The Effects of SGLT2 Inhibitors on Blood Pressure and Other Cardiometabolic Risk Factors
Abstract
1. Introduction
2. The Effect of SGLT2 Inhibitors on Blood Pressure
3. The Effect of SGLT2 Inhibitors on Body Weight
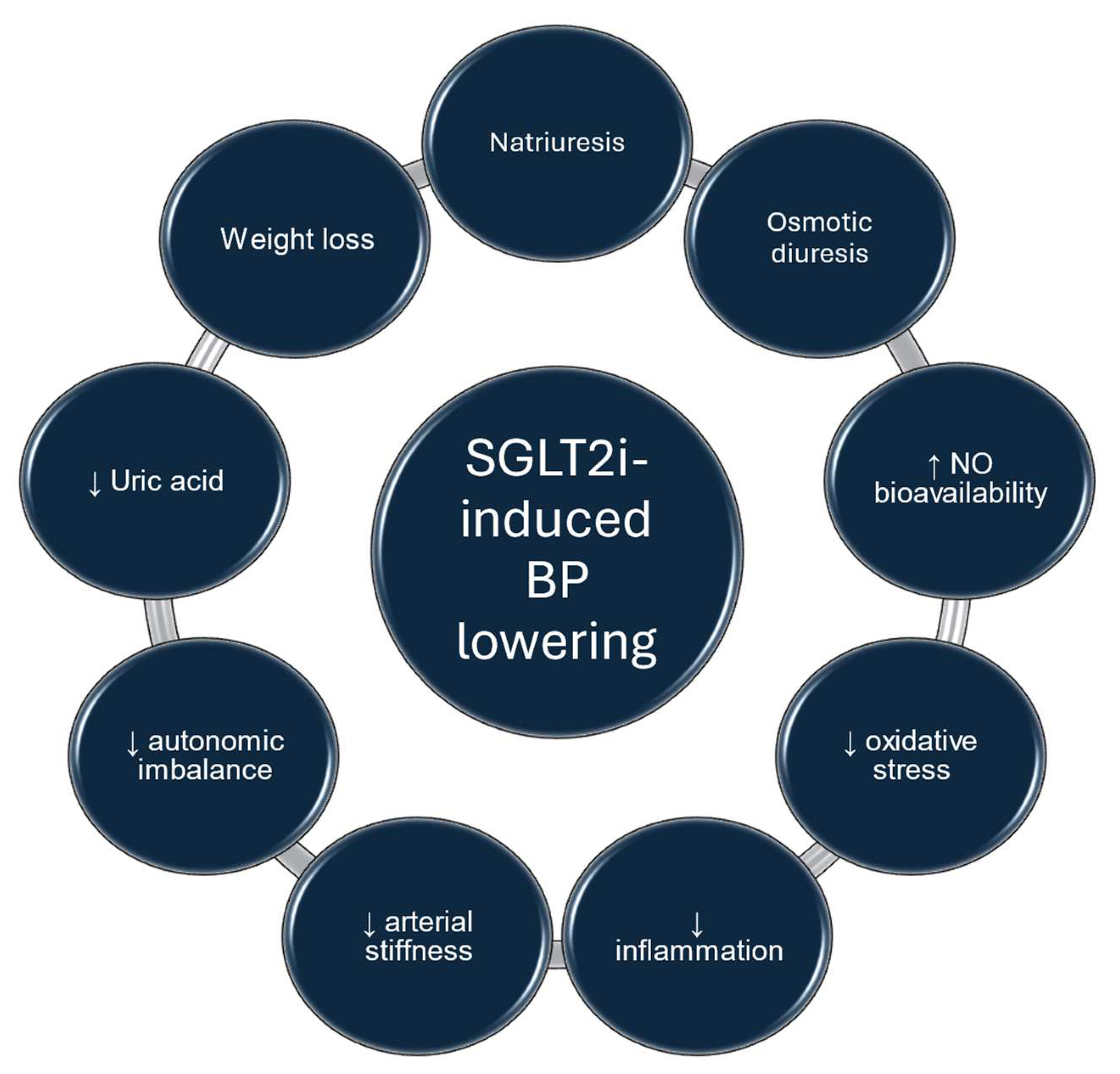
4. Pathophysiologic Mechanisms Involved in the BP-Lowering Effect of SGLT2 Inhibitors
5. Proposed Pathophysiologic Mechanisms Involved in Body Weight Reduction Though SGLT2 Inhibition
6. Effect of SGLT2 Inhibitors on Lipid Metabolism
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wright, E.M.; Loo, D.D.F.; Hirayama, B.A. Biology of Human Sodium Glucose Transporters. Physiol. Rev. 2011, 91, 733–794. [Google Scholar] [CrossRef]
- Zelniker, T.A.; Braunwald, E. Mechanisms of Cardiorenal Effects of Sodium-Glucose Cotransporter 2 Inhibitors. J. Am. Coll. Cardiol. 2020, 75, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan Sridhar, V.; Ambinathan, J.P.N.; Kretzler, M.; Pyle, L.L.; Bjornstad, P.; Eddy, S.; Cherney, D.Z.; Reich, H.N. Renal SGLT MRNA Expression in Human Health and Disease: A Study in Two Cohorts. Am. J. Physiol. Ren. Physiol. 2019, 317, F1224–F1230. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.X.; Levi, J.; Luo, Y.; Myakala, K.; Herman-Edelstein, M.; Qiu, L.; Wang, D.; Peng, Y.; Grenz, A.; Lucia, S.; et al. SGLT2 Protein Expression Is Increased in Human Diabetic Nephropathy. J. Biol. Chem. 2017, 292, 5335–5348. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Reeves, W.B.; Awad, A.S. Pathophysiology of Diabetic Kidney Disease: Impact of SGLT2 Inhibitors. Nat. Rev. Nephrol. 2021, 17, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.; Bakris, G.L.; Molitch, M.; Smulders, M.; Tian, J.; Williams, L.A.; Andress, D.L. Prevalence of Abnormal Serum Vitamin D, PTH, Calcium, and Phosphorus in Patients with Chronic Kidney Disease: Results of the Study to Evaluate Early Kidney Disease. Kidney Int. 2007, 71, 31–38. [Google Scholar] [CrossRef]
- Bakris, G.L.; Fonseca, V.A.; Sharma, K.; Wright, E.M. Renal Sodium–Glucose Transport: Role in Diabetes Mellitus and Potential Clinical Implications. Kidney Int. 2009, 75, 1272–1277. [Google Scholar] [CrossRef]
- Rahmoune, H.; Thompson, P.W.; Ward, J.M.; Smith, C.D.; Hong, G.; Brown, J. Glucose Transporters in Human Renal Proximal Tubular Cells Isolated from the Urine of Patients With Non–Insulin-Dependent Diabetes. Diabetes 2005, 54, 3427–3434. [Google Scholar] [CrossRef]
- De Vriese, A.S.; Sethi, S.; Nath, K.A.; Glassock, R.J.; Fervenza, F.C. Differentiating Primary, Genetic, and Secondary FSGS in Adults: A Clinicopathologic Approach. J. Am. Soc. Nephrol. 2018, 29, 759–774. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, J.; Han, R.; Fan, D.; Dong, X.; Luan, Z.; Xiang, R.; Zhao, M.; Yang, J. Efficacy and Safety of Sodium-glucose Cotransporter-2 Inhibitors versus Dipeptidyl Peptidase-4 Inhibitors as Monotherapy or Add-on to Metformin in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-analysis. Diabetes Obes. Metab. 2018, 20, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef] [PubMed]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner–La Rocca, H.-P.; Choi, D.-J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.V.; Claggett, B.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F.; et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2022, 387, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.-F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef]
- The EMPA-KIDNEY Collaborative Group; Herrington, W.G.; Staplin, N.; Wanner, C.; Green, J.B.; Hauske, S.J.; Emberson, J.R.; Preiss, D.; Judge, P.; Mayne, K.J.; et al. Empagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2023, 388, 117–127. [Google Scholar] [CrossRef]
- Pescariu, S.A.; Elagez, A.; Nallapati, B.; Bratosin, F.; Bucur, A.; Negru, A.; Gaita, L.; Citu, I.M.; Popa, Z.L.; Barata, P.I. Examining the Impact of Ertugliflozin on Cardiovascular Outcomes in Patients with Diabetes and Metabolic Syndrome: A Systematic Review of Clinical Trials. Pharmaceuticals 2024, 17, 929. [Google Scholar] [CrossRef] [PubMed]
