The Role of Glutamate Metabolism and the GABA Shunt in Bypassing the Tricarboxylic Acid Cycle in the Light
Abstract
:1. Introduction
| Reception of light quanta by phytochrome chromoproteins → conformational transformations of phytochrome → opening/closing of ion channels including Ca2+ channels → translocation of chromoproteins into the nucleus → interaction of chromoproteins with transcription factors with their subsequent interaction with genes → activation/inhibition of gene transcription → biological effect |
2. Results
2.1. Glutamate Dehydrogenase Activity
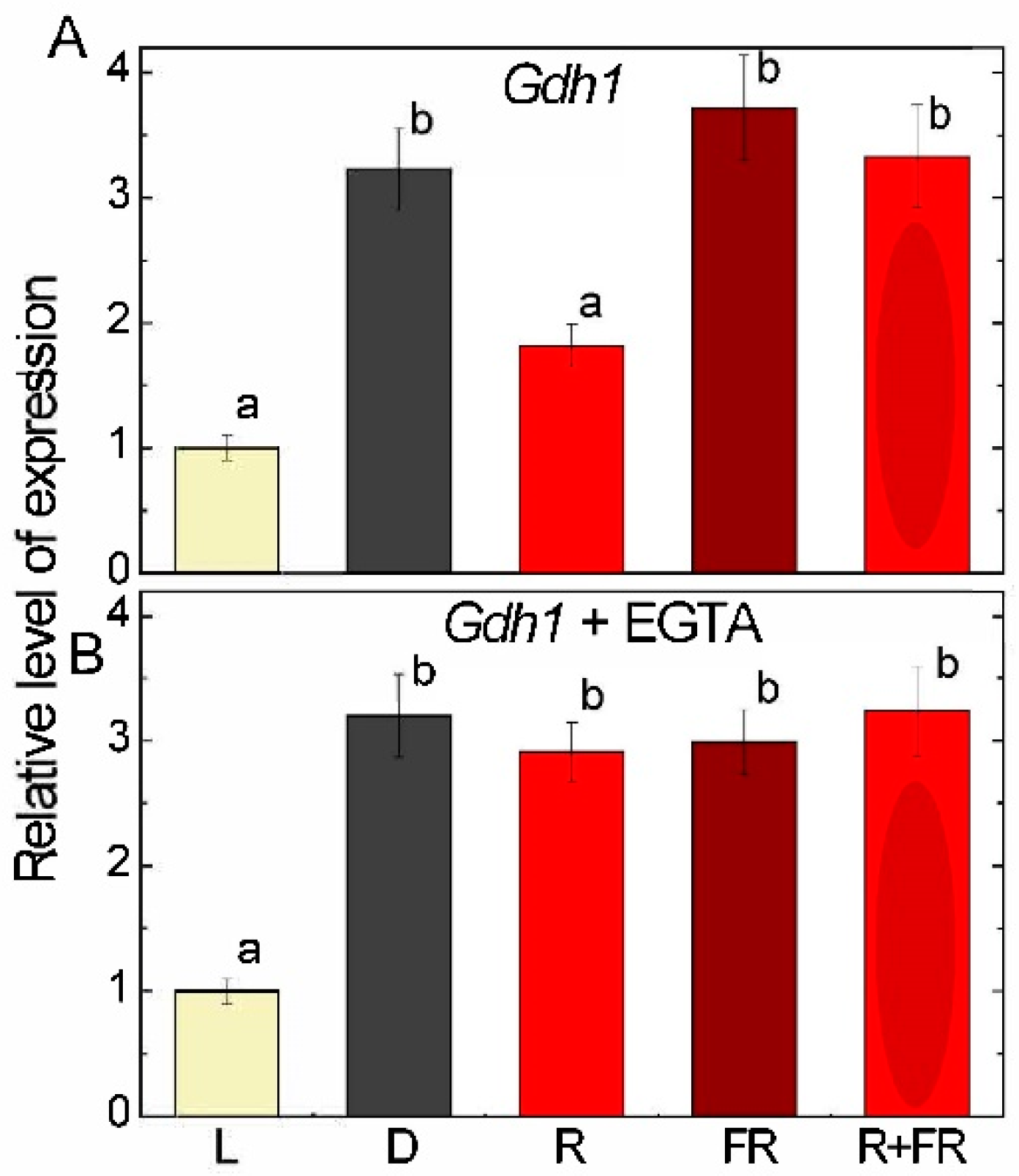
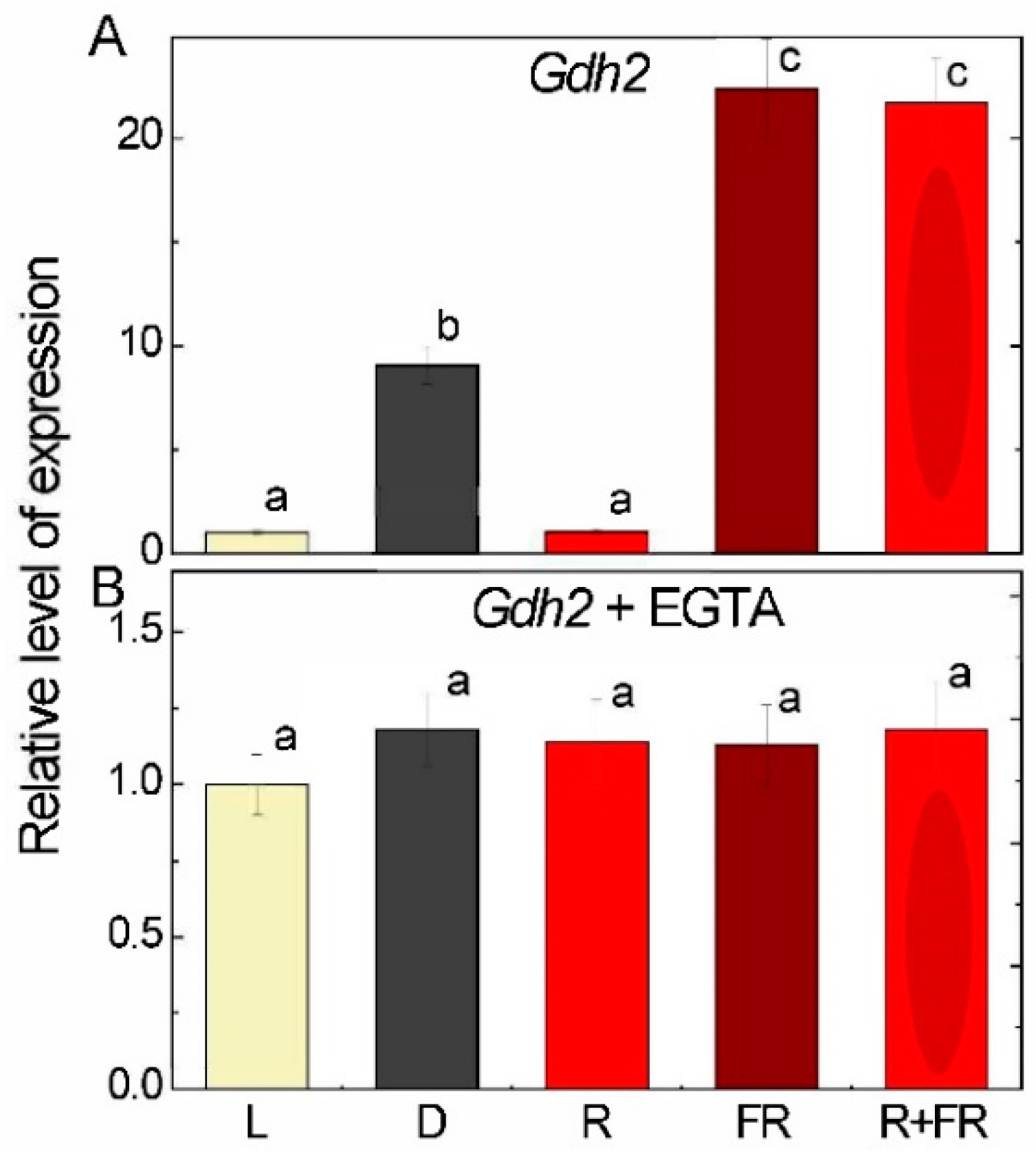
2.2. Glutamate Dehydrogenase Expression
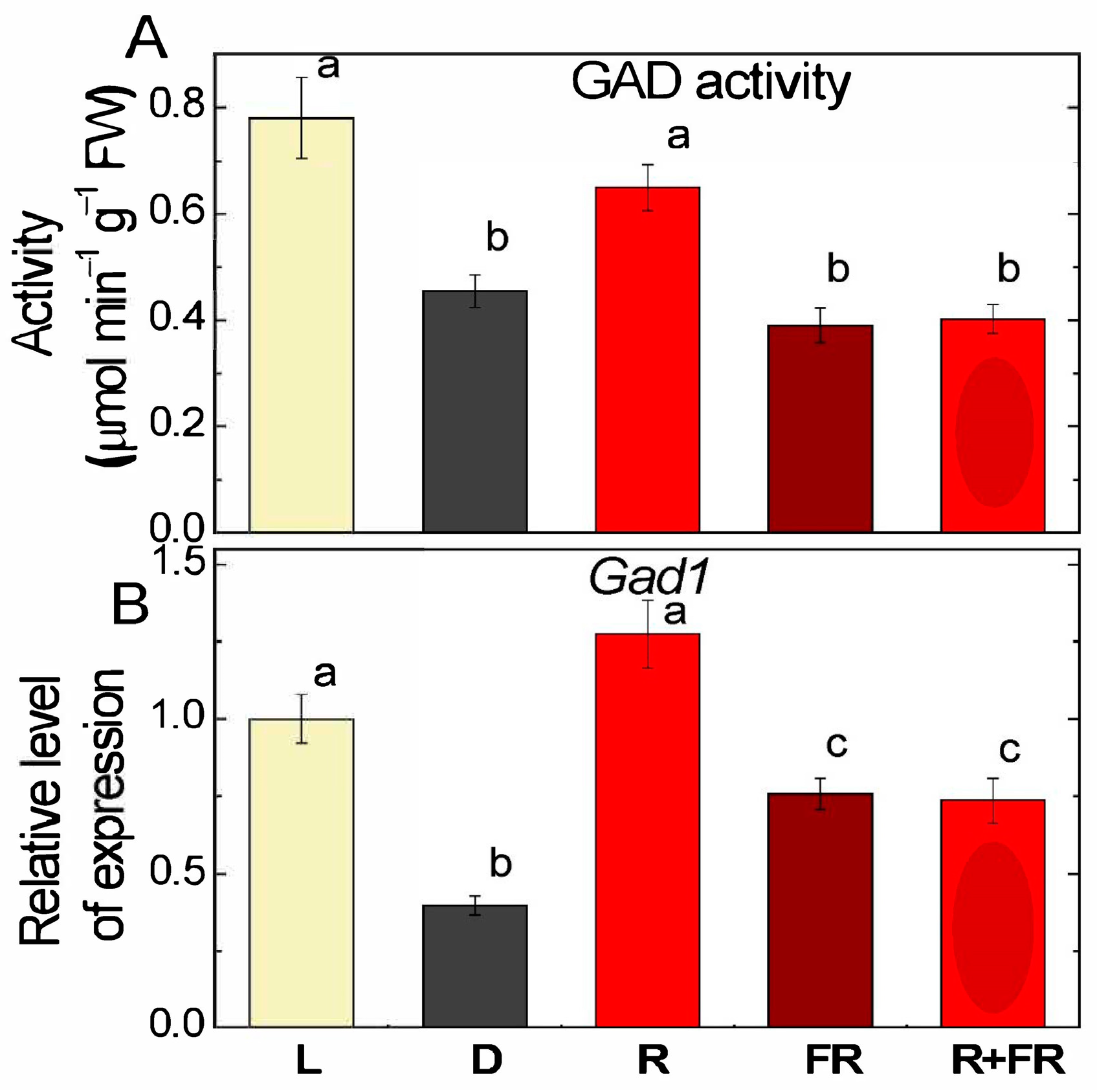
2.3. Glutamate Decarboxylase Activity and Expression
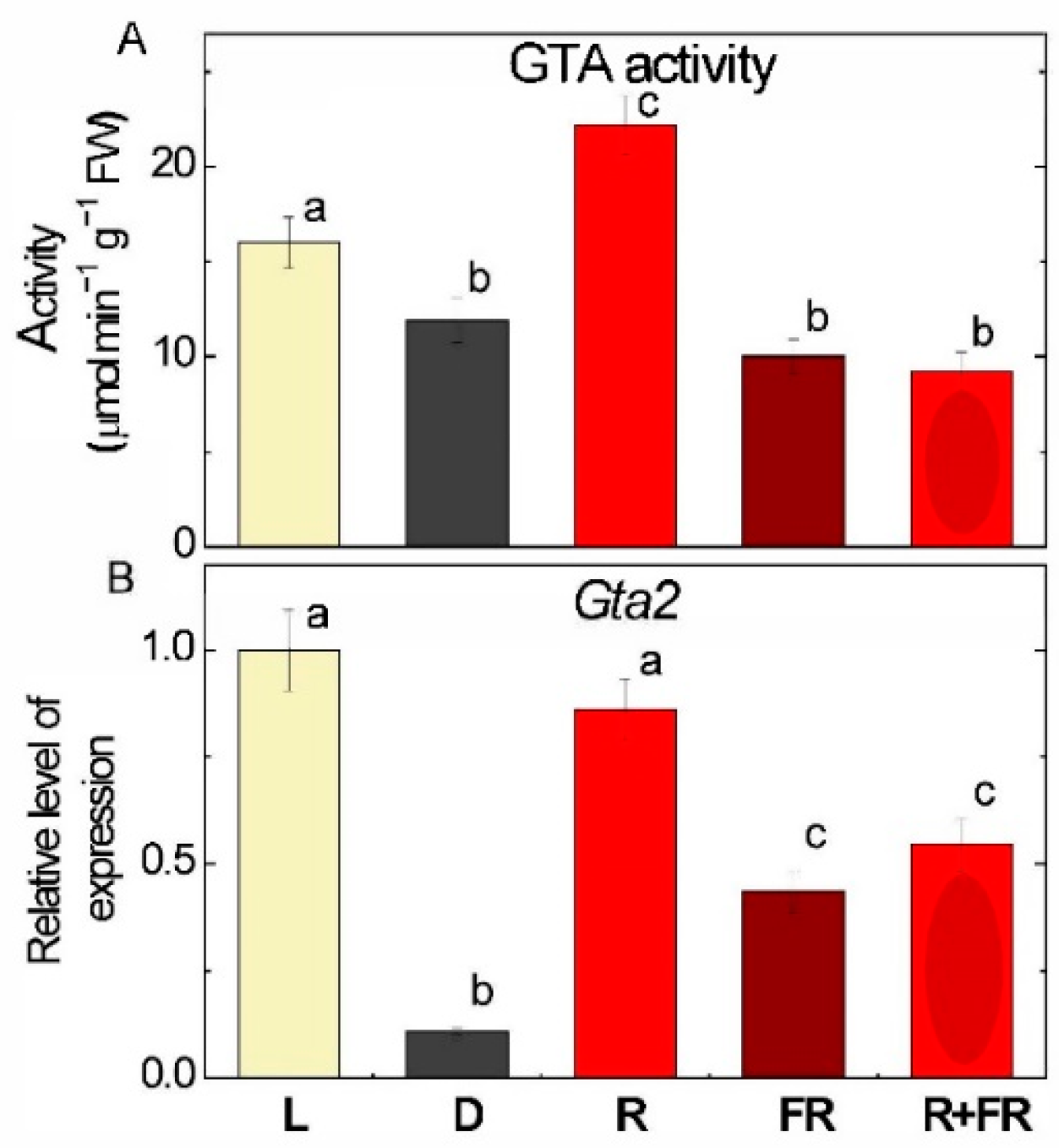
2.4. GABA Transaminase Activity and Expression
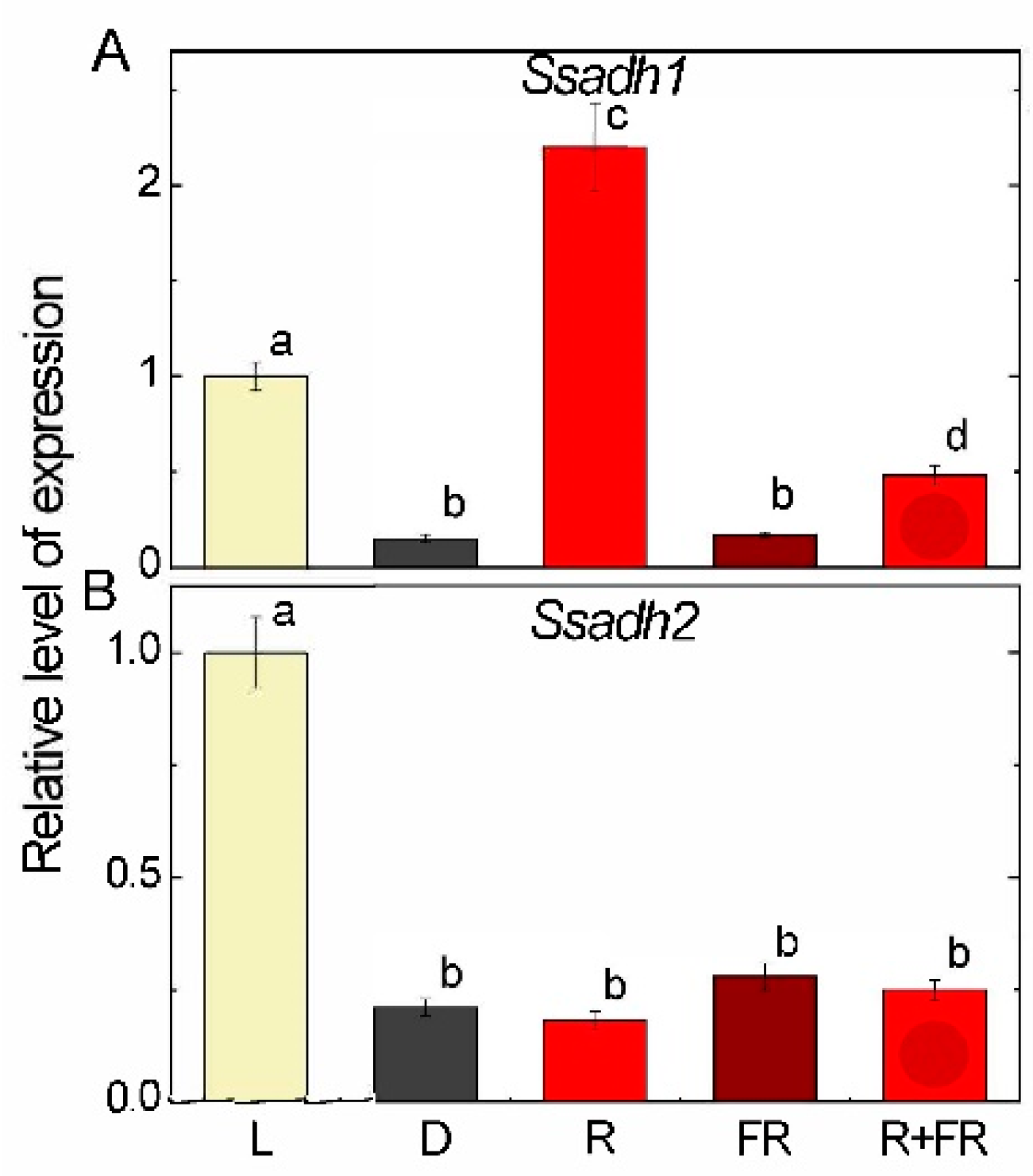
2.5. Succinic Semialdehyde Dehydrogenase Expression
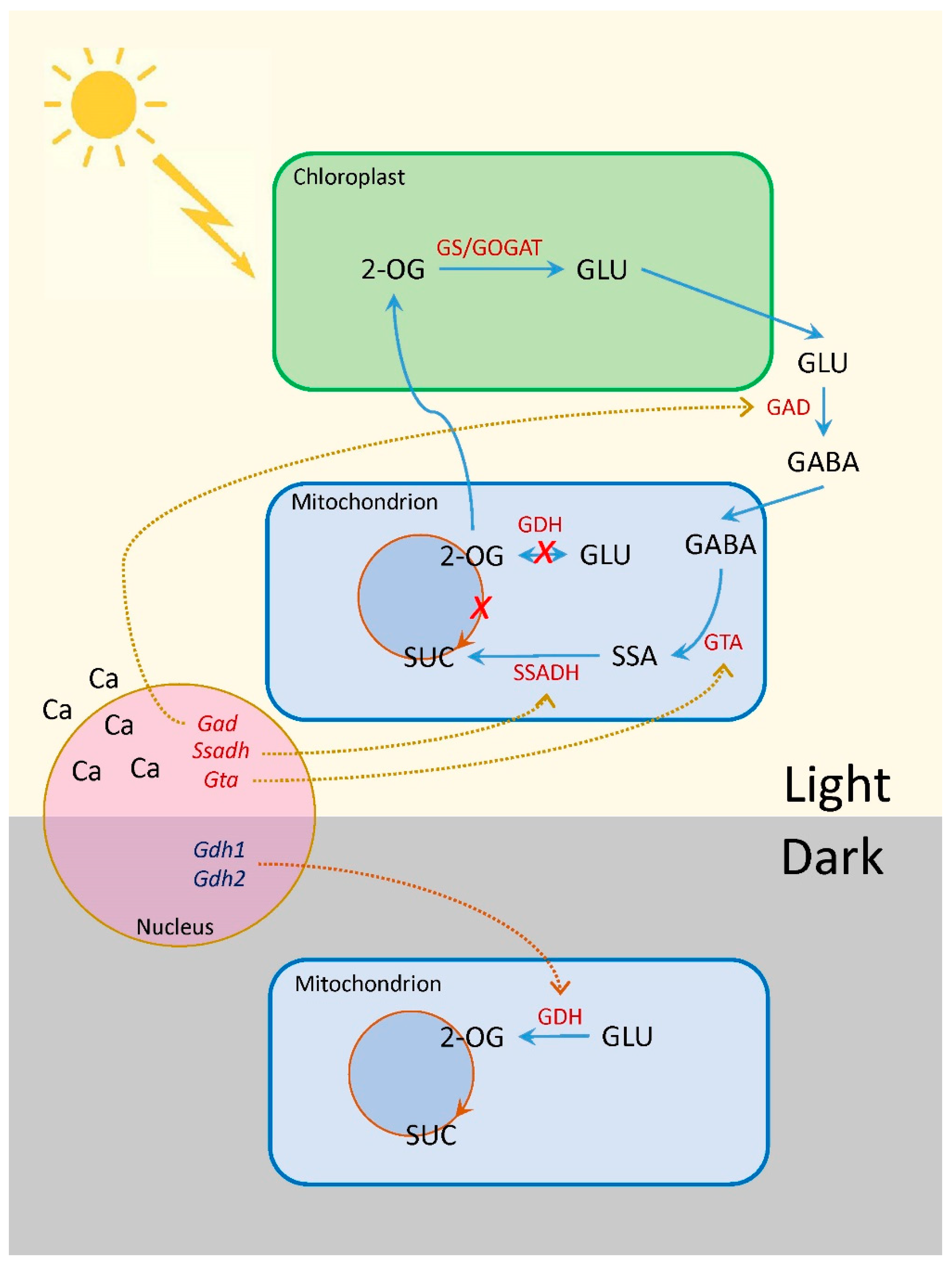
3. Discussion
4. Materials and Methods
4.1. Object of Investigation
4.2. Irradiation by Light of Different Wavelengths
4.3. Treatment with EGTA and Ruthenium Red
4.4. Isolation and Assay of Glutamate Dehydrogenase
4.5. Isolation and Assay of Glutamate Decarboxylase
4.6. Isolation and Assay of GABA Transaminase
4.7. RNA Isolation and Reverse Transcription
4.8. Polymerase Chain Reaction
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GABA | γ-aminobutyric acid |
| GAD | glutamate decarboxylase |
| GDH | glutamate dehydrogenase |
| GTA | GABA transaminase |
| 2-OG | 2-oxoglutarate |
| 2-OGDH | 2-oxoglutarate dehydrogenase |
| SSA | succinic semialdehyde |
| SSADH | succinic semialdehyde dehydrogenase |
| TCA cycle | tricarboxylic acid cycle |
References
- Kami, C.; Lorrain, S.; Hornitschek, P.; Fankhauser, C. Light-regulated plant growth and development. Curr. Top. Dev. Biol. 2010, 91, 29–66. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yao, J. Chloroplasts at the Crossroad of Photosynthesis, Pathogen Infection and Plant Defense. Int. J. Mol. Sci. 2018, 19, 3900. [Google Scholar] [CrossRef]
- Creux, N.; Harmer, S. Circadian rhythms in plants. Cold Spring Harb. Perspect. Biol. 2019, 11, a034611. [Google Scholar] [CrossRef]
- Voskresenskaya, N.P. Blue Light and Carbon Metabolism. Annu. Rev. Plant Physiol. 1972, 23, 219–234. [Google Scholar] [CrossRef]
- Voskresenskaya, N.P. Effect of Light Quality on Carbon Metabolism. In Photosynthesis II, Encyclopedia of Plant Physiology; Gibbs, M., Latzko, E., Eds.; Springer: Berlin/Heidelberg, Germany, 1979; Volume 6. [Google Scholar] [CrossRef]
- Iqbal, Z.; Iqbal, M.S.; Hashem, A.; Abd Allah, E.F.; Ansari, M.I. Plant defense responses to biotic stress and its interplay with fluctuating dark/light conditions. Front. Plant Sci. 2021, 12, 631810. [Google Scholar] [CrossRef]
