Identifying the Shared Metabolite Biomarkers and Potential Intervention Targets for Multiple Sarcopenia-Related Phenotypes
Abstract
:1. Introduction
2. Results
2.1. Metabolome-Wide MR Identified 118 Sarcopenia-Related Metabolites
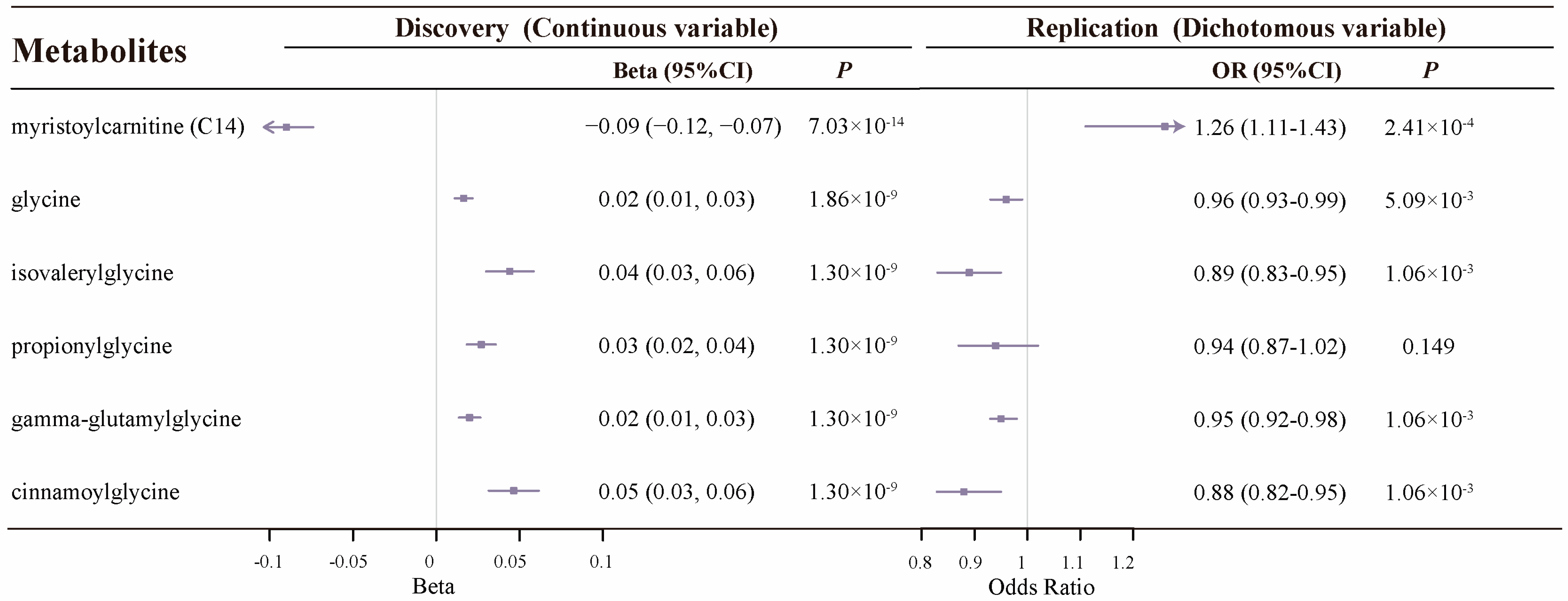
2.2. Colocalization Analysis Supports 27 Known Metabolites
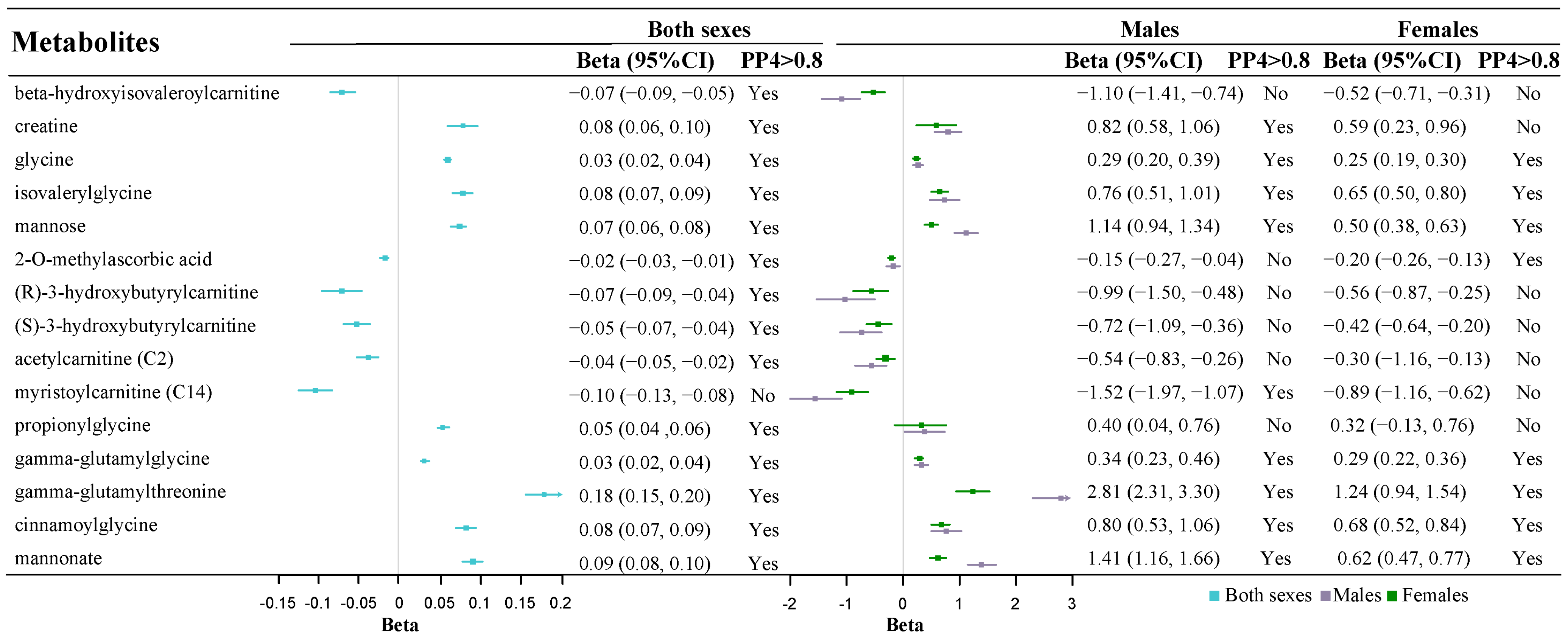
2.3. Thirteen Metabolites with Robust Colocalization Evidence Have Cross-Sarcopenia Effect
2.4. Metabolic Pathway Analysis
2.5. Six Potential Modifiable Factors Associated with Sarcopenia-Related Metabolites
3. Discussion
4. Methods and Materials
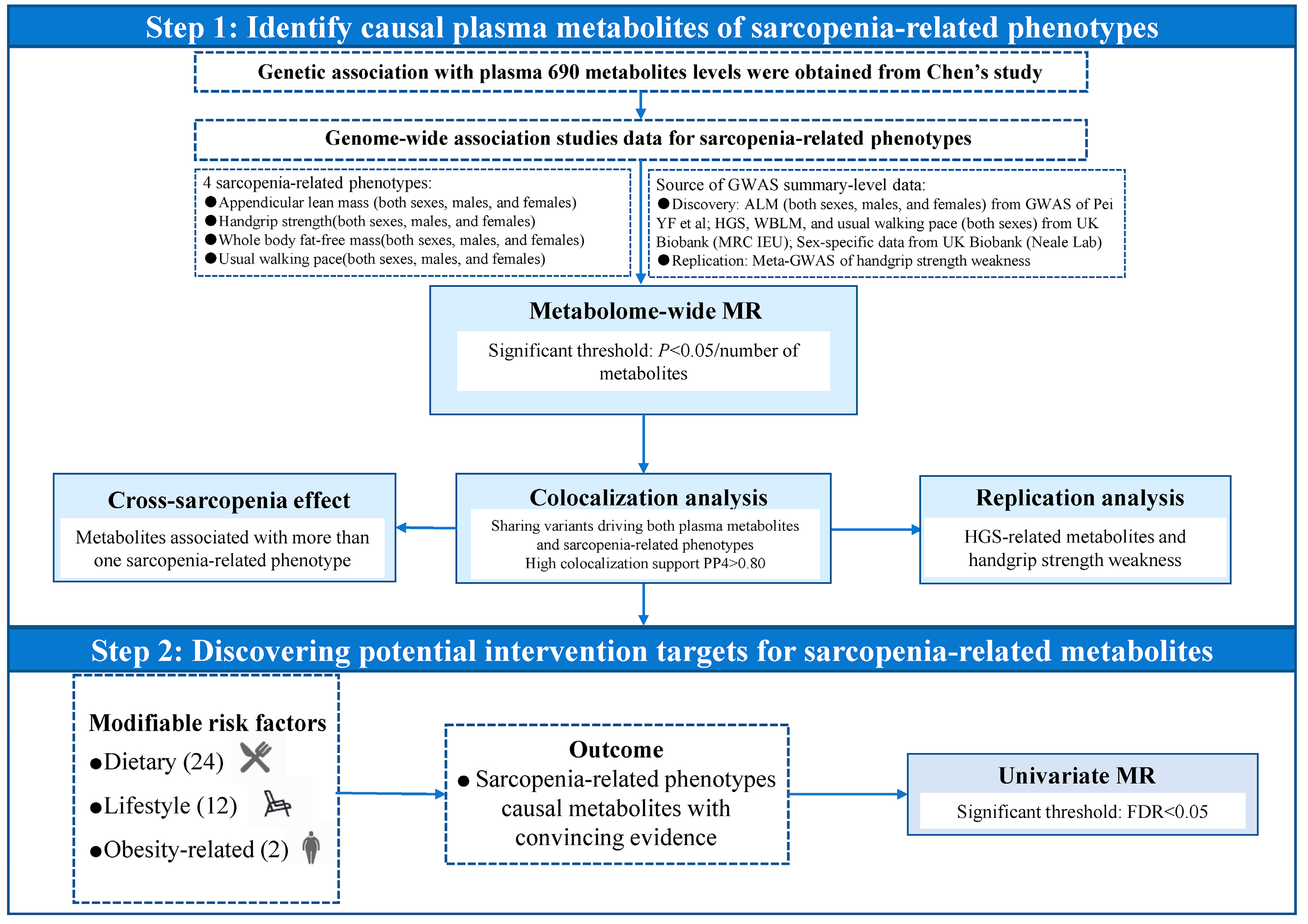
4.1. Overall Study Design
4.2. Data Sources and Study Population
4.3. Metabolome-Wide MR Analysis
4.4. Colocalization Analysis
4.5. Metabolic Pathway Analysis
4.6. Associations Between Modifiable Risk Factors and Sarcopenia-Related Metabolites
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.K.; Liu, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Bahyah, K.S.; Chou, M.Y.; Chen, L.Y.; Hsu, P.S.; Krairit, O.; et al. Sarcopenia in Asia: Consensus report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Fielding, R.A.; Vellas, B.; Evans, W.J.; Bhasin, S.; Morley, J.E.; Newman, A.B.; Abellan van Kan, G.; Andrieu, S.; Bauer, J.; Breuille, D.; et al. Sarcopenia: An undiagnosed condition in older adults. Current consensus definition: Prevalence, etiology, and consequences. International working group on sarcopenia. J. Am. Med. Dir. Assoc. 2011, 12, 249–256. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Petermann-Rocha, F.; Balntzi, V.; Gray, S.R.; Lara, J.; Ho, F.K.; Pell, J.P.; Celis-Morales, C. Global prevalence of sarcopenia and severe sarcopenia: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2022, 13, 86–99. [Google Scholar] [CrossRef]
- Yuan, S.; Larsson, S.C. Epidemiology of sarcopenia: Prevalence, risk factors, and consequences. Metab. Clin. Exp. 2023, 144, 155533. [Google Scholar] [CrossRef]
