Assessing Protein Surface-Based Scoring for Interpreting Genomic Variants
Abstract
1. Introduction
2. Results
2.1. Thermophilic and Mesophilic Enzymes Demonstrate Clear Surface Differences
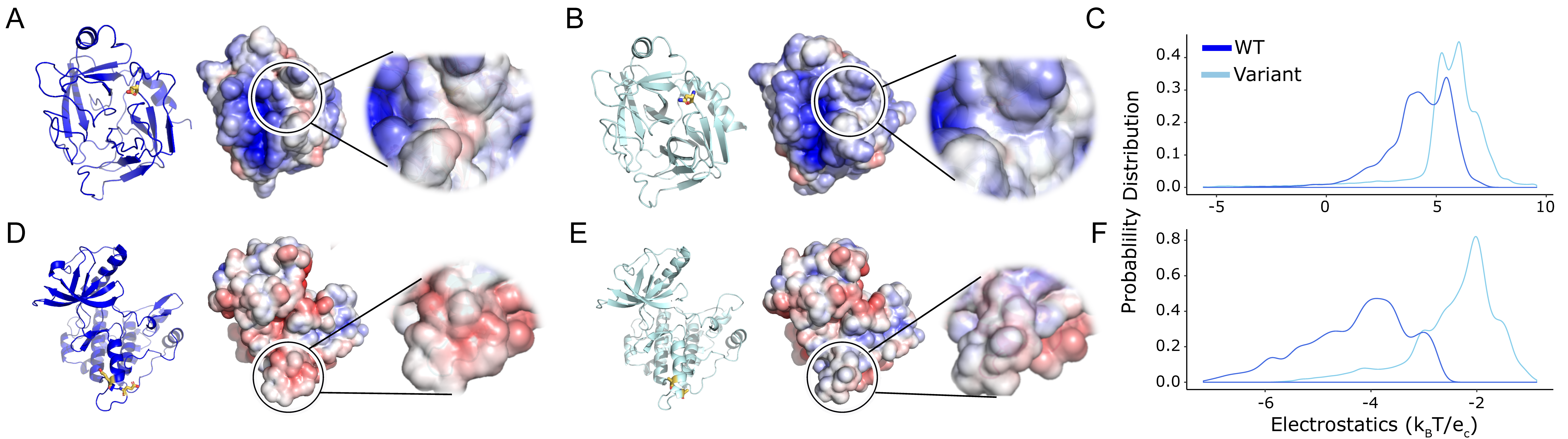
2.2. Solubilizing Protein Variants Have Significant Local Changes
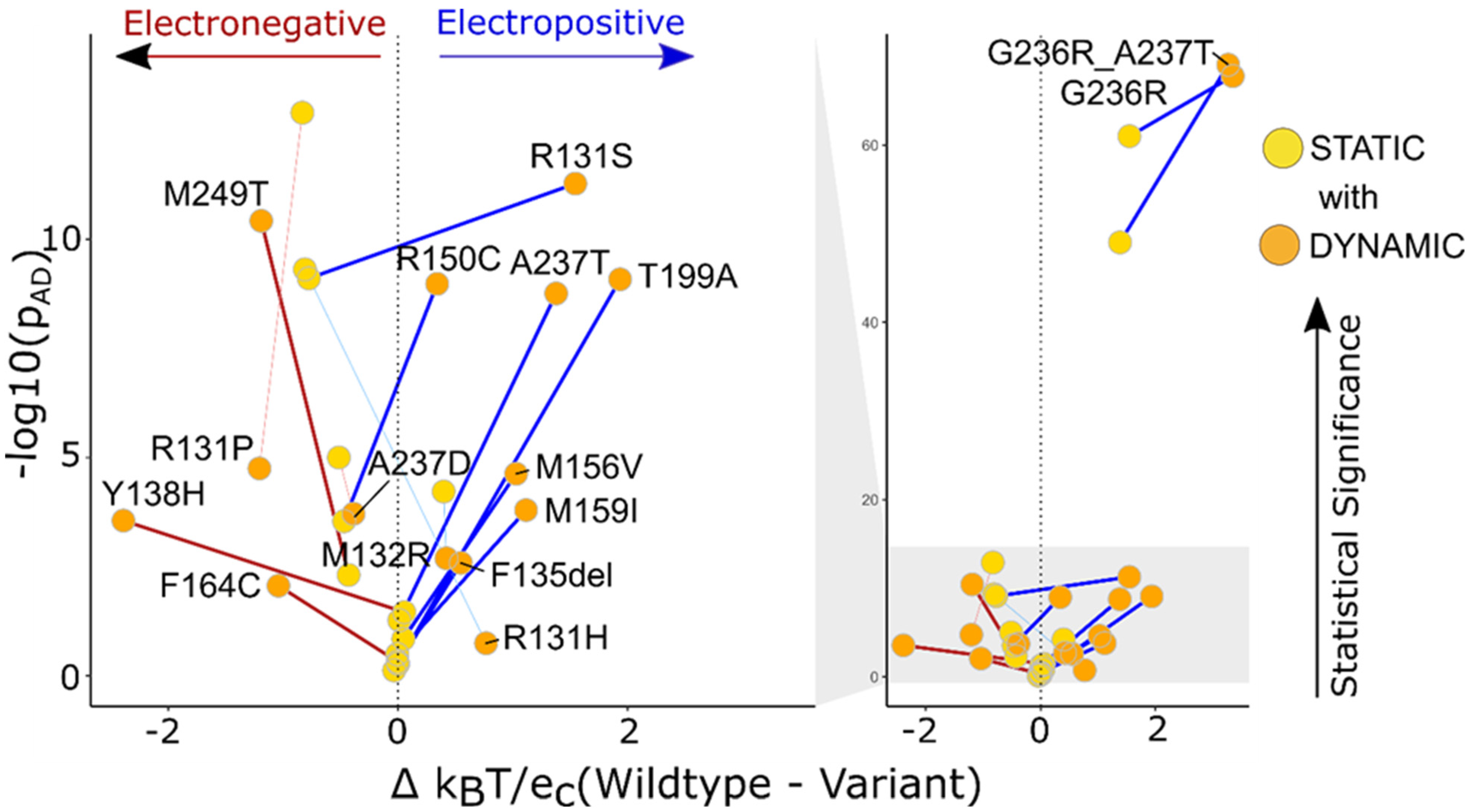
2.3. Dynamic Ensembles Provide More Explicit Mechanistic Interpretation than Static Structures
3. Discussion
4. Materials and Methods
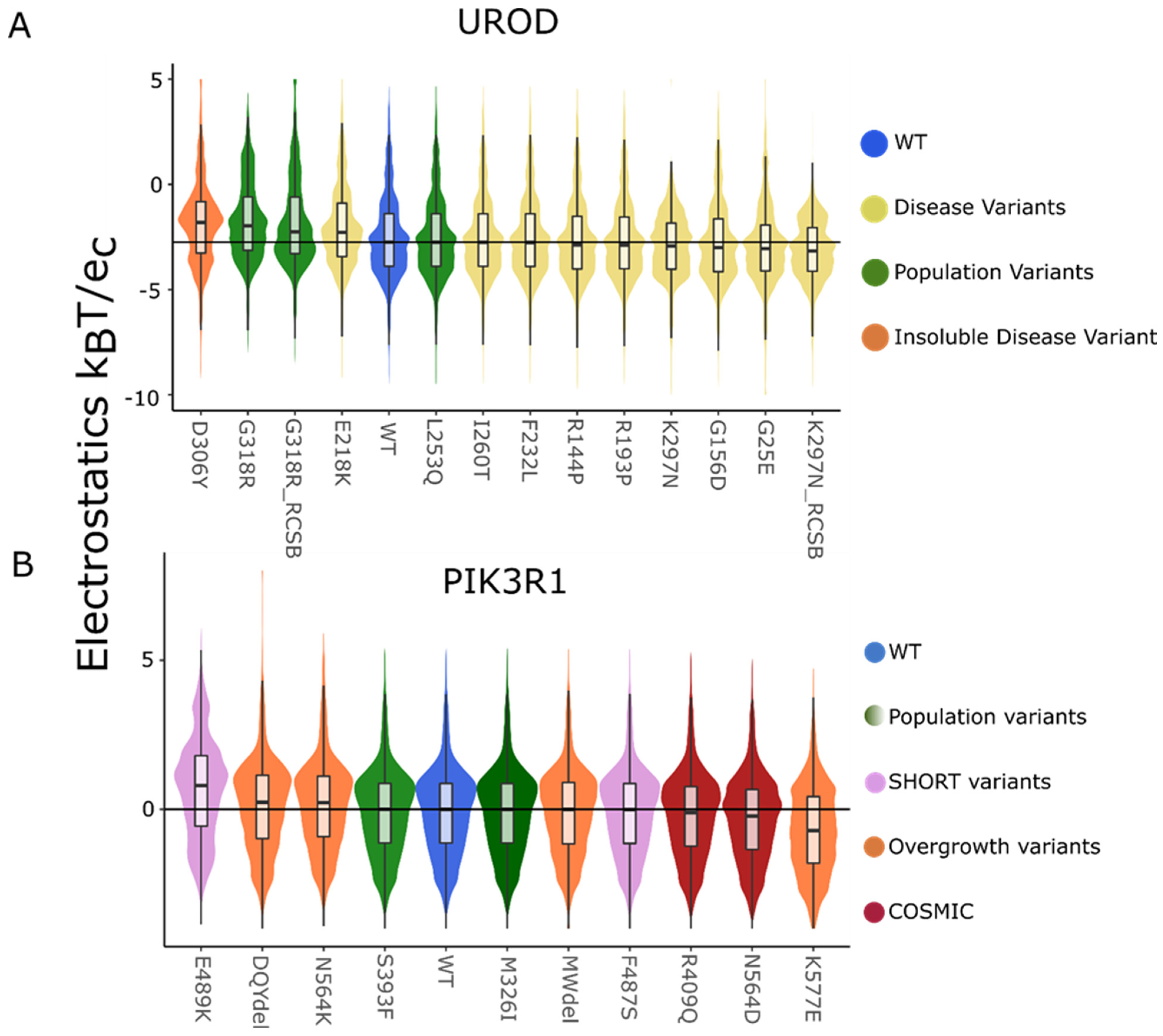
4.1. Aggregating Genomic Variants
4.2. Experimental Structures of Wild-Type and Genomic Mutations
4.3. Molecular Dynamics Simulation Used to Generate Protein Structure Ensembles
4.4. Generating Protein Surface and Local Electrostatic Scores
4.5. Statistical Assessment of Altered Surface Potentials
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Norgeot, B.; Glicksberg, B.S.; Butte, A.J. A call for deep-learning healthcare. Nat. Med. 2019, 25, 14–15. [Google Scholar] [CrossRef] [PubMed]
- Esteva, A.; Robicquet, A.; Ramsundar, B.; Kuleshov, V.; DePristo, M.; Chou, K.; Cui, C.; Corrado, G.; Thrun, S.; Dean, J. A guide to deep learning in healthcare. Nat. Med. 2019, 25, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Munoz, E.A.; Milko, L.V.; Harrison, S.M.; Azzariti, D.R.; Kurtz, C.L.; Lee, K.; Mester, J.L.; Weaver, M.A.; Currey, E.; Craigen, W.; et al. ClinGen Variant Curation Expert Panel experiences and standardized processes for disease and gene-level specification of the ACMG/AMP guidelines for sequence variant interpretation. Hum. Mutat. 2018, 39, 1614–1622. [Google Scholar] [CrossRef]
- Hu, Z.; Yu, C.; Furutsuki, M.; Andreoletti, G.; Ly, M.; Hoskins, R.; Adhikari, A.N.; Brenner, S.E. VIPdb, a genetic Variant Impact Predictor Database. Hum. Mutat. 2019, 40, 1202–1214. [Google Scholar] [CrossRef]
- Bean, L.J.H.; Hegde, M.R. Clinical implications and considerations for evaluation of in silico algorithms for use with ACMG/AMP clinical variant interpretation guidelines. Genome Med. 2017, 9, 111. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Madhavan, S.; Ritter, D.; Micheel, C.; Rao, S.; Roy, A.; Sonkin, D.; McCoy, M.; Griffith, M.; Griffith, O.L.; McGarvey, P.; et al. ClinGen Cancer Somatic Working Group—Standardizing and democratizing access to cancer molecular diagnostic data to drive translational research. Pac. Symp. Biocomput. 2018, 23, 247–258. [Google Scholar]
