Current and Potential Use of Biologically Active Compounds Derived from Cannabis sativa L. in the Treatment of Selected Diseases
Abstract
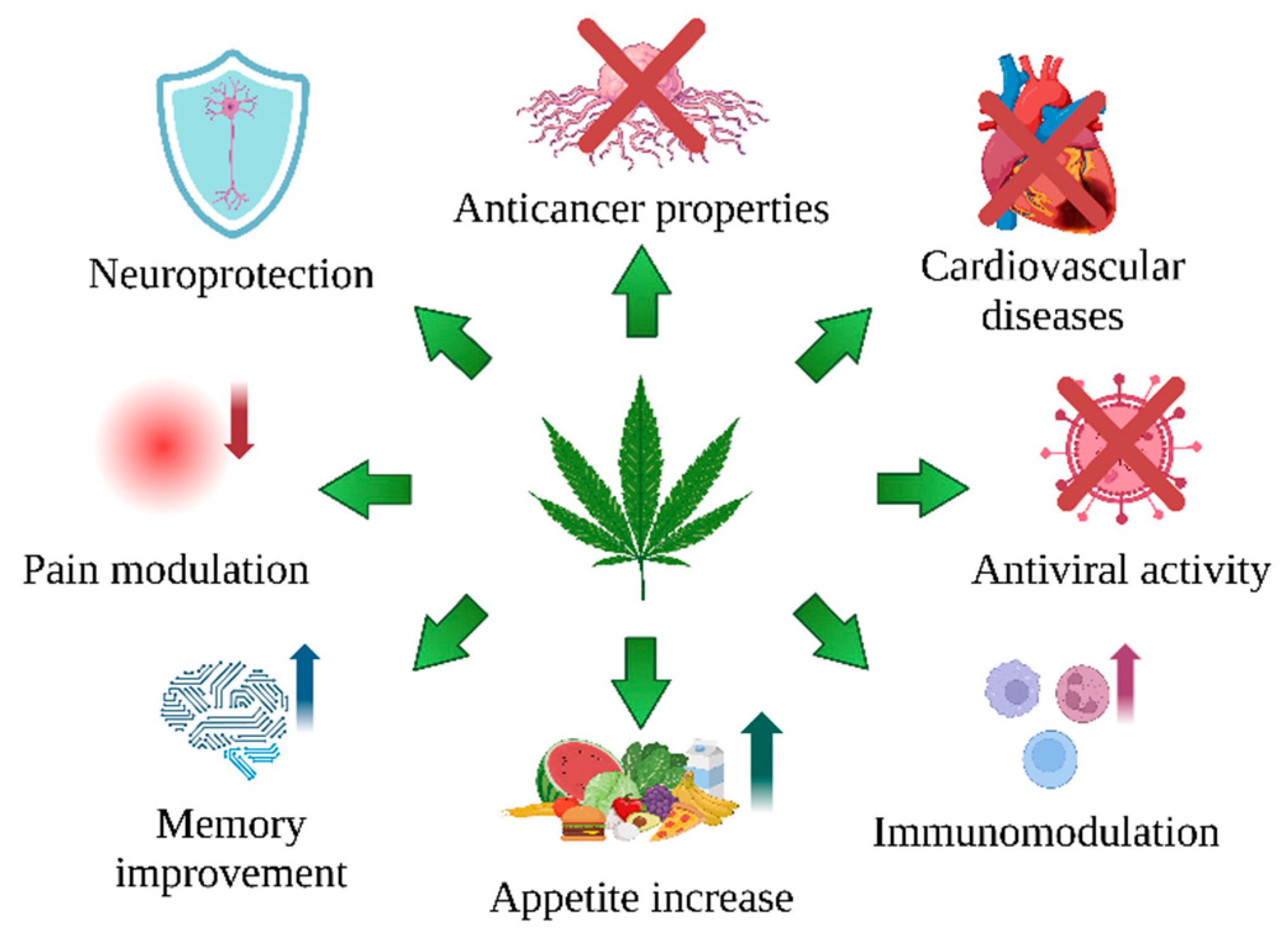
1. Introduction
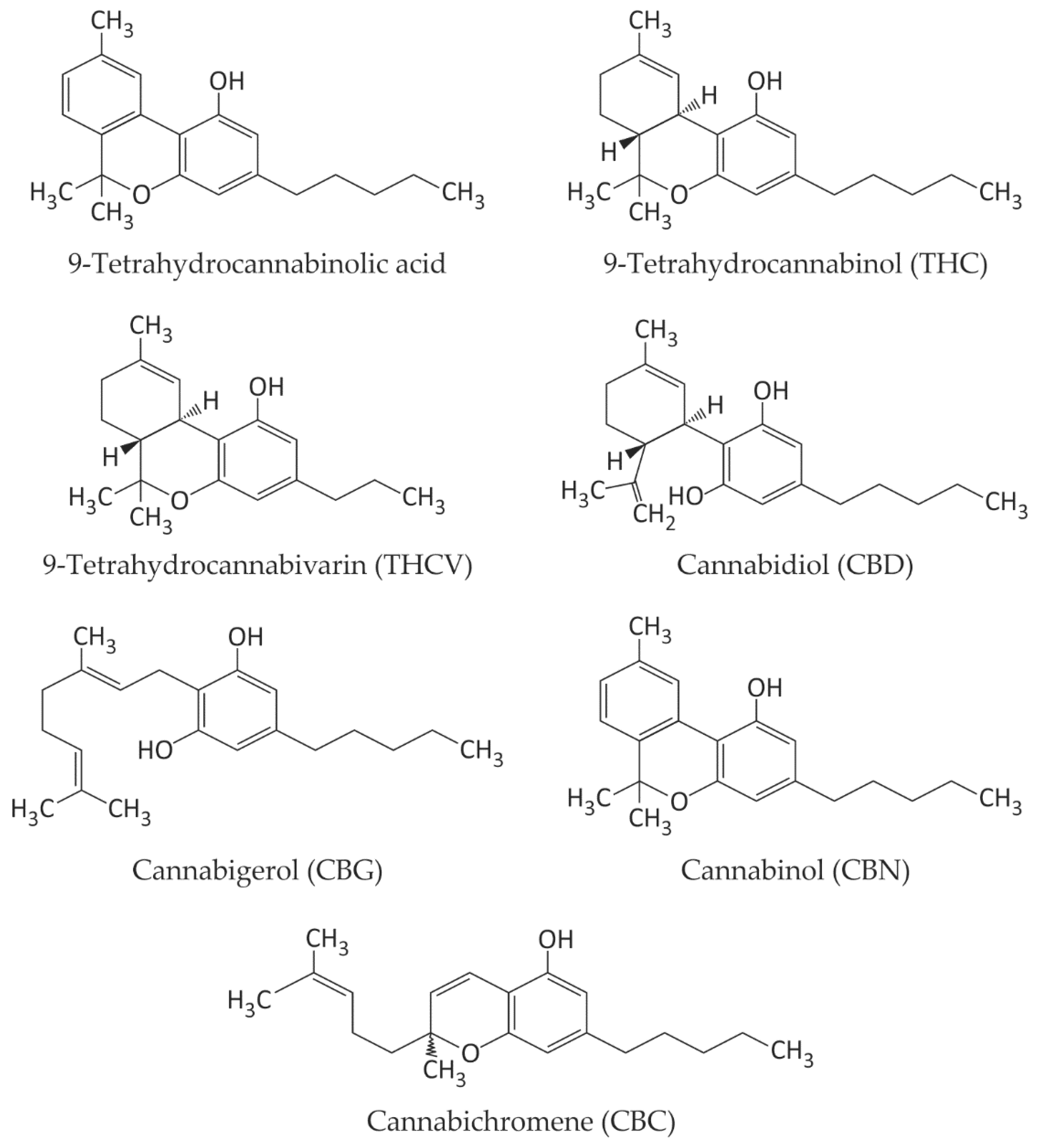
2. Biologically Active Compounds in Cannabis sativa L.
2.1. Cannabinoids—Major Natural Ligands of CB1 and CB2 Receptors, with Anti-Inflammatory Effects
2.2. Antioxidant Compounds
| Extract/Compounds | Tested System | Concentrations | References |
|---|---|---|---|
| C. sativa L. extracts and cannabinoids decrease free radicals’ level | |||
| C. sativa L. essential oil (β-caryophyllene, α-humulene, β-myrcene, α-pinene) | Chemical system | IC50 = 0.98 mg/mL for DPPH EC50 = 1.74 mg/mL for FRAP 0.101 mg AAE/g EO for TAC | [84] |
| C. sativa L. extracts of flowers (flavonoids, terpenoids, saponins, anthocyanins, tannins, and reducing sugars) | Chemical system | for DPPH Ethanol extract—IC50 = 0.23 mg/mL Hexanic extract—IC50 = 0.38 mg/mL Chloroformic extract—IC50 = 0.77 mg/mL | [79] |
| C. sativa L. extract (β-caryophyllene, its oxide, CBD, THC, α-pinene, α-humulene, 2-monolinolein, methyl eicosatetraenoate and γ-sitosterol) | Chemical system | for DPPH (at different vegetative stages) Leaf—15.03 to 35.04 mmol/L extract Roots—0.29 to 1.56 mmol/L extract | [82] |
| Hulled C. sativa L. seeds and their compounds after digestion (in vitro) | Chemical system | Percentage inhibition of radical scavenging activity (Pi) 39.97 ± 0.71% after the oral phase 50.28 ± 0.62% of the intestinal phase | [83] |
| C. sativa L. extract | In vitro— neuro-2a cell line | For DPPH IC50—0.06 mg/mL for lyophilized aqueous extract IC50—0.10 mg/mL for the residual biomass hexane extract) IC50—0.005 mg ml/L for ascorbic acid | [80] |
| C. sativa L. oil 1 mg oil/kg BW for 3 weeks | In vivo— rats with a sucrose-rich diet | For ROS levels in the liver Rats (SRD)—2.46 arbitrary units Rats (SRD + Ca)—1.45 arbitrary units | [85] |
| Cannabinoids prevented the oxidation of DHR and H2DCF. | |||
| THC and CBD | In vitro— aged pancreatic islet cells | 0.1–100 µmol/L | [86] |
| Cannabinoids: CBG, CBD, Δ9-THC, CBN, CBGA, CBDA and Δ9-THCA | chemical system | EC50 CBD—11 mmol/L EC50 THC—18 mmol/L EC50 BHT—18 mmol/L | [92] |
| THC and CBD | In vitro— rat neuronal cell cultures | EC50 of 2–4 µmol/L | [39] |
| CBD | In vitro—rat PC12 cells with β-amyloid induced toxicity | 0.1–100 µmol/L | [93] |
| C. sativa L. oil and cannabinoids reduce the lipid peroxidation | |||
| CBD | In vitro— rat PC12 cells with β-amyloid-induced toxicity | Decrease in MDA level in PC12 caused by β-amyloid 0.1–100 µmol/L | [93] |
| C. sativa L. oil 1 mg oil/kg BW for 3 weeks | In vivo— rats with a sucrose-rich diet | For TBARs in serum Rats (SRD)—3.94 μmol/L Rats (SRD + Ca)—2.99 μmol/L | [85] |
| CBD intraperitoneal injection | In vivo— Rats with traumatic spinal cord injury | 2.5–20 mg/kg | [89] |
| CBD | In vivo— rats | 50, 100, 200 ng/rat | [91] |
| C. sativa L. oil and cannabinoids increase the level of GSH | |||
| CBD | In vivo— rats chronically irradiated with UV | 120 mg/kg BW | [90] |
| CBD intraperitoneal injection | In vivo— rats with traumatic spinal cord injury | 2.5–20 mg/kg | [89] |
| Cannabis oil 1 mg oil/kg BW for 3 weeks | In vivo— rats with sucrose-rich diet | For GSH levels in the liver Rats (SRD)—45 ηmol/mg protein Rats (SRD + Ca)—61 ηmol/mg protein | [85] |
| C. sativa L. oil and cannabinoids elevated the activity of antioxidative enzymes: SOD, CAT or/and GPX and GR | |||
| CBD | In vivo— rats | Injected at 50, 100, and 200 ng/rat for five consecutive days | [91] |
| Cannabis oil 1 mg oil/kg BW for 3 weeks | In vivo— rats | For GPx and GR activities in the liver Rats (SRD)—85 and 28 mU/mg Rats (SRD + Ca)—147 and 44 mU/mg | [85] |
2.3. Potential Use of Biologically Active Compounds Derived from Cannabis sativa L. in the Treatment of Cancers
2.3.1. Lung Cancer Cells/Lung Cancer
2.3.2. Hepatocellular Carcinoma Cells
2.3.3. Prostate Cancer Cells/Prostate Cancer
2.3.4. Melanoma Cancer Cells/Malignant Melanoma Tumors
2.3.5. Glioblastoma and Neuroblastoma Cells/Malignant Gliomas
2.3.6. Studies on Humans—Clinical Trials
2.4. Antiviral Effects of Cannabinoids
2.5. Current Cannabinoid-Based Drugs Approved by the FDA
2.5.1. Epidiolex® CBD Extract as a Drug for Epilepsy
2.5.2. Sativex CBD Extract as a Drug for Treating Muscle Stiffness and Spasms Caused by MS
3. Limitations in Cannabis sativa L. Research and Use
4. Conclusions
- (1)
- C. sativa L. is an extraordinary plant that provides a valuable raw material for medical applications. Its secondary metabolites, cannabinoids, have attracted growing interest in the fight against illness, mainly due to their effect on CB1 and CB2 cannabinoid receptors.
- (2)
- (3)
- Cannabinoids and other antioxidant compounds of C. sativa L., such as cannflavin A and B, exert antioxidant potential by neutralizing free radicals, supporting metal chelation, increasing GSH level, and influencing the activity of antioxidative enzymes (Table 1).
