Vine Tea Extract (VTE) Inhibits High-Fat Diet-Induced Adiposity: Evidence of VTE’s Anti-Obesity Effects In Vitro and In Vivo
Abstract
:1. Introduction
2. Results
2.1. VTE Reduces Lipid Accumulation and Glycerol Release in 3T3–L1 Cells
2.2. VTE Regulates Protein Expression of Adipogenesis, Lipogenesis, and Lipolysis of 3T3–L1 Cells
2.3. VTE Reduces Body Weight Gain in High-Fat Diet-Induced Mice
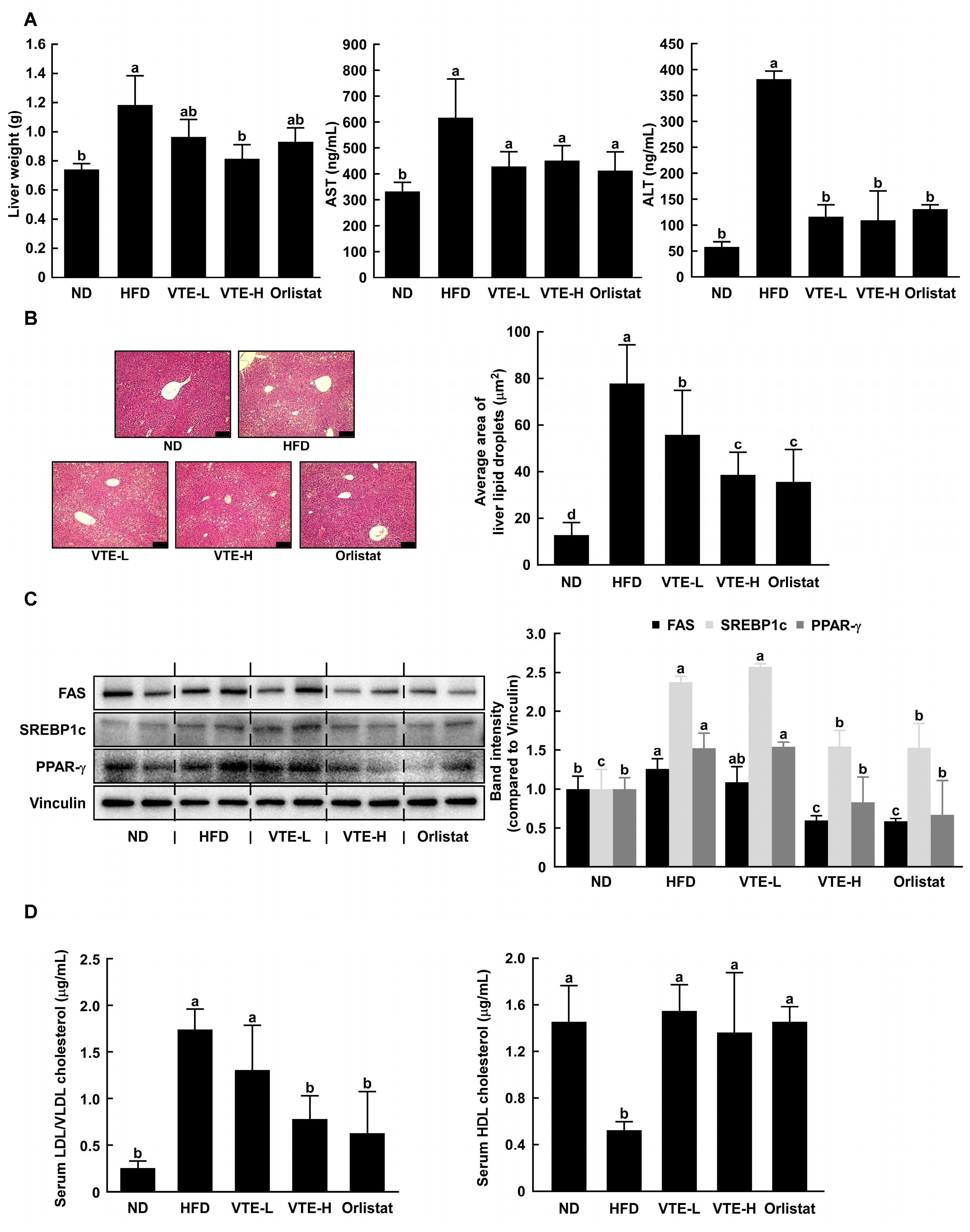
2.4. Changes Serum Biochemical Parameters in High-Fat Diet-Induced Mice
2.5. VTE Regulated Protein Expression of Adipogenesis and Lipogenesis in DIO Mice Model
2.6. Ampelopsin Was Contained in VTE and Has Potential for Anti-Obesity Effects
3. Discussion
4. Materials and Methods
4.1. Preparation of a Standardized Extract of Vine Tea (VTE)
4.2. Cell Culture, Differentiation, and Treatment
4.3. Cell Viability Assay
4.4. Oil Red O Staining
4.5. Glycerol Release
4.6. Western Blotting Analysis
4.7. Animal Experiments
4.8. In Vivo Micro-CT
4.9. H&E Staining
4.10. Detection of Serum Parameters on Enzyme-Linked Immunosorbent Assay (ELISA)
4.11. Assessment of Serum LDL/VLDL and HDL Cholesterol
4.12. Immunofluorescence
4.13. Determination of Ampelopsin in VTE
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Temple, N.J. The Origins of the Obesity Epidemic in the USA-Lessons for Today. Nutrients 2022, 14, 4253. [Google Scholar] [CrossRef] [PubMed]
- Nuttall, F.Q. Body Mass Index: Obesity, BMI, and Health: A Critical Review. Nutr. Today 2015, 50, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Pi-Sunyer, X. The medical risks of obesity. Postgrad. Med. 2009, 121, 21–33. [Google Scholar] [CrossRef]
- Safaei, M.; Sundararajan, E.A.; Driss, M.; Boulila, W.; Shapi’i, A. A systematic literature review on obesity: Understanding the causes & consequences of obesity and reviewing various machine learning approaches used to predict obesity. Comput. Biol. Med. 2021, 136, 104754. [Google Scholar]
- Abdi Beshir, S.; Ahmed Elnour, A.; Soorya, A.; Parveen Mohamed, A.; Sir Loon Goh, S.; Hussain, N.; Al Haddad, A.H.I.; Hussain, F.; Yousif Khidir, I.; Abdelnassir, Z. A narrative review of approved and emerging anti-obesity medications. Saudi Pharm. J. 2023, 31, 101757. [Google Scholar] [CrossRef] [PubMed]
- Tak, Y.J.; Lee, S.Y. Long-Term Efficacy and Safety of Anti-Obesity Treatment: Where Do We Stand? Curr. Obes. Rep. 2021, 10, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Sayed, U.F.S.M.; Moshawih, S.; Goh, H.P.; Kifli, N.; Gupta, G.; Singh, S.K.; Chellappan, D.K.; Dua, K.; Hermansyah, A.; Ser, H.L.; et al. Natural products as novel anti-obesity agents: Insights into mechanisms of action and potential for therapeutic management. Front. Pharmacol. 2023, 14, 1182937. [Google Scholar]