- Careri, G. Cooperative charge fluctuations by migrating protons in globular proteins. Prog. Biophys. Mol. Biol. 1998, 70, 223–249. [Google Scholar] [CrossRef]
- Martin, A.; Sieber, V.; Schmid, F.X. In-vitro selection of highly stabilized protein variants with optimized surface. J. Mol. Biol. 2001, 309, 717–726. [Google Scholar] [CrossRef]
- Karczewski, K.J.; Francioli, L.C.; Tiao, G.; Cummings, B.B.; Alfoldi, J.; Wang, Q.; Collins, R.L.; Laricchia, K.M.; Ganna, A.; Birnbaum, D.P.; et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 2020, 581, 434–443. [Google Scholar] [CrossRef]
- Wang, L.; Li, Q.; Wu, L.; Liu, S.; Zhang, Y.; Yang, X.; Zhu, P.; Zhang, H.; Zhang, K.; Lou, J.; et al. Identification of SERPINB1 as a physiological inhibitor of human granzyme H. J. Immunol. 2013, 190, 1319–1330. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, K.; Wu, L.; Liu, S.; Zhang, H.; Zhou, Q.; Tong, L.; Sun, F.; Fan, Z. Structural insights into the substrate specificity of human granzyme H: The functional roles of a novel RKR motif. J. Immunol. 2012, 188, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Munshi, S.; Kornienko, M.; Hall, D.L.; Reid, J.C.; Waxman, L.; Stirdivant, S.M.; Darke, P.L.; Kuo, L.C. Crystal structure of the Apo, unactivated insulin-like growth factor-1 receptor kinase. Implication for inhibitor specificity. J. Biol. Chem. 2002, 277, 38797–38802. [Google Scholar] [CrossRef] [PubMed]
- Munshi, S.; Hall, D.L.; Kornienko, M.; Darke, P.L.; Kuo, L.C. Structure of apo, unactivated insulin-like growth factor-1 receptor kinase at 1.5 A resolution. Acta Crystallogr. D Biol. Crystallogr. 2003, 59 Pt 10, 1725–1730. [Google Scholar] [CrossRef] [PubMed]
- Warby, C.A.; Phillips, J.D.; Bergonia, H.A.; Whitby, F.G.; Hill, C.P.; Kushner, J.P. Structural and kinetic characterization of mutant human uroporphyrinogen decarboxylases. Cell. Mol. Biol. 2009, 55, 40–45. [Google Scholar]