- (4)
- (5)
- In addition, their antiapoptotic, antiproliferative, antiangiogenic, and antimetastatic properties make them attractive potential cancer medications (Table 2)
- (6)
- (7)
- There is a need to build a higher number of clinical trials on humans. These studies should include larger groups of patients and should use greater standardization of products and research methodology. There is also a pressing need to understand the long-term effects and action of cannabinoids in various diseases and their interactions with other drugs.
Funding
Conflicts of Interest
Abbreviations
References
- Strzelczyk, M.; Lochynska, M.; Chudy, M. Systematics and Botanical Characteristics of Industrial Hemp Cannabis sativa L. J. Nat. Fibers 2022, 19, 5804–5826. [Google Scholar] [CrossRef]
- Pandey, Y.; Chaturvedi, T.; Swaroop, H.; Gupta, A.K.; Shanker, K.; Tiwari, G. Phytochemical and Genetic Marker (SCoT and CBDP) Based Study of Genetic Diversity and Population Structure in Natural Populations of Cannabis sativa L.: A High-Value Sustainable Biodiversity of North-Indian Himalaya. Ind. Crops Prod. 2023, 200, 116892. [Google Scholar] [CrossRef]
- Shams, R.; Azizi, A.; Hamzei, J.; Noroozisharaf, A.; Moghadam, S.; Kordrostami, M. Genetic Structure and Diversity of Iranian Cannabis Populations Based on Phytochemical, Agro-Morphological and Molecular Markers. Ind. Crops Prod. 2020, 158, 112950. [Google Scholar] [CrossRef]
- Erkelens, J.; Hazekam, A. That Which We Call Indica, by Any Other Name Would Smell as Sweet. Cannabinoids 2014, 9, 9–15. [Google Scholar]
- Martínez, V.; Iriondo De-Hond, A.; Borrelli, F.; Capasso, R.; Del Castillo, M.D.; Abalo, R. Cannabidiol and Other Non-Psychoactive Cannabinoids for Prevention and Treatment of Gastrointestinal Disorders: Useful Nutraceuticals? Int. J. Mol. Sci. 2020, 21, 3067. [Google Scholar] [CrossRef]
- Gill, A.R.; Loveys, B.R.; Cowley, J.M.; Hall, T.; Cavagnaro, T.R.; Burton, R.A. Physiological and Morphological Responses of Industrial Hemp (Cannabis sativa L.) to Water Deficit. Ind. Crops Prod. 2022, 187, 115331. [Google Scholar] [CrossRef]
- Shebaby, W.; Saliba, J.; Faour, W.H.; Ismail, J.; El Hage, M.; Daher, C.F.; Taleb, R.I.; Nehmeh, B.; Dagher, C.; Chrabieh, E.; et al. In Vivo and in Vitro Anti-Inflammatory Activity Evaluation of Lebanese Cannabis sativa L. ssp. Indica (Lam.). J. Ethnopharmacol. 2021, 270, 113743. [Google Scholar] [CrossRef]
- Eržen, M.; Čeh, B.; Kolenc, Z.; Bosancic, B.; Čerenak, A. Evaluation of Different Hemp (Cannabis sativa L.) Progenies Resulting from Crosses with Focus on Oil Content and Seed Yield. Ind. Crops Prod. 2023, 201, 116893. [Google Scholar] [CrossRef]
- Burton, R.A.; Andres, M.; Cole, M.; Cowley, J.M.; Augustin, M.A. Industrial Hemp Seed: From the Field to Value-Added Food Ingredients. J. Cannabis Res. 2022, 4, 45. [Google Scholar] [CrossRef]
- Singh, K.; Nassar, N.; Bachari, A.; Schanknecht, E.; Telukutla, S.; Zomer, R.; Piva, T.J.; Mantri, N. The Pathophysiology and the Therapeutic Potential of Cannabinoids in Prostate Cancer. Cancers 2021, 13, 4107. [Google Scholar] [CrossRef]
- Liu, M.; Thygesen, A.; Summerscales, J.; Meyer, A.S. Targeted Pre-Treatment of Hemp Bast Fibres for Optimal Performance in Biocomposite Materials: A Review. Ind. Crops Prod. 2017, 108, 660–683. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E.; Gilles, C.; Crini, N. Applications of hemp in textiles, paper industry, insulation and building materials, horticulture, animal nutrition, food and beverages, nutraceuticals, cosmetics and hygiene, medicine, agrochemistry, energy production and environment: A review. Environ. Chem. Lett. 2020, 18, 1451–1476. [Google Scholar] [CrossRef]
- Flores-Sanchez, I.J.; Choi, Y.H.; Verpoorte, R. Metabolite Analysis of Cannabis sativa L. by NMR Spectroscopy. In Functional Genomics; Kaufmann, M., Klinger, C., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2012; Volume 815, pp. 363–375. ISBN 978-1-61779-423-0. [Google Scholar]
- Cerrato, A.; Citti, C.; Cannazza, G.; Capriotti, A.L.; Cavaliere, C.; Grassi, G.; Marini, F.; Montone, C.M.; Paris, R.; Piovesana, S.; et al. Phytocannabinomics: Untargeted Metabolomics as a Tool for Cannabis Chemovar Differentiation. Talanta 2021, 230, 122313. [Google Scholar] [CrossRef]
- Cerrato, A.; Biancolillo, A.; Cannazza, G.; Cavaliere, C.; Citti, C.; Laganà, A.; Marini, F.; Montanari, M.; Montone, C.M.; Paris, R.; et al. Untargeted Cannabinomics Reveals the Chemical Differentiation of Industrial Hemp Based on the Cultivar and the Geographical Field Location. Anal. Chim. Acta 2023, 1278, 341716. [Google Scholar] [CrossRef] [PubMed]
- European Drug Report 2023. Cannabis—The Current Situation in Europe. Available online: https://www.euda.europa.eu/publications/european-drug-report/2023/cannabis_en (accessed on 1 September 2024).
