Characterization of Platelet Receptors and Their Involvement in Immune Activation of These Cells
Abstract
1. Introduction
2. Platelet Receptors and Their Involvement in the Immune Activity of These Cells
2.1. Extracellular and Intracellular Platelet TLR, NLR, and RLR Receptors
2.2. Platelet Extracellular Selectin and Integrin Receptors
2.3. Other Extracellular Platelet Receptors
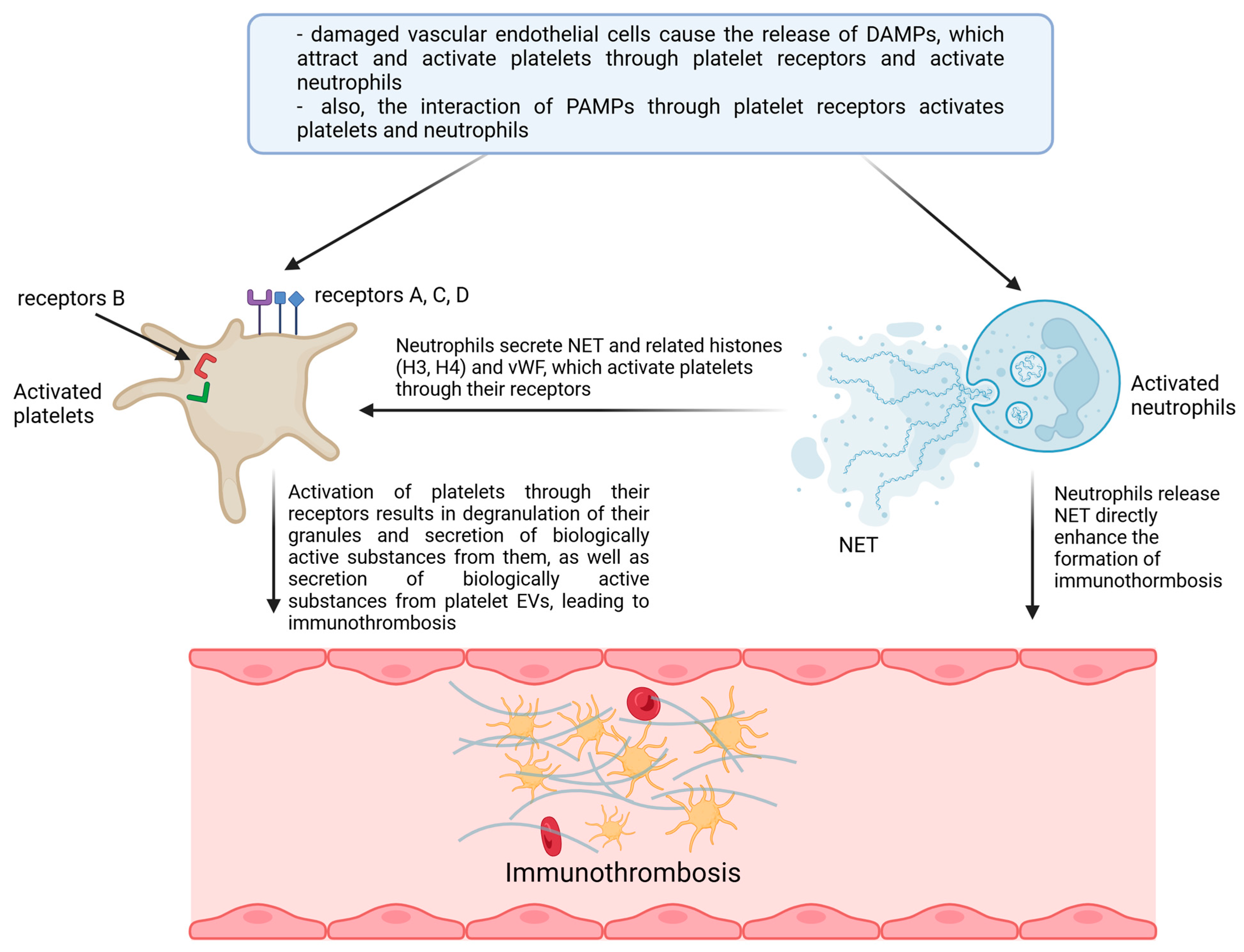
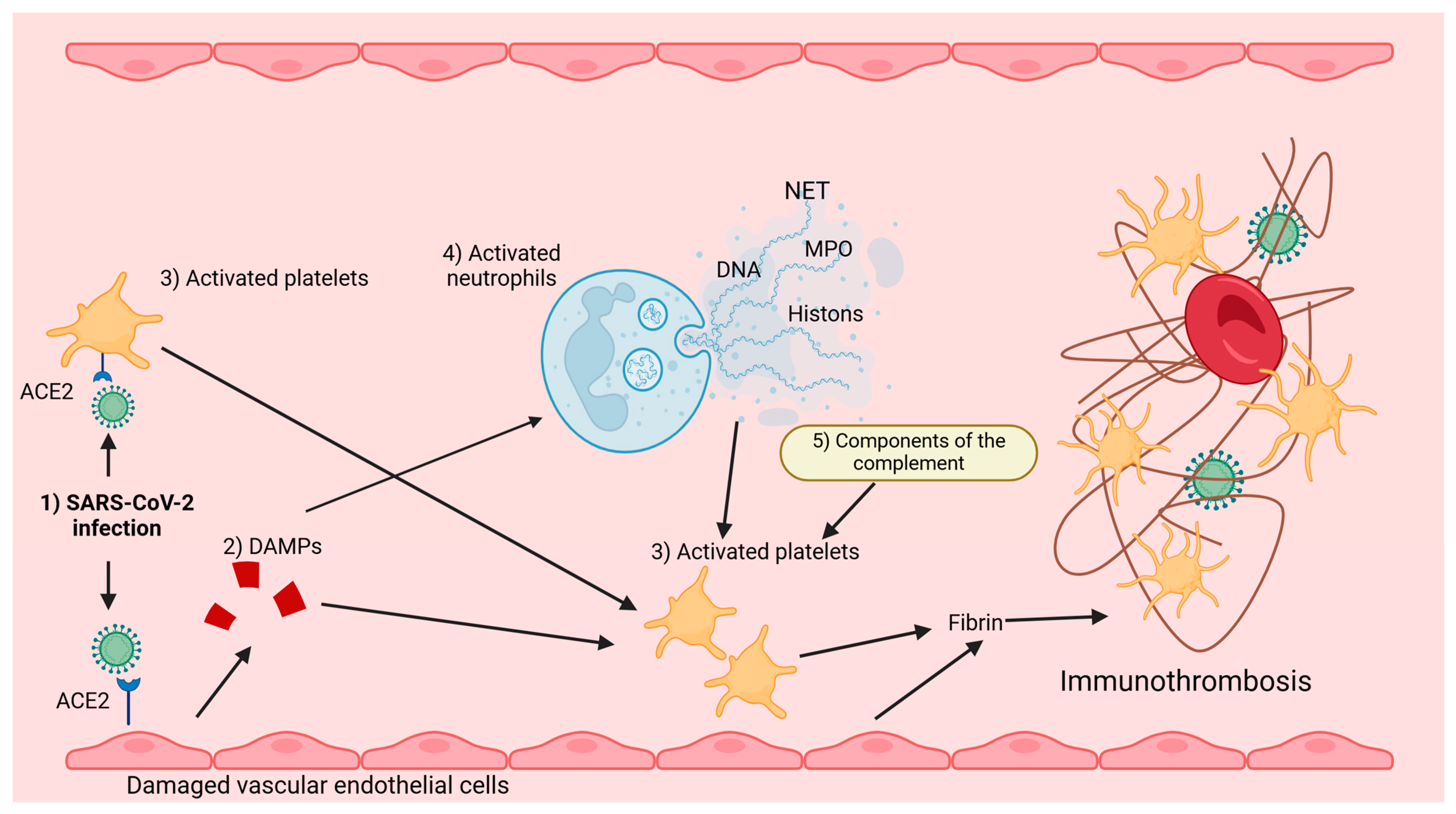
3. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Marcoux, G.; Laroche, A.; Espinoza Romero, J.; Boilard, E. Role of Platelets and Megakaryocytes in Adaptive Immunity. Platelets 2021, 32, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Mezger, M.; Nording, H.; Sauter, R.; Graf, T.; Heim, C.; von Bubnoff, N.; Ensminger, S.M.; Langer, H.F. Platelets and Immune Responses During Thromboinflammation. Front. Immunol. 2019, 10, 1731. [Google Scholar] [CrossRef] [PubMed]
- Cox, D. Sepsis—It Is All about the Platelets. Front. Immunol. 2023, 14, 1210219. [Google Scholar] [CrossRef] [PubMed]
- Gaertner, F.; Massberg, S. Patrolling the Vascular Borders: Platelets in Immunity to Infection and Cancer. Nat. Rev. Immunol. 2019, 19, 747–760. [Google Scholar] [CrossRef]
- Tokarz-Deptuła, B.; Palma, J.; Baraniecki, Ł.; Stosik, M.; Kołacz, R.; Deptuła, W. What Function Do Platelets Play in Inflammation and Bacterial and Viral Infections? Front. Immunol. 2021, 12, 770436. [Google Scholar] [CrossRef]
- Scherlinger, M.; Richez, C.; Tsokos, G.C.; Boilard, E.; Blanco, P. The Role of Platelets in Immune-Mediated Inflammatory Diseases. Nat. Rev. Immunol. 2023, 23, 495–510. [Google Scholar] [CrossRef]