- Venturutti, L.; Teater, M.; Zhai, A.; Chadburn, A.; Babiker, L.; Kim, D.; Béguelin, W.; Lee, T.C.; Kim, Y.; Chin, C.R.; et al. TBL1XR1 Mutations Drive Extranodal Lymphoma by Inducing a Pro-tumorigenic Memory Fate. Cell 2020, 182, 297–316.e27. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Liao, W.; Lu, X.; Fei, Y.; Gu, Y.; Huang, Y. Generative AI design for building structures. Autom. Constr. 2024, 157, 105187. [Google Scholar] [CrossRef]
- McCarrier JAH, D.C.; Kappes, U.; Basel, D.B.; Dsouza, N.R.; Zimmermann, M.T.; Hagelstrom, R.T. Expanding the Phenotypic Spectrum of Pierpont Syndrome—Four Patients from One Institution. In Proceedings of the ACMG Meeting 2018, Charlotte, NC, USA, 10–14 April 2018. [Google Scholar]
- Cousin, M.A.; Veale, E.L.; Dsouza, N.R.; Tripathi, S.; Holden, R.G.; Arelin, M.; Beek, G.; Bekheirnia, M.R.; Beygo, J.; Bhambhani, V.; et al. Gain and loss of TASK3 channel function and its regulation by novel variation cause KCNK9 imprinting syndrome. Genome Med. 2022, 14, 62. [Google Scholar] [CrossRef]
- Cottrell, C.E.; Bender, N.R.; Zimmermann, M.T.; Heusel, J.W.; Corliss, M.; Evenson, M.J.; Magrini, V.; Corsmeier, D.J.; Avenarius, M.; Dudley, J.N.; et al. Somatic PIK3R1 variation as a cause of vascular malformations and overgrowth. Genet. Med. 2021, 23, 1882–1888. [Google Scholar] [CrossRef]
- Kocher, J.P.; Quest, D.J.; Duffy, P.; Meiners, M.A.; Moore, R.M.; Rider, D.; Hossain, A.; Hart, S.N.; Dinu, V. The Biological Reference Repository (BioR): A rapid and flexible system for genomics annotation. Bioinformatics 2014, 30, 1920–1922. [Google Scholar] [CrossRef] [PubMed]
- Stenson, P.D.; Mort, M.; Ball, E.V.; Howells, K.; Phillips, A.D.; Thomas, N.S.; Cooper, D.N. The Human Gene Mutation Database: 2008 update. Genome Med. 2009, 1, 13. [Google Scholar] [CrossRef] [PubMed]
- Landrum, M.J.; Lee, J.M.; Riley, G.R.; Jang, W.; Rubinstein, W.S.; Church, D.M.; Maglott, D.R. ClinVar: Public archive of relationships among sequence variation and human phenotype. Nucleic Acids Res. 2014, 42, D980–D985. [Google Scholar] [CrossRef] [PubMed]
- Forbes, S.A.; Bhamra, G.; Bamford, S.; Dawson, E.; Kok, C.; Clements, J.; Menzies, A.; Teague, J.W.; Futreal, P.A.; Stratton, M.R. The Catalogue of Somatic Mutations in Cancer (COSMIC). Curr. Protoc. Hum. Genet. 2008, 57, 10.11.1–10.11.26. [Google Scholar] [CrossRef]