- State Medical Marijuana Laws. 2024. Available online: https://www.cdc.gov/cannabis/about/state-medical-cannabis-laws.html (accessed on 1 September 2024).
- Mikulic, M. Statista. 2022. Available online: https://www.statista.com/statistics/760498/total-us-cbd-sales/ (accessed on 1 September 2024).
- Tutek, K.; Masek, A. Hemp and Its Derivatives as a Universal Industrial Raw Material (with Particular Emphasis on the Polymer Industry)—A Review. Materials 2022, 15, 2565. [Google Scholar] [CrossRef]
- Abdel-Kader, M.S.; Radwan, M.M.; Metwaly, A.M.; Eissa, I.H.; Hazekamp, A.; ElSohly, M.A. Chemistry and Biological Activities of Cannflavins of the Cannabis Plant. Cannabis Cannabinoid Res. 2023, 8, 974–985. [Google Scholar] [CrossRef] [PubMed]
- Zagzoog, A.; Mohamed, K.A.; Kim, H.J.J.; Kim, E.D.; Frank, C.S.; Black, T.; Jadhav, P.D.; Holbrook, L.A.; Laprairie, R.B. In Vitro and in Vivo Pharmacological Activity of Minor Cannabinoids Isolated from Cannabis sativa. Sci. Rep. 2020, 10, 20405. [Google Scholar] [CrossRef]
- Di Sotto, A.; Gullì, M.; Acquaviva, A.; Tacchini, M.; Di Simone, S.C.; Chiavaroli, A.; Recinella, L.; Leone, S.; Brunetti, L.; Orlando, G.; et al. Phytochemical and Pharmacological Profiles of the Essential Oil from the Inflorescences of the Cannabis sativa L. Ind. Crops Prod. 2022, 183, 114980. [Google Scholar] [CrossRef]
- Orhan, I.; Sener, B. Fatty Acid Content of Selected Seed Oils. J. Herb. Pharmacother. 2002, 2, 29–33. [Google Scholar] [CrossRef]
- Da Porto, C.; Natolino, A.; Decorti, D. Effect of Ultrasound Pre-Treatment of Hemp (Cannabis sativa L.) Seed on Supercritical CO2 Extraction of Oil. J. Food Sci. Technol. 2015, 52, 1748–1753. [Google Scholar] [CrossRef]
- Konca, Y.; Cimen, B.; Yalcin, H.; Kaliber, M.; Beyzi, S.B. Effect of Hempseed (Cannabis sativa sp.) Inclusion to the Diet on Performance, Carcass and Antioxidative Activity in Japanese Quail (Coturnix Coturnix Japonica). Korean J. Food Sci. Anim. Resour. 2014, 34, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Cerino, P.; Buonerba, C.; Cannazza, G.; D’Auria, J.; Ottoni, E.; Fulgione, A.; Di Stasio, A.; Pierri, B.; Gallo, A. A Review of Hemp as Food and Nutritional Supplement. Cannabis Cannabinoid Res. 2021, 6, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Kornpointner, C.; Sainz Martinez, A.; Marinovic, S.; Haselmair-Gosch, C.; Jamnik, P.; Schröder, K.; Löfke, C.; Halbwirth, H. Chemical Composition and Antioxidant Potential of Cannabis sativa L. Roots. Ind. Crops Prod. 2021, 165, 113422. [Google Scholar] [CrossRef]
- Filer, C.N. Acidic Cannabinoid Decarboxylation. Cannabis Cannabinoid Res. 2022, 7, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Cristino, L.; Bisogno, T.; Di Marzo, V. Cannabinoids and the Expanded Endocannabinoid System in Neurological Disorders. Nat. Rev. Neurol. 2020, 16, 9–29. [Google Scholar] [CrossRef]
- Baratta, F.; Pignata, I.; Ravetto Enri, L.; Brusa, P. Cannabis for Medical Use: Analysis of Recent Clinical Trials in View of Current Legislation. Front. Pharmacol. 2022, 13, 888903. [Google Scholar] [CrossRef]
- Troyer, J.; Tanco, K. Review of the Use of Medicinal Cannabis Products in Palliative Care. Cancers 2024, 16, 1412. [Google Scholar] [CrossRef]
- Nguyen, L.C.; Yang, D.; Nicolaescu, V.; Best, T.J.; Gula, H.; Saxena, D.; Gabbard, J.D.; Chen, S.-N.; Ohtsuki, T.; Friesen, J.B.; et al. Cannabidiol Inhibits SARS-CoV-2 Replication through Induction of the Host ER Stress and Innate Immune Responses. Sci. Adv. 2022, 8, eabi6110. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, S.; Maharshi, V. Therapeutic Effects of Cannabinoids on Ulcerative Colitis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Health Sci. Med. Res. 2024, 2024, 20241041. [Google Scholar] [CrossRef]
- Paland, N.; Hamza, H.; Pechkovsky, A.; Aswad, M.; Shagidov, D.; Louria-Hayon, I. Cannabis and Rheumatoid Arthritis: A Scoping Review Evaluating the Benefits, Risks, and Future Research Directions. Rambam Maimonides Med. J. 2023, 14, e0022. [Google Scholar] [CrossRef]
- Ghazi Eid, B. Cannabinoids for Treating Cardiovascular Disorders: Putting Together a Complex Puzzle. J. Microsc. Ultrastruct. 2018, 6, 171–176. [Google Scholar] [CrossRef]
- Blázquez, C.; Casanova, M.L.; Planas, A.; Gómez del Pulgar, T.; Villanueva, C.; Fernández-Aceñero, M.J.; Aragonés, J.; Huffman, J.W.; Jorcano, J.L.; Guzmán, M. Inhibition of Tumor Angiogenesis by Cannabinoids. FASEB J. 2003, 17, 333–568. [Google Scholar] [CrossRef] [PubMed]
- Schultz, S.; Beyer, M. GW Pharmaceuticals Achieves Positive Results in Phase 2 Proof of Concept Study in Glioma. Available online: http://Ir.Gwpharm.Com/Static-Files/Cde942fe-555c-4b2f-9cc9-F34d24c7ad27 (accessed on 1 September 2024).
