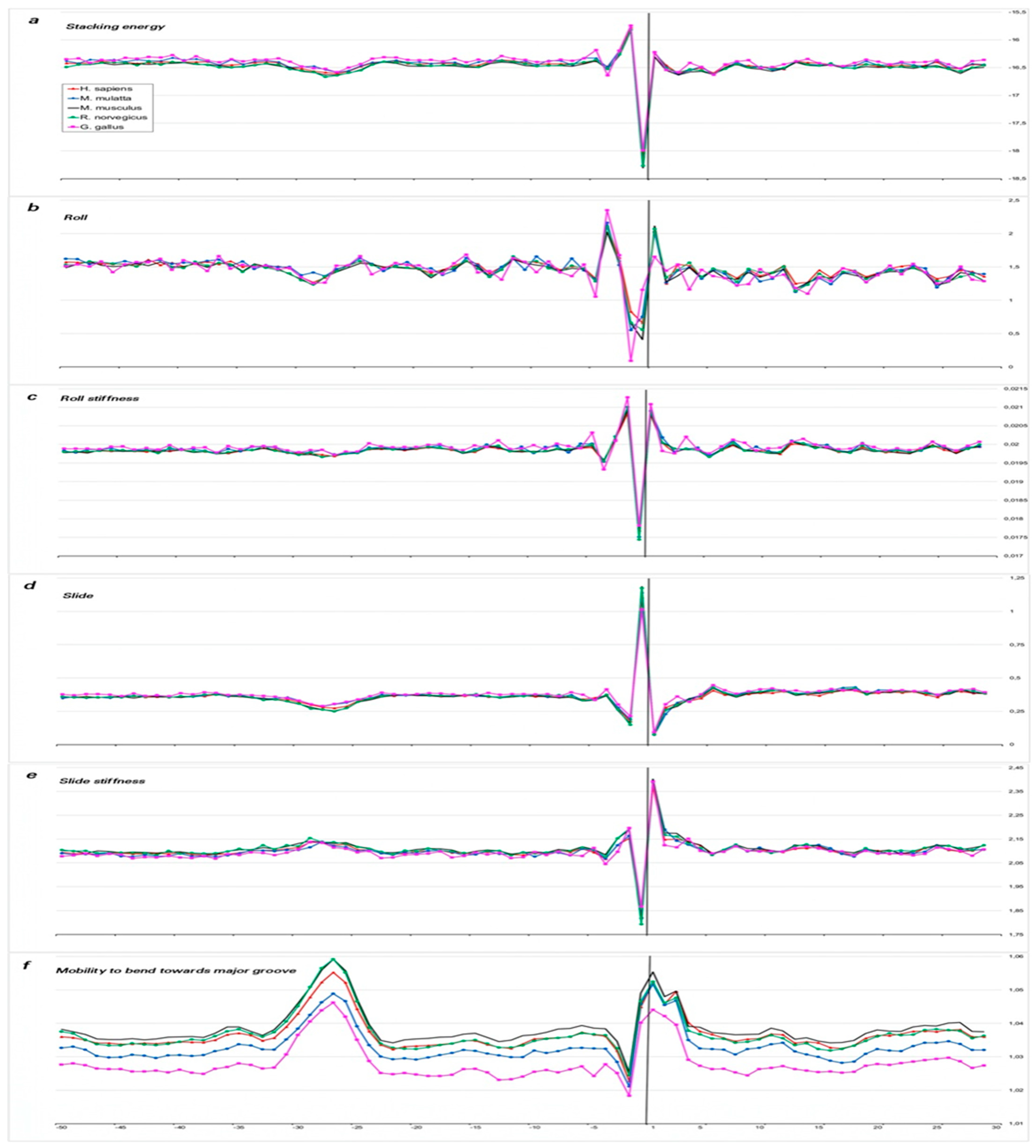
2.1.2. Characterization of Spatial Structure and Physical Properties
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Girbig, M.; Misiaszek, A.D.; Müller, C.W. Structural Insights into Nuclear Transcription by Eukaryotic DNA-Dependent RNA Polymerases. Nat. Rev. Mol. Cell Biol. 2022, 23, 603–622. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Law, J.A. RNA Pol IV and V in Gene Silencing: Rebel Polymerases Evolving Away from Pol II’s Rules. Curr. Opin. Plant Biol. 2015, 27, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Vassetzky, N.S.; Kramerov, D.A. SINEBase: A Database and Tool for SINE Analysis. Nucleic Acids Res. 2013, 41, D83–D89. [Google Scholar] [CrossRef] [PubMed]
- Werner, F. Structure and Function of Archaeal RNA Polymerases. Mol. Microbiol. 2007, 65, 1395–1404. [Google Scholar] [CrossRef]
- Langer, D.; Hain, J.; Thuriaux, P.; Zillig, W. Transcription in Archaea: Similarity to That in Eucarya. Proc. Natl. Acad. Sci. USA 1995, 92, 5768–5772. [Google Scholar] [CrossRef]
- Hamada, M.; Huang, Y.; Lowe, T.M.; Maraia, R.J. Widespread Use of TATA Elements in the Core Promoters for RNA Polymerases III, II, and I in Fission Yeast. Mol. Cell. Biol. 2001, 21, 6870–6881. [Google Scholar] [CrossRef]
- Hofstetter, H.; Kressmann, A.; Birnstiel, M.L. A Split Promoter for a Eucaryotic tRNA Gene. Cell 1981, 24, 573–585. [Google Scholar] [CrossRef]
- Schramm, L.; Hernandez, N. Recruitment of RNA Polymerase III to Its Target Promoters. Genes Dev. 2002, 16, 2593–2620. [Google Scholar] [CrossRef]
- White, R.J. Transcription by RNA Polymerase III: More Complex than We Thought. Nat. Rev. Genet. 2011, 12, 459–463. [Google Scholar] [CrossRef]
- Abascal-Palacios, G.; Ramsay, E.P.; Beuron, F.; Morris, E.; Vannini, A. Structural Basis of RNA Polymerase III Transcription Initiation. Nature 2018, 553, 301–306. [Google Scholar] [CrossRef]
- Vorländer, M.K.; Khatter, H.; Wetzel, R.; Hagen, W.J.H.; Müller, C.W. Molecular Mechanism of Promoter Opening by RNA Polymerase III. Nature 2018, 553, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Haberle, V.; Stark, A. Eukaryotic Core Promoters and the Functional Basis of Transcription Initiation. Nat. Rev. Mol. Cell Biol. 2018, 19, 621–637. [Google Scholar] [CrossRef] [PubMed]
- Il’icheva, I.A.; Khodikov, M.V.; Poptsova, M.S.; Nechipurenko, D.Y.; Nechipurenko, Y.D.; Grokhovsky, S.L. Structural Features of DNA That Determine RNA Polymerase II Core Promoter. BMC Genom. 2016, 17, 973. [Google Scholar] [CrossRef]
