Abstract
Plants are rich sources of specialized metabolites, such as alkaloids, terpenes, phenolic acids, flavonoids, coumarins, and volatile oils, which provide various health benefits including anticancer, anti-inflammatory, antiaging, skin-altering, and anti-diabetic properties. However, challenges such as low and inconsistent yields, environment and geographic factors, and species-specific production of some specialized metabolites limit the supply of raw plant material for the food, cosmetic, or pharmaceutical industries. Therefore, biotechnological approaches using plant in vitro systems offer an appealing alternative for the production of biologically active metabolites. Among these, hairy root cultures induced by Rhizobium rhizogenes have firmed up their position as “green cell factories” due to their genotypic and biosynthetic stability. Hairy roots are valuable platforms for producing high-value phytomolecules at a low cost, are amenable to pathway engineering, and can be scaled up in bioreactors, making them attractive for commercialization. This review explores the potential of hairy roots for specialized metabolites biosynthesis focusing on biotechnology tools to enhance their production. Aspects of morphological peculiarities of hairy roots, the diversity of bioreactors design, and process intensification technologies for maximizing biosynthetic capacity, as well as examples of patented plant-derived (green-labeled) products produced through hairy root cultivation at lab and industrial scales, are addressed and discussed.
1. Introduction
Plants might be regarded as an almost unlimited resource and a biochemical factory for the biosynthesis of both primary (e.g., carbohydrates, amino acids, and lipids) and highly valuable secondary/specialized metabolites (SMs), including alkaloids, terpenes, phenolic acids, flavonoids, tannins, coumarins, quinones, and volatile oils, etc. [1,2]. The SMs comprise a vast range of low-molecular-weight compounds that are not essential in the survival of plants but provide defense against biotic factors (e.g., herbivores, fungi, and bacteria) and abiotic stresses (e.g., UV radiation, temperature, and drought), and are also attracting agents during pollination and seed dispersal [3]. These SMs have diverse beneficial biological activities important for humans, including anticancer, anti-inflammatory, antiaging, skin-altering, and anti-diabetic effects, among others, and are intensively incorporated in plant-derived cosmetic products, pharmaceuticals, nutraceuticals, or food additives [4]. Some of the widely used plant-derived active ingredients with enormous commercial value that are incorporated in pharmacologically important formulations or solely administered are paclitaxel, atropine, morphine, dopamine, and artemisinin [5]. Plant SMs possess several particular features, including complex and specifically arranged aromatic rings, chiral centers, and chemical entities. These characteristics make them not only valuable targets for drug discovery but also starting points for new synthetic drugs, though this process is often very complicated and economically challenging [6]. Hence, a quarter of the drugs approved by the Food and Drug Administration (FDA) and/or the European Medicinal Agency (EMA) are plant-originated [7,8]. However, obstacles such as low production yield of the SMs, slow plant growth, dependence on anthropological, environmental, ecological, and demographic factors, as well as difficulties in the purification of the SMs and the potential presence of toxic substances, result in unavoidable fluctuations in yield and quality [9]. The broad diversity of plants and plant-derived natural products has created a considerable imbalance between their demand and availability. Consequently, there is growing global interest in establishing more effective alternative sources of important phytomolecules to address these challenges [10,11].
In the past decades, there has been a growing interest in plant in vitro systems as an appealing alternative for the production of biologically active metabolites. Plant in vitro systems offer opportunities for the production of high-value and marketable molecules through diverse biotechnological methods, possessing the following advantages: (1) production of SMs independent of internal or external factors; (2) consistency in yield and quality of natural products between different batches; (3) production under aseptic controlled conditions, improving the biosafety of the final product (free from environmental or genetic contamination); and (4) reduced production cycle time compared to the whole plant [12]. Therefore, in vitro plant-based systems are progressively considered for the large-scale production of valuable medicinal or cosmetic ingredients at lower cost [13]. The most frequently used plant in vitro systems for SMs biosynthesis have been the hairy roots (HRs) and plant cell suspension cultures. The induction and development of cell suspensions requires exogenous supplementation of plant growth regulators, and due to its more simplified bioreactor design, they are still preferred for the production of SMs at lab- or large scales. However, the somaclonal variation and genesis of undifferentiated tissues often results in limited growth and inconsistent yields of natural products [14]. Nevertheless, the production of SMs by HRs has garnered much more attention, owing to their extraordinary advantages, such as inherent genotypic, phenotypic, and biosynthetic stability [15].
Recently published reviews have limited focus on HRs mainly based on the possibilities to increase the SMs biosynthesis capacity, presenting familiar biosynthetic pathways of phenolic acids (salvianolic and rosmarinic acids) or terpenoid indole alkaloids [16,17]. Other review articles present in detail the processes of HR induction and applications [18] or have been focused on definite plant species, indicating their perspectives for biosynthesis of SMs and scale-up cultivation possibilities [19,20,21].
This review attempts to unravel the untapped potential of HRs for biosynthesis of high-value SMs and bring new insights in the biotechnology toolkit utilized to enhance SM production. An overview of various strategies to improve SM production is presented, including nutrient medium optimization, precursor feeding, biotic and abiotic elicitation, and molecular approaches such as metabolic engineering through the manipulation of single and/or multiple genes involved in the natural products’ biosynthesis pathways by CRISPR/Cas9 systems or the overexpression/suppression of transcription factors (TFs).
The review also covers some aspects of the HRs morphological peculiarities, the diversity of bioreactors design, and process intensification technologies for achieving maximum biosynthetic capacity. Special attention is given to the biosynthesis of green-labeled (plant-derived) products through HRs cultivation at both lab and industrial scales. A summary of the multiple applications and recent patents for production of SMs based on HRs cultivation is also highlighted. The workflow of this review is illustrated in Figure 1.
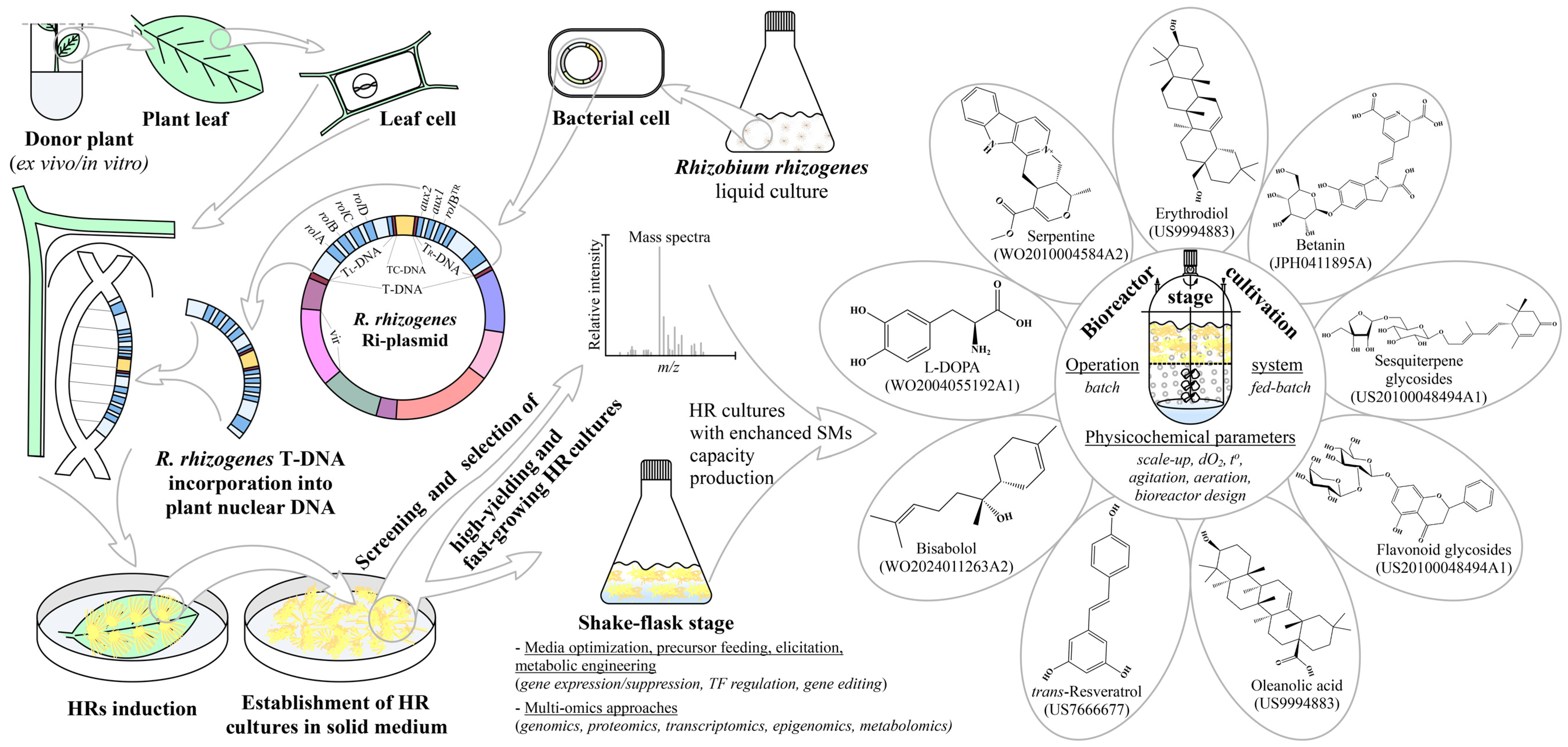
Figure 1.
Schematic presentation of the HRs induction process, biotechnological toolkit used for SMs enhancement, HRs multiple applications, and patents related to the topic. Sterile leaf explants are exposed to R. rhizogenes culture for transfer and integration of T-DNA from the bacterium Ri-plasmid to the plant genome. After HRs induction, individual lines are established and the selection of high-producing and fast-growing clones is performed. Root cultures with higher production capacity after the shake-flask stage are transferred into bioreactors with suitable design for scaling up the production process, which can finally be patented. During the shake-flask and bioreactor stages, different strategies for the enhancement of SMs biosynthesis, as well as process parameter optimizations, are applied.
2. Rhizobium rhizogenes Mediated Genetic Transformation and Hairy Roots Untapped Potential
HRs are known to be induced by genetic-mediated transformation with the soil Gram-negative bacterium Agrobacterium rhizogenes, currently known as Rhizobium rhizogenes. Although the molecular events behind this process are not fully understood, it can generally be summed up in several stages: (1) chemotaxis or activation of the attachment, during which R. rhizogenes is attracted to plant tissues after wounding and triggers the secretion of phenolic compounds (acetosyringone) that are recognized by the bacteria; (2) processing of the T-DNA (transfer DNA) component of the root-inducing plasmid (Ri-plasmid) facilitated by virulence (vir) genes on the Ri-plasmid and the chromosomal virulence (chv) genes of bacterial chromosomal DNA; (3) incorporation of the T-DNA into the host plant genome; (4) expression of the T-DNA into the host plant genome; and (5) induction of the HRs from the wound site.
The T-DNA fragment contains a set of genes responsible for the synthesis of phytohormones, such as auxin and cytokinins, which drives the characteristic development of HRs as well as genes encoding for opine synthesis (products of condensation of amino acids with ketoacids or sugars used by R. rhizogenes as a source of carbon and nitrogen) [16,17]. Among the 18 open reading frames (ORFs) in the T-DNA region, the ‘rol’ (rooting locus) genes, rolA, rolB, rolC, and rolD, are essential for HRs formation. These oncogenes modulate plant cellular processes, including the regulation of phytohormone homeostasis, as well as auxin and cytokinin metabolism and signaling pathways [18,19]. Among these, rolB is particularly important for HRs induction. For example, rolB hydrolyses bound auxins resulting in increased intracellular level of indole-3-acetic acid, which is crucial for HRs induction [20]. In a loss-of-function study, knocking out the rolB genes rendered the Ri-plasmid avirulence [21]. Conversely, the rolC gene hydrolyses cytokinin conjugates, thereby liberating cytokinin that cumulatively enhance HRs growth when expressed with rolA and rolB [22]. Rhizobium rhizogenes-mediated HRs induction can be conducted using both in vitro and in planta inoculation techniques. The current methods for in vitro HRs induction could be classified as follows: (1) Direct plant inoculation, in which R. rhizogenes liquid culture is directly injected into the plant explant and (2) Co-cultivation method, which involves simultaneous cultivating of the plant explant with the bacterial suspension. To increase the transformation efficacy, treatments such as vacuum infiltration and sonication have also been used in the in vitro inoculation method. These techniques, as well as the main steps in HRs induction, were described in more detail elsewhere [16,18,22,23,24]. The in vitro techniques are performed in an aseptic, contamination-free environment, where the R. rhizogenes strain, type, and age of explants are influential [18,25]. On the opposite, in planta techniques do not require aseptic conditions, and therefore they are faster, less labor-intensive, and more cost-effective. The main techniques here are vacuum infiltration, R. rhizogenes injection, pollen tube-mediated gene transfer, floral dip, and floral spray methods described in more detail in [24,25] and are mostly based on the protocol of [26]. The R. rhizogenes-induced HRs in vitro have the potential to facilitate the generation of transgenic plants through spontaneous organ regeneration, organogenesis, or somatic embryogenesis. Finally, these transgenic plants can be propagated in vitro or transferred to the field [18,27]. However, when the aim of HRs induction is SMs biosynthesis, they are typically cultivated in shake-flasks, and further, the cultivation is scaled up into bioreactors [28], as illustrated in Figure 1.
In conclusion, HRs typically exhibit rapid growth and lateral branching, abundant root hairs with no demand for plant hormones in the medium [16,17,23,24]. They are a suitable platform for the production of pharmaceutically valuable and complex SMs, recombinant proteins, as well as novel drugs associated with a wide range of medicinal plants, firming up their position as “green cell factories” [19]. Recombinant proteins produced by HRs are frequently secreted extracellularly, facilitating convenient purification in a well-defined, protein-deficient medium [25,26]. Amongst their many practical applications, HRs play an important role in metabolic engineering, bioreactor designing, biotransformation-mediated derivatization studies, phytoremediation, and plant-microbe interaction analyses, among others [27,28]. These versatile properties of HRs have progressively revealed numerous avenues of both fundamental and practical applications, ultimately enabling this technology to reach industrial platforms for the large-scale production of high-value-added molecules [29].
3. Plant Biotechnology Toolkit to Enhance Specialized Metabolite Production
Successful induction of HRs is influenced by several factors, including the type and development stage of the plant explant, the bacterial strain used, and the composition of culture medium [30]. The HR cultures are ideal systems for the stable and continuous production of SMs with relatively high yields. However, to meet commercial market demands, significant efforts have been invested in the selection of root cultures with higher production capacity, media composition, precursor feeding, elicitation, and metabolic engineering, as well as the development of scale-up strategies [31]. As a result, numerous HR cultures have been established for the biosynthesis of important phytochemicals, such as artemisinin from Artemisia annua L., camptothecin from Camptotheca acuminata Decne, resveratrol from Arachis hypogaea L., and indole alkaloids from Catharanthus roseus (L.) G.Don [32]. Additionally, HR cultures have been employed for the production of polyphenols, such as phenolic acids, flavonoids, and anthocyanins from Hypericum perforatum L., Nicotiana tabacum L., Solanum lycopersicum L., and Solanum melongena L. [33,34,35,36,37]; terpenes and steroids from N. tabacum L. and Withania somnifera (L.) Dunal [38,39]; as well as various alkaloids, including atropine, anisodine, hyoscyamine, scopolamine, anisodamine, and cuscohygrin from the HRs of Anisodus tanguticus (Maxim.) Pascher, Atropa baetica Willk, Duboisia myoporoides R. Br, Hyoscyamus senecionis Willd, and Scopolia japonica Maxim [40,41,42].
3.1. Bacterial Strains
The most frequently used R. rhizogenes strain for HRs induction is ATCC 15834, accounting for approximately 21% of all reported cases, followed by A4 and LBA9402, which are used in 17 and 15% of cases, respectively [43]. The HRs induced from Swainsona galegifolia (Andrews) R.Br. using the A4 strain exhibited a better genetic stability compared to those induced with LBA9402. The A4 strain maintained the chromosomes number of 2n = 32 in HRs, whereas HRs induced by LBA9402 showed an increased chromosome number count [43]. The choice of bacterial strain affects not only the HR induction frequency but also the capacity for SMs production. For example, the HR induction frequency (53%) and rosmarinic acid accumulation (45 mg/g dry weight (DW)) were higher with the ATCC 15834 strain compared to the A4 strain, which had a 37% induction frequency and a 19 mg/g DW rosmarinic acid accumulation [44]. The HRs from C. acuminata Decne induced by ATCC 15834 biosynthesized 1.0 mg/g DW of camptothecin in contrast to only 0.15 mg/g DW from HRs induced by the R-1000 strain [45]. Furthermore, HRs from Ophiorrhiza alata Craib induced by the TISTR 1450 strain accumulated 785 mg/g DW of camptothecin, which is twice as much as that found in the roots of the mother plant [46].
3.2. Nutrient Medium Optimization
Nutrient supplementation aims to enhance the SMs yield by optimizing physicochemical conditions of the culture medium. The maximum ginsenosides content (9 mg/g DW) in American ginseng (Panax quinquefolium C.A.Mey.) HRs was achieved in a liquid Gamborg B5 medium supplemented with 30 g/L sucrose [47]. Cultivating HRs in a lower-sucrose concentration (20–30 g/L) simulated the production of protopanaxadiol-type ginsenosides, while higher sucrose concentrations (50–70 g/L) favors the biosynthesis of Rg group ginsenosides [47]. A modified B5 medium (0.83 mM phosphate, 12.4 mM nitrate, and 0.5 mM ammonium) resulted in a maximum total ginsenosides content of 12.45 mg/g DW, which is 1.93 times higher compared to the standard B5 medium [48,49]. In HRs of Scutellaria baicalensis Georgi, verbascoside was the most abundant compound among martynoside and leucosceptoside A, with its maximum biosynthesis (3% DW) achieved in Murashige and Skoog (MS) medium formulated with a 50% reduction in KNO3, NH4NO3, and CaCl2 concentrations, while the concentrations of KH2PO4 and MgSO4 were doubled [50]. For W. somnifera (L.) Dunal, both the biosynthesis of withanolide A and biomass accumulation were influenced by the amount and ratio of macronutrients in the MS medium. The highest withanolide A production (15.27 mg/g DW) was observed in MS medium with double KNO3, whereas the highest biomass accumulation (13.11 g/L DW) occurred in MS medium with double KH2PO4. At an NH4+/NO3− ratio of 0.00/18.80 mM, withanolide A content was 14.68 mg/g DW, whereas an NH4+/NO3− ratio of 14.38/37.68 mM led to a biomass accumulation of 14.79 g/L DW [51].
3.3. Precursor Feeding
This approach is used to enhance the production of specific SMs and relies on plant in vitro systems to convert precursor compounds supplemented in the cultural medium into the desired SMs using pre-existing endogenous enzymes [52]. For instance, the addition of the precursor tyrosol (2.5 mM) was necessary to initiate salidroside biosynthesis in the HRs of R. kirilowii (Regel) Maxim, as tyrosol induced the activity of glucosyltransferase enzymes [53]. Supplementation of R. kirilowii HRs with cinnamic acid (CA) facilitated the biosynthesis of rosavin. The addition of 2.5 mM CA and 1% glucose on the 14th day of cultivation resulted in the accumulation of 0.7 mg/g DW rosarin along with 14.0 mg/g DW rosavin in biomass, while 500 mg/L of rosavin were released into the culture medium. Neither rosavin nor rosin was detected in the control samples without precursor supplementation [54]. The biosynthesis of certain betaxanthins, such as vulgaxanthin I, reached its maximum (a 3-fold increase compared to the control) in Beta vulgaris var. lutea HRs only when (S)-glutamate was added in the medium and sucrose was substituted by glucose [55]. Adding loganin (an iridoid glycoside) to the HR lines of C. roseus (L.) G.Don increased the biosynthesis of terpenoid indole alkaloids (TIAs), including catharanthine, ajmalicine, lochnericine, and tabersonine, by 26, 84, 119, and 225%, respectively [56]. The TIAs biogenesis in C. roseus (L.) G.Don was significantly influenced by the expression of the two key genes DXS (1-deoxy-D-xylulose synthase) and G10X (geraniol-10-hydroxylase) in the upper part of the TIA pathway. Inducible overexpression of DXS increased the production of serpentine, lochnericine, and ajmalicine by 26%, 49%, and 67%, respectively [57]. Combining genetic engineering and precursor feeding also enhanced the biosynthesis of tropane alkaloids in Hyoscyamus reticulatus L. HRs. The maximum content of phenols, flavonoids, and alkaloids were recorded in transgenic HRs fed with putrescine addition (4, 3, and 1 mM) after 48 h. Hyoscyamine and scopolamine content increased by 203.83% (125.09 µg/g DW) and 207.34% (161.88 µg/g DW) following 3 mM putrescine feeding for 24 h and 48 h in the control and transgenic HRs, respectively. Furthermore, the expression level of putrescine N-methyltransferase (PMT) and hyoscyamine-6-beta hydroxylase (H6H) genes increased 2.6- and 2.83-fold with 3 and 2 mM putrescine after 48 h of feeding in both transgenic and control HRs [58]. Simultaneous treatment with 300 µM methyl jasmonate (MeJA), together with precursors cholesterol (100 mM) and L-arginine (1 mM), improved solasodine productivity 5-fold (4.5 mg/L) in the HRs of Solanum mammosum L. compared to the control [59].