- Johnson, C.H.; Ivanisevic, J.; Siuzdak, G. Metabolomics: Beyond biomarkers and towards mechanisms. Nat. Rev. Mol. Cell Biol. 2016, 17, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Castiglione, V.; Aimo, A.; Vergaro, G.; Saccaro, L.; Passino, C.; Emdin, M. Biomarkers for the diagnosis and management of heart failure. Heart Fail. Rev. 2022, 27, 625–643. [Google Scholar] [CrossRef]
- Arnett, D.K.; Claas, S.A. Omics of Blood Pressure and Hypertension. Circ. Res. 2018, 122, 1409–1419. [Google Scholar] [CrossRef]
- Buch, A.; Carmeli, E.; Boker, L.K.; Marcus, Y.; Shefer, G.; Kis, O.; Berner, Y.; Stern, N. Muscle function and fat content in relation to sarcopenia, obesity and frailty of old age—An overview. Exp. Gerontol. 2016, 76, 25–32. [Google Scholar] [CrossRef]
- Yanai, H.; Tomono, Y.; Ito, K.; Furutani, N.; Yoshida, H.; Tada, N. The underlying mechanisms for development of hypertension in the metabolic syndrome. Nutr. J. 2008, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lin, X.; Gong, R.; Shen, H.; Qu, Z.; Zhao, Q.; Shen, J.; Xiao, H.; Deng, H. Identification and Functional Characterization of Metabolites for Skeletal Muscle Mass in Early Postmenopausal Chinese Women. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2022, 77, 2346–2355. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.Q.; Lin, X.; Shen, H.; Liu, H.M.; Qiu, X.; Li, B.Y.; Shen, W.D.; Ge, C.L.; Lv, F.Y.; Shen, J.; et al. Human gut microbiome impacts skeletal muscle mass via gut microbial synthesis of the short-chain fatty acid butyrate among healthy menopausal women. J. Cachexia Sarcopenia Muscle 2021, 12, 1860–1870. [Google Scholar] [CrossRef] [PubMed]
- Alldritt, I.; Greenhaff, P.L.; Wilkinson, D.J. Metabolomics as an Important Tool for Determining the Mechanisms of Human Skeletal Muscle Deconditioning. Int. J. Mol. Sci. 2021, 22, 13575. [Google Scholar] [CrossRef]
- Peng, W.; Xia, Z.; Guo, Y.; Li, L.; He, J.; Su, Y. The causal relationship of human blood metabolites with the components of Sarcopenia: A two-sample Mendelian randomization analysis. BMC Geriatr. 2024, 24, 339. [Google Scholar] [CrossRef]
