POLD3 as Controller of Replicative DNA Repair
Abstract
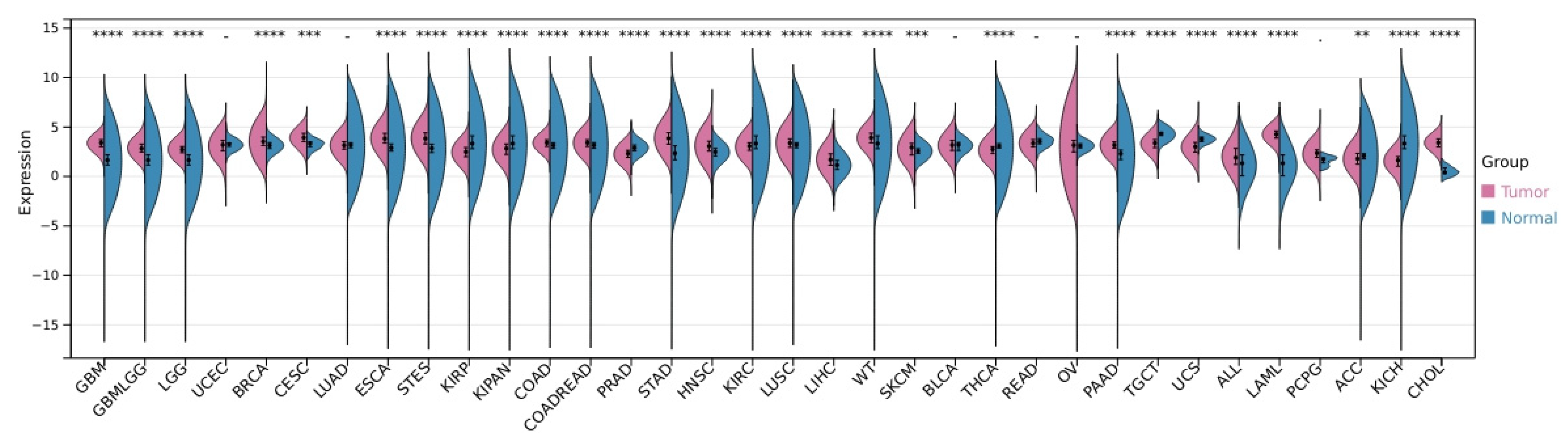
1. POLD3 in DNA Repair, Genome Stability, and Human Health
2. Discovery of POLD3 in Yeasts and Mammals
3. POLD3 Structure and Motifs
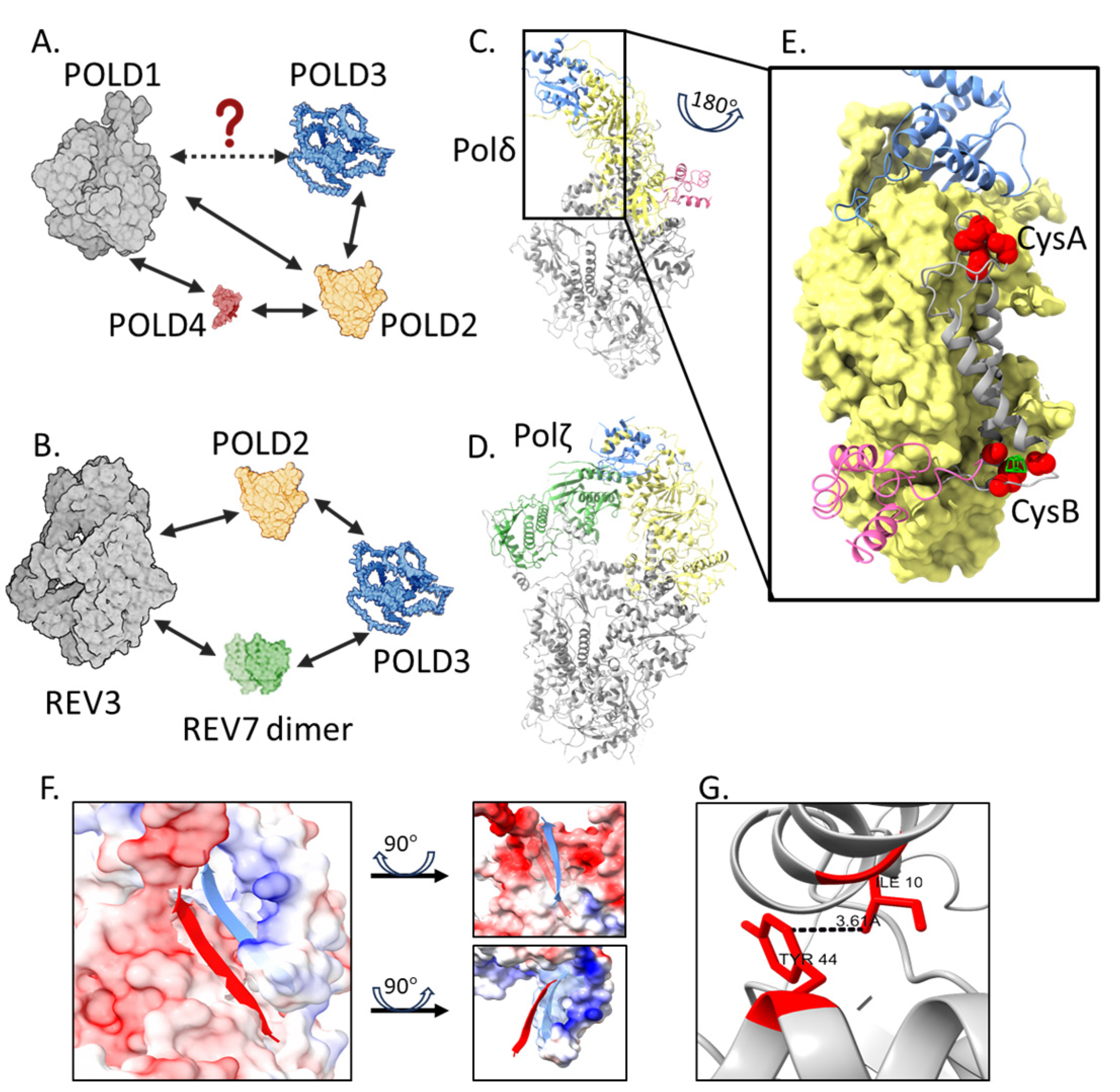
3.1. Interaction of the POLD3 wHTH Fold
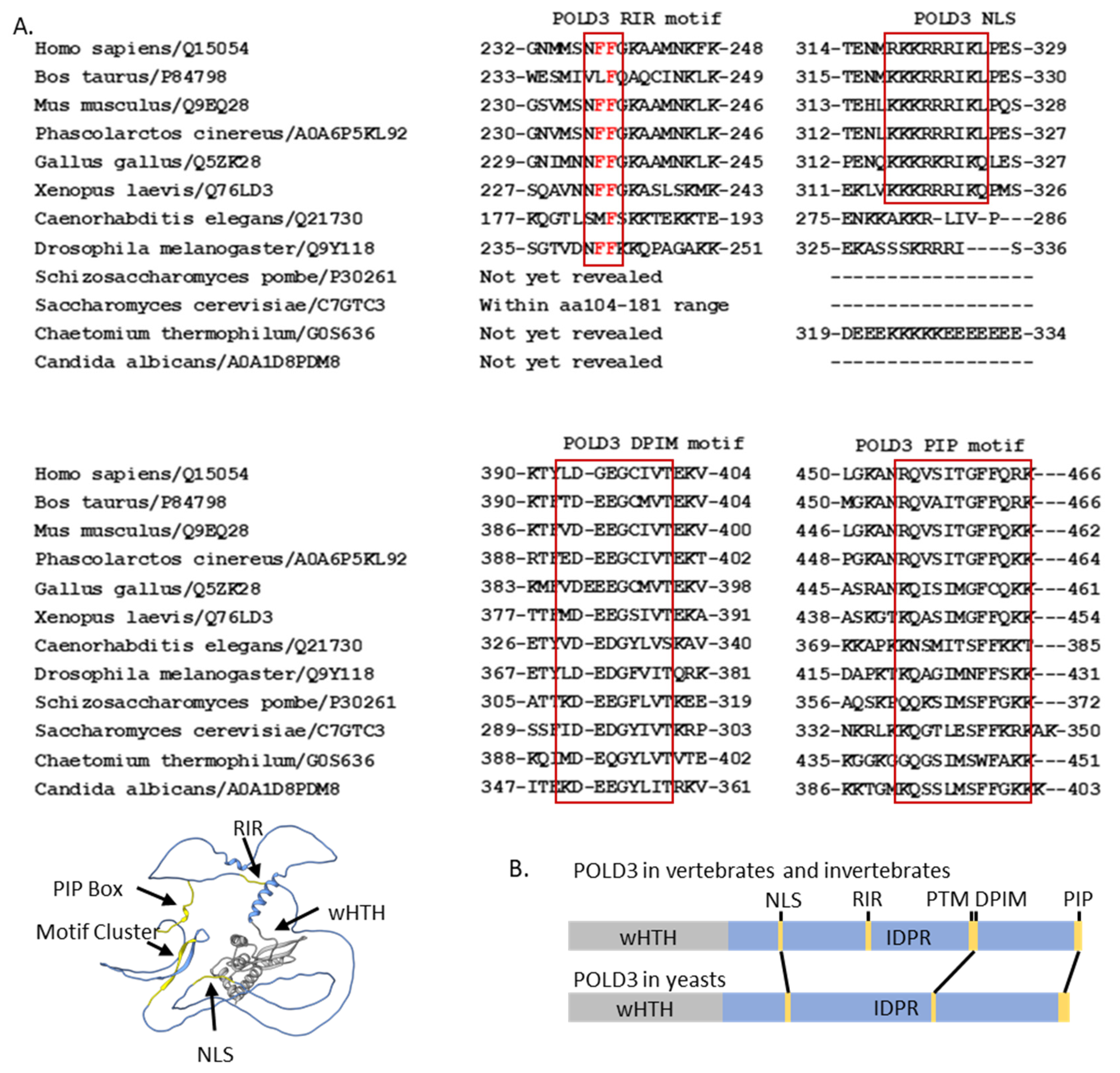
3.2. Conserved IDPR Regions of POLD3
3.2.1. The POLD3 Rev1 Interaction Region
3.2.2. The POLD3 DNA Polymerase Interacting Motif (DPIM)
3.2.3. Two Nuclear Localization Signals
3.2.4. The C-Terminal PCNA Interaction Protein (PIP) ‘Box’ of POLD3 Proteins
4. POLD3 as a Controller of DNA Synthesis by Polδ and Polζ
4.1. POLD3 Within Polδ and Polζ Complexes
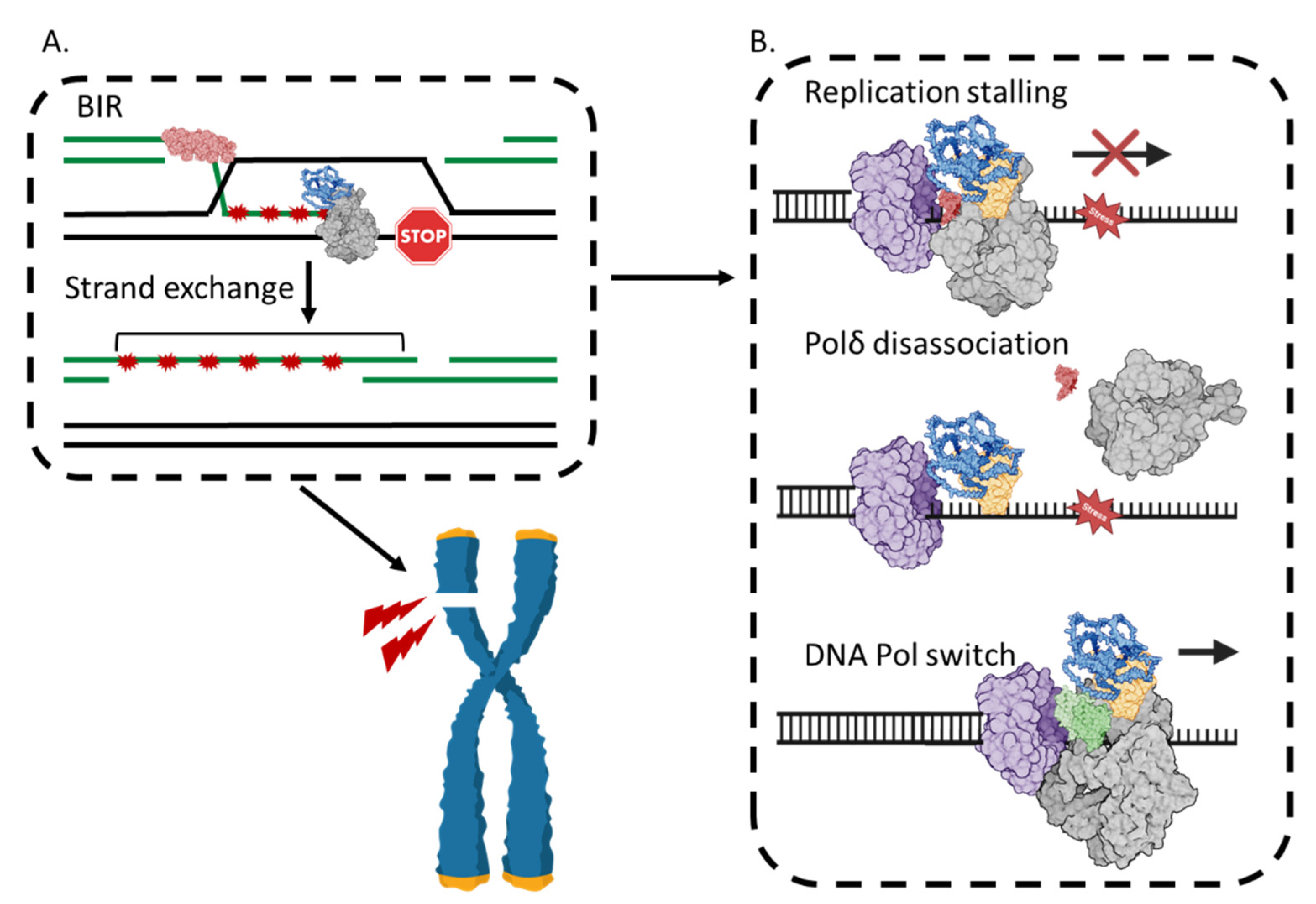
4.2. POLD3 in DNA Repair Synthesis by BIR
4.3. BIR Can Be Mutagenic
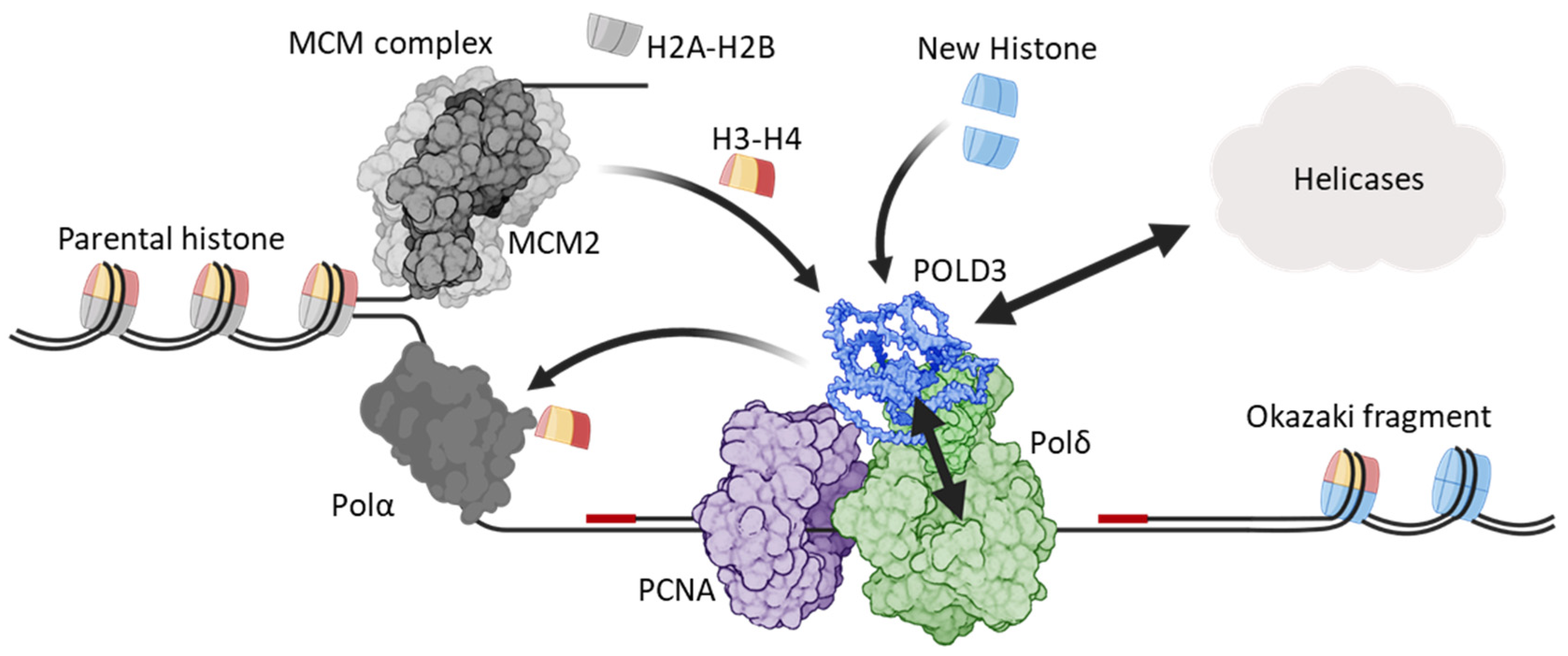
4.4. POLD3 as a Histone Chaperone During DNA Replication
5. Post-Translational Modifications of POLD3
5.1. SUMOylation of POLD3
5.2. Phosphorylation
5.3. ADP-Ribosylation
6. Interactions of Polδ and POLD3 with DNA Repair Helicases
6.1. Srs2, BLM, WRN and HELQ
6.2. Novel POLD3–Helicase Interactions Revealed During Cas9 Nuclease Screening
7. Concluding Comments
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burgers, P.M.J.; Kunkel, T.A. Eukaryotic DNA Replication Fork. Annu. Rev. Biochem. 2017, 86, 417–438. [Google Scholar] [CrossRef] [PubMed]
- Guilliam, T.A.; Yeeles, J.T.P. An updated perspective on the polymerase division of labor during eukaryotic DNA replication. Crit. Rev. Biochem. Mol. Biol. 2020, 55, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Malik, R.; Kopylov, M.; Gomez-Llorente, Y.; Jain, R.; Johnson, R.E.; Prakash, L.; Prakash, S.; Ubarretxena-Belandia, I.; Aggarwal, A.K. Structure and mechanism of B-family DNA polymerase zeta specialized for translesion DNA synthesis. Nat. Struct. Mol. Biol. 2020, 27, 913–924. [Google Scholar] [CrossRef]
- Baranovskiy, A.G.; Babayeva, N.D.; Liston, V.G.; Rogozin, I.B.; Koonin, E.V.; Pavlov, Y.I.; Vassylyev, D.G.; Tahirov, T.H. X-ray structure of the complex of regulatory subunits of human DNA polymerase delta. Cell Cycle 2008, 7, 3026–3036. [Google Scholar] [CrossRef]
- Gomez-Llorente, Y.; Malik, R.; Jain, R.; Choudhury, J.R.; Johnson, R.E.; Prakash, L.; Prakash, S.; Ubarretxena-Belandia, I.; Aggarwal, A.K. The architecture of yeast DNA polymerase zeta. Cell Rep. 2013, 5, 79–86. [Google Scholar] [CrossRef]
- Lancey, C.; Tehseen, M.; Raducanu, V.S.; Rashid, F.; Merino, N.; Ragan, T.J.; Savva, C.G.; Zaher, M.S.; Shirbini, A.; Blanco, F.J.; et al. Structure of the processive human Pol delta holoenzyme. Nat. Commun. 2020, 11, 1109. [Google Scholar] [CrossRef]
- Zheng, F.; Georgescu, R.E.; Li, H.; O’Donnell, M.E. Structure of eukaryotic DNA polymerase delta bound to the PCNA clamp while encircling DNA. Proc. Natl. Acad. Sci. USA 2020, 117, 30344–30353. [Google Scholar] [CrossRef]
- Jain, R.; Rice, W.J.; Malik, R.; Johnson, R.E.; Prakash, L.; Prakash, S.; Ubarretxena-Belandia, I.; Aggarwal, A.K. Cryo-EM structure and dynamics of eukaryotic DNA polymerase delta holoenzyme. Nat. Struct. Mol. Biol. 2019, 26, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Swan, M.K.; Johnson, R.E.; Prakash, L.; Prakash, S.; Aggarwal, A.K. Structural basis of high-fidelity DNA synthesis by yeast DNA polymerase delta. Nat. Struct. Mol. Biol. 2009, 16, 979–986. [Google Scholar] [CrossRef]
- Du Truong, C.; Craig, T.A.; Cui, G.; Botuyan, M.V.; Serkasevich, R.A.; Chan, K.Y.; Mer, G.; Chiu, P.L.; Kumar, R. Cryo-EM reveals conformational flexibility in apo DNA polymerase zeta. J. Biol. Chem. 2021, 297, 100912. [Google Scholar] [CrossRef]
- Mehawej, C.; Chouery, E.; Azar-Atallah, S.; Shebaby, W.; Delague, V.; Mansour, I.; Mustapha, M.; Lefranc, G.; Megarbane, A. POLD3 deficiency is associated with severe combined immunodeficiency, neurodevelopmental delay, and hearing impairment. Clin. Immunol. 2023, 251, 109326. [Google Scholar] [CrossRef] [PubMed]
- Riestra, M.R.; Pillay, B.A.; Willemsen, M.; Kienapfel, V.; Ehlers, L.; Delafontaine, S.; Pinton, A.; Wouters, M.; Hombrouck, A.; Sauer, K.; et al. Human Autosomal Recessive DNA Polymerase Delta 3 Deficiency Presenting as Omenn Syndrome. J. Clin. Immunol. 2023, 44, 2. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Wang, L.; Ge, F.; Gong, P.; Wang, H.; Wang, F.; Chen, L.; Liu, L. Pold3 is required for genomic stability and telomere integrity in embryonic stem cells and meiosis. Nucleic Acids Res. 2018, 46, 3468–3486. [Google Scholar] [CrossRef]
- Murga, M.; Lecona, E.; Kamileri, I.; Diaz, M.; Lugli, N.; Sotiriou, S.K.; Anton, M.E.; Mendez, J.; Halazonetis, T.D.; Fernandez-Capetillo, O. POLD3 Is Haploinsufficient for DNA Replication in Mice. Mol. Cell 2016, 63, 877–883. [Google Scholar] [CrossRef]
- Boehm, J.S.; Garnett, M.J.; Adams, D.J.; Francies, H.E.; Golub, T.R.; Hahn, W.C.; Iorio, F.; McFarland, J.M.; Parts, L.; Vazquez, F. Cancer research needs a better map. Nature 2021, 589, 514–516. [Google Scholar] [CrossRef]
