Human-Induced Pluripotent Stem Cell-Derived Neural Organoids as a Novel In Vitro Platform for Developmental Neurotoxicity Assessment
Abstract
1. Introduction
2. Results
2.1. Effects of Growth Factors on the Surface Construction of Neural Organoids
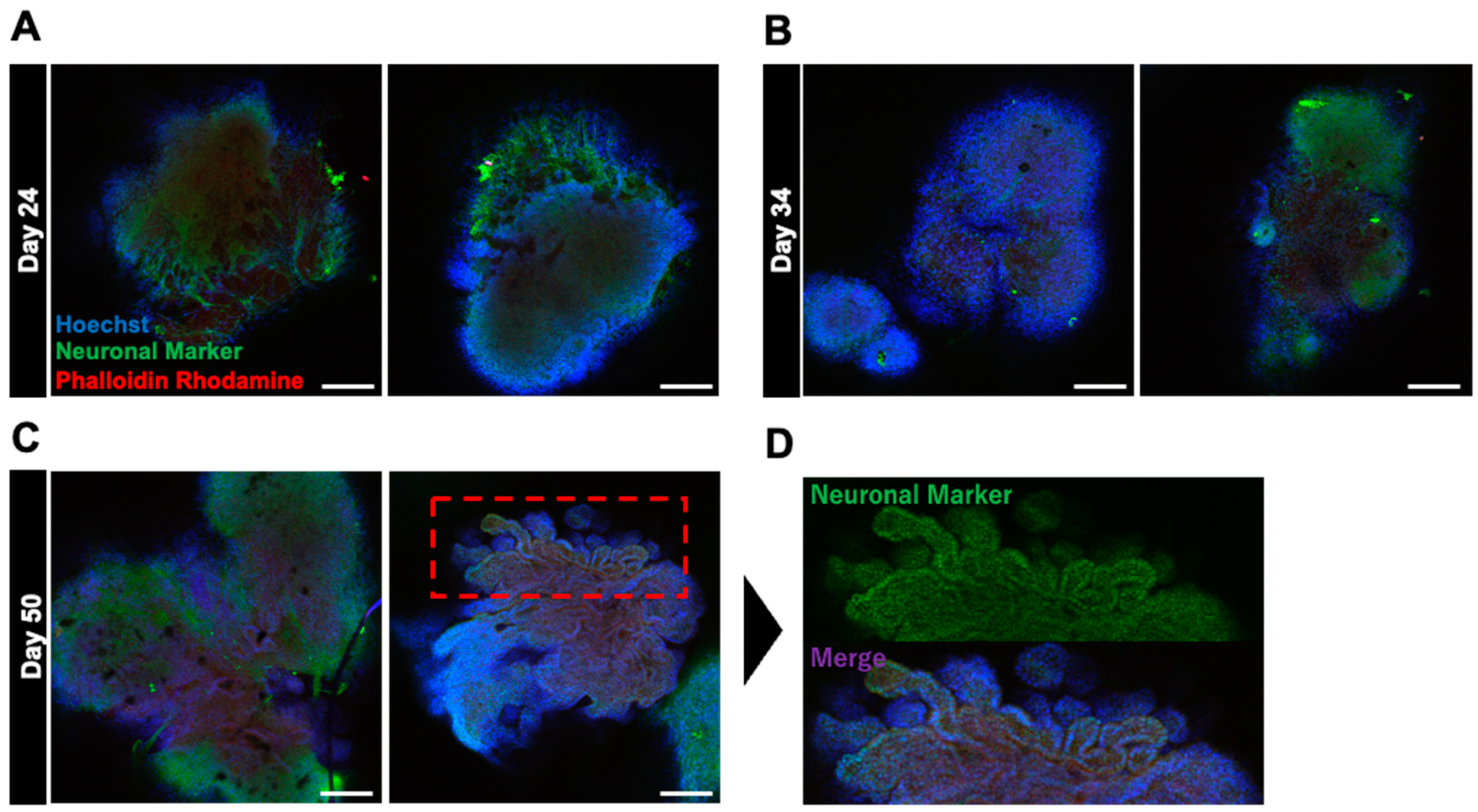
2.2. Fabrication and Analysis of the Internal Structure of Neural Organoids
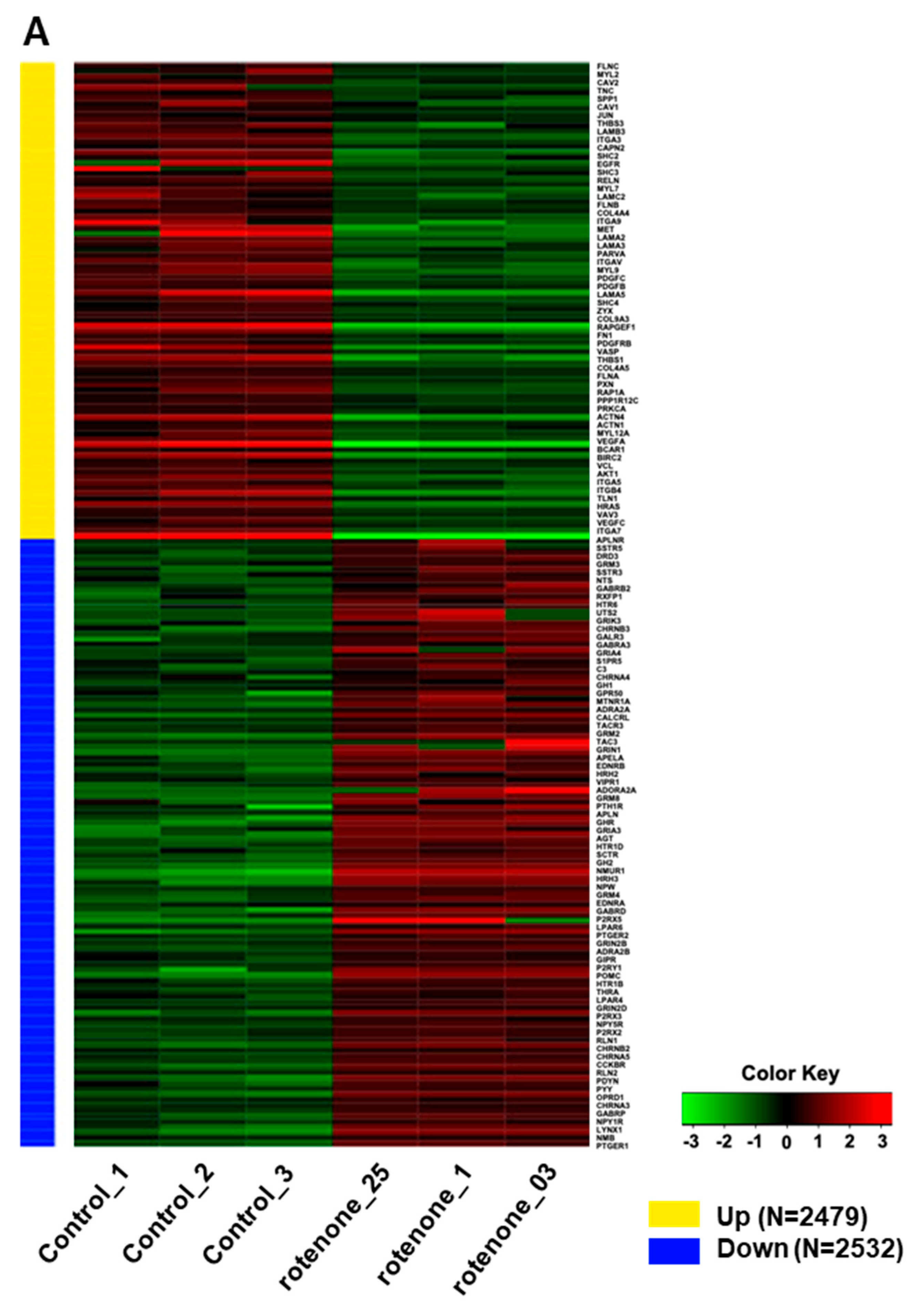
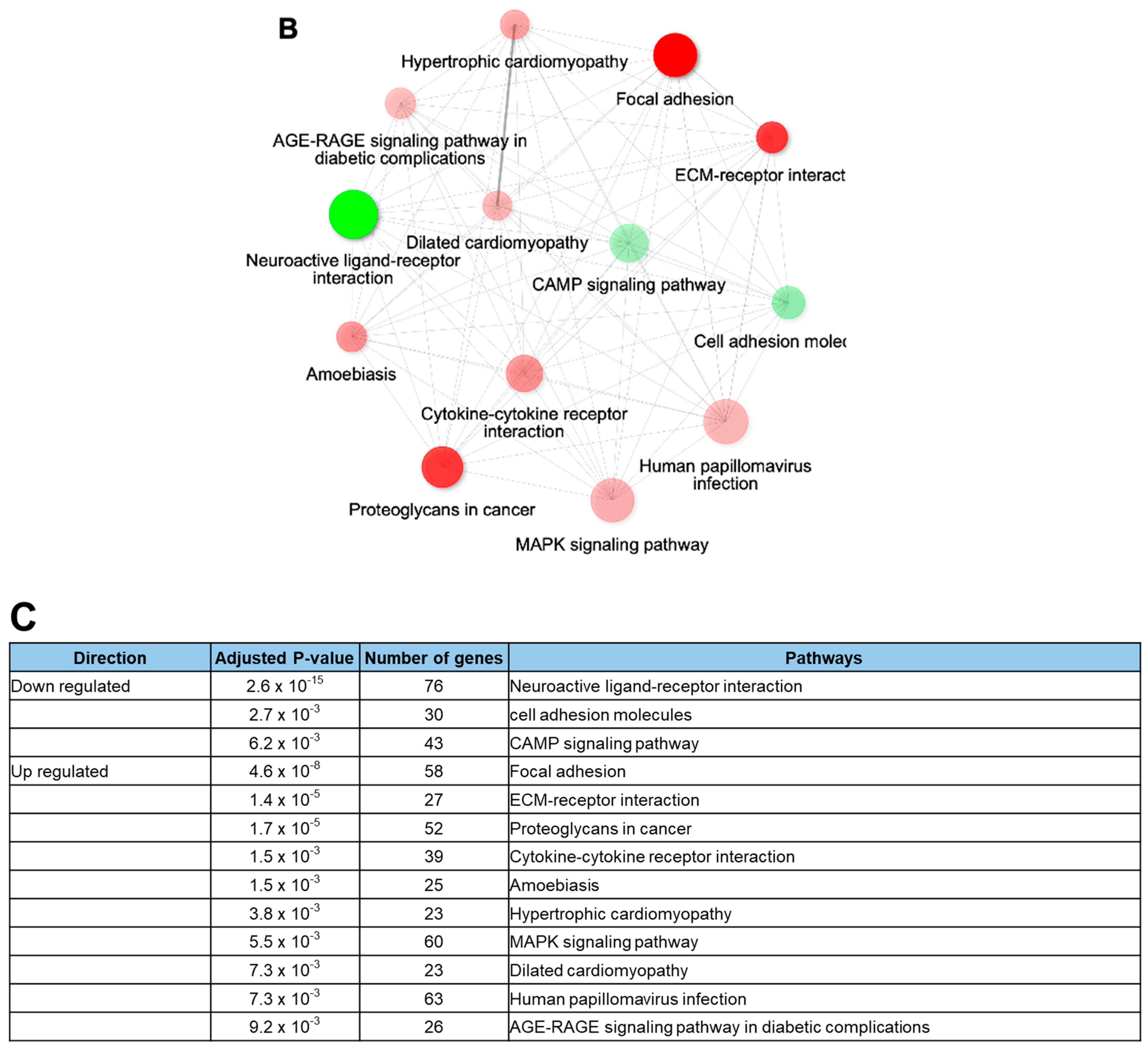
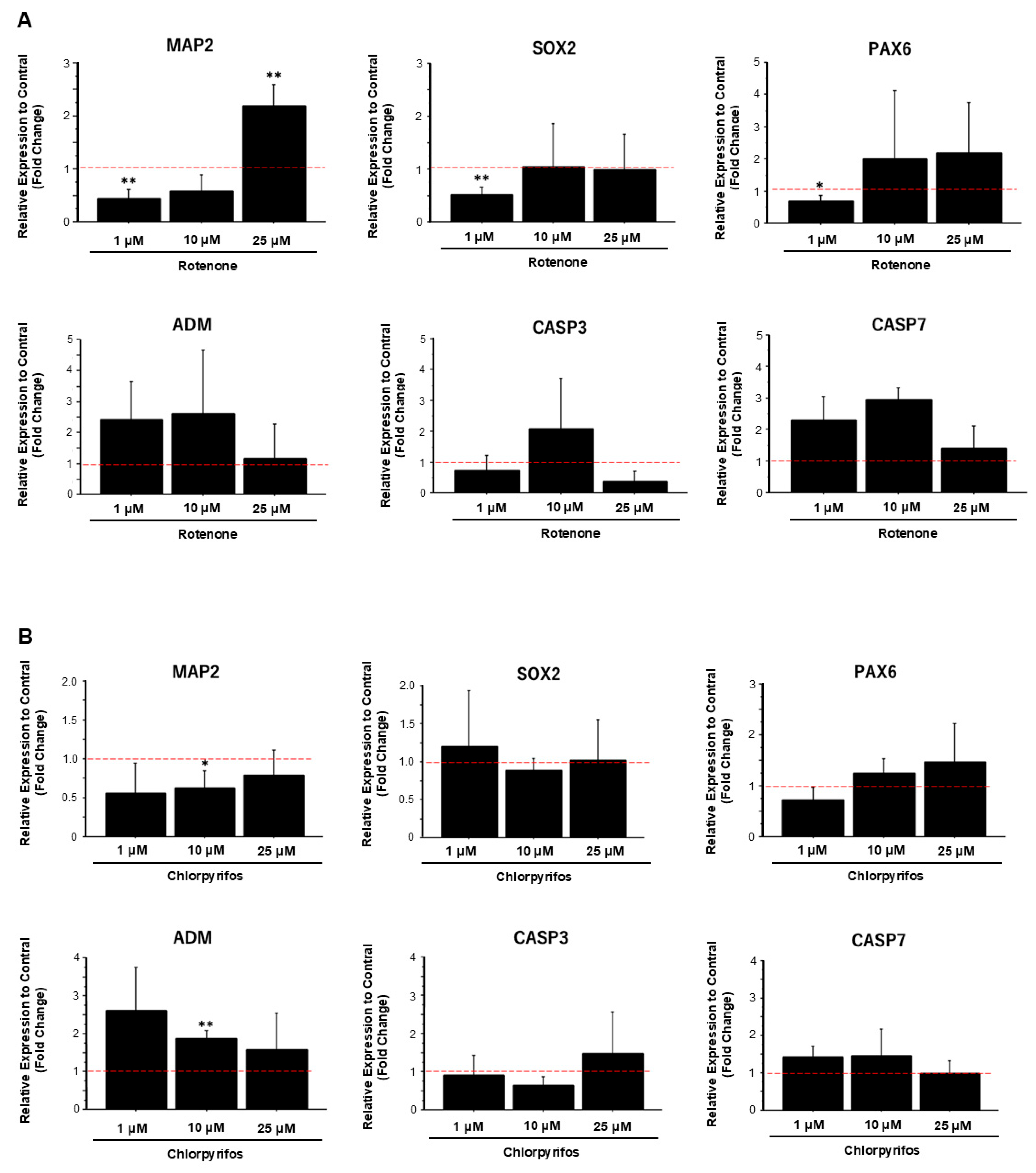
2.3. Rotenone Suppresses Neurodevelopment and the Expression of Synaptic Transmission-Related Genes
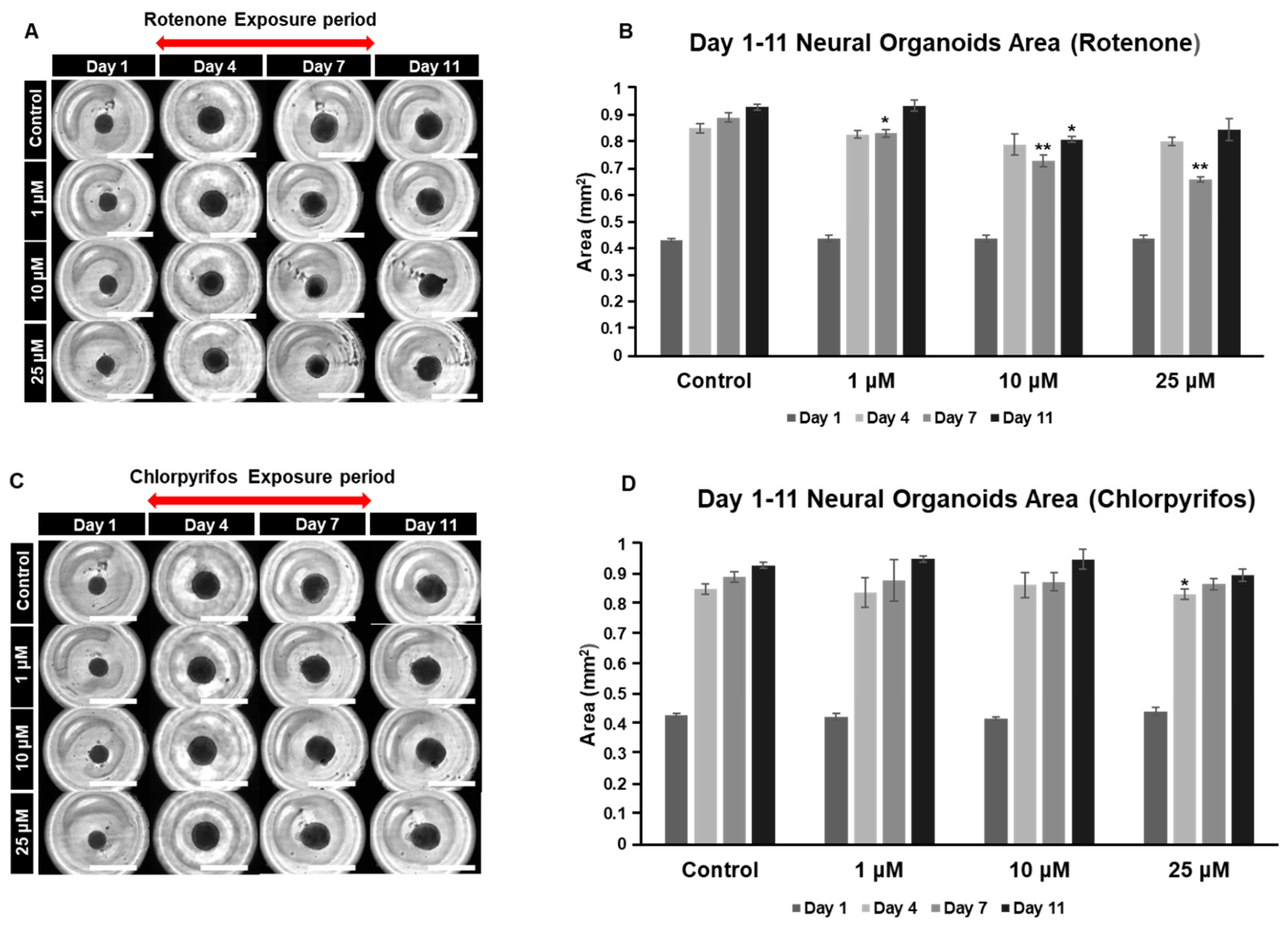
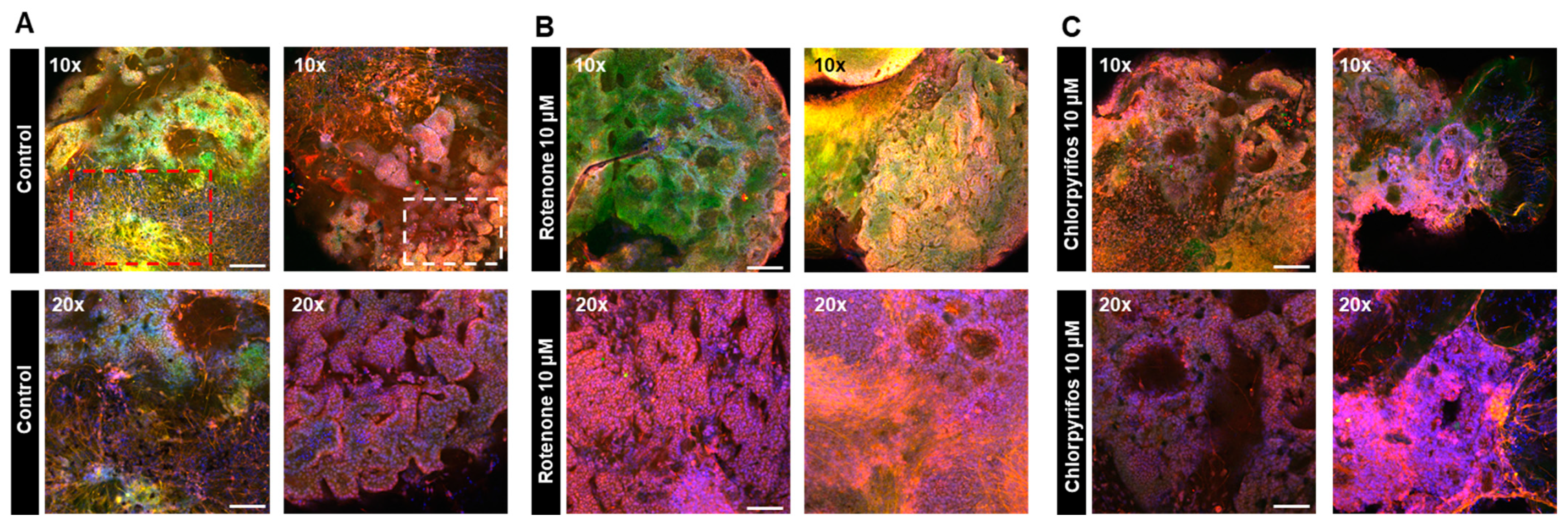
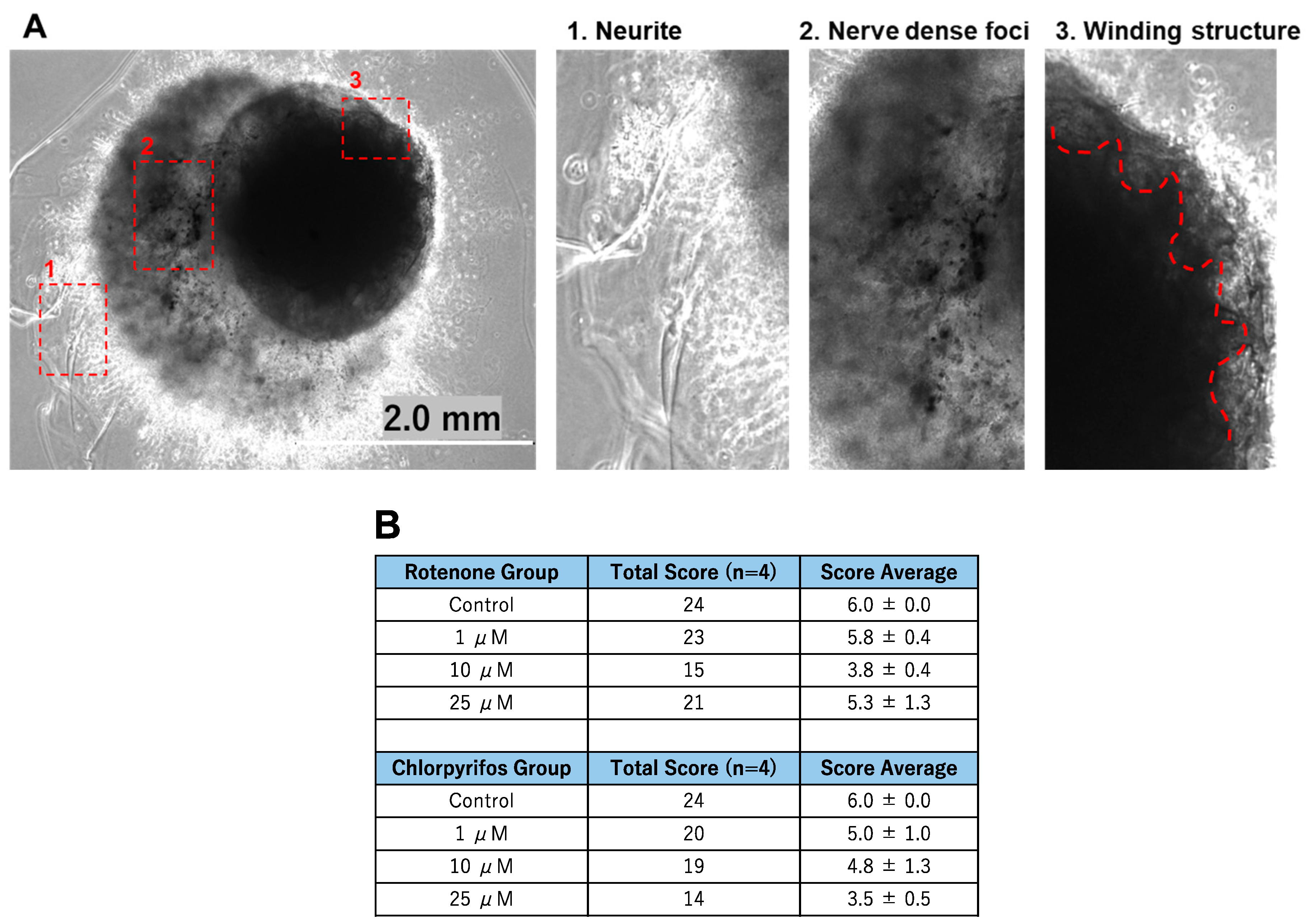
2.4. Rotenone and Chlorpyrifos Cause Neurodevelopmental Inhibition
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Maintenance Culture of Human iPSCs
4.3. Differentiation of Neural Organoids
4.4. RNA Sequencing Analysis
4.5. DNT of Rotenone and Chlorpyrifos
4.6. RNA Extraction
4.7. Gene Expression Analysis
4.8. Fluorescence Immunostaining
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Breijyeh, Z.; Karaman, R. Comprehensive review on Alzheimer’s disease: Causes and treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef] [PubMed]
- Dubonyte, U.; Asenjo-Martinez, A.; Werge, T.; Lage, K.; Kirkeby, A. Current advancements of modelling schizophrenia using patient-derived induced pluripotent stem cells. Acta Neuropathol. Commun. 2022, 10, 183. [Google Scholar] [CrossRef] [PubMed]