- Andradas, C.; Truong, A.; Byrne, J.; Endersby, R. The Role of Cannabinoids as Anticancer Agents in Pediatric Oncology. Cancers 2021, 13, 157. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Masteikova, R.; Lazauskas, R.; Bernatoniene, J. Cannabis sativa L. Bioactive Compounds and Their Protective Role in Oxidative Stress and Inflammation. Antioxidants 2022, 11, 660. [Google Scholar] [CrossRef]
- Brust, C.A.; Swanson, M.A.; Bohn, L.M. Structural and Functional Insights into the G Protein-Coupled Receptors: CB1 and CB2. Biochem. Soc. Trans. 2023, 51, 1533–1543. [Google Scholar] [CrossRef]
- Pertwee, R.G. The Diverse CB1 and CB2 Receptor Pharmacology of Three Plant Cannabinoids: Δ9-tetrahydrocannabinol, Cannabidiol and Δ9-tetrahydrocannabivarin. Br. J. Pharmacol. 2008, 153, 199–215. [Google Scholar] [CrossRef]
- Badal, S.; Smith, K.N.; Rajnarayanan, R. Analysis of Natural Product Regulation of Cannabinoid Receptors in the Treatment of Human Disease. Pharmacol. Ther. 2017, 180, 24–48. [Google Scholar] [CrossRef]
- Tham, M.; Yilmaz, O.; Alaverdashvili, M.; Kelly, M.E.M.; Denovan-Wright, E.M.; Laprairie, R.B. Allosteric and Orthosteric Pharmacology of Cannabidiol and Cannabidiol-dimethylheptyl at the Type 1 and Type 2 Cannabinoid Receptors. Br. J. Pharmacol. 2019, 176, 1455–1469. [Google Scholar] [CrossRef] [PubMed]
- Laprairie, R.B.; Bagher, A.M.; Kelly, M.E.M.; Denovan-Wright, E.M. Cannabidiol Is a Negative Allosteric Modulator of the Cannabinoid CB 1 Receptor. Br. J. Pharmacol. 2015, 172, 4790–4805. [Google Scholar] [CrossRef]
- Chung, H.; Fierro, A.; Pessoa-Mahana, C.D. Cannabidiol Binding and Negative Allosteric Modulation at the Cannabinoid Type 1 Receptor in the Presence of Delta-9-Tetrahydrocannabinol: An In Silico Study. PLoS ONE 2019, 14, e0220025. [Google Scholar] [CrossRef]
- An, D.; Peigneur, S.; Hendrickx, L.A.; Tytgat, J. Targeting Cannabinoid Receptors: Current Status and Prospects of Natural Products. Int. J. Mol. Sci. 2020, 21, 5064. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, A.; Caissutti, D.; Mattei, V.; Gado, F.; Martellucci, S.; Longo, A.; Recalchi, S.; Manganelli, V.; Riitano, G.; Garofalo, T.; et al. Anti-Inflammatory Activity of a CB2 Selective Cannabinoid Receptor Agonist: Signaling and Cytokines Release in Blood Mononuclear Cells. Molecules 2021, 27, 64. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.W.-M.; Campbell, L.M.; Sun-Suslow, N.; Hong, S.; Umlauf, A.; Ellis, R.J.; Iudicello, J.E.; Letendre, S.; Marcotte, T.D.; Heaton, R.K.; et al. Daily Cannabis Use Is Associated With Lower CNS Inflammation in People With HIV. J. Int. Neuropsychol. Soc. 2021, 27, 661–672. [Google Scholar] [CrossRef]
- Shahbazi, F.; Grandi, V.; Banerjee, A.; Trant, J.F. Cannabinoids and Cannabinoid Receptors: The Story so Far. iScience 2020, 23, 101301. [Google Scholar] [CrossRef]
- Almogi-Hazan, O.; Or, R. Cannabis, the Endocannabinoid System and Immunity—The Journey from the Bedside to the Bench and Back. Int. J. Mol. Sci. 2020, 21, 4448. [Google Scholar] [CrossRef]
- Yang, L.; Li, F.-F.; Han, Y.-C.; Jia, B.; Ding, Y. Cannabinoid Receptor CB2 Is Involved in Tetrahydrocannabinol-Induced Anti-Inflammation against Lipopolysaccharide in MG-63 Cells. Mediat. Inflamm. 2015, 2015, 362126. [Google Scholar] [CrossRef]
- Suryavanshi, S.V.; Zaiachuk, M.; Pryimak, N.; Kovalchuk, I.; Kovalchuk, O. Cannabinoids Alleviate the LPS-Induced Cytokine Storm via Attenuating NLRP3 Inflammasome Signaling and TYK2-Mediated STAT3 Signaling Pathways In Vitro. Cells 2022, 11, 1391. [Google Scholar] [CrossRef]
- Lowin, T.; Tingting, R.; Zurmahr, J.; Classen, T.; Schneider, M.; Pongratz, G. Cannabidiol (CBD): A Killer for Inflammatory Rheumatoid Arthritis Synovial Fibroblasts. Cell Death Dis. 2020, 11, 714. [Google Scholar] [CrossRef] [PubMed]
- Opęchowska, A.; Karpiuk, K.; Zahorodnii, A.; Harasim-Symbor, E.; Chabowski, A.; Konstantynowicz-Nowicka, K. Anti-Inflammatory Effects of Cannabidiol in Early Stages of Neuroinflammation Induced by High-Fat Diet in Cerebral Cortex of Rats. Toxicol. Appl. Pharmacol. 2024, 484, 116856. [Google Scholar] [CrossRef]
- Yndart Arias, A.; Kolishetti, N.; Vashist, A.; Madepalli, L.; Llaguno, L.; Nair, M. Anti-Inflammatory Effects of CBD in Human Microglial Cell Line Infected with HIV-1. Sci. Rep. 2023, 13, 7376. [Google Scholar] [CrossRef]
- Atalay, S.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Properties of Cannabidiol. Antioxidants 2019, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Markovà, J.; Essner, U.; Akmaz, B.; Marinelli, M.; Trompke, C.; Lentschat, A.; Vila, C. Sative® as Add-on Therapy vs. Further Optimized First-Line ANTispastics (SAVANT) in Resistant Multiple Sclerosis Spasticity: A Double-Blind, Placebo-Controlled Randomised Clinical Trial. Int. J. Neurosci. 2019, 129, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Navarini, L.; Bisogno, T.; Mozetic, P.; Piscitelli, F.; Margiotta, D.P.E.; Basta, F.; Afeltra, A.; Maccarrone, M. Endocannabinoid System in Systemic Lupus Erythematosus: First Evidence for a Deranged 2-Arachidonoylglycerol Metabolism. Int. J. Biochem. Cell Biol. 2018, 99, 161–168. [Google Scholar] [CrossRef]
- Ghasemi-Gojani, E.; Kovalchuk, I.; Kovalchuk, O. Cannabinoids and Terpenes for Diabetes Mellitus and Its Complications: From Mechanisms to New Therapies. Trends Endocrinol. Metab. 2022, 33, 828–849. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Huang, Y.; Yu, L.; Ji, X.; Wu, J. Impact of the Cannabinoid System in Alzheimer’s Disease. Curr. Neuropharmacol. 2023, 21, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Ning, K.; Hou, C.; Wei, X.; Zhou, Y.; Zhang, S.; Chen, Y.; Yu, H.; Dong, L.; Chen, S. Metabolomics Analysis Revealed the Characteristic Metabolites of Hemp Seeds Varieties and Metabolites Responsible for Antioxidant Properties. Front. Plant Sci. 2022, 13, 904163. [Google Scholar] [CrossRef]
- Pollastro, F.; Minassi, A.; Fresu, L.G. Cannabis Phenolics and Their Bioactivities. Curr. Med. Chem. 2018, 25, 1160–1185. [Google Scholar] [CrossRef]
- Rea, K.A.; Casaretto, J.A.; Al-Abdul-Wahid, M.S.; Sukumaran, A.; Geddes-McAlister, J.; Rothstein, S.J.; Akhtar, T.A. Biosynthesis of Cannflavins A and B from Cannabis sativa L. Phytochemistry 2019, 164, 162–171. [Google Scholar] [CrossRef]
- Bhuyan, U.; Handique, J.G. Plant Polyphenols as Potent Antioxidants: Highlighting the Mechanism of Antioxidant Activity and Synthesis/Development of Some Polyphenol Conjugates. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2022; Volume 75, pp. 243–266. ISBN 978-0-323-91250-1. [Google Scholar]
- Barrett, M.L.; Gordon, D.; Evans, F.J. Isolation from Cannabis sativa L. of Cannflavin—A Novel Inhibitor of Prostaglandin Production. Biochem. Pharmacol. 1985, 34, 2019–2024. [Google Scholar] [CrossRef]