- Koupenova, M.; Freedman, J.E. Platelets and Immunity. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1605–1607. [Google Scholar] [CrossRef]
- Cognasse, F.; Laradi, S.; Berthelot, P.; Bourlet, T.; Marotte, H.; Mismetti, P.; Garraud, O.; Hamzeh-Cognasse, H. Platelet Inflammatory Response to Stress. Front. Immunol. 2019, 10, 1478. [Google Scholar] [CrossRef]
- Guo, L.; Rondina, M.T. The Era of Thromboinflammation: Platelets Are Dynamic Sensors and Effector Cells during Infectious Diseases. Front. Immunol. 2019, 10, 2204. [Google Scholar] [CrossRef]
- Cognasse, F.; Hamzeh-Cognasse, H.; Mismetti, P.; Thomas, T.; Eglin, D.; Marotte, H. The Non-Haemostatic Response of Platelets to Stress: An Actor of the Inflammatory Environment on Regenerative Medicine? Front. Immunol. 2021, 12, 741988. [Google Scholar] [CrossRef]
- Li, C.; Li, J.; Ni, H. Crosstalk Between Platelets and Microbial Pathogens. Front. Immunol. 2020, 11, 1962. [Google Scholar] [CrossRef]
- Kerris, E.W.J.; Hoptay, C.; Calderon, T.; Freishtat, R.J. Platelets and Platelet Extracellular Vesicles in Hemostasis and Sepsis. J. Investig. Med. 2020, 68, 813–820. [Google Scholar] [CrossRef]
- Jurk, K.; Kehrel, B.E. Platelets: Physiology and Biochemistry. Semin. Thromb. Hemost. 2005, 31, 381–392. [Google Scholar] [CrossRef]
- Tokarz-Deptuła, B.; Baraniecki, Ł.; Palma, J.; Stosik, M.; Syrenicz, A.; Sobolewski, J.; Deptula, W. Platelets—Formation, Activation and Activity and Characterization of Biologically Active Substances of Granules and Membrane Microvesicles. Inter. J. Mol. Sci. 2024, in press.
