Ganglioside GM1 Alleviates Propofol-Induced Pyroptosis in the Hippocampus of Developing Rats via the PI3K/AKT/NF-κB Signaling Cascade
Abstract
1. Introduction
2. Results
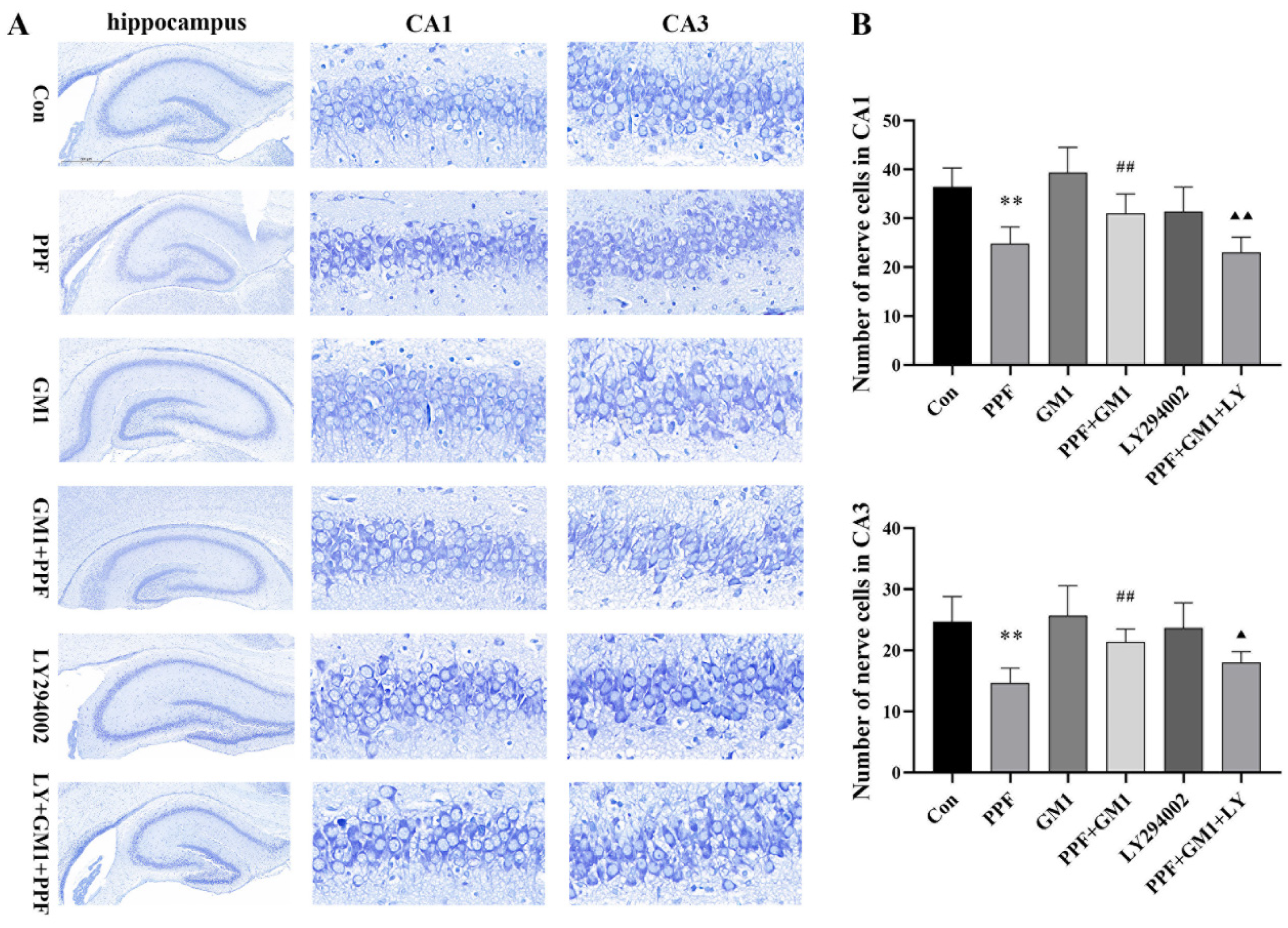
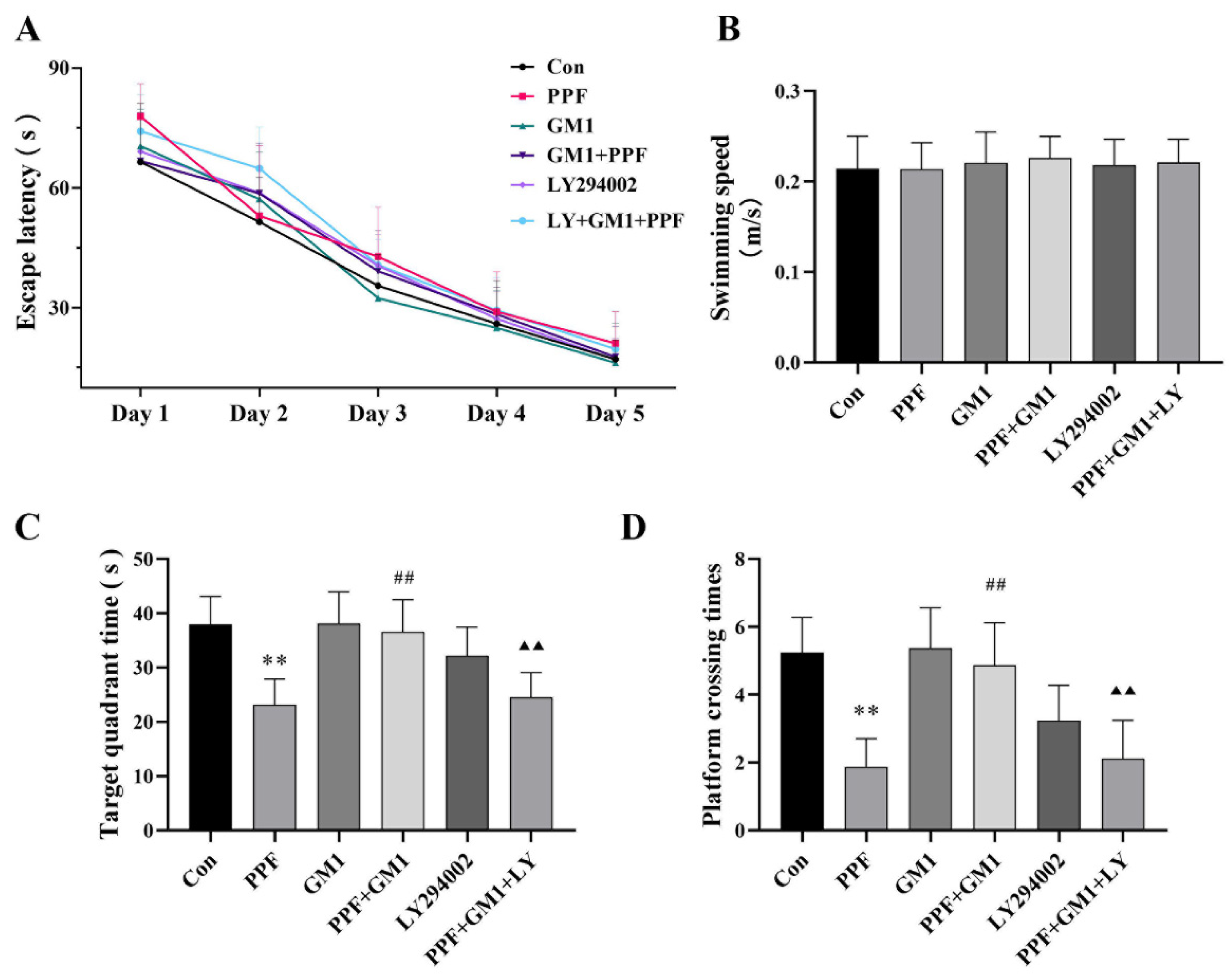
2.1. Impact of GM1 on Propofol-Induced Hippocampal Injury and Cognitive Deficits in Developing Rats
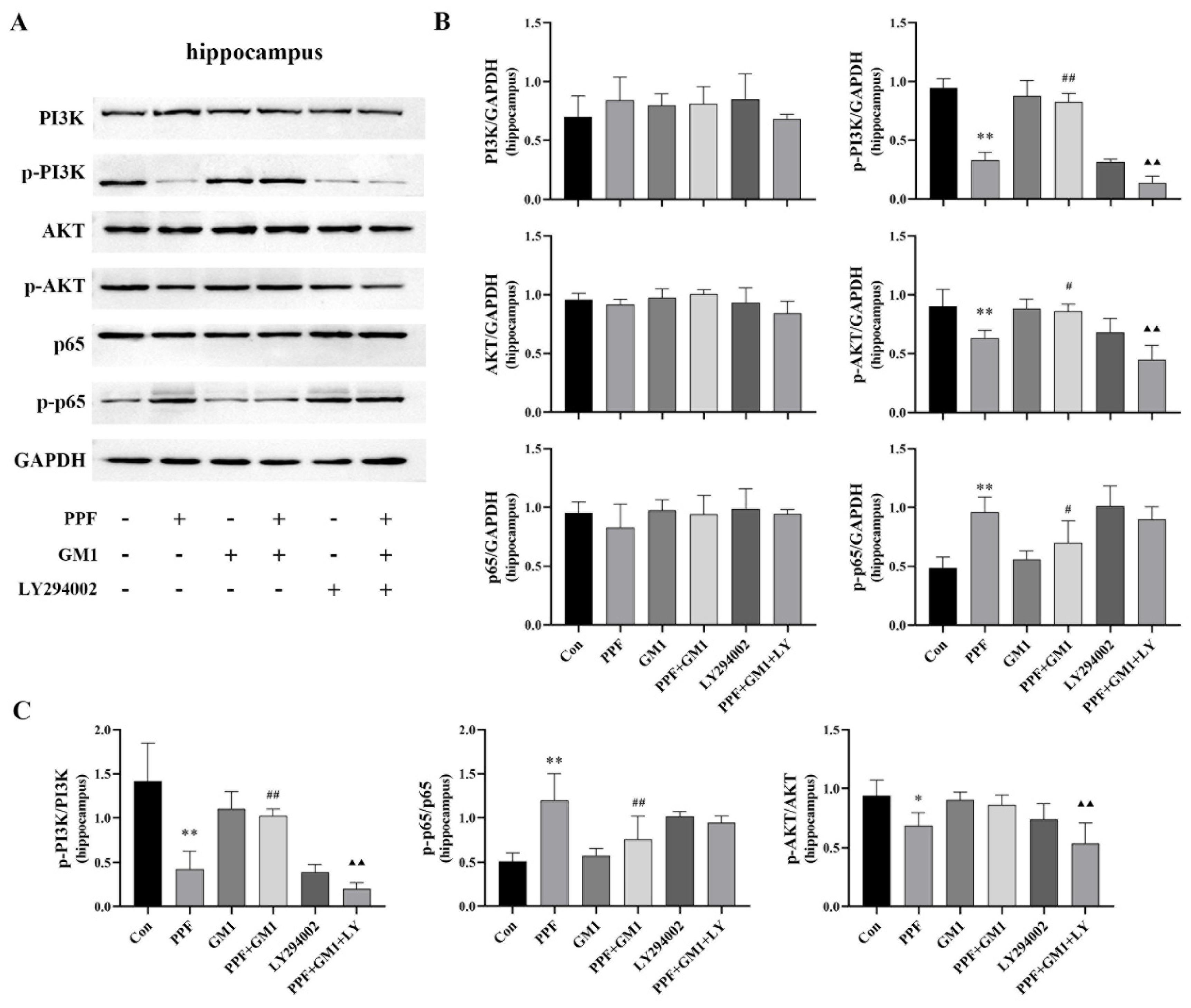
2.2. PI3K/AKT/NF-κB Pathway Involved in Propofol-Induced Hippocampal Injury
2.3. GM1 Attenuates Propofol-Induced Pyroptosis and Inflammation of the PI3K/AKT/NF-κB Pathway in the Hippocampus of Developing Rats
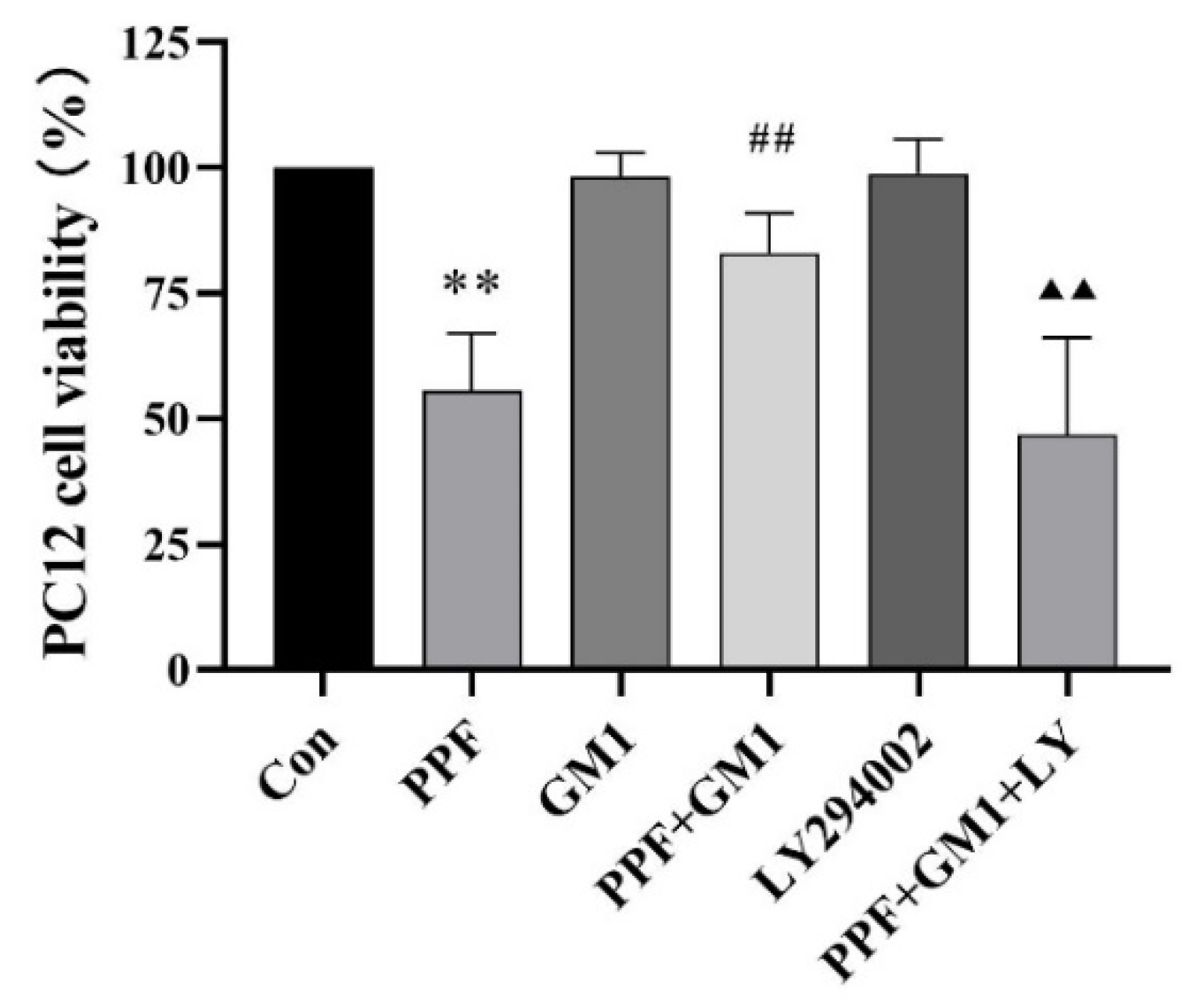
2.4. Effect of GM1 on the Survival Rate of Propofol-Administered PC12 Cells
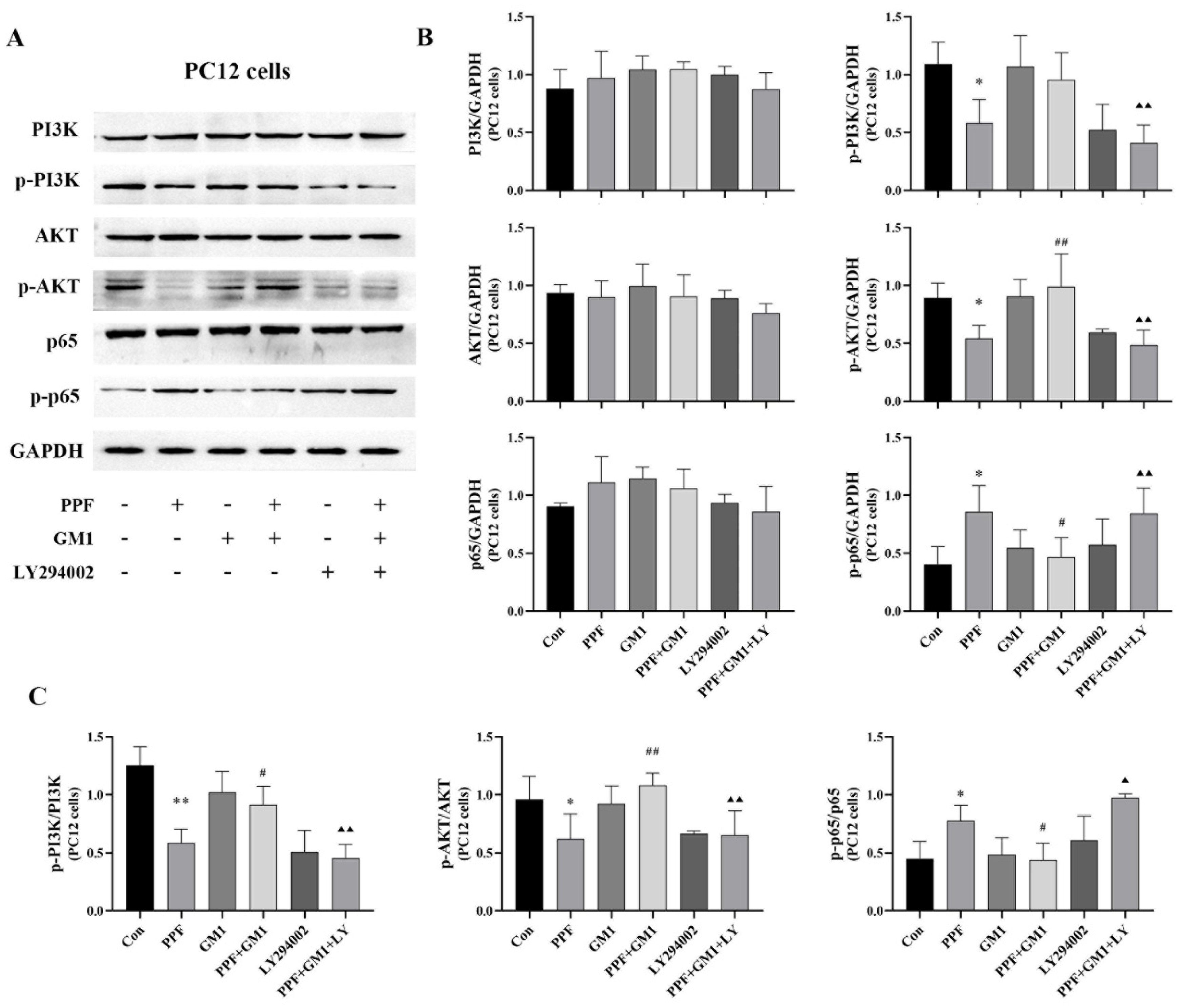
2.5. Impact of GM1 on Propofol-Induced PC12 Cell Death via the PI3K/AKT/NF-κB Pathway
2.6. GM1 Inhibits NLRP3/Caspase-1 and Propofol-Induced Pyroptosis in PC12 Cells
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Propofol Exposure and Drug Treatment
4.3. Nissl Staining Analysis
4.4. Morris Water Maze (MWM) Assay
4.5. In Vitro Experiment and Propofol Exposure
4.6. CCK-8 Test
4.7. Lactate Dehydrogenase Release Analysis
4.8. Immunofluorescence Assay
4.9. Western Blotting Analysis
4.10. ELISA Test
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Y.; Yi, J.; Pan, M.; Hu, B.; Duan, H. Edaravone alleviated propofol-induced neural injury in developing rats by BDNF/TrkB pathway. J. Cell. Mol. Med. 2021, 25, 4974–4987. [Google Scholar] [CrossRef] [PubMed]
- Jevtovic-Todorovic, V. Exposure of Developing Brain to General Anesthesia: What Is the Animal Evidence? Anesthesiology 2017, 128, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Tesic, V.; Joksimovic, S.M.; Quillinan, N.; Krishnan, K.; Covey, D.F.; Todorovic, S.M.; Jevtovic-Todorovic, V. Neuroactive steroids alphaxalone and CDNC24 are effective hypnotics and potentiators of GABAA currents, but are not neurotoxic to the developing rat brain. Br. J. Anaesth. 2020, 124, 603–613. [Google Scholar] [CrossRef]
