Overexpression of StCDPK13 in Potato Enhances Tolerance to Drought Stress
Abstract
1. Introduction
2. Results
2.1. Cloning and Sequence Analysis of StCDPK13
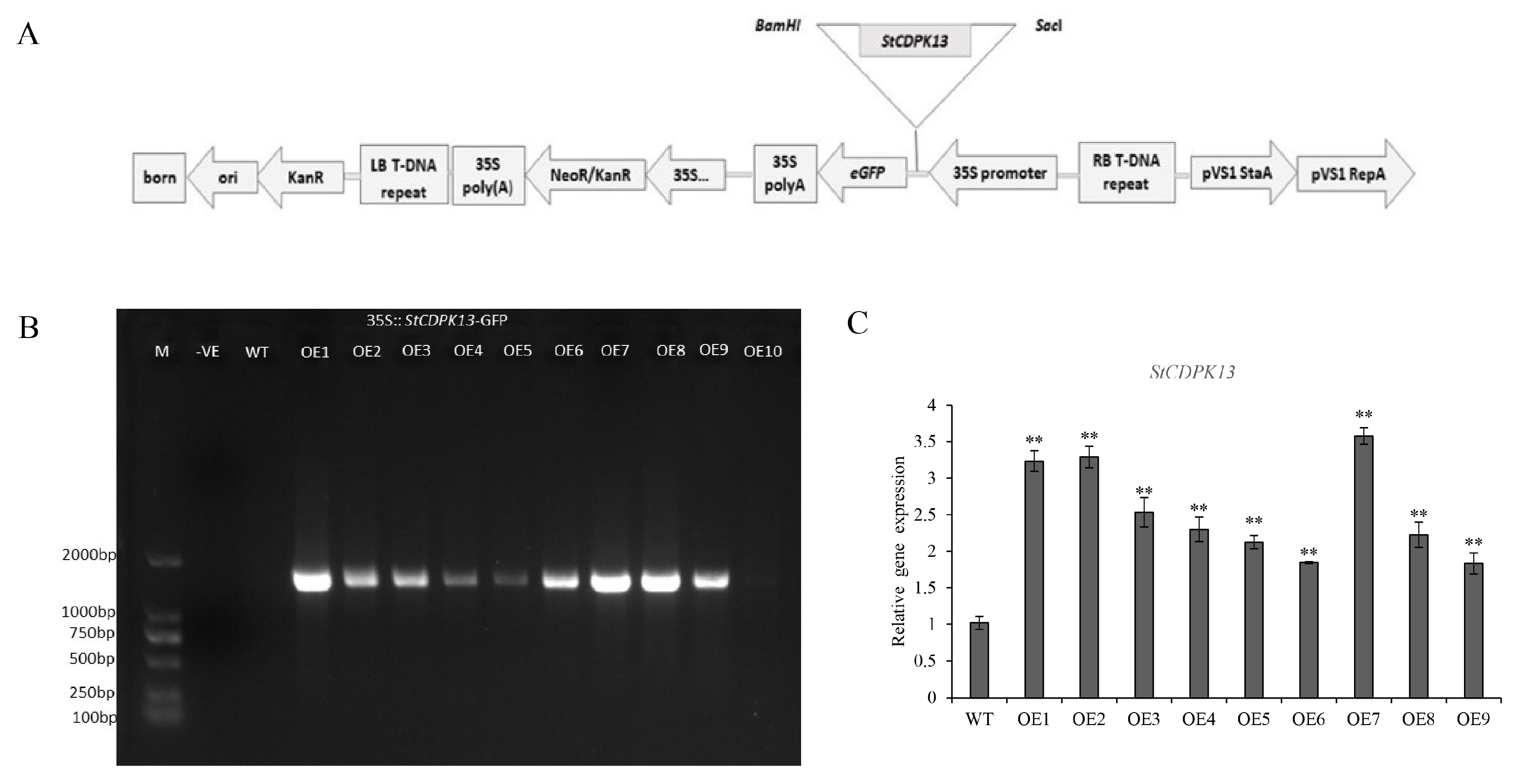
2.2. Generation of Stable Transgenic Potato Plants
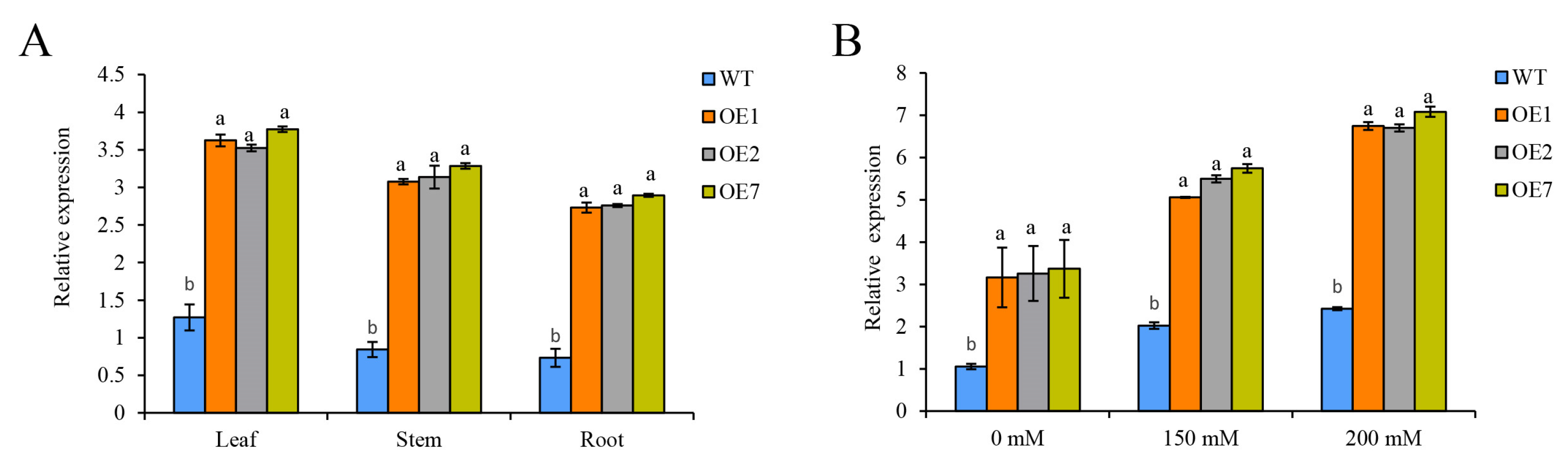
2.3. The Expression of StCDPK3 in Tissues and Response to Drought Stress in Potato
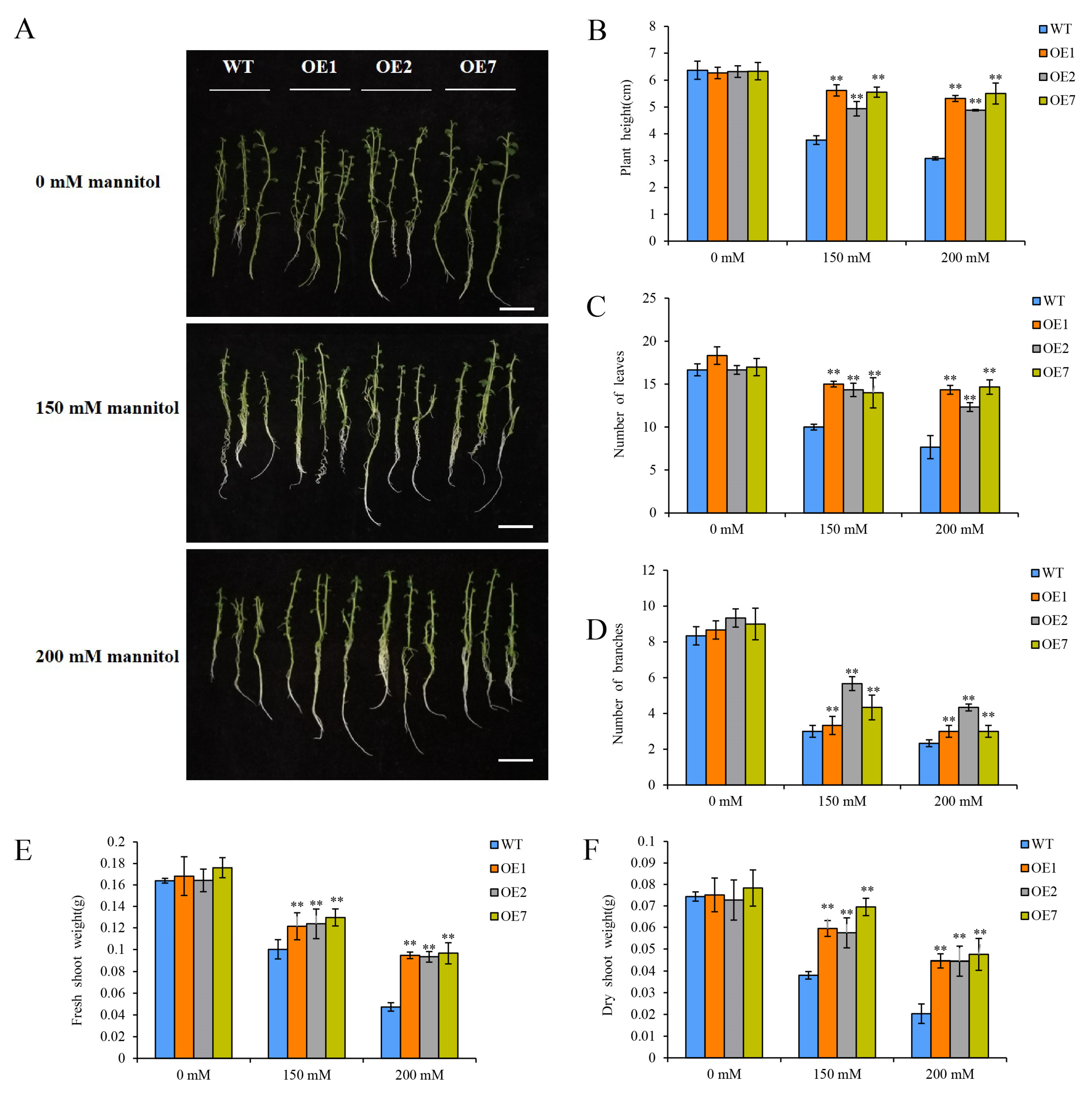
2.4. Overexpression of StCDPK3 Enhances Drought Resistance in Potato
2.5. Overexpression of StCDPK13 Promoted ROS Scavenging and Alleviated Oxidative Damage
2.6. Analysis of Stomatal Aperture and Water Loss Rate of Leaves
2.7. Expression of Drought-Rtelated Genes in StCDPK13 Transgenic Potato
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Cloning and Sequence Analysis of StCDPK13
4.3. Obtaining Transgenic Potato Plants
4.4. Phenotype Analysis of WT and Transgenic Plants Under Drought Stress
4.5. Determination of Physiological Indicators Under Drought Stress
4.6. Analysis of Stomatal Aperture and Water Loss Rate in Leaves
4.7. Expression Analysis of the StCDPK13 Gene and Stress-Related Genes
4.8. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hajibarat, Z.; Saidi, A.; Mousapour Gorji, A.; Ghaffari, M.R.; Zeinalabedini, M. Yield and morphological responses of twenty potato (Solanum tuberosom L.) genotypes in response to drought stress. Environ. Stress. Crop Sci. 2023, 16, 183–194. [Google Scholar]
- Jaleel, C.A.; Manivannan, P.; Lakshmanan, G.; Gomathinayagam, M.; Panneerselvam, R. Alterations in morphological parameters and photosynthetic pigment responses of Catharanthus roseus under soil water deficits. Colloids Surf. B Biointerfaces 2008, 61, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Sadok, W.; Lopez, J.R.; Smith, K.P. Transpiration increases under high-temperature stress: Potential mechanisms, trade-offs and prospects for crop resilience in a warming world. Plant Cell Environ. 2021, 44, 2102–2116. [Google Scholar] [CrossRef] [PubMed]
- Hanász, A.; Dobránszki, J.; Mendler-Drienyovszki, N.; Zsombik, L.; Magyar-Tábori, K. Responses of Potato (Solanum tuberosum L.) Breeding Lines to Osmotic Stress Induced in In Vitro Shoot Culture. Horticulturae 2022, 8, 591. [Google Scholar] [CrossRef]
- Ogbaga, C.; Amir, M.; Bano, H.; Chater, C.; Jellason, N. Clarity on frequently asked questions about drought measurements in plant physiology. Sci. Afr. 2020, 8, e00405. [Google Scholar] [CrossRef]