- Chen, S.; Dong, Y.; Aiheti, N.; Wang, J.; Yan, S.; Kuribanjiang, K.; Li, H.; Peng, X.; Wupuer, A.; Li, Y.; et al. Metabolome-Wide Mendelian Randomization Assessing the Causal Relationship Between Blood Metabolites and Sarcopenia-Related Traits. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2024, 79, glae051. [Google Scholar] [CrossRef]
- Sha, T.; Wang, N.; Wei, J.; He, H.; Wang, Y.; Zeng, C.; Lei, G. Genetically Predicted Levels of Serum Metabolites and Risk of Sarcopenia: A Mendelian Randomization Study. Nutrients 2023, 15, 3964. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, T.; Pettersson-Kymmer, U.; Stewart, I.D.; Butler-Laporte, G.; Nakanishi, T.; Cerani, A.; Liang, K.Y.H.; Yoshiji, S.; Willett, J.D.S.; et al. Genomic atlas of the plasma metabolome prioritizes metabolites implicated in human diseases. Nat. Genet. 2023, 55, 44–53. [Google Scholar] [CrossRef]
- Holmes, M.V.; Ala-Korpela, M.; Smith, G.D. Mendelian randomization in cardiometabolic disease: Challenges in evaluating causality. Nat. Rev. Cardiol. 2017, 14, 577–590. [Google Scholar] [CrossRef]
- Alves, A.; Bassot, A.; Bulteau, A.L.; Pirola, L.; Morio, B. Glycine Metabolism and Its Alterations in Obesity and Metabolic Diseases. Nutrients 2019, 11, 1356. [Google Scholar] [CrossRef]
- Gheller, B.J.; Blum, J.E.; Lim, E.W.; Handzlik, M.K.; Hannah Fong, E.H.; Ko, A.C.; Khanna, S.; Gheller, M.E.; Bender, E.L.; Alexander, M.S.; et al. Extracellular serine and glycine are required for mouse and human skeletal muscle stem and progenitor cell function. Mol. Metab. 2021, 43, 101106. [Google Scholar] [CrossRef] [PubMed]
- Koopman, R.; Caldow, M.K.; Ham, D.J.; Lynch, G.S. Glycine metabolism in skeletal muscle: Implications for metabolic homeostasis. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 237–242. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Wu, H.; Chen, S.; Zhu, H.; Zhang, J.; Hou, Y.; Hu, C.A.; Zhang, G. Glycine enhances muscle protein mass associated with maintaining Akt-mTOR-FOXO1 signaling and suppressing TLR4 and NOD2 signaling in piglets challenged with LPS. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, R365–R373. [Google Scholar] [CrossRef]