- Cannon, C.P.; Pratley, R.; Dagogo-Jack, S.; Mancuso, J.; Huyck, S.; Masiukiewicz, U.; Charbonnel, B.; Frederich, R.; Gallo, S.; Cosentino, F.; et al. Cardiovascular Outcomes with Ertugliflozin in Type 2 Diabetes. N. Engl. J. Med. 2020, 383, 1425–1435. [Google Scholar] [CrossRef] [PubMed]
- Stevens, P.E.; Ahmed, S.B.; Carrero, J.J.; Foster, B.; Francis, A.; Hall, R.K.; Herrington, W.G.; Hill, G.; Inker, L.A.; Kazancıoğlu, R.; et al. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef]
- Mancia, G.; Kreutz, R.; Brunström, M.; Burnier, M.; Grassi, G.; Januszewicz, A.; Muiesan, M.L.; Tsioufis, K.; Agabiti-Rosei, E.; Algharably, E.A.E.; et al. 2023 ESH Guidelines for the Management of Arterial Hypertension the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension. J. Hypertens. 2023, 41, 1874–2071. [Google Scholar] [CrossRef] [PubMed]
- Forouzanfar, M.H.; Liu, P.; Roth, G.A.; Ng, M.; Biryukov, S.; Marczak, L.; Alexander, L.; Estep, K.; Hassen Abate, K.; Akinyemiju, T.F.; et al. Global Burden of Hypertension and Systolic Blood Pressure of at Least 110 to 115 Mm Hg, 1990–2015. JAMA 2017, 317, 165. [Google Scholar] [CrossRef] [PubMed]
- Milionis, H.J.; Liberopoulos, E.N.; Achimastos, A.; Elisaf, M.S.; Mikhailidis, D.P. Statins: Another Class of Antihypertensive Agents? J. Hum. Hypertens. 2006, 20, 320–335. [Google Scholar] [CrossRef]
- Tikkanen, I.; Narko, K.; Zeller, C.; Green, A.; Salsali, A.; Broedl, U.C.; Woerle, H.J. Empagliflozin Reduces Blood Pressure in Patients With Type 2 Diabetes and Hypertension. Diabetes Care 2015, 38, 420–428. [Google Scholar] [CrossRef]
- Weber, M.A.; Mansfield, T.A.; Cain, V.A.; Iqbal, N.; Parikh, S.; Ptaszynska, A. Blood Pressure and Glycaemic Effects of Dapagliflozin versus Placebo in Patients with Type 2 Diabetes on Combination Antihypertensive Therapy: A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Study. Lancet Diabetes Endocrinol. 2016, 4, 211–220. [Google Scholar] [CrossRef]
- Baker, W.L.; Smyth, L.R.; Riche, D.M.; Bourret, E.M.; Chamberlin, K.W.; White, W.B. Effects of Sodium-Glucose Co-Transporter 2 Inhibitors on Blood Pressure: A Systematic Review and Meta-Analysis. J. Am. Soc. Hypertens. 2014, 8, 262–275.e9. [Google Scholar] [CrossRef]
- Tsapas, A.; Karagiannis, T.; Kakotrichi, P.; Avgerinos, I.; Mantsiou, C.; Tousinas, G.; Manolopoulos, A.; Liakos, A.; Malandris, K.; Matthews, D.R.; et al. Comparative Efficacy of Glucose-lowering Medications on Body Weight and Blood Pressure in Patients with Type 2 Diabetes: A Systematic Review and Network Meta-analysis. Diabetes Obes. Metab. 2021, 23, 2116–2124. [Google Scholar] [CrossRef]
- Georgianos, P.I.; Agarwal, R. Ambulatory Blood Pressure Reduction With SGLT-2 Inhibitors: Dose-Response Meta-Analysis and Comparative Evaluation with Low-Dose Hydrochlorothiazide. Diabetes Care 2019, 42, 693–700. [Google Scholar] [CrossRef]
- Theofilis, P.; Vordoni, A.; Kalaitzidis, R.G. Novel Therapeutic Approaches in the Management of Chronic Kidney Disease: A Narrative Review. Postgrad. Med. 2023, 135, 543–550. [Google Scholar] [CrossRef]
- Teo, Y.H.; Teo, Y.N.; Syn, N.L.; Kow, C.S.; Yoong, C.S.Y.; Tan, B.Y.Q.; Yeo, T.; Lee, C.; Lin, W.; Sia, C. Effects of Sodium/Glucose Cotransporter 2 (SGLT2) Inhibitors on Cardiovascular and Metabolic Outcomes in Patients Without Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized-Controlled Trials. J. Am. Heart Assoc. 2021, 10, e019463. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yi, T.; Fan, F.; Qiu, L.; Wang, Z.; Weng, H.; Ma, W.; Zhang, Y.; Huo, Y. Effect of Sodium-Glucose Cotransporter-2 Inhibitors on Blood Pressure in Patients with Heart Failure: A Systematic Review and Meta-Analysis. Cardiovasc. Diabetol. 2022, 21, 139. [Google Scholar] [CrossRef] [PubMed]