- Yao, P.; Zhang, C.; Sun, C.; Liu, Y.; Liu, Z.; Wei, J.; Su, X.; Bai, J.; Cui, J.; Bi, Z. Overexpression of Potato PYL16 Gene in Tobacco Enhances the Transgenic Plant Tolerance to Drought Stress. Int. J. Mol. Sci. 2024, 25, 8644. [Google Scholar] [CrossRef]
- Patra, N.; Hariharan, S.; Gain, H.; Maiti, M.K.; Das, A.; Banerjee, J. Typical but Delicate Ca++ re: Dissecting the essence of calcium signaling network as a robust response coordinator of versatile abiotic and biotic stimuli in plants. Front. Plant Sci. 2021, 12, 752246. [Google Scholar] [CrossRef]
- Harmon, A.C.; Gribskov, M.; Harper, J.F. CDPKs–a kinase for every Ca2+ signal? Trends Plant Sci. 2000, 5, 154–159. [Google Scholar] [CrossRef]
- Hrabak, E.M.; Chan, C.W.; Gribskov, M.; Harper, J.F.; Choi, J.H.; Halford, N.; Kudla, J.; Luan, S.; Nimmo, H.G.; Sussman, M.R.; et al. The Arabidopsis CDPK-SnRK superfamily of protein kinases. Plant Physiol. 2003, 132, 666–680. [Google Scholar] [CrossRef]
- Bender, K.W.; Zielinski, R.E.; Huber, S.C. Revisiting paradigms of Ca2+ signaling protein kinase regulation in plants. Biochem. J. 2018, 475, 207–223. [Google Scholar] [CrossRef]
- Valmonte, G.R.; Arthur, K.; Higgins, C.M.; MacDiarmid, R.M. Calcium-dependent protein kinases in plants: Evolution, expression and function. Plant Cell Physiol. 2014, 55, 551–569. [Google Scholar] [CrossRef] [PubMed]
- Bi, Z.; Wang, Y.; Li, P.; Sun, C.; Qin, T.; Bai, J. Evolution and expression analysis of CDPK genes under drought stress in two varieties of potato. Biotechnol. Lett. 2021, 43, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Dekomah, S.D.; Wang, Y.; Qin, T.; Xu, D.; Sun, C.; Yao, P.; Liu, Y.; Bi, Z.; Bai, J. Identification and expression analysis of calcium-dependent protein kinases gene family in potato under drought stress. Front. Genet. 2022, 13, 874397. [Google Scholar] [CrossRef]
- Shi, G.; Zhu, X. Genome-wide identification and functional characterization of CDPK gene family reveal their involvement in response to drought stress in Gossypium barbadense. PeerJ 2022, 10, e12883. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, W.; He, Q.; Daud, M.K.; Chen, J.; Zhu, S. Genome-wide survey and expression analysis of calcium-dependent protein kinase in Gossypium raimondii. PLoS ONE 2014, 9, e98189. [Google Scholar] [CrossRef]
- Asano, T.; Tanaka, N.; Yang, G.; Hayashi, N.; Komatsu, S. Genome-wide identification of the rice calcium-dependent protein kinase and its closely related kinase gene families: Comprehensive analysis of the CDPKs gene family in rice. Plant Cell Physiol. 2005, 46, 356–366. [Google Scholar] [CrossRef]