- Beroukhim, R.; Mermel, C.H.; Porter, D.; Wei, G.; Raychaudhuri, S.; Donovan, J.; Barretina, J.; Boehm, J.S.; Dobson, J.; Urashima, M.; et al. The landscape of somatic copy-number alteration across human cancers. Nature 2010, 463, 899–905. [Google Scholar] [CrossRef]
- Costantino, L.; Sotiriou, S.K.; Rantala, J.K.; Magin, S.; Mladenov, E.; Helleday, T.; Haber, J.E.; Iliakis, G.; Kallioniemi, O.P.; Halazonetis, T.D. Break-induced replication repair of damaged forks induces genomic duplications in human cells. Science 2014, 343, 88–91. [Google Scholar] [CrossRef]
- Johnston, D.S.; Wright, W.W.; Dicandeloro, P.; Wilson, E.; Kopf, G.S.; Jelinsky, S.A. Stage-specific gene expression is a fundamental characteristic of rat spermatogenic cells and Sertoli cells. Proc. Natl. Acad. Sci. USA 2008, 105, 8315–8320. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Song, Z.; Zhong, X.; Huang, M.; Shen, D.; Gao, P.; Qian, X.; Wang, M.; He, X.; Wang, T.; et al. Sangerbox: A comprehensive, interaction-friendly clinical bioinformatics analysis platform. Imeta 2022, 1, e36. [Google Scholar] [CrossRef]
- Payen, C.; Koszul, R.; Dujon, B.; Fischer, G. Segmental duplications arise from Pol32-dependent repair of broken forks through two alternative replication-based mechanisms. PLoS Genet. 2008, 4, e1000175. [Google Scholar] [CrossRef]
- Sakofsky, C.J.; Ayyar, S.; Deem, A.K.; Chung, W.H.; Ira, G.; Malkova, A. Translesion Polymerases Drive Microhomology-Mediated Break-Induced Replication Leading to Complex Chromosomal Rearrangements. Mol. Cell 2015, 60, 860–872. [Google Scholar] [CrossRef] [PubMed]
- Hastings, P.J.; Lupski, J.R.; Rosenberg, S.M.; Ira, G. Mechanisms of change in gene copy number. Nat. Rev. Genet. 2009, 10, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Hirota, K.; Yoshikiyo, K.; Guilbaud, G.; Tsurimoto, T.; Murai, J.; Tsuda, M.; Phillips, L.G.; Narita, T.; Nishihara, K.; Kobayashi, K.; et al. The POLD3 subunit of DNA polymerase delta can promote translesion synthesis independently of DNA polymerase zeta. Nucleic Acids Res. 2015, 43, 1671–1683. [Google Scholar] [CrossRef] [PubMed]
- Auerbach, P.A.; Demple, B. Roles of Rev1, Pol zeta, Pol32 and Pol eta in the bypass of chromosomal abasic sites in Saccharomyces cerevisiae. Mutagenesis 2010, 25, 63–69. [Google Scholar] [CrossRef]
- Pages, V.; Bresson, A.; Acharya, N.; Prakash, S.; Fuchs, R.P.; Prakash, L. Requirement of Rad5 for DNA polymerase zeta-dependent translesion synthesis in Saccharomyces cerevisiae. Genetics 2008, 180, 73–82. [Google Scholar] [CrossRef]
- Hartwell, L.H.; Mortimer, R.K.; Culotti, J.; Culotti, M. Genetic Control of the Cell Division Cycle in Yeast: V. Genetic Analysis of cdc Mutants. Genetics 1973, 74, 267–286. [Google Scholar] [CrossRef]
- Hartwell, L.H.; Culotti, J.; Reid, B. Genetic control of the cell-division cycle in yeast. I. Detection of mutants. Proc. Natl. Acad. Sci. USA 1970, 66, 352–359. [Google Scholar] [CrossRef]
- Nasmyth, K.A.; Reed, S.I. Isolation of genes by complementation in yeast: Molecular cloning of a cell-cycle gene. Proc. Natl. Acad. Sci. USA 1980, 77, 2119–2123. [Google Scholar] [CrossRef]
- Nasmyth, K.; Nurse, P. Cell division cycle mutants altered in DNA replication and mitosis in the fission yeast Schizosaccharomyces pombe. Mol. Gen. Genet. 1981, 182, 119–124. [Google Scholar] [CrossRef]
- Hughes, D.A.; MacNeill, S.A.; Fantes, P.A. Molecular cloning and sequence analysis of cdc27+ required for the G2-M transition in the fission yeast Schizosaccharomyces pombe. Mol. Gen. Genet. 1992, 231, 401–410. [Google Scholar] [CrossRef]
- Hughes, P.; Tratner, I.; Ducoux, M.; Piard, K.; Baldacci, G. Isolation and identification of the third subunit of mammalian DNA polymerase delta by PCNA-affinity chromatography of mouse FM3A cell extracts. Nucleic Acids Res. 1999, 27, 2108–2114. [Google Scholar] [CrossRef] [PubMed]
- Shikata, K.; Ohta, S.; Yamada, K.; Obuse, C.; Yoshikawa, H.; Tsurimoto, T. The human homologue of fission Yeast cdc27, p66, is a component of active human DNA polymerase delta. J. Biochem. 2001, 129, 699–708. [Google Scholar]
- Zhang, P.; Mo, J.Y.; Perez, A.; Leon, A.; Liu, L.; Mazloum, N.; Xu, H.; Lee, M.Y. Direct interaction of proliferating cell nuclear antigen with the p125 catalytic subunit of mammalian DNA polymerase delta. J. Biol. Chem. 1999, 274, 26647–26653. [Google Scholar] [CrossRef]
- Mo, J.; Liu, L.; Leon, A.; Mazloum, N.; Lee, M.Y. Evidence that DNA polymerase delta isolated by immunoaffinity chromatography exhibits high-molecular weight characteristics and is associated with the KIAA0039 protein and RPA. Biochemistry 2000, 39, 7245–7254. [Google Scholar] [CrossRef]
- Zuo, S.; Gibbs, E.; Kelman, Z.; Wang, T.S.; O’Donnell, M.; MacNeill, S.A.; Hurwitz, J. DNA polymerase delta isolated from Schizosaccharomyces pombe contains five subunits. Proc. Natl. Acad. Sci. USA 1997, 94, 11244–11249. [Google Scholar] [CrossRef]
