The Involvement of Glial Cells in Blood–Brain Barrier Damage in Neuroimmune Diseases
Abstract
:1. Introduction
2. Glial Cells in Blood–Brain Barrier Damage
2.1. Astrocytes and the Blood–Brain Barrier
2.2. Radial Glial Cells and the Blood–Brain Barrier
2.3. Reactive Astrocytes and Blood–Brain Barrier Protection
2.4. Reactive Astrocytes and Blood–Brain Barrier Damage
2.5. Microglia and the Blood–Brain Barrier
2.6. Pericytes and the Blood–Brain Barrier
3. The Involvement of Glial Cells in Blood–Brain Barrier Damage in Demyelinating Diseases
3.1. Glial Cells in Blood–Brain Barrier Damage in Multiple Sclerosis
3.1.1. Pathogenic Cell Migration in Multiple Sclerosis
3.1.2. Reactive Astrocytes and the Blood–Brain Barrier in Multiple Sclerosis
3.1.3. Microglia and the Blood–Brain Barrier in Multiple Sclerosis
3.1.4. Disease-Modifying Drugs and the Blood–Brain Barrier in Multiple Sclerosis
3.2. Glial Cells in Blood–Brain Barrier Damage in Neuromyelitis Optica Spectrum Disorders
4. Involvement of Glial Cells in Blood–Brain Barrier Damage in Other Neuroimmune Diseases
4.1. Glial Cells in Blood–Brain Barrier Damage in Anti-NMDA Receptor Antibody Encephalopathy
4.2. Glial Cells in Blood–Brain Barrier Damage in Anti-VGKC Antibody Encephalopathy
4.3. Glial Cells in Blood–Brain Barrier Damage in Neuropsychiatric Systemic Lupus Erythematosus
4.4. Glial Cells in Blood–Brain Barrier Damage in Behçet’s Disease
4.5. Glial Cells in Blood–Brain Barrier Damage in Vasculitis
4.6. Glial Cells in Blood–Brain Barrier Damage in GFAP Astrocytopathy
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kacem, K.; Lacombe, P.; Seylaz, J.; Bonvento, G. Structural organization of the perivascular astrocyte endfeet and their relationship with the endothelial glucose transporter: A confocal microscopy study. Glia 1998, 23, 1–10. [Google Scholar] [CrossRef]
- Bardehle, S.; Krüger, M.; Buggenthin, F.; Schwausch, J.; Ninkovic, J.; Clevers, H.; Snippert, H.J.; Theis, F.J.; Meyer-Luehmann, M.; Bechmann, I.; et al. Live imaging of astrocyte responses to acute injury reveals selective juxtavascular proliferation. Nat. Neurosci. 2013, 16, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S. Specialized membrane domains for water transport in glial cells: High-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J. Neurosci. 1997, 17, 171–180. [Google Scholar] [CrossRef]
- Alvarez, J.I.; Dodelet-Devillers, A.; Kebir, H.; Ifergan, I.; Fabre, P.J.; Terouz, S.; Sabbagh, M.; Wosik, K.; Bourbonnière, L.; Bernard, M.; et al. The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science 2011, 334, 1727–1731. [Google Scholar] [CrossRef]
- Kubotera, H.; Ikeshima-Kataoka, H.; Hatashita, Y.; Mascaro, A.L.A.; Pavone, F.S.; Inoue, T. Astrocytic endfeet re-cover blood vessels after removal by laser ablation. Sci. Rep. 2019, 9, 1263. [Google Scholar] [CrossRef]
- Ma, S.; Kwon, H.J.; Huang, Z. A functional requirement for astroglia in promoting blood vessel development in the early postnatal brain. PLoS ONE 2012, 7, e48001. [Google Scholar] [CrossRef]
- Mills, W.A.; Woo, A.M.; Jiang, S.; Martin, J.; Surendran, D.; Bergstresser, M.; Kimbrough, I.F.; Eyo, U.B.; Sofroniew, M.V.; Sontheimer, H. Astrocyte plasticity in mice ensures continued endfoot coverage of cerebral blood vessels following injury and declines with age. Nat. Commun. 2022, 13, 1794. [Google Scholar] [CrossRef]
- Göbel, J.; Engelhardt, E.; Pelzer, P.; Sakthivelu, V.; Jahn, H.M.; Jevtic, M.; Folz-Donahue, K.; Kukat, C.; Schauss, A.; Frese, C.K.; et al. Mitochondria-Endoplasmic Reticulum Contacts in Reactive Astrocytes Promote Vascular Remodeling. Cell Metab. 2020, 31, 791–808. [Google Scholar] [CrossRef]
- Brøchner, C.B.; Holst, C.B.; Møllgård, K. Outer brain barriers in rat and human development. Front. Neurosci. 2015, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wei, J.Y.; Li, Y.; Ban, M.; Sun, Q.; Wang, H.J.; Zhao, D.; Tong, P.G.; Wang, L.; Wang, K.J.; et al. Endothelial depletion of Atg7 triggers astrocyte–microvascular disassociation at blood–brain barrier. J. Cell Biol. 2023, 222, e202103098. [Google Scholar] [CrossRef] [PubMed]
- Lien, C.F.; Mohanta, S.K.; Frontczak-Baniewicz, M.; Swinny, J.D.; Zablocka, B.; Górecki, D.C. Absence of glial α-dystrobrevin causes abnormalities of the blood-brain barrier and progressive brain edema. J. Biol. Chem. 2012, 287, 41374–41385. [Google Scholar] [CrossRef] [PubMed]
- Sobue, K.; Yamamoto, N.; Yoneda, K.; E Hodgson, M.; Yamashiro, K.; Tsuruoka, N.; Tsuda, T.; Katsuya, H.; Miura, Y.; Asai, K.; et al. Induction of blood-brain barrier properties in immortalized bovine brain endothelial cells by astrocytic factors. Neurosci. Res. 1999, 35, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.D.; Winkler, E.A.; Singh, I.; Sagare, A.P.; Deane, R.; Wu, Z.; Holtzman, D.M.; Betsholtz, C.; Armulik, A.; Sallstrom, J.; et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature 2012, 485, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.G.; Liu, F.; Verin, A.D.; Birukova, A.; Dechert, M.A.; Gerthoffer, W.T.; Bamberg, J.R.; English, D. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J. Clin. Investig. 2001, 108, 689–701. [Google Scholar] [CrossRef]
- Ye, Q.; Jo, J.; Wang, C.-Y.; Oh, H.; Zhan, J.; Choy, T.J.; Kim, K.I.; D’alessandro, A.; Reshetnyak, Y.K.; Jung, S.Y.; et al. Astrocytic Slc4a4 regulates blood-brain barrier integrity in healthy and stroke brains via a CCL2-CCR2 pathway and NO dysregulation. Cell Rep. 2024, 43, 114193. [Google Scholar] [CrossRef]
- Liu, D.; Liao, P.; Li, H.; Tong, S.; Wang, B.; Lu, Y.; Gao, Y.; Huang, Y.; Zhou, H.; Shi, L.; et al. Regulation of blood-brain barrier integrity by Dmp1-expressing astrocytes through mitochondrial transfer. Sci. Adv. 2024, 10, eadk2913. [Google Scholar] [CrossRef]
- Jing, B.; Zhang, C.; Liu, X.; Zhou, L.; Liu, J.; Yao, Y.; Yu, J.; Weng, Y.; Pan, M.; Liu, J.; et al. Glycosylation of dentin matrix protein 1 is a novel key element for astrocyte maturation and BBB integrity. Protein Cell 2018, 9, 298–309. [Google Scholar] [CrossRef]
- Morales, J.E.; De, A.; Miller, A.A.; Chen, Z.; McCarty, J.H. Mlc1-Expressing Perivascular Astrocytes Promote Blood-Brain Barrier Integrity. J. Neurosci. 2022, 42, 1406–1416. [Google Scholar] [CrossRef]
- Chen, Z.; Kelly, J.R.; Morales, J.E.; Sun, R.C.; De, A.; Burkin, D.J.; McCarty, J.H. The alpha7 integrin subunit in astrocytes promotes endothelial blood-brain barrier integrity. Development 2023, 150, dev201356. [Google Scholar] [CrossRef]
- Yamasaki, R. Connexins in health and disease. Clin. Exp. Neuroimmunol. 2018, 9, 30–36. [Google Scholar] [CrossRef]