- Barrett, M.L.; Scutt, A.M.; Evans, F.J. Cannflavin A and B, Prenylated Flavones from Cannabis sativa L. Experientia 1986, 42, 452–453. [Google Scholar] [CrossRef]
- Erridge, S.; Mangal, N.; Salazar, O.; Pacchetti, B.; Sodergren, M.H. Cannflavins—From Plant to Patient: A Scoping Review. Fitoterapia 2020, 146, 104712. [Google Scholar] [CrossRef] [PubMed]
- Chuanphongpanich, S.; Racha, S.; Saengsitthisak, B.; Pirakitikulr, P.; Racha, K. Computational Assessment of Cannflavin A as a TAK1 Inhibitor: Implication as a Potential Therapeutic Target for Anti-Inflammation. Sci. Pharm. 2023, 91, 36. [Google Scholar] [CrossRef]
- Moreau, M.; Ibeh, U.; Decosmo, K.; Bih, N.; Yasmin-Karim, S.; Toyang, N.; Lowe, H.; Ngwa, W. Flavonoid Derivative of Cannabis Demonstrates Therapeutic Potential in Preclinical Models of Metastatic Pancreatic Cancer. Front. Oncol. 2019, 9, 660. [Google Scholar] [CrossRef]
- Izzo, L.; Pacifico, S.; Piccolella, S.; Castaldo, L.; Narváez, A.; Grosso, M.; Ritieni, A. Chemical Analysis of Minor Bioactive Components and Cannabidiolic Acid in Commercial Hemp Seed Oil. Molecules 2020, 25, 3710. [Google Scholar] [CrossRef] [PubMed]
- Bonacci, S.; Di Stefano, V.; Sciacca, F.; Buzzanca, C.; Virzì, N.; Argento, S.; Melilli, M.G. Hemp Flour Particle Size Affects the Quality and Nutritional Profile of the Enriched Functional Pasta. Foods 2023, 12, 774. [Google Scholar] [CrossRef] [PubMed]
- Nigro, E.; Crescente, G.; Formato, M.; Pecoraro, M.T.; Mallardo, M.; Piccolella, S.; Daniele, A.; Pacifico, S. Hempseed Lignanamides Rich-Fraction: Chemical Investigation and Cytotoxicity towards U-87 Glioblastoma Cells. Molecules 2020, 25, 1049. [Google Scholar] [CrossRef]
- Wu, D.; Liu, Y.; Guan, W.; Pan, J.; Kuang, H.; Yang, B. UHPLC-Orbitrap-Fusion-TMS-Based Metabolomics Study of Phenylpropionamides in the Seed of Cannabis sativa L. against Alzheimer’s Disease. Chem. Biodivers. 2023, 20, e202201047. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, S.; Ji, J.; Lou, H.; Fan, P. Hemp (Cannabis sativa L.) Seed Phenylpropionamides Composition and Effects on Memory Dysfunction and Biomarkers of Neuroinflammation Induced by Lipopolysaccharide in Mice. ACS Omega 2018, 3, 15988–15995. [Google Scholar] [CrossRef]
- Kriese, U.; Schumann, E.; Weber, W.E.; Beyer, M.; Brühl, L.; Matthäus, B. Oil Content, Tocopherol Composition and Fatty Acid Patterns of the Seeds of 51 Cannabis sativa L. Genotypes. Euphytica 2004, 137, 339–351. [Google Scholar] [CrossRef]
- Zhang, J.; Griffin, J.; Li, Y.; Wang, D.; Wang, W. Antioxidant Properties of Hemp Proteins: From Functional Food to Phytotherapy and Beyond. Molecules 2022, 27, 7924. [Google Scholar] [CrossRef]
- Wang, S.; Luo, Q.; Fan, P. Cannabisin F from Hemp (Cannabis sativa) Seed Suppresses Lipopolysaccharide-Induced Inflammatory Responses in BV2 Microglia as SIRT1 Modulator. Int. J. Mol. Sci. 2019, 20, 507. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Martin, N.M.; Montserrat-de La Paz, S.; Toscano, R.; Grao-Cruces, E.; Villanueva, A.; Pedroche, J.; Millan, F.; Millan-Linares, M.C. Hemp (Cannabis sativa L.) Protein Hydrolysates Promote Anti-Inflammatory Response in Primary Human Monocytes. Biomolecules 2020, 10, 803. [Google Scholar] [CrossRef] [PubMed]
- Ahidar, N.; Labhar, A.; Benamari, O.; Ahari, M.; Salhi, A.; Elyoussfi, A.; Amhamdi, H. Phenolic Content and Antioxidant Activity of Cannabis Sativa L. Flowers from the Ketama Region in Northern Morocco. Ecol. Eng. Environ. Technol. 2024, 25, 209–215. [Google Scholar] [CrossRef]
- Cásedas, G.; Moliner, C.; Maggi, F.; Mazzara, E.; López, V. Evaluation of Two Different Cannabis sativa L. Extracts as Antioxidant and Neuroprotective Agents. Front. Pharmacol. 2022, 13, 1009868. [Google Scholar] [CrossRef]
- Stasiłowicz-Krzemień, A.; Sip, S.; Szulc, P.; Cielecka-Piontek, J. Determining Antioxidant Activity of Cannabis Leaves Extracts from Different Varieties—Unveiling Nature’s Treasure Trove. Antioxidants 2023, 12, 1390. [Google Scholar] [CrossRef]
- Judžentienė, A.; Garjonytė, R.; Būdienė, J. Phytochemical Composition and Antioxidant Activity of Various Extracts of Fibre Hemp (Cannabis sativa L.) Cultivated in Lithuania. Molecules 2023, 28, 4928. [Google Scholar] [CrossRef]
- Frazzini, S.; Torresani, M.C.; Roda, G.; Dell’Anno, M.; Ruffo, G.; Rossi, L. Chemical and Functional Characterization of the Main Bioactive Molecules Contained in Hulled Cannabis sativa L. Seeds for Use as Functional Ingredients. J. Agric. Food Res. 2024, 16, 101084. [Google Scholar] [CrossRef]
- El-Mernissi, R.; El Menyiy, N.; Moubachir, R.; Zouhri, A.; El-Mernissi, Y.; Siddique, F.; Nadeem, S.; Ibork, H.; El Barnossi, A.; Wondmie, G.F.; et al. Cannabis sativa L. Essential Oil: Chemical Composition, Anti-Oxidant, Anti-Microbial Properties, and Acute Toxicity: In Vitro, In Vivo, and In Silico Study. Open Chem. 2024, 22, 20230214. [Google Scholar] [CrossRef]
- Degrave, V.; Vega Joubert, M.B.; Ingaramo, P.; Sedan, D.; Andrinolo, D.; D’Alessandro, M.E.; Oliva, M.E. Effects of Full-Spectrum Cannabis Oil with a Cannabidiol:Tetrahydrocannabinol 2:1 Ratio on the Mechanisms Involved in Hepatic Steatosis and Oxidative Stress in Rats Fed a Sucrose-Rich Diet. Med. Cannabis Cannabinoids 2023, 6, 170–183. [Google Scholar] [CrossRef]
- Baeeri, M.; Rahimifard, M.; Daghighi, S.M.; Khan, F.; Salami, S.A.; Moini-Nodeh, S.; Haghi-Aminjan, H.; Bayrami, Z.; Rezaee, F.; Abdollahi, M. Cannabinoids as Anti-ROS in Aged Pancreatic Islet Cells. Life Sci. 2020, 256, 117969. [Google Scholar] [CrossRef]
- Dawidowicz, A.L.; Olszowy-Tomczyk, M.; Typek, R. CBG, CBD, Δ9-THC, CBN, CBGA, CBDA and Δ9-THCA as Antioxidant Agents and Their Intervention Abilities in Antioxidant Action. Fitoterapia 2021, 152, 104915. [Google Scholar] [CrossRef] [PubMed]
- Hacke, A.C.M.; Lima, D.; De Costa, F.; Deshmukh, K.; Li, N.; Chow, A.M.; Marques, J.A.; Pereira, R.P.; Kerman, K. Probing the Antioxidant Activity of Δ9-Tetrahydrocannabinol and Cannabidiol in Cannabis sativa Extracts. Analyst 2019, 144, 4952–4961. [Google Scholar] [CrossRef] [PubMed]
- Baron-Flores, V.; Diaz-Ruiz, A.; Manzanares, J.; Rios, C.; Burelo, M.; Jardon-Guadarrama, G.; Martínez-Cárdenas, M.D.L.Á.; Mata-Bermudez, A. Cannabidiol Attenuates Hypersensitivity and Oxidative Stress after Traumatic Spinal Cord Injury in Rats. Neurosci. Lett. 2022, 788, 136855. [Google Scholar] [CrossRef] [PubMed]
- Biernacki, M.; Jastrząb, A.; Skrzydlewska, E. Changes in Hepatic Phospholipid Metabolism in Rats under UV Irradiation and Topically Treated with Cannabidiol. Antioxidants 2021, 10, 1157. [Google Scholar] [CrossRef]
- Khaksar, S.; Bigdeli, M.; Samiee, A.; Shirazi-zand, Z. Antioxidant and Anti-Apoptotic Effects of Cannabidiol in Model of Ischemic Stroke in Rats. Brain Res. Bull. 2022, 180, 118–130. [Google Scholar] [CrossRef]