- Reynolds, N.; Watt, A.; Fantes, P.A.; MacNeill, S.A. Cdm1, the smallest subunit of DNA polymerase d in the fission yeast Schizosaccharomyces pombe, is non-essential for growth and division. Curr. Genet. 1998, 34, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Gerik, K.J.; Li, X.; Pautz, A.; Burgers, P.M. Characterization of the two small subunits of Saccharomyces cerevisiae DNA polymerase delta. J. Biol. Chem. 1998, 273, 19747–19755. [Google Scholar] [CrossRef] [PubMed]
- Bauer, G.A.; Heller, H.M.; Burgers, P.M. DNA polymerase III from Saccharomyces cerevisiae. I. Purification and characterization. J. Biol. Chem. 1988, 263, 917–924. [Google Scholar] [CrossRef]
- Suszek, W.; Baranowska, H.; Zuk, J.; Jachymczyk, W.J. DNA polymerase III is required for DNA repair in Saccharomyces cerevisiae. Curr. Genet. 1993, 24, 200–204. [Google Scholar] [CrossRef]
- Budd, M.E.; Campbell, J.L. DNA polymerases required for repair of UV-induced damage in Saccharomyces cerevisiae. Mol. Cell. Biol. 1995, 15, 2173–2179. [Google Scholar] [CrossRef]
- Budd, M.E.; Campbell, J.L. Purification and enzymatic and functional characterization of DNA polymerase beta-like enzyme, POL4. expressed during yeast meiosis. Methods Enzymol. 1995, 262, 108–130. [Google Scholar]
- Giot, L.; Simon, M.; Dubois, C.; Faye, G. Suppressors of thermosensitive mutations in the DNA polymerase delta gene of Saccharomyces cerevisiae. Mol. Gen. Genet. 1995, 246, 212–222. [Google Scholar] [CrossRef]
- Huang, M.E.; Rio, A.G.; Galibert, M.D.; Galibert, F. Pol32, a subunit of Saccharomyces cerevisiae DNA polymerase delta, suppresses genomic deletions and is involved in the mutagenic bypass pathway. Genetics 2002, 160, 1409–1422. [Google Scholar] [CrossRef]
- Byrnes, J.J.; Downey, K.M.; Black, V.L.; So, A.G. A new mammalian DNA polymerase with 3′ to 5′ exonuclease activity: DNA polymerase delta. Biochemistry 1976, 15, 2817–2823. [Google Scholar] [CrossRef]
- Gillespie, D.; Saxinger, W.C.; Gallo, R.C. Information transfer in cells infected by RNA tumor viruses and extension to human neoplasia. Prog. Nucleic Acid Res. Mol. Biol. 1975, 15, 1–108. [Google Scholar]
- Lee, M.Y.; Tan, C.K.; Downey, K.M.; So, A.G. Further studies on calf thymus DNA polymerase delta purified to homogeneity by a new procedure. Biochemistry 1984, 23, 1906–1913. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, S.J.; Wu, S.M.; Lee, M.Y. Immunoaffinity purification of DNA polymerase delta. Arch. Biochem. Biophys. 1995, 320, 297–304. [Google Scholar] [CrossRef]
- Boguski, M.S.; Schuler, G.D. ESTablishing a human transcript map. Nat. Genet. 1995, 10, 369–371. [Google Scholar] [CrossRef]
- Tugendreich, S.; Boguski, M.S.; Seldin, M.S.; Hieter, P. Linking yeast genetics to mammalian genomes: Identification and mapping of the human homolog of CDC27 via the expressed sequence tag (EST) data base. Proc. Natl. Acad. Sci. USA 1993, 90, 10031–10035. [Google Scholar] [CrossRef]
- Nomura, N.; Miyajima, N.; Sazuka, T.; Tanaka, A.; Kawarabayasi, Y.; Sato, S.; Nagase, T.; Seki, N.; Ishikawa, K.; Tabata, S. Prediction of the coding sequences of unidentified human genes. I. The coding sequences of 40 new genes (KIAA0001-KIAA0040) deduced by analysis of randomly sampled cDNA clones from human immature myeloid cell line KG-1. DNA Res. 1994, 1, 27–35. [Google Scholar] [CrossRef]
- Liu, L.; Mo, J.; Rodriguez-Belmonte, E.M.; Lee, M.Y. Identification of a fourth subunit of mammalian DNA polymerase delta. J. Biol. Chem. 2000, 275, 18739–18744. [Google Scholar] [CrossRef]
- Tumini, E.; Barroso, S.; Calero, C.P.; Aguilera, A. Roles of human POLD1 and POLD3 in genome stability. Sci. Rep. 2016, 6, 38873. [Google Scholar] [CrossRef]
- Shimada, K.; Tsai-Pflugfelder, M.; Vijeh Motlagh, N.D.; Delgoshaie, N.; Fuchs, J.; Gut, H.; Gasser, S.M. The stabilized Pol31-Pol3 interface counteracts Pol32 ablation with differential effects on repair. Life Sci. Alliance 2021, 4, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Rikova, K.; Guo, A.; Zeng, Q.; Possemato, A.; Yu, J.; Haack, H.; Nardone, J.; Lee, K.; Reeves, C.; Li, Y.; et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell 2007, 131, 1190–1203. [Google Scholar] [CrossRef]
- Pustovalova, Y.; Magalhaes, M.T.; D’Souza, S.; Rizzo, A.A.; Korza, G.; Walker, G.C.; Korzhnev, D.M. Interaction between the Rev1 C-Terminal Domain and the PolD3 Subunit of Polzeta Suggests a Mechanism of Polymerase Exchange upon Rev1/Polzeta-Dependent Translesion Synthesis. Biochemistry 2016, 55, 2043–2053. [Google Scholar] [CrossRef]
- Tonzi, P.; Huang, T.T. Role of Y-family translesion DNA polymerases in replication stress: Implications for new cancer therapeutic targets. DNA Repair 2019, 78, 20–26. [Google Scholar] [CrossRef]
- Hendriks, I.A.; Larsen, S.C.; Nielsen, M.L. An Advanced Strategy for Comprehensive Profiling of ADP-ribosylation Sites Using Mass Spectrometry-based Proteomics. Mol. Cell. Proteom. 2019, 18, 1010–1026. [Google Scholar] [CrossRef]