- Vieira-de-Abreu, A.; Rondina, M.; Weyrich, A.; Zimmerman, G. Platelets in Disease: Inflammation. In Platelets; Michelson, A.D., Ed.; Elsevier: London, UK, 2013; pp. 733–766. [Google Scholar]
- Machlus, K.R.; Italiano, J.E. The Incredible Journey: From Megakaryocyte Development to Platelet Formation. J. Cell Biol. 2013, 201, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Müller-Newen, G.; Stope, M.B.; Kraus, T.; Ziegler, P. Development of Platelets during Steady State and Inflammation. J. Leukoc. Biol. 2017, 101, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Hamzeh-Cognasse, H.; Berthelot, P.; Tardy, B.; Pozzetto, B.; Bourlet, T.; Laradi, S.; Garraud, O.; Cognasse, F. Platelet Toll-like Receptors Are Crucial Sensors of Infectious Danger Moieties. Platelets 2018, 29, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Dembińska-Kieć, A.; Naskalski, J.W.; Solnica, B. Laboratory Diagnostics with Elements of Clinical Biochemistry; Edra Urban & Partner: Breslavia, Polonia, 2017. (In Polish) [Google Scholar]
- Stosik, M.P.; Deptuła, W. Selected Functions of Platelets. Med. Wet. 1998, 54, 462–465. (In Polish) [Google Scholar]
- Andrews, R.K.; Gardiner, E.E. Basic Mechanisms of Platelet Receptor Shedding. Platelets 2017, 28, 319–324. [Google Scholar] [CrossRef]
- Paul, M.; Hemshekhar, M.; Kemparaju, K.; Girish, K.S. Aggregation Is Impaired in Starved Platelets Due to Enhanced Autophagy and Cellular Energy Depletion. Platelets 2018, 30, 487–497. [Google Scholar] [CrossRef]
- Koupenova, M.; Livada, A.C.; Morrell, C.N. Platelet and Megakaryocyte Roles in Innate and Adaptive Immunity. Circ. Res. 2022, 130, 288–308. [Google Scholar] [CrossRef]
- Riley, D.R.J.; Khalil, J.S.; Naseem, K.M.; Rivero, F. Biochemical and Immunocytochemical Characterization of Coronins in Platelets. Platelets 2020, 31, 913–924. [Google Scholar] [CrossRef] [PubMed]
- Koupenova, M.; Vitseva, O.; MacKay, C.R.; Beaulieu, L.M.; Benjamin, E.J.; Mick, E.; Kurt-Jones, E.A.; Ravid, K.; Freedman, J.E. Platelet-TLR7 Mediates Host Survival and Platelet Count during Viral Infection in the Absence of Platelet-Dependent Thrombosis. Blood 2014, 124, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Provost, P. The Clinical Significance of Platelet Microparticle-Associated microRNAs. Clin. Chem. Lab. Med. 2017, 55, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Katoh, N.; Soga, F.; Nara, T.; Tamagawa-Mineoka, R.; Nin, M.; Kotani, H.; Masuda, K.; Kishimoto, S. Effect of Serotonin on the Differentiation of Human Monocytes into Dendritic Cells. Clin. Exp. Immunol. 2006, 146, 354–361. [Google Scholar] [CrossRef]
- Poli, V.; Di Gioia, M.; Sola-Visner, M.; Granucci, F.; Frelinger, A.L.; Michelson, A.D.; Zanoni, I. Inhibition of Transcription Factor NFAT Activity in Activated Platelets Enhances Their Aggregation and Exacerbates Gram-Negative Bacterial Septicemia. Immunity 2022, 55, 224–236.e5. [Google Scholar] [CrossRef]
- Gómez, R.M.; López Ortiz, A.O.; Schattner, M. Platelets and Extracellular Traps in Infections. Platelets 2021, 32, 305–313. [Google Scholar] [CrossRef]
- McDonald, B.; Dunbar, M. Platelets and Intravascular Immunity: Guardians of the Vascular Space during Bloodstream Infections and Sepsis. Front. Immunol. 2019, 10, 2400. [Google Scholar] [CrossRef]
- Yuan, H.; Liu, Y.; Zhang, J.; Dong, J.; Zhao, Z. Transcription Factors in Megakaryocytes and Platelets. Front. Immunol. 2023, 14, 1140501. [Google Scholar] [CrossRef]
- Buzas, E.I. The Roles of Extracellular Vesicles in the Immune System. Nat. Rev. Immunol. 2023, 23, 236–250. [Google Scholar] [CrossRef]
- Chen, Y.; Zhong, H.; Zhao, Y.; Luo, X.; Gao, W. Role of Platelet Biomarkers in Inflammatory Response. Biomark Res. 2020, 8, 28. [Google Scholar] [CrossRef]
- Bender, M.; Stegner, D.; Nieswandt, B. Model Systems for Platelet Receptor Shedding. Platelets 2017, 28, 325–332. [Google Scholar] [CrossRef]
- Fink, I.R.; Ribeiro, C.M.S.; Forlenza, M.; Taverne-Thiele, A.; Rombout, J.H.W.M.; Savelkoul, H.F.J.; Wiegertjes, G.F. Immune-Relevant Thrombocytes of Common Carp Undergo Parasite-Induced Nitric Oxide-Mediated Apoptosis. Dev. Comp. Immunol. 2015, 50, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Frydman, G.H.; Ellett, F.; Jorgensen, J.; Marand, A.L.; Zukerberg, L.; Selig, M.K.; Tessier, S.N.; Wong, K.H.K.; Olaleye, D.; Vanderburg, C.R.; et al. Megakaryocytes Respond during Sepsis and Display Innate Immune Cell Behaviors. Front. Immunol. 2023, 14, 1083339. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Liu, C.; Rosenberger, P. Platelet Formation and Activation Are Influenced by Neuronal Guidance Proteins. Front. Immunol. 2023, 14, 1206906. [Google Scholar] [CrossRef]
- Dib, P.R.B.; Quirino-Teixeira, A.C.; Merij, L.B.; Pinheiro, M.B.M.; Rozini, S.V.; Andrade, F.B.; Hottz, E.D. Innate Immune Receptors in Platelets and Platelet-Leukocyte Interactions. J. Leukoc. Biol. 2020, 108, 1157–1182. [Google Scholar] [CrossRef] [PubMed]
- Trzeciak-Ryczek, A.; Tokarz-Deptuła, B.; Deptuła, W. Platelets--an Important Element of the Immune System. Pol. J. Vet. Sci. 2013, 16, 407–413. [Google Scholar] [CrossRef]