- Takeuchi, H.; Suzumura, A. Gap junctions and hemichannels composed of connexins: Potential therapeutic targets for neurodegenerative diseases. Front. Cell. Neurosci. 2014, 8, 189. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Ochalski, A.; Hertzberg, E.; Nagy, J. LM and EM immunolocalization of the gap junctions protein connexin43 in rat brain. Brain Res. 1990, 508, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Ochalski, A.; Hertzberg, E.L.; Nagy, J.I. On the organization of astrocytic gap junctions in rat brain as suggested by LM and EM immunohistochemistry of connexin43 expression. J. Comp. Neurol. 1990, 302, 853–883. [Google Scholar] [CrossRef]
- Nagy, J.I.; Ochalski, P.; Li, J.; Hertzberg, E. Evidence for co-localization of another connexin-43 at astrocytic gap junctions in rat brain. Neuroscience 1997, 78, 533–548. [Google Scholar] [CrossRef]
- Nagy, J.I.; Patel, D.; Ochalski, P.A.Y.; Stelmack, G. Connexin30 in rodent, cat and human brain: Selective expression in gray matter astrocytes, co-localization with connexin30 at gap junctions and late developmental appearance. Neuroscience 1999, 88, 447–468. [Google Scholar] [CrossRef]
- Nagy, J.I.; Li, X.; Rempel, J.; Stelmack, G.; Patel, D.; Staines, W.A.; Yasumura, T.; Rash, J.E. Connexin26 in adult rodent CNS: Demonstration at astrocytic gap junctions and co-localization with connexin30 and connexin43. J. Comp. Neurol. 2001, 441, 302–323. [Google Scholar] [CrossRef]
- Meşe, G.; Richard, G.; White, T.W. Gap junctions: Basic structure and function. J. Investig. Dermatol. 2007, 127, 2516–2524. [Google Scholar] [CrossRef]
- Sáez, J.C.; Retamal, M.A.; Basilio, D.; Bukauskas, F.F.; Bennett, M.V. Connexin-based gap junction hemichannels: Gating mechanisms. Biochim. Biophys. Acta 2005, 1711, 215–224. [Google Scholar] [CrossRef]
- Alvarez, J.I.; Katayama, T.; Prat, A. Glial influence on the blood brain barrier. Glia 2013, 61, 1939–1958. [Google Scholar] [CrossRef]
- Sirko, S.; Behrendt, G.; Johansson, P.A.; Tripathi, P.; Costa, M.R.; Bek, S.; Heinrich, C.; Tiedt, S.; Colak, D.; Dichgans, M.; et al. Reactive glia in the injured brain acquire stem cell properties in response to sonic hedgehog. Cell Stem Cell 2013, 12, 426–439. [Google Scholar] [CrossRef]
- Mizee, M.R.; Nijland, P.G.; van der Pol, S.M.; Drexhage, J.A.; van Het Hof, B.; Mebius, R.; van der Valk, P.; van Horssen, J.; Reijerkerk, A.; de Vries, H.E. Astrocyte-derived retinoic acid: A novel regulator of blood–brain barrier function in multiple sclerosis. Acta Neuropathol. 2014, 128, 691–703. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.M.; Park, K.R.; Kim, E.C.; Hong, J.T. PRDX6 controls multiple sclerosis by suppressing inflammation and blood brain barrier disruption. Oncotarget 2015, 6, 20875–20884. [Google Scholar] [CrossRef] [PubMed]
- Eilam, R.; Segal, M.; Malach, R.; Sela, M.; Arnon, R.; Aharoni, R. Astrocyte disruption of neurovascular communication is linked to cortical damage in an animal model of multiple sclerosis. Glia 2018, 66, 1098–1117. [Google Scholar] [CrossRef] [PubMed]
- Prineas, J.W.; Lee, S. Multiple sclerosis: Destruction and regeneration of astrocytes in acute lesions. J. Neuropathol. Exp. Neurol. 2019, 78, 140–156. [Google Scholar] [CrossRef] [PubMed]
- Brand-Schieber, E. Connexin43, the major gap junction protein of astrocytes, is down-regulated in inflamed white matter in an animal model of multiple sclerosis. J. Neurosci. Res. 2005, 80, 798–808. [Google Scholar] [CrossRef]
- Une, H.; Yamasaki, R.; Nagata, S.; Yamaguchi, H.; Nakamuta, Y.; Indiasari, U.C.; Cui, Y.; Shinoda, K.; Masaki, K.; Götz, M.; et al. Brain gray matter astroglia-specific connexin 43 ablation attenuates spinal cord inflammatory demyelination. J. Neuroinflamm 2021, 18, 126. [Google Scholar] [CrossRef]
- Yamasaki, R. Connexins Control Glial Inflammation in Various Neurological Diseases. Int. J. Mol. Sci. 2023, 24, 16879. [Google Scholar] [CrossRef]
- Bynoe, M.S.; Viret, C.; Yan, A.; Kim, D.-G. Adenosine receptor signaling: A key to opening the blood-brain door. Fluids Barriers CNS 2015, 12, 20. [Google Scholar] [CrossRef]
- Vazana, U.; Veksler, R.; Pell, G.S.; Prager, O.; Fassler, M.; Chassidim, Y.; Roth, Y.; Shahar, H.; Zangen, A.; Raccah, R.; et al. Glutamate-mediated blood-brain barrier opening: Implications for neuroprotection and drug delivery. J. Neurosci. 2016, 36, 7727–7739. [Google Scholar] [CrossRef]
- Shi, J.; Xiao, Y.; Zhang, N.; Jiao, M.; Tang, X.; Dai, C.; Wang, C.; Xu, Y.; Tan, Z.; Gong, F.; et al. HMGB1 from astrocytes promotes EAE by influencing the immune cell infiltration-associated functions of BMECs in mice. Neurosci. Bull. 2022, 38, 1303–1314. [Google Scholar] [CrossRef]
- Xiao, M.; Xiao, Z.J.; Yang, B.; Lan, Z.; Fang, F. Blood-brain barrier: More contributor to disruption of central nervous system homeostasis than victim in neurological disorders. Front. Neurol. 2020, 14, 764. [Google Scholar] [CrossRef] [PubMed]
- Spampinato, S.F.; Merlo, S.; Costantino, G.; Sano, Y.; Kanda, T.; Sortino, M.A. Decreased astrocytic CCL2 accounts for BAF-312 effect on PBMCs transendothelial migration through a blood brain barrier in vitro model. J. Neuroimmune Pharmacol. 2022, 17, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Kimura, A.; Takekoshi, A.; Yoshikura, N.; Hayashi, Y.; Shimohata, T. Clinical characteristics of autoimmune GFAP astrocytopathy. J. Neuroimmunol. 2019, 332, 91–98. [Google Scholar] [CrossRef]
- Zheng, W.; Shen, P.; Yu, C.; Tang, Y.; Qian, C.; Yang, C.; Gao, M.; Wu, Y.; Yu, S.; Tang, W.; et al. Ginsenoside Rh1, a novel casein kinase II subunit alpha (CK2α) inhibitor, retards metastasis via disrupting HHEX/CCL20 signaling cascade involved in tumor cell extravasation across endothelial barrier. Pharmacol. Res. 2023, 198, 106986. [Google Scholar] [CrossRef]
- Chang, C.Y.; Chen, W.; Ou, Y.; Lai, C.; Hu, Y.; Wu, C.; Chang, C.; Chen, C. Disruption of in vitro endothelial barrier integrity by Japanese encephalitis virus-infected astrocytes. Glia 2015, 63, 1915–1932. [Google Scholar] [CrossRef]
- Rochfort, K.D.; Collins, L.E.; Murphy, R.P.; Cummins, P.M. Downregulation of blood-brain barrier phenotype by proinflammatory cytokines involves NADPH oxidase-dependent ROS generation: Consequences for interendothelial adherens and tight junctions. PLoS ONE 2014, 9, e101815. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, S.; Sonobe, Y.; Cheng, Y.; Horiuchi, H.; Parajuli, B.; Kawanokuchi, J.; Mizuno, T.; Takeuchi, H.; Suzumura, A. Interleukin-1β induces blood-brain barrier disruption by downregulating Sonic hedgehog in astrocytes. PLoS ONE 2014, 9, e110024. [Google Scholar] [CrossRef]
- Abadier, M.; Jahromi, N.H.; Alves, L.C.; Boscacci, R.; Vestweber, D.; Barnum, S.; Deutsch, U.; Engelhardt, B.; Lyck, R. Cell surface levels of endothelial ICAM-1 influence the transcellular or paracellular T-cell diapedesis across the blood-brain barrier. Eur. J. Immunol. 2015, 45, 1043–1058. [Google Scholar] [CrossRef]