- Ying, S.-W.; Goldstein, P.A. Propofol suppresses synaptic responsiveness of somatosensory relay neurons to excitatory input by potentiating GABA(A) receptor chloride channels. Mol. Pain 2005, 1, 2. [Google Scholar] [CrossRef] [PubMed]
- Orser, B.A.; Bertlik, M.; Wang, L.Y.; MacDonald, J.F. Inhibition by propofol (2,6 di-isopropylphenol) of the N-methyl-D-aspartate subtype of glutamate receptor in cultured hippocampal neurones. Br. J. Pharmacol. 1995, 116, 1761–1768. [Google Scholar] [CrossRef]
- Yang, B.; Liang, G.; Khojasteh, S.; Wu, Z.; Yang, W.; Joseph, D.; Wei, H. Comparison of neurodegeneration and cognitive impairment in neonatal mice exposed to propofol or isoflurane. PLoS ONE 2014, 9, e99171. [Google Scholar] [CrossRef]
- Popic, J.; Pesic, V.; Milanovic, D.; Todorovic, S.; Kanazir, S.; Jevtovic-Todorovic, V.; Ruzdijic, S. Propofol-induced changes in neurotrophic signaling in the developing nervous system in vivo. PLoS ONE 2012, 7, e34396. [Google Scholar] [CrossRef]
- Creeley, C.; Dikranian, K.; Dissen, G.; Martin, L.; Olney, J.; Brambrink, A. Propofol-induced apoptosis of neurones and oligodendrocytes in fetal and neonatal rhesus macaque brain. Br. J. Anaesth. 2013, 110 (Suppl. S1), i29–i38. [Google Scholar] [CrossRef]
- Kajimoto, M.; Atkinson, D.B.; Ledee, D.R.; Kayser, E.-B.; Morgan, P.G.; Sedensky, M.M.; Isern, N.G.; Des Rosiers, C.; Portman, M.A. Propofol compared with isoflurane inhibits mitochondrial metabolism in immature swine cerebral cortex. J. Cereb. Blood Flow Metab. 2014, 34, 514–521. [Google Scholar] [CrossRef]