- Li, A.L.; Zhu, Y.F.; Tan, X.M.; Wang, X.; Wei, B.; Guo, H.Z.; Zhang, Z.L.; Chen, X.B.; Zhao, G.Y.; Kong, X.Y.; et al. Evolutionary and functional study of the CDPK gene family in wheat (Triticum aestivum L.). Plant Mol. Biol. 2008, 66, 429–443. [Google Scholar] [CrossRef]
- Kong, X.; Lv, W.; Jiang, S.; Zhang, D.; Cai, G.; Pan, J.; Li, D. Genome-wide identification and expression analysis of calcium-dependent protein kinase in maize. BMC Genom. 2013, 14, 433. [Google Scholar] [CrossRef]
- Hu, Z.; Lv, X.; Xia, X.; Zhou, J.; Shi, K.; Yu, J.; Zhou, Y. Genome-wide identification and expression analysis of calcium-dependent protein kinase in tomato. Front. Plant Sci. 2016, 7, 469. [Google Scholar] [CrossRef]
- Zuo, R.; Hu, R.; Chai, G.; Xu, M.; Qi, G.; Kong, Y.; Zhou, G. Genome-wide identification, classification, and expression analysis of CDPK and its closely related gene families in poplar (Populus trichocarpa). Mol. Biol. Rep. 2013, 40, 2645–2662. [Google Scholar] [CrossRef]
- Xu, X.; Liu, M.; Lu, L.; He, M.; Qu, W.; Xu, Q.; Qi, X.; Chen, X. Genome-wide analysis and expression of the calcium-dependent protein kinase gene family in cucumber. Mol Genet Genom. 2015, 290, 1403–1414. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Han, Y.-T.; Zhao, F.-L.; Hu, Y.; Gao, Y.-R.; Ma, Y.-F.; Zheng, Y.; Wang, Y.-J.; Wen, Y.-Q. Genome-wide identification and expression analysis of the CDPK gene family in grape, Vitis spp. BMC Plant Biol. 2015, 15, 164. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, F.; Li, S.; Feng, Y.; Yang, J.; Zhang, N.; Si, H. Calcium-Dependent Protein Kinase 28 Maintains Potato Photosynthesis and Its Tolerance under Water Deficiency and Osmotic Stress. Int. J. Mol. Sci. 2022, 23, 8795. [Google Scholar] [CrossRef]
- Grossi, C.E.M.; Santin, F.; Quintana, S.A.; Fantino, E.; Ulloa, R.M. Calcium-dependent protein kinase 2 plays a positive role in the salt stress response in potato. Plant Cell Rep. 2022, 41, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Fantino, E.; Segretin, M.E.; Santin, F.; Mirkin, F.G.; Ulloa, R.M. Analysis of the potato calcium-dependent protein kinase family and characterization of StCDPK7, a member induced upon infection with Phytophthora infestans. Plant Cell Rep. 2017, 36, 1137–1157. [Google Scholar] [CrossRef]
- Kamiyoshihara, Y.; Iwata, M.; Fukaya, T.; Tatsuki, M.; Mori, H. Turnover of LeACS2, a wound-inducible 1-aminocyclopropane-1-carboxylic acid synthase in tomato, is regulated by phosphorylation/dephosphorylation. Plant J. 2010, 64, 140–150. [Google Scholar] [CrossRef]