3.4. Elicitation Treatment
Elicitation is part of a plant’s defense mechanism. Elicitors are biotic (e.g., microorganisms, glycoproteins, or polysaccharides derived from plant cell walls) or abiotic (e.g., chemical: organic or inorganic compounds of non-biological origin or physical: UV or low-energy ultrasound) factors that activate signal-transduction pathways, leading to the induction or enhancement of SMs production in plant cells [60,61]. Biotic elicitors are biologically derived and can be categorized as exogenous (microbial cell walls, fungal and bacterial lysates, yeast extracts, and polysaccharides such as chitin and glucans) [62] and endogenous (cell wall polysaccharides, intracellular proteins, and small molecules such as methyl jasmonate (MeJA), jasmonic acid (JA), salicylic acid (SA), acetylsalicylic acid (ASA), pectin, chitosan (CS), coronatine (COR), and yeast extract) [63].
The process of elicitation involves many interconnected events, with mechanisms that vary according to the age of the plant material, the physicochemical environment of the plant cells, and the type, dosage, and exposure time of the elicitor. In the first step, the elicitor binds to a specific receptor on the plasma membrane, which recognizes the elicitor signal and initiates a cascade of events, including ion flux, the release of reactive oxygen species, and protein activation. In the subsequent step, the activated proteins trigger the expression of key genes involved in SMs biosynthesis pathways [64]. Examples of elicitor-induced biosynthesis of various metabolite groups, such as alkaloids, phenolic acids, terpenes, iridoids, and phenylethanoids, are provided in Table 1.
The biosynthesis of hyoscyamine increased from 8.57 to 16.78 and 19.74 mg/g DW in Datura stramonium L. HRs after treatment with 100 µM SA or 100 µM ASA, respectively [65]. In Papaver orientale L. HRs, treatment with 100 µM MeJA was more effective than treatment with 100 µM SA, increasing the production of thebaine, morphine, and codeine 2.63-, 7.18-, and 3.67-fold, respectively, compared to untreated HRs [66]. The simultaneous application of two elicitors, 100 µM MeJA and 0.5 mM β-cyclodextrin, increased the content of withaferin A (steroidal alkaloid) by more than 12-fold in W. somnifera (L.) Dunal HRs [67]. Conversely, exposing W. somnifera (L.) Dunal HRs to 150 µM SA for 4 h enhanced the biosynthesis of withaferin A, withanone, and withanolide A 42-, 46-, and 58-fold, respectively, compared to the untreated control [68].
The biosynthesis of daidzin increased 2.8- and 7.3-fold after treating Psoralea corylifolia L. HRs with 1 and 10 µM of JA, respectively [69]. Among the tested elicitors, including MeJA, SA, and ASA, treatment of Astragalus membranaceus Bunge. HRs with 283 µM MeJA increased isoflavonoid yield 9.7-fold compared to the control [70]. In Plumbago indica L. HRs, elicitation with 50 µM MeJA for 48 h increased plumbagin biosynthesis 1.5-fold compared with ASA treatment [71]. Elicitation with 100 µM MeJA increased the silymarin content (1.5-fold) in the HRs of Silybum marianum (L.) Gaertn. after 48 h of treatment [72]. In Glycine max (L.) Merr HRs, treatment with 100 µM MeJA for 96 h doubled the total isoflavone production yield (53.16 mg/g DW), compared to treatment with 200 µM SA (28.79 mg/g DW), which was 65-fold more than the non-elicited control [73].


Table 1.
Examples of elicitation as a biotechnological tool for enhanced biosynthesis of SMs in HR cultures.
Table 1.
Examples of elicitation as a biotechnological tool for enhanced biosynthesis of SMs in HR cultures.
| Metabolite(s) | Plant Species | Metabolite Level, mg/g DW | Yield Increase, Folds | Elicitor, µM | Reference |
|---|---|---|---|---|---|
| Alkaloids | |||||
| Ajmalicine | Catharanthus roseus (L.) G.Don. | 15.4 | 2.5 | MeJA, 108.85 + JA, 134.08 | [74] |
| Codeine | Papaver armeniacum (L.) DC. | 0.12 | 2. | MeJA, 100 | [75] |
| Hyoscyamine | D. stramonium L. D. innoxia Mill. D. tatula L. | 11.58 8.89 17.94 | 1.4 2.8 2.1 | ASA, 100 | [65] |
| Indole alkaloids | Isatis tinctoria L. | 3.15 | 5.9 | SA, 142.61 | [76] |
| Morphine | Papaver armeniacum (L.) | 0.15 | 4.0 | MeJA, 100 | [75] |
| Paclitaxel | Taxus x media var. Hicksii | 1.43 | 3.0 | MeJA, 100 | [77] |
| Scopolamine | Anisodus luridus Link. | 0.068 | 2.0 | ASA, 100 | [78] |
| Scopolamine | Atropa acuminata Royle ex Lindl. | 10.95 | 10.0 | COR, 0.5 | [79] |
| Solasodine | Solanum trilobatum L. | 9.33 | 1.9 | MeJA, 4 | [80] |
| Wilforine | Tripterygium wilfordii Hook. f. | 0.23 | 6.7 | MeJA, 50 | [81] |
| Terpenes | |||||
| Astragaloside | Astragalus membranaceus Bunge. | 5.5 | 2.1 | MeJA, 157.4 | [82] |
| Baccatin III | Taxus x media | 0.076 | 3.0 | COR, 1 | [77] |
| Cryptotanshinone | Salvia miltiorrhiza Bunge. | 0.65 | 6.2 | MeJA, 100 | [83] |
| Friedelin Epifriedelanol | Cannabis sativa L. | 1.56 5.02 | 2.88 5.22 | SA, 50 SA, 100 | [84] |
| Ginsenosides | Panax ginseng C.A.Mey. | 0.42 | 3.8 | MeJA, 100 | [85] |
| Glycyrrhizin | Glycyrrhiza inflata Batalin. | 34.79 | 5.7 | MeJA, 100 | [86] |
| Madecassoside | Centella asiatica (L.) Urban. | 116 125 | 80 90 | COR, 1 COR, 1 + MeJA, 100 | [87] |
| Oleanolic acid glycosides | Calendula officinalis L. | 52.52 | 3.0 | MeJA, 100 | [88] |
| Rhinacanthin | Rhinacanthus nasutus (L.) Kurz. | 6.3 | 1.7 | MeJA, 10 | [89] |
| Tanshinone I | S. miltiorrhiza Bunge. | 0.46 | 3.4 | MeJA, 100 | [83] |
| Tanshinones | S. miltiorrhiza Bunge. | 11.33 | 3.1 | MeJA, 100 | [90] |
| Triptolide | T. wilfordii Hook. f. | 0.04 | 2.0 | MeJA, 50 | [81] |
| Triterpenoid saponins | Centella asiatica (L.) Urban. | 60 | 6 | MeJA, 400 | [91] |
| Triterpenoid saponins | Psammosilene tunicoides W.C.Wu & C.Y.Wu. | 15.0 | 4.55 | CS, 131.01 | [92] |
| Withaferin A | Withania somnifera (L.) Dunal. | 13.21 | 5.6 | MeJA, 15 | [93] |
| Withaferin A | W. somnifera (L.) Dunal. | 9.57 17.45 | 6.8 12.5 | MeJA, 100 β-CD 5.0 mM + MeJA, 100 | [94] |
| Phenolic compounds | |||||
| Apigenin | Ficus carica L. cv. Siah | 0.12 | 51.96 | MeJA, 300 | [95] |
| Caffeic acid | Mentha spicata L. | 0.16 | 1.47 | MeJA, 100 | [96] |
| Caffeic acid | F. carica L. | 0.18 | 9.0 | MeJA, 100 | [95] |
| Caffeic acid | S. miltiorrhiza Bunge. | 4.55 | 14.06 | COR, 0.1 | [97] |
| Chlorogenic acid | F. carica L. | 6.5 | 10.5 | MeJA, 100 | [95] |
| Chlorogenic acid | M. spicata L. | 0.015 | 1.7 | MeJA, 100 | [96] |
| Cinanmic acid | M. spicata L. | 0.043 | 1.98 | MeJA, 100 | [96] |
| Gallic acid | F. carica L. | 0.71 | 5.9 | MeJA, 100 | [95] |
| Salvianolic acid | S. miltiorrhiza Bunge | 2.15 | 7.96 | COR, 0.1 | [97] |
| Salvianolic acid | Salvia Przewalskii Maxim. | 21.5 | 8.4 | MeJA, 400 | [98] |
| Salvianolic acid | Salvia virgata Jacq. | 2.11 | 3.76 | MeJA, 100 | [99] |
| Rosmarinic acid | F. carica L. | 1.84 | 12.3 | MeJA, 200 | [95] |
| Rosmarinic acid | M. spicata L. | 11.84 | 0.06 | MeJA, 100 | [96] |
| Rosmarinic acid | Prunella vulgaris L. | 58.3 | 1.66 | MeJA, 50 | [100] |
| Rosmarinic acid | S. miltiorrhiza Bunge. | 8.01 | 18.63 | COR, 0.1 | [97] |
| Rosmarinic acid | S. przewalskii Maxim. | 67.13 | 1.9 | MeJA, 400 | [98] |
| Rosmarinic acid | Salvia virgata Jacq. | 18.45 | 1.81 | MeJA, 100 | [99] |
| Rutin | F. carica L. | 0.03 | 3.34 | MeJA, 200 | [95] |
| Quercetin | F. carica L. | 0.2 | 6.91 | MeJA, 300 | [95] |
ASA—acetylsalicylic acid; β-CD—β-cyclodextrin; COR—coronatine; JA—jasmonic acid; MeJA—methyl jasmonate; SA—salicylic acid.
The biosynthesis of the diterpenoid andrographolide increased 8- and 5-fold after treatment with 100 µM MeJA and SA, respectively [101]. Elicitation with 100 µM JA increased the intracellular accumulation of the triterpenoid oleanolic acid 20-fold in the HRs of Calendula officinalis L. and enhanced its secretion in the culture medium by 113-fold [88]. The biosynthesis of cryptotanshinone and tanshinone IIA in Salvia miltiorrhiza Bunge HRs increased 3.9-fold, while tanshinone I and dihydrotanshinone increased 3.0- and 1.3-fold, respectively, following elicitation with 100 µM MeJA [102].
The content of harpagide, catalpol, and catalposide (iridoids), isoverbascoside, and verbascoside (phenylethanoids) was significantly enhanced in response to optimal elicitation conditions (100 µM MeJA for 72 h exposure) in 23-day-old HRs of Rehmannia glutinosa (Gaertn.) DC. The biosynthesis of verbascoside and isoverbascoside increased up to 10- and 6.4-fold, respectively while the harpagide content increased 7.5-fold after 72 h elicitation with 150 µM MeJA [103]. In transgenic HRs of R. sachalinensis Boriss expressing two putative UDP-glucosyltransferases (UGT72B14 and UGT74R1), salidroside production increased by 420% and 50%, respectively, after elicitation with 300 µM MeJA, compared to the control HRs with an empty vector [104]. Tyrosine decarboxylase (TYDC) is important in salidroside biosynthesis for converting tyrosine to tyramine, while UGT is the final gene involved in salidroside biosynthesis. After SA treatment, the RcTYDC and RcUGT expressions were upregulated 49- and 36-fold compared to the control. The transgenic HR lines produced higher levels of tyramine, tyrosol, and salidroside, varying from 3.21–6.84, 1.50–2.19, and 1.27–3.47-fold, respectively, compared to their counterparts in non-transgenic lines of Rhodiola crenulata (Hook.f. & Thomson) H. Ohba [105]. The conversion of tyrosine into 4-hydroxyphenylpyruvate, a competing metabolite in salidroside biosynthesis, is done by tyrosine aminotransferase (TAT) enzymes. Downregulation of RcTAT in genetically engineered R. crenulata HRs led to a 6- to 60-fold increase in salidroside production [106].
3.5. Molecular Tools for Engineering Plant Secondary Metabolism
Metabolic engineering has been intensively utilized to enhance the biosynthesis of pharmaceutically important SMs in plant in vitro systems. These complex networks are regulated at two levels: the first level involves structural genes that encode participant enzymes of the biosynthetic pathway, while the following level of regulation is mediated by transcription factors (TFs) that control the expression of these structural genes, thereby regulating SMs biosynthesis [107].
3.5.1. Metabolic Engineering
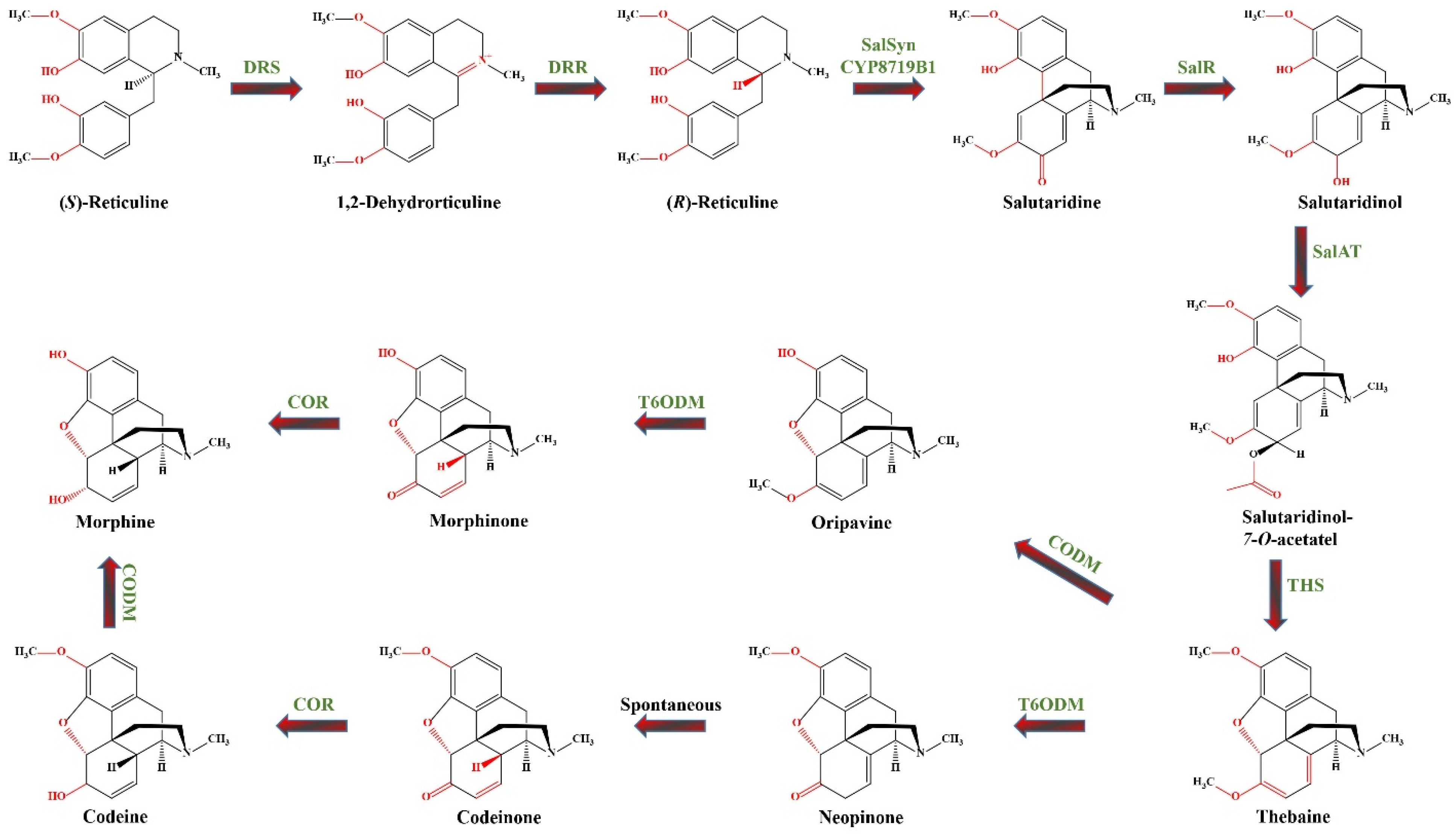
This section presents various examples demonstrating how overexpression or suppression of single or multiple genes governed by relevant TFs influence the biosynthesis of diverse groups of SMs. To better illustrate the potential of molecular tools for engineering plant secondary metabolism, we have selected a case study that explores the potential strategies for manipulating the biosynthetic pathways of morphinan alkaloids (Figure 2).
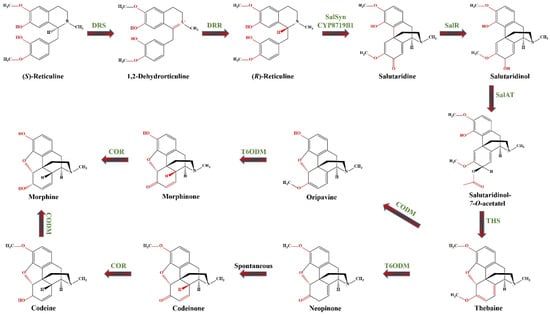
Figure 2.
The metabolic pathway of morphinan alkaloids biosynthesis, showing two routes from thebaine to morphine. DRS—1.2-dehydroreticuline synthase (EC 1.14.19.54); DRR—1.2-dehydroreticuline reductase (EC 1.5.1.27); SalSyn/CYP719B1—CYP salutaridine synthase (EC 1.14.19.67); SalR—salutaridine: NADPH 7-oxidoreductase (EC 1.1.1.253); SalAT/SAT—acetylcoenzyme A: salutaridinol-7-O-acetyltransferase (EC. 2.3.1.150); THS—thebaine synthase (EC 4.2.99.24); T6ODM—thebaine 6-O-demethylase (EC 1.14.11.31); COR—codeinone reductase (EC 1.1.1.247); CODM—codeine O-demethylase (EC 1.14.11.32).
The biosynthesis of the SMs is controlled by structural genes that encode enzymes of metabolic reactions and the genes with regulatory functions that encode the TFs regulating the structural genes upon binding their promoters [108]. Several examples of metabolic engineering of SMs in HRs are summarized in Table 2.

Table 2.
Examples of metabolic engineering strategies to enhance the SMs biosynthesis in HRs.
The biosynthesis of terpenoid indole alkaloids (TIAs) was regulated by JA-responsive Apetala2/Ethylene response factor (AP2/ERF)-domain TFs such as ORCA. Overexpression of ORCA2 elevated the amount of catharanthine and vindoline in Catharanthus roseus HRs 2.03- and 3.67-fold, respectively, compared to the control. In contrast, the increased expression of ORCA3 activates the transcription of ZCT1 and ZCT2, which are repressors of TIA pathway [117]. Treatment with 250 µM MeJA increased the expression of ORCA TF (29–40-fold), along with TIA pathway genes like G10H, strictosidine synthase (STR), tryptophan decarboxylase (TDC), and strictosidine β-glucosidase (SGD), while the levels of ZCT remained low (2–7-fold), leading to increased concentrations of TIA metabolites (secologanin, strictosidine, and tabersonine) by 150–370% compared to untreated controls in C. roseus HRs. Contrarily, at a higher dosage of 1 mM MeJA, the production of TIA metabolites was inhibited, which correlated with 40-fold higher expression level of ZCT and minimal expression of ORCA (13–19-fold) and TIA biosynthetic genes (0–6 fold) [118]. An effective strategy to increase SMs production involves the “push and pull” approach through the simultaneous expression of multiple genes. Multigene engineering was employed to enhance tropane alkaloid production. Putrescine N-methyltransferase (PMT) is considered one of the first rate-limiting enzymes upstream, while tropinone reductase I (TRI) serves as a key branch-controlling enzyme, converting tropinone to tropin. Cytochrome P450 (CYP80F1) subsequently converts littorine into hyoscyamine with the final committed step catalyzed by hyoscyamine 6β-hydroxylase (H6H), which carries out consecutive oxidation reactions such as the hydroxylation of hyoscyamine and its epoxidation to scopolamine. The co-introduction of AaPMT and AaTRI in A. acutangulus HRs increased the content of the tropane alkaloids 8.7-fold (8.104 mg/g) compared to the control sample [41]. Similarly, the co-introduction of two key genes in camptothecin biosynthesis, G10H and secologanin synthetase (SLS), increased camptothecin production 2.5-fold (3.5 mg/g DW) in Ophiorrhiza pumila Champ. ex Benth HRs [119]. The expression levels of PMT, TRI, CYP80F1, and H6H were upregulated 14.8, 6.6, 3.1, and 11.9-fold, respectively, due to elicitation of Anisodus luridus HRs with 1 mM ASA. The endogenous concentrations of hyoscyamine and scopolamine increased to 57.2 and 14.7 mg/g DW, respectively, while the secretion of scopolamine in the culture medium increased 6.2-fold (1.02 mg/L) compared to the control [78].