- Bosnjak, Z.J.; Logan, S.; Liu, Y.; Bai, X. Recent Insights into Molecular Mechanisms of Propofol-Induced Developmental Neurotoxicity: Implications for the Protective Strategies. Anesth. Analg. 2016, 123, 1286–1296. [Google Scholar] [CrossRef]
- Xu, X.-E.; Liu, L.; Wang, Y.-C.; Wang, C.-T.; Liu, X.-H. Caspase-1 inhibitor exerts brain-protective effects against sepsis-associated encephalopathy and cognitive impairments in a mouse model of sepsis. Brain Behav. Immun. 2019, 80, 859–870. [Google Scholar] [CrossRef] [PubMed]
- Adamczak, S.E.; Pablo de Rivero Vaccari, J.; Dale, G.; Brand, F.J.; Nonner, D.; Bullock, M.; Dahl, G.P.; Dietrich, W.D.; Keane, R.W. Pyroptotic neuronal cell death mediated by the AIM2 inflammasome. J. Cereb. Blood Flow Metab. 2014, 34, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Venegas, C.; Kumar, S.; Franklin, B.S.; Dierkes, T.; Brinkschulte, R.; Tejera, D.; Vieira-Saecker, A.; Schwartz, S.; Santarelli, F.; Kummer, M.P. Microglia-derived ASC specks cross-seed amyloid-β in Alzheimer’s disease. Nature 2017, 552, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Morin-Brureau, M.; Milior, G.; Royer, J.; Chali, F.; Le Duigou, C.; Savary, E.; Blugeon, C.; Jourdren, L.; Akbar, D.; Dupont, S.; et al. Microglial phenotypes in the human epileptic temporal lobe. Brain 2018, 141, 3343–3360. [Google Scholar] [CrossRef] [PubMed]
- Chitnis, T.; Weiner, H.L. CNS inflammation and neurodegeneration. J. Clin. Investig. 2017, 127, 3577–3587. [Google Scholar] [CrossRef]
- Zhong, Y.; Liang, Y.; Chen, J.; Li, L.; Qin, Y.; Guan, E.; He, D.; Wei, Y.; Xie, Y.; Xiao, Q. Propofol inhibits proliferation and induces neuroapoptosis of hippocampal neurons in vitro via downregulation of NF-κB p65 and Bcl-2 and upregulation of caspase-3. Cell Biochem. Funct. 2014, 32, 720–729. [Google Scholar] [CrossRef]