- Genton, L.; Teta, D.; Pruijm, M.; Stoermann, C.; Marangon, N.; Mareschal, J.; Bassi, I.; Wurzner-Ghajarzadeh, A.; Lazarevic, V.; Cynober, L.; et al. Glycine increases fat-free mass in malnourished haemodialysis patients: A randomized double-blind crossover trial. J. Cachexia Sarcopenia Muscle 2021, 12, 1540–1552. [Google Scholar] [CrossRef]
- Forcina, L.; Musarò, A. Rejuvenating muscle stem cells with the glutathione system. Cell Metab. 2023, 35, 379–381. [Google Scholar] [CrossRef] [PubMed]
- Martin, F.-P.; Boulange, C.L.; Rora, I.M.; Collino, S.; Dumas, M.-E.; Holmes, E.; Rezzi, S.A.D.; Nicholson, J.; Kochhar, S. Isovalerylglycine as Biomarker for the Predispositon for Weight Gain and Obesity. Google Patents G01N33/6893, 2016. [Google Scholar]
- Ticinesi, A.; Nouvenne, A.; Cerundolo, N.; Catania, P.; Prati, B.; Tana, C.; Meschi, T. Gut Microbiota, Muscle Mass and Function in Aging: A Focus on Physical Frailty and Sarcopenia. Nutrients 2019, 11, 1633. [Google Scholar] [CrossRef]
- Lustgarten, M.S.; Price, L.L.; Fielding, R.A. Analytes and Metabolites Associated with Muscle Quality in Young, Healthy Adults. Med. Sci. Sports Exerc. 2015, 47, 1659–1664. [Google Scholar] [CrossRef] [PubMed]
- Lustgarten, M.S.; Price, L.L.; Chalé, A.; Fielding, R.A. Metabolites related to gut bacterial metabolism, peroxisome proliferator-activated receptor-alpha activation, and insulin sensitivity are associated with physical function in functionally-limited older adults. Aging Cell 2014, 13, 918–925. [Google Scholar] [CrossRef]
- McCoin, C.S.; Knotts, T.A.; Adams, S.H. Acylcarnitines--old actors auditioning for new roles in metabolic physiology. Nat. Rev. Endocrinol. 2015, 11, 617–625. [Google Scholar] [CrossRef]
- Aguer, C.; McCoin, C.S.; Knotts, T.A.; Thrush, A.B.; Ono-Moore, K.; McPherson, R.; Dent, R.; Hwang, D.H.; Adams, S.H.; Harper, M.E. Acylcarnitines: Potential implications for skeletal muscle insulin resistance. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2015, 29, 336–345. [Google Scholar] [CrossRef]