The early and intermediate steps in the triterpenoids biosynthesis pathway involve the biosynthesis of isoprenyl diphosphate (IPP) by mevalonate-5-pyrophosphate decarboxylase (MVD) and the production of farnesyl diphosphate (FPP) utilizing IPP and dimethylallyl diphosphate by the activity of farnesyl pyrophosphate synthase (FPS). Overexpression of PgMVD P. ginseng HRs increased sterol concentrations (stigmasterol, sitosterol, and campesterol) up to 4.4-fold compared to the control, while ginsenoside and β-amyrin decreased. Contrariwise, overexpression of PgFPS increased both ginsenoside and sterol contents 2.4- and 4.6-fold, respectively [120]. The synthesis of triterpenoids such as madecassoside and asiaticoside in transgenic HRs of C. asiatica was slightly increased (1.15-fold) after 2 weeks of treatment with MeJA, but decreased after 4 weeks of elicitation, pointing to a feedback suppression due to PgFPS overexpression [121]. The DXS and geranylgeranyl diphosphate synthase (GGPPS) are key enzymes that provide the C20 parent structure for diterpenes, including tanshinones. The SmDXS2 is a key upstream gene in the 2-C-methyl-D-erythritol-4-phosphate (MEP) pathway, while SmGGPPS functions downstream in the tanshinone biosynthetic pathway. Overexpression of SmDXS2 and SmGGPPS together doubled tanshinone production in Salvia miltiorrhiza HRs to 12.93 mg/g DW, which is 21-fold higher than the control, whereas in single-gene-transformed lines, tanshinone production increased only 3-fold [122]. Single-gene-transformed lines expressing only SmHMGR (3-hydroxy-3-meth ylglutaryl CoA reductase) or SmDXR (1-deoxy-D xylulose 5-phosphate reductoisomerase) increased tanshinone production up to 3.25 mg/g DW in S. miltiorrhiza Bunge HRs. Co-expression of SmHMGR and SmDXR after elicitation with Ag+ increased tanshinone production to 6.72 mg/g DW [123].
The MYC and MYB1 TFs are positive regulators of pathway genes involved in the biosynthesis of phenolic acids [124,125]. Recently, a novel AP2/ERF TF responsive to MeJA treatment was found to stimulate the biosynthesis of phenolic acids [126]. Overexpression of SmMYB1 significantly increased the expression of phenylalanine ammonialyase (PAL), cinnamic acid 4-hydroxylase (C4H1), 4-hydroxyphenylpyruvate reductase (HPPR1), rosmarinic acid synthase (RAS1), and P450-dependent monooxygenase (CYP98A14) in S. miltiorrhiza Bunge HRs, resulting in a doubling of total phenolic acid content [125]. The expression of the genes increased 1.5- and 12-fold after treatment with SA (6.9 mg/L), leading to a 1.48-fold increase (58.3 mg/g DW) in rosmarinic acid content in Phaseolus vulgaris L. HRs [100]. The elevated expression levels of PAL, 4CL, TAT, HPPR, and RAS due to elicitation with 400 µM MeJA increased the content of rosmarinic and salvianolic acid B (76.1 and 21.4 mg/g DW, respectively) in the HRs of S. przewalskii Maxim [98]. After 6 h of exposure to 100 µM MeJA, the HRs of Mentha spicata L. produced 11 times more rosmarinic acid compared to the non-elicited sample, which was associated with increased expression (4.04-, 3.62-, 1.75-, and 1.45-fold) of phenylpropanoid pathway genes PAL, C4H, 4CL, and HPPR [96].
3.5.2. CRISPR/Cas9
The CRISPR/Cas9 is a powerful genome-editing tool widely utilized for engineering various plants or plant in vitro systems, often to validate the role of key genes involved in the biosynthesis of valuable SMs. For instance, the elimination of OpG10H and OpSLS genes by CRISPR/Cas9 in O. pumila HRs reduced camptothecin levels by more than 90% [119]. In S. miltiorrhiza Bunge HRs the transcription factor SmMYB98 positively regulates tanshinone and phenolic acid biosynthesis; knocking out this gene resulted in a decreased level of these metabolites [127]. The CRISPR/Cas9-mediated knockout of copper-containing amine oxidase (CuAO), specifically SoCuAO5 expressed in the roots, rendered Symphytum officinale L. HRs unable to produce pyrrolizidine alkaloids, supporting CuAO’s role in alkaloids biosynthesis [128]. N-methylputrescine oxidase (MPO) is an enzyme involved in tropane alkaloids biosynthesis. CRISPR/Cas9-mediated mutagenesis of Atropa belladonna L. HRs resulted in a 17–26% reduction in norhyoscyamine content, while HR lines expressing the MPO gene had norhyoscyamine levels 2.09- to 5.70-fold higher than the control HR line [129]. Inducing double MYC1 and MYC2 loss-of-function in tomato HRs suppressed the constitutive expression of steroidal glycoalkaloids biosynthesis genes and significantly reduced levels of α-tomatine and dehydrotomatine [130]. The silencing of the pyrrolidine ketide synthase gene reduced tropane alkaloids accumulation by 85% in A. belladonna HRs, indicating its crucial role in alkaloid biosynthesis [131]. Additionally, CRISPR/Cas9-based mutations of the homospermidine synthase (HSS) gene (the first pathway-specific enzyme in pyrrolizidine alkaloids biosynthesis) revealed that inactivation in only one of the two HSS alleles resulted in significantly reduced homospermidine and pyrrolizidine alkaloids content, whereas the HRs with both alleles inactivated, showing no detectable alkaloids [132].
In plant genome editing, CRISPR-Cas9 technology has emerged as a pivotal tool, holding the potential to revolutionize crop genetic improvement and SMs biosynthesis with its precision gene editing and regulatory capabilities [133]. Significant progress has been made in the editing of protein-coding genes. However, studies on the editing of non-coding DNA with regulatory roles are staying behind. Non-coding regulatory DNAs can be transcribed into long non-coding RNAs (lncRNAs) and miRNAs, which together with cis-regulatory elements (CREs) play crucial roles in regulating plant growth and development [134,135]. The lncRNAs are transcripts of more than 200 nucleotides in length, with minimal or no protein-coding capacity. Increasing evidence indicates that lncRNAs play important roles in the regulation of gene expression, including in the biosynthesis of secondary metabolites [135]. The triterpenoid saponin biosynthesis in Psammosilene tunicoides W.C.Wu and C.Y.Wu HRs was studied using SA as an efficient elicitor for SMs production. The analysis of the transcriptome-wide regulatory network revealed the identification of 430,117 circular consensus sequence reads, 16,375 unigenes, and 4678 lncRNAs. The SA upregulated the unigenes encoding SA-binding proteins and antioxidant enzymes compared to the control. The candidate transcription factors WRKY, NAC, and structural genes AACT (acetyl-CoA acetyltransferase), DXS, SE (squalene epoxidase), CYP72A might be the key regulators in SA-elicited saponin accumulation. The SA-elicitation promoted the yields of quillaic acid and gypsogenin compared to the controls with 35.4–125.9% and 20.1–28.4%, respectively. It may be speculated that the WRKY positively regulates triterpenoid saponin biosynthesis via binding to a W-box element in the promoter of SE [136]. In another study on S. miltiorrhiza Bunge, phenolic compounds biosynthesis was targeted by overexpression of a bZIP TF (bZIP2) that suppressed the production of phenolic acid in HRs [137]. Overexpression of bZIP2 in HRs resulted in a lower phenolic acid content while a CRISPR/Cas9-mediated knock-out of bZIP2 eliminated the negative regulatory effect of the bZIP TF that is believed to be able to physically interact with protein kinase 2s and cause a subsequent suppression of PAL gene expression, pointing to a novel TF to manipulate or enhance phenolic acids biosynthesis [137]. The overexpression of MYB36 TF in the HRs of S. miltiorrhiza enhanced the production of tanshinone, which was associated with a decline in phenolic acid levels [138]. Interestingly, transgene-free CRISPR/Cas9 protoplasts-originated MYB36 knockout S. miltiorrhiza plants resulted in white flowers, pointing to the role of these TRs as a positive regulator of anthocyanin biosynthesis [139]. The role of another bZIP TF (GbbZIP08) in promoting flavonoid biosynthesis in Ginkgo biloba L. was reported after its overexpression in transgenic Nicotiana benthamiana Domin, making it a potential candidate TF to be considered in HR transformation and gene constructs [140]. More examples of CRISPR/Cas9-mediated and other genome editing technologies for SMs’ enhancement are collected in recent review articles [141,142,143,144].
3.5.3. Case Study of the Morphinan Alkaloids Biosynthetic Pathway
This case study exemplifies the challenges and opportunities associated with the metabolic engineering of the morphinan alkaloids biosynthetic pathway.
Morphinan alkaloids are benzylisoquinoline alkaloids (BIAs) that are among the most powerful narcotic analgesics for managing moderate to severe chronic pain. This class includes natural opiates like codeine and morphine as well as semi-synthetic derivatives such as dihydromorphine and hydromorphone [75,145]. Among BIAs, the most important alkaloids are morphine and codeine, papaverine, sanguinarine, and berberine [146]. The opioids antagonist naloxone and naltrexone, widely used to combat opiate addiction and overdose, are synthesized from thebaine. Thebaine not only acts as a precursor for the biosynthesis of codeine and morphine in planta but also serves as a precursor for the chemical synthesis of the analgesics like oxycodone and buprenorphine, which offer improved side-effect profiles compared to morphine [145,147]. In plants, BIAs are produced in the roots and accumulate within specialized internal secretary structures [148].
The biosynthesis of morphinan alkaloids occurs via a multistep pathway starting with the amino acid tyrosine [149]. Figure 2 illustrates the biosynthetic pathway of morphinan alkaloids starting from (S)-reticuline, a central intermediate in the BIA pathway. Generally, biosynthesis of BIA starts by the bioconversion of tyrosine to both dopamine and 4-hydroxyphenylacetaldehyde (4HPAA).
4-hydroxyphenylacetaldehyde is formed through L-tyrosine transamination by L-tyrosine aminotransferase (TyrAT; EC 2.6.1.5) and subsequent decarboxylation by an enzyme, provisionally named 4-hydroxyphenylpyruvate decarboxylase (4HPPDC; EC 4.1.1.80). L-tyrosine and/or tyramine undergoes meta-hydroxylation by an undefined enzyme (denoted as 3′OHase) yielding L-dihydroxyphenylalanine (DOPA) and dopamine, respectively. In an alternative rout L-tyrosine and DOPA are transformed to tyramine and dopamine by tyrosine decarboxylases (TYDC; EC 4.1.1.25) [150,151]. Dopamine and 4HPAA are condensed by norcoclaurine synthase (NCS; EC 4.2.1.78) via stereoselective Pictet–Spengler condensation producing (S)-norcoclaurine, the central precursor of BIAs [150]. Subsequently, through a series of 3′-hydroxylation, O- and N-methylations, (S)-norcoclaurine is then converted to coclaurine by SAM-dependent norcoclaurine 6-O-methyltransferase (6OMT; EC 2.1.1.128), to N-methylcoclaurine by coclaurine N-methyltransferase (CNMT; EC 2.1.1.140), to 3′-hydroxy-N-methyl coclaurine by (S)-N-methylcoclaurine 3′-hydroxylase (NMCH (CYP80B1), EC1.14.14.102), and ultimately to (S)-reticuline by 3′-hydroxy N-methylcoclaurine 4′-O-methyltransferase (4′ OMT, EC 2.1.1.116) [151]. (S)-reticuline serves as the central intermediate leading to diversification of BIAs. The biosynthesis of morphine continues with the epimerization of (S)-reticuline to (R)-reticuline. The reaction proceeds via the dehydrogenation of (S)-reticuline to a 1.2-dehydroreticulinium ion by 1.2-dehydroreticuline synthase (DRS; EC 1.14.19.54), which is subsequently reduced to (R)-reticuline by 1.2-dehydroreticuline reductase (DRR; EC 1.5.1.27) [150]. (R)-reticuline is then converted by the CYP salutaridine synthase (SalSyn/CYP719B1; EC 1.14.19.67) to salutaridine, which is then reduced by the short-chain salutaridine: NADPH 7-oxidoreductase (SalR; EC 1.1.1.253) into (7S)-salutaridinol. Subsequently, (7S)-salutaridinol is O-acetylated by acetylcoenzyme A: salutaridinol-7-O-acetyltransferase (SalAT/SAT; EC. 2.3.1.150) to salutaridinol-7-O-acetate, which is then transformed into thebaine via the formation of a C-4 and C-5 oxide bridge by thebaine synthase (THS; EC 4.2.99.24). Until the discovery of THS, this cyclization step is believed to occur spontaneously [151]. Thebaine undergoes O-demethylation at position six by thebaine 6-O-demethylase (T6ODM; EC 1.14.11.31) to yield neopinone, which spontaneously rearranges to the more stable codeinone, further reduced to codeine by cytosolic NADPH-dependent aldo-keto reductase codeinone reductase (COR; EC 1.1.1.247). Finally, codeine is converted to morphine by codeine O-demethylase (CODM; EC 1.14.11.32) [149]. Alternatively, the CODM initiates a minor pathway by catalyzing 3-O-demethylation of thebaine to oripavine. This oripavine is then transformed by T6ODM into morphinone, which is subsequently reduced by COR to yield morphine [149].
Since only plants from the genus Papaver can produce morphinan alkaloids, much research has focused on exploring this biosynthetic pathway in different Papaver species. The primary commercial natural source of morphinan alkaloids is P. somniferum L. (opium poppy), from the family Papaveraceae, whose medicinal properties have been recognized since ancient times [149,150]. The alkaloid content in P. somniferum varies between 1.5–2.7% DW of harvested material [152]. Other species investigated are P. bracteatum L. (Iranian or Persian poppy) and P. orientale (Oriental poppy). However, P. bracteatum produces very low amounts of codeine and morphine due to the low activity of enzymes involved in their demethylation [153], while oriental poppy produces up to 9% thebaine and 20% oripavine but does not produce morphine and codeine, making both species economically unsuitable as commercial sources for morphinan alkaloids [66]. Research has therefore focused on using plant in vitro systems and metabolic engineering for a more efficient morphinan alkaloid production. Hairy root cultures for the production of BIAs have been initiated from P. somniferum [154], P. bracteatum [148,155], P. orientale [66], P. armenicum [75], and Macleaya cordata (Willd.) R.Br. [156]. Considerable efforts have been made to optimize HR cultures to enhance morphinan alkaloids biosynthesis. In P. bracteatum hairy roots, overexpression of SalAT increased the levels of thebaine, codeine, and morphine by 1.28, 0.02, and 0.03%, respectively, compared to the control [157]. Overexpression of COR in the HRs of the same species increased the codeine and morphine content by 0.04% and 0.28%, respectively, compared to the control [153]. In P. orientale HRs the MeJA or SA induced overexpression of COR, SalAT, SalR, T6ODM, CODM, and SalSyn, resulting in enhanced thebaine (up to 2.63-fold) and morphine (up to 6.18-fold) production compared to the control [66]. In P. armeniacum, elicitation with 100 µM MeJA increased the expression of TYDC, SalAT, T6ODM, and COR, leading to increased biosynthesis of thebaine (41 µg/g DW), codeine (120 µg/g DW), and morphine (150.67 µg/g DW) [75].
4. Bioreactor Technology for Hairy Roots Cultivation
The demand for plant-derived SMs is increasing exponentially, due to their diverse health-promoting properties. To meet this growing demand in the global market, it is necessary to produce these SMs on a large scale, utilizing bioreactor technology as the best alternative for scaling up the production of SMs from plant in vitro systems [158]. However, when the in vitro cultures are transferred from shake flasks to bioreactors and scaled-up from pilot to industrial scale, the cultivation environment of the plant cells, especially HRs, can change in terms of hydrodynamic shear forces and rheological properties. This can lead to altered effects of shear stress, oxygen supply, and gas composition, which may reduce productivity in terms of biomass and SMs [158]. A major bottleneck that hampers the industrial utilization of HRs is their complex morphology and non-uniform growth pattern [28]. The tangled and fibrous-clump nature of HRs results in the formation of an interlocked network, external boundary layers over the root surfaces, and stagnant zones within root clumps. This creates a non-homologous culture environment impeding mass transfer for the delivery of nutrients and oxygen, and leads to the formation of senescent tissues. Oxygen deficiency and gradients, resulting in decreased dissolved oxygen concentrations, are key challenges in the mass production of HRs and are considered a growth-limiting factor in HR bioreactors [28,159]. Additionally, HRs are delicate and fragile; while vigorous mixing can augment mass transfer, it also increases hydrodynamic shear stress, which reduces the vitality and productivity of the HRs due to callus formation [160]. Table 3 presents the main morphological characteristics of HRs that distinguish them from plant cell suspensions, microbial, and animal cells.

Table 3.
Comparison of plant (cell suspensions and HRs), microbial, and animal cells regarding their special characteristic correlated to bioreactors design and processes considerations according to [161,162,163,164].
Therefore, scaling up the HRs cultivation is not straightforward and requires careful consideration of factors such as oxygen supply (dissolved oxygen, dO2) and CO2 exchange, pH, medium composition, agitation, aeration, and cell density [165,166]. Continuous O2 supplementation is needed to support the biological activates of the growing HRs, while agitation ensures the homogeneity of the liquid medium. The aeration system typically consists of stainless-steel sparger, with an agitator or impellers required for mass/heat transfer and uniform air distribution. Another important consideration is the high shear and hydrodynamic stress that are experienced by HR cultures. Shear stress is caused by continuous agitation and aeration of the medium, as well as by distribution and fragmentation of the gas bubbles and bubble rupture at the liquid surface. Currently, there is no universal strategy to minimize shear stress, which is thought to be influenced by certain factors such as tissue morphology, culture age, aeration and agitation speed, and the viscosity of liquid medium [28]. Predominantly, HRs cultivation is performed at low agitation speeds between 75–150 rpm, with 10–30% dO2 (corresponding to 0.1–0.5 vvm airflow) controlled by sparger or 0.5–1.0 L/min using an airflow meter [165]. An essential parameter that provides information about the oxygen availability for HRs in liquid cultures is its transfer coefficient (kLa values), which represents the fraction dissolved in water. The reduced oxygen level is a function of the metabolic activity of the growing biomass and culture yield. HRs have a lower metabolic activity and doubling time than microbial cells, requiring a lower O2 supply. The level of dO2 in bioreactor liquid cultures can be adjusted by agitation or stirring, aeration, gas flow, and air bubble size. Temperature is another critical and controlled parameter, which is usually standardly maintained at 25–26 ˚C [165].
4.1. Bioreactor Design and Process Intensification
Considering the unique challenges of HRs cultivation, the prime focus when designing a suitable bioreactor is on achieving adequate mixing of the culture medium with minimized shear stress and optimized mass transfer while reducing the hydrodynamic pressure [28]. A bioreactor for HR cultivation must meet several key requirements upon scale-up: (1) its geometric and flow characteristic must remain effective; (2) it must provide nutrients and oxygen in sufficient concentrations and make them accessible to the roots; and (3) it should facilitate the scale-up process by having identifiable and quantifiable process parameters that can be replicated at larger scales efficiently and cost-effectively [160]. The key issue in bioreactor design and operation is to control the biochemical processes consistently and optimally for maximum productivity. This optimization can be approached on two levels: (1) the biological entity and its products, including metabolite synthesis and accumulation; and (2) physical parameters, such as oxygen supply, temperature, medium continuity, and product removal. The second level basically involves designing the reactor vessel in a way that maximizes physical parameters, maintaining them in equilibrium to achieve consistent productivity [28].
On that account, various bioreactor configurations have been employed for HR cultivation, generally classified into liquid-phase, gas-phase, and hybrid systems (a combination of both) [28]. The general advantages and disadvantages of each bioreactor type are summarized in Table 4. In liquid-phase reactors, HR biomass is fully submerged in the liquid medium. Given that mass transfer of gaseous media and high shear stress are the key limiting parameters in these bioreactors, many different vessel designs have been developed to address these challenges. The most frequently used bioreactors for HRs cultivation are presented in Figure 3.

Table 4.
Comparison of the major bioreactor types, used for HRs upscaling cultivation according to [28,159,167].