- Agostini, L.; Martinon, F.; Burns, K.; McDermott, M.F.; Hawkins, P.N.; Tschopp, J. NALP3 Forms an IL-1beta-Processing Inflammasome with Increased Activity in Muckle-Wells Autoinflammatory Disorder. Immunity 2004, 20, 319–325. [Google Scholar] [CrossRef]
- Cox, D.; Kerrigan, S.W.; Watson, S.P. Platelets and the Innate Immune System: Mechanisms of Bacterial-induced Platelet Activation. J. Thromb. Haemost. 2011, 9, 1097–1107. [Google Scholar] [CrossRef]
- Jackson, S.P. Arterial Thrombosis-Insidious, Unpredictable and Deadly. Nat. Med. 2011, 17, 1423–1436. [Google Scholar] [CrossRef]
- Jenne, C.N.; Kubes, P. Platelets in Inflammation and Infection. Platelets 2015, 26, 286–292. [Google Scholar] [CrossRef]
- Kerrigan, S.W. The Expanding Field of Platelet–Bacterial Interconnections. Platelets 2015, 26, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Maouia, A.; Rebetz, J.; Kapur, R.; Semple, J.W. The Immune Nature of Platelets Revisited. Transfus. Med. Rev. 2020, 34, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Luo, M.; Liu, B. The Role of CLEC-2 and Its Ligands in Thromboinflammation. Front. Immunol. 2021, 12, 688643. [Google Scholar] [CrossRef]
- Offermanns, S. Activation of Platelet Function through G Protein-Coupled Receptors. Circ. Res. 2006, 99, 1293–1304. [Google Scholar] [CrossRef]
- Page, M.J.; Pretorius, E. A Champion of Host Defense: A Generic Large-Scale Cause for Platelet Dysfunction and Depletion in Infection. Semin. Thromb. Hemost. 2020, 46, 302–319. [Google Scholar] [CrossRef]
- Rigg, R.A.; Healy, L.D.; Chu, T.T.; Ngo, A.T.P.; Mitrugno, A.; Zilberman-Rudenko, J.; Aslan, J.E.; Hinds, M.T.; Vecchiarelli, L.D.; Morgan, T.K.; et al. Protease-Activated Receptor 4 Activity Promotes Platelet Granule Release and Platelet-Leukocyte Interactions. Platelets 2019, 30, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Speth, C.; Löffler, J.; Krappmann, S.; Lass-Flörl, C.; Rambach, G. Platelets as Immune Cells in Infectious Diseases. Future Microbiol. 2013, 8, 1431–1451. [Google Scholar] [CrossRef]
- Warkentin, T.E.; Aird, W.C.; Rand, J.H. Platelet-Endothelial Interactions: Sepsis, HIT, and Antiphospholipid Syndrome. Hematology 2003, 2003, 497–519. [Google Scholar] [CrossRef]
- Fu, G.; Deng, M.; Neal, M.D.; Billiar, T.R.; Scott, M.J. Platelet–Monocyte Aggregates: Understanding Mechanisms and Functions in Sepsis. Shock 2021, 55, 156. [Google Scholar] [CrossRef]
- Zaid, Y.; Puhm, F.; Allaeys, I.; Naya, A.; Oudghiri, M.; Khalki, L.; Limami, Y.; Zaid, N.; Sadki, K.; Ben El Haj, R.; et al. Platelets Can Associate With SARS-CoV-2 RNA and Are Hyperactivated in COVID-19. Circ. Res. 2020, 127, 1404–1418. [Google Scholar] [CrossRef]
- Shen, S.; Zhang, J.; Fang, Y.; Lu, S.; Wu, J.; Zheng, X.; Deng, F. SARS-CoV-2 Interacts with Platelets and Megakaryocytes via ACE2-Independent Mechanism. J. Hematol. Oncol. 2021, 14, 72. [Google Scholar] [CrossRef] [PubMed]
- Etulain, J. Platelets in Wound Healing and Regenerative Medicine. Platelets 2018, 29, 556–568. [Google Scholar] [CrossRef] [PubMed]
- Geijtenbeek, T.B.H.; Gringhuis, S.I. Signalling through C-Type Lectin Receptors: Shaping Immune Responses. Nat. Rev. Immunol. 2009, 9, 465–479. [Google Scholar] [CrossRef]
- Chaipan, C.; Soilleux, E.J.; Simpson, P.; Hofmann, H.; Gramberg, T.; Marzi, A.; Geier, M.; Stewart, E.A.; Eisemann, J.; Steinkasserer, A.; et al. DC-SIGN and CLEC-2 Mediate Human Immunodeficiency Virus Type 1 Capture by Platelets. J. Virol. 2006, 80, 8951–8960. [Google Scholar] [CrossRef] [PubMed]
- Sung, P.-S.; Huang, T.-F.; Hsieh, S.-L. Extracellular Vesicles from CLEC2-Activated Platelets Enhance Dengue Virus-Induced Lethality via CLEC5A/TLR2. Nat. Commun. 2019, 10, 2402. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Waresi, M.; Zhang, W.; Han, L.; Zhao, Y.; Chen, Y.; Zhou, P.; Chang, L.; Pan, G.; Wu, B.; et al. NOD2-Mediated P2Y12 Upregulation Increases Platelet Activation and Thrombosis in Sepsis. Biochem. Pharmacol. 2021, 194, 114822. [Google Scholar] [CrossRef]
- Ed Rainger, G.; Chimen, M.; Harrison, M.J.; Yates, C.M.; Harrison, P.; Watson, S.P.; Lordkipanidzé, M.; Nash, G.B. The Role of Platelets in the Recruitment of Leukocytes during Vascular Disease. Platelets 2015, 26, 507–520. [Google Scholar] [CrossRef]
- Palacios-Acedo, A.L.; Mège, D.; Crescence, L.; Dignat-George, F.; Dubois, C.; Panicot-Dubois, L. Platelets, Thrombo-Inflammation, and Cancer: Collaborating with the Enemy. Front. Immunol. 2019, 10, 1805. [Google Scholar] [CrossRef]
- Aslam, R.; Speck, E.R.; Kim, M.; Crow, A.R.; Bang, K.W.A.; Nestel, F.P.; Ni, H.; Lazarus, A.H.; Freedman, J.; Semple, J.W. Platelet Toll-like Receptor Expression Modulates Lipopolysaccharide-Induced Thrombocytopenia and Tumor Necrosis Factor-α Production in Vivo. Blood 2006, 107, 637–641. [Google Scholar] [CrossRef]
- Cognasse, F.; Hamzeh, H.; Chavarin, P.; Acquart, S.; Genin, C.; Garraud, O. Evidence of Toll-like Receptor Molecules on Human Platelets. Immunol. Cell Biol. 2005, 83, 196–198. [Google Scholar] [CrossRef]
