The Interplay Between the MYC Oncogene and Ribosomal Proteins in Osteosarcoma Onset and Progression: Potential Mechanisms and Indication of Candidate Therapeutic Targets
Abstract
:1. Introduction
2. Unveiling RPs: Understanding Their Impact on Human Tumors
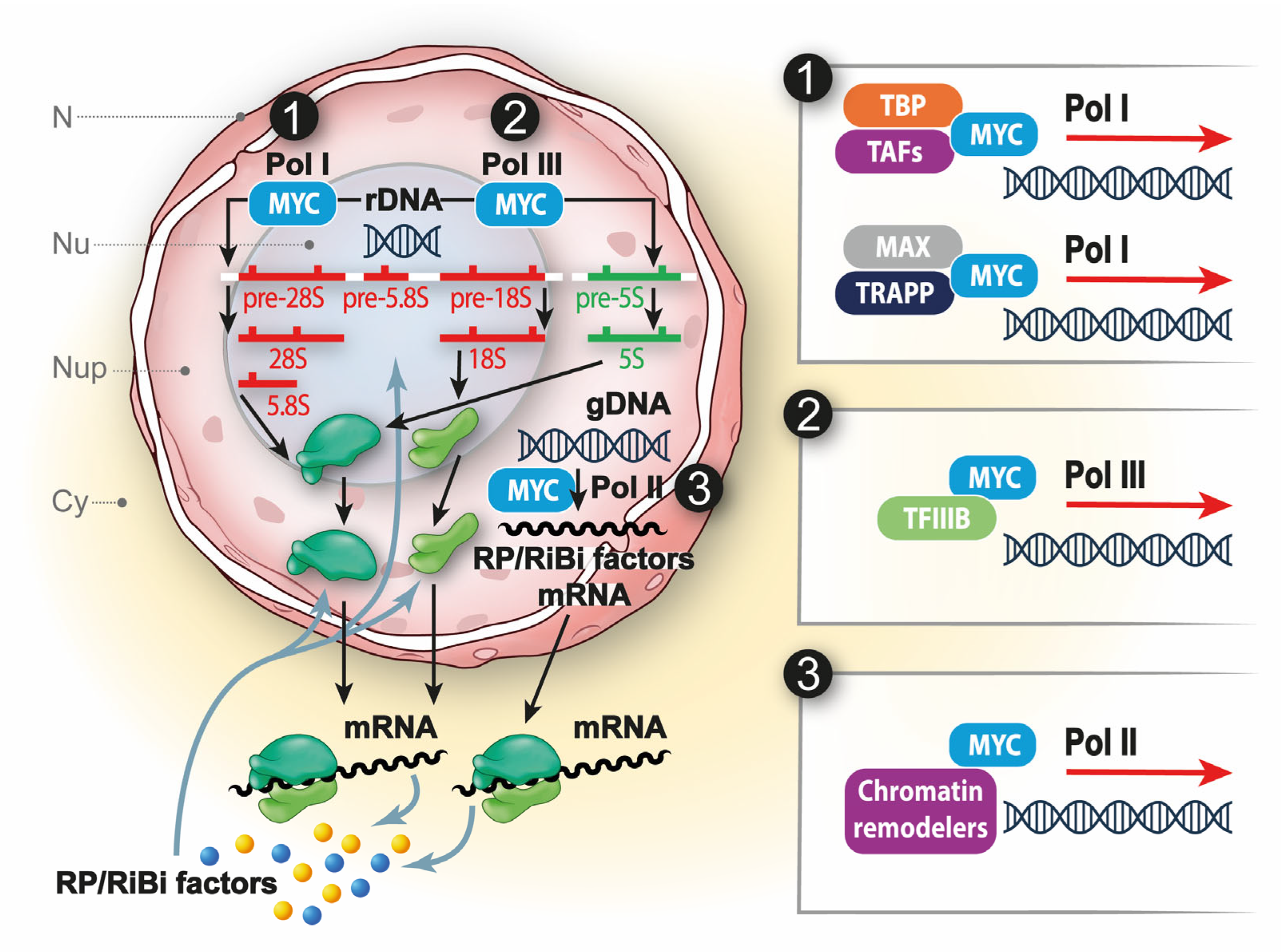
3. MYC and RP Interplay in Physiology and in Cancer
4. The Importance of MYC in OS
5. Ribosomal Proteins in Osteosarcoma
6. Targeting the Ribosome in Cancer: Hidden Therapeutic Windows for OS Patients?
6.1. Inhibition of RiBi
6.2. Inhibition of the 80S Ribosome
7. A Unique Drug That Targets MYC and Its Potential Importance in OS Treatment
8. Conclusion and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Beird, H.C.; Bielack, S.S.; Flanagan, A.M.; Gill, J.; Heymann, D.; Janeway, K.A.; Livingston, J.A.; Roberts, R.D.; Strauss, S.J.; Gorlick, R. Osteosarcoma. Nat. Rev. Dis. Prim. 2022, 8, 77. [Google Scholar] [CrossRef] [PubMed]
- Mirabello, L.; Troisi, R.J.; Savage, S.A. Osteosarcoma incidence and survival rates from 1973 to 2004. Cancer 2009, 115, 1531–1543. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, P.S.; Helman, L.J. New Horizons in the Treatment of Osteosarcoma. N. Engl. J. Med. 2021, 385, 2066–2076. [Google Scholar] [CrossRef] [PubMed]
- Hattinger, C.M.; Patrizio, M.P.; Magagnoli, F.; Luppi, S.; Serra, M. An update on emerging drugs in osteosarcoma: Towards tailored therapies? Expert Opin. Emerg. Drugs 2019, 24, 153–171. [Google Scholar] [CrossRef]
- Czarnecka, A.M.; Synoradzki, K.; Firlej, W.; Bartnik, E.; Sobczuk, P.; Fiedorowicz, M.; Grieb, P.; Rutkowski, P. Molecular Biology of Osteosarcoma. Cancers 2020, 12, 2130. [Google Scholar] [CrossRef]
- Van Riggelen, J.; Yetil, A.; Felsher, D.W. MYC as a regulator of ribosome biogenesis and protein synthesis. Nat. Rev. Cancer 2010, 10, 301–309. [Google Scholar] [CrossRef]
- Scionti, I.; Michelacci, F.; Pasello, M.; Hattinger, C.M.; Alberghini, M.; Manara, M.C.; Bacci, G.; Ferrari, S.; Scotlandi, K.; Picci, P.; et al. Clinical impact of the methotrexate resistance-associated genes C-MYC and dihydrofolate reductase (DHFR) in high-grade osteosarcoma. Ann. Oncol. 2008, 19, 1500–1508. [Google Scholar] [CrossRef]
- Hattinger, C.M.; Stoico, G.; Michelacci, F.; Pasello, M.; Scionti, I.; Remondini, D.; Castellani, G.C.; Fanelli, M.; Scotlandi, K.; Picci, P.; et al. Mechanisms of gene amplification and evidence of coamplification in drug-resistant human osteosarcoma cell lines. Genes Chromosom. Cancer 2008, 48, 289–309. [Google Scholar] [CrossRef]
- Du, M.-D.; He, K.-Y.; Qin, G.; Chen, J.; Li, J.-Y. Adriamycin resistance-associated prohibitin gene inhibits proliferation of human osteosarcoma MG63 cells by interacting with oncogenes and tumor suppressor genes. Oncol. Lett. 2016, 12, 1994–2000. [Google Scholar] [CrossRef]
- Xie, X.-K.; Yang, D.-S.; Ye, Z.-M.; Tao, H.-M. Recombinant Antisense C-MYC Adenovirus Increase in vitro Sensitivity of Osteosarcoma MG-63 Cells to Cisplatin. Cancer Investig. 2006, 24, 1–8. [Google Scholar] [CrossRef]
- Bhavsar, R.B.; Makley, L.N.; Tsonis, P.A. The other lives of ribosomal proteins. Hum. Genom. 2010, 4, 327–344. [Google Scholar] [CrossRef] [PubMed]
- Pecoraro, A.; Pagano, M.; Russo, G.; Russo, A. Ribosome Biogenesis and Cancer: Overview on Ribosomal Proteins. Int. J. Mol. Sci. 2021, 22, 5496. [Google Scholar] [CrossRef] [PubMed]
- Penzo, M.; Montanaro, L.; Treré, D.; Derenzini, M. The Ribosome Biogenesis—Cancer Connection. Cells 2019, 8, 55. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Brajanovski, N.; Chan, K.T.; Xuan, J.; Pearson, R.B.; Sanij, E. Ribosomal proteins and human diseases: Molecular mechanisms and targeted therapy. Signal Transduct. Target. Ther. 2021, 6, 323. [Google Scholar] [CrossRef] [PubMed]
- Pianese, G. Beitrag Zur Histologie Und Aetiologie Der Carcinoma. Histologische Und Experimentelle Untersuchungen. Beitr. Pathol. Anat. Allg. Pathol. 1896, 142, 1–193. [Google Scholar]
- Miller, S.C.; MacDonald, C.C.; Kellogg, M.K.; Karamysheva, Z.N.; Karamyshev, A.L. Specialized Ribosomes in Health and Disease. Int. J. Mol. Sci. 2023, 24, 6334. [Google Scholar] [CrossRef]
- Sulima, S.O.; Kampen, K.R.; De Keersmaecker, K. Cancer Biogenesis in Ribosomopathies. Cells 2019, 8, 229. [Google Scholar] [CrossRef]
- O’donohue, M.-F.; Choesmel, V.; Faubladier, M.; Fichant, G.; Gleizes, P.-E. Functional dichotomy of ribosomal proteins during the synthesis of mammalian 40S ribosomal subunits. J. Cell Biol. 2010, 190, 853–866. [Google Scholar] [CrossRef]
- Derenzini, M.; Thiry, M.; Goessens, G. Ultrastructural cytochemistry of the mammalian cell nucleolus. J. Histochem. Cytochem. 1990, 38, 1237–1256. [Google Scholar] [CrossRef]
- Fatica, A.; Tollervey, D. Making ribosomes. Curr. Opin. Cell Biol. 2002, 14, 313–318. [Google Scholar] [CrossRef]
- Molavi, G.; Samadi, N.; Hosseingholi, E.Z. The roles of moonlight ribosomal proteins in the development of human cancers. J. Cell. Physiol. 2018, 234, 8327–8341. [Google Scholar] [CrossRef] [PubMed]
- Rubbi, C.P.; Milner, J. Disruption of the nucleolus mediates stabilization of p53 in response to DNA damage and other stresses. EMBO J. 2003, 22, 6068–6077. [Google Scholar] [CrossRef] [PubMed]