- Dubrovina, A.S.; Kiselev, K.V.; Khristenko, V.S.; Aleynova, O.A. The calcium-dependent protein kinase gene VaCPK29 is involved in grapevine responses to heat and osmotic stresses. Plant Growth Regul. 2017, 82, 79–89. [Google Scholar] [CrossRef]
- Szczegielniak, J.; Borkiewicz, L.; Szurmak, B.; Lewandowska-Gnatowska, E.; Statkiewicz, M.; Klimecka, M.; Ciesla, J.; Muszynska, G. Maize calcium-dependent protein kinase (ZmCPK11): Local and systemic response to wounding, regulation by touch and components of jasmonate signaling. Physiol. Plant 2012, 146, 1–14. [Google Scholar] [CrossRef]
- Wei, S.; Hu, W.; Deng, X.; Zhang, Y.; Liu, X.; Zhao, X.; Luo, Q.; Jin, Z.; Li, Y.; Zhou, S.; et al. A rice calcium-dependent protein kinase OsCPK9 positively regulates drought stress tolerance and spikelet fertility. BMC Plant Biol. 2014, 14, 133. [Google Scholar] [CrossRef]
- Campo, S.; Baldrich, P.; Messeguer, J.; Lalanne, E.; Coca, M.; San Segundo, B. Overexpression of a Calcium-Dependent Protein Kinase Confers Salt and Drought Tolerance in Rice by Preventing Membrane Lipid Peroxidation. Plant Physiol. 2014, 165, 688–704. [Google Scholar] [CrossRef]
- Gao, X.; Chen, X.; Lin, W.; Chen, S.; Lu, D.; Niu, Y.; Li, L.; Cheng, C.; McCormack, M.; Sheen, J.; et al. Bifurcation of Arabidopsis NLR immune signaling via Ca2+-dependent protein kinases. PLoS Pathog. 2013, 9, e1003127. [Google Scholar] [CrossRef] [PubMed]
- Gao, A.; Wu, Q.; Zhang, Y.; Miao, Y.; Song, C. Arabidopsis calcium-dependent protein kinase CPK28 is potentially involved in the response to osmotic stress. Chin. Sci. Bull. 2014, 59, 1113–1122. [Google Scholar] [CrossRef]
- Yu, T.F.; Zhao, W.Y.; Fu, J.D.; Liu, Y.W.; Chen, M.; Zhou, Y.B.; Ma, Y.Z.; Xu, Z.S.; Xi, Y.J. Genome-Wide Analysis of CDPK Family in Foxtail Millet and Determination of SiCDPK24 Functions in Drought Stress. Front Plant Sci. 2018, 9, 651. [Google Scholar] [CrossRef]
- Suzuki, N.; Koussevitzky, S.; Mittler, R.; Miller, G. ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ. 2012, 35, 259–270. [Google Scholar] [CrossRef]
- Wang, S.; Zhuang, K.; Zhang, S.; Yang, M.; Kong, F.; Meng, Q. Overexpression of a tomato carotenoid ε-hydroxylase gene (SlLUT1) improved the drought tolerance of trans-genic tobacco. J. Plant Physiol. 2018, 222, 103–112. [Google Scholar] [CrossRef]
- Ranty, B.; Aldon, D.; Cotelle, V.; Galaud, J.-P.; Thuleau, P.; Mazars, C. Calcium sensors as key hubs in plant responses to biotic and abiotic stresses. Front. Plant Sci. 2016, 7, 327. [Google Scholar] [CrossRef] [PubMed]