- Huang, M.E.; Le Douarin, B.; Henry, C.; Galibert, F. The Saccharomyces cerevisiae protein YJR043C (Pol32) interacts with the catalytic subunit of DNA polymerase alpha and is required for cell cycle progression in G2/M. Mol. Gen. Genet. 1999, 260, 541–550. [Google Scholar] [CrossRef]
- Johansson, E.; Garg, P.; Burgers, P.M. The Pol32 subunit of DNA polymerase delta contains separable domains for processive replication and proliferating cell nuclear antigen (PCNA) binding. J. Biol. Chem. 2004, 279, 1907–1915. [Google Scholar] [CrossRef]
- Gray, F.C.; Pohler, J.R.; Warbrick, E.; MacNeill, S.A. Mapping and mutation of the conserved DNA polymerase interaction motif (DPIM) located in the C-terminal domain of fission yeast DNA polymerase delta subunit Cdc27. BMC Mol. Biol. 2004, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Cokol, M.; Nair, R.; Rost, B. Finding nuclear localization signals. EMBO Rep. 2000, 1, 411–415. [Google Scholar] [CrossRef]
- Pohler, J.R.; Otterlei, M.; Warbrick, E. An in vivo analysis of the localisation and interactions of human p66 DNA polymerase delta subunit. BMC Mol. Biol. 2005, 6, 17. [Google Scholar] [CrossRef]
- Brameier, M.; Krings, A.; MacCallum, R.M. NucPred--predicting nuclear localization of proteins. Bioinformatics 2007, 23, 1159–1160. [Google Scholar] [CrossRef]
- Omenn, G.S. Familial Reticuloendotheliosis with Eosinophilia. N. Engl. J. Med. 1965, 273, 427–432. [Google Scholar] [CrossRef]
- Eissenberg, J.C.; Ayyagari, R.; Gomes, X.V.; Burgers, P.M. Mutations in yeast proliferating cell nuclear antigen define distinct sites for interaction with DNA polymerase delta and DNA polymerase epsilon. Mol. Cell. Biol. 1997, 17, 6367–6378. [Google Scholar] [CrossRef]
- Warbrick, E.; Lane, D.P.; Glover, D.M.; Cox, L.S. Homologous regions of Fen1 and p21Cip1 compete for binding to the same site on PCNA: A potential mechanism to co-ordinate DNA replication and repair. Oncogene 1997, 14, 2313–2321. [Google Scholar] [CrossRef]
- Ducoux, M.; Urbach, S.; Baldacci, G.; Hubscher, U.; Koundrioukoff, S.; Christensen, J.; Hughes, P. Mediation of proliferating cell nuclear antigen (PCNA)-dependent DNA replication through a conserved p21(Cip1)-like PCNA-binding motif present in the third subunit of human DNA polymerase delta. J. Biol. Chem. 2001, 276, 49258–49266. [Google Scholar] [CrossRef] [PubMed]
- Alphey, M.S.; Wolford, C.B.; MacNeill, S.A. Canonical binding of Chaetomium thermophilum DNA polymerase delta/zeta subunit PolD3 and flap endonuclease Fen1 to PCNA. Front. Mol. Biosci. 2023, 10, 1320648. [Google Scholar] [CrossRef]
- Bruning, J.B.; Shamoo, Y. Structural and thermodynamic analysis of human PCNA with peptides derived from DNA polymerase-delta p66 subunit and flap endonuclease-1. Structure 2004, 12, 2209–2219. [Google Scholar] [CrossRef] [PubMed]
- Podust, V.N.; Chang, L.S.; Ott, R.; Dianov, G.L.; Fanning, E. Reconstitution of human DNA polymerase delta using recombinant baculoviruses: The p12 subunit potentiates DNA polymerizing activity of the four-subunit enzyme. J. Biol. Chem. 2002, 277, 3894–3901. [Google Scholar] [CrossRef] [PubMed]
- Podust, V.N.; Podust, L.M.; Muller, F.; Hubscher, U. DNA polymerase delta holoenzyme: Action on single-stranded DNA and on double-stranded DNA in the presence of replicative DNA helicases. Biochemistry 1995, 34, 5003–5010. [Google Scholar] [CrossRef]
- Masuda, Y.; Suzuki, M.; Piao, J.; Gu, Y.; Tsurimoto, T.; Kamiya, K. Dynamics of human replication factors in the elongation phase of DNA replication. Nucleic Acids Res. 2007, 35, 6904–6916. [Google Scholar] [CrossRef]
- Hirota, K.; Tsuda, M.; Mohiuddin; Tsurimoto, T.; Cohen, I.S.; Livneh, Z.; Kobayashi, K.; Narita, T.; Nishihara, K.; Murai, J.; et al. In vivo evidence for translesion synthesis by the replicative DNA polymerase delta. Nucleic Acids Res. 2016, 44, 7242–7250. [Google Scholar]
- Baranovskiy, A.G.; Lada, A.G.; Siebler, H.M.; Zhang, Y.; Pavlov, Y.I.; Tahirov, T.H. DNA polymerase delta and zeta switch by sharing accessory subunits of DNA polymerase delta. J. Biol. Chem. 2012, 287, 17281–17287. [Google Scholar] [CrossRef]
- Johnson, R.E.; Prakash, L.; Prakash, S. Pol31 and Pol32 subunits of yeast DNA polymerase delta are also essential subunits of DNA polymerase zeta. Proc. Natl. Acad. Sci. USA 2012, 109, 12455–12460. [Google Scholar] [CrossRef] [PubMed]
- Makarova, A.V.; Stodola, J.L.; Burgers, P.M. A four-subunit DNA polymerase zeta complex containing Pol delta accessory subunits is essential for PCNA-mediated mutagenesis. Nucleic Acids Res. 2012, 40, 11618–11626. [Google Scholar] [CrossRef]
- Lee, Y.S.; Gregory, M.T.; Yang, W. Human Pol zeta purified with accessory subunits is active in translesion DNA synthesis and complements Pol eta in cisplatin bypass. Proc. Natl. Acad. Sci. USA 2014, 111, 2954–2959. [Google Scholar] [CrossRef]
- Hara, K.; Shimizu, T.; Unzai, S.; Akashi, S.; Sato, M.; Hashimoto, H. Purification, crystallization and initial X-ray diffraction study of human REV7 in complex with a REV3 fragment. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2009, 65, 1302–1305. [Google Scholar] [CrossRef]