- Franklin, K.A.; Lee, S.H.; Patel, D.; Kumar, S.V.; Spartz, A.K.; Gu, C.; Ye, S.; Yu, P.; Breen, G.; Cohen, J.D.; et al. Phytochrome-interacting factor 4 (PIF4) regulates auxin biosynthesis at high temperature. Proc. Natl. Acad. Sci. USA 2011, 108, 20231–20235. [Google Scholar] [CrossRef] [PubMed]
- Franklin, K.A.; Praekelt, U.; Stoddart, W.M.; Billingham, O.E.; Halliday, K.J.; Whitelam, G.C. Phytochromes B, D, and E act redundantly to control multiple physiological responses in Arabidopsis. Plant Physiol. 2001, 131, 1340–1346. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, G.; Wang, H.; Deng, X.W. Phytochrome signaling mechanisms. Arab. Book 2011, 9, e0148. [Google Scholar] [CrossRef]
- Van Buskirk, E.K.; Decker, P.V.; Chen, M. Photobodies in light signaling. Plant Physiol. 2012, 158, 52–60. [Google Scholar] [CrossRef]
- Klose, C.; Viczián, A.; Kircher, S.; Schäfer, E.; Nagy, F. Molecular mechanisms for mediating light-dependent nucleo/cytoplasmic partitioning of phytochrome photoreceptors. New Phytol. 2015, 206, 965–971. [Google Scholar] [CrossRef]
- Zheng, P.F.; Wang, X.; Yang, Y.Y.; You, C.X.; Zhang, Z.L.; Hao, Y.J. Identification of Phytochrome-Interacting Factor Family Members and Functional Analysis of MdPIF4 in Malus domestica. Int. J. Mol. Sci. 2020, 21, 7350. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Ren, X.; Qian, J.; Li, Q.; Tao, H.; Chen, J. The phytochrome-interacting family of transcription factors in maize (Zea mays L.): Identification, evolution, and expression analysis. Acta Physiol. Plant 2019, 41, 8. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, M.; Zhang, M.; Jiang, W.; Ren, X.; Liang, E.; Zhang, D.; Zhang, C.; Xiao, N.; Li, Y.; et al. A maize phytochrome-interacting factors protein ZmPIF1 enhances drought tolerance by inducing stomatal closure and improves grain yield in Oryza sativa. Plant Biotechnol. J. 2018, 16, 1375–1387. [Google Scholar] [CrossRef] [PubMed]
- Volotovski, I.D.; Sokolovsky, S.G.; Molchan, O.V.; Knight, M.R. Second messengers mediate increases in cytosolic calcium in tobacco protoplasts. Plant Physiol. 1998, 117, 1023–1030. [Google Scholar] [CrossRef]
- Kim, J.I.; Park, J.E.; Zarate, X.; Song, P.S. Phytochrome phosphorylation in plant light signaling. Photochem. Photobiol. Sci. 2005, 4, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Volotovski, I.D. Role of calcium ions in photosignaling processes in a plant cell. Biophysics 2011, 56, 778–788. [Google Scholar] [CrossRef]
- Bossen, M.E.; Dassen, H.H.; Kendrick, R.E.; Vredenberg, W.J. The role of calcium ions in phytochrome-controlled swelling of etiolated wheat (Triticum aestivum L.) protoplasts. Planta 1988, 174, 94–100. [Google Scholar] [CrossRef]
- Volotovski, I.D. Ca2+ and intracellular signalling in plant cells: A role in phytochrome transduction. Memb. Cell Biol. 1998, 12, 721–742. [Google Scholar]