- Purebl, G.; Schnitzspahn, K.; Zsák, É. Overcoming treatment gaps in the management of depression with non-pharmacological adjunctive strategies. Front. Psychiatry 2023, 14, 1268194. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, M.A.; Renner, M.; Martin, C.-A.; Wenzel, D.; Bicknell, L.S.; Hurles, M.E.; Homfray, T.; Penninger, J.M.; Jackson, A.P.; Knoblich, J.A. Cerebral organoids model human brain development and microcephaly. Nature 2013, 501, 373–379. [Google Scholar] [CrossRef]
- Paşca, A.M.; Sloan, S.A.; Clarke, L.E.; Tian, Y.; Paşca, S.P. Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat. Methods 2015, 12, 671–678. [Google Scholar] [CrossRef]
- Quadrato, G.; Nguyen, T.; Macosko, E.Z.; Sherwood, J.L.; Min Yang, S.; Berger, D.R.; Maria, N.; Scholvin, J.; Goldman, M.; Kinney, J.P.; et al. Cell diversity and network dynamics in photosensitive human brain organoids. Nature 2017, 545, 48–53. [Google Scholar] [CrossRef]
- Xiang, Y.; Tanaka, Y.; Patterson, B.; Kang, Y.J.; Govindaiah, G.; Roselaar, N.; Cakir, B.; Kim, K.; Lombroso, A.P.; Hwang, S.; et al. Fusion of regionally specified hPSC-derived organoids models human brain development and interneuron migration. Cell Stem Cell 2017, 21, 383–398. [Google Scholar] [CrossRef]