- Melikhova, A.V.; Anashkina, A.A.; Il’icheva, I.A. Evolutionary Invariant of the Structure of DNA Double Helix in RNAP II Core Promoters. Int. J. Mol. Sci. 2022, 23, 10873. [Google Scholar] [CrossRef] [PubMed]
- Savina, E.A.; Shumilina, T.G.; Tumanyan, V.G.; Anashkina, A.A.; Il’icheva, I.A. Core Promoter Regions of Antisense and Long Intergenic Non-Coding RNAs. Int. J. Mol. Sci. 2023, 24, 8199. [Google Scholar] [CrossRef]
- Oler, A.J.; Alla, R.K.; Roberts, D.N.; Wong, A.; Hollenhorst, P.C.; Chandler, K.J.; Cassiday, P.A.; Nelson, C.A.; Hagedorn, C.H.; Graves, B.J.; et al. Human RNA Polymerase III Transcriptomes and Relationships to Pol II Promoter Chromatin and Enhancer-Binding Factors. Nat. Struct. Mol. Biol. 2010, 17, 620–628. [Google Scholar] [CrossRef]
- Stasenko, D.V.; Tatosyan, K.A.; Borodulina, O.R.; Kramerov, D.A. Nucleotide Context Can Modulate Promoter Strength in Genes Transcribed by RNA Polymerase III. Genes 2023, 14, 802. [Google Scholar] [CrossRef]
- Friedel, M.; Nikolajewa, S.; Sühnel, J.; Wilhelm, T. DiProDB: A Database for Dinucleotide Properties. Nucleic Acids Res. 2009, 37, D37–D40. [Google Scholar] [CrossRef]
- Grokhovsky, S.L.; Il’icheva, I.A.; Nechipurenko, D.Y.; Golovkin, M.V.; Panchenko, L.A.; Polozov, R.V.; Nechipurenko, Y.D. Sequence-Specific Ultrasonic Cleavage of DNA. Biophys. J. 2011, 100, 117–125. [Google Scholar] [CrossRef]
- Lazarovici, A.; Zhou, T.; Shafer, A.; Dantas Machado, A.C.; Riley, T.R.; Sandstrom, R.; Sabo, P.J.; Lu, Y.; Rohs, R.; Stamatoyannopoulos, J.A.; et al. Probing DNA Shape and Methylation State on a Genomic Scale with DNase I. Proc. Natl. Acad. Sci. USA 2013, 110, 6376–6381. [Google Scholar] [CrossRef]
- Berman, M.L.; Beckwith, J. Fusions of the Lac Operon to the Transfer RNA Gene tyrT of Escherichia Coli. J. Mol. Biol. 1979, 130, 285–301. [Google Scholar] [CrossRef] [PubMed]
- Berman, M.L.; Landy, A. Promoter Mutations in the Transfer RNA Gene tyrT of Escherichia Coli. Proc. Natl. Acad. Sci. USA 1979, 76, 4303–4307. [Google Scholar] [CrossRef] [PubMed]
- Bervoets, I.; Charlier, D. Diversity, Versatility and Complexity of Bacterial Gene Regulation Mechanisms: Opportunities and Drawbacks for Applications in Synthetic Biology. FEMS Microbiol. Rev. 2019, 43, 304–339. [Google Scholar] [CrossRef] [PubMed]
- Dieci, G.; Yukawa, Y.; Alzapiedi, M.; Guffanti, E.; Ferrari, R.; Sugiura, M.; Ottonello, S. Distinct Modes of TATA Box Utilization by the RNA Polymerase III Transcription Machineries from Budding Yeast and Higher Plants. Gene 2006, 379, 12–25. [Google Scholar] [CrossRef]