- Asano, T.; Hayashi, N.; Kobayashi, M.; Aoki, N.; Miyao, A.; Mitsuhara, I.; Ichikawa, H.; Komatsu, S.; Hirochika, H.; Kikuchi, S.; et al. A rice calcium-dependent protein kinase OsCPK12 oppositely modulates salt-stress tolerance and blast disease resistance. Plant J. 2012, 69, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.L.; Huang, L.F.; Lu, C.A.; He, S.L.; Wang, C.C.; Yu, S.P.; Chen, J.; Yu, S.M. Sugar starvation- and GA-inducible calcium-dependent protein kinase 1 feedback regulates GA biosynthesis and activates a 14-3-3 protein to confer drought tolerance in rice seedlings. Plant Mol. Biol. 2013, 81, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wan, B.; Zhou, F.; Chen, H.; Li, X.; Lin, Y. Up- and Down-regulated Expression of OsCPK25/26 Results in Increased Number of Stamens in Rice. Plant Mol. Biol. Report. 2014, 32, 1114–1128. [Google Scholar] [CrossRef]
- Chico, J.M.; Raíces, M.; Téllez-Iñón, M.T.; Ulloa, R.M. A calcium-dependent protein kinase is systemically induced upon wounding in tomato plants. Plant Physiol. 2002, 128, 256–270. [Google Scholar] [CrossRef]
- Ma, S.Y.; Wu, W.H. AtCPK23 functions in Arabidopsis responses to drought and salt stresses. Plant Mol. Biol. 2007, 65, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.H.; Liu, H.P.; Li, C.L. Calcium-dependent protein kinase CPK9 negatively functions in stomatal abscisic acid signaling by regulating ion channel activity in Arabidopsis. Plant Mol. Biol. 2019, 99, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, M.; Wu, X.; Chen, D.; Lv, H.; Shen, J.; Qiao, Z.; Zhang, W. THI1, a thiamine thiazole synthase, interacts with Ca2+-dependent protein kinase CPK33 and modulates the S-type anion channels and stomatal closure in Arabidopsis. Plant Physiol. 2016, 170, 1090–1104. [Google Scholar] [CrossRef] [PubMed]
- Franz, S.; Ehlert, B.; Liese, A.; Kurth, J.; Cazalé, A.C.; Romeis, T. Calcium-dependent protein kinase CPK21 functions in abiotic stress response in Arabidopsis thaliana. Mol. Plant 2011, 4, 83–96. [Google Scholar] [CrossRef]
- Meng, L.; Zhang, Q.; Yang, J.; Xie, G.; Liu, J.-H. PtrCDPK10 of Poncirus trifoliata functions in dehydration and drought tolerance by reducing ROS accumulation via phosphorylating PtrAPX. Plant Sci. 2020, 291, 110320. [Google Scholar] [CrossRef]
- Xu, J.; Tian, Y.S.; Peng, R.H.; Xiong, A.S.; Zhu, B.; Jin, X.F.; Gao, F.; Fu, X.Y.; Hou, X.L.; Yao, Q.H. AtCPK6, a functionally redundant and positive regulator involved in salt/drought stress tolerance in Arabidopsis. Planta 2010, 231, 1251–1260. [Google Scholar] [CrossRef]
- Bundo, M.; Coca, M. Calcium-dependent protein kinase OsCPK10 mediates both drought tolerance and blast disease resistance in rice plants. J. Exp. Bot. 2017, 68, 2963–2975. [Google Scholar] [CrossRef]