- Kuhl, P.K. Early language acquisition: Cracking the speech code. Nat. Rev. Neurosci. 2004, 5, 831–843. [Google Scholar] [CrossRef]
- Lledo, P.M.; Alonso, M.; Grubb, M.S. Adult neurogenesis and functional plasticity in neuronal circuits. Nat. Rev. Neurosci. 2006, 7, 179–193. [Google Scholar] [CrossRef]
- Gage, F.H. Neurogenesis in the adult brain. J. Neurosci. 2002, 22, 612–613. [Google Scholar] [CrossRef]
- Malave, L.; van Dijk, M.T.; Anacker, C. Early life adversity shapes neural circuit function during sensitive postnatal developmental periods. Transl. Psychiatry 2022, 12, 306. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.A.; Yamaguchi, Y.; Goto, Y. Neurodevelopmental plasticity in pre- and postnatal environmental interactions: Implications for psychiatric disorders from an evolutionary perspective. Neural Plast. 2015, 2015, 291476. [Google Scholar] [CrossRef] [PubMed]
- Hass, U. The need for developmental neurotoxicity studies in risk assessment for developmental toxicity. Reprod. Toxicol. 2006, 22, 148–156. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Imanishi, S.; Sone, H.; Nagano, R.; Qin, X.Y.; Yoshinaga, J.; Akanuma, H.; Yamane, J.; Fujibuchi, W.; Ohsako, S. Effects of methylmercury exposure on neuronal differentiation of mouse and human embryonic stem cells. Toxicol. Lett. 2012, 212, 1–10. [Google Scholar] [CrossRef]