- Kim, J.L.; Nikolov, D.B.; Burley, S.K. Co-Crystal Structure of TBP Recognizing the Minor Groove of a TATA Element. Nature 1993, 365, 520–527. [Google Scholar] [CrossRef]
- Hall, B.D.; Clarkson, S.G.; Tocchini-Valentini, G. Transcription Initiation of Eucaryotic Transfer RNA Genes. Cell 1982, 29, 3–5. [Google Scholar] [CrossRef]
- Geiduschek, E.P.; Tocchini-Valentini, G.P. Transcription by RNA Polymerase III. Annu. Rev. Biochem. 1988, 57, 873–914. [Google Scholar] [CrossRef]
- Talyzina, A.; Han, Y.; Banerjee, C.; Fishbain, S.; Reyes, A.; Vafabakhsh, R.; He, Y. Structural Basis of TFIIIC-Dependent RNA Polymerase III Transcription Initiation. Mol. Cell 2023, 83, 2641–2652.e7. [Google Scholar] [CrossRef]
- van Breugel, M.E.; Gerber, A.; van Leeuwen, F. The Choreography of Chromatin in RNA Polymerase III Regulation. Biochem. Soc. Trans. 2024, 52, 1173–1189. [Google Scholar] [CrossRef]
- Chan, P.P.; Lowe, T.M. GtRNAdb: A Database of Transfer RNA Genes Detected in Genomic Sequence. Nucleic Acids Res. 2009, 37, D93–D97. [Google Scholar] [CrossRef]
- Chan, P.P.; Lin, B.Y.; Mak, A.J.; Lowe, T.M. tRNAscan-SE 2.0: Improved Detection and Functional Classification of Transfer RNA Genes. Nucleic Acids Res. 2021, 49, 9077–9096. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savina, E.A.; Shumilina, T.G.; Porolo, V.A.; Lebedev, G.S.; Orlov, Y.L.; Anashkina, A.A.; Il’icheva, I.A. Structural Features of DNA in tRNA Genes and Their Upstream Sequences. Int. J. Mol. Sci. 2024, 25, 11758. https://doi.org/10.3390/ijms252111758
Savina EA, Shumilina TG, Porolo VA, Lebedev GS, Orlov YL, Anashkina AA, Il’icheva IA. Structural Features of DNA in tRNA Genes and Their Upstream Sequences. International Journal of Molecular Sciences. 2024; 25(21):11758. https://doi.org/10.3390/ijms252111758
Chicago/Turabian StyleSavina, Ekaterina A., Tatiana G. Shumilina, Viktoria A. Porolo, Georgy S. Lebedev, Yury L. Orlov, Anastasia A. Anashkina, and Irina A. Il’icheva. 2024. "Structural Features of DNA in tRNA Genes and Their Upstream Sequences" International Journal of Molecular Sciences 25, no. 21: 11758. https://doi.org/10.3390/ijms252111758
APA StyleSavina, E. A., Shumilina, T. G., Porolo, V. A., Lebedev, G. S., Orlov, Y. L., Anashkina, A. A., & Il’icheva, I. A. (2024). Structural Features of DNA in tRNA Genes and Their Upstream Sequences. International Journal of Molecular Sciences, 25(21), 11758. https://doi.org/10.3390/ijms252111758







