Controlled Stimulus-Responsive Delivery Systems for Osteoarthritis Treatment
Abstract
1. Introduction
2. Drug Delivery Systems
2.1. Nanoparticles
2.2. Hydrogels
2.3. Liposomes
2.4. Microspheres
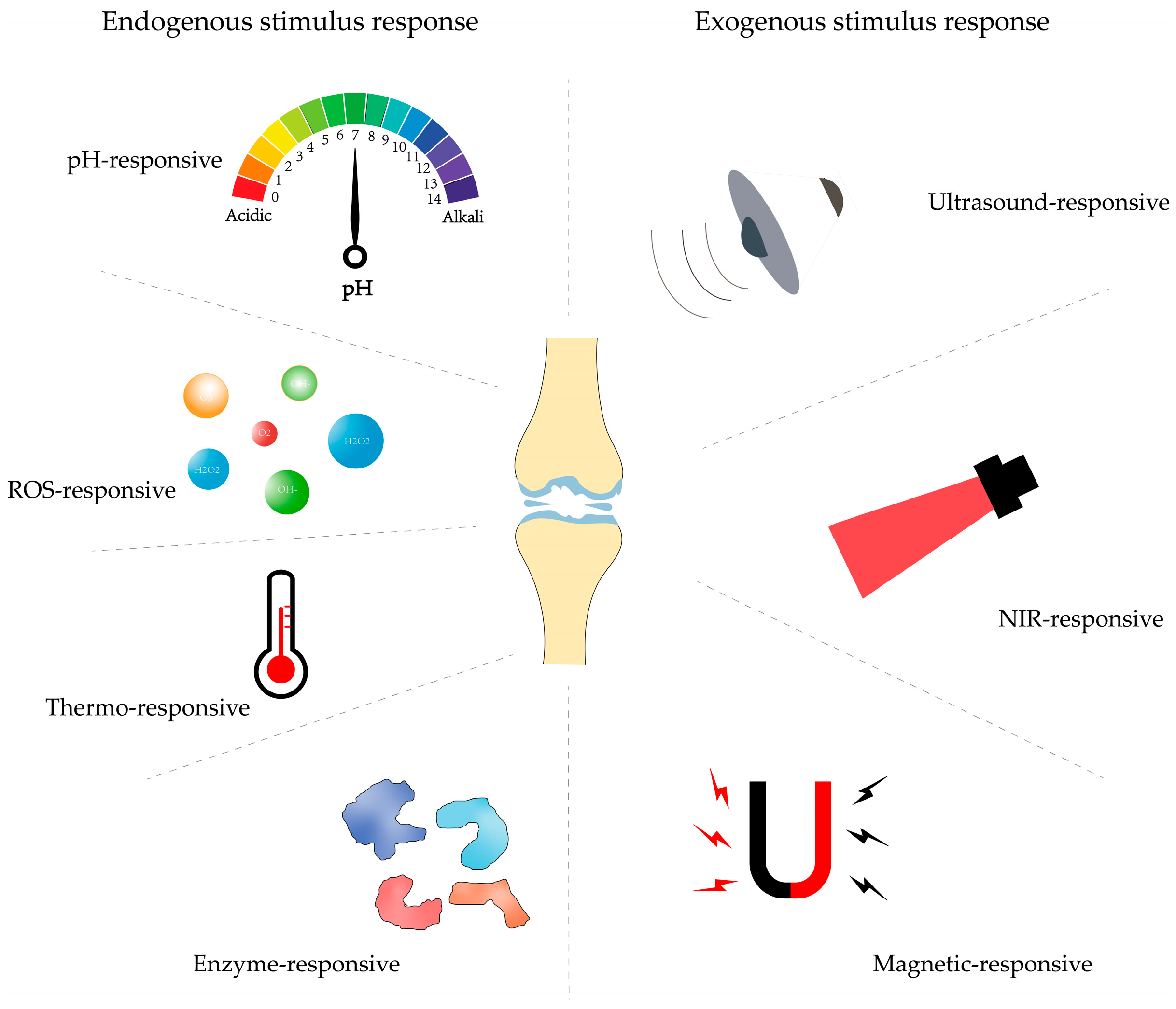
3. Controlled Stimulus Response DDSs
3.1. Endogenous Stimulus-Responsive Delivery Systems
3.1.1. Reactive Oxygen Response
3.1.2. pH Response
3.1.3. Enzyme Response
3.1.4. Temperature Response
3.2. Exogenous Stimulus-Responsive Delivery Systems
3.2.1. NIR Response
3.2.2. Magnetic Response
3.2.3. Ultrasonic Response
4. Multi-Stimulus Response

5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, W.; Kluzek, M.; Iuster, N.; Shimoni, E.; Kampf, N.; Goldberg, R.; Klein, J. Cartilage-inspired, lipid-based boundary-lubricated hydrogels. Science 2020, 370, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wu, Y.; Li, G.; Lin, Q.; Zhang, W.; Liu, H.; Su, J. Articular cartilage repair biomaterials: Strategies and applications. Mater. Today Bio 2024, 24, 100948. [Google Scholar] [CrossRef]
- Lv, B.; Lu, L.; Hu, L.; Cheng, P.; Hu, Y.; Xie, X.; Dai, G.; Mi, B.; Liu, X.; Liu, G. Recent advances in GelMA hydrogel transplantation for musculoskeletal disorders and related disease treatment. Theranostics 2023, 13, 2015–2039. [Google Scholar] [CrossRef] [PubMed]
- Ao, Y.; Zhang, E.; Liu, Y.; Yang, L.; Li, J.; Wang, F. Advanced Hydrogels with Nanoparticle Inclusion for Cartilage Tissue Engineering. Front. Bioeng. Biotechnol. 2022, 10, 951513. [Google Scholar] [CrossRef]
- Wei, W.; Ma, Y.; Yao, X.; Zhou, W.; Wang, X.; Li, C.; Lin, J.; He, Q.; Leptihn, S.; Ouyang, H. Advanced hydrogels for the repair of cartilage defects and regeneration. Bioact. Mater. 2021, 6, 998–1011. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, J.; Alahdal, M. Exosomes loaded with chondrogenic stimuli agents combined with 3D bioprinting hydrogel in the treatment of osteoarthritis and cartilage degeneration. Biomed. Pharmacother. 2023, 168, 115715. [Google Scholar] [CrossRef]
- Sharma, L. Osteoarthritis of the Knee. N. Engl. J. Med. 2021, 384, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Kalairaj, M.S.; Pradhan, R.; Saleem, W.; Smith, M.M.; Gaharwar, A.K. Intra-Articular Injectable Biomaterials for Cartilage Repair and Regeneration. Adv. Healthc. Mater. 2024, 13, e2303794. [Google Scholar] [CrossRef]
- Duan, W.L.; Zhang, L.N.; Bohara, R.; Martin-Saldaña, S.; Yang, F.; Zhao, Y.Y.; Xie, Y.; Bu, Y.Z.; Pandit, A. Adhesive hydrogels in osteoarthritis: From design to application. Mil. Med. Res. 2023, 10, 4. [Google Scholar] [CrossRef]
- Mao, L.; Wu, W.; Wang, M.; Guo, J.; Li, H.; Zhang, S.; Xu, J.; Zou, J. Targeted treatment for osteoarthritis: Drugs and delivery system. Drug Deliv. 2021, 28, 1861–1876. [Google Scholar] [CrossRef]
- Yang, D.; Xu, J.; Xu, K.; Xu, P. Skeletal interoception in osteoarthritis. Bone Res. 2024, 12, 22. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Zheng, K.; Li, W.; He, W.; Qian, C.; Lin, Z.; Xiao, H.; Yang, H.; Xu, Y.; Wei, M.; et al. Nature-Inspired Strategies for the Treatment of Osteoarthritis. Adv. Funct. Mater. 2023, 34, 2305603. [Google Scholar] [CrossRef]
- van den Bosch, M.H.J.; Blom, A.B.; van der Kraan, P.M. Inflammation in osteoarthritis: Our view on its presence and involvement in disease development over the years. Osteoarthr. Cartil. 2024, 32, 355–364. [Google Scholar] [CrossRef]
- Haq-Siddiqi, N.A.; Britton, D.; Kim Montclare, J. Protein-engineered biomaterials for cartilage therapeutics and repair. Adv. Drug Deliv. Rev. 2023, 192, 114647. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Lu, K.; Umar, M.; Zhu, Z.; Lu, W.W.; Speakman, J.R.; Chen, Y.; Tong, L.; Chen, D. Risk of metabolic abnormalities in osteoarthritis: A new perspective to understand its pathological mechanisms. Bone Res. 2023, 11, 63. [Google Scholar] [CrossRef]
- Food and Drug Administration. Osteoarthritis: Structural Endpoints for the Development of Drugs. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/osteoarthritis-structural-endpoints-development-drugs (accessed on 20 February 2022).