- Argaw, A.T.; Asp, L.; Zhang, J.; Navrazhina, K.; Pham, T.; Mariani, J.N.; Mahase, S.; Dutta, D.J.; Seto, J.; Kramer, E.G.; et al. Astrocyte-derived VEGF-A drives blood-brain barrier disruption in CNS inflammatory disease. J. Clin. Investig. 2012, 122, 2454–2468. [Google Scholar] [CrossRef]
- Chapouly, C.; Argaw, A.T.; Horng, S.; Castro, K.; Zhang, J.; Asp, L.; Loo, H.; Laitman, B.M.; Mariani, J.N.; Farber, R.S.; et al. Astrocytic TYMP and VEGFA drive blood-brain barrier opening in inflammatory central nervous system lesions. Brain 2015, 138, 1548–1567. [Google Scholar] [CrossRef]
- Bennett, M.V.L.; Garré, J.M.; Orellana, J.A.; Bukauskas, F.F.; Nedergaard, M.; Giaume, C.; Sáez, J.C. Connexin and pannexin hemichannels in inflammatory responses of glia and neurons. Brain Res. 2012, 1487, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Mizee, M.R.; Wooldrik, D.; Lakeman, K.A.M.; Hof, B.v.H.; Drexhage, J.A.R.; Geerts, D.; Bugiani, M.; Aronica, E.; Mebius, R.E.; Prat, A.; et al. Retinoic acid induces blood-brain barrier development. J. Neurosci. 2013, 33, 1660–1671. [Google Scholar] [CrossRef] [PubMed]
- Sentilhes, L.; Michel, C.; Lecourtois, M.; Catteau, J.; Bourgeois, P.; Laudenbach, V.; Marret, S.; Laquerrière, A. Vascular endothelial growth factor and its high-affinity receptor (VEGFR-2) are highly expressed in the human forebrain and cerebellum during development. J. Neuropathol. Exp. Neurol. 2010, 69, 111–128. [Google Scholar] [CrossRef]
- da Silva, S.M.; Campos, G.D.; Gomes, F.C.; Stipursky, J. Radial glia-endothelial cells’ bidirectional interactions control vascular maturation and astrocyte differentiation: Impact for blood-brain barrier formation. Curr. Neurovascular Res. 2019, 16, 291–300. [Google Scholar] [CrossRef]
- Radonjic, N.V.; Memi, F.; Ortega, J.A.; Glidden, N.; Zhan, H.; Zecevic, N. The role of sonic hedgehog in the specification of human cortical progenitors in vitro. Cereb. Cortex 2014, 26, 131–143. [Google Scholar] [CrossRef]
- Cheng, N.; Brantley, D.M.; Chen, J. The ephrins and Eph receptors in angiogenesis. Cytokine Growth Factor Rev. 2002, 13, 75–85. [Google Scholar] [CrossRef]
- Chen, F.; Liu, Z.; Peng, W.; Gao, Z.; Ouyang, H.; Yan, T.; Ding, S.; Cai, Z.; Zhao, B.; Mao, L.; et al. Activation of EphA4 induced by EphrinA1 exacerbates disruption of the blood-brain barrier following cerebral ischemia-reperfusion via the Rho/ROCK signaling pathway. Exp. Ther. Med. 2018, 16, 2651–2658. [Google Scholar] [CrossRef]
- Nishioku, T.; Matsumoto, J.; Dohgu, S.; Sumi, N.; Miyao, K.; Takata, F.; Shuto, H.; Yamauchi, A.; Kataoka, Y. Tumor necrosis factor-alpha mediates the blood-brain barrier dysfunction induced by activated microglia in mouse brain microvascular endothelial cells. J. Pharmacol. Sci. 2010, 112, 251–254. [Google Scholar] [CrossRef]
- Shigemoto-Mogami, Y.; Hoshikawa, K.; Sato, K. Activated microglia disrupt the blood-brain barrier and induce chemokines and cytokines in a rat in vitro model. Front. Cell. Neurosci. 2018, 12, 494. [Google Scholar] [CrossRef]
- Sumi, N.; Nishioku, T.; Takata, F.; Matsumoto, J.; Watanabe, T.; Shuto, H.; Yamauchi, A.; Dohgu, S.; Kataoka, Y. Lipopolysaccharide-activated microglia induce dysfunction of the blood-brain barrier in rat microvascular endothelial cells co-cultured with microglia. Cell Mol. Neurobiol. 2010, 30, 247–253. [Google Scholar] [CrossRef]
- Schreibelt, G.; Kooij, G.; Reijerkerk, A.; Doorn, R.; Gringhuis, S.I.; Pol, S.; Weksler, B.B.; Romero, I.A.; Couraud, P.; Piontek, J.; et al. Reactive oxygen species alter brain endothelial tight junction dynamics via RhoA, PI3 kinase, and PKB signaling. FASEB J. 2007, 21, 3666–3676. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Chang, H. Elevated blood and cerebrospinal fluid biomarkers of microglial activation and blood–brain barrier disruption in anti-NMDA receptor encephalitis. J. Neuroinflamm. 2023, 20, 172. [Google Scholar] [CrossRef]
- Gaengel, K.; Genové, G.; Armulik, A.; Betsholtz, C. Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 630–638. [Google Scholar] [CrossRef]
- Stark, K.; Eckart, A.; Haidari, S.; Tirniceriu, A.; Lorenz, M.; von Brühl, M.-L.; Gärtner, F.; Khandoga, A.G.; Legate, K.R.; Pless, R.; et al. Capillary and arteriolar pericytes attract innate leukocytes exiting through venules and ‘instruct’ them with pattern-recognition and motility programs. Nat. Immunol. 2013, 14, 41–51. [Google Scholar] [CrossRef]
- Li, Y. Myeloid-derived MIF drives RIPK1-mediated cerebromicrovascular endothelial cell death to exacerbate ischemic brain injury. Proc. Natl. Acad. Sci. USA 2023, 120, e2219091120. [Google Scholar] [CrossRef]
- Tasaki, A.; Shimizu, F.; Sano, Y.; Fujisawa, M.; Takahashi, T.; Haruki, H.; Abe, M.; Koga, M.; Kanda, T. Autocrine MMP-2/9 secretion increases the BBB permeability in neuromyelitis optica. J. Neurol. Neurosurg. Psychiatry 2014, 85, 419–430. [Google Scholar] [CrossRef]
- Chen, J.T.; Chen, T.G.; Chang, Y.C.; Chen, C.Y.; Chen, R.M. Roles of NMDARs in maintenance of the mouse cerebrovascular endothelial cell-constructed tight junction barrier. Toxicology 2016, 339, 40–50. [Google Scholar] [CrossRef]
- Covo-Calvo, A.; Ruiz, A.; Richard, C.; Blondel, S.; Cavagna, S.; Strazielle, N.; Ghersi-Egea, J.F.; Giraudon, P.; Marignier, R. Purified IgG from aquaporin-4 neuromyelitis optica spectrum disorder patients alters blood-brain barrier permeability. PLoS ONE 2020, 15, e0238301. [Google Scholar]
- Muller Kobold, A.C.; Van Wijk, R.T.; Franssen, C.F.; Molema, G.; Kallenberg, C.G.; Tervaert, J.W. In vitro up-regulation of E-selectin and induction of interleukin-6 in endothelial cells by autoantibodies in Wegener’s granulomatosis and microscopic polyangiitis. Clin. Exp. Rheumatol. 1999, 17, 433–440. [Google Scholar]
- Del Papa, N.; Guidali, L.; Sironi, M.; Shoenfeld, Y.; Mantovani, A.; Tincani, A.; Balestrieri, G.; Radice, A.; Sinico, R.A.; Meroni, P.L. Anti-endothelial cell IgG antibodies from patients with Wegener’s granulomatosis bind to human endothelial cells in vitro and induce adhesion molecule expression and cytokine secretion. Arthritis Rheum. 1996, 39, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, N.; Zhang, J.; Liu, H.; Liu, J.; Xia, X.; Sun, C.; Feng, X.; Gu, J.; Du, C.; et al. Enolase of streptococcus suis serotype 2 enhances blood-brain barrier permeability by inducing IL-8 release. Inflammation 2016, 39, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Mao, F.; Nong, W.; Gong, Z.; Lao, D.; Huang, W. Inhibiting caveolin-1-related Akt/mTOR signaling pathway protects against N-methyl-D-aspartate receptor activation-mediated dysfunction of blood-brain barrier in vitro. Mol. Neurobiol. 2024, 61, 4166–4177. [Google Scholar] [CrossRef]
- McDermott, K.W.; Barry, D.S.; McMahon, S.S. Role of radial glia in cytogenesis, patterning and boundary formation in the developing spinal cord. J. Anat. 2005, 207, 241–250. [Google Scholar] [CrossRef]