- Hampson, A.J.; Grimaldi, M.; Axelrod, J.; Wink, D. Cannabidiol and (−)Δ9-Tetrahydrocannabinol Are Neuroprotective Antioxidants. Proc. Natl. Acad. Sci. USA 1998, 95, 8268–8273. [Google Scholar] [CrossRef]
- Iuvone, T.; Esposito, G.; Esposito, R.; Santamaria, R.; Di Rosa, M.; Izzo, A.A. Neuroprotective Effect of Cannabidiol, a Non-psychoactive Component from Cannabis Sativa, on Β-amyloid-induced Toxicity in PC12 Cells. J. Neurochem. 2004, 89, 134–141. [Google Scholar] [CrossRef]
- Marinho, A.M.D.N.; Silva-Neto, R.W.G.D. Anti-Inflammatory Effects of Cannabinoids. Braz. J. Pain 2023, 6, S31–S37. [Google Scholar] [CrossRef]
- Sawtelle, L.; Holle, L.M. Use of Cannabis and Cannabinoids in Patients With Cancer. Ann. Pharmacother. 2021, 55, 870–890. [Google Scholar] [CrossRef]
- Simmerman, E.; Qin, X.; Yu, J.C.; Baban, B. Cannabinoids as a Potential New and Novel Treatment for Melanoma: A Pilot Study in a Murine Model. J. Surg. Res. 2019, 235, 210–215. [Google Scholar] [CrossRef]
- Ramer, R.; Heinemann, K.; Merkord, J.; Rohde, H.; Salamon, A.; Linnebacher, M.; Hinz, B. COX-2 and PPAR-γ Confer Cannabidiol-Induced Apoptosis of Human Lung Cancer Cells. Mol. Cancer Ther. 2013, 12, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Solinas, M.; Massi, P.; Cinquina, V.; Valenti, M.; Bolognini, D.; Gariboldi, M.; Monti, E.; Rubino, T.; Parolaro, D. Cannabidiol, a Non-Psychoactive Cannabinoid Compound, Inhibits Proliferation and Invasion in U87-MG and T98G Glioma Cells through a Multitarget Effect. PLoS ONE 2013, 8, e76918. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Matsabisa, M. In Silico Investigation of Cannabinoids from Cannabis Sativa Leaves as a Potential Anticancer Drug to Inhibit MAPK-ERK Signaling Pathway and EMT Induction. Silico Pharmacol. 2024, 12, 41. [Google Scholar] [CrossRef] [PubMed]
- Motadi, L.R.; Jantjies, Z.E.; Moleya, B. Cannabidiol and Cannabis Sativa as a Potential Treatment in Vitro Prostate Cancer Cells Silenced with RBBp6 and PC3 Xenograft. Mol. Biol. Rep. 2023, 50, 4039–4047. [Google Scholar] [CrossRef] [PubMed]
- Pongking, T.; Intuyod, K.; Thongpon, P.; Thanan, R.; Sitthirach, C.; Chaidee, A.; Kongsintaweesuk, S.; Klungsaeng, S.; Hongsrichan, N.; Sakonsinsiri, C.; et al. Cannabidiol Suppresses Proliferation and Induces Cell Death, Autophagy and Senescence in Human Cholangiocarcinoma Cells via the PI3K/AKT/mTOR Pathway. J. Tradit. Complement. Med. 2024, 14, 622–634. [Google Scholar] [CrossRef]
- Wyrobnik, I.; Steinberg, M.; Gelfand, A.; Rosenblum, R.; Eid Mutlak, Y.; Sulimani, L.; Procaccia, S.; Ofran, Y.; Novak-Kotzer, H.; Meiri, D. Decreased Melanoma CSF-1 Secretion by Cannabigerol Treatment Reprograms Regulatory Myeloid Cells and Reduces Tumor Progression. OncoImmunology 2023, 12, 2219164. [Google Scholar] [CrossRef]
- Bachari, A.; Nassar, N.; Telukutla, S.; Zomer, R.; Piva, T.J.; Mantri, N. Evaluating the Mechanism of Cell Death in Melanoma Induced by the Cannabis Extract PHEC-66. Cells 2024, 13, 268. [Google Scholar] [CrossRef]
- Poommarapan, K.; Rummaneethorn, P.; Srisubat, A.; Suwanpidokkul, N.; Leenutaphong, P.; Nararatwanchai, T.; Srihirun, S.; Phetchengkao, W.; Suriyachan, K.; Tancharoen, S. Gene Profiling of Cannabis sativa-Mediated Apoptosis in Human Melanoma Cells. Anticancer Res. 2023, 43, 1221–1237. [Google Scholar] [CrossRef]
- Ramer, R.; Hinz, B. Inhibition of Cancer Cell Invasion by Cannabinoids via Increased Expression of Tissue Inhibitor of Matrix Metalloproteinases-1. JNCI J. Natl. Cancer Inst. 2008, 100, 59–69. [Google Scholar] [CrossRef]
- Dobovišek, L.; Krstanović, F.; Borštnar, S.; Debeljak, N. Cannabinoids and Hormone Receptor-Positive Breast Cancer Treatment. Cancers 2020, 12, 525. [Google Scholar] [CrossRef]
- Myint, Z.W.; St. Clair, W.H.; Strup, S.E.; Yan, D.; Li, N.; Allison, D.B.; McLouth, L.E.; Ellis, C.S.; Wang, P.; James, A.C.; et al. A Phase I Dose Escalation and Expansion Study of Epidiolex (Cannabidiol) in Patients with Biochemically Recurrent Prostate Cancer. Cancers 2023, 15, 2505. [Google Scholar] [CrossRef] [PubMed]
- Velasco, G.; Carracedo, A.; Blázquez, C.; Lorente, M.; Aguado, T.; Haro, A.; Sánchez, C.; Galve-Roperh, I.; Guzmán, M. Cannabinoids and Gliomas. Mol. Neurobiol. 2007, 36, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Dumitru, C.A.; Sandalcioglu, I.E.; Karsak, M. Cannabinoids in Glioblastoma Therapy: New Applications for Old Drugs. Front. Mol. Neurosci. 2018, 11, 159. [Google Scholar] [CrossRef] [PubMed]
- Richtig, G.; Kienzl, M.; Rittchen, S.; Roula, D.; Eberle, J.; Sarif, Z.; Pichler, M.; Hoefler, G.; Heinemann, A. Cannabinoids Reduce Melanoma Cell Viability and Do Not Interfere with Commonly Used Targeted Therapy in Metastatic Melanoma In Vivo and In Vitro. Biology 2023, 12, 706. [Google Scholar] [CrossRef]
- Mashabela, M.D.; Kappo, A.P. Anti-Cancer and Anti-Proliferative Potential of Cannabidiol: A Cellular and Molecular Perspective. Int. J. Mol. Sci. 2024, 25, 5659. [Google Scholar] [CrossRef]
- Le, T.Q.; Meesiripan, N.; Sanggrajang, S.; Suwanpidokkul, N.; Prayakprom, P.; Bodhibukkana, C.; Khaowroongrueng, V.; Suriyachan, K.; Thanasitthichai, S.; Srisubat, A.; et al. Anti-Proliferative and Apoptotic Effect of Cannabinoids on Human Pancreatic Ductal Adenocarcinoma Xenograft in BALB/c Nude Mice Model. Sci. Rep. 2024, 14, 6515. [Google Scholar] [CrossRef]
- Jeon, Y.; Kim, T.; Kwon, H.; Kim, J.-K.; Park, Y.-T.; Ham, J.; Kim, Y.-J. Cannabidiol Enhances Cabozantinib-Induced Apoptotic Cell Death via Phosphorylation of P53 Regulated by ER Stress in Hepatocellular Carcinoma. Cancers 2023, 15, 3987. [Google Scholar] [CrossRef]
- Zhong, N.; Li, D.; Wang, B.; Kovalchuk, O.; Kovalchuk, I. Cannabinol Inhibits Cell Growth and Triggers Cell Cycle Arrest and Apoptosis in Cancer Cells. Biocatal. Agric. Biotechnol. 2023, 48, 102627. [Google Scholar] [CrossRef]
- Go, Y.Y.; Kim, S.R.; Kim, D.Y.; Chae, S.-W.; Song, J.-J. Cannabidiol Enhances Cytotoxicity of Anti-Cancer Drugs in Human Head and Neck Squamous Cell Carcinoma. Sci. Rep. 2020, 10, 20622. [Google Scholar] [CrossRef]
- Xu, Y.; Luo, J.; Guo, Y.; Zhou, J.; Shen, L.; Gu, F.; Shi, C.; Yao, L.; Hua, M. Chemical Compounds, Anti-Tumor and Anti-Neuropathic Pain Effect of Hemp Essential Oil In Vivo. Fitoterapia 2024, 177, 106092. [Google Scholar] [CrossRef]
- Ramer, R.; Merkord, J.; Rohde, H.; Hinz, B. Cannabidiol Inhibits Cancer Cell Invasion via Upregulation of Tissue Inhibitor of Matrix Metalloproteinases-1. Biochem. Pharmacol. 2010, 79, 955–966. [Google Scholar] [CrossRef] [PubMed]
- Ramer, R.; Rohde, A.; Merkord, J.; Rohde, H.; Hinz, B. Decrease of Plasminogen Activator Inhibitor-1 May Contribute to the Anti-Invasive Action of Cannabidiol on Human Lung Cancer Cells. Pharm. Res. 2010, 27, 2162–2174. [Google Scholar] [CrossRef] [PubMed]