- Wang, L.; Yu, C.; Xu, S.; Zhu, Y.; Huang, W. OsDi19-4 acts downstream of OsCDPK14 to positively regulate ABA response in rice. Plant Cell Environ. 2016, 39, 2740–2753. [Google Scholar] [CrossRef]
- Wang, C.; Song, W. Calcium-dependent protein kinase gene ZmCPK12 from maize confers tolerance to drought and salt stresses in transgenic plants. Acta Physiol. Plant 2013, 35, 1659–1666. [Google Scholar] [CrossRef]
- Chen, J.; Xue, B.; Xia, X.; Yin, W. A novel calcium-dependent protein kinase gene from Populus euphratica, confers both drought and cold stress tolerance. Biochem. Biophys. Res. Commun. 2013, 441, 630–636. [Google Scholar] [CrossRef]
- Vivek, P.; Tuteja, N.; Soniya, E. CDPK1 from Ginger Promotes Salinity and Drought Stress Tolerance without Yield Penalty by Improving Growth and Photosynthesis in Nicotiana tabacum. PLoS ONE 2013, 8, e76392. [Google Scholar] [CrossRef] [PubMed]
- Henry, A.; Cal, A.J.; Batoto, T.C.; Torres, R.O.; Serraj, R. Root attributes affecting water uptake of rice (Oryza sativa) under drought. J. Exp. Bot. 2012, 63, 4751–4763. [Google Scholar] [CrossRef]
- Narayanan, S.; Mohan, A.; Gill, K.S.; Prasad, P.V. Variability of root traits in spring wheat germplasm. PLoS ONE 2014, 9, e100317. [Google Scholar] [CrossRef]
- Bandurska, H. Drought stress responses: Coping strategy and resistance. Plants 2022, 11, 922. [Google Scholar] [CrossRef] [PubMed]
- Jaleel, C.A.; Riadh, K.; Gopi, R.; Manivannan, P.; Ines, J.; Al-Juburi, H.J.; Chang-Xing, Z.; Hong-Bo, S.; Panneerselvam, R. Antioxidant defense responses: Physiological plasticity in higher plants under abiotic constraints. Acta Physiol. Planta-Rum 2009, 31, 427–436. [Google Scholar] [CrossRef]
- Hou, L.; Zhao, M.; Huang, C.; He, Q.; Zhang, L.; Zhang, J. Alternative oxidase gene induced by nitric oxide is involved in the regulation of ROS and enhances the resistance of Pleurotus ostreatus to heat stress. Microb. Cell Factories 2021, 20, 137. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Wu, C.; Luo, C.; Wei, M.; Qu, S.; Wang, S. Overexpression of MdCPK1a gene, a calcium dependent protein kinase in apple, increase tobacco cold tolerance via scavenging ROS accumulation. PLoS ONE 2020, 15, e0242139. [Google Scholar] [CrossRef]
- Franks, P.J.; Beerling, D.J. CO2-forced evolution of plant gas exchange capacity and water-use efficiency over the Phanerozoic. Geobiology 2009, 7, 227–236. [Google Scholar] [CrossRef]