- Goldshmit, Y.; Homman-Ludiye, J.; Bourne, J.A. EphA4 is associated with multiple cell types in the marmoset primary visual cortex throughout the lifespan. Eur. J. Neurosci. 2014, 39, 1419–1428. [Google Scholar] [CrossRef]
- Ma, S.; Kwon, H.J.; Johng, H.; Zang, K.; Huang, Z. Radial glial neural progenitors regulate nascent brain vascular network stabilization via inhibition of Wnt signaling. PLoS Biol. 2013, 11, e1001469. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, J.; Sha, W.M.; Zhang, X.P.; Mai, J.Y.; Bartlett, P.F.; Hou, S.T. Inhibition of EphA4 reduces vasogenic edema after experimental stroke in mice by protecting the blood-brain barrier integrity. J. Cereb. Blood Flow Metab. 2024, 44, 419–433. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Z.; Xia, Z.; Rallo, M.; Duffy, A.; Matise, M.P. Inactivation of Hedgehog signal transduction in adult astrocytes results in region-specific blood-brain barrier defects. Proc. Natl. Acad. Sci. USA 2021, 118, e2017779118. [Google Scholar] [CrossRef]
- Didier, N.; Romero, I.A.; Créminon, C.; Wijkhuisen, A.; Grassi, J.; Mabondzo, A. Secretion of interleukin-1beta by astrocytes mediates endothelin-1 and tumour necrosis factor-alpha effects on human brain microvascular endothelial cell permeability. J. Neurochem. 2003, 86, 246–254. [Google Scholar] [CrossRef]
- Marchetti, L.; Francisco, D.; Soldati, S.; Haghayegh Jahromi, N.; Barcos, S.; Gruber, I.; Pareja, J.R.; Thiriot, A.; von Andrian, U.; Deutsch, U.; et al. ACKR1 favors transcellular over paracellular T-cell diapedesis across the blood-brain barrier in neuroinflammation in vitro. Eur. J. Immunol. 2021, 52, 161–177. [Google Scholar] [CrossRef]
- Almolda, B.; Villacampa, N.; Manders, P.; Hidalgo, J.; Campbell, I.L.; González, B.; Castellano, B. Effects of astrocyte-targeted production of interleukin-6 in the mouse on the host response to nerve injury. Glia 2014, 62, 1142–1161. [Google Scholar] [CrossRef] [PubMed]
- Korff, T.; Dandekar, G.; Pfaff, D.; Fuller, T.; Goettsch, W.; Morawietz, H.; Schaffner, F.; Augustin, H.G. Endothelial ephrinB2 is controlled by microenvironmental determinants and associates context-dependently with CD31. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Mondo, E.; Becker, S.C.; Kautzman, A.G.; Schifferer, M.; Baer, C.E.; Chen, J.; Huang, E.J.; Simons, M.; Schafer, D.P. A Developmental Analysis of Juxtavascular Microglia Dynamics and Interactions with the Vasculature. J. Neurosci. 2020, 40, 6503–6521. [Google Scholar] [CrossRef] [PubMed]
- Császár, E.; Lénárt, N.; Cserép, C.; Környei, Z.; Fekete, R.; Pósfai, B.; Balázsfi, D.; Hangya, B.; Schwarcz, A.D.; Szabadits, E.; et al. Microglia modulate blood flow, neurovascular coupling, and hypoperfusion via purinergic actions. J. Exp. Med. 2022, 219, e20211071. [Google Scholar] [CrossRef]
- Bisht, K.; Okojie, K.A.; Sharma, K.; Lentferink, D.H.; Sun, Y.-Y.; Chen, H.-R.; Uweru, J.O.; Amancherla, S.; Calcuttawala, Z.; Campos-Salazar, A.B.; et al. Capillary-associated microglia regulate vascular structure and function through PANX1-P2RY12 coupling in mice. Nat. Commun. 2021, 12, 5289. [Google Scholar] [CrossRef]
- Mills, S.A.; Jobling, A.I.; Dixon, M.A.; Bui, B.V.; Vessey, K.A.; Phipps, J.A.; Greferath, U.; Venables, G.; Wong, V.H.Y.; Wong, C.H.Y.; et al. Fractalkine-induced microglial vasoregulation occurs within the retina and is altered early in diabetic retinopathy. Proc. Natl. Acad. Sci. USA 2021, 118, e2112561118. [Google Scholar] [CrossRef]
- Hartmann, D.A.; Berthiaume, A.-A.; Grant, R.I.; Harrill, S.A.; Koski, T.; Tieu, T.; McDowell, K.P.; Faino, A.V.; Kelly, A.L.; Shih, A.Y. Brain capillary pericytes exert a substantial but slow influence on blood flow. Nat. Neurosci. 2021, 24, 633–645. [Google Scholar] [CrossRef]
- Argaw, A.T.; Gurfein, B.T.; Zhang, Y.; Zameer, A.; John, G.R. VEGF-mediated disruption of endothelial CLN-5 promotes blood-brain barrier breakdown. Proc. Natl. Acad. Sci. USA 2009, 106, 1977–1982. [Google Scholar] [CrossRef]
- Armulik, A.; Genové, G.; Mäe, M.; Nisancioglu, M.H.; Wallgard, E.; Niaudet, C.; He, L.; Norlin, J.; Lindblom, P.; Strittmatter, K.; et al. Pericytes regulate the blood-brain barrier. Nature 2010, 468, 557–561. [Google Scholar] [CrossRef]
- Armulik, A.; Genové, G.; Betsholtz, C. Pericytes: Developmental, physiological, and pathological perspectives, problems, and promises. Dev. Cell 2011, 21, 193–215. [Google Scholar] [CrossRef]
- Gundersen, G.A.; Vindedal, G.F.; Skare, Ø.; Nagelhus, E.A. Evidence that pericytes regulate aquaporin-4 polarization in mouse cortical astrocytes. Brain Struct. Funct. 2014, 219, 2181–2186. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Muramatsu, M.; Azuma, E.; Ikutani, M.; Nagai, Y.; Sagara, H.; Koo, B.-N.; Kita, S.; O’donnell, E.; Osawa, T.; et al. A subset of cerebrovascular pericytes originates from mature macrophages in the very early phase of vascular development in CNS. Sci. Rep. 2017, 7, 3855. [Google Scholar] [CrossRef] [PubMed]
- Hutter-Schmid, B.; Humpel, C. Primary mouse brain pericytes isolated from transgenic Alzheimer mice spontaneously differentiate into a CD11b+ microglial-like cell type in vitro. Exp. Gerontol. 2018, 112, 30–37. [Google Scholar] [CrossRef]
- Klement, W.; Garbelli, R.; Zub, E.; Rossini, L.; Tassi, L.; Girard, B.; Blaquiere, M.; Bertaso, F.; Perroy, J.; de Bock, F.; et al. Seizure progression and inflammatory mediators promote pericytosis and pericyte-microglia clustering at the cerebrovasculature. Neurobiol. Dis. 2018, 113, 70–81. [Google Scholar] [CrossRef]
- Ozen, I.; Deierborg, T.; Miharada, K.; Padel, T.; Englund, E.; Genové, G.; Paul, G. Brain pericytes acquire a microglial phenotype after stroke. Acta Neuropathol. 2014, 128, 381–396. [Google Scholar] [CrossRef]
- Villasenor, R.; Kuennecke, B.; Ozmen, L.; Ammann, M.; Kugler, C.; Grüninger, F.; Loetscher, H.; Freskgård, P.-O.; Collin, L. Region-specific permeability of the blood-brain barrier upon pericyte loss. J. Cereb. Blood Flow. Metab. 2017, 37, 3683–3694. [Google Scholar] [CrossRef]
- Chow, B.W.; Gu, C. Gradual Suppression of Transcytosis Governs Functional Blood-Retinal Barrier Formation. Neuron 2017, 93, 1325–1333. [Google Scholar] [CrossRef]
- Lecuyer, M.A.; Kebir, H.; Prat, A. Glial influences on BBB functions and molecular players in immune cell trafficking. Biochim. Biophys. Acta 2016, 1862, 472–482. [Google Scholar] [CrossRef]
- Cannella, B. The adhesion molecule and cytokine profile of multiple sclerosis lesions. Ann. Neurol. 1995, 37, 424–435. [Google Scholar] [CrossRef]
- Losy, J. Increased serum levels of soluble PECAM-1 in multiple sclerosis patients with brain gadolinium-enhancing lesions. J. Neuroimmunol. 1999, 99, 169–172. [Google Scholar] [CrossRef]