- Rizzo, A.A.; Vassel, F.M.; Chatterjee, N.; D’Souza, S.; Li, Y.; Hao, B.; Hemann, M.T.; Walker, G.C.; Korzhnev, D.M. Rev7 dimerization is important for assembly and function of the Rev1/Polzeta translesion synthesis complex. Proc. Natl. Acad. Sci. USA 2018, 115, E8191–E8200. [Google Scholar] [CrossRef]
- Burgers, P.M.; Gerik, K.J. Structure and processivity of two forms of Saccharomyces cerevisiae DNA polymerase delta. J. Biol. Chem. 1998, 273, 19756–19762. [Google Scholar] [CrossRef]
- Netz, D.J.; Stith, C.M.; Stumpfig, M.; Kopf, G.; Vogel, D.; Genau, H.M.; Stodola, J.L.; Lill, R.; Burgers, P.M.; Pierik, A.J. Eukaryotic DNA polymerases require an iron-sulfur cluster for the formation of active complexes. Nat. Chem. Biol. 2011, 8, 125–132. [Google Scholar] [CrossRef]
- Nicolas, E.; Golemis, E.A.; Arora, S. POLD1: Central mediator of DNA replication and repair, and implication in cancer and other pathologies. Gene 2016, 590, 128–141. [Google Scholar] [CrossRef]
- Sakofsky, C.J.; Malkova, A. Break induced replication in eukaryotes: Mechanisms, functions, and consequences. Crit. Rev. Biochem. Mol. Biol. 2017, 52, 395–413. [Google Scholar] [CrossRef]
- Lee, R.S.; Twarowski, J.M.; Malkova, A. Stressed? Break-induced replication comes to the rescue! DNA Repair 2024, 142, 103759. [Google Scholar] [CrossRef]
- Liu, L.; Malkova, A. Break-induced replication: Unraveling each step. Trends Genet. 2022, 38, 752–765. [Google Scholar] [CrossRef]
- Epum, E.A.; Haber, J.E. DNA replication: The recombination connection. Trends Cell Biol. 2022, 32, 45–57. [Google Scholar] [CrossRef]
- Brenner, K.A.; Nandakumar, J. Consequences of telomere replication failure: The other end-replication problem. Trends Biochem. Sci. 2022, 47, 506–517. [Google Scholar] [CrossRef]
- Cesare, A.J.; Reddel, R.R. Alternative lengthening of telomeres: Models, mechanisms and implications. Nat. Rev. Genet. 2010, 11, 319–330. [Google Scholar] [CrossRef]
- Musmaker, K.; Wells, J.; Tsai, M.C.; Comeron, J.M.; Malkova, A. Alternative Lengthening of Telomeres in Yeast: Old Questions and New Approaches. Biomolecules 2024, 14, 113. [Google Scholar] [CrossRef]
- Minocherhomji, S.; Ying, S.; Bjerregaard, V.A.; Bursomanno, S.; Aleliunaite, A.; Wu, W.; Mankouri, H.W.; Shen, H.; Liu, Y.; Hickson, I.D. Replication stress activates DNA repair synthesis in mitosis. Nature 2015, 528, 286–290. [Google Scholar] [CrossRef]
- Ozer, O.; Hickson, I.D. Pathways for maintenance of telomeres and common fragile sites during DNA replication stress. Open Biol. 2018, 8, 180018. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.E.; Lam, A.F.; Symington, L.S. Aberrant double-strand break repair resulting in half crossovers in mutants defective for Rad51 or the DNA polymerase delta complex. Mol. Cell. Biol. 2009, 29, 1432–1441. [Google Scholar] [CrossRef] [PubMed]
- Kane, D.P.; Shusterman, M.; Rong, Y.; McVey, M. Competition between replicative and translesion polymerases during homologous recombination repair in Drosophila. PLoS Genet. 2012, 8, e1002659. [Google Scholar] [CrossRef] [PubMed]
- Donnianni, R.A.; Zhou, Z.X.; Lujan, S.A.; Al-Zain, A.; Garcia, V.; Glancy, E.; Burkholder, A.B.; Kunkel, T.A.; Symington, L.S. DNA Polymerase Delta Synthesizes Both Strands during Break-Induced Replication. Mol. Cell 2019, 76, 371–381 e374. [Google Scholar] [CrossRef]
- Wright, W.D.; Shah, S.S.; Heyer, W.D. Homologous recombination and the repair of DNA double-strand breaks. J. Biol. Chem. 2018, 293, 10524–10535. [Google Scholar] [CrossRef]
- Elbakry, A.; Lobrich, M. Homologous Recombination Subpathways: A Tangle to Resolve. Front. Genet. 2021, 12, 723847. [Google Scholar] [CrossRef]
- Bosco, G.; Haber, J.E. Chromosome break-induced DNA replication leads to nonreciprocal translocations and telomere capture. Genetics 1998, 150, 1037–1047. [Google Scholar] [CrossRef]
- Lydeard, J.R.; Jain, S.; Yamaguchi, M.; Haber, J.E. Break-induced replication and telomerase-independent telomere maintenance require Pol32. Nature 2007, 448, 820–823. [Google Scholar] [CrossRef]
- Bhandari, J.; Karg, T.; Golic, K.G. Homolog-Dependent Repair Following Dicentric Chromosome Breakage in Drosophila melanogaster. Genetics 2019, 212, 615–630. [Google Scholar] [CrossRef]
- Kramara, J.; Osia, B.; Malkova, A. Break-induced replication: An unhealthy choice for stress relief? Nat. Struct. Mol. Biol. 2017, 24, 11–12. [Google Scholar] [CrossRef]
- Strathern, J.N.; Shafer, B.K.; McGill, C.B. DNA synthesis errors associated with double-strand-break repair. Genetics 1995, 140, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.S.; Bruschi, C.V. Diploid yeast cells yield homozygous spontaneous mutations. Curr. Genet. 1993, 23, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Layer, J.V.; Debaize, L.; Van Scoyk, A.; House, N.C.; Brown, A.J.; Liu, Y.; Stevenson, K.E.; Hemann, M.; Roberts, S.A.; Price, B.D.; et al. Polymerase delta promotes chromosomal rearrangements and imprecise double-strand break repair. Proc. Natl. Acad. Sci. USA 2020, 117, 27566–27577. [Google Scholar] [CrossRef]