- Kampen, K.R.; Sulima, S.O.; Vereecke, S.; De Keersmaecker, K. Hallmarks of ribosomopathies. Nucleic Acids Res. 2019, 48, 1013–1028. [Google Scholar] [CrossRef] [PubMed]
- Vlachos, A.; Rosenberg, P.S.; Atsidaftos, E.; Kang, J.; Onel, K.; Sharaf, R.N.; Alter, B.P.; Lipton, J.M. Increased risk of colon cancer and osteogenic sarcoma in Diamond-Blackfan anemia. Blood 2018, 132, 2205–2208. [Google Scholar] [CrossRef] [PubMed]
- Passweg, J.R. Anämie bei Knochenmarksaplasie, paroxysmaler nächtlicher Hämoglobinurie und Myelodysplastischen Syndromen. Ther. Umsch. 2010, 67, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Beroukhim, R.; Mermel, C.H.; Porter, D.; Wei, G.; Raychaudhuri, S.; Donovan, J.; Barretina, J.; Boehm, J.S.; Dobson, J.; Urashima, M.; et al. The landscape of somatic copy-number alteration across human cancers. Nature 2010, 463, 899–905. [Google Scholar] [CrossRef]
- D’Avola, A.; Kluckova, K.; Finch, A.J.; Riches, J.C. Spotlight on New Therapeutic Opportunities for MYC-Driven Cancers. OncoTargets Ther. 2023, 16, 371–383. [Google Scholar] [CrossRef]
- Amati, B.; Dalton, S.; Brooks, M.W.; Littlewood, T.D.; Evan, G.I.; Land, H. Transcriptional activation by the human c-MYC oncoprotein in yeast requires interaction with Max. Nature 1992, 359, 423–426. [Google Scholar] [CrossRef]
- Kadauke, S.; Blobel, G.A. Mitotic bookmarking by transcription factors. Epigenetics Chromatin 2013, 6, 6. [Google Scholar] [CrossRef]
- Poortinga, G.; Wall, M.; Sanij, E.; Siwicki, K.; Ellul, J.; Brown, D.; Holloway, T.P.; Hannan, R.D.; McArthur, G.A. c-MYC coordinately regulates ribosomal gene chromatin remodeling and Pol I availability during granulocyte differentiation. Nucleic Acids Res. 2011, 39, 3267–3281. [Google Scholar] [CrossRef]
- Liu, P.; Cheng, H.; Santiago, S.; Raeder, M.; Zhang, F.; Isabella, A.; Yang, J.; Semaan, D.J.; Chen, C.; Fox, E.A.; et al. Oncogenic PIK3CA-driven mammary tumors frequently recur via PI3K pathway–dependent and PI3K pathway–independent mechanisms. Nat. Med. 2011, 17, 1116–1120. [Google Scholar] [CrossRef] [PubMed]
- Destefanis, F.; Manara, V.; Bellosta, P. MYC as a Regulator of Ribosome Biogenesis and Cell Competition: A Link to Cancer. Int. J. Mol. Sci. 2020, 21, 4037. [Google Scholar] [CrossRef] [PubMed]
- Edwards-Hicks, J.; Su, H.; Mangolini, M.; Yoneten, K.K.; Wills, J.; Rodriguez-Blanco, G.; Young, C.; Cho, K.; Barker, H.; Muir, M.; et al. MYC sensitises cells to apoptosis by driving energetic demand. Nat. Commun. 2022, 13, 4674. [Google Scholar] [CrossRef] [PubMed]
- Kress, T.R.; Sabò, A.; Amati, B. MYC: Connecting selective transcriptional control to global RNA production. Nat. Rev. Cancer 2015, 15, 593–607. [Google Scholar] [CrossRef]
- Pelletier, J.; Thomas, G.; Volarević, S. Ribosome biogenesis in cancer: New players and therapeutic avenues. Nat. Rev. Cancer 2017, 18, 51–63. [Google Scholar] [CrossRef]
- Barna, M.; Pusic, A.; Zollo, O.; Costa, M.; Kondrashov, N.; Rego, E.; Rao, P.H.; Ruggero, D. Suppression of MYC oncogenic activity by ribosomal protein haploinsufficiency. Nature 2008, 456, 971–975. [Google Scholar] [CrossRef]
- Morcelle, C.; Menoyo, S.; Morón-Duran, F.D.; Tauler, A.; Kozma, S.C.; Thomas, G.; Gentilella, A. Oncogenic MYC Induces the Impaired Ribosome Biogenesis Checkpoint and Stabilizes p53 Independent of Increased Ribosome Content. Cancer Res. 2019, 79, 4348–4359. [Google Scholar] [CrossRef]
- Grandori, C.; Gomez-Roman, N.; Felton-Edkins, Z.A.; Ngouenet, C.; Galloway, D.A.; Eisenman, R.N.; White, R.J. c-MYC binds to human ribosomal DNA and stimulates transcription of rRNA genes by RNA polymerase I. Nat. Cell Biol. 2005, 7, 311–318. [Google Scholar] [CrossRef]
- Arabi, A.; Wu, S.; Ridderstråle, K.; Bierhoff, H.; Shiue, C.; Fatyol, K.; Fahlén, S.; Hydbring, P.; Söderberg, O.; Grummt, I.; et al. c-MYC associates with ribosomal DNA and activates RNA polymerase I transcription. Nat. Cell Biol. 2005, 7, 303–310. [Google Scholar] [CrossRef]
- Gomez-Roman, N.; Grandori, C.; Eisenman, R.N.; White, R.J. Direct activation of RNA polymerase III transcription by c-MYC. Nature 2003, 421, 290–294. [Google Scholar] [CrossRef]
- McMahon, S.B.; Wood, M.A.; Cole, M.D. The Essential Cofactor TRRAP Recruits the Histone Acetyltransferase hGCN5 to c-MYC. Mol. Cell. Biol. 2000, 20, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Schlosser, I.; Hölzel, M.; Mürnseer, M.; Burtscher, H.; Weidle, U.H.; Eick, D. A role for c-MYC in the regulation of ribosomal RNA processing. Nucleic Acids Res. 2003, 31, 6148–6156. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.H.; Du, Y.; Ard, P.G.; Phillips, C.; Carella, B.; Chen, C.-J.; Rakowski, C.; Chatterjee, C.; Lieberman, P.M.; Lane, W.S.; et al. The c-MYC Oncoprotein Is a Substrate of the Acetyltransferases hGCN5/PCAF and TIP60. Mol. Cell. Biol. 2004, 24, 10826–10834. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.C.; Wims, M.; Spotts, G.D.; Hann, S.R.; Bradley, A. A null c-MYC mutation causes lethality before 10.5 days of gestation in homozygotes and reduced fertility in heterozygous female mice. Genes Dev. 1993, 7, 671–682. [Google Scholar] [CrossRef]