- Losy, J. Is MS an inflammatory or primary degenerative disease? Neural Transm. 2013, 120, 1459–1462. [Google Scholar] [CrossRef] [PubMed]
- Charabati, M.; Grasmuck, C.; Ghannam, S.; Bourbonnière, L.; Fournier, A.P.; Lécuyer, M.A.; Tastet, O.; Kebir, H.; Rébillard, R.M.; Hoornaert, C.; et al. DICAM promotes TH17 lymphocyte trafficking across the blood-brain barrier during autoimmune neuroinflammation. Sci. Transl. Med. 2022, 14, eabj0473. [Google Scholar] [CrossRef]
- Charabati, M.; Zandee, S.; Fournier, A.P.; Tastet, O.; Thai, K.; Zaminpeyma, R.; Lécuyer, M.A.; Bourbonnière, L.; Larouche, S.; Klement, W.; et al. MCAM+ brain endothelial cells contribute to neuroinflammation by recruiting pathogenic CD4+ T lymphocytes. Brain 2023, 146, 1483–1495. [Google Scholar] [CrossRef]
- Yonezawa, T.; Ohtsuka, A.; Yoshitaka, T.; Hirano, S.; Nomoto, H.; Yamamoto, K.; Ninomiya, Y. Limitrin, a novel immunoglobulin superfamily protein localized to glia limitans formed by astrocyte endfeet. Glia 2003, 44, 190–204. [Google Scholar] [CrossRef]
- Puthenparampil, M.; Marin, A.; Zanotelli, G.; Mauceri, V.; De Napoli, F.; Gaggiola, M.; Miscioscia, A.; Ponzano, M.; Bovis, F.; Perini, P.; et al. Blood-brain barrier damage associates with glia-related cytokines in the cerebrospinal fluid of patients with multiple sclerosis. Mult. Scler. Relat. Disord. 2024, 82, 105403. [Google Scholar] [CrossRef]
- Nagata, S.; Yamasaki, R.; Takase, E.O.; Iida, K.; Watanabe, M.; Masaki, K.; Wijering, M.H.C.; Yamaguchi, H.; Kira, J.-I.; Isobe, N. Iguratimod ameliorates the severity of secondary progressive multiple sclerosis in model mice by directly inhibiting IL-6 production and Th17 cell migration via mitigation of glial inflammation. Biology 2023, 12, 1217. [Google Scholar] [CrossRef]
- Ashley, S.L.; Pretto, C.D.; Stier, M.T.; Kadiyala, P.; Castro-Jorge, L.; Hsu, T.-H.; Doherty, R.; Carnahan, K.E.; Castro, M.G.; Lowenstein, P.R.; et al. Matrix metalloproteinase activity in infections by an encephalitic virus, mouse adenovirus type 1. J. Virol. 2017, 91, e01412–e01416. [Google Scholar] [CrossRef]
- Szychowski, K.A.; Gmiński, J. Impact of elastin-derived VGVAPG peptide on bidirectional interaction between peroxisome proliferator-activated receptor gamma (Pparγ) and beta-galactosidase (β-Gal) expression in mouse cortical astrocytes in vitro. Naunyn Schmiedebergs Arch. Pharmacol. 2019, 392, 405–413. [Google Scholar] [CrossRef]
- Wójtowicz, A.K.; Sitarz-Głownia, A.M.; Wnuk, A.; Kajta, M.; Szychowski, K.A. Involvement of the peroxisome proliferator-activated receptor gamma (Pparγ) and matrix metalloproteinases-2 and -9 (Mmp-2 and -9) in the mechanism of action of di(2-ethylhexyl)phthalate (DEHP) in cultured mouse brain astrocytes and neurons. Toxicol. In Vitro 2023, 92, 105639. [Google Scholar] [CrossRef]
- Szychowski, K.A.; Skóra, B.; Wójtowicz, A.K. Elastin-Derived Peptides in the Central Nervous System: Friend or Foe. Cell. Mol. Neurobiol. 2022, 42, 2473–2487. [Google Scholar] [CrossRef]
- Gallo, V.; Armstrong, R.C. Myelin repair strategies: A cellular view. Curr. Opin. Neurol. 2008, 21, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Ballester, A.; Guijarro, A.; Bravo, B.; Hernández, J.; Murillas, R.; Gallego, M.I.; Ballester, S. Hedgehog Signalling Modulates Immune Response and Protects against Experimental Autoimmune Encephalomyelitis. Int. J. Mol. Sci. 2022, 23, 3171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Qu, Y.; Song, H.H.; Cheng, G.; Lu, F.; Cui, T.T.; Gong, Y.; Ding, X.L.; Yang, Y.; Zhang, Q.; et al. Isoliquiritigenin alleviates experimental autoimmune encephalomyelitis by modulating in-flammatory and neuroprotective reactive astrocytes. Biomed. Pharmacother. 2024, 178, 117188. [Google Scholar] [CrossRef]
- Xu, N.; Bai, Y.; Han, X.; Yuan, J.; Wang, L.; He, Y.; Yang, L.; Wu, H.; Shi, H.; Wu, X. Taurochenodeoxycholic acid reduces astrocytic neuroinflammation and alleviates experimental autoimmune encephalomyelitis in mice. Immunobiology 2023, 228, 152388. [Google Scholar] [CrossRef]
- Masaki, K.; Suzuki, S.O.; Matsushita, T.; Matsuoka, T.; Imamura, S.; Yamasaki, R.; Suzuki, M.; Suenaga, T.; Iwaki, T.; Kira, J.-I. Connexin 43 astrocytopathy linked to rapidly progressive multiple sclerosis and neuromyelitis optica. PLoS ONE 2013, 8, e72919. [Google Scholar] [CrossRef]
- Markoullis, K.; Sargiannidou, I.; Schiza, N.; Hadjisavvas, A.; Roncaroli, F.; Reynolds, R.; Kleopa, K.A. Gap junction pathology in multiple sclerosis lesions and normal-appearing white matter. Acta Neuropathol. 2012, 123, 873–886. [Google Scholar] [CrossRef]
- Takase, E.O.; Yamasaki, R.; Nagata, S.; Watanabe, M.; Masaki, K.; Yamaguchi, H.; Kira, J.-I.; Takeuchi, H.; Isobe, N. Astroglial connexin 43 is a novel therapeutic target for chronic multiple sclerosis model. Sci. Rep. 2024, 14, 10877. [Google Scholar] [CrossRef]
- Oh, J.; Kwon, T.W.; Choi, J.H.; Kim, Y.; Moon, S.K.; Nah, S.Y.; Cho, I.H. Ginsenoside-Re inhibits experimental autoimmune encephalomyelitis as a mouse model of multiple sclerosis by downregulating TLR4/MyD88/NF-κB signaling pathways. Phytomedicine 2024, 122, 155065. [Google Scholar] [CrossRef]
- Gao, D.; Zheng, C.C.; Hao, J.P.; Yang, C.C.; Hu, C.Y. Icariin ameliorates behavioral deficits and neuropathology in a mouse model of multiple sclerosis. Brain Res. 2023, 1804, 148267. [Google Scholar] [CrossRef]
- Hong, S.; Niu, M.; Meng, D.; Li, A.; Dong, Q.; Zhang, J.; Tian, X.; Lu, S.; Wang, Y. High-density lipoprotein reduces microglia activation and protects against experimental autoimmune encephalomyelitis in mice. Int. Immunopharmacol. 2022, 105, 108566. [Google Scholar] [CrossRef]
- Liu, N.; Yu, W.; Sun, M.; Li, X.; Zhang, W.; Wang, M. Dabrafenib mitigates the neuroinflammation caused by ferroptosis in experimental autoimmune encephalomyelitis by up regulating Axl receptor. Eur. J. Pharmacol. 2024, 973, 176600. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Lu, Q.; Zhao, Y.; Wang, X.; Ding, G.; Wang, Y.; Cheng, X. Microglia-astrocyte crosstalk is regulated by Astragalus polysaccharides mediated through suppression of Sema4D-PlexinB2 signaling in experimental autoimmune encephalomyelitis. Brain Res. 2024, 1845, 149275. [Google Scholar] [CrossRef] [PubMed]
- Balasa, R.; Barcutean, L.; Mosora, O.; Manu, D. Reviewing the Significance of Blood-Brain Barrier Disruption in Multiple Sclerosis Pathology and Treatment. Int. J. Mol. Sci. 2021, 22, 8370. [Google Scholar] [CrossRef] [PubMed]
- Pyka-Fosciak, G.; Lis, G.J.; Litwin, J.A. Effect of natalizumab treatment on metalloproteinases and their inhibitors in a mouse model of multiple sclerosis. J. Physiol. Pharmacol. 2020, 71, 265–273. [Google Scholar]
- Bloomgren, G.; Richman, S.; Hotermans, C.; Subramanyam, M.; Goelz, S.; Natarajan, A.; Lee, S.; Plavina, T.; Scanlon, J.V.; Sandrock, A.; et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N. Engl. J. Med. 2012, 366, 1870–1880. [Google Scholar] [CrossRef]