- Alonso, A.L.; Cox, D. Platelet Interactions with Viruses and Parasites. Platelets 2015, 26, 317–323. [Google Scholar] [CrossRef]
- Claushuis, T.A.M.; Van Der Veen, A.I.P.; Horn, J.; Schultz, M.J.; Houtkooper, R.H.; Van ’t Veer, C.; Van Der Poll, T. Platelet Toll-like Receptor Expression and Activation Induced by Lipopolysaccharide and Sepsis. Platelets 2019, 30, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Martinod, K.; Deppermann, C. Immunothrombosis and Thromboinflammation in Host Defense and Disease. Platelets 2021, 32, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Zindel, J.; Kubes, P. DAMPs, PAMPs, and LAMPs in Immunity and Sterile Inflammation. Annu. Rev. Pathol. Mech. Dis. 2020, 15, 493–518. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Jenne, C.N. Role of Platelets in Neutrophil Extracellular Trap (NET) Production and Tissue Injury. Semin. Immunol. 2016, 28, 546–554. [Google Scholar] [CrossRef]
- Berthet, J.; Damien, P.; Hamzeh-Cognasse, H.; Arthaud, C.-A.; Eyraud, M.-A.; Zéni, F.; Pozzetto, B.; McNicol, A.; Garraud, O.; Cognasse, F. Human Platelets Can Discriminate between Various Bacterial LPS Isoforms via TLR4 Signaling and Differential Cytokine Secretion. Clin. Immunol. 2012, 145, 189–200. [Google Scholar] [CrossRef]
- Zhang, G.; Han, J.; Welch, E.J.; Ye, R.D.; Voyno-Yasenetskaya, T.A.; Malik, A.B.; Du, X.; Li, Z. Lipopolysaccharide Stimulates Platelet Secretion and Potentiates Platelet Aggregation via TLR4/MyD88 and the cGMP-Dependent Protein Kinase Pathway. J. Immunol. 2009, 182, 7997–8004. [Google Scholar] [CrossRef]
- Koessler, J.; Niklaus, M.; Weber, K.; Koessler, A.; Kuhn, S.; Boeck, M.; Kobsar, A. The Role of Human Platelet Preparation for Toll-Like Receptors 2 and 4 Related Platelet Responsiveness. TH Open 2019, 03, e94–e102. [Google Scholar] [CrossRef]
- Schattner, M. Platelet TLR4 at the Crossroads of Thrombosis and the Innate Immune Response. J. Leukoc. Biol. 2019, 105, 873–880. [Google Scholar] [CrossRef]
- Damås, J.K.; Jensenius, M.; Ueland, T.; Otterdal, K.; Yndestad, A.; Frøland, S.S.; Rolain, J.-M.; Myrvang, B.; Raoult, D.; Aukrust, P. Increased Levels of Soluble CD40L in African Tick Bite Fever: Possible Involvement of TLRs in the Pathogenic Interaction between Rickettsia Africae, Endothelial Cells, and Platelets1. J. Immunol. 2006, 177, 2699–2706. [Google Scholar] [CrossRef]
- Liu, X.; Liu, H.; Luo, X.; Zhang, P.; Gao, Y.; Xie, S.; Xu, K.; Chang, J.; Ma, L. Strains of Group B Streptococci from Septic Patients Induce Platelet Activation via Toll-like Receptor 2. Clin. Exp. Pharmacol. Physiol. 2017, 44, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Rex, S.; Beaulieu, L.M.; Perlman, D.H.; Vitseva, O.; Blair, P.S.; McComb, M.E.; Costello, C.E.; Freedman, J.E. Immune versus Thrombotic Stimulation of Platelets Differentially Regulates Signalling Pathways, Intracellular Protein-Protein Interactions, and Alpha-Granule Release. Thromb. Haemost. 2009, 102, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Blair, P.; Rex, S.; Vitseva, O.; Beaulieu, L.; Tanriverdi, K.; Chakrabarti, S.; Hayashi, C.; Genco, C.A.; Iafrati, M.; Freedman, J.E. Stimulation of Toll-like Receptor 2 in Human Platelets Induces a Thromboinflammatory Response through Activation of Phosphoinositide 3-Kinase. Circ. Res. 2009, 104, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.R.; Ma, A.C.; Tavener, S.A.; McDonald, B.; Goodarzi, Z.; Kelly, M.M.; Patel, K.D.; Chakrabarti, S.; McAvoy, E.; Sinclair, G.D.; et al. Platelet TLR4 Activates Neutrophil Extracellular Traps to Ensnare Bacteria in Septic Blood. Nat. Med. 2007, 13, 463–469. [Google Scholar] [CrossRef]
- Kraemer, B.F.; Campbell, R.A.; Schwertz, H.; Cody, M.J.; Franks, Z.; Tolley, N.D.; Kahr, W.H.A.; Lindemann, S.; Seizer, P.; Yost, C.C.; et al. Novel Anti-Bacterial Activities of β-Defensin 1 in Human Platelets: Suppression of Pathogen Growth and Signaling of Neutrophil Extracellular Trap Formation. PLoS Pathog. 2011, 7, e1002355. [Google Scholar] [CrossRef]
- Matoszka, N.; Działo, J.; Tokarz-Deptuła, B.; Deptuła, W. NET and NETosis—New Phenomenon in Immunology. Postępy Hig. Med. Dosw. 2012, 66, 437–445. (In Polish) [Google Scholar] [CrossRef]
- Soehnlein, O.; Steffens, S.; Hidalgo, A.; Weber, C. Neutrophils as Protagonists and Targets in Chronic Inflammation. Nat. Rev. Immunol. 2017, 17, 248–261. [Google Scholar] [CrossRef]
- Leroy, J.; Bortolus, C.; Lecointe, K.; Parny, M.; Charlet, R.; Sendid, B.; Jawhara, S. Fungal Chitin Reduces Platelet Activation Mediated via TLR8 Stimulation. Front. Cell. Infect. Microbiol. 2019, 9, 383. [Google Scholar] [CrossRef]
- Koupenova, M.; Corkrey, H.A.; Vitseva, O.; Manni, G.; Pang, C.J.; Clancy, L.; Yao, C.; Rade, J.; Levy, D.; Wang, J.P.; et al. The Role of Platelets in Mediating a Response to Human Influenza Infection. Nat. Commun. 2019, 10, 1780. [Google Scholar] [CrossRef]
- Koupenova, M.; Mick, E.; Mikhalev, E.; Benjamin, E.J.; Tanriverdi, K.; Freedman, J.E. Sex Differences in Platelet Toll-Like Receptors and Their Association With Cardiovascular Risk Factors. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1030–1037. [Google Scholar] [CrossRef]