- Pelengaris, S.; Khan, M.; Evan, G.I. Suppression of MYC-Induced Apoptosis in β Cells Exposes Multiple Oncogenic Properties of MYC and Triggers Carcinogenic Progression. Cell 2002, 109, 321–334. [Google Scholar] [CrossRef]
- Pelengaris, S.; Littlewood, T.; Khan, M.; Elia, G.; Evan, G. Reversible Activation of c-MYC in Skin. Mol. Cell 1999, 3, 565–577. [Google Scholar] [CrossRef]
- Kim, S.; Li, Q.; Dang, C.V.; Lee, L.A. Induction of ribosomal genes and hepatocyte hypertrophy by adenovirus-mediated expression of c-MYC in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 11198–11202. [Google Scholar] [CrossRef]
- Devlin, J.R.; Hannan, K.M.; Hein, N.; Cullinane, C.; Kusnadi, E.; Ng, P.Y.; George, A.; Shortt, J.; Bywater, M.J.; Poortinga, G.; et al. Combination Therapy Targeting Ribosome Biogenesis and mRNA Translation Synergistically Extends Survival in MYC-Driven Lymphoma. Cancer Discov. 2016, 6, 59–70. [Google Scholar] [CrossRef]
- Gabay, M.; Li, Y.; Felsher, D.W. MYC Activation Is a Hallmark of Cancer Initiation and Maintenance. Cold Spring Harb. Perspect. Med. 2014, 4, a014241. [Google Scholar] [CrossRef]
- Huang, Z.; Traugh, J.A.; Bishop, J.M. Negative Control of the MYC Protein by the Stress-Responsive Kinase Pak2. Mol. Cell. Biol. 2004, 24, 1582–1594. [Google Scholar] [CrossRef]
- Eilers, M.; Eisenman, R.N. MYC’s Broad Reach. Genes Dev. 2008, 22, 2755–2766. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Zhao, J.; Fowdur, M.; Wang, K.; Jiang, T.; He, M. Highly expressed ribosomal protein L34 indicates poor prognosis in osteosarcoma and its knockdown suppresses osteosarcoma proliferation probably through translational control. Sci. Rep. 2016, 6, 37690. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Lu, H. Crosstalk between c-MYC and ribosome in ribosomal biogenesis and cancer. J. Cell. Biochem. 2008, 105, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.-S.; Sears, R.; Lu, H. Feedback Regulation of c-MYC by Ribosomal Protein L11. Cell Cycle 2007, 6, 2735–2741. [Google Scholar] [CrossRef]
- De Noon, S.; Ijaz, J.; Coorens, T.H.; Amary, F.; Ye, H.; Strobl, A.; Lyskjær, I.; Flanagan, A.M.; Behjati, S. MYC amplifications are common events in childhood osteosarcoma. J. Pathol. Clin. Res. 2021, 7, 425–431. [Google Scholar] [CrossRef]
- Hattinger, C.M.; Reverter-Branchat, G.; Remondini, D.; Castellani, G.C.; Benini, S.; Pasello, M.; Manara, M.C.; Scotlandi, K.; Picci, P.; Serra, M. Genomic imbalances associated with methotrexate resistance in human osteosarcoma cell lines detected by comparative genomic hybridization-based techniques. Eur. J. Cell Biol. 2003, 82, 483–493. [Google Scholar] [CrossRef]
- Kinnaman, M.D.; Zaccaria, S.; Makohon-Moore, A.; Arnold, B.; Levine, M.F.; Gundem, G.; Ossa, J.E.A.; Glodzik, D.; Rodríguez-Sánchez, M.I.; Bouvier, N.; et al. Subclonal Somatic Copy-Number Alterations Emerge and Dominate in Recurrent Osteosarcoma. Cancer Res. 2023, 83, 3796–3812. [Google Scholar] [CrossRef]
- Wu, X.; Cai, Z.-D.; Lou, L.-M.; Zhu, Y.-B. Expressions of p53, c-MYC, BCL-2 and apoptotic index in human osteosarcoma and their correlations with prognosis of patients. Cancer Epidemiol. 2012, 36, 212–216. [Google Scholar] [CrossRef]
- Reed, D.R.; Grohar, P.; Rubin, E.; Binitie, O.; Krailo, M.; Davis, J.; DuBois, S.G.; Janeway, K.A. Children’s Oncology Group’s 2023 blueprint for research: Bone tumors. Pediatr. Blood Cancer 2023, 70, e30583. [Google Scholar] [CrossRef]
- Marinoff, A.E.; Spurr, L.F.; Fong, C.; Li, Y.Y.; Forrest, S.J.; Ward, A.; Doan, D.; Corson, L.; Mauguen, A.; Pinto, N.; et al. Clinical Targeted Next-Generation Panel Sequencing Reveals MYC Amplification Is a Poor Prognostic Factor in Osteosarcoma. JCO Precis. Oncol. 2023, 7, e2200334. [Google Scholar] [CrossRef]
- Chi, X.; Ji, T.; Li, J.; Xu, J.; Tang, X.; Xie, L.; Meng, F.; Guo, W. Genomic Analysis Revealed Mutational Traits Associated with Clinical Outcomes in Osteosarcoma. Cancer Manag. Res. 2021, 13, 5101–5111. [Google Scholar] [CrossRef] [PubMed]
- Kuijjer, M.L.; Rydbeck, H.; Kresse, S.H.; Buddingh, E.P.; Lid, A.B.; Roelofs, H.; Bürger, H.; Myklebost, O.; Hogendoorn, P.C.W.; Meza-Zepeda, L.A.; et al. Identification of osteosarcoma driver genes by integrative analysis of copy number and gene expression data. Genes Chromosom. Cancer 2012, 51, 696–706. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhao, Z.; Huang, Z.; Chen, D.-C.; Zhu, X.-X.; Wang, Y.-Z.; Yan, Y.-W.; Tang, S.; Madhavan, S.; Ni, W.; et al. Super enhancer inhibitors suppress MYC driven transcriptional amplification and tumor progression in osteosarcoma. Bone Res. 2018, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Massó-Vallés, D.; Soucek, L. Blocking MYC to Treat Cancer: Reflecting on Two Decades of Omomyc. Cells 2020, 9, 883. [Google Scholar] [CrossRef]
- Nirala, B.K.; Patel, T.D.; Kurenbekova, L.; Shuck, R.; Dasgupta, A.; Rainusso, N.; Coarfa, C.; Yustein, J.T. MYC regulates CSF1 expression via microRNA 17/20a to modulate tumor-associated macrophages in osteosarcoma. J. Clin. Investig. 2023, 8. [Google Scholar] [CrossRef]