- Shen, Y.; Yuan, M.; Luo, H.; Yang, Z.; Liang, M.; Gan, J. Rare variant of TBL1XR1 in West syndrome: A case report. Mol. Genet. Genom. Med. 2022, 10, e1991. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Xu, C.; Tempel, W.; He, H.; Wu, X.; Bountra, C.; Arrowsmith, C.H.; Edwards, A.M.; Min, J. Crystal structure of TBL1XR1 WD40 repeats. 2014; to be published. [Google Scholar] [CrossRef]
- Kallberg, M.; Margaryan, G.; Wang, S.; Ma, J.; Xu, J. RaptorX server: A resource for template-based protein structure modeling. Methods Mol. Biol. 2014, 1137, 17–27. [Google Scholar] [CrossRef]
- Biasini, M.; Bienert, S.; Waterhouse, A.; Arnold, K.; Studer, G.; Schmidt, T.; Kiefer, F.; Cassarino, T.G.; Bertoni, M.; Bordoli, L.; et al. SWISS-MODEL: Modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 2014, 42, W252–W258. [Google Scholar] [CrossRef]
- Zhang, Y. I-TASSER server for protein 3D structure prediction. BMC Bioinform. 2008, 9, 40. [Google Scholar] [CrossRef]
- Miller, A.N.; Long, S.B. Crystal structure of the human two-pore domain potassium channel K2P1. Science 2012, 335, 432–436. [Google Scholar] [CrossRef]
- Brohawn, S.G.; del Marmol, J.; MacKinnon, R. Crystal structure of the human K2P TRAAK, a lipid- and mechano-sensitive K+ ion channel. Science 2012, 335, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Hoegenauer, K.; Soldermann, N.; Stauffer, F.; Furet, P.; Graveleau, N.; Smith, A.B.; Hebach, C.; Hollingworth, G.J.; Lewis, I.; Gutmann, S.; et al. Discovery and Pharmacological Characterization of Novel Quinazoline-Based PI3K Delta-Selective Inhibitors. ACS Med. Chem. Lett. 2016, 7, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Schymkowitz, J.; Borg, J.; Stricher, F.; Nys, R.; Rousseau, F.; Serrano, L. The FoldX web server: An online force field. Nucleic Acids Res. 2005, 33, W382–W388. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.C.; Braun, R.; Wang, W.; Gumbart, J.; Tajkhorshid, E.; Villa, E.; Chipot, C.; Skeel, R.D.; Kale, L.; Schulten, K. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005, 26, 1781–1802. [Google Scholar] [CrossRef]