- Malheiro, R.F.; Carmo, H.; Carvalho, F.; Silva, J.P. Cannabinoid-Mediated Targeting of Mitochondria on the Modulation of Mitochondrial Function and Dynamics. Pharmacol. Res. 2023, 187, 106603. [Google Scholar] [CrossRef] [PubMed]
- Rocha, F.C.M.; dos Santos Júnior, J.G.; Stefano, S.C.; da Silveira, D.X. Systematic Review of the Literature on Clinical and Experimental Trials on the Antitumor Effects of Cannabinoids in Gliomas. J. Neurooncol. 2014, 116, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Khodadadi, H.; Salles, É.L.; Alptekin, A.; Mehrabian, D.; Rutkowski, M.; Arbab, A.S.; Yeudall, W.A.; Yu, J.C.; Morgan, J.C.; Hess, D.C.; et al. Inhalant Cannabidiol Inhibits Glioblastoma Progression Through Regulation of Tumor Microenvironment. Cannabis Cannabinoid Res. 2023, 8, 824–834. [Google Scholar] [CrossRef]
- Gross, C.; Ramirez, D.A.; McGrath, S.; Gustafson, D.L. Cannabidiol Induces Apoptosis and Perturbs Mitochondrial Function in Human and Canine Glioma Cells. Front. Pharmacol. 2021, 12, 725136. [Google Scholar] [CrossRef]
- Ellert-Miklaszewska, A.; Ciechomska, I.A.; Kaminska, B. Synthetic Cannabinoids Induce Autophagy and Mitochondrial Apoptotic Pathways in Human Glioblastoma Cells Independently of Deficiency in TP53 or PTEN Tumor Suppressors. Cancers 2021, 13, 419. [Google Scholar] [CrossRef]
- Vrechi, T.A.M.; Leão, A.H.F.F.; Morais, I.B.M.; Abílio, V.C.; Zuardi, A.W.; Hallak, J.E.C.; Crippa, J.A.; Bincoletto, C.; Ureshino, R.P.; Smaili, S.S.; et al. Cannabidiol Induces Autophagy via ERK1/2 Activation in Neural Cells. Sci. Rep. 2021, 11, 5434. [Google Scholar] [CrossRef]
- Guggisberg, J.; Schumacher, M.; Gilmore, G.; Zylla, D.M. Cannabis as an Anticancer Agent: A Review of Clinical Data and Assessment of Case Reports. Cannabis Cannabinoid Res. 2022, 7, 24–33. [Google Scholar] [CrossRef]
- Sea, Y.L.; Gee, Y.J.; Lal, S.K.; Choo, W.S. Cannabis as Antivirals. J. Appl. Microbiol. 2023, 134, lxac036. [Google Scholar] [CrossRef]
- Van Breemen, R.B.; Simchuk, D. Antiviral Activities of Hemp Cannabinoids. Clin. Sci. 2023, 137, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; Pesce, M.; Seguella, L.; Sanseverino, W.; Lu, J.; Corpetti, C.; Sarnelli, G. The Potential of Cannabidiol in the COVID-19 Pandemic. Br. J. Pharmacol. 2020, 177, 4967–4970. [Google Scholar] [CrossRef] [PubMed]
- Costiniuk, C.T.; Jenabian, M.-A. Cannabinoids and Inflammation: Implications for People Living with HIV. AIDS 2019, 33, 2273–2288. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Kovalchuk, A.; Li, D.; Rodriguez-Juarez, R.; Ilnytskyy, Y.; Kovalchuk, I.; Kovalchuk, O. In Search of Preventative Strategies: Novel High-CBD Cannabis Sativa Extracts Modulate ACE2 Expression in COVID-19 Gateway Tissues. Aging 2020, 12, 22425. [Google Scholar] [CrossRef]
- Classen, N.; Pitakbut, T.; Schöfbänker, M.; Kühn, J.; Hrincius, E.R.; Ludwig, S.; Hensel, A.; Kayser, O. Cannabigerol and Cannabicyclol Block SARS-CoV-2 Cell Fusion. Planta Med. 2024, 90, 717–725. [Google Scholar] [CrossRef]
- Raj, V.; Park, J.G.; Cho, K.-H.; Choi, P.; Kim, T.; Ham, J.; Lee, J. Assessment of Antiviral Potencies of Cannabinoids against SARS-CoV-2 Using Computational and in Vitro Approaches. Int. J. Biol. Macromol. 2021, 168, 474–485. [Google Scholar] [CrossRef] [PubMed]
- Salles, É.L.; Khodadadi, H.; Jarrahi, A.; Ahluwalia, M.; Paffaro, V.A.; Costigliola, V.; Yu, J.C.; Hess, D.C.; Dhandapani, K.M.; Baban, B. Cannabidiol (CBD) Modulation of Apelin in Acute Respiratory Distress Syndrome. J. Cell. Mol. Med. 2020, 24, 12869–12872. [Google Scholar] [CrossRef]
- Beyerstedt, S.; Casaro, E.B.; Rangel, É.B. COVID-19: Angiotensin-Converting Enzyme 2 (ACE2) Expression and Tissue Susceptibility to SARS-CoV-2 Infection. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 905–919. [Google Scholar] [CrossRef]
- Muthumalage, T.; Rahman, I. Cannabidiol Differentially Regulates Basal and LPS-Induced Inflammatory Responses in Macrophages, Lung Epithelial Cells, and Fibroblasts. Toxicol. Appl. Pharmacol. 2019, 382, 114713. [Google Scholar] [CrossRef]
- van Breemen, R.B.; Muchiri, R.N.; Bates, T.A.; Weinstein, J.B.; Leier, H.C.; Farley, S.; Tafesse, F.G. Cannabinoids Block Cellular Entry of SARS-CoV-2 and the Emerging Variants. J. Nat. Prod. 2022, 85, 176–184. [Google Scholar] [CrossRef]
- Likar, R.; Nahler, G. The Use of Cannabis in Supportive Care and Treatment of Brain Tumor. Neuro-Oncol. Pract. 2017, 4, 151–160. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hidding, U.; Mainka, T.; Buhmann, C. Therapeutic Use of Medical Cannabis in Neurological Diseases: A Clinical Update. J. Neural Transm. 2024, 131, 117–126. [Google Scholar] [CrossRef] [PubMed]
- EPIDIOLEX® (Cannabidiol) Offers Flexible Dosing for Tolerability and Response Optimization. 2024. Available online: https://www.epidiolexhcp.com/dosing/dosing-and-administration (accessed on 1 September 2024).
- VanDolah, H.J.; Bauer, B.A.; Mauck, K.F. Clinicians’ Guide to Cannabidiol and Hemp Oils. Mayo Clin. Proc. 2019, 94, 1840–1851. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, D.; Langley, J.; Hartkopf, K.; Hawk, L.; Margolis, A.; Struck, A.; Felton, E.; Hsu, D.; Gidal, B.E. Real-World, Long-Term Evaluation of the Tolerability and Therapy Retention of Epidiolex® (Cannabidiol) in Patients with Refractory Epilepsy. Epilepsy Behav. 2023, 141, 109159. [Google Scholar] [CrossRef] [PubMed]
- Szaflarski, J.P.; Devinsky, O.; Lopez, M.; Park, Y.D.; Zentil, P.P.; Patel, A.D.; Thiele, E.A.; Wechsler, R.T.; Checketts, D.; Sahebkar, F. Long-term Efficacy and Safety of Cannabidiol in Patients with Treatment-resistant Epilepsies: Four-year Results from the Expanded Access Program. Epilepsia 2023, 64, 619–629. [Google Scholar] [CrossRef]
- Devinsky, O.; Cross, J.H.; Laux, L.; Marsh, E.; Miller, I.; Nabbout, R.; Scheffer, I.E.; Thiele, E.A.; Wright, S. Trial of Cannabidiol for Drug-Resistant Seizures in the Dravet Syndrome. N. Engl. J. Med. 2017, 376, 2011–2020. [Google Scholar] [CrossRef]
- Thiele, E.A.; Marsh, E.D.; French, J.A.; Mazurkiewicz-Beldzinska, M.; Benbadis, S.R.; Joshi, C.; Lyons, P.D.; Taylor, A.; Roberts, C.; Sommerville, K.; et al. Cannabidiol in Patients with Seizures Associated with Lennox-Gastaut Syndrome (GWPCARE4): A Randomised, Double-Blind, Placebo-Controlled Phase 3 Trial. Lancet 2018, 391, 1085–1096. [Google Scholar] [CrossRef]
- Fazlollahi, A.; Zahmatyar, M.; ZareDini, M.; Golabi, B.; Nejadghaderi, S.A.; Sullman, M.J.M.; Gharagozli, K.; Kolahi, A.-A.; Safiri, S. Adverse Events of Cannabidiol Use in Patients With Epilepsy: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2023, 6, e239126. [Google Scholar] [CrossRef]
- Cannabis Extract (Sativex®). 2024. Available online: https://www.panmerseyapc.nhs.uk/media/2377/cannabis_support.pdf (accessed on 1 September 2024).