- Yılmaz, H.; Şengelen, A.; Demirgan, S.; Paşaoğlu, H.E.; Çağatay, M.; Erman, İ.E.; Bay, M.; Güneyli, H.C.; Önay-Uçar, E. Acutely increased aquaporin-4 exhibits more potent protective effects in the cortex against single and repeated isoflurane-induced neurotoxicity in the developing rat brain. Toxicol. Mech. Methods 2023, 33, 279–292. [Google Scholar] [CrossRef]
- Gao, J.; Wang, N.; Zong, F.; Dong, J.; Lin, Y.; Zhang, H.; Zhang, F. TIPE2 regulates the response of BV2 cells to lipopolysaccharide by the crosstalk between PI3K/AKT signaling and microglia M1/M2 polarization. Int. Immunopharmacol. 2023, 120, 110389. [Google Scholar] [CrossRef]
- Hua, F.-Z.; Ying, J.; Zhang, J.; Wang, X.-F.; Hu, Y.-H.; Liang, Y.-P.; Liu, Q.; Xu, G.-H. Naringenin pre-treatment inhibits neuroapoptosis and ameliorates cognitive impairment in rats exposed to isoflurane anesthesia by regulating the PI3/Akt/PTEN signalling pathway and suppressing NF-κB-mediated inflammation. Int. J. Mol. Med. 2016, 38, 1271–1280. [Google Scholar] [CrossRef]
- Posse de Chaves, E.; Sipione, S. Sphingolipids and gangliosides of the nervous system in membrane function and dysfunction. FEBS Lett. 2010, 584, 1748–1759. [Google Scholar] [CrossRef]
- Sipione, S.; Monyror, J.; Galleguillos, D.; Steinberg, N.; Kadam, V. Gangliosides in the Brain: Physiology, Pathophysiology and Therapeutic Applications. Front. Neurosci. 2020, 14, 572965. [Google Scholar] [CrossRef] [PubMed]
- Ledeen, R.; Wu, G. Gangliosides of the Nervous System. Methods Mol. Biol. 2018, 1804, 19–55. [Google Scholar] [CrossRef] [PubMed]