- Fujii, T.; Yamada, S.; Yamaguchi, N.; Fujimoto, K.; Suzuki, T.; Kawashima, K. Species differences in the concentration of acetylcholine, a neurotransmitter, in whole blood and plasma. Neurosci. Lett. 1995, 201, 207–210. [Google Scholar] [CrossRef]
- Fitzgerald, P.J. Neuromodulating mice and men: Are there functional species differences in neurotransmitter concentration? Neurosci. Biobehav. Rev. 2009, 33, 1037–1041. [Google Scholar] [CrossRef]
- Bal-Price, A.; Pistollato, F.; Sachana, M.; Bopp, S.K.; Munn, S.; Worth, A. Strategies to improve the regulatory assessment of developmental neurotoxicity (DNT) using in vitro methods. Toxicol. Appl. Pharmacol. 2018, 354, 7–18. [Google Scholar] [CrossRef]
- Schantz, S.L.; Eskenazi, B.; Buckley, J.P.; Braun, J.M.; Sprowles, J.N.; Bennett, D.H.; Cordero, J.; Frazier, J.A.; Lewis, J.; Hertz-Picciotto, I.; et al. A framework for assessing the impact of chemical exposures on neurodevelopment in ECHO: Opportunities and challenges. Environ. Res. 2020, 188, 109709. [Google Scholar] [CrossRef]
- Kortenkamp, A. Ten years of mixing cocktails: A review of combination effects of endocrine-disrupting chemicals. Environ. Health Perspect. 2007, 115, 98–105. [Google Scholar] [CrossRef]
- Petrović, D.J.; Jagečić, D.; Krasić, J.; Sinčić, N.; Mitrečić, D. Effect of Fetal Bovine Serum or Basic Fibroblast Growth Factor on Cell Survival and the Proliferation of Neural Stem Cells: The Influence of Homocysteine Treatment. Int. J. Mol. Sci. 2023, 24, 14161. [Google Scholar] [CrossRef]