- Rutkowsky, J.M.; Knotts, T.A.; Ono-Moore, K.D.; McCoin, C.S.; Huang, S.; Schneider, D.; Singh, S.; Adams, S.H.; Hwang, D.H. Acylcarnitines activate proinflammatory signaling pathways. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E1378–E1387. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Light, A.R.; Hoppel, C.L.; Campbell, C.; Chandler, C.J.; Burnett, D.J.; Souza, E.C.; Casazza, G.A.; Hughen, R.W.; Keim, N.L.; et al. Acylcarnitines as markers of exercise-associated fuel partitioning, xenometabolism, and potential signals to muscle afferent neurons. Exp. Physiol. 2017, 102, 48–69. [Google Scholar] [CrossRef] [PubMed]
- Rinaldo, P.; Matern, D.; Bennett, M.J. Fatty acid oxidation disorders. Annu. Rev. Physiol. 2002, 64, 477–502. [Google Scholar] [CrossRef]
- McCoin, C.S.; Knotts, T.A.; Ono-Moore, K.D.; Oort, P.J.; Adams, S.H. Long-chain acylcarnitines activate cell stress and myokine release in C2C12 myotubes: Calcium-dependent and -independent effects. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E990–E1000. [Google Scholar] [CrossRef]
- Wax, B.; Kerksick, C.M.; Jagim, A.R.; Mayo, J.J.; Lyons, B.C.; Kreider, R.B. Creatine for Exercise and Sports Performance, with Recovery Considerations for Healthy Populations. Nutrients 2021, 13, 1915. [Google Scholar] [CrossRef] [PubMed]
- Butts, J.; Jacobs, B.; Silvis, M. Creatine Use in Sports. Sports Health 2018, 10, 31–34. [Google Scholar] [CrossRef]
- Slupsky, C.M.; Rankin, K.N.; Wagner, J.; Fu, H.; Chang, D.; Weljie, A.M.; Saude, E.J.; Lix, B.; Adamko, D.J.; Shah, S.; et al. Investigations of the effects of gender, diurnal variation, and age in human urinary metabolomic profiles. Anal. Chem. 2007, 79, 6995–7004. [Google Scholar] [CrossRef]
- Thévenot, E.A.; Roux, A.; Xu, Y.; Ezan, E.; Junot, C. Analysis of the Human Adult Urinary Metabolome Variations with Age, Body Mass Index, and Gender by Implementing a Comprehensive Workflow for Univariate and OPLS Statistical Analyses. J. Proteome Res. 2015, 14, 3322–3335. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, J.K.; Siwach, A.; Sachdeva, S.N. Metabolomics and cellular altered pathways in cancer biology: A review. J. Biochem. Mol. Toxicol. 2024, 38, e23807. [Google Scholar] [CrossRef]