- Ledeen, R.W.; Wu, G. Gangliosides, α-Synuclein, and Parkinson’s Disease. Prog. Mol. Biol. Transl. Sci. 2018, 156, 435–454. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Dong, S.; Quan, S.; Ding, S.; Zhou, X.; Yu, Y.; Wu, Y.; Huang, W.; Shi, Q.; Li, Q. Nuciferine reduces inflammation induced by cerebral ischemia-reperfusion injury through the PI3K/Akt/NF-κB pathway. Phytomedicine 2024, 125, 155312. [Google Scholar] [CrossRef]
- Qian, H.; Chen, A.; Lin, D.; Deng, J.; Gao, F.; Wei, J.; Wu, X.; Huang, Y.; Cai, D.; Chen, X.; et al. Activation of the CD200/CD200R1 axis improves cognitive impairment by enhancing hippocampal neurogenesis via suppression of M1 microglial polarization and neuroinflammation in hypoxic-ischemic neonatal rats. Int. Immunopharmacol. 2024, 128, 111532. [Google Scholar] [CrossRef]
- Smith, M.C.; Williamson, J.; Yaster, M.; Boyd, G.J.C.; Heitmiller, E.S. Off-label use of medications in children undergoing sedation and anesthesia. Anesth. Analg. 2012, 115, 1148–1154. [Google Scholar] [CrossRef]
- Twaroski, D.M.; Yan, Y.; Olson, J.M.; Bosnjak, Z.J.; Bai, X. Down-regulation of microRNA-21 is involved in the propofol-induced neurotoxicity observed in human stem cell-derived neurons. Anesthesiology 2014, 121, 786–800. [Google Scholar] [CrossRef]
- Sall, J.W.; Stratmann, G.; Leong, J.; Woodward, E.; Bickler, P.E. Propofol at clinically relevant concentrations increases neuronal differentiation but is not toxic to hippocampal neural precursor cells in vitro. Anesthesiology 2012, 117, 1080–1090. [Google Scholar] [CrossRef]
- Kahraman, S.; Zup, S.L.; McCarthy, M.M.; Fiskum, G. GABAergic mechanism of propofol toxicity in immature neurons. J. Neurosurg. Anesthesiol. 2008, 20, 233–240. [Google Scholar] [CrossRef]
- Cattano, D.; Young, C.; Straiko, M.M.W.; Olney, J.W. Subanesthetic doses of propofol induce neuroapoptosis in the infant mouse brain. Anesth. Analg. 2008, 106, 1712–1714. [Google Scholar] [CrossRef]