- Konstantinidi, M.; Koutelidakis, A.E. Functional Foods and Bioactive Compounds: A Review of Its Possible Role on Weight Management and Obesity’s Metabolic Consequences. Medicines 2019, 6, 94. [Google Scholar] [CrossRef]
- Wu, R.R.; Li, X.; Cao, Y.H.; Peng, X.; Liu, G.F.; Liu, Z.K.; Yang, Z.; Liu, Z.Y.; Wu, Y. China Medicinal Plants of the Ampelopsis grossedentata—A Review of Their Botanical Characteristics, Use, Phytochemistry, Active Pharmacological Components, and Toxicology. Molecules 2023, 28, 7145. [Google Scholar] [CrossRef]
- Hou, L.; Jiang, F.; Huang, B.; Zheng, W.; Jiang, Y.; Cai, G.; Liu, D.; Hu, C.Y.; Wang, C. Dihydromyricetin Ameliorates Inflammation-Induced Insulin Resistance via Phospholipase C-CaMKK-AMPK Signal Pathway. Oxid. Med. Cell. Longev. 2021, 2021, 8542809. [Google Scholar] [CrossRef]
- Yang, Y.; Qiu, W.; Xiao, J.; Sun, J.; Ren, X.; Jiang, L. Dihydromyricetin ameliorates hepatic steatosis and insulin resistance via AMPK/PGC-1alpha and PPARalpha-mediated autophagy pathway. J. Transl. Med. 2024, 22, 309. [Google Scholar] [CrossRef] [PubMed]
- Wueest, S.; Rapold, R.A.; Schumann, D.M.; Rytka, J.M.; Schildknecht, A.; Nov, O.; Chervonsky, A.V.; Rudich, A.; Schoenle, E.J.; Donath, M.Y.; et al. Deletion of Fas in adipocytes relieves adipose tissue inflammation and hepatic manifestations of obesity in mice. J. Clin. Investig. 2010, 120, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Christe, M.; Hirzel, E.; Lindinger, A.; Kern, B.; von Flue, M.; Peterli, R.; Peters, T.; Eberle, A.N.; Lindinger, P.W. Obesity affects mitochondrial citrate synthase in human omental adipose tissue. ISRN Obes. 2013, 2013, 826027. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Xiaoli, A.M.; Yang, F. Regulation and Metabolic Significance of De Novo Lipogenesis in Adipose Tissues. Nutrients 2018, 10, 1383. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R.E.; Ahmadian, M.; Jaworski, K.; Sarkadi-Nagy, E.; Sul, H.S. Regulation of lipolysis in adipocytes. Annu. Rev. Nutr. 2007, 27, 79–101. [Google Scholar] [CrossRef]
- Prusty, D.; Park, B.H.; Davis, K.E.; Farmer, S.R. Activation of MEK/ERK signaling promotes adipogenesis by enhancing peroxisome proliferator-activated receptor gamma (PPARgamma) and C/EBPalpha gene expression during the differentiation of 3T3–L1 preadipocytes. J. Biol. Chem. 2002, 277, 46226–46232. [Google Scholar] [CrossRef]
- Li, J.; Wu, H.; Liu, Y.; Yang, L. High fat diet induced obesity model using four strains of mice: Kunming, C57BL/6, BALB/c and ICR. Exp. Anim. 2020, 69, 326–335. [Google Scholar] [CrossRef]
- McGill, M.R. The past and present of serum aminotransferases and the future of liver injury biomarkers. EXCLI J. 2016, 15, 817–828. [Google Scholar]