- Ho, P.R.; Koendgen, H.; Campbell, N.; Haddock, B.; Richman, S.; Chang, I. Risk of natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: A retrospective analysis of data from four clinical studies. Lancet Neurol. 2017, 16, 925–933. [Google Scholar] [CrossRef]
- Oshima, Y.; Tanimoto, T.; Yuji, K.; Tojo, A. Drug-associated progressive multifocal leukoencephalopathy in multiple sclerosis patients. Mult. Scler. 2019, 25, 1141–1149. [Google Scholar] [CrossRef]
- Major, E.O.; Yousry, T.A.; Clifford, D.B. Pathogenesis of progressive multifocal leukoencephalopathy and risks associated with treatments for multiple sclerosis: A decade of lessons learned. Lancet Neurol. 2018, 17, 467–480. [Google Scholar] [CrossRef]
- Cortese, I.; Reich, D.S.; Nath, A. Progressive multifocal leukoencephalopathy and the spectrum of JC virus-related disease. Nat. Rev. Neurol. 2021, 17, 37–51. [Google Scholar] [CrossRef]
- Bernard-Valnet, R.; Koralnik, I.J.; Du Pasquier, R. Advances in Treatment of Progressive Multifocal Leukoencephalopathy. Ann. Neurol. 2021, 90, 865–873. [Google Scholar] [CrossRef]
- Janosschka, C.; Lindner, M.; Koppers, N.; Starost, L.; Liebmann, M.; Eschborn, M.; Schneider-Hohendorf, T.; Windener, F.; Schafflick, D.; Fleck, A.K.; et al. Enhanced pathogenicity of Th17 cells due to natalizumab treatment: Implications for MS disease rebound. Proc. Natl. Acad. Sci. USA 2023, 120, e2209944120. [Google Scholar] [CrossRef] [PubMed]
- Sica, F.; Centonze, D.; Buttari, F. Fingolimod Immune Effects Beyond Its Sequestration Ability. Neurol. Ther. 2019, 8, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Spampinato, S.F.; Merlo, S.; Sano, Y.; Kanda, T.; Sortino, M.A. Protective effect of the sphingosine-1 phosphate receptor agonist siponimod on disrupted blood brain barrier function. Biochem. Pharmacol. 2021, 186, 114465. [Google Scholar] [CrossRef] [PubMed]
- Spampinato, S.F.; Obermeier, B.; Cotleur, A.; Love, A.; Takeshita, Y.; Sano, Y.; Kanda, T.; Ransohoff, R.M. Sphingosine 1 Phosphate at the Blood Brain Barrier: Can the Modulation of S1P Receptor 1 Influence the Response of Endothelial Cells and Astrocytes to Inflammatory Stimuli? PLoS ONE 2015, 10, e0133392. [Google Scholar] [CrossRef]
- Lennon, V.A.; Wingerchuk, D.M.; Kryzer, T.J.; Pittock, S.J.; Lucchinetti, C.F.; Fujihara, K.; Nakashima, I.; Weinshenker, B.G. A serum autoantibody marker of neuromyelitis optica: Distinction from multiple sclerosis. Lancet 2004, 364, 2106–2112. [Google Scholar] [CrossRef]
- Lennon, V.A.; Kryzer, T.J.; Pittock, S.J.; Verkman, A.; Hinson, S.R. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J. Exp. Med. 2005, 202, 473–477. [Google Scholar] [CrossRef]
- Sabater, L.; Giralt, A.; Boronat, A.; Hankiewicz, K.; Blanco, Y.; Llufriu, S.; Alberch, J.; Graus, F.; Saiz, A. Cytotoxic effect of neuromyelitis optica antibody (NMO-IgG) to astrocytes: An in vitro study. J. Neuroimmunol. 2009, 215, 31–35. [Google Scholar] [CrossRef]
- Guo, Y.; Weigand, S.D.; Popescu, B.F.; Lennon, V.A.; Parisi, J.E.; Pittock, S.J.; Parks, N.E.; Clardy, S.L.; Howe, C.L.; Lucchinetti, C.F. Pathogenic implications of cerebrospinal fluid barrier pathology in neuromyelitis optica. Acta Neuropathol. 2017, 133, 597–612. [Google Scholar] [CrossRef]
- Uchida, T.; Mori, M.; Uzawa, A.; Masuda, H.; Muto, M.; Ohtani, R.; Kuwabara, S. Increased cerebrospinal fluid metalloproteinase-2 and interleukin-6 are associated with albumin quotient in neuromyelitis optica: Their possible role on blood-brain barrier disruption. Mult. Scler. 2017, 23, 1072–1084. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Chang, H.; Wang, H.; Xu, W.; Cong, H.; Zhang, X.; Liu, J.; Yin, L. NMO-IgG Induce Interleukin-6 Release via Activation of the NF-κB Signaling Pathway in Astrocytes. Neuroscience 2022, 496, 96–104. [Google Scholar] [CrossRef]
- Wang, Y.; Bian, J.; Yao, M.; Du, L.; Xu, Y.; Chang, H.; Cong, H.; Wei, Y.; Xu, W.; Wang, H.; et al. Targeting chemoattractant chemokine (C-C motif) ligand 2 derived from astrocytes is a promising therapeutic approach in the treatment of neuromyelitis optica spectrum disorders. Front. Immunol. 2023, 14, 1144532. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, F.; Schaller, K.L.; Owens, G.P.; Cotleur, A.C.; Kellner, D.; Takeshita, Y.; Obermeier, B.; Kryzer, T.J.; Sano, Y.; Kanda, T.; et al. Glucose-regulated protein 78 autoantibody associates with blood-brain barrier disruption in neuromyelitis optica. Sci. Transl. Med. 2017, 9, eaai9111. [Google Scholar] [CrossRef]
- Shimizu, F.; Takeshita, Y.; Hamamoto, Y.; Nishihara, H.; Sano, Y.; Honda, M.; Sato, R.; Maeda, T.; Takahashi, T.; Fujikawa, S.; et al. GRP 78 antibodies are associated with clinical phenotype in neuromyelitis optica. Ann. Clin. Transl. Neurol. 2019, 6, 2079–2087. [Google Scholar] [CrossRef]
- Gu, Y.; Zhong, M.; He, L.; Li, W.; Huang, Y.; Liu, J.; Chen, Y.; Xiao, Z. Epidemiology of antibody-positive autoimmune encephalitis in southwest China: A multicenter study. Front. Immunol. 2019, 10, 2611. [Google Scholar] [CrossRef]
- Gable, M.S.; Sheriff, H.; Dalmau, J.; Tilley, D.H.; Glaser, C.A. The frequency of autoimmune N-methyl-D-aspartate receptor encephalitis surpasses that of individual viral etiologies in young individuals enrolled in the California Encephalitis Project. Clin. Infect. Dis. 2012, 54, 899–904. [Google Scholar] [CrossRef]
- Wandinger, K.P.; Saschenbrecker, S.; Stoecker, W.; Dalmau, J. Anti-NMDA-receptor encephalitis: A severe, multistage, treatable disorder presenting with psychosis. J. Neuroimmunol. 2011, 231, 86–91. [Google Scholar] [CrossRef]
- Dalmau, J.; Tüzün, E.; Wu, H.Y.; Masjuan, J.; Rossi, J.E.; Voloschin, A.; Baehring, J.M.; Shimazaki, H.; Koide, R.; King, D.; et al. araneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann. Neurol. 2007, 61, 25–36. [Google Scholar] [CrossRef]
- Bersier, M.G.; Pena, C.; Rodríguez de Lores Arnaiz, G. The expression of NMDA receptor subunits in cerebral cortex and hippocampus is differentially increased by administration of endobain E, a Na+, K+-ATPase inhibitor. Neurochem. Res. 2008, 33, 66–72. [Google Scholar] [CrossRef]
- Hughes, E.G.; Peng, X.; Gleichman, A.J.; Lai, M.; Zhou, L.; Tsou, R.; Parsons, T.D.; Lynch, D.R.; Dalmau, J.; Balice-Gordon, R.J. Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. J. Neurosci. 2010, 30, 5866–5875. [Google Scholar] [CrossRef]
- Wagnon, I.; Hélie, P.; Bardou, I.; Regnauld, C.; Lesec, L.; Leprince, J.; Naveau, M.; Delaunay, B.; Toutirais, O.; Lemauff, B.; et al. Autoimmune encephalitis mediated by B-cell response against N-methyl-d-aspartate receptor. Brain 2020, 143, 2957–2972. [Google Scholar] [CrossRef]