- Palmerini, E.; Meazza, C.; Tamburini, A.; Bisogno, G.; Ferraresi, V.; Asaftei, S.D.; Milano, G.M.; Coccoli, L.; Manzitti, C.; Luksch, R.; et al. Phase 2 study for nonmetastatic extremity high-grade osteosarcoma in pediatric and adolescent and young adult patients with a risk-adapted strategy based on ABCB1/P-glycoprotein expression: An Italian Sarcoma Group trial (ISG/OS-2). Cancer 2022, 128, 1958–1966. [Google Scholar] [CrossRef]
- Múdry, P.; Kýr, M.; Rohleder, O.; Mahdal, M.; Zambo, I.S.; Ježová, M.; Tomáš, T.; Štěrba, J. Improved osteosarcoma survival with addition of mifamurtide to conventional chemotherapy—Observational prospective single institution analysis. J. Bone Oncol. 2021, 28, 100362. [Google Scholar] [CrossRef]
- Zheng, S.-E.; Yao, Y.; Dong, Y.; Lin, F.; Zhao, H.; Shen, Z.; Sun, Y.-J.; Tang, L.-N. Down-regulation of ribosomal protein L7A in human osteosarcoma. J. Cancer Res. Clin. Oncol. 2009, 135, 1025–1031. [Google Scholar] [CrossRef]
- Nagao, H.; Ijiri, K.; Hirotsu, M.; Ishidou, Y.; Yamamoto, T.; Nagano, S.; Takizawa, T.; Nakashima, K.; Komiya, S.; Setoguchi, T. Role of GLI2 in the growth of human osteosarcoma. J. Pathol. 2011, 224, 169–179. [Google Scholar] [CrossRef]
- Nagao-Kitamoto, H.; Setoguchi, T.; Kitamoto, S.; Nakamura, S.; Tsuru, A.; Nagata, M.; Nagano, S.; Ishidou, Y.; Yokouchi, M.; Kitajima, S.; et al. Ribosomal protein S3 regulates GLI2-mediated osteosarcoma invasion. Cancer Lett. 2015, 356, 855–861. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, W. New molecular insights into osteosarcoma targeted therapy. Curr. Opin. Oncol. 2013, 25, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Liu, J.-Y.; Zhang, J.-T. eIF3d: A driver of noncanonical cap–dependent translation of specific mRNAs and a trigger of biological/pathological processes. J. Biol. Chem. 2023, 299, 104658. [Google Scholar] [CrossRef] [PubMed]
- Volegova, M.P.; Hermosillo, C.; Cate, J.H.D. The Helix-Loop-Helix motif of human EIF3A regulates translation of proliferative cellular mRNAs. PLoS ONE 2023, 18, e0292080. [Google Scholar] [CrossRef]
- Huang, P.; Zhao, J.; Fowdur, M.; Liu, Y.; Wu, H.; He, M. Knockdown of RPL34 suppresses osteosarcoma cell proliferation likely through EIF3/FAU signaling pathway. Transl. Cancer Res. 2019, 8, 848–855. [Google Scholar] [CrossRef]
- Shen, D.-W.; Liang, X.-J.; Suzuki, T.; Gottesman, M.M. Identification by Functional Cloning from a Retroviral cDNA Library of cDNAs for Ribosomal Protein L36 and the 10-kDa Heat Shock Protein that Confer Cisplatin Resistance. Mol. Pharmacol. 2006, 69, 1383–1388. [Google Scholar] [CrossRef]
- Cheng, D.-D.; Zhu, B.; Li, S.-J.; Yuan, T.; Yang, Q.-C.; Fan, C.-Y. Down-regulation of RPS9 Inhibits Osteosarcoma Cell Growth through Inactivation of MAPK Signaling Pathway. J. Cancer 2017, 8, 2720–2728. [Google Scholar] [CrossRef]
- Steffner, R.J.; Jang, E.S. Staging of Bone and Soft-tissue Sarcomas. J. Am. Acad. Orthop. Surg. 2018, 26, e269–e278. [Google Scholar] [CrossRef]
- Wang, T.; Wang, Z.-Y.; Zeng, L.-Y.; Gao, Y.-Z.; Yan, Y.-X.; Zhang, Q. Down-Regulation of Ribosomal Protein RPS21 Inhibits Invasive Behavior of Osteosarcoma Cells Through the Inactivation of MAPK Pathway. Cancer Manag. Res. 2020, 12, 4949–4955. [Google Scholar] [CrossRef]
- Xu, W.; Li, Y.; Ye, X.; Ji, Y.; Chen, Y.; Zhang, X.; Li, Z. TMED3/RPS15A Axis promotes the development and progression of osteosarcoma. Cancer Cell Int. 2021, 21, 1–13. [Google Scholar] [CrossRef]
- Liang, C.; Zhou, J.; Wang, Y.; Sun, Y.; Zhou, J.; Shao, L.; Zhang, Z.; Yan, W.; Liu, Z.; Dong, Y. Essential genes analysis reveals small ribosomal subunit protein eS28 may be a prognostic factor and potential vulnerability in osteosarcoma. J. Bone Oncol. 2023, 44, 100517. [Google Scholar] [CrossRef]
- Gilles, A.; Frechin, L.; Natchiar, K.; Biondani, G.; von Loeffelholz, O.; Holvec, S.; Malaval, J.-L.; Winum, J.-Y.; Klaholz, B.P.; Peyron, J.-F. Targeting the Human 80S Ribosome in Cancer: From Structure to Function and Drug Design for Innovative Adjuvant Therapeutic Strategies. Cells 2020, 9, 629. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Ran, X.; Yuan, J.; Wu, H.; Wang, Y.; Li, H.; Teng, H.; Sun, Z. Genomic hallmarks and therapeutic targets of ribosome biogenesis in cancer. Brief. Bioinform. 2024, 25, bbae023. [Google Scholar] [CrossRef]
- Temaj, G.; Chichiarelli, S.; Telkoparan-Akillilar, P.; Saha, S.; Nuhii, N.; Hadziselimovic, R.; Saso, L. P53: A key player in diverse cellular processes including nuclear stress and ribosome biogenesis, highlighting potential therapeutic compounds. Biochem. Pharmacol. 2024, 226, 116332. [Google Scholar] [CrossRef] [PubMed]
- Haddach, M.; Schwaebe, M.K.; Michaux, J.; Nagasawa, J.; O’Brien, S.E.; Whitten, J.P.; Pierre, F.; Kerdoncuff, P.; Darjania, L.; Stansfield, R.; et al. Discovery of CX-5461, the First Direct and Selective Inhibitor of RNA Polymerase I, for Cancer Therapeutics. ACS Med. Chem. Lett. 2012, 3, 602–606. [Google Scholar] [CrossRef]
- Mars, J.-C.; Tremblay, M.G.; Valere, M.; Sibai, D.S.; Sabourin-Felix, M.; Lessard, F.; Moss, T. The chemotherapeutic agent CX-5461 irreversibly blocks RNA polymerase I initiation and promoter release to cause nucleolar disruption, DNA damage and cell inviability. NAR Cancer 2020, 2, zcaa032. [Google Scholar] [CrossRef]