- Dubovskaya, L.V.; Molchan, O.V.; Volotovsky, I.D. Photoregulation of the endogenous cGMP content in oat seedlings. Russ. J. Plant Physiol. 2001, 48, 19–22. [Google Scholar] [CrossRef]
- Ikura, M.; Ames, J.B. Genetic polymorphism and protein conformational plasticity in the calmodulin superfamily: Two ways to promote multifunctionality. Proc. Natl. Acad. Sci. USA 2006, 103, 1159–1164. [Google Scholar] [CrossRef]
- Reddy, A.S. Calcium: Silver bullet in signaling. Plant Sci. 2001, 160, 381–404. [Google Scholar] [CrossRef]
- Snedden, W.A.; Fromm, H. Calmodulin as a versatile calcium signal transducer in plants. New Phytol. 2001, 151, 35–66. [Google Scholar] [CrossRef]
- Luan, S.; Kudla, J.; Rodriguez-Concepcion, M.; Yalovsky, S.; Gruissem, W. Calmodulins and calcineurin B-like proteins: Calcium sensors for specific signal response coupling in plants. Plant Cell 2002, 14, S389–S400. [Google Scholar] [CrossRef]
- Eprintsev, A.T.; Fedorin, D.N.; Sazonova, O.V.; Igamberdiev, A.U. Light inhibition of fumarase in Arabidopsis leaves is phytochrome A-dependent and mediated by calcium. Plant Physiol. Biochem. 2016, 102, 161–166. [Google Scholar] [CrossRef]
- Astegno, A.; Capitani, G.; Dominici, P. Functional roles of the hexamer organization of plant glutamate decarboxylase. Biochim. Biophys. Acta 2015, 1854, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Snedden, W.A.; Arazi, T.; Fromm, H.; Shelp, B.J. Calcium/Calmodulin Activation of Soybean Glutamate Decarboxylase. Plant Physiol. 1995, 108, 543–549. [Google Scholar] [CrossRef]
- Snedden, W.A. Regulation of the γ-aminobutyrate-synthesizing enzyme, glutamate decarboxylase, by calcium-calmodulin: A mechanism for rapid activation in response to stress. In Plant Responses to Environmental Stresses; Lerner, H.R., Ed.; Routledge: London, UK, 2018; pp. 549–574. [Google Scholar] [CrossRef]
- Busch, K.B.; Fromm, H. Plant succinic semialdehyde dehydrogenase. Cloning, purification, localization in mitochondria, and regulation by adenine nucleotides. Plant Physiol. 1999, 121, 589–597. [Google Scholar] [CrossRef]
- Eprintsev, A.T.; Selivanova, N.V.; Fedorin, D.N.; Bashkin, S.S.; Selezneva, E.A.; Dadakina, I.V.; Makhmud Ali, S. The role of calcium cations in the mechanism of phytochrome-dependent regulation of the sdh1-2 gene expression and succinate dehydrogenase activity in maize leaves. Biochem. Mosc. 2012, 6, 310–313. [Google Scholar] [CrossRef]
- Tcherkez, G.; Mahé, A.; Gauthier, P.; Mauve, C.; Gout, E.; Bligny, R.; Cornic, G.; Hodges, M. In folio respiratory fluxomics revealed by 13C isotopic labeling and H/D isotope effects highlight the noncyclic nature of the tricarboxylic acid “cycle” in illuminated leaves. Plant Physiol. 2009, 151, 620–630. [Google Scholar] [CrossRef]
- Tcherkez, G.; Boex-Fontvieille, E.; Mahé, A.; Hodges, M. Respiratory carbon fluxes in leaves. Curr. Opin. Plant Biol. 2012, 15, 308–314. [Google Scholar] [CrossRef]