- Gelber, A.C. Knee Osteoarthritis. Ann. Intern. Med. 2024, 177, Itc129–Itc144. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Sun, A.R.; Young, R.S.E.; Afara, I.O.; Hamilton, B.R.; Ong, L.J.Y.; Crawford, R.; Prasadam, I. Spatial analysis of the osteoarthritis microenvironment: Techniques, insights, and applications. Bone Res. 2024, 12, 7. [Google Scholar] [CrossRef]
- Fan, W.J.; Liu, D.; Pan, L.Y.; Wang, W.Y.; Ding, Y.L.; Zhang, Y.Y.; Ye, R.X.; Zhou, Y.; An, S.B.; Xiao, W.F. Exosomes in osteoarthritis: Updated insights on pathogenesis, diagnosis, and treatment. Front. Cell Dev. Biol. 2022, 10, 949690. [Google Scholar] [CrossRef]
- Li, G.; Liu, S.; Chen, Y.; Zhao, J.; Xu, H.; Weng, J.; Yu, F.; Xiong, A.; Udduttula, A.; Wang, D.; et al. An injectable liposome-anchored teriparatide incorporated gallic acid-grafted gelatin hydrogel for osteoarthritis treatment. Nat. Commun. 2023, 14, 3159. [Google Scholar] [CrossRef]
- Yu, H.; Gao, R.; Liu, Y.; Fu, L.; Zhou, J.; Li, L. Stimulus-Responsive Hydrogels as Drug Delivery Systems for Inflammation Targeted Therapy. Adv. Sci. 2024, 11, e2306152. [Google Scholar] [CrossRef]
- Silverstein, F.E.; Faich, G.; Goldstein, J.L.; Simon, L.S.; Pincus, T.; Whelton, A.; Makuch, R.; Eisen, G.; Agrawal, N.M.; Stenson, W.F.; et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: The CLASS study: A randomized controlled trial. Celecoxib Long-term Arthritis Safety Study. JAMA-J. Am. Med. Assoc. 2000, 284, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Atwal, A.; Dale, T.P.; Snow, M.; Forsyth, N.R.; Davoodi, P. Injectable hydrogels: An emerging therapeutic strategy for cartilage regeneration. Adv. Colloid Interface Sci. 2023, 321, 103030. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.N.; Arant, K.R.; Loeser, R.F. Diagnosis and Treatment of Hip and Knee Osteoarthritis: A Review. JAMA 2021, 325, 568–578. [Google Scholar] [CrossRef]
- Bruno, M.C.; Cristiano, M.C.; Celia, C.; d’Avanzo, N.; Mancuso, A.; Paolino, D.; Wolfram, J.; Fresta, M. Injectable Drug Delivery Systems for Osteoarthritis and Rheumatoid Arthritis. ACS Nano 2022, 16, 19665–19690. [Google Scholar] [CrossRef] [PubMed]
- DeJulius, C.R.; Walton, B.L.; Colazo, J.M.; d’Arcy, R.; Francini, N.; Brunger, J.M.; Duvall, C.L. Engineering approaches for RNA-based and cell-based osteoarthritis therapies. Nat. Rev. Rheumatol. 2024, 20, 81–100. [Google Scholar] [CrossRef]
- Brown, S.; Kumar, S.; Sharma, B. Intra-articular targeting of nanomaterials for the treatment of osteoarthritis. Acta Biomater. 2019, 93, 239–257. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, S. Stimulus-Responsive Drug Delivery Nanoplatforms for Osteoarthritis Therapy. Small 2023, 19, e2206929. [Google Scholar] [CrossRef]
- Majumder, J.; Minko, T. Multifunctional and stimuli-responsive nanocarriers for targeted therapeutic delivery. Expert Opin. Drug Deliv. 2021, 18, 205–227. [Google Scholar] [CrossRef]
- Raza, A.; Rasheed, T.; Nabeel, F.; Hayat, U.; Bilal, M.; Iqbal, H.M.N. Endogenous and Exogenous Stimuli-Responsive Drug Delivery Systems for Programmed Site-Specific Release. Molecules 2019, 24, 1117. [Google Scholar] [CrossRef]
- Wang, X.; Li, C.; Wang, Y.; Chen, H.; Zhang, X.; Luo, C.; Zhou, W.; Li, L.; Teng, L.; Yu, H.; et al. Smart drug delivery systems for precise cancer therapy. Acta Pharm. Sin. B 2022, 12, 4098–4121. [Google Scholar] [CrossRef]
- Thorn, C.R.; Howell, P.L.; Wozniak, D.J.; Prestidge, C.A.; Thomas, N. Enhancing the therapeutic use of biofilm-dispersing enzymes with smart drug delivery systems. Adv. Drug Deliv. Rev. 2021, 179, 113916. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Ma, Y.; Tao, Y.; Lin, W.; Wang, P. Intra-Articular Drug Delivery for Osteoarthritis Treatment. Pharmaceutics 2021, 13, 2166. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, M.; Charmi, G.; Matyjaszewski, K.; Banquy, X.; Pietrasik, J. Recent developments in natural and synthetic polymeric drug delivery systems used for the treatment of osteoarthritis. Acta Biomater. 2021, 123, 31–50. [Google Scholar] [CrossRef] [PubMed]
- Nakkala, J.R.; Li, Z.; Ahmad, W.; Wang, K.; Gao, C. Immunomodulatory biomaterials and their application in therapies for chronic inflammation-related diseases. Acta Biomater. 2021, 123, 1–30. [Google Scholar] [CrossRef]
- Li, X.; Dai, B.; Guo, J.; Zheng, L.; Guo, Q.; Peng, J.; Xu, J.; Qin, L. Nanoparticle-Cartilage Interaction: Pathology-Based Intra-articular Drug Delivery for Osteoarthritis Therapy. Nano-Micro Lett. 2021, 13, 149. [Google Scholar] [CrossRef]
- Wei, Y.; Yan, L.; Luo, L.; Gui, T.; Jang, B.; Amirshaghaghi, A.; You, T.; Tsourkas, A.; Qin, L.; Cheng, Z. Phospholipase A(2) inhibitor-loaded micellar nanoparticles attenuate inflammation and mitigate osteoarthritis progression. Sci. Adv. 2021, 7, eabe6374. [Google Scholar] [CrossRef]
- Liang, H.; Yan, Y.; Sun, W.; Ma, X.; Su, Z.; Liu, Z.; Chen, Y.; Yu, B. Preparation of Melatonin-Loaded Nanoparticles with Targeting and Sustained Release Function and Their Application in Osteoarthritis. Int. J. Mol. Sci. 2023, 24, 8740. [Google Scholar] [CrossRef]
- Cai, C.; Zhang, X.; Li, Y.; Liu, X.; Wang, S.; Lu, M.; Yan, X.; Deng, L.; Liu, S.; Wang, F.; et al. Self-Healing Hydrogel Embodied with Macrophage-Regulation and Responsive-Gene-Silencing Properties for Synergistic Prevention of Peritendinous Adhesion. Adv. Mater. 2022, 34, e2106564. [Google Scholar] [CrossRef]