- Bywater, M.J.; Poortinga, G.; Sanij, E.; Hein, N.; Peck, A.; Cullinane, C.; Wall, M.; Cluse, L.; Drygin, D.; Anderes, K.; et al. Inhibition of RNA Polymerase I as a Therapeutic Strategy to Promote Cancer-Specific Activation of p53. Cancer Cell 2012, 22, 51–65. [Google Scholar] [CrossRef]
- Tsoi, H.; You, C.-P.; Leung, M.-H.; Man, E.P.S.; Khoo, U.-S. Targeting Ribosome Biogenesis to Combat Tamoxifen Resistance in ER+ve Breast Cancer. Cancers 2022, 14, 1251. [Google Scholar] [CrossRef]
- Sanij, E.; Hannan, K.M.; Xuan, J.; Yan, S.; Ahern, J.E.; Trigos, A.S.; Brajanovski, N.; Son, J.; Chan, K.T.; Kondrashova, O.; et al. CX-5461 activates the DNA damage response and demonstrates therapeutic efficacy in high-grade serous ovarian cancer. Nat. Commun. 2020, 11, 2641. [Google Scholar] [CrossRef]
- Yan, S.; Frank, D.; Son, J.; Hannan, K.M.; Hannan, R.D.; Chan, K.T.; Pearson, R.B.; Sanij, E. The Potential of Targeting Ribosome Biogenesis in High-Grade Serous Ovarian Cancer. Int. J. Mol. Sci. 2017, 18, 210. [Google Scholar] [CrossRef]
- Lawrence, M.G.; Porter, L.H.; Choo, N.; Pook, D.; Grummet, J.P.; Pezaro, C.J.; Sandhu, S.; Ramm, S.; Luu, J.; Bakshi, A.; et al. CX-5461 Sensitizes DNA Damage Repair–proficient Castrate-resistant Prostate Cancer to PARP Inhibition. Mol. Cancer Ther. 2021, 20, 2140–2150. [Google Scholar] [CrossRef]
- Mohapatra, P.; Mohanty, S.; Ansari, S.A.; Shriwas, O.; Ghosh, A.; Rath, R.; Das Majumdar, S.K.; Swain, R.K.; Raghav, S.K.; Dash, R. CMTM6 attenuates cisplatin-induced cell death in OSCC by regulating AKT/c-MYC-driven ribosome biogenesis. FASEB J. 2022, 36, e22566. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Luo, H.; Wang, L.; Li, H.; Liang, Y.; Xia, J.; Wang, Z.; Cheng, B.; Huang, L.; Liao, G.; et al. Combined inhibition of RNA polymerase I and mTORC1/2 synergize to combat oral squamous cell carcinoma. Biomed. Pharmacother. 2020, 133, 110906. [Google Scholar] [CrossRef] [PubMed]
- Behrens, K.; Brajanovski, N.; Xu, Z.; Viney, E.M.; DiRago, L.; Hediyeh-Zadeh, S.; Davis, M.J.; Pearson, R.B.; Sanij, E.; Alexander, W.S.; et al. ERG and c-MYC regulate a critical gene network in BCR: ABL1-driven B cell acute lymphoblastic leukemia. Sci. Adv. 2024, 10, eadj8803. [Google Scholar] [CrossRef]
- Negi, S.S.; Brown, P. Transient rRNA synthesis inhibition with CX-5461 is sufficient to elicit growth arrest and cell death in acute lymphoblastic leukemia cells. Oncotarget 2015, 6, 34846–34858. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Wang, H.; Baladandayuthapani, V.; Lin, H.; He, J.; Jones, R.J.; Kuiatse, I.; Gu, D.; Wang, Z.; Ma, W.; et al. RNA Polymerase I Inhibition with CX-5461 as a Novel Therapeutic Strategy to Target MYC in Multiple Myeloma. Br. J. Haematol. 2017, 177, 80–94. [Google Scholar] [CrossRef]
- Kang, C.-W.; Blackburn, A.C.; Loh, A.H.P.; Hong, K.C.; Goh, J.Y.; Hein, N.; Drygin, D.; Parish, C.R.; Hannan, R.D.; Hannan, K.M.; et al. Targeting RNA Polymerase I Transcription Activity in Osteosarcoma: Pre-Clinical Molecular and Animal Treatment Studies. Biomedicines 2023, 11, 1133. [Google Scholar] [CrossRef]
- Kang, C.-W.; Hannan, K.M.; Blackburn, A.C.; Loh, A.H.P.; Hong, K.C.; Yuan, G.J.; Hein, N.; Drygin, D.; Hannan, R.D.; Coupland, L.A. The therapeutic potential of RNA Polymerase I transcription inhibitor, CX-5461, in uterine leiomyosarcoma. Investig. New Drugs 2022, 40, 529–536. [Google Scholar] [CrossRef]
- Hilton, J.; Gelmon, K.; Bedard, P.L.; Tu, D.; Xu, H.; Tinker, A.V.; Goodwin, R.; Laurie, S.A.; Jonker, D.; Hansen, A.R.; et al. Results of the phase I CCTG IND.231 trial of CX-5461 in patients with advanced solid tumors enriched for DNA-repair deficiencies. Nat. Commun. 2022, 13, 3607. [Google Scholar] [CrossRef]
- Khot, A.; Brajanovski, N.; Cameron, D.P.; Hein, N.; Maclachlan, K.H.; Sanij, E.; Lim, J.; Soong, J.; Link, E.; Blombery, P.; et al. First-in-Human RNA Polymerase I Transcription Inhibitor CX-5461 in Patients with Advanced Hematologic Cancers: Results of a Phase I Dose-Escalation Study. Cancer Discov. 2019, 9, 1036–1049. [Google Scholar] [CrossRef]
- Koh, G.C.C.; Boushaki, S.; Zhao, S.J.; Pregnall, A.M.; Sadiyah, F.; Badja, C.; Memari, Y.; Georgakopoulos-Soares, I.; Nik-Zainal, S. The chemotherapeutic drug CX-5461 is a potent mutagen in cultured human cells. Nat. Genet. 2023, 56, 23–26. [Google Scholar] [CrossRef]
- Colis, L.; Peltonen, K.; Sirajuddin, P.; Liu, H.; Sanders, S.; Ernst, G.; Barrow, J.C.; Laiho, M. DNA intercalator BMH-21 inhibits RNA polymerase I independent of DNA damage response. Oncotarget 2014, 5, 4361–4369. [Google Scholar] [CrossRef] [PubMed]
- Peltonen, K.; Colis, L.; Liu, H.; Trivedi, R.; Moubarek, M.S.; Moore, H.M.; Bai, B.; Rudek, M.A.; Bieberich, C.J.; Laiho, M. A Targeting Modality for Destruction of RNA Polymerase I that Possesses Anticancer Activity. Cancer Cell 2014, 25, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, S.; Terrosu, S.; Yusupova, G.; Yusupov, M. Inhibition of the Eukaryotic 80S Ribosome as a Potential Anticancer Therapy: A Structural Perspective. Cancers 2021, 13, 4392. [Google Scholar] [CrossRef] [PubMed]
- Ciriello, G.; Gallina, C.; Guerra, C. Analysis of interactions between ribosomal proteins and RNA structural motifs. BMC Bioinform. 2010, 11, S41. [Google Scholar] [CrossRef]