- Igamberdiev, A.U.; Eprintsev, A.T.; Fedorin, D.N.; Popov, V.N. Phytochrome-mediated regulation of plant respiration and photorespiration. Plant Cell Environ. 2014, 37, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Igamberdiev, A.U.; Bykova, N.V. Mitochondria in photosynthetic cells: Coordinating redox control and energy balance. Plant Physiol. 2023, 191, 2104–2119. [Google Scholar] [CrossRef] [PubMed]
- Eprintsev, A.T.; Fedorin, D.N.; Igamberdiev, A.U. Ca2+ is involved in phytochrome A-dependent regulation of the succinate dehydrogenase gene sdh1-2 in Arabidopsis. J. Plant Physiol. 2013, 170, 1349–1352. [Google Scholar] [CrossRef]
- Eprintsev, A.T.; Fedorin, D.N.; Dobychina, M.A.; Igamberdiev, A.U. Regulation of expression of the mitochondrial and peroxisomal forms of citrate synthase in maize during germination and in response to light. Plant Sci. 2018, 272, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Eprintsev, A.T.; Fedorin, D.N.; Cherkasskikh, M.V.; Igamberdiev, A.U. Expression of succinate dehydrogenase and fumarase genes in maize leaves is mediated by cryptochrome. J. Plant Physiol. 2018, 221, 81–84. [Google Scholar] [CrossRef]
- Eprintsev, A.T.; Fedorin, D.N.; Cherkasskikh, M.V.; Igamberdiev, A.U. Regulation of expression of the mitochondrial and cytosolic forms of aconitase in maize leaves via phytochrome. Plant Physiol. Biochem. 2020, 146, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Eprintsev, A.T.; Fedorin, D.N.; Anokhina, G.B.; Sedykh, A.V. Molecular and Biochemical Aspects of Light Regulation of 2-Oxoglutarate Dehydrogenase in Plants. Russ. J. Plant Physiol. 2020, 67, 378–385. [Google Scholar] [CrossRef]
- Eprintsev, A.T.; Fedorin, D.N.; Igamberdiev, A.U. Light-Dependent Expression and Promoter Methylation of the Genes Encoding Succinate Dehydrogenase, Fumarase, and NAD-Malate Dehydrogenase in Maize (Zea mays L.) Leaves. Int. J. Mol. Sci. 2023, 24, 10211. [Google Scholar] [CrossRef]
- Araújo, W.L.; Tohge, T.; Nunes-Nesi, A.; Daloso, D.M.; Nimick, M.; Krahnert, I.; Bunik, V.I.; Moorhead, G.B.; Fernie, A.R. Phosphonate analogs of 2-oxoglutarate perturb metabolism and gene expression in illuminated Arabidopsis leaves. Front. Plant Sci. 2012, 3, 114. [Google Scholar] [CrossRef]
- Quail, P.H.; Boylan, M.T.; Parks, B.M.; Short, T.W.; Xu, Y.; Wagner, D. Phytochromes: Photosensory perception and signal transduction. Science 1995, 268, 675–680. [Google Scholar] [CrossRef]
- Igamberdiev, A.U.; Kleczkowski, L.A. The Glycerate and Phosphorylated Pathways of Serine Synthesis in Plants: The Branches of Plant Glycolysis Linking Carbon and Nitrogen Metabolism. Front. Plant Sci. 2018, 9, 318. [Google Scholar] [CrossRef]