- Chen, H.; Chen, F.; Hu, F.; Li, Y.; Zhang, M.; Zhou, Q.; Ding, T.; Tulufu, N.; Ye, T.; Wang, F.; et al. MicroRNA-224-5p nanoparticles balance homeostasis via inhibiting cartilage degeneration and synovial inflammation for synergistic alleviation of osteoarthritis. Acta Biomater. 2023, 167, 401–415. [Google Scholar] [CrossRef]
- Gui, T.; Luo, L.; Chhay, B.; Zhong, L.; Wei, Y.; Yao, L.; Yu, W.; Li, J.; Nelson, C.L.; Tsourkas, A.; et al. Superoxide dismutase-loaded porous polymersomes as highly efficient antioxidant nanoparticles targeting synovium for osteoarthritis therapy. Biomaterials 2022, 283, 121437. [Google Scholar] [CrossRef]
- Saeedi, T.; Alotaibi, H.F.; Prokopovich, P. Polymer colloids as drug delivery systems for the treatment of arthritis. Adv. Colloid Interface Sci. 2020, 285, 102273. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Adjei, I.M.; Brown, S.B.; Liseth, O.; Sharma, B. Manganese dioxide nanoparticles protect cartilage from inflammation-induced oxidative stress. Biomaterials 2019, 224, 119467. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Zhao, S.; Li, Y.; Xu, J.; Yan, W.; Guo, B.; Xu, J.; Jiang, L.; Zhang, Y.; Wei, H.; et al. Engineered MgO nanoparticles for cartilage-bone synergistic therapy. Adv. Sci. 2024, 10, eadk6084. [Google Scholar] [CrossRef]
- Jahanbekam, S.; Mozafari, N.; Bagheri-Alamooti, A.; Mohammadi-Samani, S.; Daneshamouz, S.; Heidari, R.; Azarpira, N.; Ashrafi, H.; Azadi, A. Ultrasound-responsive hyaluronic acid hydrogel of hydrocortisone to treat osteoarthritis. Int. J. Biol. Macromol. 2023, 240, 124449. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Wang, H.; Ling, C.; Vermerris, W.; Wang, B.; Tong, Z. Cellulose-based injectable hydrogel composite for pH-responsive and controllable drug delivery. Carbohydr. Polym. 2019, 225, 115207. [Google Scholar] [CrossRef]
- Yan, X.; Yang, B.; Chen, Y.; Song, Y.; Ye, J.; Pan, Y.; Zhou, B.; Wang, Y.; Mao, F.; Dong, Y.; et al. Anti-Friction MSCs Delivery System Improves the Therapy for Severe Osteoarthritis. Adv. Mater. 2021, 33, e2104758. [Google Scholar] [CrossRef]
- Hu, W.; Yao, X.; Li, Y.; Li, J.; Zhang, J.; Zou, Z.; Kang, F.; Dong, S. Injectable hydrogel with selenium nanoparticles delivery for sustained glutathione peroxidase activation and enhanced osteoarthritis therapeutics. Mater. Today Bio 2023, 23, 100864. [Google Scholar] [CrossRef]
- Manivong, S.; Cullier, A.; Audigié, F.; Banquy, X.; Moldovan, F.; Demoor, M.; Roullin, V.G. New trends for osteoarthritis: Biomaterials, models and modeling. Drug Discov. Today 2023, 28, 103488. [Google Scholar] [CrossRef]
- Demoor, M.; Ollitrault, D.; Gomez-Leduc, T.; Bouyoucef, M.; Hervieu, M.; Fabre, H.; Lafont, J.; Denoix, J.M.; Audigié, F.; Mallein-Gerin, F.; et al. Cartilage tissue engineering: Molecular control of chondrocyte differentiation for proper cartilage matrix reconstruction. Biochim. Biophys. Acta 2014, 1840, 2414–2440. [Google Scholar] [CrossRef]
- Mohanty, S.; Swarup, J.; Priya, S.; Jain, R.; Singhvi, G. Exploring the potential of polysaccharide-based hybrid hydrogel systems for their biomedical and therapeutic applications: A review. Int. J. Biol. Macromol. 2024, 256 Pt 1, 128348. [Google Scholar] [CrossRef]
- Shentu, C.Y.; Wang, H.B.; Peng, X.; Xu, D.C.; Qian, L.N.; Chen, Y.; Peng, L.H. Progress and Challenges of Topical Delivery Technologies Meditated Drug Therapy for Osteoarthritis. Int. J. Nanomed. 2024, 19, 8337–8352. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Zhou, F.; Sheng, S.; Wei, Y.; Chen, X.; Su, J. Intra-articular nanodrug delivery strategies for treating osteoarthritis. Drug Discov. Today 2023, 28, 103482. [Google Scholar] [CrossRef] [PubMed]
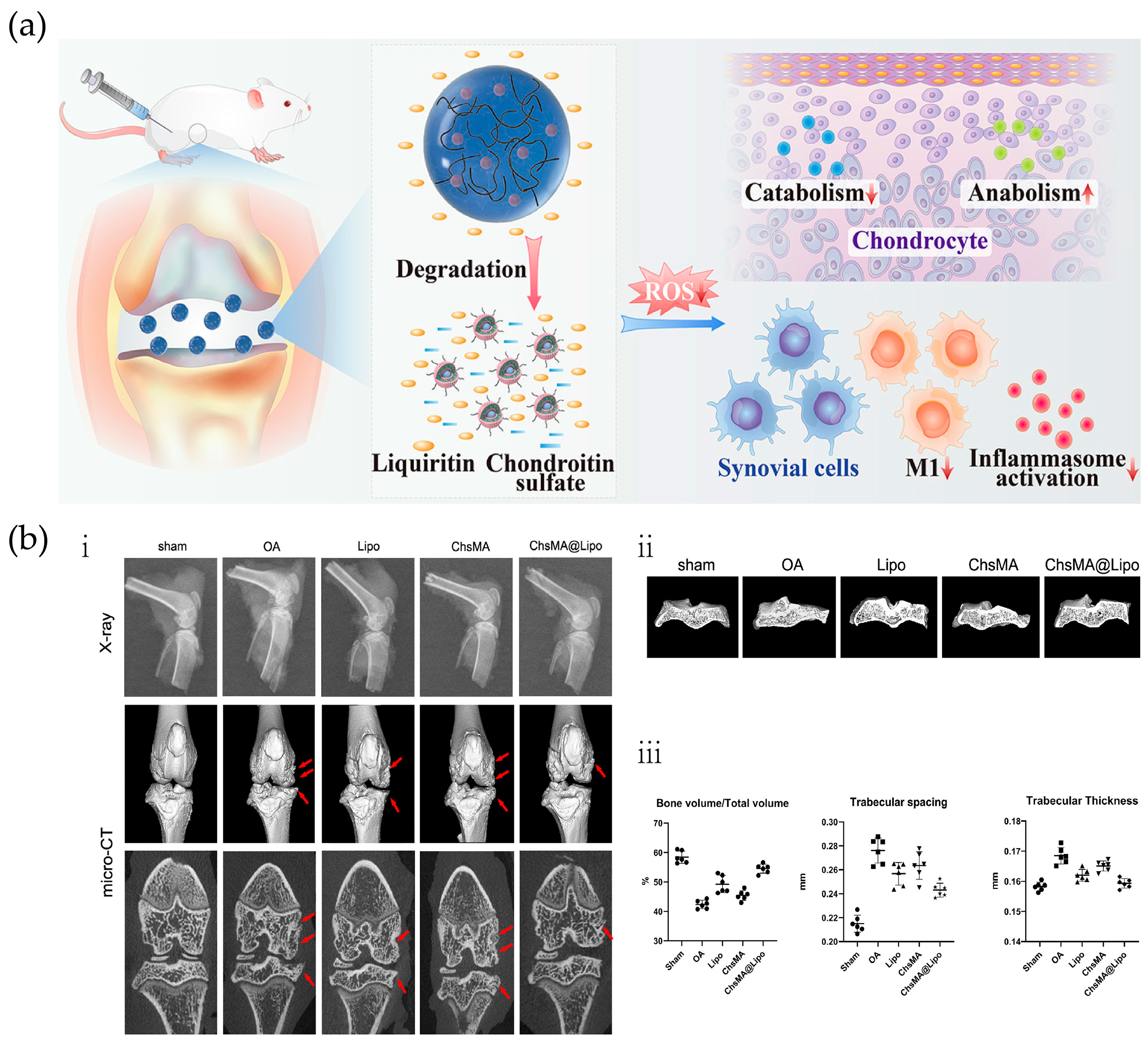
- He, Y.; Sun, M.; Wang, J.; Yang, X.; Lin, C.; Ge, L.; Ying, C.; Xu, K.; Liu, A.; Wu, L. Chondroitin sulfate microspheres anchored with drug-loaded liposomes play a dual antioxidant role in the treatment of osteoarthritis. Acta Biomater. 2022, 151, 512–527. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yan, R.; Chen, Q.; Wang, Y.; Zhong, X.; Liu, S.; Xie, R.; Ren, L. Functional nano drug delivery system with dual lubrication and immune escape for treating osteoarthritis. J. Colloid Interface Sci. 2023, 652 Pt B, 2167–2179. [Google Scholar] [CrossRef]
- Liu, L.; Xian, Y.; Wang, W.; Huang, L.; Fan, J.; Ma, W.; Li, Y.; Liu, H.; Yu, J.K.; Wu, D. Meniscus-Inspired Self-Lubricating and Friction-Responsive Hydrogels for Protecting Articular Cartilage and Improving Exercise. ACS Nano 2023, 17, 24308–24319. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.Z. Current Nanoparticle-Based Technologies for Osteoarthritis Therapy. Nanomaterials 2020, 10, 2368. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Tan, P.; Fu, S.; Tian, X.; Zhang, H.; Ma, X.; Gu, Z.; Luo, K. Preparation and application of pH-responsive drug delivery systems. J. Control. Release 2022, 348, 206–238. [Google Scholar] [CrossRef]
- Yao, Y.; Wei, G.; Deng, L.; Cui, W. Visualizable and Lubricating Hydrogel Microspheres Via NanoPOSS for Cartilage Regeneration. Adv. Sci. 2023, 10, e2207438. [Google Scholar] [CrossRef]
- Han, Y.; Yang, J.; Zhao, W.; Wang, H.; Sun, Y.; Chen, Y.; Luo, J.; Deng, L.; Xu, X.; Cui, W.; et al. Biomimetic injectable hydrogel microspheres with enhanced lubrication and controllable drug release for the treatment of osteoarthritis. Bioact. Mater. 2021, 6, 3596–3607. [Google Scholar] [CrossRef]