- Fresno, M.; Jiménez, A.; Vázquez, D. Inhibition of Translation in Eukaryotic Systems by Harringtonine. Eur. J. Biochem. 1977, 72, 323–330. [Google Scholar] [CrossRef]
- Takemura, Y.; Ohnuma, T.; Chou, T.-C.; Okano, T.; Holland, J.F. Biologic and pharmacologic effects of harringtonine on human leukemia-lymphoma cells. Cancer Chemother. Pharmacol. 1985, 14, 206–210. [Google Scholar] [CrossRef]
- Khatua, S.; Nandi, S.; Nag, A.; Sen, S.; Chakraborty, N.; Naskar, A.; Gürer, E.S.; Calina, D.; Acharya, K.; Sharifi-Rad, J. Homoharringtonine: Updated insights into its efficacy in hematological malignancies, diverse cancers and other biomedical applications. Eur. J. Med Res. 2024, 29, 269. [Google Scholar] [CrossRef]
- Ajani, J.A.; Dimery, I.; Chawla, S.P.; Pinnamaneni, K.; Benjamin, R.S.; Legha, S.S.; Krakoff, I.H. Phase II Studies of Homoharringtonine in Patients with Advanced Malignant Melanoma; Sarcoma; and Head and Neck, Breast, and Colorectal Carcinomas. Cancer Treat. Rep. 1986, 70, 375–379. [Google Scholar]
- Kantarjian, H.M.; O’Brien, S.; Cortes, J. Homoharringtonine/Omacetaxine Mepesuccinate: The Long and Winding Road to Food and Drug Administration Approval. Clin. Lymphoma Myeloma Leuk. 2013, 13, 530–533. [Google Scholar] [CrossRef]
- Hale, K.J.; Domostoj, M.M.; El-Tanani, M.; Campbell, C.F.; Mason, C.K. Total Synthesis and Mechanism of Action Studies on the Antitumor Alkaloid, (−)-Agelastatin, A. In Strategies and Tactics in Organic Synthesis; Academic Press: Cambridge, UK, 2005; pp. 352–394. [Google Scholar]
- Mason, C.K.; McFarlane, S.; Johnston, P.G.; Crowe, P.; Erwin, P.J.; Domostoj, M.M.; Campbell, F.C.; Manaviazar, S.; Hale, K.J.; El-Tanani, M. Agelastatin A: A novel inhibitor of osteopontin-mediated adhesion, invasion, and colony formation. Mol. Cancer Ther. 2008, 7, 548–558. [Google Scholar] [CrossRef]
- Antropow, A.H.; Xu, K.; Buchsbaum, R.J.; Movassaghi, M. Synthesis and Evaluation of Agelastatin Derivatives as Potent Modulators for Cancer Invasion and Metastasis. J. Org. Chem. 2017, 82, 7720–7731. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Siegel, D.S.; Morrison, K.C.; Hergenrother, P.J.; Movassaghi, M. Synthesis and Anticancer Activity of All Known (−)-Agelastatin Alkaloids. J. Org. Chem. 2013, 78, 11970–11984. [Google Scholar] [CrossRef] [PubMed]
- Stout, E.P.; Choi, M.Y.; Castro, J.E.; Molinski, T.F. Potent Fluorinated Agelastatin Analogues for Chronic Lymphocytic Leukemia: Design, Synthesis, and Pharmacokinetic Studies. J. Med. Chem. 2014, 57, 5085–5093. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Svatek, H.; Bertonha, A.F.; Reisenauer, K.; Robinson, J.; Kim, M.; Ingros, A.; Ho, M.; Taube, J.; Romo, D. Synthesis of agelastatin A and derivatives premised on a hidden symmetry element leading to analogs displaying anticancer activity. Tetrahedron 2021, 94, 132340. [Google Scholar] [CrossRef] [PubMed]
- Jouanneau, M.; McClary, B.; Reyes, J.C.P.; Chen, R.; Chen, Y.; Plunkett, W.; Cheng, X.; Milinichik, A.Z.; Albone, E.F.; Liu, J.O.; et al. Derivatization of agelastatin A leading to bioactive analogs and a trifunctional probe. Bioorg. Med. Chem. Lett. 2016, 26, 2092–2097. [Google Scholar] [CrossRef]
- Cahlíková, L.; Kawano, I.; Řezáčová, M.; Blunden, G.; Hulcová, D.; Havelek, R. The Amaryllidaceae alkaloids haemanthamine, haemanthidine and their semisynthetic derivatives as potential drugs. Phytochem. Rev. 2020, 20, 303–323. [Google Scholar] [CrossRef]
- Pellegrino, S.; Meyer, M.; Zorbas, C.; Bouchta, S.A.; Saraf, K.; Pelly, S.C.; Yusupova, G.; Evidente, A.; Mathieu, V.; Kornienko, A.; et al. The Amaryllidaceae Alkaloid Haemanthamine Binds the Eukaryotic Ribosome to Repress Cancer Cell Growth. Structure 2018, 26, 416–425.e4. [Google Scholar] [CrossRef]
- Uher, M.; Hroch, M.; Peřinová, R.; Havelek, R.; Křoustková, J.; Řezáčová, M.; Muthná, D.; Koutová, D.; Kuneš, J.; Cahlíková, L. Semisynthetic derivatives of haemanthamine and their in vitro antiproliferative activity evaluation against a panel of human cell lines. Arab. J. Chem. 2022, 15, 103746. [Google Scholar] [CrossRef]
- Si, Y.; Chen, K.; Ngo, H.G.; Guan, J.S.; Totoro, A.; Zhou, Z.; Kim, S.; Kim, T.; Zhou, L.; Liu, X. Targeted EV to Deliver Chemotherapy to Treat Triple-Negative Breast Cancers. Pharmaceutics 2022, 14, 146. [Google Scholar] [CrossRef]
- Palanivel, K.; Kanimozhi, V.; Kadalmani, B.; Akbarsha, M.A. Verrucarin A induces apoptosis through ROS-mediated EGFR/MAPK/Akt signaling pathways in MDA-MB-231 breast cancer cells. J. Cell. Biochem. 2014, 115, 2022–2032. [Google Scholar] [CrossRef]
- Palanivel, K.; Kanimozhi, V.; Kadalmani, B. Verrucarin A alters cell-cycle regulatory proteins and induces apoptosis through reactive oxygen species-dependent p38MAPK activation in the human breast cancer cell line MCF-7. Tumor Biol. 2014, 35, 10159–10167. [Google Scholar] [CrossRef] [PubMed]
- Palanivel, K.; Kanimozhi, V.; Kadalmani, B.; Akbarsha, M.A. Verrucarin A, a protein synthesis inhibitor, induces growth inhibition and apoptosis in breast cancer cell lines MDA-MB-231 and T47D. Biotechnol. Lett. 2013, 35, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Guan, J.; Xu, Y.; Chen, K.; Kim, S.; Zhou, L.; Jaskula-Sztul, R.; Liu, X.M. Dual-Targeted Extracellular Vesicles to Facilitate Combined Therapies for Neuroendocrine Cancer Treatment. Pharmaceutics 2020, 12, 1079. [Google Scholar] [CrossRef] [PubMed]