- Kleczkowski, L.A.; Igamberdiev, A.U. Multiple Roles of Glycerate Kinase-From Photorespiration to Gluconeogenesis, C4 Metabolism, and Plant Immunity. Int. J. Mol. Sci. 2024, 25, 3258. [Google Scholar] [CrossRef] [PubMed]
- Sweetlove, L.J.; Beard, K.F.; Nunes-Nesi, A.; Fernie, A.R.; Ratcliffe, R.G. Not just a circle: Flux modes in the plant TCA cycle. Trends Plant Sci. 2010, 15, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Araújo, W.L.; Nunes-Nesi, A.; Trenkamp, S.; Bunik, V.I.; Fernie, A.R. Inhibition of 2-oxoglutarate dehydrogenase in potato tuber suggests the enzyme is limiting for respiration and confirms its importance in nitrogen assimilation. Plant Physiol. 2008, 148, 1782–1796. [Google Scholar] [CrossRef]
- Hodges, M. Enzyme redundancy and the importance of 2-oxoglutarate in plant ammonium assimilation. J. Exp. Bot. 2002, 53, 905–916. [Google Scholar] [CrossRef]
- Lea, P.J.; Miflin, B.J. Glutamate synthase and the synthesis of glutamate in plants. Plant Physiol. Biochem. 2003, 41, 555–564. [Google Scholar] [CrossRef]
- Hoekenga, O.A.; Maron, L.G.; Piñeros, M.A.; Cançado, G.M.; Shaff, J.; Kobayashi, Y.; Ryan, P.R.; Dong, B.; Delhaize, E.; Sasaki, T.; et al. AtALMT1, which encodes a malate transporter, is identified as one of several genes critical for aluminum tolerance in Arabidopsis. Proc. Natl. Acad. Sci. USA 2006, 103, 9738–9743. [Google Scholar] [CrossRef]
- Yu, B.; Wu, Q.; Li, X.; Zeng, R.; Min, Q.; Huang, J. GLUTAMATE RECEPTOR-like gene OsGLR3.4 is required for plant growth and systemic wound signaling in rice (Oryza sativa). New Phytol. 2022, 233, 1238–1256. [Google Scholar] [CrossRef]
- Woodrow, P.; Ciarmiello, L.F.; Annunziata, M.G.; Pacifico, S.; Iannuzzi, F.; Mirto, A.; D’Amelia, L.; Dell’Aversana, E.; Piccolella, S.; Fuggi, A.; et al. Durum wheat seedling responses to simultaneous high light and salinity involve a fine reconfiguration of amino acids and carbohydrate metabolism. Physiol. Plant. 2017, 159, 290–312. [Google Scholar] [CrossRef]
- Ji, J.; Shi, Z.; Xie, T.; Zhang, X.; Chen, W.; Du, C.; Sun, J.; Yue, J.; Zhao, X.; Jiang, Z.; et al. Responses of GABA shunt coupled with carbon and nitrogen metabolism in poplar under NaCl and CdCl2 stresses. Ecotoxicol. Environ. Saf. 2020, 193, 110322. [Google Scholar] [CrossRef]
- Bouché, N.; Fait, A.; Bouchez, D.; Møller, S.G.; Fromm, H. Mitochondrial succinic-semialdehyde dehydrogenase of the gamma-aminobutyrate shunt is required to restrict levels of reactive oxygen intermediates in plants. Proc. Natl. Acad. Sci. USA 2003, 100, 6843–6848. [Google Scholar] [CrossRef] [PubMed]
- Esmaelpour, S.; Iranbakhsh, A.; Dilmaghani, K.; Marandi, S.J.; Oraghi Ardebili, Z. The potential contribution of the WRKY53 transcription factor, gamma-aminobutyric acid (GABA) transaminase, and histone deacetylase in regulating growth, organogenesis, photosynthesis, and transcriptional responses of tomato to different light-emitting diodes (LEDs). J. Photochem. Photobiol. B 2022, 229, 112413. [Google Scholar] [CrossRef]
