Identification of Diagnostic and Prognostic Subnetwork Biomarkers for Women with Breast Cancer Using Integrative Genomic and Network-Based Analysis
Abstract
1. Introduction
2. Results
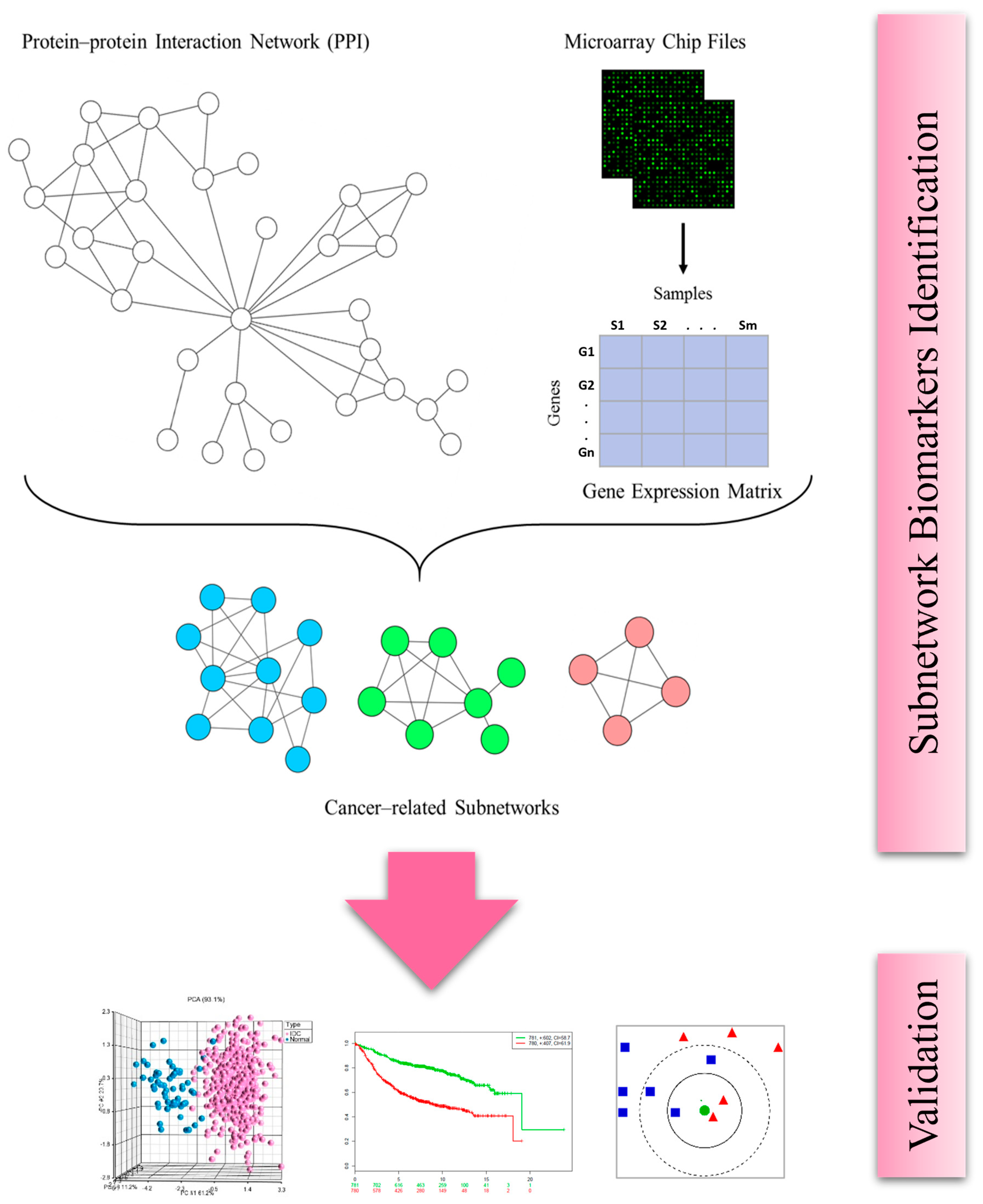
2.1. Identification of Subnetwork Biomarkers
2.2. Diagnostic Significance of the Identified Subnetwork Biomarkers
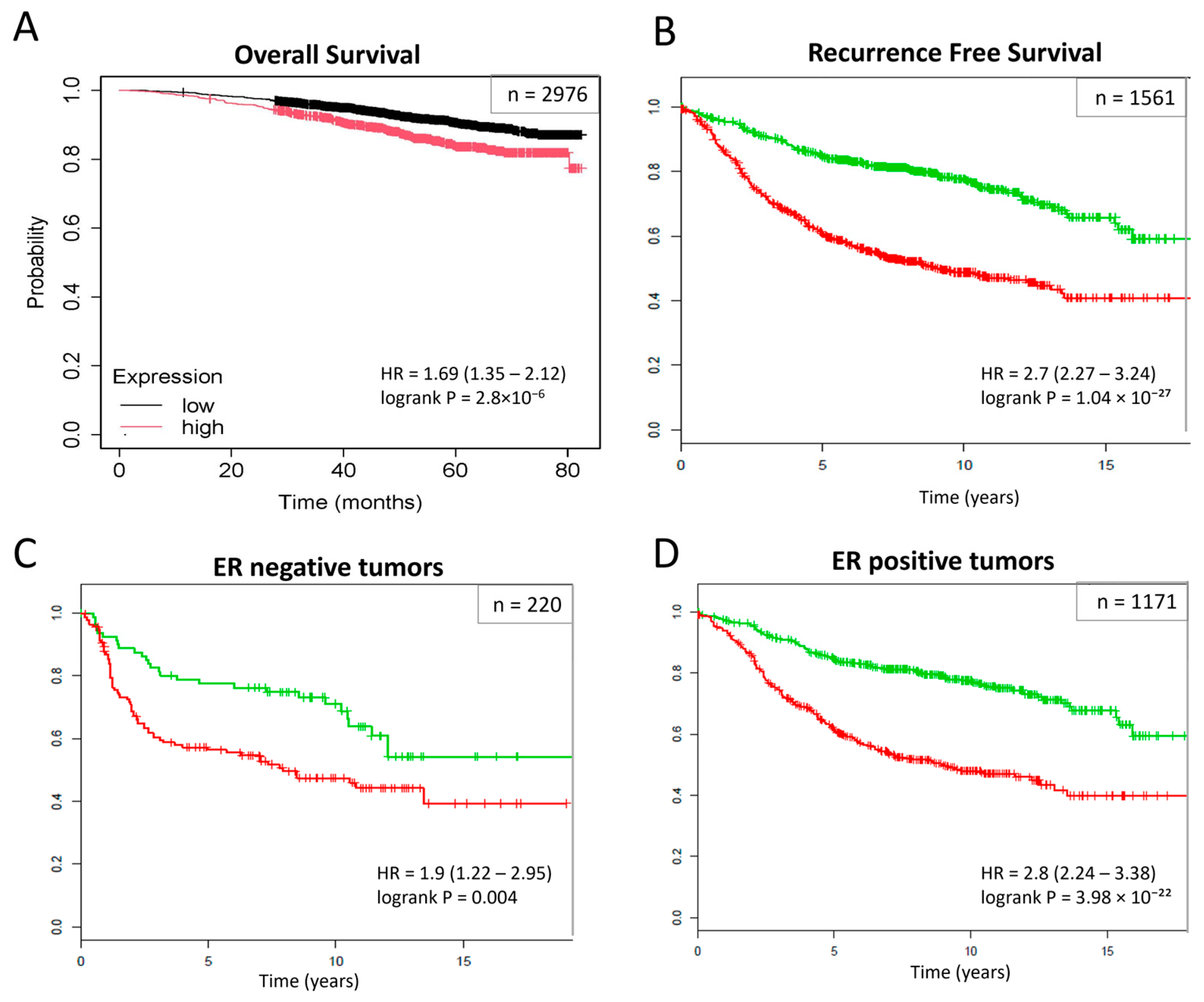
2.3. Prognostic Significance of the Identified Subnetwork Biomarkers
3. Discussion
4. Materials and Methods
4.1. Gene Expression Datasets
4.2. Subnetwork Identification
4.3. Classification
4.4. Survival Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Denkert, C.; Liedtke, C.; Tutt, A.; von Minckwitz, G. Molecular alterations in triple-negative breast cancer-the road to new treatment strategies. Lancet 2017, 389, 2430–2442. [Google Scholar] [CrossRef] [PubMed]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat. Rev. Dis. Primers 2019, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- Perou, C.M.; Sorlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Curtis, C.; Shah, S.P.; Chin, S.F.; Turashvili, G.; Rueda, O.M.; Dunning, M.J.; Speed, D.; Lynch, A.G.; Samarajiwa, S.; Yuan, Y.Y.; et al. The genomic and transcriptomic architecture of 2000 breast tumours reveals novel subgroups. Nature 2012, 486, 346–352. [Google Scholar] [CrossRef]