- Deeb, D.; Gao, X.; Liu, Y.; Zhang, Y.; Shaw, J.; Valeriote, F.A.; Gautam, S.C. The inhibition of cell proliferation and induction of apoptosis in pancreatic ductal adenocarcinoma cells by verrucarin A, a macrocyclic trichothecene, is associated with the inhibition of Akt/NF-κB/mTOR prosurvival signaling. Int. J. Oncol. 2016, 49, 1139–1147. [Google Scholar] [CrossRef]
- Woldemichael, G.M.; Turbyville, T.J.; Vasselli, J.R.; Linehan, W.M.; McMahon, J.B. Lack of a Functional VHL Gene Product Sensitizes Renal Cell Carcinoma Cells to the Apoptotic Effects of the Protein Synthesis Inhibitor Verrucarin A. Neoplasia 2012, 14, 771–777. [Google Scholar] [CrossRef]
- Carter, K.; Rameshwar, P.; Ratajczak, M.Z.; Kakar, S.S. Verrucarin J inhibits ovarian cancer and targets cancer stem cells. Oncotarget 2017, 8, 92743–92756. [Google Scholar] [CrossRef]
- Udoh, K.; Parte, S.; Carter, K.; Mack, A.; Kakar, S.S. Targeting of Lung Cancer Stem Cell Self-Renewal Pathway by a Small Molecule Verrucarin, J. Stem Cell Rev. Rep. 2019, 15, 601–611. [Google Scholar] [CrossRef]
- Pal, D.; Tyagi, A.; Chandrasekaran, B.; Alattasi, H.; Ankem, M.K.; Sharma, A.K.; Damodaran, C. Suppression of Notch1 and AKT mediated epithelial to mesenchymal transition by Verrucarin J in metastatic colon cancer. Cell Death Dis. 2018, 9, 798. [Google Scholar] [CrossRef]
- Burres, N.; Clement, J. Antitumor-Activity and Mechanism of Action of the Novel Marine Natural-Products Mycalamide-A and Mycalamide-B and Onnamide. Cancer Res. 1989, 49, 2935–2940. [Google Scholar]
- Guzmán, E.A.; Harmody, D.; Pitts, T.P.; Vera-Diaz, B.; Winder, P.L.; Yu, Y.; Wright, A.E. Inhibition of IL-8 secretion on BxPC-3 and MIA PaCa-2 cells and induction of cytotoxicity in pancreatic cancer cells with marine natural products. Anti-Cancer Drugs 2016, 28, 153–160. [Google Scholar] [CrossRef]
- Mosey, R.A.; Floreancig, P.E. Isolation, biological activity, synthesis, and medicinal chemistry of the pederin/mycalamide family of natural products. Nat. Prod. Rep. 2012, 29, 980–995. [Google Scholar] [CrossRef] [PubMed]
- Polikanov, Y.S.; Osterman, I.A.; Szal, T.; Tashlitsky, V.N.; Serebryakova, M.V.; Kusochek, P.; Bulkley, D.; Malanicheva, I.A.; Efimenko, T.A.; Efremenkova, O.V.; et al. Amicoumacin A Inhibits Translation by Stabilizing mRNA Interaction with the Ribosome. Mol. Cell 2014, 56, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Prokhorova, I.V.; Akulich, K.A.; Makeeva, D.S.; Osterman, I.A.; Skvortsov, D.A.; Sergiev, P.V.; Dontsova, O.A.; Yusupova, G.; Yusupov, M.M.; Dmitriev, S.E. Amicoumacin A induces cancer cell death by targeting the eukaryotic ribosome. Sci. Rep. 2016, 6, 27720. [Google Scholar] [CrossRef] [PubMed]
- Bucher, K.; Skogerson, L. Cryptopleurine—An inhibitor of translocation. Biochemistry 1976, 15, 4755–4759. [Google Scholar] [CrossRef]
- Melnikov, S.V.; Söll, D.; Steitz, T.A.; Polikanov, Y.S. Insights into RNA binding by the anticancer drug cisplatin from the crystal structure of cisplatin-modified ribosome. Nucleic Acids Res. 2016, 44, 4978–4987. [Google Scholar] [CrossRef]
- Thuy, A.D.T.; Thanh, V.T.T.; Mai, H.D.T.; Le, H.T.; Litaudon, M.; Nguyen, V.H.; Chau, V.M.; Pham, V.C. Cytotoxic Alkaloids from Leaves of Pilea aff. martinii. Planta Medica 2019, 85, 496–502. [Google Scholar] [CrossRef]
- Lai, C.-Y.; Pan, S.-L.; Yang, X.-M.; Chang, L.-H.; Chang, Y.-L.; Yang, P.-C.; Lee, K.-H.; Teng, C.-M. Depletion of 4E-BP1 and regulation of autophagy lead to YXM110-induced anticancer effects. Carcinogenesis 2013, 34, 2050–2060. [Google Scholar] [CrossRef]
- Yang, X.; Shi, Q.; Yang, S.-C.; Chen, C.-Y.; Yu, S.-L.; Bastow, K.F.; Morris-Natschke, S.L.; Wu, P.-C.; Lai, C.-Y.; Wu, T.-S.; et al. Antitumor Agents 288: Design, Synthesis, SAR, and Biological Studies of Novel Heteroatom-Incorporated Antofine and Cryptopleurine Analogues as Potent and Selective Antitumor Agents. J. Med. Chem. 2011, 54, 5097–5107. [Google Scholar] [CrossRef]
- Kwon, Y.; Song, J.; Lee, H.; Kim, E.-Y.; Lee, K.; Lee, S.K.; Kim, S. Design, Synthesis, and Biological Activity of Sulfonamide Analogues of Antofine and Cryptopleurine as Potent and Orally Active Antitumor Agents. J. Med. Chem. 2015, 58, 7749–7762. [Google Scholar] [CrossRef]
- Bidou, L.; Allamand, V.; Rousset, J.-P.; Namy, O. Sense from nonsense: Therapies for premature stop codon diseases. Trends Mol. Med. 2012, 18, 679–688. [Google Scholar] [CrossRef]
- Chowdhury, H.M.; Siddiqui, M.A.; Kanneganti, S.; Sharmin, N.; Chowdhury, M.W.; Nasim, M.T. Aminoglycoside-mediated promotion of translation readthrough occurs through a non-stochastic mechanism that competes with translation termination. Hum. Mol. Genet. 2017, 27, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Dabrowski, M.; Bukowy-Bieryllo, Z.; Zietkiewicz, E. Advances in therapeutic use of a drug-stimulated translational readthrough of premature termination codons. Mol. Med. 2018, 24, 25. [Google Scholar] [CrossRef] [PubMed]