- Banerjee, M.; Huang, Y.; Joshi, S.; Popa, G.J.; Mendenhall, M.D.; Wang, Q.J.; Garvy, B.A.; Myint, T.; Whiteheart, S.W. Platelets Endocytose Viral Particles and Are Activated via TLR (Toll-Like Receptor) Signaling. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1635–1650. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, S.; Nagasaki, M.; Kunishima, S.; Sawaguchi, A.; Sakata, A.; Sakaguchi, H.; Ohmori, T.; Manabe, I.; Italiano, J.E., Jr.; Ryu, T.; et al. IL-1α Induces Thrombopoiesis through Megakaryocyte Rupture in Response to Acute Platelet Needs. J. Cell Biol. 2015, 209, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhao, Y.; Wang, K.; Shi, X.; Wang, Y.; Huang, H.; Zhuang, Y.; Cai, T.; Wang, F.; Shao, F. Cleavage of GSDMD by Inflammatory Caspases Determines Pyroptotic Cell Death. Nature 2015, 526, 660–665. [Google Scholar] [CrossRef]
- Au, A.E.; Josefsson, E.C. Regulation of Platelet Membrane Protein Shedding in Health and Disease. Platelets 2017, 28, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Baroni, G.; Banzato, A.; Bison, E.; Denas, G.; Zoppellaro, G.; Pengo, V. The Role of Platelets in Antiphospholipid Syndrome. Platelets 2017, 28, 762–766. [Google Scholar] [CrossRef]
- Théorêt, J.-F.; Yacoub, D.; Hachem, A.; Gillis, M.-A.; Merhi, Y. P-Selectin Ligation Induces Platelet Activation and Enhances Microaggregate and Thrombus Formation. Thromb. Res. 2011, 128, 243–250. [Google Scholar] [CrossRef]
- Heffron, S.P.; Marier, C.; Parikh, M.; Fisher, E.A.; Berger, J.S. Severe Obesity and Bariatric Surgery Alter the Platelet mRNA Profile. Platelets 2019, 30, 967–974. [Google Scholar] [CrossRef]
- Eisinger, F.; Langer, H.F. The Mutual Relation of Platelet Activation and Innate Immunity. Hamostaseologie 2018, 38, 186–202. [Google Scholar] [CrossRef]
- Kannan, M.; Ahmad, F.; Shankar, E.M. Editorial: Innate Immunity: Platelets and Their Interaction with Other Cellular Elements in Host Defense and Disease Pathogenesis. Front. Immunol. 2023, 14, 1292316. [Google Scholar] [CrossRef]
- Stegner, D.; Klaus, V.; Nieswandt, B. Platelets as Modulators of Cerebral Ischemia/Reperfusion Injury. Front. Immunol. 2019, 10, 2505. [Google Scholar] [CrossRef]
- Lefrançais, E.; Ortiz-Muñoz, G.; Caudrillier, A.; Mallavia, B.; Liu, F.; Sayah, D.M.; Thornton, E.E.; Headley, M.B.; David, T.; Coughlin, S.R.; et al. The Lung Is a Site of Platelet Biogenesis and a Reservoir for Haematopoietic Progenitors. Nature 2017, 544, 105–109. [Google Scholar] [CrossRef]
- Gando, S.; Wada, T. Thromboplasminflammation in COVID-19 Coagulopathy: Three Viewpoints for Diagnostic and Therapeutic Strategies. Front. Immunol. 2021, 12, 649122. [Google Scholar] [CrossRef]
- Fitzgerald, J.R.; Foster, T.J.; Cox, D. The Interaction of Bacterial Pathogens with Platelets. Nat. Rev. Microbiol. 2006, 4, 445–457. [Google Scholar] [CrossRef]
- Pagel, O.; Walter, E.; Jurk, K.; Zahedi, R.P. Taking the Stock of Granule Cargo: Platelet Releasate Proteomics. Platelets 2017, 28, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Edelstein, L.C. The Role of Platelet Microvesicles in Intercellular Communication. Platelets 2017, 28, 222–227. [Google Scholar] [CrossRef]
- Chandraratne, S.; von Bruehl, M.-L.; Pagel, J.-I.; Stark, K.; Kleinert, E.; Konrad, I.; Farschtschi, S.; Coletti, R.; Gärtner, F.; Chillo, O.; et al. Critical Role of Platelet Glycoprotein Ibα in Arterial Remodeling. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 589–597. [Google Scholar] [CrossRef]
- Verschoor, A.; Neuenhahn, M.; Navarini, A.A.; Graef, P.; Plaumann, A.; Seidlmeier, A.; Nieswandt, B.; Massberg, S.; Zinkernagel, R.M.; Hengartner, H.; et al. A Platelet-Mediated System for Shuttling Blood-Borne Bacteria to CD8α+ Dendritic Cells Depends on Glycoprotein GPIb and Complement C3. Nat. Immunol. 2011, 12, 1194–1201. [Google Scholar] [CrossRef]
- Andrews, R.K.; Gardiner, E.E.; Shen, Y.; Whisstock, J.C.; Berndt, M.C. Glycoprotein Ib-IX-V. Int. J. Biochem. Cell Biol. 2003, 35, 1170–1174. [Google Scholar] [CrossRef]
- Gros, A.; Ollivier, V.; Ho-Tin-Noé, B. Platelets in Inflammation: Regulation of Leukocyte Activities and Vascular Repair. Front. Immunol. 2015, 5, 678. [Google Scholar] [CrossRef] [PubMed]
- Landau, M.; Rosenberg, N. Molecular Insight into Human Platelet Antigens: Structural and Evolutionary Conservation Analyses Offer New Perspective to Immunogenic Disorders. Transfusion 2011, 51, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Irrazabal, T.; So, C.C.; Berru, M.; Du, L.; Lam, E.; Ling, A.K.; Gommerman, J.L.; Pan-Hammarström, Q.; Martin, A. The H2B Deubiquitinase Usp22 Promotes Antibody Class Switch Recombination by Facilitating Non-Homologous End Joining. Nat. Commun. 2018, 9, 1006. [Google Scholar] [CrossRef] [PubMed]
- Elzey, B.D.; Tian, J.; Jensen, R.J.; Swanson, A.K.; Lees, J.R.; Lentz, S.R.; Stein, C.S.; Nieswandt, B.; Wang, Y.; Davidson, B.L.; et al. Platelet-Mediated Modulation of Adaptive Immunity: A Communication Link between Innate and Adaptive Immune Compartments. Immunity 2003, 19, 9–19. [Google Scholar] [CrossRef]