- Szebényi, K.; Wenger, L.M.D.; Sun, Y.; Dunn, A.W.E.; Limegrover, C.A.; Gibbons, G.M.; Conci, E.; Paulsen, O.; Mierau, S.B.; Balmus, G.; et al. Human ALS/FTD brain organoid slice cultures display distinct early astrocyte and targetable neuronal pathology. Nat. Neurosci. 2021, 24, 1542–1554. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Sun, G.; Tian, E.; Zhang, M.; Davtyan, H.; Beach, T.G.; Reiman, E.M.; Blurton-Jones, M.; Holtzman, D.M.; Shi, Y. Modeling sporadic Alzheimer’s disease in human brain organoids under serum exposure. Adv. Sci. 2021, 8, e2101462. [Google Scholar] [CrossRef] [PubMed]
- Hogberg, H.T.; Smirnova, L. The future of 3D brain cultures in developmental neurotoxicity testing. Front. Toxicol. 2022, 4, 808620. [Google Scholar] [CrossRef] [PubMed]
- Chesnut, M.; Hartung, T.; Hogberg, H.; Pamies, D. Human oligodendrocytes and myelin in vitro to evaluate developmental neurotoxicity. Int. J. Mol. Sci. 2021, 22, 7929. [Google Scholar] [CrossRef]
- Kim, S.H.; Chang, M.Y. Application of human brain organoids-opportunities and challenges in modeling human brain development and neurodevelopmental diseases. Int. J. Mol. Sci. 2023, 24, 12528. [Google Scholar] [CrossRef]