- Iraqui, I.; Chekkal, Y.; Jmari, N.; Pietrobon, V.; Freon, K.; Costes, A.; Lambert, S.A. Recovery of arrested replication forks by homologous recombination is error-prone. PLoS Genet. 2012, 8, e1002976. [Google Scholar] [CrossRef]
- Deem, A.; Keszthelyi, A.; Blackgrove, T.; Vayl, A.; Coffey, B.; Mathur, R.; Chabes, A.; Malkova, A. Break-induced replication is highly inaccurate. PLoS Biol. 2011, 9, e1000594. [Google Scholar] [CrossRef]
- Malkova, A.; Naylor, M.L.; Yamaguchi, M.; Ira, G.; Haber, J.E. RAD51-dependent break-induced replication differs in kinetics and checkpoint responses from RAD51-mediated gene conversion. Mol. Cell. Biol. 2005, 25, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.A.; Gordenin, D.A. Hypermutation in human cancer genomes: Footprints and mechanisms. Nat. Rev. Cancer 2014, 14, 786–800. [Google Scholar] [CrossRef]
- Sakofsky, C.J.; Roberts, S.A.; Malc, E.; Mieczkowski, P.A.; Resnick, M.A.; Gordenin, D.A.; Malkova, A. Break-induced replication is a source of mutation clusters underlying kataegis. Cell Rep. 2014, 7, 1640–1648. [Google Scholar] [CrossRef]
- Elango, R.; Osia, B.; Harcy, V.; Malc, E.; Mieczkowski, P.A.; Roberts, S.A.; Malkova, A. Repair of base damage within break-induced replication intermediates promotes kataegis associated with chromosome rearrangements. Nucleic Acids Res. 2019, 47, 9666–9684. [Google Scholar] [CrossRef]
- Osia, B.; Alsulaiman, T.; Jackson, T.; Kramara, J.; Oliveira, S.; Malkova, A. Cancer cells are highly susceptible to accumulation of templated insertions linked to MMBIR. Nucleic Acids Res. 2021, 49, 8714–8731. [Google Scholar] [CrossRef]
- Shaw, C.J.; Lupski, J.R. Implications of human genome architecture for rearrangement-based disorders: The genomic basis of disease. Hum. Mol. Genet. 2004, 13, R57–R64. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Carvalho, C.M.; Hastings, P.J.; Lupski, J.R. Mechanisms for recurrent and complex human genomic rearrangements. Curr. Opin. Genet. Dev. 2012, 22, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Sakofsky, C.J.; Ayyar, S.; Malkova, A. Break-induced replication and genome stability. Biomolecules 2012, 2, 483–504. [Google Scholar] [CrossRef]
- Setton, J.; Hadi, K.; Choo, Z.N.; Kuchin, K.S.; Tian, H.; Da Cruz Paula, A.; Rosiene, J.; Selenica, P.; Behr, J.; Yao, X.; et al. Long-molecule scars of backup DNA repair in BRCA1- and BRCA2-deficient cancers. Nature 2023, 621, 129–137. [Google Scholar] [CrossRef]
- Nick McElhinny, S.A.; Gordenin, D.A.; Stith, C.M.; Burgers, P.M.; Kunkel, T.A. Division of labor at the eukaryotic replication fork. Mol. Cell 2008, 30, 137–144. [Google Scholar] [CrossRef]
- Pursell, Z.F.; Isoz, I.; Lundstrom, E.B.; Johansson, E.; Kunkel, T.A. Yeast DNA polymerase epsilon participates in leading-strand DNA replication. Science 2007, 317, 127–130. [Google Scholar] [CrossRef]
- Fuchs, J.; Cheblal, A.; Gasser, S.M. Underappreciated Roles of DNA Polymerase delta in Replication Stress Survival. Trends Genet. 2021, 37, 476–487. [Google Scholar] [CrossRef]
- Saini, N.; Ramakrishnan, S.; Elango, R.; Ayyar, S.; Zhang, Y.; Deem, A.; Ira, G.; Haber, J.E.; Lobachev, K.S.; Malkova, A. Migrating bubble during break-induced replication drives conservative DNA synthesis. Nature 2013, 502, 389–392. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, H.; Jehi, S.; Li, J.; Liu, S.; Wang, Z.; Truong, L.; Chiba, T.; Wang, Z.; Wu, X. PIF1 helicase promotes break-induced replication in mammalian cells. EMBO J. 2021, 40, e104509. [Google Scholar] [CrossRef]
- Anand, R.; Buechelmaier, E.; Belan, O.; Newton, M.; Vancevska, A.; Kaczmarczyk, A.; Takaki, T.; Rueda, D.S.; Powell, S.N.; Boulton, S.J. HELQ is a dual-function DSB repair enzyme modulated by RPA and RAD51. Nature 2022, 601, 268–273. [Google Scholar] [CrossRef]
- Buzovetsky, O.; Kwon, Y.; Pham, N.T.; Kim, C.; Ira, G.; Sung, P.; Xiong, Y. Role of the Pif1-PCNA Complex in Pol delta-Dependent Strand Displacement DNA Synthesis and Break-Induced Replication. Cell Rep. 2017, 21, 1707–1714. [Google Scholar] [CrossRef] [PubMed]
- Kotenko, O.; Makovets, S. The functional significance of the RPA- and PCNA-dependent recruitment of Pif1 to DNA. EMBO Rep. 2024, 25, 1734–1751. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, S.; Kockler, Z.; Evans, R.; Downing, B.D.; Malkova, A. Single-strand annealing between inverted DNA repeats: Pathway choice, participating proteins, and genome destabilizing consequences. PLoS Genet. 2018, 14, e1007543. [Google Scholar] [CrossRef] [PubMed]
- Osia, B.; Twarowski, J.; Jackson, T.; Lobachev, K.; Liu, L.; Malkova, A. Migrating bubble synthesis promotes mutagenesis through lesions in its template. Nucleic Acids Res. 2022, 50, 6870–6889. [Google Scholar] [CrossRef] [PubMed]