- Higashi, Y. Endothelial Function in Dyslipidemia: Roles of LDL-Cholesterol, HDL-Cholesterol and Triglycerides. Cells 2023, 12, 1293. [Google Scholar] [CrossRef]
- Fernandez, M.L.; Webb, D. The LDL to HDL Cholesterol Ratio as a Valuable Tool to Evaluate Coronary Heart Disease Risk. J. Am. Coll. Nutr. 2008, 27, 1–5. [Google Scholar] [CrossRef]
- Brunton, S.A.; Wysham, C.H. GLP-1 receptor agonists in the treatment of type 2 diabetes: Role and clinical experience to date. Postgrad. Med. 2020, 132, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Bost, F.; Aouadi, M.; Caron, L.; Binétruy, B. The role of MAPKs in adipocyte differentiation and obesity. Biochimie 2005, 87, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, R.; Ye, L.; Baek, N.; Teixeira, G.; O’Keefe, S. Vine tea (Ampelopsis grossedentata): A review of chemical composition, functional properties, and potential food applications. J. Funct. Food. 2021, 76, 104317. [Google Scholar] [CrossRef]
- Zhang, Q.L.; Zhao, Y.F.; Zhang, M.Y.; Zhang, Y.L.; Ji, H.F.; Shen, L. Recent advances in research on vine tea, a potential and functional herbal tea with dihydromyricetin and myricetin as major bioactive compounds. J. Pharm. Anal. 2021, 11, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Miyasaka, K.; Yamada, W.; Takeda, S.; Shimizu, N.; Shimoda, H. The Anti-Adiposity Mechanisms of Ampelopsin and Vine Tea Extract in High Fat Diet and Alcohol-Induced Fatty Liver Mouse Models. Molecules 2022, 27, 607. [Google Scholar] [CrossRef]
- Romieu, I.; Dossus, L.; Barquera, S.; Blottière, H.M.; Franks, P.W.; Gunter, M.; Hwalla, N.; Hursting, S.D.; Leitzmann, M.; Margetts, B.; et al. Energy balance and obesity: What are the main drivers? Cancer Causes Control 2017, 28, 247–258. [Google Scholar] [CrossRef]
- Choe, S.S.; Huh, J.Y.; Hwang, I.J.; Kim, J.I.; Kim, J.B. Adipose Tissue Remodeling: Its Role in Energy Metabolism and Metabolic Disorders. Front. Endocrinol. 2016, 7, 30. [Google Scholar] [CrossRef]
- Kintscher, U.; Foryst-Ludwig, A.; Haemmerle, G.; Zechner, R. The Role of Adipose Triglyceride Lipase and Cytosolic Lipolysis in Cardiac Function and Heart Failure. Cell Rep. Med. 2020, 1, 100001. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, J.E.; Choi, Y.J.; Gong, J.E.; Jin, Y.J.; Lee, D.W.; Choi, Y.W.; Hwang, D.Y. Anti-Obesity Effect of alpha-Cubebenol Isolated from Schisandra chinensis in 3T3–L1 Adipocytes. Biomolecules 2021, 11, 1650. [Google Scholar] [CrossRef]
- Lee, M.R.; Kim, J.E.; Choi, J.Y.; Park, J.J.; Kim, H.R.; Song, B.R.; Park, J.W.; Kang, M.J.; Choi, Y.W.; Kim, K.M.; et al. Morusin Functions as a Lipogenesis Inhibitor as Well as a Lipolysis Stimulator in Differentiated 3T3–L1 and Primary Adipocytes. Molecules 2018, 23, 2004. [Google Scholar] [CrossRef]