- Gilliham, M.; Tyerman, S.D. Linking Metabolism to Membrane Signaling: The GABA-Malate Connection. Trends Plant Sci. 2016, 21, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Garnik, E.Y.; Vilyanen, D.V.; Vlasova, A.A.; Tarasenko, V.I.; Konstantinov, Y.M. Arabidopsis GDH1 and GDH2 genes double knock-out results in a stay-green phenotype during dark-induced senescence. Physiol. Mol. Biol. Plants 2024, 30, 1631–1642. [Google Scholar] [CrossRef]
- Garnik, E.Y.; Belkov, V.I.; Tarasenko, V.I.; Rudikovskii, A.V.; Konstantinov, Y.M. Expression of glutamate dehydrogenase genes in Arabidopsis thaliana depends on the redox state of plastoquinone pool. Plant Cell Tissue Organ Cult. 2021, 147, 107–116. [Google Scholar] [CrossRef]
- Miyashita, Y.; Good, A.G. NAD(H)-dependent glutamate dehydrogenase is essential for the survival of Arabidopsis thaliana during dark-induced carbon starvation. J. Exp. Bot. 2008, 59, 667–680. [Google Scholar] [CrossRef]
- Li, Y.; Lee, K.K.; Walsh, S.; Smith, C.; Hadingham, S.; Sorefan, K.; Cawley, G.; Bevan, M.W. Establishing glucose- and ABA-regulated transcription networks in Arabidopsis by microarray analysis and promoter classification using a Relevance Vector Machine. Genome Res. 2006, 16, 414–427. [Google Scholar] [CrossRef]
- Tcherkez, G.; Bligny, R.; Gout, E.; Mahé, A.; Hodges, M.; Cornic, G. Respiratory metabolism of illuminated leaves depends on CO2 and O2 conditions. Proc. Natl. Acad. Sci. USA 2008, 105, 797–802. [Google Scholar] [CrossRef]
- Yue, J.; Du, C.; Ji, J.; Xie, T.; Chen, W.; Chang, E.; Chen, L.; Jiang, Z.; Shi, S. Inhibition of α-ketoglutarate dehydrogenase activity affects adventitious root growth in poplar via changes in GABA shunt. Planta 2018, 248, 963–979. [Google Scholar] [CrossRef]
- Xie, T.; Ji, J.; Chen, W.; Yue, J.; Du, C.; Sun, J.; Chen, L.; Jiang, Z.; Shi, S. GABA negatively regulates adventitious root development in poplar. J. Exp. Bot. 2020, 71, 1459–1474. [Google Scholar] [CrossRef]
- Ludewig, F.; Hüser, A.; Fromm, H.; Beauclair, L.; Bouché, N. Mutants of GABA transaminase (POP2) suppress the severe phenotype of succinic semialdehyde dehydrogenase (ssadh) mutants in Arabidopsis. PLoS ONE 2008, 3, e3383. [Google Scholar] [CrossRef] [PubMed]
- Carillo, P.; Parisi, D.; Woodrow, P.; Pontecorvo, G.; Massaro, G.; Annunziata, M.G.; Fuggi, A.; Sulpice, R. Salt-induced accumulation of glycine betaine is inhibited by high light in durum wheat. Funct. Plant Biol. 2011, 38, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Gardeström, P.; Igamberdiev, A.U. The origin of cytosolic ATP in photosynthetic cells. Physiol. Plant. 2016, 157, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Craig, D.W.; Wedding, R.T. Regulation of the 2-oxoglutarate dehydrogenase lipoate succinyltransferase complex from cauliflower by nucleotide. Steady-state kinetic studies. J. Biol. Chem. 1980, 255, 5763–5768. [Google Scholar] [CrossRef]