- Li, J.; Spletter, M.L.; Johnson, D.A.; Wright, L.S.; Svendsen, C.N.; Johnson, J.A. Rotenone-induced caspase 9/3-independent and -dependent cell death in undifferentiated and differentiated human neural stem cells. J. Neurochem. 2005, 92, 462–476. [Google Scholar] [CrossRef]
- Rocha, E.M.; Hatcher, N.G.; Greenamyre, J.T. LRRK2 inhibition prevents endolysosomal deficits seen in human Parkinson’s disease. Neurobiol. Dis. 2020, 134, 104626. [Google Scholar] [CrossRef]
- Tanner, C.M.; Kamel, F.; Ross, G.W.; Hoppin, J.A.; Goldman, S.M.; Korell, M.; Marras, C.; Bhudhikanok, G.S.; Kasten, M.; Chade, A.R.; et al. Rotenone, paraquat, and Parkinson’s disease. Environ. Health Perspect. 2011, 119, 866–872. [Google Scholar] [CrossRef]
- Betarbet, R.; Sherer, T.B.; MacKenzie, G.; Garcia-Osuna, M.; Panov, A.V.; Greenamyre, J.T. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat. Neurosci. 2000, 3, 1301–1306. [Google Scholar] [CrossRef]
- Sindhu, K.M.; Saravanan, K.S.; Mohanakumar, K.P. Behavioral differences in a rotenone-induced hemiparkinsonian rat model developed following intranigral or median forebrain bundle infusion. Brain Res. 2005, 1051, 25–34. [Google Scholar] [CrossRef]
- Zagoura, D.; Canovas-Jorda, D.; Pistollato, F.; Bremer-Hoffmann, S.; Bal-Price, A. Evaluation of the rotenone-induced activation of the Nrf2 pathway in a neuronal model derived from human induced pluripotent stem cells. Neurochem. Int. 2017, 106, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Fleming, S.M.; Zhu, C.; Fernagut, P.