- Garralda, E.; Beaulieu, M.-E.; Moreno, V.; Casacuberta-Serra, S.; Martínez-Martín, S.; Foradada, L.; Alonso, G.; Massó-Vallés, D.; López-Estévez, S.; Jauset, T.; et al. MYC targeting by OMO-103 in solid tumors: A phase 1 trial. Nat. Med. 2024, 30, 762–771. [Google Scholar] [CrossRef] [PubMed]
- Galardi, S.; Savino, M.; Scagnoli, F.; Pellegatta, S.; Pisati, F.; Zambelli, F.; Illi, B.; Annibali, D.; Beji, S.; Orecchini, E.; et al. Resetting cancer stem cell regulatory nodes upon MYC inhibition. EMBO Rep. 2016, 17, 1872–1889. [Google Scholar] [CrossRef] [PubMed]

| Ribosomal Protein (RP) | Expression Levels in Patients’ Tissues and Cell Lines | Association with Clinicopathological Features | Up-/Down-Stream Signaling Pathway Involved | Cell Line Models * | Ref. |
|---|---|---|---|---|---|
| RPL7A | Low | Yes (poor survival in case of lung metastasis) | Not indicated | MG63 | [68] |
| RPS3 | High | Yes (increased in case of lung metastasis, shorter survival rate) | GLI2 | 143B, HS-Os-1 | [69] |
| RPL8 | Gene amplification | Not indicated | MYC | N.A. | [70] |
| RPL34 | High | Yes (poor prognosis) | MYC, eIF3 | SaOS-2 | [52,71] |
| RPS9 | High | Yes (advanced Enneking stage and disease recurrence) | MAPK pathway | MNNG/ HOS, MG63, U2OS | [72] |
| RPS21 | High | Yes (shorter survival rate) | MAPK pathway | MG63 | [73] |
| RPS15A | High | Yes (disease progression) | TMED3 | MNNG/ HOS, U2OS | [74] |
| RPS28 | High | Yes (shorter overall and progression free survival rates) | MAPK pathway, MYC | 143B, MG63 | [75] |
| Compound | Target | Mechanism of Action | Stage of Development (Preclinical/ Clinical) | Clinical Trial Details
| Ref. |
|---|---|---|---|---|---|
| CX-5461(PidnalurexTM, Tucson, AZ, USA) | RNA Pol I | Selective inhibitor of RNA Pol I activity | Clinical |
| [82] |
| CX-5461 | RNA Pol I | Selective inhibitor of RNA Pol I activity | 1. Hematologic cancers 2. 12613001061729 3. Australia 4. Phase I 5. Completed 6. 27 June 2013–4 May 2016 | [83] | |
| CX-5461 (Pidnalurex™) NB: in combination with Talazoparib | RNA Pol I | Selective inhibitor of RNA Pol I activity | Clinical |
| N/A |
| BMH-21 | RNA Pol I | Selective inhibitor of RNA Pol I activity, GC-rich DNA intercalator | Preclinical | N/A | [84,85] |
| Homoarringtonine (HHT, or Omacetaxine Mepesuccinate, Synribo™) | 80S ribosome (PTC) | Translation elongation inhibition | Clinical | 1. Advanced solid tumors (i.e., breast, lung, head/neck, colorectal, melanoma, and sarcoma) and leukemia 2. NCT01844869 3. Netherlands 4. Phase I 5. Completed 6. July 2013–December 2014 | N/A |
| Agelastatin A | 80S ribosome (PTC) | Translation elongation inhibition | Preclinical | N/A | [86,87,88,89,90,91] |
| Haemanthamine | 80S ribosome (PTC) | Translation elongation inhibition | Preclinical | N/A | [92,93,94] |
| Verrucarins (Verrucarin A, Verrucarin J, deoxynivalenol) | 80S ribosome (A-site) | Translation elongation inhibition | Preclinical | N/A | [95,96,97,98,99,100,101,102] |
| MYCalamides (MYC A, MYC B) | 80S ribosome (E-site) | Translation elongation inhibition | Preclinical | N/A | [103,104] |
| Amicoumacin A | 80S ribosome (mRNA path) | Translation elongation inhibition | Preclinical | N/A | [105,106] |
| Cryptopleurine | 80S ribosome (mRNA path) | Translation elongation inhibition | Preclinical | N/A | [107,108,109] |
| Ataluren (PTC124, TranslarnaTM) NB: In combination with Pembrolizumab | 80S ribosome (decoding center) | Stop codon readthrough | Clinical | 1. Metastatic colorectal and endometrial carcinomas 2. NCT04014530 3. Netherlands 4. Phase I/II 5. Recruiting 6. 1 August 2019–ongoing | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerrieri, A.N.; Hattinger, C.M.; Marchesini, F.; Melloni, M.; Serra, M.; Ibrahim, T.; Penzo, M. The Interplay Between the MYC Oncogene and Ribosomal Proteins in Osteosarcoma Onset and Progression: Potential Mechanisms and Indication of Candidate Therapeutic Targets. Int. J. Mol. Sci. 2024, 25, 12031. https://doi.org/10.3390/ijms252212031
Guerrieri AN, Hattinger CM, Marchesini F, Melloni M, Serra M, Ibrahim T, Penzo M. The Interplay Between the MYC Oncogene and Ribosomal Proteins in Osteosarcoma Onset and Progression: Potential Mechanisms and Indication of Candidate Therapeutic Targets. International Journal of Molecular Sciences. 2024; 25(22):12031. https://doi.org/10.3390/ijms252212031
Chicago/Turabian StyleGuerrieri, Ania Naila, Claudia Maria Hattinger, Federica Marchesini, Martina Melloni, Massimo Serra, Toni Ibrahim, and Marianna Penzo. 2024. "The Interplay Between the MYC Oncogene and Ribosomal Proteins in Osteosarcoma Onset and Progression: Potential Mechanisms and Indication of Candidate Therapeutic Targets" International Journal of Molecular Sciences 25, no. 22: 12031. https://doi.org/10.3390/ijms252212031
APA StyleGuerrieri, A. N., Hattinger, C. M., Marchesini, F., Melloni, M., Serra, M., Ibrahim, T., & Penzo, M. (2024). The Interplay Between the MYC Oncogene and Ribosomal Proteins in Osteosarcoma Onset and Progression: Potential Mechanisms and Indication of Candidate Therapeutic Targets. International Journal of Molecular Sciences, 25(22), 12031. https://doi.org/10.3390/ijms252212031