- Alavi, S.E.; Alharthi, S.; Alavi, S.Z.; Raza, A.; Ebrahimi Shahmabadi, H. Bioresponsive drug delivery systems. Drug Discov. Today 2024, 29, 103849. [Google Scholar] [CrossRef]
- He, Q.; Chen, J.; Yan, J.; Cai, S.; Xiong, H.; Liu, Y.; Peng, D.; Mo, M.; Liu, Z. Tumor microenvironment responsive drug delivery systems. Expert Opin. Drug Deliv. 2020, 15, 416–448. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Hu, W.; Cai, C.; Wu, Y.; Li, J.; Dong, S. Advanced application of stimuli-responsive drug delivery system for inflammatory arthritis treatment. Mater. Today Bio 2022, 14, 100223. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Li, Y.; Li, Y.; Tan, Y.; Liu, Y.; Pan, W.; Tan, G. Stimuli-responsive electrospun nanofibers for drug delivery, cancer therapy, wound dressing, and tissue engineering. J. Nanobiotechnol. 2023, 21, 237. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Lovell, J.F.; Zhang, L.; Zhang, Y. Stimulus-Responsive Nanomedicines for Disease Diagnosis and Treatment. Int. J. Mol. Sci. 2020, 21, 6380. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Liu, Y.; Liu, J. Smart stimuli-responsive drug delivery systems based on cyclodextrin: A review. Carbohydr. Polym. 2021, 251, 116871. [Google Scholar] [CrossRef]
- Li, X.; Rommelaere, S.; Kondo, S.; Lemaitre, B. Renal Purge of Hemolymphatic Lipids Prevents the Accumulation of ROS-Induced Inflammatory Oxidized Lipids and Protects Drosophila from Tissue Damage. Immunity 2020, 52, 374–387. [Google Scholar] [CrossRef]
- Yang, C.Y.; Chanalaris, A.; Troeberg, L. ADAMTS and ADAM metalloproteinases in osteoarthritis—Looking beyond the ‘usual suspects’. Osteoarthr. Cartil. 2017, 25, 1000–1009. [Google Scholar] [CrossRef]
- Klimak, M.; Nims, R.J.; Pferdehirt, L.; Collins, K.H.; Harasymowicz, N.S.; Oswald, S.J.; Setton, L.A.; Guilak, F. Immunoengineering the next generation of arthritis therapies. Acta Biomater. 2021, 133, 74–86. [Google Scholar] [CrossRef]
- Haseeb, A.; Haqqi, T.M. Immunopathogenesis of osteoarthritis. Clin. Immunol. 2013, 146, 185–196. [Google Scholar] [CrossRef]
- Wu, J.; Qin, Z.; Jiang, X.; Fang, D.; Lu, Z.; Zheng, L.; Zhao, J. ROS-responsive PPGF nanofiber membrane as a drug delivery system for long-term drug release in attenuation of osteoarthritis. NPJ Regen. Med. 2022, 7, 66. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, H.; Zhang, Z.; Pan, J.; Yuan, H. Cartilage targeting therapy with reactive oxygen species-responsive nanocarrier for osteoarthritis. J. Nanobiotechnol. 2022, 20, 419. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Song, C.C.; Su, S.; Du, F.S.; Li, Z.C. ROS-Activated Ratiometric Fluorescent Polymeric Nanoparticles for Self-Reporting Drug Delivery. ACS Appl. Mater. Interfaces 2018, 10, 7798–7810. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, P.; Cheng, J.; Xu, Q.; Lu, B.; Han, C.; Huo, W. ROS-Sensitive Nanoparticles Co-delivering Dexamethasone and CDMP-1 for the Treatment of Osteoarthritis Through Chondrogenic Differentiation Induction and Inflammation Inhibition. Front. Bioeng. Biotechnol. 2021, 9, 608150. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Gao, M.; Chen, H.; Zhan, Y.; Lan, Q.; Li, Z.; Xiong, W.; Qin, Z.; Zheng, L.; Zhao, J. Reactive oxygen species (ROS)-responsive nanoprobe for bioimaging and targeting therapy of osteoarthritis. J. Nanobiotechnol. 2021, 19, 395. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Gao, Y.; Wang, W.; Wang, Y.; Liu, P.; Shou, Z.; Yang, R.; Jin, C.; Zan, X.; Wang, C.; et al. Self-Report Amphiphilic Polymer-Based Drug Delivery System with ROS-Triggered Drug Release for Osteoarthritis Therapy. ACS Macro Lett. 2024, 13, 58–64. [Google Scholar] [CrossRef]
- Xiong, F.; Qin, Z.; Chen, H.; Lan, Q.; Wang, Z.; Lan, N.; Yang, Y.; Zheng, L.; Zhao, J.; Kai, D. pH-responsive and hyaluronic acid-functionalized metal-organic frameworks for therapy of osteoarthritis. J. Nanobiotechnol. 2020, 18, 139. [Google Scholar] [CrossRef]
- He, M.; Qin, Z.; Liang, X.; He, X.; Zhu, B.; Lu, Z.; Wei, Q.; Zheng, L. A pH-responsive mesoporous silica nanoparticles-based drug delivery system with controlled release of andrographolide for OA treatment. Regen. Biomater. 2021, 8, rbab020. [Google Scholar] [CrossRef]
- Zhang, M.; Peng, X.; Ding, Y.; Ke, X.; Ren, K.; Xin, Q.; Qin, M.; Xie, J.; Li, J. A cyclic brush zwitterionic polymer based pH-responsive nanocarrier-mediated dual drug delivery system with lubrication maintenance for osteoarthritis treatment. Mater. Horiz. 2023, 10, 2554–2567. [Google Scholar] [CrossRef]
- Zerrillo, L.; Que, I.; Vepris, O.; Morgado, L.N.; Chan, A.; Bierau, K.; Li, Y.; Galli, F.; Bos, E.; Censi, R.; et al. pH-responsive poly(lactide-co-glycolide) nanoparticles containing near-infrared dye for visualization and hyaluronic acid for treatment of osteoarthritis. J. Control. Release 2019, 309, 265–276. [Google Scholar] [CrossRef]
- Jin, T.; Wu, D.; Liu, X.M.; Xu, J.T.; Ma, B.J.; Ji, Y.; Jin, Y.Y.; Wu, S.Y.; Wu, T.; Ma, K. Intra-articular delivery of celastrol by hollow mesoporous silica nanoparticles for pH-sensitive anti-inflammatory therapy against knee osteoarthritis. J. Nanobiotechnol. 2020, 18, 94. [Google Scholar] [CrossRef]