- Huang, J.; MacKerell, A.D., Jr. CHARMM36 all-atom additive protein force field: Validation based on comparison to NMR data. J. Comput. Chem. 2013, 34, 2135–2145. [Google Scholar] [CrossRef]
- Zimmermann, M.T.; Urrutia, R.; Oliver, G.R.; Blackburn, P.R.; Cousin, M.A.; Bozeck, N.J.; Klee, E.W. Molecular modeling and molecular dynamic simulation of the effects of variants in the TGFBR2 kinase domain as a paradigm for interpretation of variants obtained by next generation sequencing. PLoS ONE 2017, 12, e0170822. [Google Scholar] [CrossRef]
- Zimmermann, M.T.; Urrutia, R.; Cousin, M.A.; Oliver, G.R.; Klee, E.W. Assessing Human Genetic Variations in Glucose Transporter SLC2A10 and Their Role in Altering Structural and Functional Properties. Front. Genet. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Cheng, X.; Swails, J.M.; Yeom, M.S.; Eastman, P.K.; Lemkul, J.A.; Wei, S.; Buckner, J.; Jeong, J.C.; Qi, Y.; et al. CHARMM-GUI Input Generator for NAMD, GROMACS, AMBER, OpenMM, and CHARMM/OpenMM Simulations Using the CHARMM36 Additive Force Field. J. Chem. Theory Comput. 2016, 12, 405–413. [Google Scholar] [CrossRef]
- Brooks, B.R.; Brooks, C.L., III; Mackerell, A.D., Jr.; Nilsson, L.; Petrella, R.J.; Roux, B.; Won, Y.; Archontis, G.; Bartels, C.; Boresch, S. CHARMM: The biomolecular simulation program. J. Comput. Chem. 2009, 30, 1545–1614. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, Y. Generating triangulated macromolecular surfaces by Euclidean Distance Transform. PLoS ONE 2009, 4, e8140. [Google Scholar] [CrossRef]
- Jurrus, E.; Engel, D.; Star, K.; Monson, K.; Brandi, J.; Felberg, L.E.; Brookes, D.H.; Wilson, L.; Chen, J.; Liles, K.; et al. Improvements to the APBS biomolecular solvation software suite. Protein Sci. 2018, 27, 112–128. [Google Scholar] [CrossRef] [PubMed]
- Dolinsky, T.J.; Czodrowski, P.; Li, H.; Nielsen, J.E.; Jensen, J.H.; Klebe, G.; Baker, N.A. PDB2PQR: Expanding and upgrading automated preparation of biomolecular structures for molecular simulations. Nucleic Acids Res. 2007, 35, W522–W525. [Google Scholar] [CrossRef] [PubMed]