- Sun, S.; Li, X.; Gao, S.; Nie, N.; Zhang, H.; Yang, Y.; He, S.; Liu, Q.; Zhai, H. A Novel WRKY Transcription Factor from Ipomoea trifida, ItfWRKY70, Confers Drought Tolerance in Sweet Potato. Int. J. Mol. Sci. 2022, 23, 686. [Google Scholar] [CrossRef]
- Mori, I.; Murata, Y.; Yang, Y.; Munemasa, S.; Wang, Y.; Andreoli, S.; Tiriac, H.; Alonso, J.; Harper, J.; Ecker, J.; et al. CDPKs CPK6 and CPK3 function in ABA regulation of guard cell S-type anion- and Ca(2+)-permeable channels and stomatal closure. PLoS Biol. 2006, 4, e327. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Liu, B.; Zhang, L.; Chen, J.; Lu, M. Genome-wide analysis of the Populus Hsp90 gene family reveals differential expression patterns, localization, and heat stress responses. BMC Genom. 2013, 14, 532. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Wang, D.; Wang, R.; Kong, N.; Zhang, C.; Yang, C.; Wu, W.; Ma, H.; Chen, Q. Genome-wide analysis of the potato Hsp20 gene family: Identification, genomic organization and expression profiles in response to heat stress. BMC Genom. 2018, 19, 61. [Google Scholar] [CrossRef] [PubMed]
- Dumont, S.; Bykova, N.V.; Khaou, A.; Besserour, Y.; Dorval, M.; Rivoal, J. Arabidopsis thaliana alcohol dehydrogenase is differently affected by several redox modifications. PLoS ONE 2018, 13, e0204530. [Google Scholar] [CrossRef]
- Chen, Y.; Li, C.; Zhang, B.; Yi, J.; Yang, Y.; Kong, C.; Lei, C.; Gong, M. The role of the late embryogenesis-abundant (LEA) protein family in development and the abiotic stress response: A comprehensive expression analysis of potato (Solanum tuberosum). Genes 2019, 10, 148. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, B.; Ma, C.; Zhang, G.; Chang, J.; Si, H.; Wang, D. Transcriptome characterization and sequencing-based identification of drought-responsive genes in potato. Mol. Biol. Rep. 2014, 41, 505–517. [Google Scholar] [CrossRef]
- Pieczynski, M.; Wyrzykowska, A.; Milanowska, K.; Boguszewska-Mankowska, D.; Zagdanska, B.; Karlowski, W.; Jarmolowski, A.; Szweykowska-Kulinska, Z. Genomewide identification of genes involved in the potato response to drought indicates functional evolutionary conservation with Arabidopsis plants. Plant Biotechnol. J. 2018, 16, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Lim, C.W.; Lee, S.C. The pepper CaOSR1 protein regulates the osmotic stress response via abscisic acid signaling. Front. Plant Sci. 2016, 7, 890. [Google Scholar] [CrossRef]
- Wang, Y.; Qin, T.; Pu, Z.; Dekomah, S.D.; Yao, P.; Sun, C.; Liu, Y.; Bi, Z.; Bai, J. Foliar Application of Chelated Sugar Alcohol Calcium Improves Photosynthesis and Tuber Quality under Drought Stress in Potatoes (Solanum tuberosum L.). Int. J. Mol. Sci. 2023, 24, 12216. [Google Scholar] [CrossRef]