- Dubois, F.; Tercé-Laforgue, T.; Gonzalez-Moro, M.-B.; Estavillo, J.-M.; Sangwan, R.; Gallais, A.; Hirel, B. Glutamate dehydrogenase in plants: Is there a new story for an old enzyme? Plant Physiol. Biochem. 2003, 41, 565–576. [Google Scholar] [CrossRef]
- Tercé-Laforgue, T.; Lothier, J.; Limami, A.M.; Rouster, J.; Lea, P.J.; Hirel, B. The Key Role of Glutamate Dehydrogenase 2 (GDH2) in the Control of Kernel Production in Maize (Zea mays L.). Plants 2023, 12, 2612. [Google Scholar] [CrossRef]
- Baum, G.; Lev-Yadun, S.; Fridmann, Y.; Arazi, T.; Katsnelson, H.; Zik, M.; Fromm, H. Calmodulin binding to glutamate decarboxylase is required for regulation of glutamate and GABA metabolism and normal development in plants. EMBO J. 1996, 15, 2988–2996. [Google Scholar] [CrossRef]
- Bouché, N.; Lacombe, B.; Fromm, H. GABA signaling: A conserved and ubiquitous mechanism. Trends Cell Biol. 2003, 13, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.I.; Lee, R.-H.; Chen, S.-C.G. A novel senescence-associated gene encoding γ-aminobutyric acid (GABA): Pyruvate transaminase is upregulated during rice leaf senescence. Physiol. Plant. 2005, 123, 1–8. [Google Scholar] [CrossRef]
- Michaeli, S.; Fait, A.; Lagor, K.; Nunes-Nesi, A.; Grillich, N.; Yellin, A.; Bar, D.; Khan, M.; Fernie, A.R.; Turano, F.J.; et al. A mitochondrial GABA permease connects the GABA shunt and the TCA cycle, and is essential for normal carbon metabolism. Plant J. 2011, 67, 485–498. [Google Scholar] [CrossRef]
- Mei, X.; Chen, Y.; Zhang, L.; Fu, X.; Wei, Q.; Grierson, D.; Zhou, Y.; Huang, Y.; Dong, F.; Yang, Z. Dual mechanisms regulating glutamate decarboxylases and accumulation of gamma-aminobutyric acid in tea (Camellia sinensis) leaves exposed to multiple stresses. Sci. Rep. 2016, 6, 23685. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.A.; Tyerman, S.D.; Gilliham, M.; Xu, B. γ-Aminobutyric acid (GABA) signalling in plants. Cell. Mol. Life Sci. 2017, 74, 1577–1603. [Google Scholar] [CrossRef] [PubMed]
- Carillo, P. GABA shunt in durum wheat. Front. Plant Sci. 2018, 9, 100. [Google Scholar] [CrossRef] [PubMed]
- Trewavas, A.; Knight, M. Mechanical signalling, calcium and plant form. Plant Mol. Biol. 1994, 26, 1329–1341. [Google Scholar] [CrossRef]
- Pathuri, I.P.; Reitberger, I.E.; Hückelhoven, R.; Proels, R.K. Alcohol dehydrogenase 1 of barley modulates susceptibility to the parasitic fungus Blumeria graminis f.sp. hordei. J. Exp. Bot. 2011, 62, 3449–3457. [Google Scholar] [CrossRef]
- Jelski, W.; Laniewska-Dunaj, M.; Orywal, K.; Kochanowicz, J.; Rutkowski, R.; Szmitkowski, M. The activity of alcohol dehydrogenase (ADH) isoenzymes and aldehyde dehydrogenase (ALDH) in the sera of patients with brain cancer. Neuroch. Res. 2014, 39, 2313–2318. [Google Scholar] [CrossRef]