- Dolinsky, T.J.; Nielsen, J.E.; McCammon, J.A.; Baker, N.A. PDB2PQR: An automated pipeline for the setup of Poisson-Boltzmann electrostatics calculations. Nucleic Acids Res. 2004, 32, W665–W667. [Google Scholar] [CrossRef] [PubMed]

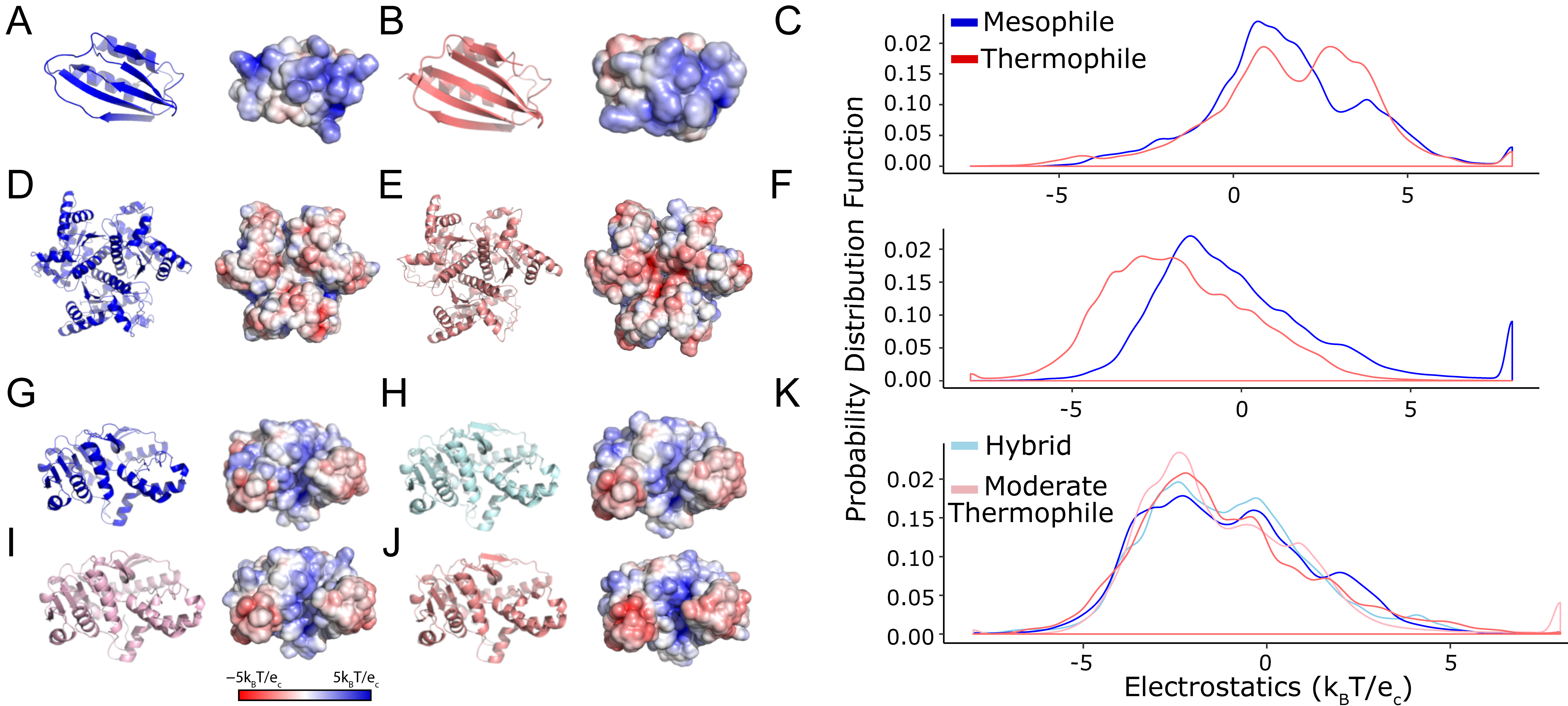

| Protein | PDB ID | Thermodynamic Class | pT | pAD | pKS |
|---|---|---|---|---|---|
| Acyl Phosphatase | 3TOQ | Mesophile | NA | NA | NA |
| Acyl Phosphatase | 3TNV | Thermophile | 4.38 × 10−1 | 1.06 × 10−7 | 4.64 × 10−9 |
| Adenylate Kinase | 1KHT | Mesophile | NA | NA | NA |
| Adenylate Kinase | 1KI9 | Thermophile | 1.48 × 10−6 | 1.80 × 10−51 | 0 |
| Malate Dehydrogenase | 1GV1 | Mesophile | NA | NA | NA |
| Malate Dehydrogenase | 1GV0 | Moderate Thermophile | 4.87 × 10−1 | 2.91 × 10−3 | 1.34 × 10−1 |
| Malate Dehydrogenase | 1GUZ | Hybrid | 4.99 × 10−1 | 9.52 × 10−3 | 9.13 × 10−1 |
| Malate Dehydrogenase | 1GUY | Thermophile | 4.82 × 10−1 | 9.44 × 10−3 | 1.64 × 10−1 |
| Protein | Surface Region | PDB ID | Variant | pT | pAD | pKS |
|---|---|---|---|---|---|---|
| Granzyme H | Global | 3TK9 | D102N | 7.23 × 10−2 | 6.22 × 10−10 | 8.25 × 10−10 |
| Granzyme H | Local | D102N | 3.89 × 10−8 | 1.46 × 10−148 | 0 | |
| Granzyme H | Reference | 4GAW | WT | NA | NA | NA |
| IGF1R | Global | 1P4O | E1067A and E1069A | 5.01 × 10−2 | 8.16 × 10−10 | 2.16 × 10−12 |
| IGF1R | Local | E1067A and E1069A | 8.71 × 10−34 | 3.55 × 10−299 | 0 | |
| IGF1R | Reference | 1M7N | WT | NA | NA | NA |
| UROD | Global | D306Y | 3.76 × 10−1 | 7.62 × 10−4 | 6.16 × 10−2 | |
| UROD | Local | D306Y | 2.32 × 10−2 | 6.28 × 10−19 | 0 | |
| UROD | Reference | 3GVQ | WT | NA | NA | NA |
| Protein | Variant | pT | pAD | pKS |
|---|---|---|---|---|
| UROD | D306Y | 2.3 × 10−2 | 6.3 × 10−19 | 0.0 × 100 |
| E218K | 1.0 × 10−1 | 3.9 × 10−11 | 2.3 × 10−6 | |
| F232L | 4.8 × 10−1 | 8.7 × 10−1 | 3.3 × 10−2 | |
| G25E | 1.5 × 10−1 | 5.4 × 10−5 | 2.6 × 10−7 | |
| G156D | 3.4 × 10−1 | 3.3 × 10−4 | 6.9 × 10−2 | |
| G318R | 7.0 × 10−3 | 3.4 × 10−23 | 1.1 × 10−16 | |
| G318R * | 1.5 × 10−2 | 1.3 × 10−22 | 5.4 × 10−8 | |
| I260T | 5.0 × 10−1 | 3.1 × 10−1 | 5.7 × 10−1 | |
| K297N | 9.7 × 10−2 | 2.6 × 10−7 | 1.5 × 10−7 | |
| K297N * | 1.2 × 10−2 | 2.8 × 10−15 | 5.8 × 10−12 | |