- Jakab, J.; Miskic, B.; Miksic, S.; Juranic, B.; Cosic, V.; Schwarz, D.; Vcev, A. Adipogenesis as a Potential Anti-Obesity Target: A Review of Pharmacological Treatment and Natural Products. Diabet. Metab. Synd. Ob. 2021, 14, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Arimura, N.; Horiba, T.; Imagawa, M.; Shimizu, M.; Sato, R. The peroxisome proliferator-activated receptor gamma regulates expression of the perilipin gene in adipocytes. J. Biol. Chem. 2004, 279, 10070–10076. [Google Scholar] [CrossRef] [PubMed]
- Sawada, T.; Miyoshi, H.; Shimada, K.; Suzuki, A.; Okamatsu-Ogura, Y.; Perfield, J.W., 2nd; Kondo, T.; Nagai, S.; Shimizu, C.; Yoshioka, N.; et al. Perilipin overexpression in white adipose tissue induces a brown fat-like phenotype. PLoS ONE 2010, 5, e14006. [Google Scholar] [CrossRef]
- Choi, H.M.; Doss, H.M.; Kim, K.S. Multifaceted Physiological Roles of Adiponectin in Inflammation and Diseases. Int. J. Mol. Sci. 2020, 21, 1219. [Google Scholar] [CrossRef] [PubMed]
- Begum, M.; Choubey, M.; Tirumalasetty, M.B.; Arbee, S.; Mohib, M.M.; Wahiduzzaman, M.; Mamun, M.A.; Uddin, M.B.; Mohiuddin, M.S. Adiponectin: A Promising Target for the Treatment of Diabetes and Its Complications. Life 2023, 13, 2213. [Google Scholar] [CrossRef]
- Son, Y.H.; Ka, S.; Kim, A.Y.; Kim, J.B. Regulation of Adipocyte Differentiation via MicroRNAs. Endocrinol. Metab. 2014, 29, 122–135. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, W.; Choi, S.; Kim, J.; Baek, K.-S.; Park, M.; Lee, G.; Lim, T.-G. Vine Tea Extract (VTE) Inhibits High-Fat Diet-Induced Adiposity: Evidence of VTE’s Anti-Obesity Effects In Vitro and In Vivo. Int. J. Mol. Sci. 2024, 25, 12042. https://doi.org/10.3390/ijms252212042
Lim W, Choi S, Kim J, Baek K-S, Park M, Lee G, Lim T-G. Vine Tea Extract (VTE) Inhibits High-Fat Diet-Induced Adiposity: Evidence of VTE’s Anti-Obesity Effects In Vitro and In Vivo. International Journal of Molecular Sciences. 2024; 25(22):12042. https://doi.org/10.3390/ijms252212042
Chicago/Turabian StyleLim, Wonchul, Seongmin Choi, Jinhak Kim, Kwang-Soo Baek, Minkuk Park, Gakyung Lee, and Tae-Gyu Lim. 2024. "Vine Tea Extract (VTE) Inhibits High-Fat Diet-Induced Adiposity: Evidence of VTE’s Anti-Obesity Effects In Vitro and In Vivo" International Journal of Molecular Sciences 25, no. 22: 12042. https://doi.org/10.3390/ijms252212042
APA StyleLim, W., Choi, S., Kim, J., Baek, K.-S., Park, M., Lee, G., & Lim, T.-G. (2024). Vine Tea Extract (VTE) Inhibits High-Fat Diet-Induced Adiposity: Evidence of VTE’s Anti-Obesity Effects In Vitro and In Vivo. International Journal of Molecular Sciences, 25(22), 12042. https://doi.org/10.3390/ijms252212042