- Cherney, D.Z.I.; Cooper, M.E.; Tikkanen, I.; Pfarr, E.; Johansen, O.E.; Woerle, H.J.; Broedl, U.C.; Lund, S.S. Pooled Analysis of Phase III Trials Indicate Contrasting Influences of Renal Function on Blood Pressure, Body Weight, and HbA1c Reductions with Empagliflozin. Kidney Int. 2018, 93, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Zanchi, A.; Pruijm, M.; Muller, M.-E.; Ghajarzadeh-Wurzner, A.; Maillard, M.; Dufour, N.; Bonny, O.; Wuerzner, G.; Burnier, M. Twenty-Four Hour Blood Pressure Response to Empagliflozin and Its Determinants in Normotensive Non-Diabetic Subjects. Front. Cardiovasc. Med. 2022, 9, 854230. [Google Scholar] [CrossRef] [PubMed]
- Kario, K.; Okada, K.; Kato, M.; Nishizawa, M.; Yoshida, T.; Asano, T.; Uchiyama, K.; Niijima, Y.; Katsuya, T.; Urata, H.; et al. Twenty-Four-Hour Blood Pressure–Lowering Effect of a Sodium-Glucose Cotransporter 2 Inhibitor in Patients with Diabetes and Uncontrolled Nocturnal Hypertension. Circulation 2019, 139, 2089–2097. [Google Scholar] [CrossRef]
- Kario, K.; Hoshide, S.; Okawara, Y.; Tomitani, N.; Yamauchi, K.; Ohbayashi, H.; Itabashi, N.; Matsumoto, Y.; Kanegae, H. Effect of Canagliflozin on Nocturnal Home Blood Pressure in Japanese Patients with Type 2 Diabetes Mellitus: The SHIFT-J Study. J. Clin. Hypertens. 2018, 20, 1527–1535. [Google Scholar] [CrossRef] [PubMed]
- Ferdinand, K.C.; Izzo, J.L.; Lee, J.; Meng, L.; George, J.; Salsali, A.; Seman, L. Antihyperglycemic and Blood Pressure Effects of Empagliflozin in Black Patients with Type 2 Diabetes Mellitus and Hypertension. Circulation 2019, 139, 2098–2109. [Google Scholar] [CrossRef]
- Papadopoulou, E.; Loutradis, C.; Tzatzagou, G.; Kotsa, K.; Zografou, I.; Minopoulou, I.; Theodorakopoulou, M.P.; Tsapas, A.; Karagiannis, A.; Sarafidis, P. Dapagliflozin Decreases Ambulatory Central Blood Pressure and Pulse Wave Velocity in Patients with Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J. Hypertens. 2021, 39, 749–758. [Google Scholar] [CrossRef]
- Kario, K.; Okada, K.; Murata, M.; Suzuki, D.; Yamagiwa, K.; Abe, Y.; Usui, I.; Tsuchiya, N.; Iwashita, C.; Harada, N.; et al. Effects of Luseogliflozin on Arterial Properties in Patients with Type 2 Diabetes Mellitus: The Multicenter, Exploratory LUSCAR Study. J. Clin. Hypertens. 2020, 22, 1585–1593. [Google Scholar] [CrossRef]
- Saito, Y.; Tanaka, A.; Imai, T.; Nakamura, I.; Kanda, J.; Matsuhisa, M.; Uehara, H.; Kario, K.; Kobayashi, Y.; Node, K.; et al. Long-Term Effects of Ipragliflozin on Blood Pressure in Patients with Type 2 Diabetes: Insights from the Randomized PROTECT Trial. Hypertens. Res. 2024, 47, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Ye, N.; Jardine, M.J.; Oshima, M.; Hockham, C.; Heerspink, H.J.L.; Agarwal, R.; Bakris, G.; Schutte, A.E.; Arnott, C.; Chang, T.I.; et al. Blood Pressure Effects of Canagliflozin and Clinical Outcomes in Type 2 Diabetes and Chronic Kidney Disease. Circulation 2021, 143, 1735–1749. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, M.; Toto, R.D.; Vart, P.; Umanath, K.; Luis Górriz, J.; Mark, P.B.; Mann, J.F.E.; Chertow, G.M.; McMurray, J.J.V.; Correa-Rotter, R.; et al. POS-255 Effect of Dapagliflozin on Blood Pressure in Patients with CKD: A Pre-Specified Analysis from DAPA-CKD. Kidney Int. Rep. 2022, 7, S112. [Google Scholar] [CrossRef]
- Mayne, K.J.; Staplin, N.; Keane, D.F.; Wanner, C.; Brenner, S.; Cejka, V.; Stegbauer, J.; Judge, P.K.; Preiss, D.; Emberson, J.; et al. Effects of Empagliflozin on Fluid Overload, Weight, and Blood Pressure in CKD. J. Am. Soc. Nephrol. 2024, 35, 202–215. [Google Scholar] [CrossRef]
- Barnett, A.H.; Mithal, A.; Manassie, J.; Jones, R.; Rattunde, H.; Woerle, H.J.; Broedl, U.C. Efficacy and Safety of Empagliflozin Added to Existing Antidiabetes Treatment in Patients with Type 2 Diabetes and Chronic Kidney Disease: A Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Diabetes Endocrinol. 2014, 2, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Kinguchi, S.; Wakui, H.; Ito, Y.; Kondo, Y.; Azushima, K.; Osada, U.; Yamakawa, T.; Iwamoto, T.; Yutoh, J.; Misumi, T.; et al. Improved Home BP Profile with Dapagliflozin Is Associated with Amelioration of Albuminuria in Japanese Patients with Diabetic Nephropathy: The Yokohama Add-on Inhibitory Efficacy of Dapagliflozin on Albuminuria in Japanese Patients with Type 2 Diabetes Study (Y-AIDA Study). Cardiovasc. Diabetol. 2019, 18, 110. [Google Scholar] [CrossRef]