- Ait Saada, A.; Guo, W.; Costa, A.B.; Yang, J.; Wang, J.; Lobachev, K.S. Widely spaced and divergent inverted repeats become a potent source of chromosomal rearrangements in long single-stranded DNA regions. Nucleic Acids Res. 2023, 51, 3722–3734. [Google Scholar] [CrossRef]
- Holbeck, S.L.; Strathern, J.N. A role for REV3 in mutagenesis during double-strand break repair in Saccharomyces cerevisiae. Genetics 1997, 147, 1017–1024. [Google Scholar] [CrossRef]
- Lawrence, C.W. Cellular roles of DNA polymerase zeta and Rev1 protein. DNA Repair 2002, 1, 425–435. [Google Scholar] [CrossRef]
- Martin, S.K.; Wood, R.D. DNA polymerase zeta in DNA replication and repair. Nucleic Acids Res. 2019, 47, 8348–8361. [Google Scholar] [CrossRef]
- Malik, R.; Johnson, R.E.; Prakash, L.; Prakash, S.; Ubarretxena-Belandia, I.; Aggarwal, A.K. Cryo-EM structure of translesion DNA synthesis polymerase zeta with a base pair mismatch. Nat. Commun. 2022, 13, 1050. [Google Scholar] [CrossRef]
- Escobar, T.M.; Loyola, A.; Reinberg, D. Parental nucleosome segregation and the inheritance of cellular identity. Nat. Rev. Genet. 2021, 22, 379–392. [Google Scholar] [CrossRef]
- Tian, C.; Zhang, Q.; Jia, J.; Zhou, J.; Zhang, Z.; Karri, S.; Jiang, J.; Dickinson, Q.; Yao, Y.; Tang, X.; et al. DNA polymerase delta governs parental histone transfer to DNA replication lagging strand. Proc. Natl. Acad. Sci. USA 2024, 121, e2400610121. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Yang, C.; Wu, J.; Lei, Y.; Hu, J.; Feng, J.; Li, Q. DNA polymerase delta subunit Pol32 binds histone H3-H4 and couples nucleosome assembly with Okazaki fragment processing. Sci. Adv. 2024, 10, eado1739. [Google Scholar] [CrossRef] [PubMed]
- Serra-Cardona, A.; Hua, X.; McNutt, S.W.; Zhou, H.; Toda, T.; Jia, S.; Chu, F.; Zhang, Z. The PCNA-Pol delta complex couples lagging strand DNA synthesis to parental histone transfer for epigenetic inheritance. Sci. Adv. 2024, 10, eadn5175. [Google Scholar] [CrossRef]
- Liu, G.; Warbrick, E. The p66 and p12 subunits of DNA polymerase delta are modified by ubiquitin and ubiquitin-like proteins. Biochem. Biophys. Res. Commun. 2006, 349, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, I.A.; Lyon, D.; Su, D.; Skotte, N.H.; Daniel, J.A.; Jensen, L.J.; Nielsen, M.L. Site-specific characterization of endogenous SUMOylation across species and organs. Nat. Commun. 2018, 9, 2456. [Google Scholar] [CrossRef]
- Impens, F.; Radoshevich, L.; Cossart, P.; Ribet, D. Mapping of SUMO sites and analysis of SUMOylation changes induced by external stimuli. Proc. Natl. Acad. Sci. USA 2014, 111, 12432–12437. [Google Scholar] [CrossRef]
- Bursomanno, S.; Beli, P.; Khan, A.M.; Minocherhomji, S.; Wagner, S.A.; Bekker-Jensen, S.; Mailand, N.; Choudhary, C.; Hickson, I.D.; Liu, Y. Proteome-wide analysis of SUMO2 targets in response to pathological DNA replication stress in human cells. DNA Repair 2015, 25, 84–96. [Google Scholar] [CrossRef]
- Lopez-Contreras, A.J.; Ruppen, I.; Nieto-Soler, M.; Murga, M.; Rodriguez-Acebes, S.; Remeseiro, S.; Rodrigo-Perez, S.; Rojas, A.M.; Mendez, J.; Munoz, J.; et al. A proteomic characterization of factors enriched at nascent DNA molecules. Cell Rep. 2013, 3, 1105–1116. [Google Scholar] [CrossRef]
- Lemmens, L.; Urbach, S.; Prudent, R.; Cochet, C.; Baldacci, G.; Hughes, P. Phosphorylation of the C subunit (p66) of human DNA polymerase delta. Biochem. Biophys. Res. Commun. 2008, 367, 264–270. [Google Scholar] [CrossRef]
- Litchfield, D.W. Protein kinase CK2: Structure, regulation and role in cellular decisions of life and death. Biochem. J. 2003, 369, 1–15. [Google Scholar] [CrossRef]
- Rahmeh, A.A.; Zhou, Y.; Xie, B.; Li, H.; Lee, E.Y.; Lee, M.Y. Phosphorylation of the p68 subunit of Pol delta acts as a molecular switch to regulate its interaction with PCNA. Biochemistry 2012, 51, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Olsen, J.V.; Blagoev, B.; Gnad, F.; Macek, B.; Kumar, C.; Mortensen, P.; Mann, M. Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell 2006, 127, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Larsen, S.C.; Hendriks, I.A.; Lyon, D.; Jensen, L.J.; Nielsen, M.L. Systems-wide Analysis of Serine ADP-Ribosylation Reveals Widespread Occurrence and Site-Specific Overlap with Phosphorylation. Cell Rep. 2018, 24, 2493–2505 e2494. [Google Scholar] [CrossRef]
- Buch-Larsen, S.C.; Hendriks, I.A.; Lodge, J.M.; Rykaer, M.; Furtwangler, B.; Shishkova, E.; Westphall, M.S.; Coon, J.J.; Nielsen, M.L. Mapping Physiological ADP-Ribosylation Using Activated Ion Electron Transfer Dissociation. Cell Rep. 2020, 32, 108176. [Google Scholar] [CrossRef] [PubMed]
- Richards, F.; Llorca-Cardenosa, M.J.; Langton, J.; Buch-Larsen, S.C.; Shamkhi, N.F.; Sharma, A.B.; Nielsen, M.L.; Lakin, N.D. Regulation of Rad52-dependent replication fork recovery through serine ADP-ribosylation of PolD3. Nat. Commun. 2023, 14, 4310. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.E.; de Calignon, A.; Nicolas, A.; Galibert, F. POL32, a subunit of the Saccharomyces cerevisiae DNA polymerase delta, defines a link between DNA replication and the mutagenic bypass repair pathway. Curr. Genet. 2000, 38, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Kamath-Loeb, A.S.; Johansson, E.; Burgers, P.M.; Loeb, L.A. Functional interaction between the Werner Syndrome protein and DNA polymerase delta. Proc. Natl. Acad. Sci. USA 2000, 97, 4603–4608. [Google Scholar] [CrossRef]