- Furman, M.I.; Krueger, L.A.; Linden, M.D.; Barnard, M.R.; Frelinger, A.L.; Michelson, A.D. Release of Soluble CD40L from Platelets Is Regulated by Glycoprotein IIb/IIIa and Actin Polymerization. J. Am. Coll. Cardiol. 2004, 43, 2319–2325. [Google Scholar] [CrossRef] [PubMed]
- Melki, I.; Tessandier, N.; Zufferey, A.; Boilard, E. Platelet Microvesicles in Health and Disease. Platelets 2017, 28, 214–221. [Google Scholar] [CrossRef]
- Bye, A.P.; Hoepel, W.; Mitchell, J.L.; Jégouic, S.; Loureiro, S.; Sage, T.; Vidarsson, G.; Nouta, J.; Wuhrer, M.; de Taeye, S.; et al. Aberrant Glycosylation of Anti-SARS-CoV-2 Spike IgG Is a Prothrombotic Stimulus for Platelets. Blood 2021, 138, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- Chapman, L.M.; Aggrey, A.A.; Field, D.J.; Srivastava, K.; Ture, S.; Yui, K.; Topham, D.J.; Baldwin, W.M.; Morrell, C.N. Platelets Present Antigen in the Context of MHC Class I. J. Immunol. 2012, 189, 916–923. [Google Scholar] [CrossRef]
- Seizer, P.; May, A.E. Platelets and Matrix Metalloproteinases. Thromb. Haemost. 2013, 110, 903–909. [Google Scholar] [CrossRef]
- Rachidi, S.; Metelli, A.; Riesenberg, B.; Wu, B.X.; Nelson, M.H.; Wallace, C.; Paulos, C.M.; Rubinstein, M.P.; Garrett-Mayer, E.; Hennig, M.; et al. Platelets Subvert T Cell Immunity against Cancer via GARP-TGFβ Axis. Sci. Immunol. 2017, 2, eaai7911. [Google Scholar] [CrossRef]
- Cognasse, F.; Nguyen, K.A.; Damien, P.; McNicol, A.; Pozzetto, B.; Hamzeh-Cognasse, H.; Garraud, O. The Inflammatory Role of Platelets via Their TLRs and Siglec Receptors. Front. Immunol. 2015, 6, 83. [Google Scholar] [CrossRef]
- van Holten, T.C.; Bleijerveld, O.B.; Wijten, P.; de Groot, P.G.; Heck, A.J.R.; Barendrecht, A.D.; Merkx, T.H.; Scholten, A.; Roest, M. Quantitative Proteomics Analysis Reveals Similar Release Profiles Following Specific PAR-1 or PAR-4 Stimulation of Platelets. Cardiovasc. Res. 2014, 103, 140–146. [Google Scholar] [CrossRef]
- Gołąb, J.; Jakóbsiak, M.; Stokłasa, T.; Lasek, W. Immunology, 7th ed.; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2017. (In Polish) [Google Scholar]
- Bordon, Y. A Platelet-Derived Factor Protects the Ageing Brain. Nat. Rev. Immunol. 2023, 23, 614. [Google Scholar] [CrossRef] [PubMed]
- Sauter, R.J.; Sauter, M.; Reis, E.S.; Emschermann, F.N.; Nording, H.; Ebenhöch, S.; Kraft, P.; Münzer, P.; Mauler, M.; Rheinlaender, J.; et al. Functional Relevance of the Anaphylatoxin Receptor C3aR for Platelet Function and Arterial Thrombus Formation Marks an Intersection Point Between Innate Immunity and Thrombosis. Circulation 2018, 138, 1720–1735. [Google Scholar] [CrossRef] [PubMed]
- Nording, H.; Baron, L.; Haberthür, D.; Emschermann, F.; Mezger, M.; Sauter, M.; Sauter, R.; Patzelt, J.; Knoepp, K.; Nording, A.; et al. The C5a/C5a Receptor 1 Axis Controls Tissue Neovascularization through CXCL4 Release from Platelets. Nat. Commun. 2021, 12, 3352. [Google Scholar] [CrossRef] [PubMed]
- Schrottmaier, W.C.; Schmuckenschlager, A.; Pirabe, A.; Assinger, A. Platelets in Viral Infections—Brave Soldiers or Trojan Horses. Front. Immunol. 2022, 13, 856713. [Google Scholar] [CrossRef]
| Lp. | Extracellular and intracellular TLR, NLR, and RLR receptors |
|
| 1. | ||
| 2. | Extracellular selectin and integrin receptors |
|
| 3. | Other extracellular receptors | Lectin receptor type C; it is the CLR in this CLEC-2 and DC SIGN, and also CD116, CD18, CD40, FcγRIIa, MHC class I, GARP, LPA1, LPA2, LPA3, P2Y1, P2Y12, PAR-1,3,4, ADP, TxA, IL1β, IL1R1, IL18Ra, TNF, IFNγ, TGFβR, CCL-1, 3, 4, 5, CXCL-1, 2, 3, 4, 6, 7, 8, 12, 16, 17, 22, 24, 26, ICAM-1, ICAM-2, JAM-A, JAM-C, PECAM1, C1q, C8, C9, C3a, C3aR, and ACE-2 |
| Platelet Receptors | Pathways of Influence on Platelets | Action Effect | Involvement in Pathological States/Diseases | |
|---|---|---|---|---|
| Extracellular and intracellular | TLR-1, 2, 4, 6 | Increase the synthesis of pro-inflammatory cytokines, MHC class II markers, CD40, P-selectin, ligand for CD40L, CCL5, and aggregation, adhesion, migration, and chemotaxis, through the NF-κB factor, Myd88, and TRIF pathway. Also enhance phagocytosis and NET of platelets and endocytosis of viruses by these cells and leukocytes. | Enhance antibacterial and antiviral immunity, non-infectious and metabolic diseases | G+ and G- bacterial and viral infections, e.g., Dengue virus, and reaction to DAMP and LAMP patterns. |
| TLR-3,7,8,9 | Enhance aggregation, platelet adhesion, and the expression of TNFR1, selectin, and CD receptors on these cells, through factor NF-κB. Stimulate secretion of substances secreted from platelet dense granules and T cells, and increase the cidal capacity and NET of PMN cells. | Enhance antiviral and antimicrobial immunity, mainly against intracellular bacteria and non-infectious and metabolic diseases | Infections with ssRNA, dsRNA, DNA viruses, and intracellular bacteria and reaction to DAMP and LAMP patterns | |