- Joshi, N.; Yan, J.; Levy, S.; Bhagchandani, S.; Slaughter, K.V.; Sherman, N.E.; Amirault, J.; Wang, Y.; Riegel, L.; He, X.; et al. Towards an arthritis flare-responsive drug delivery system. Nat. Commun. 2018, 9, 1275. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Xu, Z.; Liu, Q.; Zhou, H.; Yuan, L.; Li, D.; Zhao, L.; Mu, C.; Ge, L. Matrix metalloproteinase-responsive collagen-oxidized hyaluronic acid injectable hydrogels for osteoarthritic therapy. Biomater. Adv. 2022, 137, 212804. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.B.; Kwon, Y.; Kim, J.; Hong, K.H.; Kim, S.E.; Song, H.R.; Kim, Y.M.; Song, S.C. Injectable polymeric nanoparticle hydrogel system for long-term anti-inflammatory effect to treat osteoarthritis. Bioact. Mater. 2022, 7, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Han, S.; Zeng, X.; Zhu, C.; Pu, Y.; Sun, Y. Multifunctional thermo-sensitive hydrogel for modulating the microenvironment in Osteoarthritis by polarizing macrophages and scavenging RONS. J. Nanobiotechnol. 2022, 20, 221. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, H.; Shu, X.; Wu, M.; Liu, J.; Hao, T.; Cui, H.; Zheng, L. Intra-articular delivery of flurbiprofen sustained release thermogel: Improved therapeutic outcome of collagenase II-induced rat knee osteoarthritis. Drug Deliv. 2020, 27, 1034–1043. [Google Scholar] [CrossRef]
- Jung, Y.S.; Park, W.; Park, H.; Lee, D.K.; Na, K. Thermo-sensitive injectable hydrogel based on the physical mixing of hyaluronic acid and Pluronic F-127 for sustained NSAID delivery. Carbohydr. Polym. 2017, 156, 403–408. [Google Scholar] [CrossRef]
- Ding, D.F.; Xue, Y.; Wu, X.C.; Zhu, Z.H.; Ding, J.Y.; Song, Y.J.; Xu, X.L.; Xu, J.G. Recent Advances in Reactive Oxygen Species (ROS)-Responsive Polyfunctional Nanosystems 3.0 for the Treatment of Osteoarthritis. J. Inflamm. Res. 2022, 15, 5009–5026. [Google Scholar] [CrossRef]
- Saravanakumar, G.; Kim, J.; Kim, W.J. Reactive-Oxygen-Species-Responsive Drug Delivery Systems: Promises and Challenges. Adv. Sci. 2017, 4, 1600124. [Google Scholar] [CrossRef]
- Wu, Z.; Yuan, K.; Zhang, Q.; Guo, J.J.; Yang, H.; Zhou, F. Antioxidant PDA-PEG nanoparticles alleviate early osteoarthritis by inhibiting osteoclastogenesis and angiogenesis in subchondral bone. J. Nanobiotechnol. 2022, 20, 479. [Google Scholar] [CrossRef]
- Cao, Z.; Li, D.; Wang, J.; Yang, X. Reactive oxygen species-sensitive polymeric nanocarriers for synergistic cancer therapy. Acta Biomater. 2021, 130, 17–31. [Google Scholar] [CrossRef]
- Liu, J.; Jia, B.; Li, Z.; Li, W. Reactive oxygen species-responsive polymer drug delivery systems. Front. Bioeng. Biotechnol. 2023, 11, 1115603. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.; Li, C.; Li, L.; Guo, J.; Zhang, J. Bioresponsive drug delivery systems for the treatment of inflammatory diseases. J. Control. Release 2020, 327, 641–666. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Prajapati, B.G.; Singh, S. A critical review on the dissemination of PH and stimuli-responsive polymeric nanoparticular systems to improve drug delivery in cancer therapy. Crit. Rev. Oncol. Hematol. 2023, 185, 103961. [Google Scholar] [CrossRef] [PubMed]
- Najjari, Z.; Sadri, F.; Varshosaz, J. Smart stimuli-responsive drug delivery systems in spotlight of COVID-19. Asian J. Pharm. Sci. 2023, 18, 100873. [Google Scholar] [CrossRef]
- Abbasi, Y.F.; Bera, H.; Cun, D.; Yang, M. Recent advances in pH/enzyme-responsive polysaccharide-small-molecule drug conjugates as nanotherapeutics. Carbohydr. Polym. 2023, 312, 120797. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Han, Y.; Hu, Y.; Geng, Z.; Su, J. Targeted and responsive biomaterials in osteoarthritis. Theranostics 2023, 13, 931–954. [Google Scholar] [CrossRef]
- Liu, D.; Yang, F.; Xiong, F.; Gu, N. The Smart Drug Delivery System and Its Clinical Potential. Theranostics 2016, 6, 1306–1323. [Google Scholar] [CrossRef]
- Armenia, I.; Cuestas Ayllón, C.; Torres Herrero, B.; Bussolari, F.; Alfranca, G.; Grazú, V.; Martínez de la Fuente, J. Photonic and magnetic materials for on-demand local drug delivery. Adv. Drug Deliv. Rev. 2022, 191, 114584. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, N.; Feng, X. The role of internal and external stimuli in the rational design of skin-specific drug delivery systems. Int. J. Pharm. 2021, 592, 120081. [Google Scholar] [CrossRef]
- Xue, S.; Zhou, X.; Sang, W.; Wang, C.; Lu, H.; Xu, Y.; Zhong, Y.; Zhu, L.; He, C.; Ma, J. Cartilage-targeting peptide-modified dual-drug delivery nanoplatform with NIR laser response for osteoarthritis therapy. Bioact. Mater. 2021, 6, 2372–2389. [Google Scholar] [CrossRef]
- Shi, G.; Jiang, H.; Yang, F.; Lin, Z.; Li, M.; Guo, J.; Liao, X.; Lin, Y.; Cai, X.; Li, D. NIR-responsive molybdenum (Mo)-based nanoclusters enhance ROS scavenging for osteoarthritis therapy. Pharmacol. Res. 2023, 192, 106768. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Bu, P.; Xu, K.; Peng, R.; Xiong, W.; Cheng, P.; Cui, J.; Chen, A.; Mo, H.; Zhang, X.; et al. Remodeling of the pro-inflammatory microenvironment in osteoarthritis via hydrogel-based photothermal therapy. Adv. Compos. Hybrid Mater. 2024, 7, 36. [Google Scholar] [CrossRef]