- Bonizzi, G.; Karin, M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004, 25, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Withoff, S.; Verma, I.M. Inflammation-associated cancer: NF-κB is the lynchpin. Trends Immunol. 2005, 26, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.; Natoli, G.; Ghosh, G. Transcriptional regulation via the NF-kappaB signaling module. Oncogene 2006, 25, 6706–6716. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.S.; Ghosh, S. Signaling to NF-kappaB. Genes Dev. 2004, 18, 2195. [Google Scholar] [CrossRef]
- Place, D.E.; Kanneganti, T.-D. Recent advances in inflammasome biology. Curr. Opin. Immunol. 2018, 50, 32–38. [Google Scholar] [CrossRef]
- Ding, J.; Shao, F. SnapShot: The Noncanonical Inflammasome. Cell 2017, 168, 544.e1. [Google Scholar] [CrossRef]
- Liu, P.-F.; Gao, T.; Li, T.-Z.; Yang, Y.-T.; Xu, Y.-X.; Xu, Z.-P.; Mi, W.-D. Repeated propofol exposure-induced neuronal damage and cognitive impairment in aged rats by activation of NF-κB pathway and NLRP3 inflammasome. Neurosci. Lett. 2021, 740, 135461. [Google Scholar] [CrossRef]
- Ledeen, R.W.; Wu, G.; Lu, Z.H.; Kozireski-Chuback, D.; Fang, Y. The role of GM1 and other gangliosides in neuronal differentiation. Overview and new finding. Ann. N. Y. Acad. Sci. 1998, 845, 161–175. [Google Scholar] [CrossRef]
- Whitehead, S.N.; Chan, K.H.N.; Gangaraju, S.; Slinn, J.; Li, J.; Hou, S.T. Imaging mass spectrometry detection of gangliosides species in the mouse brain following transient focal cerebral ischemia and long-term recovery. PLoS ONE 2011, 6, e20808. [Google Scholar] [CrossRef]