- Al-Diwani, A.; Theorell, J.; Damato, V.; Bull, J.; McGlashan, N.; Green, E.; Kienzler, A.K.; Harrison, R.; Hassanali, T.; Campo, L.; et al. Cervical lymph nodes and ovarian teratomas as germinal centres in NMDA receptor-antibody encephalitis. Brain 2022, 145, 2742–2754. [Google Scholar] [CrossRef]
- Pan, H.; Oliveira, B.; Saher, G.; Dere, E.; Tapken, D.; Mitjans, M.; Seidel, J.; Wesolowski, J.; Wakhloo, D.; Klein-Schmidt, C.; et al. Uncoupling the widespread occurrence of anti-NMDAR1 autoantibodies from neuropsychiatric disease in a novel autoimmune model. Mol. Psychiatry 2019, 24, 1489–1501. [Google Scholar] [CrossRef]
- Gong, X.; Wang, N.; Zhu, H.; Tang, N.; Wu, K.; Meng, Q. Anti-NMDAR antibodies, the blood-brain barrier, and anti-NMDAR encephalitis. Front. Neurol. 2023, 14, 1283511. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, Y.; Cao, X.; Li, J.; Liao, X.; Wei, J.; Huang, W. The Clinical Features and Prognosis of Anti-NMDAR Encephalitis Depends on Blood Brain Barrier Integrity. Mult. Scler. Relat. Disord. 2021, 47, 102604. [Google Scholar] [CrossRef]
- Sharp, C.D.; Hines, I.; Houghton, J.; Warren, A.; Jackson, T.H.; Jawahar, A.; Nanda, A.; Elrod, J.W.; Long, A.; Chi, A.; et al. Glutamate causes a loss in human cerebral endothelial barrier integrity through activation of NMDA receptor. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H2592–H2598. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, Y.; Wei, J.; Huang, F.; Mao, F.; Nong, W.; Cao, X.; Huang, W. NMDA mediates disruption of blood-brain barrier permeability via Rho/ROCK signaling pathway. Neurochem. Int. 2022, 154, 105278. [Google Scholar] [CrossRef]
- Shu, Y.; Peng, F.; Zhao, B.; Liu, C.; Li, Q.; Li, H.; Wang, Y.; Jiang, Y.; Lu, T.; Wang, Q.; et al. Transfer of patient’s peripheral blood mononuclear cells (PBMCs) disrupts blood-brain barrier and induces anti-NMDAR encephalitis: A study of novel humanized PBMC mouse model. J. Neuroinflammation 2023, 20, 164. [Google Scholar] [CrossRef]
- Gong, Z.; Lao, D.; Wu, Y.; Li, T.; Lv, S.; Mo, X.; Huang, W. Inhibiting PI3K/Akt-Signaling pathway improves neurobehavior changes in anti-NMDAR encephalitis mice by ameliorating blood-brain barrier disruption and neuronal damage. Cell. Mol. Neurobiol. 2023, 43, 3623–3637. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Kirchhoff, F. NMDA Receptors in glia. Neuroscientist 2007, 13, 28–37. [Google Scholar] [CrossRef]
- Skowronska, K.; Obara-Michlewska, M.; Zielińska, M.; Albrecht, J. NMDA Receptors in Astrocytes: In Search for Roles in Neurotransmission and Astrocytic Homeostasis. Int. J. Mol. Sci. 2019, 20, 309. [Google Scholar] [CrossRef]
- Ismail, F.S.; Faustmann, P.M. Astrocytes and their potential role in anti-NMDA receptor encephalitis. Med. Hypotheses 2020, 139, 109612. [Google Scholar] [CrossRef] [PubMed]
- Ariño, H.; Armangué, T.; Petit-Pedrol, M.; Sabater, L.; Martinez-Hernandez, E.; Hara, M.; Lancaster, E.; Saiz, A.; Dalmau, J.; Graus, F. Anti-LGI1-associated cognitive impairment: Presentation and long-term outcome. Neurology 2016, 87, 759–765. [Google Scholar] [CrossRef] [PubMed]
- van Sonderen, A.; Thijs, R.D.; Coenders, E.C.; Jiskoot, L.C.; Sanchez, E.; De Bruijn, M.A.; van Coevorden-Hameete, M.H.; Wirtz, P.W.; Schreurs, M.W.; Sillevis Smitt, P.A.; et al. Anti-LGI1 encephalitis: Clinical syndrome and long-term follow-up. Neurology 2016, 87, 1449–1456. [Google Scholar] [CrossRef]
- Bien, C.G.; Vincent, A.; Barnett, M.H.; Becker, A.J.; Blümcke, I.; Graus, F.; Jellinger, K.A.; Reuss, D.E.; Ribalta, T.; Schlegel, J.; et al. Immunopathology of autoantibody-associated encephalitides: Clues for pathogenesis. Brain 2012, 135, 1622–1638. [Google Scholar] [CrossRef]
- Tröscher, A.R.; Klang, A.; French, M.; Quemada-Garrido, L.; Kneissl, S.M.; Bien, C.G.; Pákozdy, Á.; Bauer, J. Selective limbic blood-brain barrier breakdown in a feline model of limbic encephalitis with LGI1 antibodies. Front. Immunol. 2017, 8, 1364. [Google Scholar] [CrossRef]
- Qiao, S.; Li, H.; Cui, C.; Zhang, C.; Wang, A.; Jiang, W.; Zhang, S. CSF Findings in Chinese patients with NMDAR, LGI1 and GABABR antibody-associated encephalitis. J. Inflamm. Res. 2024, 17, 1765–1776. [Google Scholar] [CrossRef]
- Jeltsch-David, H.; Muller, S. Neuropsychiatric systemic lupus erythematosus: Pathogenesis and biomarkers. Nat. Rev. Neurol. 2014, 10, 575–596. [Google Scholar] [CrossRef]
- Bertsias, G.K.; Boumpas, D.T. Pathogenesis, diagnosis and management of neuropsychiatric SLE manifestations. Nat. Rev. Rheumatol. 2010, 6, 358–367. [Google Scholar] [CrossRef]
- Stock, A.D.; Wen, J.; Putterman, C. Neuropsychiatric lupus, the blood brain barrier, and the TWEAK/Fn14 pathway. Front. Immunol. 2013, 4, 484. [Google Scholar] [CrossRef]
- Mader, S.; Brimberg, L.; Diamond, B. The role of brain-reactive autoantibodies in brain pathology and cognitive iImpairment. Front. Immunol. 2017, 8, 1101. [Google Scholar] [CrossRef]
- Kowal, C.; DeGiorgio, L.A.; Nakaoka, T.; Hetherington, H.; Huerta, P.T.; Diamond, B.; Volpe, B.T. Cognition and immunity; antibody impairs memory. Immunity 2004, 21, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Hirohata, S.; Arinuma, Y.; Yanagida, T.; Yoshio, T. Blood-brain barrier damages and intrathecal synthesis of anti-N-methyl-D-aspartate receptor NR2 antibodies in diffuse psychiatric/neuropsychological syndromes in systemic lupus erythematosus. Arthritis Res. Ther. 2014, 16, R77. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.; Wang, X.; Xu, J.; Jin, C.; Chen, J.; Wang, X.; Wang, J.; Qin, L.; Yang, P. Pristane induced lupus mice as a model for neuropsychiatric lupus (NPSLE). Behav. Brain Funct. 2023, 19, 3. [Google Scholar] [CrossRef]
- Nikolopoulos, D.; Manolakou, T.; Polissidis, A.; Filia, A.; Bertsias, G.; Koutmani, Y.; Boumpas, D.T. Microglia activation in the presence of intact blood-brain barrier and disruption of hippocampal neurogenesis via IL-6 and IL-18 mediate early diffuse neuropsychiatric lupus. Ann. Rheum. Dis. 2023, 82, 646–657. [Google Scholar] [CrossRef]
- Karla, S.; Silman, A.; Akman-Demir, G.; Bohlega, S.; Borhani-Haghighi, A.; Constantinescu, C.S.; Houman, H.; Mahr, A.; Salvarani, C.; Sfikakis, P.P.; et al. Diagnosis and management of Neuro-Behçet’s disease: International consensus recommendations. J. Neurol. 2014, 261, 1662–1676. [Google Scholar]
- Borhani-Haghighi, A.; Kardeh, B.; Banerjee, S.; Yadollahikhales, G.; Safari, A.; Sahraian, M.A.; Shapiro, L. Neuro-Behcet’s disease: An update on diagnosis, differential diagnoses, and treatment. Mult. Scler. Relat. Disord. 2020, 39, 101906. [Google Scholar] [CrossRef]
- Hirohata, S. Histopathology of central nervous system lesions in Behçet’s disease. J. Neurol. Sci. 2008, 267, 41–47. [Google Scholar] [CrossRef]