| L253Q | 5.1 × 10−1 | 5.4 × 10−1 | 3.1 × 10−1 | |
| R144P | 4.3 × 10−1 | 6.5 × 10−1 | 3.3 × 10−2 | |
| R193P | 4.3 × 10−1 | 6.1 × 10−4 | 3.8 × 10−2 | |
| PIK3R1 | E489K | 4.5 × 10−4 | 2.1 × 10−42 | 0.0 × 100 |
| DQYdel | 2.7 × 10−1 | 3.3 × 10−6 | 6.1 × 10−5 | |
| N564K | 2.1 × 10−1 | 1.5 × 10−1 | 7.3 × 10−4 | |
| S393F | 5.0 × 10−1 | 4.2 × 10−1 | 5.0 × 10−1 | |
| M326I | 5.2 × 10−1 | 8.8 × 10−1 | 2.5 × 10−1 | |
| MWdel | 4.8 × 10−1 | 5.6 × 10−1 | 6.5 × 10−1 | |
| F487S | 5.1 × 10−1 | 4.3 × 10−1 | 2.0 × 10−2 | |
| R409Q | 4.7 × 10−1 | 4.6 × 10−2 | 7.8 × 10−2 | |
| N564D | 3.0 × 10−1 | 2.4 × 10−3 | 1.1 × 10−6 | |
| K567E | 4.7 × 10−3 | 1.2 × 10−17 | 0.0 × 100 |
| Protein | Variant | Static or Dynamic | pT | pAD | pKS |
|---|---|---|---|---|---|
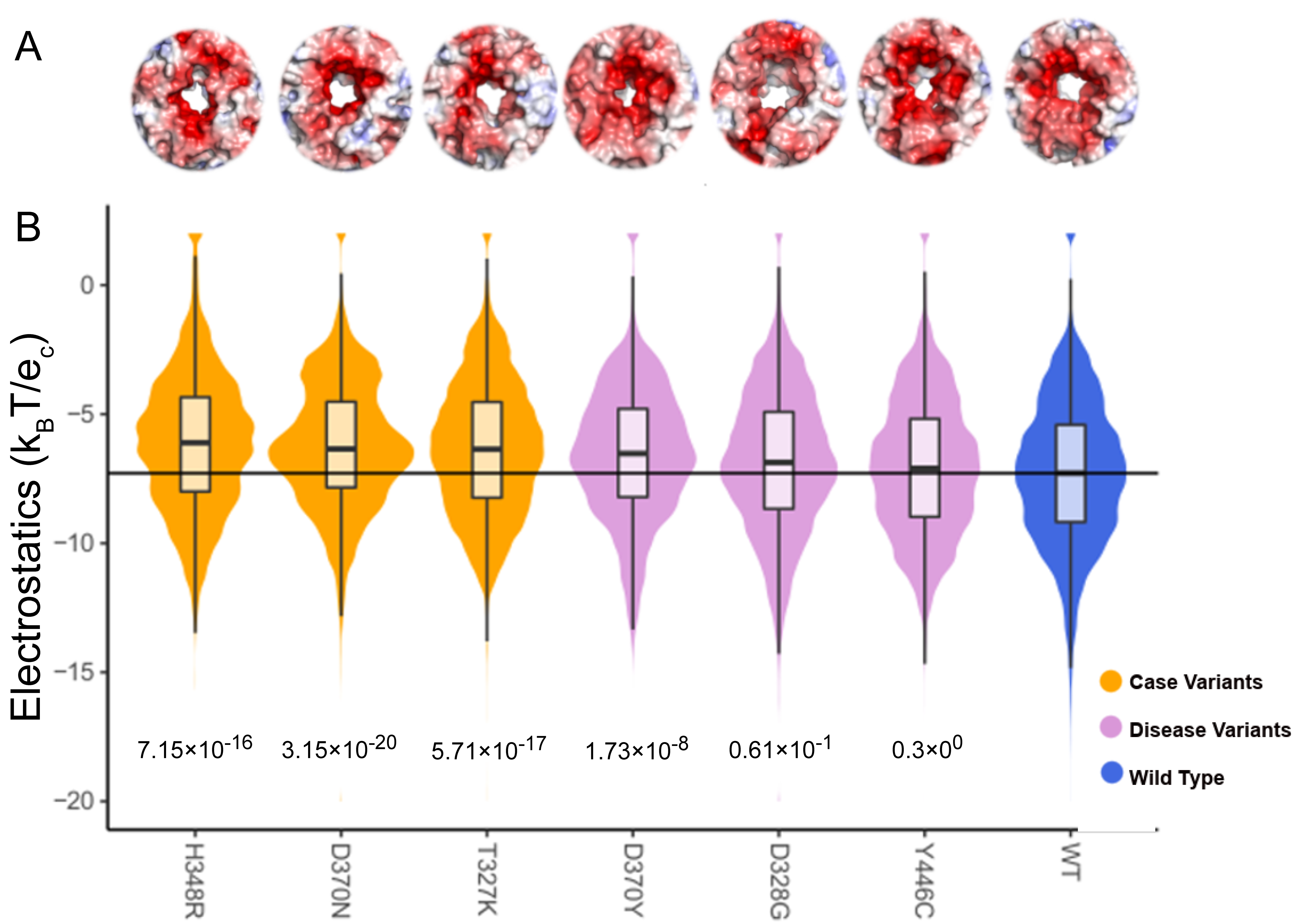
| TBL1XR1 | H348R | Dynamic | 4.7 × 10−3 | 7.2 × 10−19 | 1.7 × 10−14 |
| Static | 4.0 × 10−1 | 2.3 × 10−5 | 8.4 × 10−3 | ||
| D370N | Dynamic | 9.3 × 10−3 | 3.2 × 10−20 | 5.7 × 10−15 | |
| Static | 3.2 × 10−1 | 1.3 × 10−5 | 4.8 × 10−2 | ||
| T327K | Dynamic | 1.8 × 10−2 | 5.7 × 10−17 | 3.5 × 10−9 | |
| Static | 1.1 × 10−1 | 1.0 × 10−7 | 1.5 × 10−3 | ||
| D370Y | Dynamic | 5.9 × 10−2 | 1.7 × 10−8 | 6.1 × 10−10 | |
| Static | 4.8 × 10−1 | 2.6 × 10−3 | 9.7 × 10−3 | ||
| D328G | Dynamic | 2.9 × 10−1 | 6.0 × 10−2 | 4.0 × 10−3 | |
| Static | 2.9 × 10−1 | 3.9 × 10−5 | 5.5 × 10−2 | ||
| Y446C | Dynamic | 4.2 × 10−1 | 3.0 × 10−1 | 2.0 × 10−1 | |
| Static | 4.8 × 10−1 | 5.1 × 10−1 | 9.1 × 10−1 | ||
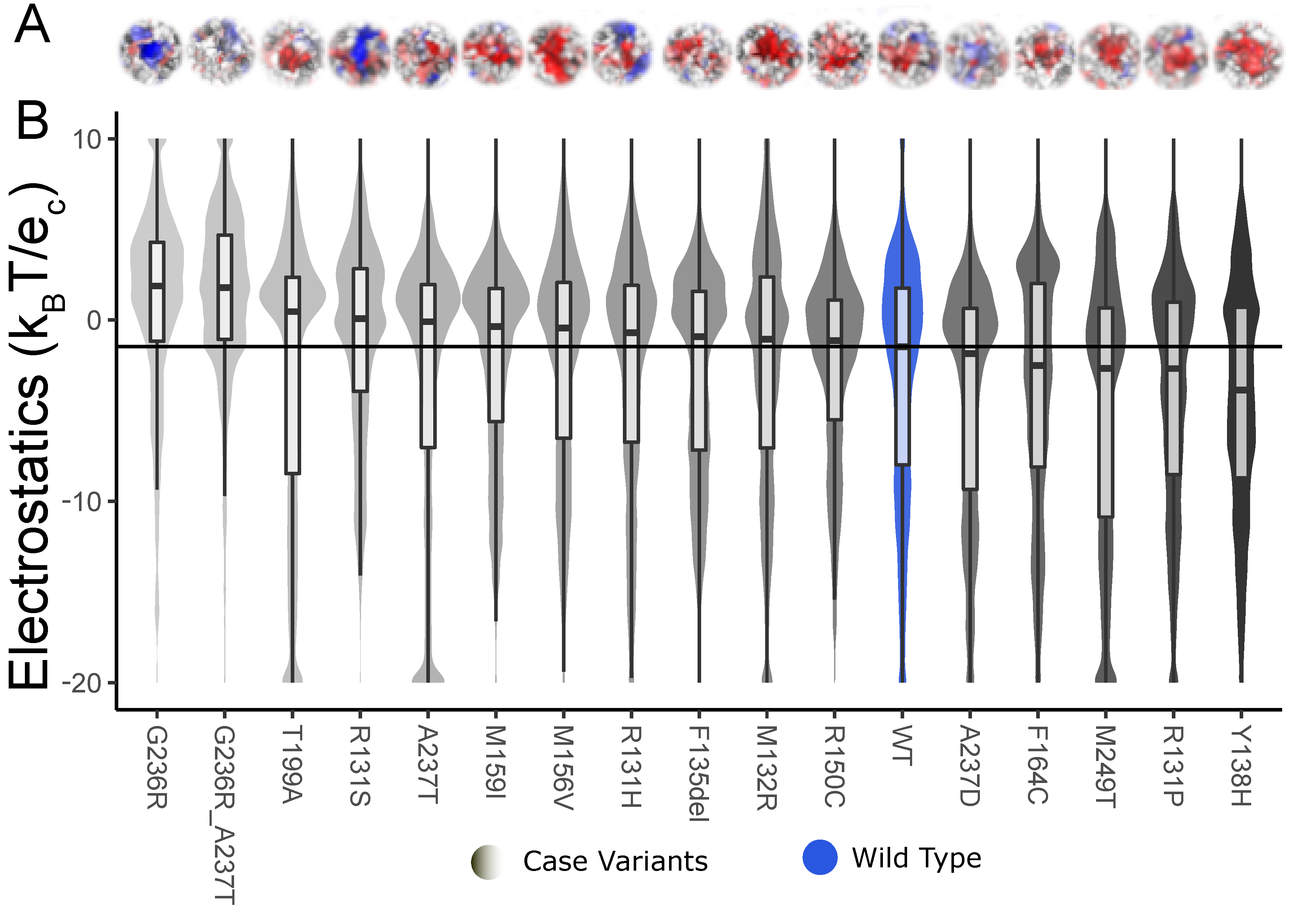
| KCNK9 | G236R | Dynamic | 3.1 × 10−6 | 1.7 × 10−68 | 0.0 × 100 |
| Static | 3.2 × 10−6 | 5.3 × 10−55 | 0.0 × 100 | ||
| G236R, A237T | Dynamic | 1.1 × 10−7 | 8.6 × 10−70 | 0.0 × 100 | |
| Static | 1.6 × 10−5 | 7.1 × 10−51 | 0.0 × 100 | ||
| T199A | Dynamic | 5.1 × 10−1 | 8.3 × 10−10 | 1.4 × 10−4 | |
| Static | 4.9 × 10−1 | 9.1 × 10−1 | 6.5 × 10−1 | ||
| R131S | Dynamic | 1.1 × 10−2 | 5.3 × 10−12 | 2.9 × 10−11 | |
| Static | 1.5 × 10−1 | 3.6 × 10−8 | 1.1 × 10−6 | ||
| A237T | Dynamic | 5.0 × 10−1 | 1.7 × 10−9 | 1.5 × 10−3 | |
| Static | 1.2 × 10−2 | 7.0 × 10−1 | 4.2 × 10−7 | ||
| M159I | Dynamic | 2.1 × 10−1 | 1.6 × 10−4 | 8.7 × 10−4 | |