- Xiao, Q.; Moore, S.C.; Keadle, S.K.; Xiang, Y.B.; Zheng, W.; Peters, T.M.; Leitzmann, M.F.; Ji, B.T.; Sampson, J.N.; Shu, X.O.; et al. Objectively measured physical activity and plasma metabolomics in the Shanghai Physical Activity Study. Int. J. Epidemiol. 2016, 45, 1433–1444. [Google Scholar] [CrossRef]
- Bruyère, O.; Reginster, J.Y.; Beaudart, C. Lifestyle approaches to prevent and retard sarcopenia: A narrative review. Maturitas 2022, 161, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhou, D.; Li, J.; Wang, M.; Jia, L.; Lan, D.; Song, H.; Ji, X.; Meng, R. The causal relationship between sarcopenia-related traits and ischemic stroke: Insights from univariable and multivariable Mendelian randomization analyses. CNS Neurosci. Ther. 2024, 30, e14759. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.F.; Chen, T.; Gu, X.J.; Su, W.M.; Jiang, Z.; Lu, S.J.; Cao, B.; Chi, L.Y.; Gao, X.; Chen, Y.P. Systematic druggable genome-wide Mendelian randomization identifies therapeutic targets for sarcopenia. J. Cachexia Sarcopenia Muscle 2024, 15, 1324–1334. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.F.; Liu, Y.Z.; Yang, X.L.; Zhang, H.; Feng, G.J.; Wei, X.T.; Zhang, L. The genetic architecture of appendicular lean mass characterized by association analysis in the UK Biobank study. Commun. Biol. 2020, 3, 608. [Google Scholar] [CrossRef] [PubMed]
- Elsworth, B.L.; Mitchell, R.; Raistrick, C.A.; Paternoster, L.; Hemani, G.; Gaunt, T.R. MRC IEU UK Biobank GWAS Pipeline Version; University of Bristol: Bristol, UK, 2017. [Google Scholar] [CrossRef]
- Vikberg, S.; Sörlén, N.; Brandén, L.; Johansson, J.; Nordström, A.; Hult, A.; Nordström, P. Effects of Resistance Training on Functional Strength and Muscle Mass in 70-Year-Old Individuals with Pre-sarcopenia: A Randomized Controlled Trial. J. Am. Med. Dir. Assoc. 2019, 20, 28–34. [Google Scholar] [CrossRef]
- Bohannon, R.W. Are hand-grip and knee extension strength reflective of a common construct? Percept. Mot. Ski. 2012, 114, 514–518. [Google Scholar] [CrossRef]
- UK Biobank GWAS Round 2 Results (Neale Lab). Available online: http://www.nealelab.is/uk-biobank (accessed on 10 August 2023).