- Chatur, S.; Cunningham, J.W.; Vaduganathan, M.; Mc Causland, F.R.; Claggett, B.L.; Desai, A.S.; Miao, Z.M.; Jhund, P.S.; de Boer, R.A.; Hernandez, A.F.; et al. Renal and Blood Pressure Effects of Dapagliflozin in Recently Hospitalized Patients with Heart Failure with Mildly Reduced or Preserved Ejection Fraction: Insights from the DELIVER Trial. Eur. J. Heart Fail. 2023, 25, 1170–1175. [Google Scholar] [CrossRef]
- Braunwald, E. Gliflozins in the Management of Cardiovascular Disease. N. Engl. J. Med. 2022, 386, 2024–2034. [Google Scholar] [CrossRef]
- Meraz-Muñoz, A.Y.; Weinstein, J.; Wald, R. EGFR Decline after SGLT2 Inhibitor Initiation: The Tortoise and the Hare Reimagined. Kidney360 2021, 2, 1042–1047. [Google Scholar] [CrossRef]
- Onishi, A.; Fu, Y.; Patel, R.; Darshi, M.; Crespo-Masip, M.; Huang, W.; Song, P.; Freeman, B.; Kim, Y.C.; Soleimani, M.; et al. A Role for Tubular Na+/H+ Exchanger NHE3 in the Natriuretic Effect of the SGLT2 Inhibitor Empagliflozin. Am. J. Physiol.-Ren. Physiol. 2020, 319, F712–F728. [Google Scholar] [CrossRef]
- Patoulias, D.; Papadopoulos, C.; Kassimis, G.; Fragakis, N.; Vassilikos, V.; Karagiannis, A.; Doumas, M. Effect of Sodium-Glucose Co-Transporter-2 Inhibitors on Arterial Stiffness: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Vasc. Med. 2022, 27, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Zanchi, A.; Burnier, M.; Muller, M.; Ghajarzadeh-Wurzner, A.; Maillard, M.; Loncle, N.; Milani, B.; Dufour, N.; Bonny, O.; Pruijm, M. Acute and Chronic Effects of SGLT2 Inhibitor Empagliflozin on Renal Oxygenation and Blood Pressure Control in Nondiabetic Normotensive Subjects: A Randomized, Placebo-Controlled Trial. J. Am. Heart Assoc. 2020, 9, e016173. [Google Scholar] [CrossRef] [PubMed]
- Patoulias, D.; Papadopoulos, C.; Kassimis, G.; Vassilikos, V.; Karagiannis, A.; Doumas, M. Meta-Analysis Addressing the Effect of Sodium-Glucose Cotransporter 2 Inhibitors on Flow-Mediated Dilation in Patients with Type 2 Diabetes Mellitus. Am. J. Cardiol. 2022, 165, 133–135. [Google Scholar] [CrossRef]
- Theofilis, P.; Sagris, M.; Oikonomou, E.; Antonopoulos, A.S.; Siasos, G.; Tsioufis, K.; Tousoulis, D. Pleiotropic effects of SGLT2 inhibitors and heart failure outcomes. Diabetes Res. Clin. Pract. 2022, 188, 109927. [Google Scholar] [CrossRef] [PubMed]
- Theofilis, P.; Sagris, M.; Oikonomou, E.; Antonopoulos, A.S.; Siasos, G.; Tsioufis, C.; Tousoulis, D. Inflammatory Mechanisms Contributing to Endothelial Dysfunction. Biomedicines 2021, 9, 781. [Google Scholar] [CrossRef] [PubMed]
- Nakao, M.; Shimizu, I.; Katsuumi, G.; Yoshida, Y.; Suda, M.; Hayashi, Y.; Ikegami, R.; Hsiao, Y.T.; Okuda, S.; Soga, T.; et al. Empagliflozin Maintains Capillarization and Improves Cardiac Function in a Murine Model of Left Ventricular Pressure Overload. Sci. Rep. 2021, 11, 18384. [Google Scholar] [CrossRef]
- Moustafa Ahmed, Y.; Shehata Messiha, B.A.; El-Sayed El-Daly, M.; Abo-Saif, A.A. Effects of Ticagrelor, Empagliflozin and Tamoxifen against Experimentally-Induced Vascular Reactivity Defects in Rats in Vivo and in Vitro. Pharmacol. Rep. 2019, 71, 1034–1043. [Google Scholar] [CrossRef]
- Li, H.; Shin, S.E.; Seo, M.S.; An, J.R.; Choi, I.-W.; Jung, W.-K.; Firth, A.L.; Lee, D.-S.; Yim, M.-J.; Choi, G.; et al. The Anti-Diabetic Drug Dapagliflozin Induces Vasodilation via Activation of PKG and Kv Channels. Life Sci. 2018, 197, 46–55. [Google Scholar] [CrossRef]
- Sano, M.; Goto, S. Possible Mechanism of Hematocrit Elevation by Sodium Glucose Cotransporter 2 Inhibitors and Associated Beneficial Renal and Cardiovascular Effects. Circulation 2019, 139, 1985–1987. [Google Scholar] [CrossRef]