- Syed, Y.Y.; McKeage, K.; Scott, L.J. Delta-9-Tetrahydrocannabinol/Cannabidiol (Sativex®): A Review of Its Use in Patients with Moderate to Severe Spasticity Due to Multiple Sclerosis. Drugs 2014, 74, 563–578. [Google Scholar] [CrossRef]
- Santarossa, T.M.; So, R.; Smyth, D.P.; Gustavsen, D.S.; Tsuyuki, D.R.T. Medical Cannabis Use in Canadians with Multiple Sclerosis. Mult. Scler. Relat. Disord. 2022, 59, 103638. [Google Scholar] [CrossRef]
- Rekand, T. THC:CBD Spray and MS Spasticity Symptoms: Data from Latest Studies. Eur. Neurol. 2014, 71, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Jazz Pharmaceuticals. A Randomized Controlled Trial of Cannabidiol (GWP42003-P, CBD) for Seizures in Tuberous Sclerosis Complex (GWPCARE6); Jazz Pharmaceuticals: Dublin, Ireland, 2022. [Google Scholar]
- Jazz Pharmaceuticals. Announces Initiation of Phase 3 Trial Evaluating Epidiolex®/Epidyolex® (Cannabidiol) for Patients with Epilepsy with Myoclonic-Atonic Seizures; Jazz Pharmaceuticals: Dublin, Ireland, 2022. [Google Scholar]
- Pauli, C.S.; Conroy, M.; Vanden Heuvel, B.D.; Park, S.-H. Cannabidiol Drugs Clinical Trial Outcomes and Adverse Effects. Front. Pharmacol. 2020, 11, 63. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.; Winterstein, A. Potential Adverse Drug Events and Drug–Drug Interactions with Medical and Consumer Cannabidiol (CBD) Use. J. Clin. Med. 2019, 8, 989. [Google Scholar] [CrossRef] [PubMed]
- Van Ours, J.C.; Williams, J.; Fergusson, D.; Horwood, L.J. Cannabis Use and Suicidal Ideation. J. Health Econ. 2013, 32, 524–537. [Google Scholar] [CrossRef] [PubMed]
- Woerdenbag, H.J.; Olinga, P.; Kok, E.A.; Brugman, D.A.P.; Van Ark, U.F.; Ramcharan, A.S.; Lebbink, P.W.; Hoogwater, F.J.H.; Knapen, D.G.; De Groot, D.J.A.; et al. Potential, Limitations and Risks of Cannabis-Derived Products in Cancer Treatment. Cancers 2023, 15, 2119. [Google Scholar] [CrossRef]
- Blal, K.; Besser, E.; Procaccia, S.; Schwob, O.; Lerenthal, Y.; Abu Tair, J.; Meiri, D.; Benny, O. The Effect of Cannabis Plant Extracts on Head and Neck Squamous Cell Carcinoma and the Quest for Cannabis-Based Personalized Therapy. Cancers 2023, 15, 497. [Google Scholar] [CrossRef]
- United Nations Office on Drugs and Crime World Drug Report Drug Use and Health Consequences. 2020. Available online: https://wdr.unodc.org/wdr2020/field/WDR20_Booklet_2.pdf (accessed on 1 September 2024).


| Extract, Compounds /Concentrations | Tested System | Mechanism of Action | References |
|---|---|---|---|
| C. sativa L. extracts and cannabinoids induction of apoptosis | |||
| CBD 3 μmol/L | A549, H460 human lung cancer cells | Upregulates COX-2 and PPAR-γ | [97] |
| C. sativa L. extracts—30 μmol/L CBD—10 μmol/L | PC3 prostate cancer cells | Increase caspase 3/7 activity, upregulate TP53 and Bax expression, and induce silencing of RBBP6. | [100] |
| CBD 3.125–50.0 μmol/L | HCC—Hepatocellular carcinoma cells | Enhances p53 activation via ER stress | [113] |
| Extract from C. sativa 50% and 100% of their corresponding IC50 values | Melanoma cell lines MM418-C1, MM329, MM96L | Increases pro-apoptotic markers (Bax) expression Decreases anti-apoptotic markers (Bcl-2-) expression | [103] |
| CBD and THC 6, 10, 15 μmol/L | A375, A2058 and SK-Mel-28 melanoma cell lines | Release of mitochondrial cytochrome c and activate caspase-3/7 | [110] |
| Cannabinol (CBN) | A172 glioblastoma, HepG2 liver cancer and HCC1806 breast cancer cell lines | Downregulates p21 and p27 arrested cell cycle in G1 or S phase | [114] |
| CBD induction of autophagy | |||
| CBD 2–20 μmol/L | Human cholangiocarcinoma cells (KKU-213B, KKU-100, KKU-055) | Upregulates LC3BII, downregulates p62, inhibits p-PI3K, p-AKT, and p-mTOR pathways | [101] |
| CBD 3, 6, or 10 μmol/L | Head and neck squamous cell carcinoma (FaDu, SNU899, SCC15, Hep2) | Increases in Beclin- and LC3II-coding gene expression | [115] |
| C. sativa L. extracts and cannabinoids induction of tumor regression | |||
| C. sativa L. essential oil extracted from flowers and leaves | Lewis lung cancer grafted mice model | Inhibits tumor growth, decreases of TNF-α and IL-6, increases in CD4+, CD8+ T lymphocytes count | [116] |
| CBD 5 mg/kg BW | Mice C57BL/6 with B16F10 murine melanoma tumor | Reduces tumor size | [96] |
| CBD—10 mg/kg BW THC—10 mg/kg BW | NSG mice | Deplete tumor growth | [110] |
| Cannabigerrol (CBG) 2.5 mg/kg or 3.75 mg/kg | Tumor-bearing mice | Decreases tumor progression by combining CBG and αPD-L1, decreases colony-stimulating factor 1 (CSF-1) level | [102] |
| PHEC-66 extract from C. sativa L. | MM418-C1, MM329, and MM96L melanoma cell lines | Induces DNA fragmentation, and arrests cell progression at the G1 cell cycle checkpoint. | [103] |
| Cannabinoids decrease tumor proliferation. | |||
| CBD and THC 3.9–500 µg/mL | A375 human melanoma cells | Inhibit ERK1/2 signaling pathway phosphorylation, which is responsible for the regulation of cell proliferation. | [104] |
| Cannabinol (CBN) | A172 glioblastoma, HepG2 liver cancer and HCC1806 breast cancer cell lines | Decreases cancer cell proliferation | [114] |
| Cannabinoids induce suppression of cell invasion/inhibition of angiogenesis | |||
| R(+)-methanandamide analog 0.1 µmol/L THC—0.01 µmol/L | HeLa, C33A human cervical carcinoma, A549 human lung cancer cells | Increase expression of TIMP-1, which mediates an anti-invasive effect of cannabinoids. | [105] |
| Cannabinoid JWH-133 8 days at 50 µg/day | Mice with malignant gliomas | Inhibits expression of proangiogenic factors: vascular endothelial growth factor, and angiopoietin 2 | [36] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bukowska, B. Current and Potential Use of Biologically Active Compounds Derived from Cannabis sativa L. in the Treatment of Selected Diseases. Int. J. Mol. Sci. 2024, 25, 12738. https://doi.org/10.3390/ijms252312738
Bukowska B. Current and Potential Use of Biologically Active Compounds Derived from Cannabis sativa L. in the Treatment of Selected Diseases. International Journal of Molecular Sciences. 2024; 25(23):12738. https://doi.org/10.3390/ijms252312738
Chicago/Turabian StyleBukowska, Bożena. 2024. "Current and Potential Use of Biologically Active Compounds Derived from Cannabis sativa L. in the Treatment of Selected Diseases" International Journal of Molecular Sciences 25, no. 23: 12738. https://doi.org/10.3390/ijms252312738
APA StyleBukowska, B. (2024). Current and Potential Use of Biologically Active Compounds Derived from Cannabis sativa L. in the Treatment of Selected Diseases. International Journal of Molecular Sciences, 25(23), 12738. https://doi.org/10.3390/ijms252312738