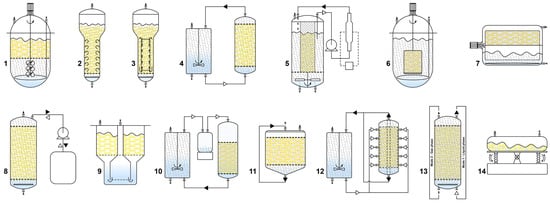
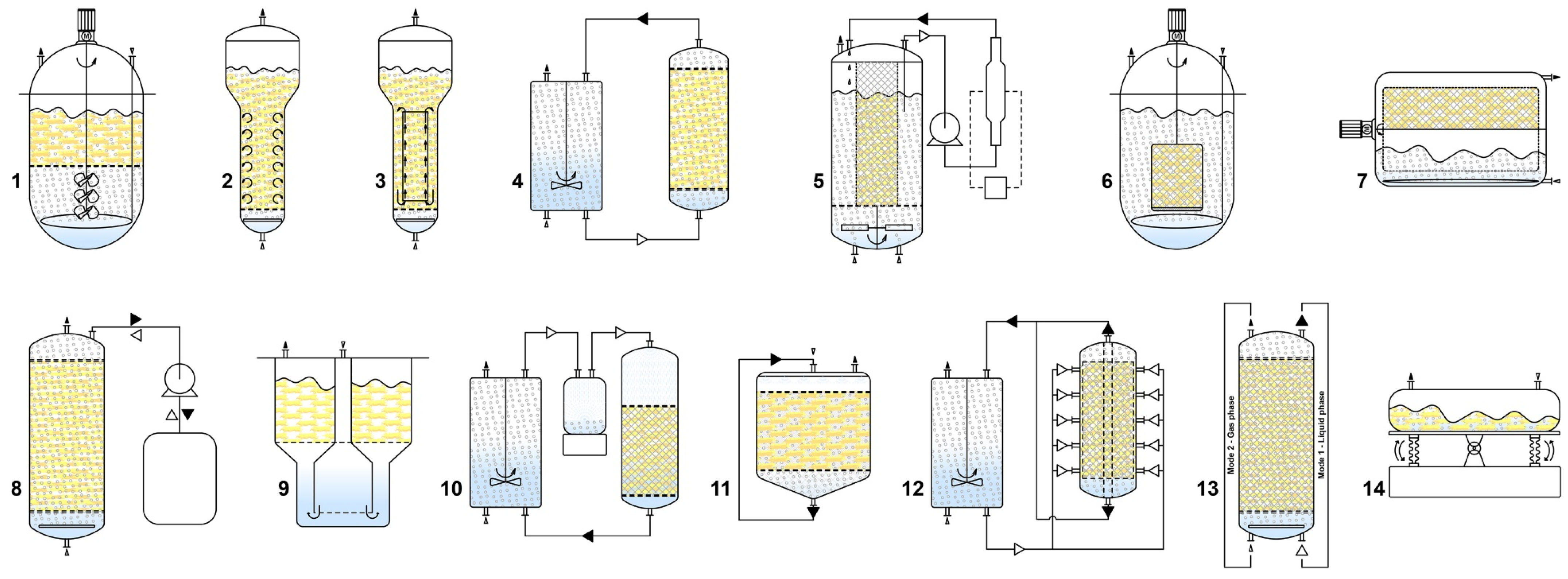
Figure 3.
Various types of liquid-phase (1—modified stirred tank; 2—bubble column; 3—airlift; 4—convective flow; 5—turbine blade; 6—spin filter; 7—rotating drum; 8—ebb and flow), 9—temporary immersion system (RITA® bioreactor), gas-phase (10—nutrient mist; 11—trickle bed; 12—radial flow), hybrid (13—hybrid reactor, working as a liquid-phase reactor in mode 1 and a gas-phase reactor in mode 2), and single-use (14—wave-mixed) bioreactors, used for upscaling of hairy root cultures.
Among the most frequently used bioreactors for HR cultivation are the mechanically driven stirred tank reactors (STRs), pneumatically driven bubble column (BCRs), airlift reactors (ALRs), and convective flow reactor (CFRs) [159]. STRs are conventional bioreactors known for their characteristic mass and heat transfer properties. They consist of impeller or turbine blades and a sparger that regulate aeration, bubble size, and medium currency [168]. Although the STRs provide excellent control of temperature, dO2, and nutrient concentration, the impellers cause higher shear stress on the roots than other bioreactor types. The specific morphology of the HRs often leads to damage from the rotating impellers, resulting in wounding, callus formation, and a negative effect on biomass accumulation and SMs biosynthesis [168]. Therefore, HR cultivation in STRs should be conducted with restricted impeller input and speed, and several adaptations, such as impeller modifications or the use of steel cage or perforated mesh to separate HRs from the impeller, may be employed [169].
Bubble column reactor consist of a vertical column with a sparger at the bottom facilitating the release of air or a gas mixture for both aeration and mixing. These reactors are easy to scale up, have low capital and operational costs, and significantly minimize HR shear stress due to the use of bubbles. However, the undefined flow patterns and non-uniform mixing in high-density cultures can lead to reduced growth performance, often due to a decrease in gas-liquid interface areas. Introducing multiple spargers, dividing the bubble column into segments, or increasing the aeration rate alongside HR growth and density, may mitigate these disadvantages [28,159]. ALRs have a similar construction to BCRs but include an external or internal draft tube. This tube separates upward and downward flows, providing liquid circulation by directing airflow through a sparger at the bottom for gas exchange. The density difference between the flows facilitates liquid circulation, prevents bubbles coalescence, and enhances oxygen mass transfer due to the increased number of bubbles [167]. The turbine blade reactor unites features of STRs and ALRs, where the cultivation space is separated from the agitation one by a stainless-steel mesh, significantly reducing the shear stress by preventing HR contact with the impeller. Air is delivered through the bottom of the reactor and dispersed by an eight-blade stirrer impeller [169]. The spin filter reactor (SFR) uses a rotating filter to the cultures and allows simultaneous removal of the spent medium and the addition of a fresh one [169]. Spin filter reactor consist of an STR with an external culture chamber for HR growth, and the medium circulates through both chambers. Although this bioreactor type may offer improved performance, it is difficult to scale up due to the high pressure required to circulate the culture medium [28,159]. The rotating drum reactor (RDR) operates similarly to a fill-and-drain reactor. It consists of a drum-shaped container placed on rollers providing support and rotation. The drum rotates at slow speed (2–6 rpm) to minimize the shear pressure on the HRs, and a polyurethane foam sheet onto the drum surface is used to attach (immobilize) the HRs without breaking. The rotating motion facilitates proper gas-liquid mixing and promotes efficient oxygen transfer to biomass at high densities [28,159]. Another cultivation system that uses repeating cycles of liquid and gas phases is the ebb and flow reactor (EFBR). It is designed for repetitive cycles of filling and draining between the reactor vessel and medium reservoir [28]. This bioreactor type offers improved mixing and mass transfer capacity, including effective manipulation of the gas composition, such as the addition or removal of O2, CO2, or ethylene [160]. Due to its advantages, such as limited shear damage, reduced hypertension (pressure stress), facilitated medium change, and improved balance between oxygen and nutrients access, temporary immersions systems (TIS) have been introduced for HR cultivation. HRs are placed on the netting of the cultivation chamber, while the nutrient tank is located at the bottom of the vessel. These systems are based on the concept of temporary contact between the cultured tissue and liquid medium, and optimizing the frequency and time of immersion can enhance HRs growth and SMs production. One of the most frequently used TIS is the RITA® (Recipient for Automated Temporary Immersion System) from the French company Vitropic [170].
In gas-phase reactors, HRs are fixed (immobilized) in the culture vessel on horizontal sheets or rings of inert material. HRs are exposed to a mixture of ambient air and nutrient medium, which is dispersed or trickled over the HR bed in a spray, mist, or droplet mode, while the unused medium is collected and recirculated [28]. Cultivation in gas-phase reactors comprises a two-stage cultivation system, where the first stage is a liquid-phase reactor regime, allowing HRs to disperse and grow on the support matrix [17]. Gas-phase bioreactors solve key challenges associated with liquid-phase reactors, such as oxygen mass transfer, and provide a lower shear stress environment and complete control of gases in the culture environment [159]. However, a major disadvantage is the channeling of liquid through the bed, often forming a viscous liquid film coating the roots, creating a high mass transfer barrier [17]. Some of the most frequently used gas-phase reactors include nutrient mist (NMRs), trickle bed (TBRs), droplet reactors, and radial flow (RFR) reactors [159]. In NMRs, the HRs are immobilized in a growth chamber, and the culture medium is delivered from the top over the root bed as an aerosol (nutrient mist) produced by ultrasonic methods, nozzles, or compressed air where spent medium is drained from the bottom to a reservoir and recirculated. The droplet sizes in NMRs are usually 0.01–10 μm, which decrease the thickness of the liquid film deposition on the root tissue surface, facilitating nutrient and gas exchange. NMRs offer unique advantages, including low pressure drops and shear rates, high nutrient transfer rates (because of the large mass transfer area), rapid replenishment of nutrients, removal of toxic metabolites, easy control of gas composition, operation, and scale-up [28,159,167]. In contrast, TBRs have a larger droplet size (over 10 μm), resulting in a thicker liquid film on the plant tissue surface, which hinders gas transfer. The tendency for the root bed to accumulate liquid and the absence of agitation are major limitations for large-scale design of TBR [167]. RFRs use a specific cross-sectional area where the air-saturated medium enters a sidewall in the reactor and exits through ports at the center of the top and bottom plates. This design improves the oxygen supply and is suitable for high-density HR culture [159].
Hybrid bioreactors for HR cultivation aim to overcome some of the main disadvantages observed in liquid-phase reactors such as the formation of stagnant zones with inadequate nutrient transfer and oxygen depletion, and gas-phase reactors like the manual distribution of the growth chamber. In hybrid bioreactors, during the initial cultivation stage, the reactor operates in a liquid-phase regime allowing the roots to attach uniformly to the anchoring system before switching to the gas-phase reactor for more effective HR cultivation. Well-accepted hybrid reactors include combinations BCR and TBR, and BCR and NMR [28].
Hairy root cultures have been successively cultivated in STRs or modified STRs for the biosynthesis of various secondary metabolites, achieving concentrations of SMs and growth characteristics similar to or superior to those obtained in shake flasks demonstrating their suitability for HR cultivation [171,172]. For example, the cultivation of C. roseus (L.) G. Don HRs in a 5-L STR (4 L/min flow rate equivalent to 40% dO2, marine-type impeller working at 100–120 rpm, 25 °C, and illumination of 300 lux) resulted in an 11-fold increase in biomass and a 2-fold increase in ajmalicine content (0.029 mg/g DW) [172]. Several modification of STRs, such as the use of perforated Teflon disk [173,174], nylon, or stainless-steel mesh supports, have been employed [171]. Examples illustrating HR cultivation and SMs biosynthesis in different bioreactor configurations, including modifications and cultivation parameters, are presented in Table 5. The HR cultivation of the medicinal herb Boerhaavia diffusa L. in a 10-L STR with a nylon mesh, which facilitated high-density HR cultivation and reduced the shear stress, resulted in 6.1- and 2.1-fold increases in boeravinone B and eupalitin accumulation, respectively, compared to the shake flask cultivation, without negatively effecting biomass yield [171]. HR growth has been protected from the shear forces of the impellers in a STR by the addition of perforated Teflon disk. Batch cultivation of A. annua L. HRs in this setup increased biomass accumulation 1.5-fold, while artemisinin production increased 1.4-fold [173]. However, using the same bioreactor configuration in a fed-batch mode, artemisinin production increased almost 4.0-fold compared to the batch mode of cultivation [174].

Table 5.
Examples of SMs biosynthesis and HRs cultivation in bioreactors.
Another strategy to enhance SMs production involves elicitation and precursor feeding. For instance, the application of 10 mg/L MeJA improved artemisinin content 2.4-fold during batch cultivation in an STR, compared to the non-elicited cultivation [173]. In a similar manner, the addition of appropriate elicitors and precursors, although to a lesser extent, increased the total alkaloid production in Vinca minor L. HRs, cultivated in a 5-L STR [178]. Metabolic engineering aimed at increasing the expression of key genes in the curcumin biosynthetic pathway in A. belladonna HRs resulted in a 2.3-fold improvement of curcumin yield in a modified STR, compared to the shake-flask cultivation [176].
Bubble column reactors (BCRs) bioreactors are also suitable for HR cultivation, providing sufficient oxygen and nutrients. A balloon-type BCR was effective for scaling up P. ginseng C.A. Mey. HR cultivation from 5 to 20-L volumes [196]. Scaling up the cultivation of Clitoria ternatea L. HRs in BCRs resulted in a 4-fold increase in total alkaloid content (34.8 mg/g DW) in comparison to the shake-flask cultivation [197]. Elicitation with 15 mg/L pullulan at the late exponential growth phase during B. vulgaris L. HR cultivation in a BCR with a plastic basket for HR anchoring resulted in 47% higher betalaine production compared to the non-elicited control, whereas betalaine content was even lower in the shake-flask experiment [186]. Likewise, using MeJA increased betalaine content 1.4-fold (36.13 mg/g DW) during B. vulgaris L. HR cultivation in a 3-L BCR. Simultaneous feeding with spermidine and putrescine, both at concentrations of 0.75 mM, resulted in a similar increase of 1.27-fold in betalaine content (32.9 mg/g DW), compared to the control [187].
The importance of bioreactor modifications, especially the supportive matrices for HR anchoring, was highlighted by comparing azadirachtin biosynthesis in A. indica L. HRs cultivated in conventional STRs and BCRs, and modified BCRs with a polypropylene mesh or foam as root support. In conventional STRs and BCRs, HR growth was not significant compared to the initial inoculum, and azadirachtin production was not reported likely due to high shear stress on the HRs, as indicated by elevated phenolic levels. In the modified BCR, biomass accumulation was 9.5 g/L DW with polypropylene mesh and 9.2 g/L DW with polyurethane foam. However, azadirachtin content was higher in BCR with polyurethane foam (28.52 mg/L) than with polypropylene mesh (20.23 mg/L) due to polyurethane foam’s superior porosity and absorbency, which more effectively facilitates oxygen and mass transfer [183].
Similarly, C. roseus (L.) G. Don HR biomass accumulation and ajmalicine biosynthesis were highest in a BCR with polyurethane foam. Biomass accumulation in this bioreactor was 2.33-, 4.53-, and 1.22-fold higher than in the conventional BCR, rotating drum reactor (RDR), and modified BCR with polypropylene mesh, respectively. Ajmalicine content was 2.19-, 7.39-, and 1.13-fold higher in BCR with polyurethane foam compared to the other bioreactor types [188]. ALR have been proven to be suitable for HR growth [198] and have also been integrated for the production of diverse SMs, including phenolic acids [192], flavonoids [199], terpenes [194], and even human growth hormone (hGH1) [200]. The biosynthesis of puerarin from the HRs of Pueraria phaseoloides (Roxb.) Benth was approximately 200-fold higher (5.59 mg/g DW) when cultivated in 2.5-L disposable ALR than in shake flasks [199]. Cone ALR produced the highest levels of betacyanin (27 mg/g DW) and biomass (6 g/L) from B. vulgaris L. HRs compared to other bioreactor constructions, such as balloon, bulb, drum, and column reactors [195]. The insertion of stainless-steel mesh in ALR is a common modification that positively affects the HR growth and SMs biosynthesis. The production of hGH1 increased 1.53-fold when the HRs of Brassica oleracea var. italica were grown in an ALR with a mesh compared to flask cultivation [200]. The biosynthesis of sotolone and 3-amino-4,5-dimethyl-2(5H)-furanone increased by up to 1.7- and 17% of the volatile fraction in Trigonella foenum-graecum L. HRs cultivated in an ALR with a mesh [201]. Gas-phase reactors, such as nutrient mist reactors (NMR), provide better growth and biosynthetic characteristics than liquid-phase reactors. For example, artemisinin production from A. annua L. HRs was 3-fold higher than in BCR [182]. Acoustic NMR showed the best performance in terms of increased specific growth rate and esculin content, which was twice as high as in the bubble-column and nutrient-sprinkle reactor. This effect was attributed to the growth of the HRs in acoustic NMR on mesh anchorage, which supports high mass transfer with increased absorption rates in the dispersed phase [189]. Thiophene (a sulfur-containing aromatic compound) content was also twice higher in Tagetes patula L. HRs cultivated in acoustic NMR compared to nutrient-sprinkle reactors [202]. Due to the nano-size nutrient mist particles generated in the NMRs, which promote uniform growth during high-density cultivation of A. indica L. HRs, azadirachtin production was 2.5-fold higher than in BCR [177]. Artemisinin production from A. annua L. HRs was nearly 5-fold higher in NMR than the BCR [190]. The production of mouse interleukin-12 was 5.3 µg/g FW in NMR, which was 49.5% higher compared to the ALR [203].
The advantages of the TIS concept also appear beneficial for HR cultivation. The cultivation of Centaurium maritimum (L.) Fritch in RITA® bioreactors resulted in up to a 4-fold increase in biomass accumulation and an 8-fold higher total secoiridoid glycosides production compared to shake-flask cultivation [204]. Similarly, cultivation of Gentiana dinarica Beck HRs in RITA® bioreactors yielded the highest xanthones production (56.82 mg/vessel), compared to 18.08 mg/vessel in a BCR system [205]. RITA® systems achieved the highest biomass accumulation of Agastache rugosa (Fisch. & C.A.Mey.) Kuntze HRs. Although the rosmarinic acid content (4.49 mg/g DW) was half that in the nutrient-sprinkle bioreactor, it was comparable to the content (4.65 mg/g DW) achieved in shake flasks [206].
Driven by the market demand and the need for cost minimization, disposable/single-use bioreactors (SUB) have gained significant attention in plant cell-based processes over the past few decades [207]. Unlike traditional glass or stainless-steel bioreactors, SUBs cultivation containers are made of FDA-approved disposable biocompatible plastics (polyethylene, polystyrene, polytetrafluorethylene, or polypropylene). The pre-sterile cultivation containers remove the necessity for cleaning and sterilization and are discarded after product harvest. While challenges such as proper mixing, mass transfer, and process measurement and control may arise, these vessels are equipped with necessary miniaturized sensors, sampling ports, and piping sections. Therefore, SUBs offer high flexibility in terms of time and costs, typically reducing costs by 30–40% [207,208,209]. Currently, the most popular SUBs include STR, NMR, and pneumatically driven SUBs, which are similar in principle to conventional reactors; orbitally shaken SUBs, which provide mixing through orbital oscillations of the cylindrically shaped vessel; and rocking SUBs (wave-mixed bioreactors), which use an agitation mechanism originating from the rocking of a platform with a disposable bag-like container [203,207]. For HRs, the most used SUBs are wave-mixed and NMRs [203,210,211,212].
Wave-mixed bioreactors consist of a disposable bag containing cells and media mounted on the bioreactor platform. Headspace aeration inflates the cell bag, and the rocking motion of the platform (which can be one, two, or three-dimensional) provides mixing and mass transfer. The dO2 and pH are typically measured by optical probes, and gas blending is supplied using mass flow and integrated controllers [209]. The wave-mixed bioreactor system provided a suitable environment for the growth and SMs biosynthesis of Rindera graeca (A.DC.) Boiss. & Heldr HRs. Root growth was 2-fold higher than in shake-flask cultivation, while deoxyshikonin production was observed only in the wave-mixed bioreactor [211]. The wave bioreactor was the most efficient in promoting HR growth of P. ginseng C.A. Mey. compared to conventional spray reactor and shake flask cultivation, with ginsenoside content reaching 145.6 mg/L, almost 3-fold higher than that obtained during shake-flask culturing [210]. The disposable NMR was also suitable for the HR cultivation of A. annua L. and A. hypogaea, even when scaled from 1- to 20-L, resulting in a biomass accumulation almost 1.2-fold higher than in shake flasks [212]. Similarly, the disposable NMR exhibited better growth and biosynthetic characteristics than the conventional ALR when total production of the mouse IL-12 from tobacco HRs was approximately 50% higher [203].
4.2. Hairy Roots Multiple Applications, Recent Patents, and Commercial Examples
The combined efforts in basic and applied research focusing on initiation and selection of fast-growing and highly productive HR lines, nutrient medium optimization, increased knowledge of cellular processes and factors regulating SMs biosynthetic pathways, and the development of suitable bioreactors with optimized physical parameters, have paved new avenues for designing highly efficient HRs for the biosynthesis of valuable SMs and effectively operating bioreactor systems, leading to novel technologies subject to patenting [170]. The patents cover the production of various SMs (with potential application in cosmetics, dietary supplements, and drugs) from HR cultures as well as the technical solutions for large-scale HR cultivation through modifications of existing bioreactors or the design of a new ones. Several technologies for SM biosynthesis and isolation from HRs have been described, including the production of four new flavonoid glycosides and two new sesqueterpene glycosides from C. roseus (L.) G. Don HRs [213], the anti-diabetic compound serpentine from the same plant species [214], betanin production from B. vulgaris L. HRs [215], the neurotransmitter precursor L-DOPA, used to relieve Parkinson’s disease symptoms biosynthesized by B. vulgaris L. HRs [216], and the triterpenoid sapogenin from Medicago truncatula Gaertn HRs, which, when elicited with 25 µM β-cyclodextrine, increased the biosynthesis of erythrodiol and oleanolic acid by 150- and 2-fold, respectively [217]. The combined use of elicitors and suitable TIS for the biosynthesis of stilbenes and stilbene derivatives from Arachis hypogaea L. HRs has been reported. Elicitation of the HRs with 10.2 mM sodium acetate during cultivation in an intermittent immersion vessel resulted in a 60-fold increase in trans-resveratrol compared to the non-elicited control [218]. The same cultivation system was used for ginsenosides production from P. ginseng C.A. Mey. HRs [219].