- Burgess, S.; Thompson, S.G. Bias in causal estimates from Mendelian randomization studies with weak instruments. Stat. Med. 2011, 30, 1312–1323. [Google Scholar] [CrossRef]
- Hemani, G.; Zheng, J.; Elsworth, B.; Wade, K.H.; Haberland, V.; Baird, D.; Laurin, C.; Burgess, S.; Bowden, J.; Langdon, R.; et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife 2018, 7, e34408. [Google Scholar] [CrossRef]
- Jones, G.; Trajanoska, K.; Santanasto, A.J.; Stringa, N.; Kuo, C.L.; Atkins, J.L.; Lewis, J.R.; Duong, T.; Hong, S.; Biggs, M.L.; et al. Genome-wide meta-analysis of muscle weakness identifies 15 susceptibility loci in older men and women. Nat. Commun. 2021, 12, 654. [Google Scholar] [CrossRef]
- Giambartolomei, C.; Vukcevic, D.; Schadt, E.E.; Franke, L.; Hingorani, A.D.; Wallace, C.; Plagnol, V. Bayesian test for colocalisation between pairs of genetic association studies using summary statistics. PLoS Genet. 2014, 10, e1004383. [Google Scholar] [CrossRef]
- Sun, J.; Zhao, J.; Jiang, F.; Wang, L.; Xiao, Q.; Han, F.; Chen, J.; Yuan, S.; Wei, J.; Larsson, S.C.; et al. Identification of novel protein biomarkers and drug targets for colorectal cancer by integrating human plasma proteome with genome. Genome Med. 2023, 15, 75. [Google Scholar] [CrossRef] [PubMed]
- Kia, D.A.; Zhang, D.; Guelfi, S.; Manzoni, C.; Hubbard, L.; Reynolds, R.H.; Botía, J.; Ryten, M.; Ferrari, R.; Lewis, P.A.; et al. Identification of Candidate Parkinson Disease Genes by Integrating Genome-Wide Association Study, Expression, and Epigenetic Data Sets. JAMA Neurol. 2021, 78, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Psychogios, N.; Young, N.; Wishart, D.S. MetaboAnalyst: A web server for metabolomic data analysis and interpretation. Nucleic Acids Res. 2009, 37, W652–W660. [Google Scholar] [CrossRef] [PubMed]

| Phenotypes | Stratified Analysis | Number of Metabolites | ||
|---|---|---|---|---|
| with Available IVs | p < 0.05 | p < Bonferroni-Corrected Threshold | ||
| ALM | Both sexes | 665 | 239 | 95 |
| Male | 665 | 184 | 60 | |
| Female | 665 | 211 | 77 | |
| HGS | Both sexes | 645 | 86 | 18 |
| Male | 657 | 86 | 5 | |
| Female | 657 | 92 | 12 | |
| WBLM | Both sexes | 645 | 198 | 64 |
| Male | 657 | 142 | 36 | |
| Female | 657 | 123 | 31 | |
| Usual walking pace | Both sexes | 645 | 73 | 2 |
| Male | 657 | 25 | 2 | |
| Female | 657 | 49 | 0 | |
| Metabolites | Association Between Metabolites with Sarcopenia-Related Traits | |||
|---|---|---|---|---|
| Sarcopenia-Related Traits | SNP | Beta (95%CI) | p Value | |
| myristoylcarnitine | ALM * | 1 | −0.23 (−0.28, −0.18) | 1.6 × 10−20 |
| WBLM * | 1 | −1.52 (−1.97, −1.07) | 2.91 × 10−11 | |
| HGS | 1 | −0.09 (−0.12, −0.07) | 7.03 × 10−14 | |
| glycine | ALM | 2 | 0.04 (0.03, 0.05) | 7.52 × 10−29 |
| WBLM | 2 | 0.03 (0.02, 0.04) | 4.38 × 10−38 | |
| HGS | 2 | 0.02 (0.01, 0.03) | 1.86 × 10−9 | |
| isovalerylglycine | ALM * | 1 | 0.04 (0.03, 0.06) | 7.33 × 10−9 |
| WBLM | 1 | 0.08 (0.07, 0.09) | 6.02 × 10−37 | |
| HGS | 1 | 0.04 (0.03, 0.06) | 1.3 × 10−9 | |
| propionylglycine | ALM * | 2 | 0.05 (0.03, 0.06) | 2.12 × 10−9 |
| WBLM | 2 | 0.05 (0.04, 0.06) | 6.02 × 10−37 | |
| HGS | 2 | 0.03 (0.02, 0.04) | 1.3 × 10−9 | |
| gamma-glutamylglycine | ALM | 1 | 0.30 (0.27, 0.34) | 2.29 × 10−31 |
| WBLM | 1 | 0.03 (0.02, 0.04) | 6.02 × 10−37 | |