- Elkazzaz, S.K.; Khodeer, D.M.; El Fayoumi, H.M.; Moustafa, Y.M. Role of Sodium Glucose Cotransporter Type 2 Inhibitors Dapagliflozin on Diabetic Nephropathy in Rats; Inflammation, Angiogenesis and Apoptosis. Life Sci. 2021, 280, 119018. [Google Scholar] [CrossRef]
- Castoldi, G.; Carletti, R.; Ippolito, S.; Colzani, M.; Barzaghi, F.; Stella, A.; Zerbini, G.; Perseghin, G.; Zatti, G.; di Gioia, C.R.T. Sodium-Glucose Cotransporter 2 Inhibition Prevents Renal Fibrosis in Cyclosporine Nephropathy. Acta Diabetol. 2021, 58, 1059–1070. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.H.; Al Salam, S.; Al Suleimani, Y.; Al Za’abi, M.; Abdelrahman, A.M.; Ashique, M.; Manoj, P.; Adham, S.A.; Hartmann, C.; Schupp, N.; et al. Effects of the SGLT-2 Inhibitor Canagliflozin on Adenine-Induced Chronic Kidney Disease in Rats. Cell. Physiol. Biochem. 2019, 52, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Li, X.; He, L.; Zhu, S.; Lai, S.; Zhang, X.; Huang, Z.; Yu, B.; Cui, C.; Wang, Q. Empagliflozin Improves Renal Ischemia–Reperfusion Injury by Reducing Inflammation and Enhancing Mitochondrial Fusion through AMPK–OPA1 Pathway Promotion. Cell Mol. Biol. Lett. 2023, 28, 42. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Kitada, M.; Ogura, Y.; Liu, H.; Koya, D. Dapagliflozin Restores Impaired Autophagy and Suppresses Inflammation in High Glucose-Treated HK-2 Cells. Cells 2021, 10, 1457. [Google Scholar] [CrossRef] [PubMed]
- García-Sánchez, A.; Miranda-Díaz, A.G.; Cardona-Muñoz, E.G. The Role of Oxidative Stress in Physiopathology and Pharmacological Treatment with Pro- and Antioxidant Properties in Chronic Diseases. Oxid. Med. Cell. Longev. 2020, 2020, 2082145. [Google Scholar] [CrossRef]
- Behnammanesh, G.; Durante, G.L.; Khanna, Y.P.; Peyton, K.J.; Durante, W. Canagliflozin Inhibits Vascular Smooth Muscle Cell Proliferation and Migration: Role of Heme Oxygenase-1. Redox Biol. 2020, 32, 101527. [Google Scholar] [CrossRef]
- Takahashi, H.; Nomiyama, T.; Terawaki, Y.; Horikawa, T.; Kawanami, T.; Hamaguchi, Y.; Tanaka, T.; Motonaga, R.; Fukuda, T.; Tanabe, M.; et al. Combined Treatment with DPP-4 Inhibitor Linagliptin and SGLT2 Inhibitor Empagliflozin Attenuates Neointima Formation after Vascular Injury in Diabetic Mice. Biochem. Biophys. Rep. 2019, 18, 100640. [Google Scholar] [CrossRef]
- Herat, L.Y.; Magno, A.L.; Rudnicka, C.; Hricova, J.; Carnagarin, R.; Ward, N.C.; Arcambal, A.; Kiuchi, M.G.; Head, G.A.; Schlaich, M.P.; et al. SGLT2 Inhibitor–Induced Sympathoinhibition. JACC Basic. Transl. Sci. 2020, 5, 169–179. [Google Scholar] [CrossRef]
- Zhang, L.; Li, J.; Zhang, L.; Guo, L.; Li, H.; Li, D. Association and Interaction Analysis of Body Mass Index and Triglycerides Level with Blood Pressure in Elderly Individuals in China. Biomed. Res. Int. 2018, 2018, 8934534. [Google Scholar] [CrossRef]
- Yusni, Y.; Rahman, S.; Naufal, I. Positive Correlation between Body Weight and Body Mass Index with Blood Pressure in Young Adults. Narra J. 2024, 4, e533. [Google Scholar] [CrossRef]
- Horie, I.; Abiru, N.; Hongo, R.; Nakamura, T.; Ito, A.; Haraguchi, A.; Natsuda, S.; Sagara, I.; Ando, T.; Kawakami, A. Increased Sugar Intake as a Form of Compensatory Hyperphagia in Patients with Type 2 Diabetes under Dapagliflozin Treatment. Diabetes Res. Clin. Pract. 2018, 135, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Cefalu, W.T.; Leiter, L.A.; Yoon, K.-H.; Arias, P.; Niskanen, L.; Xie, J.; Balis, D.A.; Canovatchel, W.; Meininger, G. Efficacy and Safety of Canagliflozin versus Glimepiride in Patients with Type 2 Diabetes Inadequately Controlled with Metformin (CANTATA-SU): 52 Week Results from a Randomised, Double-Blind, Phase 3 Non-Inferiority Trial. Lancet 2013, 382, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Matthews, J.R.; Herat, L.Y.; Magno, A.L.; Gorman, S.; Schlaich, M.P.; Matthews, V.B. SGLT2 Inhibitor-Induced Sympathoexcitation in White Adipose Tissue: A Novel Mechanism for Beiging. Biomedicines 2020, 8, 514. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Nagata, N.; Nagashimada, M.; Zhuge, F.; Ni, Y.; Chen, G.; Mayoux, E.; Kaneko, S.; Ota, T. SGLT2 Inhibition by Empagliflozin Promotes Fat Utilization and Browning and Attenuates Inflammation and Insulin Resistance by Polarizing M2 Macrophages in Diet-Induced Obese Mice. EBioMedicine 2017, 20, 137–149. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Q.; Li, Y.; Ding, Y.; Zhao, Y.; Tang, Q.; Wu, T.; Chen, L.; Pu, S.; Cheng, S.; et al. Inhibition of the Sodium-Glucose Co-Transporter SGLT2 by Canagliflozin Ameliorates Diet-Induced Obesity by Increasing Intra-Adipose Sympathetic Innervation. Br. J. Pharmacol. 2021, 178, 1756–1771. [Google Scholar] [CrossRef]
- Lv, Y.; Zhao, C.; Jiang, Q.; Rong, Y.; Ma, M.; Liang, L.; Li, W.; Zhang, J.; Xu, N.; Wu, H. Dapagliflozin Promotes Browning of White Adipose Tissue through the FGFR1-LKB1-AMPK Signaling Pathway. Mol. Biol. Rep. 2024, 51, 562. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Liu, M.; Xiang, G.; Yue, L.; Zhang, J.; Xu, X.; Dong, J. Dapagliflozin Promotes White Adipose Tissue Browning Though Regulating Angiogenesis in High Fat Induced Obese Mice. BMC Pharmacol. Toxicol. 2024, 25, 26. [Google Scholar] [CrossRef]
- Xu, L.; Nagata, N.; Chen, G.; Nagashimada, M.; Zhuge, F.; Ni, Y.; Sakai, Y.; Kaneko, S.; Ota, T. Empagliflozin Reverses Obesity and Insulin Resistance through Fat Browning and Alternative Macrophage Activation in Mice Fed a High-Fat Diet. BMJ Open Diabetes Res. Care 2019, 7, e000783. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, M.J.; Kang, D.; Khang, A.R.; Bae, J.H.; Kim, J.Y.; Kim, S.H.; Kang, Y.H.; Yi, D. Effects of Sodium-Glucose Cotransporter 2 Inhibitors on Transcription Regulation of AgRP and POMC Genes. Curr. Issues Mol. Biol. 2024, 46, 7505–7515. [Google Scholar] [CrossRef]
- Filippas-Ntekouan, S.; Tsimihodimos, V.; Filippatos, T.; Dimitriou, T.; Elisaf, M. SGLT-2 Inhibitors: Pharmacokinetics Characteristics and Effects on Lipids. Expert. Opin. Drug Metab. Toxicol. 2018, 14, 1113–1121. [Google Scholar] [CrossRef]
- Sánchez-García, A.; Simental-Mendía, M.; Millán-Alanís, J.M.; Simental-Mendía, L.E. Effect of Sodium-Glucose Co-Transporter 2 Inhibitors on Lipid Profile: A Systematic Review and Meta-Analysis of 48 Randomized Controlled Trials. Pharmacol. Res. 2020, 160, 105068. [Google Scholar] [CrossRef] [PubMed]
- Bechmann, L.E.; Emanuelsson, F.; Nordestgaard, B.G.; Benn, M. SGLT2-Inhibition Increases Total, LDL, and HDL Cholesterol and Lowers Triglycerides: Meta-Analyses of 60 Randomized Trials, Overall and by Dose, Ethnicity, and Drug Type. Atherosclerosis 2024, 394, 117236. [Google Scholar] [CrossRef] [PubMed]
- Tewari, J.; Qidwai, K.A.; Rana, A.; Tewari, A.; Tewari, V.; Maheshwari, A. Safety and Efficacy of Remogliflozin in People with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Cureus 2024, 16, e66145. [Google Scholar] [CrossRef] [PubMed]
- Lingli, X.; Wenfang, X. Characteristics and Molecular Mechanisms through Which SGLT2 Inhibitors Improve Metabolic Diseases: A Mechanism Review. Life Sci. 2022, 300, 120543. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Fukui, T.; Nakanishi, N.; Yamamoto, S.; Tomoyasu, M.; Osamura, A.; Ohara, M.; Yamamoto, T.; Ito, Y.; Hirano, T. Dapagliflozin Decreases Small Dense Low-Density Lipoprotein-Cholesterol and Increases High-Density Lipoprotein 2-Cholesterol in Patients with Type 2 Diabetes: Comparison with Sitagliptin. Cardiovasc. Diabetol. 2017, 16, 8. [Google Scholar] [CrossRef]
- Katsuyama, H.; Horinaka, S.; Hakoshima, M.; Adachi, H.; Yanai, H. Retrospective Longitudinal Observational Study on the Long-Term Effects of Sodium-Glucose Cotransporter-2 Inhibitors on the Development of Metabolic Dysfunction-Associated Steatotic Liver Disease in Type 2 Diabetic Japanese Patients. J. Clin. Med. 2024, 13, 4929. [Google Scholar] [CrossRef]