- Liu, J.-R.; Ding, M.-P.; Wei, E.-Q.; Luo, J.-H.; Song, Y.; Huang, J.-Z.; Ge, Q.-F.; Hu, H.; Zhu, L.-J. GM1 stabilizes expression of NMDA receptor subunit 1 in the ischemic hemisphere of MCAo/reperfusion rat. J. Zhejiang Univ. Sci. B 2005, 6, 254–258. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, W.; Shen, M.; Ma, X.; Li, R.; Jin, X.; Bai, H.; Gao, L. Protective Effect of GM1 Attenuates Hippocampus and Cortex Apoptosis After Ketamine Exposure in Neonatal Rat via PI3K/AKT/GSK3β Pathway. Mol. Neurobiol. 2021, 58, 3471–3483. [Google Scholar] [CrossRef] [PubMed]
- Meng, C.; Yao, X.-Q.; Chang, R.-J.; Wang, S.-L.; Wang, X.; Ma, D.-Q.; Li, Q.; Wang, X.-Y. Exogenous GM1 Ganglioside Attenuates Ketamine-Induced Neurocognitive Impairment in the Developing Rat Brain. Anesth. Analg. 2020, 130, 505–517. [Google Scholar] [CrossRef]
- Fruman, D.A.; Chiu, H.; Hopkins, B.D.; Bagrodia, S.; Cantley, L.C.; Abraham, R.T. The PI3K Pathway in Human Disease. Cell 2017, 170, 605–635. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-F.; Xu, R.; Ouyang, Z.-J.; Qian, C.; Shen, Y.; Wu, X.-D.; Gu, Y.-H.; Xu, Q.; Sun, Y. Beauvericin ameliorates experimental colitis by inhibiting activated T cells via downregulation of the PI3K/Akt signaling pathway. PLoS ONE 2013, 8, e83013. [Google Scholar] [CrossRef]
- Li, L.; Jiang, W.; Yu, B.; Liang, H.; Mao, S.; Hu, X.; Feng, Y.; Xu, J.; Chu, L. Quercetin improves cerebral ischemia/reperfusion injury by promoting microglia/macrophages M2 polarization via regulating PI3K/Akt/NF-κB signaling pathway. Biomed. Pharmacother. 2023, 168, 115653. [Google Scholar] [CrossRef]
- Kargaran, P.; Lenglet, S.; Montecucco, F.; Mach, F.; Copin, J.-C.; Vutskits, L. Impact of propofol anaesthesia on cytokine expression profiles in the developing rat brain: A randomised placebo-controlled experimental in-vivo study. Eur. J. Anaesthesiol. 2015, 32, 336–345. [Google Scholar] [CrossRef]