- Kamath-Loeb, A.S.; Loeb, L.A.; Johansson, E.; Burgers, P.M.; Fry, M. Interactions between the Werner syndrome helicase and DNA polymerase delta specifically facilitate copying of tetraplex and hairpin structures of the d(CGG)n trinucleotide repeat sequence. J. Biol. Chem. 2001, 276, 16439–16446. [Google Scholar] [CrossRef]
- Cohen, S.; Guenole, A.; Lazar, I.; Marnef, A.; Clouaire, T.; Vernekar, D.V.; Puget, N.; Rocher, V.; Arnould, C.; Aguirrebengoa, M.; et al. A POLD3/BLM dependent pathway handles DSBs in transcribed chromatin upon excessive RNA:DNA hybrid accumulation. Nat. Commun. 2022, 13, 2012. [Google Scholar] [CrossRef]
- He, L.; Lever, R.; Cubbon, A.; Tehseen, M.; Jenkins, T.; Nottingham, A.O.; Horton, A.; Betts, H.; Fisher, M.; Hamdan, S.M.; et al. Interaction of human HelQ with DNA polymerase delta halts DNA synthesis and stimulates DNA single-strand annealing. Nucleic Acids Res. 2023, 51, 1740–1749. [Google Scholar] [CrossRef]
- Szekely, A.M.; Chen, Y.H.; Zhang, C.; Oshima, J.; Weissman, S.M. Werner protein recruits DNA polymerase delta to the nucleolus. Proc. Natl. Acad. Sci. USA 2000, 97, 11365–11370. [Google Scholar] [CrossRef] [PubMed]
- Kolesar, P.; Altmannova, V.; Silva, S.; Lisby, M.; Krejci, L. Pro-recombination Role of Srs2 Protein Requires SUMO (Small Ubiquitin-like Modifier) but Is Independent of PCNA (Proliferating Cell Nuclear Antigen) Interaction. J. Biol. Chem. 2016, 291, 7594–7607. [Google Scholar] [CrossRef]
- Zhang, J.M.; Genois, M.M.; Ouyang, J.; Lan, L.; Zou, L. Alternative lengthening of telomeres is a self-perpetuating process in ALT-associated PML bodies. Mol. Cell 2021, 81, 1027–1042 e1024. [Google Scholar] [CrossRef]
- Min, J.; Wright, W.E.; Shay, J.W. Clustered telomeres in phase-separated nuclear condensates engage mitotic DNA synthesis through BLM and RAD52. Genes. Dev. 2019, 33, 814–827. [Google Scholar] [CrossRef] [PubMed]
- Sobinoff, A.P.; Allen, J.A.; Neumann, A.A.; Yang, S.F.; Walsh, M.E.; Henson, J.D.; Reddel, R.R.; Pickett, H.A. BLM and SLX4 play opposing roles in recombination-dependent replication at human telomeres. EMBO J. 2017, 36, 2907–2919. [Google Scholar] [CrossRef]
- Ward, J.D.; Muzzini, D.M.; Petalcorin, M.I.; Martinez-Perez, E.; Martin, J.S.; Plevani, P.; Cassata, G.; Marini, F.; Boulton, S.J. Overlapping mechanisms promote postsynaptic RAD-51 filament disassembly during meiotic double-strand break repair. Mol. Cell 2010, 37, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, T.; Northall, S.J.; Ptchelkine, D.; Lever, R.; Cubbon, A.; Betts, H.; Taresco, V.; Cooper, C.D.O.; McHugh, P.J.; Soultanas, P.; et al. The HelQ human DNA repair helicase utilizes a PWI-like domain for DNA loading through interaction with RPA, triggering DNA unwinding by the HelQ helicase core. NAR Cancer 2021, 3, zcaa043. [Google Scholar] [CrossRef]
- Kamp, J.A.; Lemmens, B.; Romeijn, R.J.; Changoer, S.C.; van Schendel, R.; Tijsterman, M. Helicase Q promotes homology-driven DNA double-strand break repair and prevents tandem duplications. Nat. Commun. 2021, 12, 7126. [Google Scholar] [CrossRef]
- Reint, G.; Li, Z.; Labun, K.; Keskitalo, S.; Soppa, I.; Mamia, K.; Tolo, E.; Szymanska, M.; Meza-Zepeda, L.A.; Lorenz, S.; et al. Rapid genome editing by CRISPR-Cas9-POLD3 fusion. eLife 2021, 10, e75415. [Google Scholar] [CrossRef]
- Killelea, T.; Kemm, F.E.; He, L.; Rudolph, C.J.; Bolt, E.L. Repurposing Proximity-Dependent Protein Labeling (BioID2) for Protein Interaction Mapping in E. coli. Methods Mol. Biol. 2024, 2828, 87–106. [Google Scholar]
- Havugimana, P.C.; Goel, R.K.; Phanse, S.; Youssef, A.; Padhorny, D.; Kotelnikov, S.; Kozakov, D.; Emili, A. Scalable multiplex co-fractionation/mass spectrometry platform for accelerated protein interactome discovery. Nat. Commun. 2022, 13, 4043. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alli, N.; Lou-Hing, A.; Bolt, E.L.; He, L. POLD3 as Controller of Replicative DNA Repair. Int. J. Mol. Sci. 2024, 25, 12417. https://doi.org/10.3390/ijms252212417
Alli N, Lou-Hing A, Bolt EL, He L. POLD3 as Controller of Replicative DNA Repair. International Journal of Molecular Sciences. 2024; 25(22):12417. https://doi.org/10.3390/ijms252212417
Chicago/Turabian StyleAlli, Nabilah, Anna Lou-Hing, Edward L. Bolt, and Liu He. 2024. "POLD3 as Controller of Replicative DNA Repair" International Journal of Molecular Sciences 25, no. 22: 12417. https://doi.org/10.3390/ijms252212417
APA StyleAlli, N., Lou-Hing, A., Bolt, E. L., & He, L. (2024). POLD3 as Controller of Replicative DNA Repair. International Journal of Molecular Sciences, 25(22), 12417. https://doi.org/10.3390/ijms252212417