The suitability of the 15, 250, 350, and 1000-L bubble-column bioreactor for HR cultivation [220], the betalaines biosynthesis from B. vulgaris L. in a 3-L BCR [221], and the production of the anti-inflammatory compound bisabolol from Matricaria recutita L HRs in conventional mechanically and pneumatically driven bioreactors were reported [222]. Alongside conventional bioreactors, the cultivation of Taxus chinensis (Rehder and E.H. Wilson) Rehder in 5- to 300-L external circulation NMRs has been patented [223]. A flexible wall bioreactor using a small droplet-size mist unit and a flexible culture chamber was found suitable for increasing HR growth and density [224]. In another patent, an NMR with a rotatable culture bed provided optimal condition for HR growth [225], even when scaled up to 1000-L [226].
Despite the potential and patents for HR cultivation and production, sustainable industrial cultivation remained unexplored. The only company producing a wide range of products for cosmetics and nutrition from HRs was RooTec, Switzerland. Their portfolio included the production of atropine, gingsenosides, coumarines, flavonoids, alkaloids, camptothechin, anabasine, and nicotine from HRs of Atropa belladonna, Carlina acaulis, Nicotiana glauca, and Panax gingsen [29,166]. The Swiss Company Mibelle Biochemistry Mibelle AG developed RootBioTec™ HW, a product based on basil hairy root extract designed to promote fuller and denser hair [227]. Ocimum basilicum L. HR extract was incorporated in the Sunumbra® sport natural sunscreen, produced by Organic Products LLC, USA [228]. A concentrated mixture of cell wall fraction and hydro-ethanolic extract, both derived from Brassica rapa HRs, was used in Vita Genesis White product of Vitalab s.r.l., Italy, a product dedicated to safely inhibiting pigmentation, reducing melanin synthesis, and regenerating the extracellular matrix for a luminous and even skin tone [229]. Researchers from Samabriva, SA Company, France, investigated the capacity of B. rapa HRs for producing the recombinant protein alpha-L-iduronidase (IDUA), clinically important as an enzyme-replacement pharmaceutical for treating mucopolysaccharidosis type I (MPS I), a progressive lysosomal storage disorder. Due to the optimized secretion medium, IDUA activity increased more than 150-fold compared to non-induced medium [230]. The B. rapa HR-based expression system was successfully transferred to a pilot-scale 25-L airlift bioreactor, demonstrating that recombinant protein IDUA has highly homogeneous posttranslational profiles, ensuring strong batch-to-batch reproducibility [231]. Funded in France in 2011 with an R&D center focused on developing bioproduction processes for customers, Samabriva, SA, established a biomanufacturing unit in Belgium in 2023 aimed at industrial-scale production of high-value molecules. The company is now focused on industrializing HR-based expression systems for the sustainable production of natural products, including recombinant proteins in proprietary bioreactors of at least 350-L [232].
5. Conclusions and Future Perspectives
For decades, plant in vitro systems have offered a suitable alternative to whole plants for continuous production of safe, superior-in-yield, and high-quality natural products at a lower cost. Although chromosomal changes and fluctuating yields of SMs are reported in plant in vitro systems (e.g., cell suspensions), HRs, in particular, are genetically stable and offer a biosynthetically consistent platform with a high growth and productivity rate. Therefore, HRs are gaining increasing attention as a bioproduction system and are now considered a valuable approach for both basic and applied research on plant-derived natural products biosynthesis. When developing commercial processes for SMs biosynthesis based on HRs, several factors regarding sustained production and efficiency must be considered. Strategies like nutrient medium optimization, precursor feeding, elicitation, and metabolic engineering (e.g., editing of non-coding regulatory DNA/RNA sequences, overexpression of biosynthetic genes and TFs, or suppression of catabolic or competing pathway genes) have been employed to boost the biosynthesis of these low-volume, high-value SMs. Recent advancements in genomic engineering, particularly CRISPR/Cas9 technology, open new possibilities for modifying metabolic pathways and creating custom, high-yielding HR lines capable of producing functional molecules for application in food, cosmetic, and pharmaceutical industries. The combination of CRISPR/Cas technology and non-coding regulatory DNA has great potential to generate novel alleles that affect various agronomic traits of crops or medicinal plants for precise regulation of the biosynthesis of SMs. However, commercial exploitation of these SMs requires scaling up of the HRs in bioreactors. Considering the unique morphological peculiarities and sensitivity of HRs, the suitable bioreactor design must provide optimized oxygen transfer, adequate growth conditions, appropriate agitation, aeration, a homogeneous culture environment, and a minimized shear stress. Various liquid-phase, gas-phase, and hybrid bioreactors with numerous modifications have been designed to meet these challenges.
The major bottleneck that hampers the industrial production of SMs based on HRs cultivation is the poor understanding of the SMs metabolic pathways and their regulation, which further reflects on their competitive economic value. Therefore, the future focus should be on the integration of different approaches, including omics (genomics, transcriptomics, proteomics, and metabolomics) technologies to understand metabolite synthesis pathways through the identification of missing/unknown metabolite pathway enzymes or to identify enzyme variants with higher activity that are expected to obtain higher productivity of SMs. Along with that, the initial investments and overall operating costs during the cultivation process are correlated with the appropriate design of the bioreactor and the optimized process parameters. The use of disposable bioreactors is the preferred choice for the reduction of the production costs, while the application of mathematical models and real-time monitoring can be harnessed to optimize the cell-cultivation process and enhance the productivity in plant cell bioreactors at a commercial scale.
Author Contributions
Conceptualization, A.S.M.; Writing—original draft preparation, A.S.M., I.M. and A.K.S.; Writing—review and editing—A.S.M., I.M. and A.K.S.; Visualization, A.K.S.; Supervision, A.S.M.; Project administration, A.S.M.; Funding, A.S.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received funding from the Bulgarian National Science Fund (contract number KΠ-06-H61/4) and was supported by the Research Excellence Programme of the Hungarian University of Agriculture and Life Sciences 2024.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| ALR | Airlift reactor |
| ASA | Acetylsalicylic acid |
| BCR | Bubble column reactor |
| CFR | Convective flow reactor |
| COR | Coronatine |
| CRISPR/Cas9 | Clustered regularly interspaced short palindromic repeats/CRISPR-associated protein 9 |
| CS | Chitosan |
| DW | Dry weight |
| EFBR | Ebb and flow reactor |
| EMA | European medicinal agency |
| FDA | Food and drug administration |
| hGH1 | Human growth hormone |
| HRs | Hairy roots |
| JA | Jasmonic acid |
| L-DOPA | L-dihydroxyphenylalanine |
| MeJA | Methyl jasmonate |
| ORFs | Open reading frames |
| RDR | Rotating drum reactor |
| RFR | Radial flow reactor |
| Ri-plasmid | Root-inducing plasmid |
| SA | Salicylic acid |
| SMs | Specialized metabolites |
| STR | Stirred tank reactor |
| TBR | Trickle bed reactor |
| T-DNA | Transfer DNA |
| TIAs | Terpenoid indole alkaloids |
| TFs | Transcription factors |
References
- Halder, M.; Sarkar, S.; Jha, S. Elicitation: A Biotechnological Tool for Enhanced Production of Secondary Metabolites in Hairy Root Cultures. Eng. Life Sci. 2019, 19, 880–895. [Google Scholar] [CrossRef]
- Biswas, D.; Chakraborty, A.; Mukherjee, S.; Ghosh, B. Hairy Root Culture: A Potent Method for Improved Secondary Metabolite Production of Solanaceous Plants. Front. Plant Sci. 2023, 14, 1197555. [Google Scholar] [CrossRef]
- Kaur, K.; Pati, P.K. Stress-Induced Metabolite Production Utilizing Plant Hairy Roots. In Hairy Roots; Srivastava, V., Mehrotra, S., Mishra, S., Eds.; Springer: Singapore, 2018; pp. 123–145. [Google Scholar] [CrossRef]
- Trehan, S.; Michniak-Kohn, B.; Beri, K. Plant Stem Cells in Cosmetics: Current Trends and Future Directions. Future Sci. OA 2017, 3, FSO226. [Google Scholar] [CrossRef]
- Katyal, P. Plant Secondary Metabolites: Functions in Plants and Pharmacological Importance. In Plant Secondary Metabolites; Sharma, A.K., Sharma, A., Eds.; Springer Nature: Singapore, 2022; pp. 437–457. [Google Scholar] [CrossRef]
- Koehn, F.E.; Carter, G.T. The Evolving Role of Natural Products in Drug Discovery. Nat. Rev. Drug Discov. 2005, 4, 206–220. [Google Scholar] [CrossRef]
- Agarwal, G.; Carcache, P.J.B.; Addo, E.M.; Kinghorn, A.D. Current Status and Contemporary Approaches to the Discovery of Antitumor Agents from Higher Plants. Biotechnol. Adv. 2020, 38, 107337. [Google Scholar] [CrossRef]
- Thomford, N.E.; Senthebane, D.A.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural Products for Drug Discovery in the 21st Century: Innovations for Novel Drug Discovery. Int. J. Mol. Sci. 2018, 19, 1578. [Google Scholar] [CrossRef]
- Sharma, S.B.; Gupta, R. Drug Development from Natural Resource: A Systematic Approach. Mini-Rev. Med. Chem. 2015, 15, 52–57. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products ss Sources of New Drugs over the 30 Years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Namdeo, A.G.; Ingawale, D.K. Ashwagandha: Advances in Plant Biotechnological Approaches for Propagation and Production of Bioactive Compounds. J. Ethnopharmacol. 2021, 271, 113709. [Google Scholar] [CrossRef]
- Han, J.-E.; Park, Y.-J.; Lee, H.; Jeong, Y.-J.; Park, S.-Y. Increased Brazzein Expression by Abiotic Stress and Bioreactor Culture System for the Production of Sweet Protein, Brazzein. Plant Biotechnol. Rep. 2020, 14, 459–466. [Google Scholar] [CrossRef]
- Schmitz, C.; Fritsch, L.; Fischer, R.; Schillberg, S.; Rasche, S. Statistical Experimental Designs for the Production of Secondary Metabolites in Plant Cell Suspension Cultures. Biotechnol. Lett. 2016, 38, 2007–2014. [Google Scholar] [CrossRef]
- Wang, J.W.; Wu, J.Y. Effective Elicitors and Process Strategies for Enhancement of Secondary Metabolite Production in Hairy Root Cultures. In Biotechnology of Hairy Root Systems; Doran, P.M., Ed.; Advances in Biochemical Engineering/Biotechnology; Springer: Berlin/Heidelberg, Germany, 2013; Volume 134, pp. 55–89. [Google Scholar] [CrossRef]
- Awere, C.O.; Rakkammal, K.; Mwaura, M.M.; Anadebe, V.C.; Ramesh, M. Hairy-Root Technology: A Metabolic Engineering Tool and Specialized Metabolite Pathway Elucidation and Production of Secondary Metabolites. A Review. Results Eng. 2024, 23, 102697. [Google Scholar] [CrossRef]
- Morey, K.J.; Peebles, C.A.M. Hairy Roots: An Untapped Potential for Production of Plant Products. Front. Plant Sci. 2022, 13, 937095. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, X.; Wen, Y.; Wang, L.; Wang, Y.; Liao, C.; Zhao, M.; Li, T.; Liu, D.; Li, B.; et al. Plant Hairy Roots: Induction, Applications, Limitations and Prospects. Ind. Crops Prod. 2024, 219, 119104. [Google Scholar] [CrossRef]
- Aghaali, Z.; Naghavi, M.R.; Zargar, M. Collaboration of Hairy Root Culture and Scale-up Strategies for Enhancing the Biosynthesis of Medicinal and Defensive Alkaloids in Papaver sp. Curr. Plant Biol. 2024, 40, 100381. [Google Scholar] [CrossRef]
- Banadka, A.; Narasimha, S.W.; Dandin, V.S.; Naik, P.M.; Vennapusa, A.R.; Melmaiee, K.; Vemanna, R.S.; Al-Khayri, J.M.; Thiruvengadam, M.; Nagella, P. Biotechnological Approaches for the Production of Camptothecin. Appl. Microbiol. Biotechnol. 2024, 108, 382. [Google Scholar] [CrossRef]
- Murthy, H.N.; Yadav, G.G.; Paek, K.Y.; Park, S.Y. Curcuminoid Production from Plant Cells and Organ Cultures for Application in Food and Pharmaceutical Industries. Food Rev. Int. 2024, 1–19. [Google Scholar] [CrossRef]
- Huang, P.; Lu, M.; Li, X.; Sun, H.; Cheng, Z.; Miao, Y.; Fu, Y.; Zhang, X. An Efficient Agrobacterium rhizogenes-Mediated Hairy Root Transformation Method in a Soybean Root Biology Study. Int. J. Mol. Sci. 2022, 23, 12261. [Google Scholar] [CrossRef]
- Gantait, S.; Mukherjee, E. Hairy Root Culture Technology: Applications, Constraints and Prospect. Appl. Microbiol. Biotechnol. 2021, 105, 35–53. [Google Scholar] [CrossRef]
- Niazian, M.; Sadat Noori, S.A.; Galuszka, P.; Mortazavian, S.M.M. Tissue Culture-Based Agrobacterium-Mediated and in Planta Transformation Methods. Czech, J. Genet. Plant Breed. 2017, 53, 133–143. [Google Scholar] [CrossRef]
- Niazian, M.; Belzile, F.; Torkamaneh, D. CRISPR/Cas9 in Planta Hairy Root Transformation: A Powerful Platform for Functional Analysis of Root Traits in Soybean. Plants 2022, 11, 1044. [Google Scholar] [CrossRef]
- Kereszt, A.; Li, D.; Indrasumunar, A.; Nguyen, C.D.; Nontachaiyapoom, S.; Kinkema, M.; Gresshoff, P.M. Agrobacterium rhizogenes-Mediated Transformation of Soybean to Study Root Biology. Nat. Protoc. 2007, 2, 948–952. [Google Scholar] [CrossRef]
- Ying, W.; Wen, G.; Xu, W.; Liu, H.; Ding, W.; Zheng, L.; He, Y.; Yuan, H.; Yan, D.; Cui, F.; et al. Agrobacterium rhizogenes: Paving the Road to Research and Breeding for Woody Plants. Front. Plant Sci. 2023, 14, 1196561. [Google Scholar] [CrossRef]
- Mehrotra, S.; Mishra, S.; Srivastava, V. Bioreactor Technology for Hairy Roots Cultivation. In Bioprocessing of Plant In Vitro Systems; Pavlov, A., Bley, T., Eds.; Reference Series in Phytochemistry; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Talano, M.A.; Oller, A.L.W.; González, P.S.; Agostini, E. Hairy roots, their Multiple Applications and Recent Patents. Recent Pat. Biotechnol. 2012, 6, 115–133. [Google Scholar] [CrossRef]
- Hu, Z.; Du, M. Hairy Root and Its Application in Plant Genetic Engineering. J. Integr. Plant Biol. 2006, 48, 121–127. [Google Scholar] [CrossRef]
- Halder, M.; Roychowdhury, D.; Jha, S. A Critical Review on Biotechnological Interventions for Production and Yield Enhancement of Secondary Metabolites in Hairy Root Cultures. In Hairy Roots; Srivastava, V., Mehrotra, S., Mishra, S., Eds.; Springer: Singapore, 2018; pp. 21–44. [Google Scholar] [CrossRef]
- Ono, N.N.; Tian, L. The Multiplicity of Hairy Root Cultures: Prolific Possibilities. Plant Sci. 2011, 180, 439–446. [Google Scholar] [CrossRef]
- Chen, Y.-K.; Li, X.-S.; Yang, G.-Y.; Chen, Z.-Y.; Hu, Q.-F.; Miao, M.-M. Phenolic Compounds from Nicotiana tabacum and Their Biological Activities. J. Asian Nat. Prod. Res. 2012, 14, 450–456. [Google Scholar] [CrossRef]
- Mishra, B.B.; Gautam, S.; Sharma, A. Purification and Characterisation of Polyphenol Oxidase (PPO) from Eggplant (Solanum melongena). Food Chem. 2012, 134, 1855–1861. [Google Scholar] [CrossRef]
- Tusevski, O.; Stanoeva, J.; Stefova, M.; Kungulovski, D.; Pancevska, N.; Sekulovski, N.; Panov, S.; Simic, S. Hairy Roots of Hypericum perforatum L.: A Promising System for Xanthone Production. Open Life Sci. 2013, 8, 1010–1022. [Google Scholar] [CrossRef]
- Anton, D.; Bender, I.; Kaart, T.; Roasto, M.; Heinonen, M.; Luik, A.; Püssa, T. Changes in Polyphenols Contents and Antioxidant Capacities of Organically and Conventionally Cultivated Tomato (Solanum lycopersicum L.) Fruits during Ripening. Int. J. Anal. Chem. 2017, 2017, 2367453. [Google Scholar] [CrossRef]
- Sena, L.M.; Zappelli, C.; Apone, F.; Barbulova, A.; Tito, A.; Leone, A.; Oliviero, T.; Ferracane, R.; Fogliano, V.; Colucci, G. Brassica rapa Hairy Root Extracts Promote Skin Depigmentation by Modulating Melanin Production and Distribution. J. Cosmet. Dermatol. 2018, 17, 246–257. [Google Scholar] [CrossRef]
- Ritala, A.; Dong, L.; Imseng, N.; Seppänen-Laakso, T.; Vasilev, N.; Van Der Krol, S.; Rischer, H.; Maaheimo, H.; Virkki, A.; Brändli, J.; et al. Evaluation of Tobacco (Nicotiana tabacum L. Cv. Petit Havana SR1) Hairy Roots for the Production of Geraniol, the First Committed Step in Terpenoid Indole Alkaloid Pathway. J. Biotechnol. 2014, 176, 20–28. [Google Scholar] [CrossRef]
- Rogowska, A.; Szakiel, A. Enhancement of Phytosterol and Triterpenoid Production in Plant Hairy Root Cultures—Simultaneous Stimulation or Competition? Plants 2021, 10, 2028. [Google Scholar] [CrossRef]