| HGS | 1 | 0.02 (0.01, 0.03) | 1.3 × 10−9 | |
| cinnamoylglycine | ALM | 1 | 0.08 (0.05, 0.11) | 2.29 × 10−31 |
| WBLM | 1 | 0.08 (0.07, 0.09) | 6.02 × 10−37 | |
| HGS | 1 | 0.05 (0.03, 0.06) | 1.30 × 10−9 | |
| mannose | ALM | 1 | 0.12 (0.11, 0.14) | 8.21 × 10−65 |
| WBLM | 1 | 0.07 (0.06, 0.08) | 1.1 × 10−50 | |
| creatine | ALM | 2 | 0.11 (0.10, 0.13) | 4.27 × 10−39 |
| WBLM | 2 | 0.08 (0.06, 0.10) | 3.39 × 10−17 | |
| mannonate | ALM | 1 | 0.15 (0.14, 0.17) | 8.21 × 10−65 |
| WBLM | 1 | 0.09 (0.08, 0.10) | 1.1 × 10−50 | |
| beta-hydroxyisovaleroylcarnitine | ALM | 2 | −0.17 (−0.21, −0.13) | 7.65 × 10−26 |
| WBLM | 2 | −0.07 (−0.09, −0.05) | 1.45 × 10−18 | |
| (R)-3-hydroxybutyrylcarnitine | ALM | 1 | −0.14 (−0.17, −0.10) | 1.79 × 10−13 |
| WBLM | 1 | −0.07 (−0.09, −0.04) | 1.48 × 10−8 | |
| (S)-3-hydroxybutyrylcarnitine | ALM | 1 | −0.09 (−0.12, −0.07) | 4.51 × 10−14 |
| WBLM | 1 | −0.05 (−0.07, −0.04) | 2.27 × 10−9 | |
| acetylcarnitine | ALM | 1 | −0.08 (−0.10, −0.05) | 1.79 × 10−13 |
| WBLM | 1 | −0.04 (−0.05, −0.02) | 1.48 × 10−8 | |
| Metabolites | Modifiable Factors | Method | nSNP | Beta (95%CI) | p | FDR |
|---|---|---|---|---|---|---|
| 1-lignoceroyl-GPC (24:0) | Television watching | IVW | 88 | −0.44 (−0.66, −0.22) | 1.07 × 10−4 | 4.05 × 10−3 |
| beta-hydroxyisovaleroylcarnitine | Sleep duration | IVW | 58 | −0.007 (−0.010, −003) | 1.13 × 10−3 | 2.63 × 10−2 |
| beta-hydroxyisovaleroylcarnitine | Smoking initiation | IVW | 192 | 0.20 (0.08, 0.32) | 1.38 × 10−3 | 2.63 × 10−2 |
| gamma-glutamylglycine | WHRadjBMI | IVW | 292 | −0.21 (−0.32, −0.10) | 2.68 × 10−4 | 1.02 × 10−2 |
| glycine | WHRadjBMI | IVW | 292 | −0.23 (−0.35, −0.12) | 3.89 × 10−5 | 1.48 × 10−3 |
| levulinoylcarnitine | Tea consumption | IVW | 12 | 1.82 (0.83, 2.81) | 3.19 × 10−4 | 1.21 × 10−2 |
| mannose | Smoking initiation | IVW | 192 | 0.22 (0.10, 0.35) | 2.98 × 10−4 | 1.13 × 10−2 |
| mannose | Milk intake | Wald ratio | 1 | 0.08 (0.03, 0.13) | 2.41 × 10−3 | 4.59 × 10−2 |
| sphingomyelin (d18:2/14:0, 18:1/14:1) | WHRadjBMI | IVW | 292 | −0.22 (−0.31, −0.12) | 7.77 × 10−6 | 2.95 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, J.; Li, J.; Wang, W.; Zhang, R.; Zhang, D. Identifying the Shared Metabolite Biomarkers and Potential Intervention Targets for Multiple Sarcopenia-Related Phenotypes. Int. J. Mol. Sci. 2024, 25, 12310. https://doi.org/10.3390/ijms252212310
Luo J, Li J, Wang W, Zhang R, Zhang D. Identifying the Shared Metabolite Biomarkers and Potential Intervention Targets for Multiple Sarcopenia-Related Phenotypes. International Journal of Molecular Sciences. 2024; 25(22):12310. https://doi.org/10.3390/ijms252212310
Chicago/Turabian StyleLuo, Jia, Jingxian Li, Weijing Wang, Ronghui Zhang, and Dongfeng Zhang. 2024. "Identifying the Shared Metabolite Biomarkers and Potential Intervention Targets for Multiple Sarcopenia-Related Phenotypes" International Journal of Molecular Sciences 25, no. 22: 12310. https://doi.org/10.3390/ijms252212310
APA StyleLuo, J., Li, J., Wang, W., Zhang, R., & Zhang, D. (2024). Identifying the Shared Metabolite Biomarkers and Potential Intervention Targets for Multiple Sarcopenia-Related Phenotypes. International Journal of Molecular Sciences, 25(22), 12310. https://doi.org/10.3390/ijms252212310