- Li, Y.; Yang, J.; Chen, X.; Hu, H.; Lan, N.; Zhao, J.; Zheng, L. Mitochondrial-targeting and NIR-responsive Mn3O4@PDA@Pd-SS31 nanozymes reduce oxidative stress and reverse mitochondrial dysfunction to alleviate osteoarthritis. Biomaterials 2024, 305, 122449. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Liu, H.; Wang, S.; Hu, Y.; Huang, B.; Li, M.; Gao, J.Y.; Wang, X.; Su, J. Neutrophil-erythrocyte hybrid membrane-coated hollow copper sulfide nanoparticles for targeted and photothermal/anti-inflammatory therapy of osteoarthritis. Compos. Part B Eng. 2022, 237, 109855. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhang, Z.; Li, S.; Lin, S.; Yuan, H. Magnetically Guided Intracartilaginous Delivery of Kartogenin Improves Stem Cell-Targeted Degenerative Arthritis Therapy. Int. J. Nanomed. 2022, 17, 5511–5524. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, C.; Zhou, Y.; Zhang, F.; Duan, X.; Liu, Y.; Zhao, X.; Liu, J.; Shuai, X.; Wang, J.; et al. MRI-visible mesoporous polydopamine nanoparticles with enhanced antioxidant capacity for osteoarthritis therapy. Biomaterials 2023, 295, 122030. [Google Scholar] [CrossRef]
- Yang, L.; Li, W.; Zhao, Y.; Shang, L. Magnetic Polysaccharide Mesenchymal Stem Cells Exosomes Delivery Microcarriers for Synergistic Therapy of Osteoarthritis. ACS Nano 2024, 18, 20101–20110. [Google Scholar] [CrossRef]
- Yuan, F.Z.; Wang, H.F.; Guan, J.; Fu, J.N.; Yang, M.; Zhang, J.Y.; Chen, Y.R.; Wang, X.; Yu, J.K. Fabrication of Injectable Chitosan-Chondroitin Sulfate Hydrogel Embedding Kartogenin-Loaded Microspheres as an Ultrasound-Triggered Drug Delivery System for Cartilage Tissue Engineering. Pharmaceutics 2021, 13, 1487. [Google Scholar] [CrossRef]
- Hu, B.; Zhang, Y.; Zhang, G.; Li, Z.; Jing, Y.; Yao, J.; Sun, S. Research progress of bone-targeted drug delivery system on metastatic bone tumors. J. Control. Release 2022, 350, 377–388. [Google Scholar] [CrossRef]
- Ni, D.; Jiang, D.; Kutyreff, C.J.; Lai, J.; Yan, Y.; Barnhart, T.E.; Yu, B.; Im, H.-J.; Kang, L.; Cho, S.Y.; et al. Molybdenum-based nanoclusters act as antioxidants and ameliorate acute kidney injury in mice. Nat. Commun. 2018, 9, 5421. [Google Scholar] [CrossRef]
- Gumerova, N.I.; Rompel, A. Synthesis, structures and applications of electron-rich polyoxometalates. Nat. Rev. Chem. 2018, 2, 0112. [Google Scholar] [CrossRef]
- Edis, Z.; Wang, J.; Waqas, M.K.; Ijaz, M.; Ijaz, M. Nanocarriers-Mediated Drug Delivery Systems for Anticancer Agents: An Overview and Perspectives. Int. J. Nanomed. 2021, 16, 1313–1330. [Google Scholar] [CrossRef] [PubMed]
- Lima-Tenório, M.K.; Pineda, E.A.; Ahmad, N.M.; Fessi, H.; Elaissari, A. Magnetic nanoparticles: In vivo cancer diagnosis and therapy. Int. J. Pharm. 2015, 493, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Yi, W.; Chen, S.; Cai, Z.; Zhu, Y.; Han, W.; Guo, X.; Shen, J.; Cui, W.; Bai, D. Ultrasound-responsive smart composite biomaterials in tissue repair. Nano Today 2023, 49, 101804. [Google Scholar] [CrossRef]
- Zhao, Z.; Saiding, Q.; Cai, Z.; Cai, M.; Cui, W. Ultrasound technology and biomaterials for precise drug therapy. Mater. Today 2023, 63, 210–238. [Google Scholar] [CrossRef]
- Yi, Y.; Song, J.; Zhou, P.; Shu, Y.; Liang, P.; Liang, H.; Liu, Y.; Yuan, X.; Shan, X.; Wu, X. An ultrasound-triggered injectable sodium alginate scaffold loaded with electrospun microspheres for on-demand drug delivery to accelerate bone defect regeneration. Carbohydr. Polym. 2024, 334, 122039. [Google Scholar] [CrossRef]
- Vinikoor, T.; Dzidotor, G.K.; Le, T.T.; Liu, Y.; Kan, H.M.; Barui, S.; Chorsi, M.T.; Curry, E.J.; Reinhardt, E.; Wang, H.; et al. Injectable and biodegradable piezoelectric hydrogel for osteoarthritis treatment. Nat. Commun. 2023, 14, 6257. [Google Scholar] [CrossRef]
- Qiu, L.Y.; Yan, L.; Zhang, L.; Jin, Y.M.; Zhao, Q.H. Folate-modified poly(2-ethyl-2-oxazoline) as hydrophilic corona in polymeric micelles for enhanced intracellular doxorubicin delivery. Int. J. Pharm. 2013, 456, 315–324. [Google Scholar] [CrossRef]
- Li, C.; Zhang, X.; Chen, Q.; Zhang, J.; Li, W.; Hu, H.; Zhao, X.; Qiao, M.; Chen, D. Synthetic Polymeric Mixed Micelles Targeting Lymph Nodes Trigger Enhanced Cellular and Humoral Immune Responses. ACS Appl. Mater. Interfaces 2018, 10, 2874–2889. [Google Scholar] [CrossRef]
- Lan, Q.; Lu, R.; Chen, H.; Pang, Y.; Xiong, F.; Shen, C.; Qin, Z.; Zheng, L.; Xu, G.; Zhao, J. MMP-13 enzyme and pH responsive theranostic nanoplatform for osteoarthritis. J. Nanobiotechnol. 2020, 18, 117. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, H.; Wang, S.; Sun, J.; Hu, Y.; Liu, H.; Liu, J.; Chen, X.; Zhou, F.; Bai, L.; et al. Ultrasound-triggered in situ gelation with ROS-controlled drug release for cartilage repair. Mater. Horiz. 2023, 10, 3507–3522. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Qin, Z.; Zhao, J.; He, Y.; Ren, E.; Zhu, Y.; Liu, G.; Mao, C.; Zheng, L. Cartilage-targeting and dual MMP-13/pH responsive theranostic nanoprobes for osteoarthritis imaging and precision therapy. Biomaterials 2019, 225, 119520. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Lou, J.; Wang, F.; Fan, D.; Qin, Z. Recent Advances in Nano-Therapeutic Strategies for Osteoarthritis. Front. Pharmacol. 2022, 13, 924387. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Wu, X.; Chen, X.; Wang, B.; Xu, W. Intellective and stimuli-responsive drug delivery systems in eyes. Int. J. Pharm. 2021, 602, 120591. [Google Scholar] [CrossRef]
- Adepu, S.; Ramakrishna, S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef]
- Long, J.; Liang, X.; Ao, Z.; Tang, X.; Li, C.; Yan, K.; Yu, X.; Wan, Y.; Li, Y.; Li, C.; et al. Stimulus-responsive drug delivery nanoplatforms for inflammatory bowel disease therapy. Acta Biomater. 2024, 188, 27–47. [Google Scholar] [CrossRef]




| Internal Stimulus | Materials | Responsive Shell | Bioactive Agent | Effects | Reference |
|---|---|---|---|---|---|