- El Jaber-Vazdekis, N.; Barres, M.L.; Ravelo, Á.G.; Zárate, R. Effects of Elicitors on Tropane Alkaloids and Gene Expression in Atropa baetica Transgenic Hairy Roots. J. Nat. Prod. 2008, 71, 2026–2031. [Google Scholar] [CrossRef]
- Kai, G.; Yang, S.; Luo, X.; Zhou, W.; Fu, X.; Zhang, A.; Zhang, Y.; Xiao, J. Co-Expression of AaPMT and AaTRI Effectively Enhances the Yields of Tropane Alkaloids in Anisodus Acutangulus Hairy Roots. BMC Biotechnol. 2011, 11, 43. [Google Scholar] [CrossRef]
- Dehghan, E.; Reed, D.W.; Covello, P.S.; Hasanpour, Z.; Palazon, J.; Oksman-Caldentey, K.-M.; Ahmadi, F.S. Genetically Engineered Hairy Root Cultures of Hyoscyamus Senecionis and H. Muticus: Ploidy as a Promising Parameter in the Metabolic Engineering of Tropane Alkaloids. Plant Cell Rep. 2017, 36, 1615–1626. [Google Scholar] [CrossRef]
- Banerjee, S. Voyaging through Chromosomal Studies in Hairy Root Cultures towards Unravelling Their Relevance to Medicinal Plant Research: An Updated Review. Nucleus 2018, 61, 3–18. [Google Scholar] [CrossRef]
- Grzegorczyk, I.; Królicka, A.; Wysokińska, H. Establishment of Salvia Officinalis, L. Hairy Root Cultures for the Production of Rosmarinic Acid. Z. Für Naturforschung C 2006, 61, 351–356. [Google Scholar] [CrossRef]
- Wang, Q.; Pi, Y.; Hou, R.; Jiang, K.; Huang, Z.; Hsieh, M.; Sun, X.; Tang, K. Molecular Cloning and Characterization of 1-Hydroxy-2-Methyl-2-(E)-Butenyl 4-Diphosphate Reductase (CaHDR) from Camptotheca acuminata and Its Functional Identification in Escherichia coli. BMB Rep. 2008, 41, 112–118. [Google Scholar] [CrossRef]
- Ya-ut, P.; Chareonsap, P.; Sukrong, S. Micropropagation and Hairy Root Culture of Ophiorrhiza alata Craib for Camptothecin Production. Biotechnol. Lett. 2011, 33, 2519–2526. [Google Scholar] [CrossRef]
- Kochan, E.; Szymańska, G.; Szymczyk, P. Effect of Sugar Concentration on Ginsenoside Biosynthesis in Hairy Root Cultures of Panax quinquefolium Cultivated in Shake Flasks and Nutrient Sprinkle Bioreactor. Acta Physiol. Plant. 2014, 36, 613–619. [Google Scholar] [CrossRef]
- Kochan, E.; Szymczyk, P.; Kuźma, Ł.; Szymańska, G. Nitrogen and Phosphorus as the Factors Affecting Ginsenoside Production in Hairy Root Cultures of Panax quinquefolium Cultivated in Shake Flasks and Nutrient Sprinkle Bioreactor. Acta Physiol. Plant. 2016, 38, 149. [Google Scholar] [CrossRef]
- Kochan, E.; Szymczyk, P.; Kuźma, Ł.; Szymańska, G.; Wajs-Bonikowska, A.; Bonikowski, R.; Sienkiewicz, M. The Increase of Triterpene Saponin Production Induced by Trans-Anethole in Hairy Root Cultures of Panax quinquefolium. Molecules 2018, 23, 2674. [Google Scholar] [CrossRef]
- Nishikawa, K.; Furukawa, H.; Fujioka, T.; Fujii, H.; Mihashi, K.; Shimomura, K.; Ishimaru, K. Flavone Production in Transformed Root Cultures of Scutellaria baicalensis Georgi. Phytochemistry 1999, 52, 885–890. [Google Scholar] [CrossRef]
- Praveen, N.; Murthy, H.N. Withanolide A Production from Withania Somnifera Hairy Root Cultures with Improved Growth by Altering the Concentrations of Macro Elements and Nitrogen Source in the Medium. Acta Physiol. Plant. 2013, 35, 811–816. [Google Scholar] [CrossRef]
- Javid, A.; Gampe, N.; Gelana, F.; György, Z. Enhancing the Accumulation of Rosavins in Rhodiola rosea L. Plants Grown In Vitro by Precursor Feeding. Agronomy 2021, 11, 2531. [Google Scholar] [CrossRef]
- Grech-Baran, M.; Sykłowska-Baranek, K.; Giebułtowicz, J.; Wroczyński, P.; Pietrosiuk, A. Tyrosol Glucosyltransferase Activity and Salidroside Production in Natural and Transformed Root Cultures of Rhodiola kirilowii (Regel) Regel et Maximowicz. Acta Biol. Cracoviensia Ser. Bot. 2013, 55, 126–133. [Google Scholar] [CrossRef]
- Grech-Baran, M.; Sykłowska-Baranek, K.; Krajewska-Patan, A.; Wyrwał, A.; Pietrosiuk, A. Biotransformation of Cinnamyl Alcohol to Rosavins by Non-Transformed Wild Type and Hairy Root Cultures of Rhodiola kirilowii. Biotechnol. Lett. 2014, 36, 649–656. [Google Scholar] [CrossRef]
- Böhm, H.; Mäck, G. Betaxanthin Formation and Free Amino Acids in Hairy Roots of Beta vulgaris Var. Lutea Depending on Nutrient Medium and Glutamate or Glutamine Feeding. Phytochemistry 2004, 65, 1361–1368. [Google Scholar] [CrossRef]
- Peebles, C.A.M.; Hong, S.; Gibson, S.I.; Shanks, J.V.; San, K. Effects of Terpenoid Precursor Feeding on Catharanthus roseus Hairy Roots Over-expressing the Alpha or the Alpha and Beta Subunits of Anthranilate Synthase. Biotechnol. Bioeng. 2006, 93, 534–540. [Google Scholar] [CrossRef]
- Peebles, C.A.M.; Sander, G.W.; Hughes, E.H.; Peacock, R.; Shanks, J.V.; San, K.-Y. The Expression of 1-Deoxy-d-Xylulose Synthase and Geraniol-10-Hydroxylase or Anthranilate Synthase Increases Terpenoid Indole Alkaloid Accumulation in Catharanthus roseus Hairy Roots. Metab. Eng. 2011, 13, 234–240. [Google Scholar] [CrossRef]
- Shameh, S.; Hosseini, B.; Palazon, J. Genetic Engineering of Tropane Alkaloid Biosynthesis of Hyoscyamus reticulatus L. Hairy Roots by Pmt Gene Overexpression and Feeding with Putrescine. Ind. Crops Prod. 2021, 170, 113716. [Google Scholar] [CrossRef]
- Ooi, C.T.; Syahida, A.; Stanslas, J.; Maziah, M. The Influence of Methyl Jasmonate, Cholesterol and l -arginine on Solasodine Production in Hairy Root Culture of Solanum mammosum. Eng. Life Sci. 2016, 16, 432–442. [Google Scholar] [CrossRef]
- Shakya, P.; Marslin, G.; Siram, K.; Beerhues, L.; Franklin, G. Elicitation as a Tool to Improve the Profiles of High-Value Secondary Metabolites and Pharmacological Properties of Hypericum perforatum. J. Pharm. Pharmacol. 2018, 71, 70–82. [Google Scholar] [CrossRef]
- Radman, R.; Saez, T.; Bucke, C.; Keshavarz, T. Elicitation of Plants and Microbial Cell Systems. Biotechnol. Appl. Biochem. 2003, 37, 91–102. [Google Scholar] [CrossRef]
- Ochoa-Meza, L.C.; Quintana-Obregón, E.A.; Vargas-Arispuro, I.; Falcón-Rodríguez, A.B.; Aispuro-Hernández, E.; Virgen-Ortiz, J.J.; Martínez-Téllez, M.Á. Oligosaccharins as Elicitors of Defense Responses in Wheat. Polymers 2021, 13, 3105. [Google Scholar] [CrossRef]
- Ramirez-Estrada, K.; Vidal-Limon, H.; Hidalgo, D.; Moyano, E.; Golenioswki, M.; Cusidó, R.; Palazon, J. Elicitation, an Effective Strategy for the Biotechnological Production of Bioactive High-Added Value Compounds in Plant Cell Factories. Molecules 2016, 21, 182. [Google Scholar] [CrossRef]
- Seybold, H.; Trempel, F.; Ranf, S.; Scheel, D.; Romeis, T.; Lee, J. Ca2+ Signalling in Plant Immune Response: From Pattern Recognition Receptors to Ca2+ Decoding Mechanisms. New Phytol. 2014, 204, 782–790. [Google Scholar] [CrossRef]
- Harfi, B.; Khelifi, L.; Khelifi-Slaoui, M.; Assaf-Ducrocq, C.; Gontier, E. Tropane Alkaloids GC/MS Analysis and Low Dose Elicitors’ Effects on Hyoscyamine Biosynthetic Pathway in Hairy Roots of Algerian Datura Species. Sci. Rep. 2018, 8, 17951. [Google Scholar] [CrossRef]
- Hashemi, S.M.; Naghavi, M.R. Production and Gene Expression of Morphinan Alkaloids in Hairy Root Culture of Papaver orientale L. Using Abiotic Elicitors. Plant Cell Tissue Organ Cult. PCTOC 2016, 125, 31–41. [Google Scholar] [CrossRef]
- Karami, M.; Naghavi, M.R.; Nasiri, J.; Farzin, N. Methyl Jasmonate and β-Cyclodextrin Shake Hands to Boost Withaferin A Production from the Hairy Root Culture of Withania somnifera. Preprint 2023. [Google Scholar] [CrossRef]
- Sivanandhan, G.; Kapil Dev, G.; Jeyaraj, M.; Rajesh, M.; Arjunan, A.; Muthuselvam, M.; Manickavasagam, M.; Selvaraj, N.; Ganapathi, A. Increased Production of Withanolide A, Withanone, and Withaferin A in Hairy Root Cultures of Withania somnifera (L.) Dunal Elicited with Methyl Jasmonate and Salicylic Acid. Plant Cell Tissue Organ Cult. PCTOC 2013, 114, 121–129. [Google Scholar] [CrossRef]
- Zaheer, M.; Reddy, V.D.; Giri, C.C. Enhanced Daidzin Production from Jasmonic and Acetyl Salicylic Acid Elicited Hairy Root Cultures of Psoralea corylifolia L. (Fabaceae). Nat. Prod. Res. 2016, 30, 1542–1547. [Google Scholar] [CrossRef]
- Gai, Q.-Y.; Jiao, J.; Luo, M.; Wang, W.; Gu, C.-B.; Fu, Y.-J.; Ma, W. Tremendous Enhancements of Isoflavonoid Biosynthesis, Associated Gene Expression and Antioxidant Capacity in Astragalus Membranaceus Hairy Root Cultures Elicited by Methyl Jasmonate. Process Biochem. 2016, 51, 642–649. [Google Scholar] [CrossRef]
- Martin, K.P.; Sabovljevic, A.; Madassery, J. High-Frequency Transgenic Plant Regeneration and Plumbagin Production through Methyl Jasmonate Elicitation from Hairy Roots of Plumbago indica L.J. Crop Sci. Biotechnol. 2011, 14, 205–212. [Google Scholar] [CrossRef]
- Gharechahi, J.; Khalili, M.; Hasanloo, T.; Salekdeh, G.H. An Integrated Proteomic Approach to Decipher the Effect of Methyl Jasmonate Elicitation on the Proteome of Silybum marianum L. Hairy Roots. Plant Physiol. Biochem. 2013, 70, 115–122. [Google Scholar] [CrossRef]
- Theboral, J.; Sivanandhan, G.; Subramanyam, K.; Arun, M.; Selvaraj, N.; Manickavasagam, M.; Ganapathi, A. Enhanced Production of Isoflavones by Elicitation in Hairy Root Cultures of Soybean. Plant Cell Tissue Organ Cult. PCTOC 2014, 117, 477–481. [Google Scholar] [CrossRef]
- Thakore, D.; Srivastava, A.K.; Sinha, A.K. Model Based Fed Batch Cultivation and Elicitation for the Overproduction of Ajmalicine from Hairy Roots of Catharanthus roseus. Biochem. Eng. J. 2015, 97, 73–80. [Google Scholar] [CrossRef]
- Sharifzadeh Naeini, M.; Naghavi, M.R.; Bihamta, M.R.; Sabokdast, M.; Salehi, M. Production of Some Benzylisoquinoline Alkaloids in Papaver armeniacum L. Hairy Root Cultures Elicited with Salicylic Acid and Methyl Jasmonate. In Vitro Cell. Dev. Biol. Plant 2021, 57, 261–271. [Google Scholar] [CrossRef]
- Gai, Q.-Y.; Jiao, J.; Wang, X.; Zang, Y.-P.; Niu, L.-L.; Fu, Y.-J. Elicitation of Isatis Tinctoria, L. Hairy Root Cultures by Salicylic Acid and Methyl Jasmonate for the Enhanced Production of Pharmacologically Active Alkaloids and Flavonoids. Plant Cell Tissue Organ Cult. PCTOC 2019, 137, 77–86. [Google Scholar] [CrossRef]
- Sykłowska-Baranek, K.; Pilarek, M.; Bonfill, M.; Kafel, K.; Pietrosiuk, A. Perfluorodecalin-Supported System Enhances Taxane Production in Hairy Root Cultures of Taxus x Media Var. Hicksii Carrying a Taxadiene Synthase Transgene. Plant Cell Tissue Organ Cult. PCTOC 2015, 120, 1051–1059. [Google Scholar] [CrossRef]
- Qin, B.; Ma, L.; Wang, Y.; Chen, M.; Lan, X.; Wu, N.; Liao, Z. Effects of Acetylsalicylic Acid and UV-B on Gene Expression and Tropane Alkaloid Biosynthesis in Hairy Root Cultures of Anisodus Luridus. Plant Cell Tissue Organ Cult. PCTOC 2014, 117, 483–490. [Google Scholar] [CrossRef]
- Fattahi, F.; Shojaeiyan, A.; Palazon, J.; Moyano, E.; Torras-Claveria, L. Methyl-β-cyclodextrin and Coronatine as New Elicitors of Tropane Alkaloid Biosynthesis in Atropa acuminata and Atropa belladonna Hairy Root Cultures. Physiol. Plant. 2021, 172, 2098–2111. [Google Scholar] [CrossRef]
- Shilpha, J.; Satish, L.; Kavikkuil, M.; Joe Virgin Largia, M.; Ramesh, M. Methyl Jasmonate Elicits the Solasodine Production and Anti-Oxidant Activity in Hairy Root Cultures of Solanum trilobatum L. Ind. Crops Prod. 2015, 71, 54–64. [Google Scholar] [CrossRef]
- Zhu, C.; Miao, G.; Guo, J.; Huo, Y.; Zhang, X.; Xie, J.; Feng, J. Establishment of Tripterygium wilfordii Hook. f. Hairy Root Culture and Optimization of Its Culture Conditions for the Production of Triptolide and Wilforine. J. Microbiol. Biotechnol. 2014, 24, 823–834. [Google Scholar] [CrossRef]
- Jiao, J.; Gai, Q.-Y.; Wang, W.; Luo, M.; Zu, Y.-G.; Fu, Y.-J.; Ma, W. Enhanced Astragaloside Production and Transcriptional Responses of Biosynthetic Genes in Astragalus Membranaceus Hairy Root Cultures by Elicitation with Methyl Jasmonate. Biochem. Eng. J. 2016, 105, 339–346. [Google Scholar] [CrossRef]
- Wang, C.H.; Zheng, L.P.; Tian, H.; Wang, J.W. Synergistic Effects of Ultraviolet-B and Methyl Jasmonate on Tanshinone Biosynthesis in Salvia miltiorrhiza Hairy Roots. J. Photochem. Photobiol. B 2016, 159, 93–100. [Google Scholar] [CrossRef]
- Mahendran, G.; Vimolmangkang, S. Effect of Carbon Source and Elicitors on Biomass Production and Accumulation of Friedelin and Epifriedelanol in Hairy Roots of Hemp (Cannabis sativa L.). Plant Cell Tissue Organ Cult. PCTOC 2024, 156, 61. [Google Scholar] [CrossRef]
- Kim, O.T.; Yoo, N.H.; Kim, G.S.; Kim, Y.C.; Bang, K.H.; Hyun, D.Y.; Kim, S.H.; Kim, M.Y. Stimulation of Rg3 Ginsenoside Biosynthesis in Ginseng Hairy Roots Elicited by Methyl Jasmonate. Plant Cell Tissue Organ Cult. PCTOC 2013, 112, 87–93. [Google Scholar] [CrossRef]
- Wongwicha, W.; Tanaka, H.; Shoyama, Y.; Putalun, W. Methyl Jasmonate Elicitation Enhances Glycyrrhizin Production in Glycyrrhiza inflata Hairy Roots Cultures. Z. Für Naturforschung C 2011, 66, 423–428. [Google Scholar] [CrossRef]
- Alcalde, M.A.; Cusido, R.M.; Moyano, E.; Palazon, J.; Bonfill, M. Metabolic Gene Expression and Centelloside Production in Elicited Centella Asiatica Hairy Root Cultures. Ind. Crops Prod. 2022, 184, 114988. [Google Scholar] [CrossRef]
- Alsoufi, A.S.M.; Pączkowski, C.; Szakiel, A.; Długosz, M. Effect of Jasmonic Acid and Chitosan on Triterpenoid Production in Calendula officinalis Hairy Root Cultures. Phytochem. Lett. 2019, 31, 5–11. [Google Scholar] [CrossRef]
- Cheruvathur, M.K.; Thomas, T.D. Effect of Plant Growth Regulators and Elicitors on Rhinacanthin Accumulation in Hairy Root Cultures of Rhinacanthus nasutus (L.) Kurz. Plant Cell Tissue Organ Cult. PCTOC 2014, 118, 169–177. [Google Scholar] [CrossRef]
- Hao, X.; Shi, M.; Cui, L.; Xu, C.; Zhang, Y.; Kai, G. Effects of Methyl Jasmonate and Salicylic Acid on Tanshinone Production and Biosynthetic Gene Expression in Transgenic Salvia miltiorrhiza Hairy Roots. Biotechnol. Appl. Biochem. 2015, 62, 24–31. [Google Scholar] [CrossRef]
- Baek, S.; Ho, T.-T.; Lee, H.; Jung, G.; Kim, Y.E.; Jeong, C.-S.; Park, S.-Y. Enhanced Biosynthesis of Triterpenoids in Centella asiatica Hairy Root Culture by Precursor Feeding and Elicitation. Plant Biotechnol. Rep. 2020, 14, 45–53. [Google Scholar] [CrossRef]
- Qiu, H.; Su, L.; Wang, H.; Zhang, Z. Chitosan Elicitation of Saponin Accumulation in Psammosilene Tunicoides Hairy Roots by Modulating Antioxidant Activity, Nitric Oxide Production and Differential Gene Expression. Plant Physiol. Biochem. 2021, 166, 115–127. [Google Scholar] [CrossRef]
- Saxena, P.; Ahlawat, S.; Ali, A.; Khan, S.; Abdin, M.Z. Gene Expression Analysis of the Withanolide Biosynthetic Pathway in Hairy Root Cultures of Withania somnifera Elicited with Methyl Jasmonate and the Fungus Piriformospora Indica. Symbiosis 2017, 71, 143–154. [Google Scholar] [CrossRef]
- Karami, M.; Naghavi, M.R.; Nasiri, J.; Farzin, N.; Ignea, C. Enhanced Production of Withaferin A from the Hairy Root Culture of Withania somnifera via Synergistic Effect of Methyl Jasmonate and β-Cyclodextrin. Plant Physiol. Biochem. 2024, 208, 108440. [Google Scholar] [CrossRef]
- Amani, S.; Mohebodini, M.; Khademvatan, S.; Jafari, M.; Kumar, V. Modifications in Gene Expression and Phenolic Compounds Content by Methyl Jasmonate and Fungal Elicitors in Ficus Carica. Cv. Siah Hairy Root Cultures. BMC Plant Biol. 2024, 24, 520. [Google Scholar] [CrossRef]
- Yousefian, S.; Lohrasebi, T.; Farhadpour, M.; Haghbeen, K. Effect of Methyl Jasmonate on Phenolic Acids Accumulation and the Expression Profile of Their Biosynthesis-Related Genes in Mentha spicata Hairy Root Cultures. Plant Cell Tissue Organ Cult. PCTOC 2020, 142, 285–297. [Google Scholar] [CrossRef]
- Rastegarnejad, F.; Mirjalili, M.H.; Bakhtiar, Z. Enhanced Production of Tanshinone and Phenolic Compounds in Hairy Roots Culture of Salvia miltiorrhiza Bunge by Elicitation. Plant Cell Tissue Organ Cult. PCTOC 2024, 156, 4. [Google Scholar] [CrossRef]
- Li, J.; Li, B.; Luo, L.; Cao, F.; Yang, B.; Gao, J.; Yan, Y.; Zhang, G.; Peng, L.; Hu, B. Increased Phenolic Acid and Tanshinone Production and Transcriptional Responses of Biosynthetic Genes in Hairy Root Cultures of Salvia przewalskii Maxim. Treated with Methyl Jasmonate and Salicylic Acid. Mol. Biol. Rep. 2020, 47, 8565–8578. [Google Scholar] [CrossRef] [PubMed]
- Attaran Dowom, S.; Abrishamchi, P.; Radjabian, T.; Salami, S.A. Elicitor-Induced Phenolic Acids Accumulation in Salvia virgata Jacq. Hairy Root Cultures. Plant Cell Tissue Organ Cult. PCTOC 2022, 148, 107–117. [Google Scholar] [CrossRef]
- Ru, M.; An, Y.; Wang, K.; Peng, L.; Li, B.; Bai, Z.; Wang, B.; Liang, Z. Prunella vulgaris L. Hairy Roots: Culture, Growth, and Elicitation by Ethephon and Salicylic Acid. Eng. Life Sci. 2016, 16, 494–502. [Google Scholar] [CrossRef]
- Zaheer, M.; Giri, C.C. Enhanced Diterpene Lactone (Andrographolide) Production from Elicited Adventitious Root Cultures of Andrographis paniculata. Res. Chem. Intermed. 2017, 43, 2433–2444. [Google Scholar] [CrossRef]