| Static | 4.8 × 10−1 | 3.2 × 10−1 | 9.1 × 10−1 | ||
| M156V | Dynamic | 2.7 × 10−1 | 2.4 × 10−5 | 4.9 × 10−5 | |
| Static | 4.7 × 10−1 | 8.6 × 10−2 | 6.1 × 10−1 | ||
| R131H | Dynamic | 4.4 × 10−1 | 1.8 × 10−1 | 2.0 × 10−2 | |
| Static | 1.3 × 10−1 | 6.6 × 10−5 | 2.6 × 10−9 | ||
| F135del | Dynamic | 4.7 × 10−1 | 2.6 × 10−3 | 6.2 × 10−2 | |
| Static | 4.7 × 10−1 | 2.4 × 10−1 | 8.9 × 10−1 | ||
| M132R | Dynamic | 4.4 × 10−1 | 2.0 × 10−3 | 4.2 × 10−4 | |
| Static | 2.4 × 10−1 | 8.5 × 10−6 | 8.8 × 10−7 | ||
| R150C | Dynamic | 3.3 × 10−1 | 1.1 × 10−9 | 3.5 × 10−4 | |
| Static | 2.2 × 10−1 | 5.7 × 10−3 | 3.9 × 10−3 | ||
| A237D | Dynamic | 2.6 × 10−1 | 1.9 × 10−4 | 6.9 × 10−7 | |
| Static | 1.3 × 10−1 | 1.0 × 10−5 | 9.2 × 10−8 | ||
| F164C | Dynamic | 5.2 × 10−1 | 8.7 × 10−3 | 3.3 × 10−2 | |
| Static | 4.9 × 10−1 | 3.4 × 10−1 | 7.6 × 10−1 | ||
| M249T | Dynamic | 7.1 × 10−2 | 3.8 × 10−11 | 4.5 × 10−6 | |
| Static | 4.7 × 10−1 | 9.2 × 10−2 | 1.4 × 10−6 | ||
| R131P | Dynamic | 2.6 × 10−1 | 1.8 × 10−5 | 4.2 × 10−4 | |
| Static | 6.0 × 10−2 | 1.8 × 10−11 | 5.8 × 10−12 | ||
| Y138H | Dynamic | 2.1 × 10−1 | 2.8 × 10−4 | 4.0 × 10−13 | |
| Static | 5.4 × 10−1 | 3.1 × 10−2 | 6.9 × 10−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dsouza, N.R.; Haque, N.; Tripathi, S.; Zimmermann, M.T. Assessing Protein Surface-Based Scoring for Interpreting Genomic Variants. Int. J. Mol. Sci. 2024, 25, 12018. https://doi.org/10.3390/ijms252212018
Dsouza NR, Haque N, Tripathi S, Zimmermann MT. Assessing Protein Surface-Based Scoring for Interpreting Genomic Variants. International Journal of Molecular Sciences. 2024; 25(22):12018. https://doi.org/10.3390/ijms252212018
Chicago/Turabian StyleDsouza, Nikita R., Neshatul Haque, Swarnendu Tripathi, and Michael T. Zimmermann. 2024. "Assessing Protein Surface-Based Scoring for Interpreting Genomic Variants" International Journal of Molecular Sciences 25, no. 22: 12018. https://doi.org/10.3390/ijms252212018
APA StyleDsouza, N. R., Haque, N., Tripathi, S., & Zimmermann, M. T. (2024). Assessing Protein Surface-Based Scoring for Interpreting Genomic Variants. International Journal of Molecular Sciences, 25(22), 12018. https://doi.org/10.3390/ijms252212018