| NLR | They enhance platelet aggregation and clot formation and stimulate the synthesis of IL-1β and IL-18 and the formation of “controlled” inflammation, with the help of the NOD2 receptor and NLRP3. | Enhance antibacterial and antiviral immunity and non-infectious and metabolic diseases | Infections with intracellular bacteria and viruses, mainly RNA viruses, and reaction to DAMP and LAMP patterns | |
| RLR | Stimulate antiviral immune mechanisms. | Enhance anti-infective immunity and non-infectious and metabolic diseases | Infections with ssRNA and dsRNA viruses and reaction to DAMP and LAMP patterns | |
| Extracellular | Selectin | Activate vascular endothelial elements including fibrinogen, PSGL-1 ligand, and CD receptors and enhance Th1, DC, PMN, and MN cell activity, as well as platelet aggregation and adhesion. | Optimize intravascular homeostasis and enhance antiviral immunity in infections and vascular diseases | Viral infections |
| Integrin | Enhance the activity of vascular endothelial cells and blood leukocytes through, among others, ITAM receptors and affect platelet aggregation and adhesion, as well as their secretion of ADP and TxA2. Enhance the activity of vascular endothelial cells and blood leukocytes through, among others, ITAM receptors and affect platelet aggregation and adhesion, as well as their secretion of ADP and TxA2. | Enhance antibacterial and antiviral immunity and stabilize intravascular homeostasis, vascular diseases, and autoimmune diseases | Systemic lupus erythematosus (SLE), Bernard–Soulier syndrome (BSS), SFTS syndrome, autoantibody production | |
| Other extracellular | Lectin receptor type C | Increase the synthesis of pro-inflammatory cytokines by platelets and leukocytes, as well as increase the secretion of substances from α-granules, dense, and EV platelets | Enhance anti-infective immunity mainly against viruses in infections and non-infectious and metabolic diseases | Dengue virus infections, Ebola, Marburg, influenza, SARS-CoV-2, HIV, and reaction to DAMP patterns |
| CD receptors | They affect isotype switching from IgM to IgG and IgA, increase TCD8 and platelet activity and vascular endothelial cell secreting, as well as IL-2, IL-8, CCL-2, and selectin G and P. | Enhance anti-infective immunity mainly against viruses in infections and vascular diseases | Adenovirus infections | |
| FcγRIIa, MHC class I, GARP, and lysophosphatidic acid (LPA1, LPA2, LPA3) | Activate platelets and Treg cells and enhance TGFβ secretion through, among others, the CD86 receptor. | Enhance anti-infective immunity in infections and parasitic infections and vascular diseases | Bacterial infections, influenza virus, SARS-CoV-2 infections, and Plasmodium sp. infections and atherosclerotic lesions | |
| P2Y1, P2Y12 receptors | They activate platelet aggregation via NFAT and stimulate immune cells and cause platelet shape changes with the contribution of coronins. | Enhance anti-bacterial immunity in bacterial infections | G- bacterial infections | |
| PAR 1, 3, 4, TxA receptors | Enhance the defensive attributes of platelets, cause a change in vascular permeability and a conformational change in integrin receptors and platelet pseudopodia. | Enhance anti-bacterial immunity in bacterial infections and vascular diseases | Bacterial infections | |
| Receptors for IL-1β, IL-1R1, IL-18α, TNF, INFγ, TGFβR, CCL-1,3,4,5, CXCL-1,2,3,4,6,7,8,12, 17,22,24,26, ICAM1 i 2, JAM-A i C, PECAM1, C i ACE-2 | Activate aggregation, adhesion, engulfment and cidal capacity and secretion of platelet EV substances and increase TF, vWF release, and blood coagulation. Increase leukocyte chemotaxis, activate Treg toward synthesis of, among others, TNF-α, and activate blood vessel cells toward angiogenic. | Enhance antiviral and anti-bacterial immunity—mainly natural in infections and vascular diseases | Viral and bacterial infections | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tokarz-Deptuła, B.; Baraniecki, Ł.; Palma, J.; Stosik, M.; Deptuła, W. Characterization of Platelet Receptors and Their Involvement in Immune Activation of These Cells. Int. J. Mol. Sci. 2024, 25, 12611. https://doi.org/10.3390/ijms252312611
Tokarz-Deptuła B, Baraniecki Ł, Palma J, Stosik M, Deptuła W. Characterization of Platelet Receptors and Their Involvement in Immune Activation of These Cells. International Journal of Molecular Sciences. 2024; 25(23):12611. https://doi.org/10.3390/ijms252312611
Chicago/Turabian StyleTokarz-Deptuła, Beata, Łukasz Baraniecki, Joanna Palma, Michał Stosik, and Wiesław Deptuła. 2024. "Characterization of Platelet Receptors and Their Involvement in Immune Activation of These Cells" International Journal of Molecular Sciences 25, no. 23: 12611. https://doi.org/10.3390/ijms252312611
APA StyleTokarz-Deptuła, B., Baraniecki, Ł., Palma, J., Stosik, M., & Deptuła, W. (2024). Characterization of Platelet Receptors and Their Involvement in Immune Activation of These Cells. International Journal of Molecular Sciences, 25(23), 12611. https://doi.org/10.3390/ijms252312611