- Liang, Z.-S.; Yang, D.-F.; Liang, X.; Zhang, Y.-J.; Liu, Y.; Liu, F.-H. Roles of Reactive Oxygen Species in Methyl Jasmonate and Nitric Oxide-Induced Tanshinone Production in Salvia miltiorrhiza Hairy Roots. Plant Cell Rep. 2012, 31, 873–883. [Google Scholar] [CrossRef]
- Piątczak, E.; Kuźma, Ł.; Wysokińska, H. The Influence of Methyl Jasmonate and Salicylic Acid on Secondary Metabolite Production in Rehmannia glutinosa Libosch. Hairy Root Culture. Acta Biol. Cracoviensia Bot. 2016, 58, 57–65. [Google Scholar] [CrossRef]
- Yu, H.-S.; Ma, L.-Q.; Zhang, J.-X.; Shi, G.-L.; Hu, Y.-H.; Wang, Y.-N. Characterization of Glycosyltransferases Responsible for Salidroside Biosynthesis in Rhodiola sachalinensis. Phytochemistry 2011, 72, 862–870. [Google Scholar] [CrossRef]
- Lan, X.; Chang, K.; Zeng, L.; Liu, X.; Qiu, F.; Zheng, W.; Quan, H.; Liao, Z.; Chen, M.; Huang, W.; et al. Engineering Salidroside Biosynthetic Pathway in Hairy Root Cultures of Rhodiola crenulata Based on Metabolic Characterization of Tyrosine Decarboxylase. PLoS ONE 2013, 8, e75459. [Google Scholar] [CrossRef]
- Liu, X.; Tang, Y.; Zeng, J.; Qin, J.; Lin, M.; Chen, M.; Liao, Z.; Lan, X. Biochemical Characterization of Tyrosine Aminotransferase and Enhancement of Salidroside Production by Suppressing Tyrosine Aminotransferase in Rhodiola crenulata. Ind. Crops Prod. 2021, 173, 114075. [Google Scholar] [CrossRef]
- Marchev, A.S.; Yordanova, Z.P.; Georgiev, M.I. Green (Cell) Factories for Advanced Production of Plant Secondary Metabolites. Crit. Rev. Biotechnol. 2020, 40, 443–458. [Google Scholar] [CrossRef] [PubMed]
- Marchev, A.S.; Georgiev, M.I. Plant In Vitro Systems as a Sustainable Source of Active Ingredients for Cosmeceutical Application. Molecules 2020, 25, 2006. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.; Wen, S.; Wang, W.; Wang, X.; Xu, H.; Kai, G. Enhancement of Camptothecin Production in Camptotheca acuminata Hairy Roots by Overexpressing ORCA3 Gene. J. Appl. Pharm. Sci. 2011, 1, 85–88. [Google Scholar]
- Wang, C.-T.; Liu, H.; Gao, X.-S.; Zhang, H.-X. Overexpression of G10H and ORCA3 in the Hairy Roots of Catharanthus roseus Improves Catharanthine Production. Plant Cell Rep. 2010, 29, 887–894. [Google Scholar] [CrossRef]
- Guo, Z.; Tan, H.; Lv, Z.; Ji, Q.; Huang, Y.; Liu, J.; Chen, D.; Diao, Y.; Si, J.; Zhang, L. Targeted Expression of Vitreoscilla Hemoglobin Improves the Production of Tropane Alkaloids in Hyoscyamus niger Hairy Roots. Sci. Rep. 2018, 8, 17969. [Google Scholar] [CrossRef]
- Li, Q.; Zhu, T.; Zhang, R.; Bu, Q.; Yin, J.; Zhang, L.; Chen, W. Molecular Cloning and Functional Analysis of Hyoscyamine 6β-Hydroxylase (H6H) in the Poisonous and Medicinal Plant Datura innoxia Mill. Plant Physiol. Biochem. 2020, 153, 11–19. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Kim, J.K.; Kim, Y.B.; Lee, S.; Kim, S.-U.; Park, S.U. Enhanced Accumulation of Phytosterol and Triterpene in Hairy Root Cultures of Platycodon Grandiflorum by Overexpression of Panax Ginseng 3-Hydroxy-3-Methylglutaryl-Coenzyme A Reductase. J. Agric. Food Chem. 2013, 61, 1928–1934. [Google Scholar] [CrossRef]
- Kai, G.; Xu, H.; Zhou, C.; Liao, P.; Xiao, J.; Luo, X.; You, L.; Zhang, L. Metabolic Engineering Tanshinone Biosynthetic Pathway in Salvia miltiorrhiza Hairy Root Cultures. Metab. Eng. 2011, 13, 319–327. [Google Scholar] [CrossRef]
- Sitarek, P.; Kowalczyk, T.; Rijo, P.; Białas, A.J.; Wielanek, M.; Wysokińska, H.; Garcia, C.; Toma, M.; Śliwiński, T.; Skała, E. Over-Expression of AtPAP1 Transcriptional Factor Enhances Phenolic Acid Production in Transgenic Roots of Leonurus sibiricus L. and Their Biological Activities. Mol. Biotechnol. 2018, 60, 74–82. [Google Scholar] [CrossRef]
- Grzegorczyk-Karolak, I.; Krzemińska, M.; Grąbkowska, R.; Gomulski, J.; Żekanowski, C.; Gaweda-Walerych, K. Accumulation of Polyphenols and Associated Gene Expression in Hairy Roots of Salvia viridis Exposed to Methyl Jasmonate. Int. J. Mol. Sci. 2024, 25, 764. [Google Scholar] [CrossRef] [PubMed]
- Dong Hui, L.; Wei Wei, R.; Li Jie, C.; Li Da, Z.; Xiao Fen, S.; Ke Xuan, T. Enhanced Accumulation of Catharanthine and Vindoline in Catharanthus roseus Hairy Roots by Overexpression of Transcriptional Factor ORCA2. Afr. J. Biotechnol. 2011, 10, 3260–3268. [Google Scholar] [CrossRef]
- Goklany, S.; Rizvi, N.F.; Loring, R.H.; Cram, E.J.; Lee-Parsons, C.W.T. Jasmonate-dependent Alkaloid Biosynthesis in Catharanthus roseus Hairy Root Cultures Is Correlated with the Relative Expression of Orca and Zct Transcription Factors. Biotechnol. Prog. 2013, 29, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Gong, H.; Cui, L.; Wang, Q.; Wang, C.; Wang, Y.; Kai, G. Targeted Metabolic Engineering of Committed Steps Improves Anti-Cancer Drug Camptothecin Production in Ophiorrhiza pumila Hairy Roots. Ind. Crops Prod. 2020, 148, 112277. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Kim, Y.B.; Uddin, M.R.; Lee, S.; Kim, S.-U.; Park, S.U. Enhanced Triterpene Accumulation in Panax ginseng Hairy Roots Overexpressing Mevalonate-5-Pyrophosphate Decarboxylase and Farnesyl Pyrophosphate Synthase. ACS Synth. Biol. 2014, 3, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.T.; Kim, S.H.; Ohyama, K.; Muranaka, T.; Choi, Y.E.; Lee, H.Y.; Kim, M.Y.; Hwang, B. Upregulation of Phytosterol and Triterpene Biosynthesis in Centella asiatica Hairy Roots Overexpressed Ginseng Farnesyl Diphosphate Synthase. Plant Cell Rep. 2010, 29, 403–411. [Google Scholar] [CrossRef]
- Shi, M.; Luo, X.; Ju, G.; Li, L.; Huang, S.; Zhang, T.; Wang, H.; Kai, G. Enhanced Diterpene Tanshinone Accumulation and Bioactivity of Transgenic Salvia miltiorrhiza Hairy Roots by Pathway Engineering. J. Agric. Food Chem. 2016, 64, 2523–2530. [Google Scholar] [CrossRef]
- Shi, M.; Luo, X.; Ju, G.; Yu, X.; Hao, X.; Huang, Q.; Xiao, J.; Cui, L.; Kai, G. Increased Accumulation of the Cardio-Cerebrovascular Disease Treatment Drug Tanshinone in Salvia miltiorrhiza Hairy Roots by the Enzymes 3-Hydroxy-3-Methylglutaryl CoA Reductase and 1-Deoxy-d-Xylulose 5-Phosphate Reductoisomerase. Funct. Integr. Genom. 2014, 14, 603–615. [Google Scholar] [CrossRef]
- Zhou, Y.; Sun, W.; Chen, J.; Tan, H.; Xiao, Y.; Li, Q.; Ji, Q.; Gao, S.; Chen, L.; Chen, S.; et al. SmMYC2a and SmMYC2b Played Similar but Irreplaceable Roles in Regulating the Biosynthesis of Tanshinones and Phenolic Acids in Salvia miltiorrhiza. Sci. Rep. 2016, 6, 22852. [Google Scholar] [CrossRef]
- Zhou, W.; Shi, M.; Deng, C.; Lu, S.; Huang, F.; Wang, Y.; Kai, G. The Methyl Jasmonate-Responsive Transcription Factor SmMYB1 Promotes Phenolic Acid Biosynthesis in Salvia miltiorrhiza. Hortic. Res. 2021, 8, 10. [Google Scholar] [CrossRef]
- Sun, M.; Shi, M.; Wang, Y.; Huang, Q.; Yuan, T.; Wang, Q.; Wang, C.; Zhou, W.; Kai, G. The Biosynthesis of Phenolic Acids Is Positively Regulated by the JA-Responsive Transcription Factor ERF115 in Salvia miltiorrhiza. J. Exp. Bot. 2019, 70, 243–254. [Google Scholar] [CrossRef]
- Hao, X.; Pu, Z.; Cao, G.; You, D.; Zhou, Y.; Deng, C.; Shi, M.; Nile, S.H.; Wang, Y.; Zhou, W.; et al. Tanshinone and Salvianolic Acid Biosynthesis Are Regulated by SmMYB98 in Salvia miltiorrhiza Hairy Roots. J. Adv. Res. 2020, 23, 1–12. [Google Scholar] [CrossRef]
- Zakaria, M.M.; Kruse, L.H.; Engelhardt, A.; Ober, D. Seeing Double: Two Different Homospermidine Oxidases Are Involved in Pyrrolizidine Alkaloid Biosynthesis in Different Organs of Comfrey (Symphytum officinale). Plant J. 2024, 119, 1272–1288. [Google Scholar] [CrossRef]
- Zhang, F.; Qiu, F.; Zeng, J.; Xu, Z.; Tang, Y.; Zhao, T.; Gou, Y.; Su, F.; Wang, S.; Sun, X.; et al. Revealing Evolution of Tropane Alkaloid Biosynthesis by Analyzing Two Genomes in the Solanaceae Family. Nat. Commun. 2023, 14, 1446. [Google Scholar] [CrossRef]
- Swinnen, G.; De Meyer, M.; Pollier, J.; Molina-Hidalgo, F.J.; Ceulemans, E.; Venegas-Molina, J.; De Milde, L.; Fernández-Calvo, P.; Ron, M.; Pauwels, L.; et al. The Basic helix–loop–helix Transcription Factors MYC1 and MYC2 Have a Dual Role in the Regulation of Constitutive and stress-inducible Specialized Metabolism in Tomato. New Phytol. 2022, 236, 911–928. [Google Scholar] [CrossRef]
- Hasebe, F.; Yuba, H.; Hashimoto, T.; Saito, K.; Funa, N.; Shoji, T. CRISPR/Cas9-Mediated Disruption of the PYRROLIDINE KETIDE SYNTHASE Gene Reduces the Accumulation of Tropane Alkaloids in Atropa belladonna Hairy Roots. Biosci. Biotechnol. Biochem. 2021, 85, 2404–2409. [Google Scholar] [CrossRef]
- Zakaria, M.M.; Schemmerling, B.; Ober, D. CRISPR/Cas9-Mediated Genome Editing in Comfrey (Symphytum Officinale) Hairy Roots Results in the Complete Eradication of Pyrrolizidine Alkaloids. Molecules 2021, 26, 1498. [Google Scholar] [CrossRef]
- Chen, X.; Zhong, Z.; Tang, X.; Yang, S.; Zhang, Y.; Wang, S.; Liu, Y.; Zhang, Y.; Zheng, X.; Zhang, Y.; et al. Advancing PAM-Less Genome Editing in Soybean Using CRISPR-SpRY. Hortic. Res. 2024, 11, uhae160. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Lu, J.; Yang, X.; Huang, L.-C.; Zhang, C.-Q.; Liu, Q.-Q.; Li, Q.-F. Gene Editing of Non-Coding Regulatory DNA and Its Application in Crop Improvement. J. Exp. Bot. 2023, 74, 6158–6175. [Google Scholar] [CrossRef]
- Wang, L.; Zou, P.; Liu, F.; Liu, R.; Yan, Z.-Y.; Chen, X. Integrated Analysis of lncRNAs, mRNAs, and TFs to Identify Network Modules Underlying Diterpenoid Biosynthesis in Salvia miltiorrhiza. PeerJ 2023, 11, e15332. [Google Scholar] [CrossRef]
- Su, L.; Li, S.; Qiu, H.; Wang, H.; Wang, C.; He, C.; Xu, M.; Zhang, Z. Full-Length Transcriptome Analyses of Genes Involved in Triterpenoid Saponin Biosynthesis of Psammosilene tunicoides Hairy Root Cultures with Exogenous Salicylic Acid. Front. Genet. 2021, 12, 657060. [Google Scholar] [CrossRef]
- Shi, M.; Du, Z.; Hua, Q.; Kai, G. CRISPR/Cas9-Mediated Targeted Mutagenesis of bZIP2 in Salvia miltiorrhiza Leads to Promoted Phenolic Acid Biosynthesis. Ind. Crops Prod. 2021, 167, 113560. [Google Scholar] [CrossRef]
- Li, Q.; Fang, X.; Zhao, Y.; Cao, R.; Dong, J.; Ma, P. The SmMYB36-SmERF6/SmERF115 Module Regulates the Biosynthesis of Tanshinones and Phenolic Acids in Salvia miltiorrhiza Hairy Roots. Hortic. Res. 2023, 10, uhac238. [Google Scholar] [CrossRef]
- Hsu, C.; Chiu, C.; Hsiao, P.; Lin, C.; Cheng, S.; Lin, Y.; Yang, Y.; Wu, F.; Harn, H.; Lin, S.; et al. Transgene-free CRISPR/Cas9-mediated Gene Editing through Protoplast-to-plant Regeneration Enhances Active Compounds in Salvia miltiorrhiza. Plant Biotechnol. J. 2024, 22, 1549–1551. [Google Scholar] [CrossRef]
- Han, H.; Dong, L.; Zhang, W.; Liao, Y.; Wang, L.; Wang, Q.; Ye, J.; Xu, F. Ginkgo Biloba GbbZIP08 Transcription Factor Is Involved in the Regulation of Flavonoid Biosynthesis. J. Plant Physiol. 2023, 287, 154054. [Google Scholar] [CrossRef]
- Das, S.; Kwon, M.; Kim, J.-Y. Enhancement of Specialized Metabolites Using CRISPR/Cas Gene Editing Technology in Medicinal Plants. Front. Plant Sci. 2024, 15, 1279738. [Google Scholar] [CrossRef]
- Mitra, S.; Anand, U.; Ghorai, M.; Kant, N.; Kumar, M.; Radha; Jha, N.K.; Swamy, M.K.; Proćków, J.; De La Lastra, J.M.P.; et al. Genome Editing Technologies, Mechanisms and Improved Production of Therapeutic Phytochemicals: Opportunities and Prospects. Biotechnol. Bioeng. 2023, 120, 82–94. [Google Scholar] [CrossRef]
- Bagal, D.; Chowdhary, A.A.; Mehrotra, S.; Mishra, S.; Rathore, S.; Srivastava, V. Metabolic Engineering in Hairy Roots: An Outlook on Production of Plant Secondary Metabolites. Plant Physiol. Biochem. 2023, 201, 107847. [Google Scholar] [CrossRef]
- Shelake, R.M.; Jadhav, A.M.; Bhosale, P.B.; Kim, J.-Y. Unlocking secrets of nature’s chemists: Potential of CRISPR/Cas-based tools in plant metabolic engineering for customized nutraceutical and medicinal profiles. Plant Physiol. Biochem. 2023, 203, 108070. [Google Scholar] [CrossRef]
- Fossati, E.; Narcross, L.; Ekins, A.; Falgueyret, J.-P.; Martin, V.J.J. Synthesis of Morphinan Alkaloids in Saccharomyces Cerevisiae. PLoS ONE 2015, 10, e0124459. [Google Scholar] [CrossRef]
- Khaldari, I.; Naghavi, M.R.; Motamedi, E.; Zargar, M. The Effects of Green and Chemically-Synthesized Copper Oxide Nanoparticles on the Production and Gene Expression of Morphinan Alkaloids in Oriental Poppy. Sci. Rep. 2024, 14, 6000. [Google Scholar] [CrossRef]
- Allen, R.S.; Miller, J.A.C.; Chitty, J.A.; Fist, A.J.; Gerlach, W.L.; Larkin, P.J. Metabolic Engineering of Morphinan Alkaloids by Over-expression and RNAi Suppression of Salutaridinol 7-O-acetyltransferase in Opium Poppy. Plant Biotechnol. J. 2008, 6, 22–30. [Google Scholar] [CrossRef]
- Sharafi, A.; Sohi, H.H.; Mousavi, A.; Azadi, P.; Razavi, K.; Ntui, V.O. A Reliable and Efficient Protocol for Inducing Hairy Roots in Papaver bracteatum. Plant Cell Tissue Organ Cult. PCTOC 2013, 113, 1–9. [Google Scholar] [CrossRef]
- Hagel, J.M.; Facchini, P.J. Dioxygenases Catalyze the O-Demethylation Steps of Morphine Biosynthesis in Opium Poppy. Nat. Chem. Biol. 2010, 6, 273–275. [Google Scholar] [CrossRef] [PubMed]
- Beaudoin, G.A.W.; Facchini, P.J. Benzylisoquinoline Alkaloid Biosynthesis in Opium Poppy. Planta 2014, 240, 19–32. [Google Scholar] [CrossRef]
- Sato, F. Plant Alkaloid Engineering. In Comprehensive Natural Products III; Elsevier: Amsterdam, The Netherlands, 2020; pp. 700–755. [Google Scholar] [CrossRef]
- Larkin, P.J.; Miller, J.A.C.; Allen, R.S.; Chitty, J.A.; Gerlach, W.L.; Frick, S.; Kutchan, T.M.; Fist, A.J. Increasing Morphinan Alkaloid Production by Over-expressing Codeinone Reductase in Transgenic Papaver somniferum. Plant Biotechnol. J. 2007, 5, 26–37. [Google Scholar] [CrossRef]
- Sharafi, A.; Sohi, H.H.; Mousavi, A.; Azadi, P.; Khalifani, B.H.; Razavi, K. Metabolic Engineering of Morphinan Alkaloids by Over-Expression of Codeinone Reductase in Transgenic Hairy Roots of Papaver bracteatum, the Iranian Poppy. Biotechnol. Lett. 2013, 35, 445–453. [Google Scholar] [CrossRef]
- Park, S.-U. Agrobacterium rhizogenes-Mediated Transformation of Opium Poppy, Papaver somniferum L., and California Poppy, Eschscholzia californica Cham., Root Cultures. J. Exp. Bot. 2000, 51, 1005–1016. [Google Scholar] [CrossRef]
- Rostampour, S.; Hashemi Sohi, H.; Jourabchi, E.; Ansari, E. Influence of Agrobacterium rhizogenes on Induction of Hairy Roots and Benzylisoquinoline Alkaloids Production in Persian Poppy (Papaver rracteatum Lindl.): Preliminary Report. World, J. Microbiol. Biotechnol. 2009, 25, 1807–1814. [Google Scholar] [CrossRef]
- Huang, P.; Xia, L.; Liu, W.; Jiang, R.; Liu, X.; Tang, Q.; Xu, M.; Yu, L.; Tang, Z.; Zeng, J. Hairy Root Induction and Benzylisoquinoline Alkaloid Production in Macleaya cordata. Sci. Rep. 2018, 8, 11986. [Google Scholar] [CrossRef]
- Sharafi, A.; Hashemi Sohi, H.; Mousavi, A.; Azadi, P.; Dehsara, B.; Hosseini Khalifani, B. Enhanced Morphinan Alkaloid Production in Hairy Root Cultures of Papaver bracteatum by Over-Expression of Salutaridinol 7-o-Acetyltransferase Gene via Agrobacterium rhizogenes Mediated Transformation. World J. Microbiol. Biotechnol. 2013, 29, 2125–2131. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.J.; Abbas, Y.; Nazli, N.; Fatima, S.; Drouet, S.; Hano, C.; Abbasi, B.H. Root Cultures, a Boon for the Production of Valuable Compounds: A Comparative Review. Plants 2022, 11, 439. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Srivastava, A.K. Hairy Root Culture for Mass-Production of High-Value Secondary Metabolites. Crit. Rev. Biotechnol. 2007, 27, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Cuello, J.L.; Yue, L.C. Ebb-and-Flow Bioreactor Regime and Electrical Elicitation: Novel Strategies for Hairy Root Biochemical Production. Electron. J. Integr. Biosci 2008, 3, 45–56. [Google Scholar]
- Xu, J.; Zhang, N. On the Way to Commercializing Plant Cell Culture Platform for Biopharmaceuticals: Present Status and Prospect. Pharm. Bioprocess. 2014, 2, 499–518. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Farkya, S.; Srivastava, A.K.; Bisaria, V.S. Bioprocess Considerations for Production of Secondary Metabolites by Plant Cell Suspension Cultures. Biotechnol. Bioprocess. Eng. 2002, 7, 138–149. [Google Scholar] [CrossRef]
- Su, W.W.; Lee, K.-T. Chapter 10. Plant Cell and Hairy Root Cultures—Process Characteristics, Products, and Applications. Bioprocess. Value-Added Prod. Renew. Resour. 2007, 263–292. [Google Scholar] [CrossRef]
- Srikantan, C.; Srivastava, S. Bioreactor Design and Analysis for Large- Scale Plant Cell and Hairy Root Cultivation. Hairy Roots Eff. Tool Plant Biotechnol. 2018, 147–182. [Google Scholar]