- Bessho, R.; Takiyama, Y.; Takiyama, T.; Kitsunai, H.; Takeda, Y.; Sakagami, H.; Ota, T. Hypoxia-Inducible Factor-1α Is the Therapeutic Target of the SGLT2 Inhibitor for Diabetic Nephropathy. Sci. Rep. 2019, 9, 14754. [Google Scholar] [CrossRef]
- Yang, Z.; Li, T.; Xian, J.; Chen, J.; Huang, Y.; Zhang, Q.; Lin, X.; Lu, H.; Lin, Y. SGLT2 Inhibitor Dapagliflozin Attenuates Cardiac Fibrosis and Inflammation by Reverting the HIF-2α Signaling Pathway in Arrhythmogenic Cardiomyopathy. FASEB J. 2022, 36, e22410. [Google Scholar] [CrossRef]
- Packer, M. Alleviation of Anemia by SGLT2 Inhibitors in Patients with CKD: Mechanisms and Results of Long-Term Placebo-Controlled Trials. Clin. J. Am. Soc. Nephrol. 2023, 19, 531–534. [Google Scholar] [CrossRef]
| Author/Study Design | Population | Ν | BP Assessment | Intervention vs. Control | Study Duration | Change in SBP/DBP |
|---|---|---|---|---|---|---|
| Non-diabetic patients | ||||||
| Zanchi et al. [35] Double-blind RCT | Normotensive non-diabetic patients | 39 | 24 h ABPM | 10 mg empagliflozin vs. placebo | 1 m | Change in 24 h SBP/DBP: Empagliflozin: −5/−2 Placebo: 3/2 Change in daytime SBP/DBP: Empagliflozin: −4/−1 Placebo: 1/1 Change in nighttime SBP/DBP: Empagliflozin: −6/−4 Placebo: 5/1 |
| Teo et al. [32] Meta-analysis of eight RCTs | Non-diabetic patients | 5233 | Office BP | Dapagliflozin, empagliflozin, canagliflozin vs. control group | 12 w–18 m | Change in SBP/DBP vs. control: −1.9/0.27 |
| Patients with T2DM and hypertension | ||||||
| Kario et al. [36] SACRA study Multicenter double-blind RCT | T2DM patients under stable antihypertensive treatment including an ARB and uncontrolled nocturnal HTN | 132 | 24 h ABPM | 10 mg empagliflozin vs. placebo | 12 w | Between-group differences: 24 h SBP/DBP: −7.7/−2.9 (p < 0.05) Daytime SBP/DBP: −9.5/−3.9 (p < 0.05) Nighttime SBP/DBP: −4.3/−1.6 (p > 0.05) |
| Kario et al. [37] SHIFT-J study Open-label RCT | T2DM patients with uncontrolled HTN under stable antihypertensive treatment | 84 | Office and home BP | 100 mg canagliflozin vs. control group | 8 w | Change in nighttime home SBP: Canagliflozin: −5.23 Control group: −1.04 (p = 0.078) Change in evening home SBP: Canagliflozin: −8.7 Control group: 2.4 (p = 0.012) |
| Ferdinand et al. [38] Double-blind RCT | Black T2DM patients with uncontrolled HTN | 150 | Office BP and 24 h ABPM | 25 mg empagliflozin vs. placebo | 24 w | Between-group differences: 24 h SBP: −8.39 Office SBP/DBP: −7.43/−4.25 |
| Tikkanen et al. [26] Double-blind RCT | T2DM patients with stage 1 HTN | 825 | Office BP and 24 h ABPM | Empagliflozin 10 mg and 25 mg vs. placebo | 12 w | Between-group difference in 24 h SBP/DBP: −3.44/−1.36 for empagliflozin 10 mg, −4.16/−1.72 for empagliflozin 25 mg Between-group difference in office SBP/DBP: −3.92/−1.93 for empagliflozin 10 mg, −4.8/−1.89 for empagliflozin 25 mg |
| Patients with T2DM | ||||||
| Papadopoulou et al. [39] Double-blind RCT | T2DM patients | 85 | 24 h ABPM | Dapagliflozin 10 mg vs. placebo | 12 w | 24 h SBP/DBP: Dapagliflozin: −5.8/−2.2 Placebo: −0.1/0.1 Central 24 h SBP: Dapagliflozin: −4.1 Placebo: −0.7 |
| Kario et al. [40] LUSCAR Study Multicenter trial | T2DM patients | 47 | Office and home BP | Luseogliflozin 2.5 mg | 12 w | Change in morning home SBP/DBP: −5.2/−2.5 Change in evening home SBP/DBP: −5.5/−2.9 |
| Saito et al. [41] PROTECT study Multicenter open-label RCT | T2DM patients | 232 | Office BP | Ipragliflozin 50–100 mg vs. control group | 24 m | Between-group difference in SBP: −3.6 mmHg (−6.2 to −1 mmHg) |
| Baker et al. [28] Meta-analysis of 27 RCTs | T2DM patients | 12.960 | Office BP | Canagliflozin Dapagliflozin Empagliflozin Ipragliflozin Remogliflozin vs. placebo (21 studies) or other antidiabetic treatment (6 studies) | 3–52 w | Mean between-group difference in SBP/DBP: −4/−1.6 |
| Tsapas et al. [29] Meta-analysis of 204 RCTs | T2DM patients | 165.639 | Office BP | SGLT2i vs. control group | >24 w | Between-group difference in SBP/DBP: −2.89/−1.44 |