- Zhan, H.; Cheng, L.; Liu, Y.; Xu, H.; Feng, X.; Liu, Y.; Li, H.; Li, Z.; Wang, S.; Jin, H.; et al. Significance of immunoglobulins synthesis with central nervous system involvement in Neuro-Behçet’s disease. Clin. Chim. Acta 2024, 559, 119681. [Google Scholar] [CrossRef]
- Tobón, G.J.; Alard, J.É.; Youinou, P.; Jamin, C. Are autoantibodies triggering endothelial cell apoptosis really pathogenic? Autoimmun. Rev. 2009, 8, 605–610. [Google Scholar] [CrossRef]
- Chauhan, S.K.; Tripathy, N.K.; Nityanand, S. Antigenic targets and pathogenicity of anti-aortic endothelial cell antibodies in Takayasu arteritis. Arthritis Rheum. 2006, 54, 2326–2333. [Google Scholar] [CrossRef]
- Jamin, C.; Dugué, C.; Alard, J.É.; Jousse, S.; Saraux, A.; Guillevin, L.; Piette, J.C.; Youinou, P. Induction of endothelial cell apoptosis by the binding of anti-endothelial cell antibodies to Hsp60 in vasculitis-associated systemic autoimmune diseases. Arthritis Rheum. 2005, 52, 4028–4038. [Google Scholar] [CrossRef] [PubMed]
- Regent, A.; Lofek, S.; Dib, H.; Bussone, G.; Tamas, N.; Federici, C.; Broussard, C.; Guillevin, L.; Mouthon, L. Identification of target antigens of anti-endothelial cell antibodies in patients with anti-neutrophil cytoplasmic antibody-associated vasculitides: A proteomic approach. Clin. Immunol. 2014, 153, 123–135. [Google Scholar] [CrossRef]
- Fang, B.; McKeon, A.; Hinson, S.R.; Kryzer, T.J.; Pittock, S.J.; Aksamit, A.J.; Lennon, V.A. Autoimmune Glial Fibrillary Acidic Protein Astrocytopathy: A Novel Meningoencephalomyelitis. JAMA Neurol. 2016, 73, 1297–1307. [Google Scholar] [CrossRef]
- Flanagan, E.P.; Hinson, S.R.; Lennon, V.A.; Fang, B.; Aksamit, A.J.; Morris, P.P.; Basal, E.; Honorat, J.A.; Alfugham, N.B.; Linnoila, J.J.; et al. Glial fibrillary acidic protein immunoglobulin G as biomarker of autoimmune astrocytopathy: Analysis of 102 patients. Ann. Neurol. 2017, 81, 298–309. [Google Scholar] [CrossRef]
- Iorio, R.; Damato, V.; Evoli, A.; Gessi, M.; Gaudino, S.; Di Lazzaro, V.; Spagni, G.; A Sluijs, J.; Hol, E.M. Clinical and immunological characteristics of the spectrum of GFAP autoimmunity: A case series of 22 patients. J. Neurol. Neurosurg. Psychiatry 2018, 89, 138–146. [Google Scholar] [CrossRef]
- Liao, H.; Chen, Q.; Zhang, M.; Chen, W. MRI features and evolution of autoimmune glial fibrillary acidic protein astrocytopathy: A retrospective cross-sectional and longitudinal study. Mult. Scler. Relat. Disord. 2022, 58, 103512. [Google Scholar] [CrossRef]
- Long, Y.; Liang, J.; Xu, H.; Huang, Q.; Yang, J.; Gao, C.; Qiu, W.; Lin, S.; Chen, X. Autoimmune glial fibrillary acidic protein astrocytopathy in Chinese patients: A retrospective study. Eur. J. Neurol. 2018, 25, 477–483. [Google Scholar] [CrossRef]
- Chen, A.Q.; Fang, Z.; Chen, X.L.; Yang, S.; Zhou, Y.F.; Mao, L.; Xia, Y.P.; Jin, H.J.; Li, Y.N.; You, M.F.; et al. Microglia-derived TNF-α mediates endothelial necroptosis aggravating blood brain-barrier disruption after ischemic stroke. Cell Death Dis. 2019, 10, 487. [Google Scholar] [CrossRef]
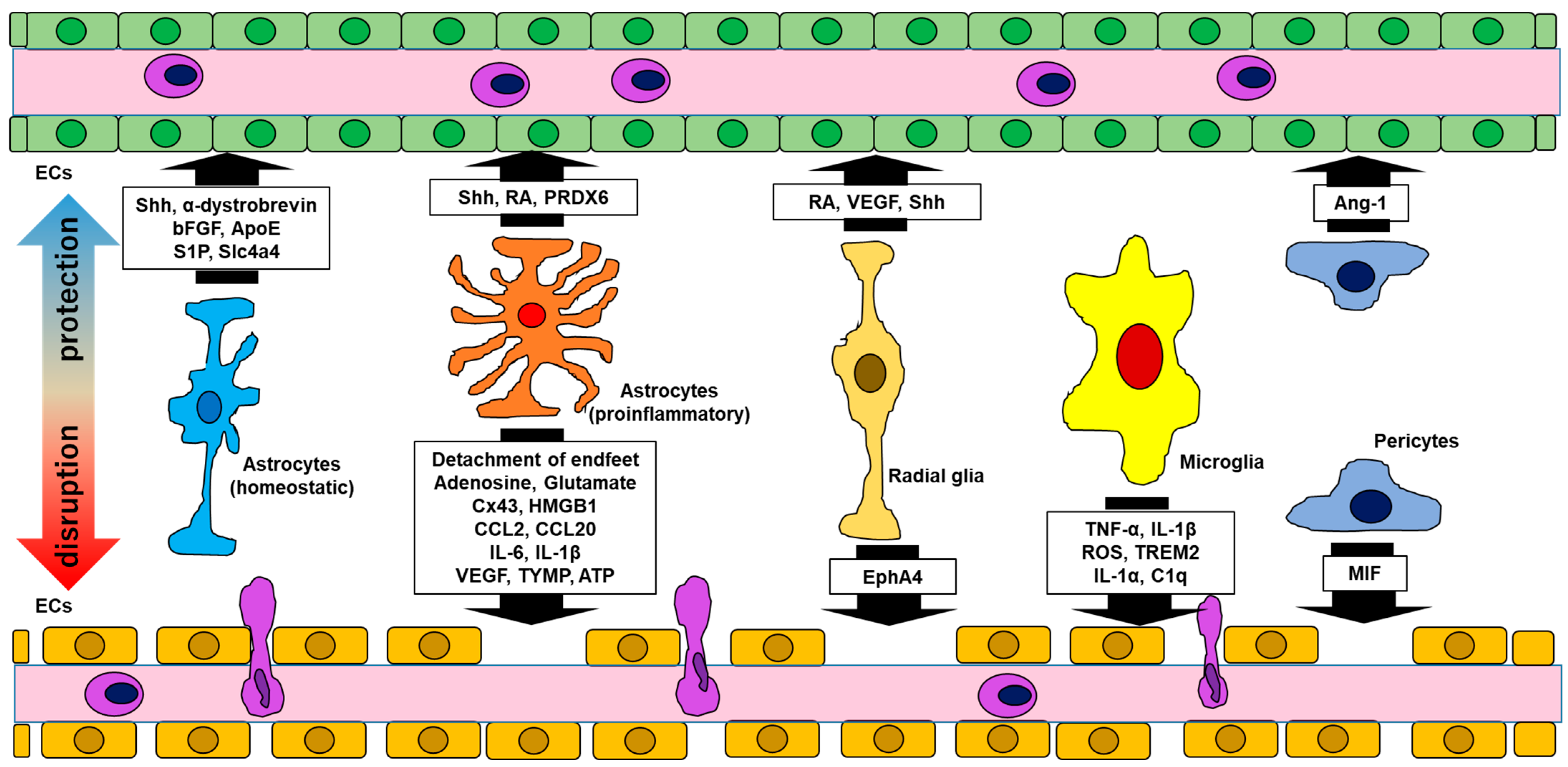
| Functional Molecules | Impact on the BBB | Related Diseases | Reference | |
|---|---|---|---|---|
| Astrocytes (homeostatic) | Shh α-dystrobrevin bFGF ApoE S1P Slc4a4 | BBB integrity ↑ BBB integrity ↑ L-glucose permeability ↓ BBB stability BBB integrity ↑ BBB stability (related to CCL2 ↓) | - - - - - - | Alvarez 2011 [4] Lien 2012 [11] Sobue 1999 [12] Bell 2012 [13] Garcia 2001 [14] Ye 2024 [15] |
| Astrocytes (proinflammatory) | Shh ↑ RA ↑ PRDX6 ↑ ------------------------------------------ Detachment of endfeet Cx43 ↓(acute phase) Cx43 ↑, Adenosine ↑, Glutamate ↑ (chronic phase) HMGB1 ↑ CCL2 ↑ CCL20 ↑ IL-6 ↑ IL-1β ↑ VEGF ↑, TYMP ↑ ATP ↑ | BBB integrity ↑ BBB protection (integration ↑) BBB protection --------------------------------------------------- BBB permeability ↑ BBB permeability ↑ or ↓ BBB permeability ↑ Claudin5 ↓, ICAM-1 ↑, VCAM-1 ↑ BBB integrity ↓ BBB integrity ↓ VE-cadherin ↓, occluding ↓, claudin5 ↓ (BBB integrity ↓) Shh ↓, ICAM-1 ↑ BBB integrity ↓ (claudin5 ↓, occluding ↓) BBB permeability ↑ | MS MS MS MS MS, NMOSD MS, NMOSD MS MS, NMOSD GFAPA MS MS MS NMDARE | Alvarez 2011 [4], 2013 [29]; Sirko 2013 [30] Mizee 2014 [31] Yun 2015 [32] Eilam 2018 [33]; Prineas & Lee 2019 [34] Brand-Schieber 2005 [35]; Une 2021 [36] Yamasaki 2023 [37]; Bynoe 2015 [38]; Vazana 2016 [39] Shi 2022 [40] Xiao 2020 [41]; Spanpinato 2022 [42] Kimura 2019 [43]; Zheng 2023 [44] Chang 2015 [45]; Rochfort 2014 [46] Wang 2014 [47], Abadier 2015 [48] Argaw 2012 [49]; Chapouly 2015 [50] Bennett 2012 [51]; Yamaski 2023 [37]; Bynoe 2015 [38] |
| Radial glia | RA VEGF Shh ------------------------------------------ EphA4 | BBB development BBB development BBB integrity↑ -------------------------------------------------- BBB dysfunction | - - - - | Mizee 2013 [52] Sentilhes 2010 [53]; Silva 2019 [54] Radonjic 2014 [55] Cheng 2002 [56]; Chen 2018 [57] |
| Microglia | TNF-α ↑, IL-1β ↑ ROS ↑ IL-1α ↑, TNF-α ↑, C1q ↑ TREM2↑ | BBB integrity ↓ (ZO-1 ↓, occluding ↓) BBB integrity ↓ (VE-cadherin ↓, occludin↓, claudin5 ↓) (inducing proinflammatory astrocytes) BBB integrity ↓ | MS MS MS NMDARE | Nishioku 2010 [58]; Shigemoto-Mogami 2018 [59] Sumi 2010 [60]; Rochfort 2014 [46]; Schreibelt 2007 [61] Liddelow 2017 [62] Chang 2023 [63] |
| Pericytes | Ang-1 ↑ MIF ↑ | BBB stability ↑ Endothelial cell apoptosis ↑ | - (Inflammation) | Gaengel 2009 [64] Stark 2013 [65]; Li 2023 [66] |
| Endothelial cells | MMP2/MMP9 ↑ IL-6 ↑ IL-1β ↑ IL-8 ↑ CCL2 ↑ NMDAR ↑ | VCAM-1 ↑, BBB integrity ↓ VE-cadherin ↓, occluding ↓, claudin5 ↓ (BBB integrity ↓) Shh ↓, ICAM-1 ↑ BBB integrity ↓ BBB integrity ↓ BBB integrity ↓ | NMOSD, NMDARE NMOSD, Vasculitis NMOSD, Vasculitis Vasculitis Vasculitis NMDARE | Tasaki 2014 [67]; Chen 2016 [68] Covo-Calvo 2020 [69]; Muller Kobold 1999 [70]; Del Papa 1996 [71] Del Papa 1996 [71] Del Papa 1996 [71]; Sun 2016 [72] Del Papa 1996 [71] Huang 2024 [73] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagata, S.; Yamasaki, R. The Involvement of Glial Cells in Blood–Brain Barrier Damage in Neuroimmune Diseases. Int. J. Mol. Sci. 2024, 25, 12323. https://doi.org/10.3390/ijms252212323
Nagata S, Yamasaki R. The Involvement of Glial Cells in Blood–Brain Barrier Damage in Neuroimmune Diseases. International Journal of Molecular Sciences. 2024; 25(22):12323. https://doi.org/10.3390/ijms252212323
Chicago/Turabian StyleNagata, Satoshi, and Ryo Yamasaki. 2024. "The Involvement of Glial Cells in Blood–Brain Barrier Damage in Neuroimmune Diseases" International Journal of Molecular Sciences 25, no. 22: 12323. https://doi.org/10.3390/ijms252212323
APA StyleNagata, S., & Yamasaki, R. (2024). The Involvement of Glial Cells in Blood–Brain Barrier Damage in Neuroimmune Diseases. International Journal of Molecular Sciences, 25(22), 12323. https://doi.org/10.3390/ijms252212323