- Ruffoni, B.; Pistelli, L.; Bertoli, A.; Pistelli, L. Plant Cell Cultures: Bioreactors for Industrial Production. Adv. Exp. Med. Biol. 2010, 698, 203–221. [Google Scholar] [CrossRef]
- Ochoa-Villarreal, M.; Howat, S.; Hong, S.; Jang, M.O.; Jin, Y.-W.; Lee, E.-K.; Loake, G.J. Plant Cell Culture Strategies for the Production of Natural Products. BMB Rep. 2016, 49, 149–158. [Google Scholar] [CrossRef]
- Stiles, A.R.; Liu, C.-Z. Hairy Root Culture: Bioreactor Design and Process Intensification. Adv. Biochem. Eng. Biotechnol. 2013, 134, 91–114. [Google Scholar] [CrossRef]
- Choi, Y.E.; Kim, Y.S.; Paek, K.Y. Types And Designs Of Bioreactors For Hairy Root Culture. In Plant Tissue Culture Engineering. Focus on Biotechnology; Gupta, S.D., Ibaraki, Y., Eds.; Springer: Dordrecht, The Netherlands, 2008; Volume 6. [Google Scholar] [CrossRef]
- Giri, A.; Narasu, M.L. Transgenic Hairy Roots. Recent Trends and Applications. Biotechnol. Adv. 2000, 18, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, T.; Merecz-Sadowska, A.; Picot, L.; Brčić Karačonji, I.; Wieczfinska, J.; Śliwiński, T.; Sitarek, P. Genetic Manipulation and Bioreactor Culture of Plants as a Tool for Industry and Its Applications. Molecules 2022, 27, 795. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Pandey, P.; Singh, S.; Singh, D.K.; Saxena, A.; Luqman, S.; Bawankule, D.U.; Banerjee, S. Advances in Boerhaavia Diffusa Hairy Root Technology: A Valuable Pursuit for Identifying Strain Sensitivity and up-Scaling Factors to Refine Metabolite Yield and Bioactivity Potentials. Protoplasma 2016, 253, 1145–1158. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Mathur, A.K.; Shanker, K. Growth, Alkaloid Production, rol Genes Integration, Bioreactor Up-Scaling and Plant Regeneration Studies in Hairy Root Lines of Catharanthus roseus. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2012, 146 (Suppl. S1), 27–40. [Google Scholar] [CrossRef]
- Patra, N.; Srivastava, A.K. Enhanced Production of Artemisinin by Hairy Root Cultivation of Artemisia Annua in a Modified Stirred Tank Reactor. Appl. Biochem. Biotechnol. 2014, 174, 2209–2222. [Google Scholar] [CrossRef]
- Patra, N.; Srivastava, A.K. Use of Model-Based Nutrient Feeding for Improved Production of Artemisinin by Hairy Roots of Artemisia annua in a Modified Stirred Tank Bioreactor. Appl. Biochem. Biotechnol. 2015, 177, 373–388. [Google Scholar] [CrossRef]
- Jeong, G.-T.; Park, D.-H.; Hwang, B.; Park, K.; Kim, S.-W.; Woo, J.-C. Studies on Mass Production of Transformed Panax ginseng Hairy Roots in Bioreactor. Appl. Biochem. Biotechnol. 2002, 98–100, 1115–1128. [Google Scholar] [CrossRef]
- Singh, S.; Pandey, P.; Akhtar, M.Q.; Negi, A.S.; Banerjee, S. A New Synthetic Biology Approach for the Production of Curcumin and Its Glucoside in Atropa belladonna Hairy Roots. J. Biotechnol. 2021, 328, 23–33. [Google Scholar] [CrossRef]
- Srivastava, S.; Srivastava, A.K. In Vitro Azadirachtin Production by Hairy Root Cultivation of Azadirachta indica in Nutrient Mist Bioreactor. Appl. Biochem. Biotechnol. 2012, 166, 365–378. [Google Scholar] [CrossRef]
- Verma, P.; Khan, S.A.; Mathur, A.K.; Shanker, K.; Lal, R.K. Regulation of Vincamine Biosynthesis and Associated Growth Promoting Effects through Abiotic Elicitation, Cyclooxygenase Inhibition, and Precursor Feeding of Bioreactor Grown Vinca minor Hairy Roots. Appl. Biochem. Biotechnol. 2014, 173, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Cardillo, A.B.; Otálvaro, A.Á.M.; Busto, V.D.; Talou, J.R.; Velásquez, L.M.E.; Giulietti, A.M. Scopolamine, Anisodamine and Hyoscyamine Production by Brugmansia candida Hairy Root Cultures in Bioreactors. Process Biochem. 2010, 45, 1577–1581. [Google Scholar] [CrossRef]
- Patra, N.; Srivastava, A.K. Mass Scale Artemisinin Production in a Stirred Tank Bioreactor Using Hairy Roots of Artemisia annua. Int. J. Biosci. Biochem. Bioinform. 2014, 4, 467–474. [Google Scholar] [CrossRef]
- Verma, P.C.; Singh, H.; Negi, A.S.; Saxena, G.; Rahman, L.; Banerjee, S. Yield Enhancement Strategies for the Production of Picroliv from Hairy Root Culture of Picrorhiza kurroa Royle Ex Benth. Plant Signal. Behav. 2015, 10, e1023976. [Google Scholar] [CrossRef]
- Kim, Y.; Wyslouzil, B.; Weathers, P. A Comparative Study of Mist and Bubble Column Reactors in the in Vitro Production of Artemisinin. Plant Cell Rep. 2001, 20, 451–455. [Google Scholar] [CrossRef]
- Srivastava, S.; Srivastava, A.K. Production of the Biopesticide Azadirachtin by Hairy Root Cultivation of Azadirachta indica in Liquid-Phase Bioreactors. Appl. Biochem. Biotechnol. 2013, 171, 1351–1361. [Google Scholar] [CrossRef]
- Kareem, Z.J.; Su, L.; Rathgeb, A.; Sirrenberg, A.; Hadacek, F.; Rashid, A.H.A.H.; Karlovsky, P. Small-Scale Bioreactor for Sterile Hydroponics and Hairy Roots: Metabolic Diversity and Salicylic Acid Exudation by Hairy Roots of Hyoscyamus niger. Appl. Sci. 2019, 9, 3044. [Google Scholar] [CrossRef]
- Jaremicz, Z.; Luczkiewicz, M.; Kokotkiewicz, A.; Krolicka, A.; Sowinski, P. Production of Tropane Alkaloids in Hyoscyamus niger (Black Henbane) Hairy Roots Grown in Bubble-Column and Spray Bioreactors. Biotechnol. Lett. 2014, 36, 843–853. [Google Scholar] [CrossRef]
- Savitha, B.C.; Thimmaraju, R.; Bhagyalakshmi, N.; Ravishankar, G.A. Different Biotic and Abiotic Elicitors Influence Betalain Production in Hairy Root Cultures of Beta vulgaris in Shake-Flask and Bioreactor. Process Biochem. 2006, 41, 50–60. [Google Scholar] [CrossRef]
- Suresh, B.; Thimmaraju, R.; Bhagyalakshmi, N.; Ravishankar, G.A. Polyamine and Methyl Jasmonate-Influenced Enhancement of Betalaine Production in Hairy Root Cultures of Beta vulgaris Grown in a Bubble Column Reactor and Studies on Efflux of Pigments. Process Biochem. 2004, 39, 2091–2096. [Google Scholar] [CrossRef]
- Thakore, D.; Srivastava, A.K. Mass Scale Hairy Root Cultivation of Catharanthus roseus in Bioreactor for Indole Alkaloid Production. In Plant Cell and Tissue Differentiation and Secondary Metabolites; Ramawat, K.G., Ekiert, H.M., Goyal, S., Eds.; Reference Series in Phytochemistry; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Pal Bais, H.; Suresh, B.; Rachavarao, K.S.M.S.; Ravishankar, G.A. Performance of Hairy Root Cultures of Cichorium intybus L. In Bioreactors of Different Configurations. In Vitro Cell. Dev. Biol. Plant 2002, 38, 573–580. [Google Scholar] [CrossRef]
- Patra, N.; Srivastava, A.K. Artemisinin Production by Plant Hairy Root Cultures in Gas- and Liquid-Phase Bioreactors. Plant Cell Rep. 2016, 35, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Heredia, A.A.; Marín-López, R.; Ponce-Noyola, T.; Cerda-García-Rojas, C.M.; Trejo-Tapia, G.; Ramos-Valdivia, A.C. Oxidative Stress Induces Alkaloid Production in Uncaria tomentosa Root and Cell Cultures in Bioreactors. Eng. Life Sci. 2009, 9, 211–218. [Google Scholar] [CrossRef]
- Abbasi, B.H.; Liu, R.; Saxena, P.K.; Liu, C.-Z. Cichoric Acid Production from Hairy Root Cultures of Echinacea purpurea Grown in a Modified Airlift Bioreactor. J. Chem. Technol. Biotechnol. 2009, 84, 1697–1701. [Google Scholar] [CrossRef]
- Caspeta, L.; Nieto, I.; Zamilpa, A.; Alvarez, L.; Quintero, R.; Villarreal, M.L. Solanum chrysotrichum Hairy Root Cultures: Characterization, Scale-Up and Production of Five Antifungal Saponins for Human Use. Planta Med. 2005, 71, 1084–1087. [Google Scholar] [CrossRef]
- Du, M.; Wu, X.J.; Ding, J.; Hu, Z.B.; White, K.N.; Branford-White, C.J. Astragaloside IV and Polysaccharide Production by Hairy Roots of Astragalus membranaceus in Bioreactors. Biotechnol. Lett. 2003, 25, 1853–1856. [Google Scholar] [CrossRef]
- Shin, K.S.; Murthy, H.N.; Ko, J.Y.; Paek, K.Y. Growth and betacyanin production by hairy roots of Beta vulgaris in airlift bioreactors. Biotechnol. Lett. 2003, 24, 2067–2069. [Google Scholar] [CrossRef]
- Sivakumar, G.; Yu, K.W.; Hahn, E.J.; Paek, K.Y. Optimization of Organic Nutrients for Ginseng Hairy Roots Production in Large-Scale Bioreactors. Curr. Sci. 2005, 89, 641–649. [Google Scholar]
- Malabadi, R. Alkaloid Biosynthesis Influenced by Agrobacterium rhizogenes Mediated-Transformation and Bioreactor in Clitoria ternatea (Linn.). Plant Cell Biotechnol. Mol. Biol. 2003, 4, 169–178. [Google Scholar]
- Caspeta, L.; Quintero, R.; Villarreal, M.L. Novel Airlift Reactor Fitting for Hairy Root Cultures: Developmental and Performance Studies. Biotechnol. Prog. 2005, 21, 735–740. [Google Scholar] [CrossRef]
- Kintzios, S.; Makri, O.; Pistola, E.; Matakiadis, T.; Shi, H.P.; Economou, A. Scale-up Production of Puerarin from Hairy Roots of Pueraria phaseoloides in an Airlift Bioreactor. Biotechnol. Lett. 2004, 26, 1057–1059. [Google Scholar] [CrossRef] [PubMed]
- López, E.G.; Ramírez, E.G.R.; Gúzman, O.G.; Calva, G.C.; Ariza-Castolo, A.; Pérez-Vargas, J.; Rodríguez, H.G.M. MALDI-TOF Characterization of hGH1 Produced by Hairy Root Cultures of Brassica oleracea Var. Italica Grown in an Airlift with Mesh Bioreactor. Biotechnol. Prog. 2014, 30, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Peraza-Luna, F.; Rodríguez-Mendiola, M.; Arias-Castro, C.; Bessiere, J.M.; Calva-Calva, G. Sotolone Production by Hairy Root Cultures of Trigonella foenum-graecum in Airlift with Mesh Bioreactors. J. Agric. Food Chem. 2001, 49, 6012–6019. [Google Scholar] [CrossRef] [PubMed]
- Suresh, B.; Bais, H.P.; Raghavarao, K.S.M.S.; Ravishankar, G.A.; Ghildyal, N.P. Comparative Evaluation of Bioreactor Design Using Tagetes patula L. Hairy Roots as a Model System. Process Biochem. 2005, 40, 1509–1515. [Google Scholar] [CrossRef]
- Liu, C.; Towler, M.J.; Medrano, G.; Cramer, C.L.; Weathers, P.J. Production of Mouse Interleukin-12 Is Greater in Tobacco Hairy Roots Grown in a Mist Reactor than in an Airlift Reactor. Biotechnol. Bioeng. 2009, 102, 1074–1086. [Google Scholar] [CrossRef]
- Mišić, D. Secoiridoid Glycosides Production by Centaurium maritimum (L.) Fritch Hairy Root Cultures in Temporary Immersion Bioreactor. Process Biochem. 2013, 48, 1587–1591. [Google Scholar] [CrossRef]
- Vinterhalter, B.; Banjac, N.; Vinterhalter, D.; Krstić-Milošević, D. Xanthones Production in Gentiana Dinarica Beck Hairy Root Cultures Grown in Simple Bioreactors. Plants 2021, 10, 1610. [Google Scholar] [CrossRef]
- Kozłowska, W.; Piątczak, E.; Kolniak-Ostek, J.; Kochan, E.; Pencakowski, B.; Stafiniak, M.; Bielecka, M.; Płachno, B.J.; Strzemski, M.; Matkowski, A.; et al. Upscaling Biomass Production of Rosmarinic Acid-Rich Hairy Root Cultures of Agastache rugosa (Fisch. & C.A.Mey.) Kuntze. Plant Cell Tissue Organ Cult. PCTOC 2024, 156, 41. [Google Scholar] [CrossRef]
- Bartczak, M.; Wierzchowski, K.; Pilarek, M. Mixing Performance in a Litre-Scale Rocking Disposable Bioreactor: DoE-Based Investigation of Mixing Time Dependence on Operational Parameters. Chem. Eng. J. 2022, 431, 133288. [Google Scholar] [CrossRef]
- Eibl, R.; Werner, S.; Eibl, D. Disposable Bioreactors for Plant Liquid Cultures at Litre-scale. Eng. Life Sci. 2009, 9, 156–164. [Google Scholar] [CrossRef]
- Oosterhuis, N.M.G.; Hudson, T.; D’Avino, A.; Zijlstra, G.M.; Amanullah, A. Disposable Bioreactors. In Comprehensive Biotechnology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 361–373. [Google Scholar] [CrossRef]
- Palazón, J.; Mallol, A.; Eibl, R.; Lettenbauer, C.; Cusidó, R.M.; Piñol, M.T. Growth and Ginsenoside Production in Hairy Root Cultures of Panax ginseng Using a Novel Bioreactor. Planta Med. 2003, 69, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Wierzchowski, K.; Kawka, M.; Sykłowska-Baranek, K.; Pilarek, M. Intensification of Rindera graeca Transgenic Roots Proliferation and Deoxyshikonin Secretion in Wave-Agitated Disposable Bioreactor. Chem. Eng. Process. Process Intensif. 2024, 203, 109905. [Google Scholar] [CrossRef]
- Sivakumar, G.; Liu, C.; Towler, M.J.; Weathers, P.J. Biomass Production of Hairy Roots of Artemisia annua and Arachis hypogaea in a Scaled-up Mist Bioreactor. Biotechnol. Bioeng. 2010, 107, 802–813. [Google Scholar] [CrossRef] [PubMed]
- San, K.; Chung, I.; Ahmad, A. Natural Products from Vinca. US20100048494A1. 2010. Available online: https://patents.google.com/patent/US20100048494A1/en?oq=US20100048494A1 (accessed on 26 August 2024).
- Asis, D.; Samir, B.; Nikas, P.; Jayanti, S.; Suman, D.; Anindita, B.; Jyoti, B. Process of Production of Anti-Diabetic Compound in Root Culture of Catharanthus Roseus. WO2010004584A2. 2010. Available online: https://patents.google.com/patent/WO2010004584A2/en?oq=WO2010004584A2 (accessed on 26 August 2024).
- Minamii, Y.; Miura, Y.; Inoue, H.; Matsui, C. Production of Betanin. JPH0411895A. 1992. Available online: https://patents.google.com/patent/JPH0411895A/en?oq=JPH0411895A (accessed on 26 August 2024).
- Gokare, R.; Bhamidi, S.; Rao, R. Process for Preparation of Dopa and Dopamine from Hairy Root Cultures of Beta vulgaris. WO2004055192A1. 2002. Available online: https://patents.google.com/patent/WO2004055192A1/en?oq=WO2004055192A1 (accessed on 26 August 2024).
- Goossens, A.; Moses, T.; Pollier, J.; Romero, J. Triterpenoid Sapogenin Production in Plant and Microbial Cultures. US9994883. 2018. Available online: https://patents.google.com/patent/US9994883B2/en?oq=US9994883 (accessed on 26 August 2024).
- Medina-Bolivar, L.; Dolan, M.; Bennett, S.; Condori, J.; Hubstenberger, J. Production of Stilbenes in Plant Hairy Root Cultures. US7666677. 2010. Available online: https://patents.google.com/patent/US7666677B2/en?oq=US7666677 (accessed on 26 August 2024).
- Chen, J.; Jin, M.; Zhang, B.; Ouyang, P.; Jin, L. Method for Continuously Obtaining Secondary Ginseng Hairy Root Metabolites Through Tissue Culture. CN104026019A. 2014. Available online: https://patents.google.com/patent/CN104026019A/en?oq=CN104026019A (accessed on 26 August 2024).
- Cardon, F.; Lemasson, C.; Guillet, M. Optimized Hairy Roots Bioreactor. WO2023187185A1. 2023. Available online: https://patents.google.com/patent/WO2023187185A1/en?oq=WO2023187185A1 (accessed on 26 August 2024).
- Thimmaraju, R.; Ravishankar, G.; Bhagyalakshmi, N.; Suresh, B.; Narayan, M. Process for Effluxing Betalaines from Beta vulgaris Hairy Root Cultures. WO2003080819A1. 2003. Available online: https://patents.google.com/patent/WO2003080819A1/en?oq=WO2003080819A1 (accessed on 26 August 2024).
- Demorset, Z.; Dudley, Q.; Mayers, J.; Kurtz, B.; Harrison, L.; Jordan, A.; Lapham, R. Producing Sesquiterpenes and Other Terpenes Using Plant-Based Biomasses. WO2024011263A2. 2024. Available online: https://patents.google.com/patent/WO2024011263A2/en?oq=WO2024011263A2 (accessed on 26 August 2024).
- Ye, Q.; Zhang, Z.; Liu, T.; Yu, Z.; Li, Z.; Sun, G.; Wang, Z. External Circulation Mist-Type Bioreactor for Large-Scale Cultivation of Plant Hairy Roots. CN102783414A. Available online: https://patents.google.com/patent/CN102783414A/en?oq=CN102783414A (accessed on 26 August 2024).
- Weathers, P.; Towler, M. Scalable Wall Bioreactor for Culture of Plant and Animal Tissues. US2009017529A1. 2009. Available online: https://patents.google.com/patent/US20090017529A1/en?oq=US2009017529A1 (accessed on 26 August 2024).
- Shiau, Y. Bioreactor for Growing Fungus, Plant Cell, Tissue, Organ, Hairy Roots and Plantlet. US7531350. 2009. Available online: https://patents.google.com/patent/US7531350B2/en?oq=US7531350 (accessed on 26 August 2024).
- Brillmann, M.; Krautwaschl, P.; Reichelt, W.; Herwig, C. Bioreactors for Root Organ Cultures. US12018242. 2024. Available online: https://patents.google.com/patent/US12018242B2/en?oq=US120182422 (accessed on 26 August 2024).
- Mibellebiochemistry. Available online: https://mibellebiochemistry.com/rootbiotectm-hw (accessed on 26 August 2024).
- Sunumbra. Available online: https://www.sunumbra.com/basilicum-hairy-root.html (accessed on 26 August 2024).
- Vitalabactive. Available online: https://vitalabactive.com/project/radiant-skin/ (accessed on 26 August 2024).
- Gutierrez-Valdes, N.; Häkkinen, S.T.; Lemasson, C.; de Groot, J.; Ele-Ekouna, J.-P.; Guillet, M.; Cardon, F.; Ritala, A. Improving Yield of a Recombinant Biologic in a Brassica Hairy Root Manufacturing Process. Biotechnol. Bioeng. 2022, 119, 2831–2841. [Google Scholar] [CrossRef]
- Cardon, F.; Pallisse, R.; Bardor, M.; Caron, A.; Vanier, J.; Ele Ekouna, J.P.; Lerouge, P.; Boitel-Conti, M.; Guillet, M. Brassica rapa Hairy Root Based Expression System Leads to the Production of Highly Homogenous and Reproducible Profiles of Recombinant Human Alpha-L-Iduronidase. Plant Biotechnol. J. 2019, 17, 505–516. [Google Scholar] [CrossRef]
- Samabriva. Available online: https://samabriva.com/the-process/ (accessed on 26 August 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).