| ROS | Poly (ethylene glycol diacrylate) (PEGDA)-1,2-ethylenedithiol (EDT) copolymer (PEGDA-EDT); reduced graphene oxide (rGO) | PEGDA-EDT | rGO | 1. Inhibit the expression of inflammatory cytokines with strong anti-inflammatory effect 2. Upregulation of key antioxidant factors has a strong antioxidant effect | [71] |
| Mesoporous silica NPs (MSN) modified with methoxy polyethylene glycol-thioketal (TK), loaded with the small molecule compound oltipraz (OL) | MSN | OL | MSN-OL significantly activated the Nrf2/HO-1 signaling pathway and exhibited better ROS scavenging and stronger antiapoptotic ability to protect the mitochondrial membrane potential of chondrocytes. | [72] | |
| The NPs consisted of an amphiphilic block copolymer composed of PEG and oxidatively reactive hydrophobic blocks (acrylic monomer of phenylboronic ester and 1,8-naphthalimide fluorescent monomer) encapsulating doxorubicin (DOX) | PEG, phenylboronic ester and naphthalimide | DOX | 1. Both copolymer NPs and their degradation products are cytocompatible. 2. ROS stimulated the release of nanoparticle-loaded DOX and allowed polymer degradation to be monitored by scaled fluorescence imaging. | [73] | |
| Synthesis of nanoparticulate DLNPs with -SeSe- moiety as ROS-responsive component, anti-inflammatory drug dexamethasone (DEX) and chondrogenic differentiation factor chondrogenic derivative -luminescent protein-1 as main pharmacophore | -SeSe-group | DEX, CDMP-1 | 1. Effectively inhibit the proliferation of activated macrophages, induce macrophage apoptosis, with anti-inflammatory effect, so that BMSCs differentiate into chondrocytes. 2. High concentration of ROS in the joint cavity leads to -SeSe- breakage. The slow release of DEX reduces pain and inflammation. | [74] | |
| PEG micelles prepared with ROS-sensitive thioketal (TK) and cartilage-targeting peptide (known as TKCP) modified PEG micelles were then encapsulated with DEX to form TKCP@DEX NPs. | TK | DEX | 1. Nanoprobes can intelligently “turn on” in response to excessive ROS and “turn off” in normal joints. 2. TKCP@DEX can effectively respond to ROS and slow-release DEX to significantly reduce cartilage damage in OA joints. | [75] | |
| Pinacol borate, PEG and naphthylamide monomers with encapsulated hydrophobic curcumin | polymethacrylate | Cur | Both in vitro and in vivo studies validated real-time visualization of drug release and ROS clearance, as well as therapeutic efficacy in OA. | [76] | |
| pH | HA-modified metal–organic frameworks (MOFs) system loaded with anti-inflammatory protocatechuic acid (PCA) | MOFs | PCA | 1. Significantly reduced IL-1β-induced synovial inflammation in chondrocytes and OA joints. 2. Downregulates the expression of OA inflammatory markers and promotes the expression of cartilage-specific genes. | [77] |
| Modified mesoporous silica NPs (MSNs) and polyacrylic acid (PAA), loaded with andrographolide (AG) | PAA | AG | Shows stronger anti-arthritic efficacy and cartilage protection, as evidenced by lower expression of inflammatory factors and better prevention of proteoglycan loss. | [78] | |
| Cyclic brushed amphiphilic polymer (CB) with SBMA and DMAEMA as brushes and cyclic polymer (c-P(HEMA)) as core template, loaded with hydrophobic curcumin (Cur) and hydrophilic loxoprofen (LXP) | DMAEMA | Cur, LXP | 1. Hydrophilic and hydrophobic anti-inflammatory drugs can be co-loaded with higher drug loading efficiency. 2. With over-lubrication, sequence-controlled release and anti-inflammatory effects, it can effectively treat OA. | [79] | |
| PLGA, ammonium bicarbonate (NH4HCO3), HA | NH4HCO3 | HA | 1. The NPs are non-toxic to chondrocytes and have no negative impact on joints. 2. Exhibits extracellular burst release behavior and higher chondrocyte viability. | [80] | |
| Hollow mesoporous silica NPs (HMSN), chitosan (Cs) as coating, loaded with celastrol (CSL) | Cs | CSL | 1. High biocompatibility for intra-articular injection. 2. Downregulate the expression of inflammatory factors, improve joint surface erosion and joint effusion. | [81] | |
| Enzyme | Triglyceride monostearate (TG-18), corticosteroid triamcinolone acetonide (TA) | TG-18 | TA | Provide the optimal amount of therapeutic medication when needed, thereby maximizing the therapeutic effect and prolonging the duration of the therapeutic effect. | [82] |
| Schiff base cross-linking between oxidized hyaluronic acid (OHA) and type I collagen to form collagen-based hydrogels (Col-OHA); dexamethasone sodium phosphate (DSP)-loaded | Col-OHA | DSP | It has a significant inhibitory effect on the production of synovial inflammatory cytokines and provides effective and sustained relief of OA symptoms. | [83] | |
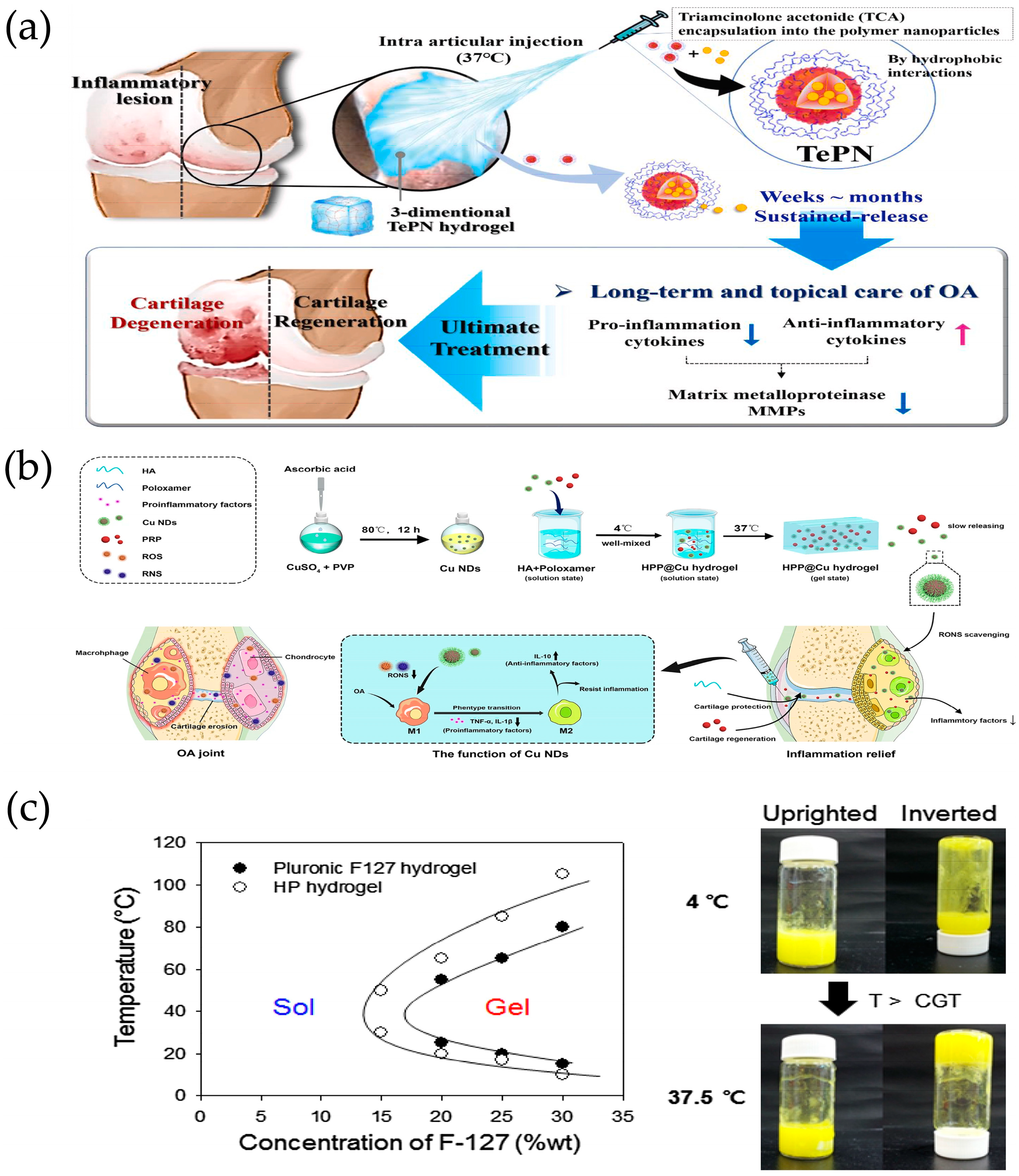
| Temperature | Poly (organophosphorus nitrile) NPs (TePN), encapsulated tretinoin (TCA) | TePN | TCA | OA is treated by inhibiting MMP expression in cartilage by decreasing pro-inflammatory cytokine expression and increasing anti-inflammatory cytokine expression. | [84] |
| Poloxamer 407 (P407), HA, copper nanodots (Cu NDs), platelet rich plasma (PRP) | P407 | Cu NDs, HA, PRP | 1. It can remove RON in the joint microenvironment and block the destructive effect of RONS on chondrocytes 2. It can reverse the M1 polarization of macrophages and promote the production of M2 macrophages. | [85] | |