| Georgianos et al. [30] Meta-analysis of seven RCTs | T2DM patients | 2.381 | 24 h ABPM | Canagliflozin Dapagliflozin Empagliflozin Ertugliflozin vs. placebo (five studies) and low-dose Hydrochlorothiazide (two studies) | 4–12 w | Between-group difference in 24 h SBP/DBP: −3.62/−1.7 Between-group difference in daytime SBP/DBP: −4.32/−2.03 Between-group difference in nighttime SBP/DBP: −2.62/−1.39 |
| Patients with CKD | ||||||
| Ye et al. [42] CREDENCE study Multicenter double-blind RCT | Patients with T2DM and CKD (eGFR 30–90 mL/min/1.73 m2 and UACR 300–5000 mg/dL) | 4401 | Office BP | 100 mg canagliflozin vs. placebo | 2.6 y | Between-group difference in office SBP: −3.3 (p = 0.84) |
| Provenzano et al. [43] DAPA-CKD study Double-blind RCT | Patients with CKD (eGFR 25–75 mL/min/1.73 m2 and UACR 200–5000 mg/dL) | 4304 | Office BP | Dapagliflozin 10 mg vs. placebo | 2.4 y | Between-group difference in office SBP: −2.9 mmHg |
| Mayne et al. [44] EMPA-KIDNEY study Double-blind RCT | Patients with CKD (eGFR 20–45 mL/min/1.73 m2 or 45–90 mL/min/1.73 m2 and UACR 200 mg/dL) | 6609 | Office BP | Empagliflozin 10 mg vs. placebo | 2 y | Between-group difference in office SBP/DBP: −2.6/−0.5 |
| Barnett et al. [45] EMPAREG RENAAL study Multicenter double-blind RCT | Patients with T2DM and CKD stages 2 and 3 | 290 with stage 2 CKD, 374 with stage 3 CKD, 74 with stage 4 CKD | Office BP | Empagliflozin 10 mg and 25 mg vs. placebo | 52 w | With stage 2 CKD: Between-group difference in SBP/DBP: −3.3/−2.7 for empagliflozin 10 mg, −7.8/−4.5 for empagliflozin 25 mg With stage 3 CKD: Between-group difference in SBP/DBP: −4.3/−1.5 for empagliflozin 25 mg With stage 4 CKD: Between-group difference in SBP/DBP: −11.2/−4.3 for empagliflozin 25 mg |
| Kinguchi et al. [46] Y-AIDA study Prospective multicenter study | Patients with T2DM, eGFR ≥ 45 mL/min/1.73 m2 and UACR ≥30 mg/g creatinine | 86 | Home BP | Dapagliflozin 10 mg | 24 w | Change in morning SBP/DBP: −8.32/−4.18, p = 0.001 Change in evening SBP/DBP: −9.57/−4.48, p = 0.001 Change in nighttime SBP/DBP: −2.38/−1.17, p < 0.05 |
| Patients with heart failure | ||||||
| Li et al. [33] Meta-analysis of 16 RCTs | Patients with HFrEF (eight studies), HFpEF (four studies) or with HF regardless of EF (four studies) | 7.696 | Office BP | Dapagliflozin Empagliflozin Canagliflozin Luseogliflozin or placebo | 6 w–26.2 m | Change in SBP: −1.68 for SGLT2i |
| Chatur et al. [47] DELIVER study Double-blind RCT | Symptomatic HF with EF > 40% and hospitalization in the previous 30 days for decompensated HF | 654 | Office BP | Dapagliflozin 10 mg vs. placebo | 1 m | Placebo: +1.4 mmHg Dapagliflozin: +0.2 mmHg Between-group difference: −1.3 mmHg (−3.6 to 0.9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katsimardou, A.; Theofilis, P.; Vordoni, A.; Doumas, M.; Kalaitzidis, R.G. The Effects of SGLT2 Inhibitors on Blood Pressure and Other Cardiometabolic Risk Factors. Int. J. Mol. Sci. 2024, 25, 12384. https://doi.org/10.3390/ijms252212384
Katsimardou A, Theofilis P, Vordoni A, Doumas M, Kalaitzidis RG. The Effects of SGLT2 Inhibitors on Blood Pressure and Other Cardiometabolic Risk Factors. International Journal of Molecular Sciences. 2024; 25(22):12384. https://doi.org/10.3390/ijms252212384
Chicago/Turabian StyleKatsimardou, Alexandra, Panagiotis Theofilis, Aikaterini Vordoni, Michael Doumas, and Rigas G. Kalaitzidis. 2024. "The Effects of SGLT2 Inhibitors on Blood Pressure and Other Cardiometabolic Risk Factors" International Journal of Molecular Sciences 25, no. 22: 12384. https://doi.org/10.3390/ijms252212384
APA StyleKatsimardou, A., Theofilis, P., Vordoni, A., Doumas, M., & Kalaitzidis, R. G. (2024). The Effects of SGLT2 Inhibitors on Blood Pressure and Other Cardiometabolic Risk Factors. International Journal of Molecular Sciences, 25(22), 12384. https://doi.org/10.3390/ijms252212384