- Briner, A.; Nikonenko, I.; De Roo, M.; Dayer, A.; Muller, D.; Vutskits, L. Developmental Stage-dependent persistent impact of propofol anesthesia on dendritic spines in the rat medial prefrontal cortex. Anesthesiology 2011, 115, 282–293. [Google Scholar] [CrossRef]
- Zhang, Z.; Bai, H.; Ma, X.; Shen, M.; Li, R.; Qiu, D.; Li, S.; Gao, L. Blockade of the NLRP3/caspase-1 axis attenuates ketamine-induced hippocampus pyroptosis and cognitive impairment in neonatal rats. J. Neuroinflamm. 2021, 18, 239. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Du, S.; Chen, X.; Qiu, D.; Li, S.; Han, L.; Bai, H.; Gao, R. Ganglioside GM1 Alleviates Propofol-Induced Pyroptosis in the Hippocampus of Developing Rats via the PI3K/AKT/NF-κB Signaling Cascade. Int. J. Mol. Sci. 2024, 25, 12662. https://doi.org/10.3390/ijms252312662
Zhang Z, Du S, Chen X, Qiu D, Li S, Han L, Bai H, Gao R. Ganglioside GM1 Alleviates Propofol-Induced Pyroptosis in the Hippocampus of Developing Rats via the PI3K/AKT/NF-κB Signaling Cascade. International Journal of Molecular Sciences. 2024; 25(23):12662. https://doi.org/10.3390/ijms252312662
Chicago/Turabian StyleZhang, Zhiheng, Shan Du, Xinzhang Chen, Di Qiu, Siyao Li, Lin Han, Hui Bai, and Ruifeng Gao. 2024. "Ganglioside GM1 Alleviates Propofol-Induced Pyroptosis in the Hippocampus of Developing Rats via the PI3K/AKT/NF-κB Signaling Cascade" International Journal of Molecular Sciences 25, no. 23: 12662. https://doi.org/10.3390/ijms252312662
APA StyleZhang, Z., Du, S., Chen, X., Qiu, D., Li, S., Han, L., Bai, H., & Gao, R. (2024). Ganglioside GM1 Alleviates Propofol-Induced Pyroptosis in the Hippocampus of Developing Rats via the PI3K/AKT/NF-κB Signaling Cascade. International Journal of Molecular Sciences, 25(23), 12662. https://doi.org/10.3390/ijms252312662