| Poly(ε-caprolactone-propionate)-b-poly (ethylene glycol)-b-poly(ε-caprolactone-propionate) (PCLA-PEG-PCLA) triblock copolymer, flurbiprofen | PCLA-PEG-PCLA | flurbiprofen | 1. Intra-articular administration can effectively prolong the analgesic time. 2. Significantly reduce the inflammatory response of rat OA model by downregulating the expression of inflammatory factors. | [86] | |
| HA, Pluronic F-127, piroxicam (PX) | Pluronic F-127 | PX | It has excellent mechanical strength and also induces sustained drug release behavior. | [87] |
| External Stimulus | Materials | Responsive Shell | Bioactive Agent | Effects | Reference |
|---|---|---|---|---|---|
| NIR | Metal Organic Framework (MOF) modified mesoporous polydopamine (MPDA), collagen II targeting peptide (WYRGRL), loaded with rapamycin (Rap) and bilirubin (Br) | PDA | Rap, Br | 1. With excellent near infrared laser stimulation responsive drug release effect. 2. Has excellent MR imaging properties to monitor its in vivo therapeutic effects. 3. Enhanced energy metabolism of chondrocytes, further rescued apoptosis in vitro and inhibited cartilage degeneration in vivo. | [101] |
| molybdenum (Mo)-based polyoxometalate clusters (POM) | POM | - | 1. It has excellent antioxidant activity, biological safety and good anti-inflammatory effect. 2. Effectively alleviate the symptoms of OA mice, prevent cartilage erosion, reduce inflammatory cytokines, and reduce articular cartilage decomposition and metabolism proteases. | [102] | |
| Preparation of HSC hydrogels by mixing HA-thiourea (NCSN) solution with Cu2+ | NCSN, Cu2+ | NCSN | Effectively promotes chondrocyte anabolism while reducing IL-1β-induced catabolism and inflammation. | [103] | |
| Mitochondria-targeted and sod-mimicking Mn3O4@PDA@Pd-SS31 nanoenzymes | PDA | Pd, Mn3O4 | 1. It has good biocompatibility and photodynamic effect. 2. Effective removal of ROS in mitochondria, thus improving the oxidative stress microenvironment. | [104] | |
| Neutrophil erythrocyte hybrid membrane-encapsulated dexamethasone sodium phosphate (Dexp) loaded hollow copper sulf ide NPs (D-CuS@NR NPs) | CuS | Dexp | 1. It has excellent photothermal conversion ability, drug release behavior control and good cytocompatibility. 2. It can target the inflamed joint parts with stronger anti-inflammatory effect. 3. After NIR treatment can effectively inhibit cartilage degeneration through photothermal therapy and downregulation of synovial inflammation. | [105] | |
| Magnetic | KGN-loaded magnetic NPs (KGN-MNPs) were synthesized by encapsulating KGN on the surface of iron oxide NPs using poly(propylene lactone) (PLA) | MNP | KGN | 1. Significantly increased the retention of KGN in MSCs and improved the utilization of KGN. 2. Inhibited the generation of inflammation, induced the chondrogenic differentiation of MSCs and can prevent articular cartilage degeneration. | [106] |
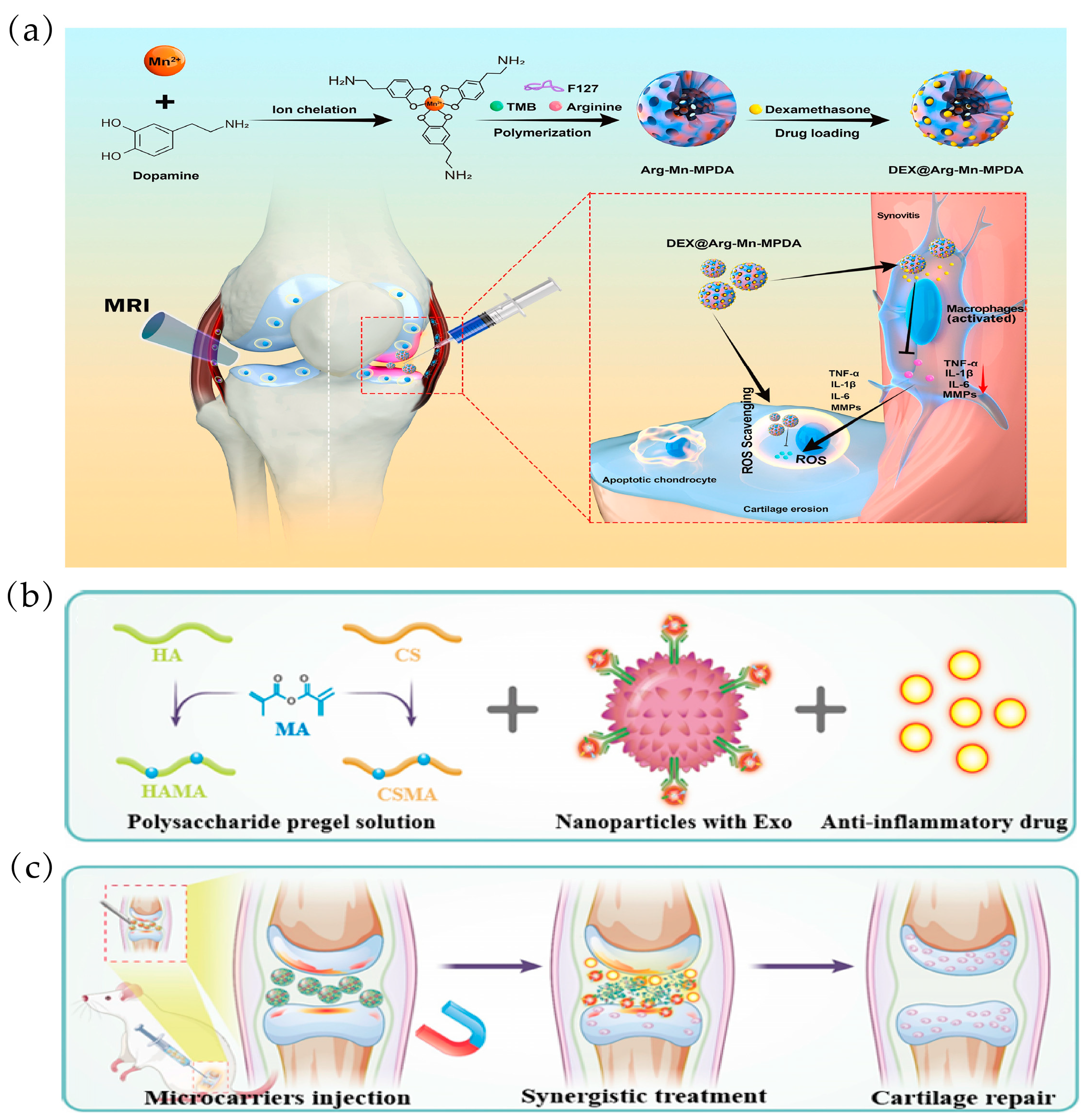
| Mesoporous polydopamine NPs (DAMM NPs) doped with arginine and manganese (Mn) ions, loaded with dexamethasone (DEX) | DAMM NP | DEX | 1. Prolonged DEX release can directly ameliorate OA progression by inhibiting macrophage-induced synovial inflammation. 2. It can directly reduce ROS-induced chondrocyte apoptosis. 3. Displaying magnetic resonance imaging (MRI) sensitive signals, thus enabling real-time visualization of damaged articular cartilage. | [107] | |
| Modified natural polysaccharide hyaluronic acid (HAMA) and chondroitin sulphate (CSMA), loaded with magnetic NPs (MPM) and the anti-inflammatory drug DS | Fe3O4@MgSiO3 | DS | 1. It is mono-disperse, porous and facilitates slow drug release, and its magnetic properties give it controlled drug delivery. 2. Can effectively alleviate cartilage degradation in OA rats | [108] | |
| Ultrasonic | Pluronic® F-127, HA, gelatin, loaded with hydrocortisone | Pluronic | hydrocortisone | 1. Has thermosensitive properties and is capable of sustaining drug release. 2. Has controlled on-demand drug release and provides higher hydrocortisone concentrations in OA. 3. Possesses ultrasound, thermal response and stability. | [45] |
| Carboxymethyl chitosan oxidized chondroitin sulphate (CMC-OCS) hydrogel-embedded KGN loaded PLGA MPs (MPs@KGN) (CMC-OCS@MPs@KGN) | PLGA | KGN | 1. Exhibits faster gelation, lower swelling ratio and lower in vitro degradation. 2. Has the ability to increase COL-2 synthesis, which facilitates cartilage repair. 3. Can respond to ultrasound by controlled KGN burst release. | [109] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, Q.; Zhang, M.; Li, S.; Liu, W.; Xu, C.; Li, Y.; Xie, R. Controlled Stimulus-Responsive Delivery Systems for Osteoarthritis Treatment. Int. J. Mol. Sci. 2024, 25, 11799. https://doi.org/10.3390/ijms252111799
Ye Q, Zhang M, Li S, Liu W, Xu C, Li Y, Xie R. Controlled Stimulus-Responsive Delivery Systems for Osteoarthritis Treatment. International Journal of Molecular Sciences. 2024; 25(21):11799. https://doi.org/10.3390/ijms252111799
Chicago/Turabian StyleYe, Qianwen, Mingshuo Zhang, Shuyue Li, Wenyue Liu, Chunming Xu, Yumei Li, and Renjian Xie. 2024. "Controlled Stimulus-Responsive Delivery Systems for Osteoarthritis Treatment" International Journal of Molecular Sciences 25, no. 21: 11799. https://doi.org/10.3390/ijms252111799
APA StyleYe, Q., Zhang, M., Li, S., Liu, W., Xu, C., Li, Y., & Xie, R. (2024). Controlled Stimulus-Responsive Delivery Systems for Osteoarthritis Treatment. International Journal of Molecular Sciences, 25(21), 11799. https://doi.org/10.3390/ijms252111799