-O.; Mehta, A.; DiCarlo, C.D.; Seaman, R.L.; Chesselet, M.-F. Behavioral and immunohistochemical effects of chronic intravenous and subcutaneous infusions of varying doses of rotenone. Exp. Neurol. 2004, 187, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Richter, F.; Hamann, M.; Richter, A. Chronic rotenone treatment induces behavioral effects but no pathological signs of parkinsonism in mice. J. Neurosci. Res. 2007, 85, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Pamies, D.; Block, K.; Lau, P.; Gribaldo, L.; Pardo, C.A.; Barreras, P.; Smirnova, L.; Wiersma, D.; Zhao, L.; Harris, G.; et al. Rotenone exerts developmental neurotoxicity in a human brain spheroid model. Toxicol. Appl. Pharmacol. 2018, 354, 101–114. [Google Scholar] [CrossRef]
- Di Consiglio, E.; Pistollato, F.; Mendoza-De Gyves, E.; Bal-Price, A.; Testai, E. Integrating biokinetics and in vitro studies to evaluate developmental neurotoxicity induced by chlorpyrifos in human iPSC-derived neural stem cells undergoing differentiation towards neuronal and glial cells. Reprod. Toxicol. 2020, 98, 174–188. [Google Scholar] [CrossRef]
- Grandjean, P.; Landrigan, P.J. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014, 13, 330–338. [Google Scholar] [CrossRef]