- Eprintsev, A.T.; Fedorin, D.N.; Selivanova, N.V.; Wu, T.L.; Makhmud, A.S.; Popov, V.N. The role of promoter methylation in the regulation of genes encoding succinate dehydrogenase in maize seedlings. Russ. J. Plant Physiol. 2012, 59, 299–306. [Google Scholar] [CrossRef]
- Sarasketa, A.; González-Moro, M.B.; González-Murua, C.; Marino, D. Nitrogen source and external medium pH interaction differentially affects root and shoot metabolism in Arabidopsis. Front. Plant Sci. 2016, 7, 29. [Google Scholar] [CrossRef]
- Yu, K.; Hu, S.; Huang, J.; Mei, L.H. A high-throughput colorimetric assay to measure the activity of glutamate decarboxylase. Enzyme Microb. Technology 2011, 49, 272–276. [Google Scholar] [CrossRef]
- Eprintsev, A.T.; Anokhina, G.B.; Selivanova, P.S.; Moskvina, P.P.; Igamberdiev, A.U. Biochemical and Epigenetic Regulation of Glutamate Metabolism in Maize (Zea mays L.) Leaves under Salt Stress. Plants 2024, 13, 2651. [Google Scholar] [CrossRef]
- Award, R.; Levac, D.; Cybulska, P.; Merali, Z.; Trudeau, V.L.; Arnason, J.T. Effects of traditionally used anxiolytic botanicals on enzymes of the γ-aminobutyric acid (GABA) system. Can. J. Physiol. Pharmacol. 2007, 85, 933–942. [Google Scholar]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Matz, M.V. Amplification of representative cDNA samples from microscopic amounts of invertebrate tissue to search for new genes. Methods Mol. Biol. 2002, 183, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis; Pearson: Upper Saddle River, NJ, USA, 1999; ISBN 978-0130815422. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eprintsev, A.T.; Anokhina, G.B.; Shakhov, Z.N.; Moskvina, P.P.; Igamberdiev, A.U. The Role of Glutamate Metabolism and the GABA Shunt in Bypassing the Tricarboxylic Acid Cycle in the Light. Int. J. Mol. Sci. 2024, 25, 12711. https://doi.org/10.3390/ijms252312711
Eprintsev AT, Anokhina GB, Shakhov ZN, Moskvina PP, Igamberdiev AU. The Role of Glutamate Metabolism and the GABA Shunt in Bypassing the Tricarboxylic Acid Cycle in the Light. International Journal of Molecular Sciences. 2024; 25(23):12711. https://doi.org/10.3390/ijms252312711
Chicago/Turabian StyleEprintsev, Alexander T., Galina B. Anokhina, Zakhar N. Shakhov, Polina P. Moskvina, and Abir U. Igamberdiev. 2024. "The Role of Glutamate Metabolism and the GABA Shunt in Bypassing the Tricarboxylic Acid Cycle in the Light" International Journal of Molecular Sciences 25, no. 23: 12711. https://doi.org/10.3390/ijms252312711
APA StyleEprintsev, A. T., Anokhina, G. B., Shakhov, Z. N., Moskvina, P. P., & Igamberdiev, A. U. (2024). The Role of Glutamate Metabolism and the GABA Shunt in Bypassing the Tricarboxylic Acid Cycle in the Light. International Journal of Molecular Sciences, 25(23), 12711. https://doi.org/10.3390/ijms252312711