- Hamurcu, M.; Sekmen, A.H.; Turkan, I.; Gezgin, S.; Demiral, T.; Bell, R.W. Induced anti-oxidant activity in soybean alleviates oxidative stress under moderate boron toxicity. Plant Growth Regul. 2013, 70, 217–226. [Google Scholar] [CrossRef]
- Huang, C.; Zhao, S.; Wang, L.; Anjum, S.A.; Chen, M.; Zhou, H.; Zou, C. Alteration in chlorophyll fluorescence, lipid peroxidation and antioxidant enzymes activities in hybrid ramie (‘Boehmeria nivea’ L.) Under drought stress. Aust. J. Crop Sci. 2013, 7, 594–599. [Google Scholar]
- Braik, M.; Barsan, M.M.; Dridi, C.; Ali, M.B.; Brett, C.M. Highly sensitive amperometric enzyme biosensor for detection of superoxide based on conducting polymer/CNT modified electrodes and superoxide dismutase. Sens. Actuators B Chem. 2016, 236, 574–582. [Google Scholar] [CrossRef]
- Junglee, S.; Urban, L.; Sallanon, H.; Lopez-Lauri, F. Optimized assay for hydrogen peroxide determination in plant tissue using potassium iodide. Am. J. Anal. Chem. 2014, 5, 730. [Google Scholar] [CrossRef]
- Kong, F.; Deng, Y.; Zhou, B.; Wang, G.; Wang, Y.; Meng, Q. A chloroplast targeted DnaJ protein contributes to maintenance of photosystem II under chilling stress. J. Exp. Bot. 2014, 65, 143–158. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bi, Z.; Dekomah, S.D.; Wang, Y.; Pu, Z.; Wang, X.; Dormatey, R.; Sun, C.; Liu, Y.; Liu, Z.; Bai, J.; et al. Overexpression of StCDPK13 in Potato Enhances Tolerance to Drought Stress. Int. J. Mol. Sci. 2024, 25, 12620. https://doi.org/10.3390/ijms252312620
Bi Z, Dekomah SD, Wang Y, Pu Z, Wang X, Dormatey R, Sun C, Liu Y, Liu Z, Bai J, et al. Overexpression of StCDPK13 in Potato Enhances Tolerance to Drought Stress. International Journal of Molecular Sciences. 2024; 25(23):12620. https://doi.org/10.3390/ijms252312620
Chicago/Turabian StyleBi, Zhenzhen, Simon Dontoro Dekomah, Yihao Wang, Zhuanfang Pu, Xiangdong Wang, Richard Dormatey, Chao Sun, Yuhui Liu, Zhen Liu, Jiangping Bai, and et al. 2024. "Overexpression of StCDPK13 in Potato Enhances Tolerance to Drought Stress" International Journal of Molecular Sciences 25, no. 23: 12620. https://doi.org/10.3390/ijms252312620
APA StyleBi, Z., Dekomah, S. D., Wang, Y., Pu, Z., Wang, X., Dormatey, R., Sun, C., Liu, Y., Liu, Z., Bai, J., & Yao, P. (2024). Overexpression of StCDPK13 in Potato Enhances Tolerance to Drought Stress. International Journal of Molecular Sciences, 25(23), 12620. https://doi.org/10.3390/ijms252312620