- Balogh, A.; Bódi-Jakus, M.; Karl, V.R.; Bellák, T.; Széky, B.; Farkas, J.; Lamberto, F.; Novak, D.; Fehér, A.; Zana, M.; et al. Establishment of human pluripotent stem cell-derived cortical neurosphere model to study pathomechanisms and chemical toxicity in Kleefstra syndrome. Sci. Rep. 2024, 14, 22572. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hongen, T.; Sakai, K.; Ito, T.; Qin, X.-Y.; Sone, H. Human-Induced Pluripotent Stem Cell-Derived Neural Organoids as a Novel In Vitro Platform for Developmental Neurotoxicity Assessment. Int. J. Mol. Sci. 2024, 25, 12523. https://doi.org/10.3390/ijms252312523
Hongen T, Sakai K, Ito T, Qin X-Y, Sone H. Human-Induced Pluripotent Stem Cell-Derived Neural Organoids as a Novel In Vitro Platform for Developmental Neurotoxicity Assessment. International Journal of Molecular Sciences. 2024; 25(23):12523. https://doi.org/10.3390/ijms252312523
Chicago/Turabian StyleHongen, Tsunehiko, Kenta Sakai, Tomohiro Ito, Xian-Yang Qin, and Hideko Sone. 2024. "Human-Induced Pluripotent Stem Cell-Derived Neural Organoids as a Novel In Vitro Platform for Developmental Neurotoxicity Assessment" International Journal of Molecular Sciences 25, no. 23: 12523. https://doi.org/10.3390/ijms252312523
APA StyleHongen, T., Sakai, K., Ito, T., Qin, X.-Y., & Sone, H. (2024). Human-Induced Pluripotent Stem Cell-Derived Neural Organoids as a Novel In Vitro Platform for Developmental Neurotoxicity Assessment. International Journal of Molecular Sciences, 25(23), 12523. https://doi.org/10.3390/ijms252312523