- Colak, D.; Nofal, A.; AlBakheet, A.; Nirmal, M.; Jeprel, H.; Eldali, A.; AL-Tweigeri, T.; Tulbah, A.; Ajarim, D.; Al Malik, O.; et al. Age-Specific Gene Expression Signatures for Breast Tumors and Cross-Species Conserved Potential Cancer Progression Markers in Young Women. PLoS ONE 2013, 8, e63204. [Google Scholar] [CrossRef]
- Barabási, A.L.; Gulbahce, N.; Loscalzo, J. Network medicine: A network-based approach to human disease. Nat. Rev. Genet. 2011, 12, 56–68. [Google Scholar] [CrossRef]
- Al-Harazi, O.; El Allali, A.; Colak, D. Biomolecular Databases and Subnetwork Identification Approaches of Interest to Big Data Community: An Expert Review. Omics 2019, 23, 138–151. [Google Scholar] [CrossRef]
- Al-Harazi, O.; Kaya, I.H.; El Allali, A.; Colak, D. A Network-Based Methodology to Identify Subnetwork Markers for Diagnosis and Prognosis of Colorectal Cancer. Front. Genet. 2021, 12, 721949. [Google Scholar] [CrossRef]
- Kim, M.; Lee, S.; Lim, S.; Lee, D.Y.; Kim, S. Subnetwork representation learning for discovering network biomarkers in predicting lymph node metastasis in early oral cancer. Sci. Rep. 2022, 12, 488. [Google Scholar] [CrossRef]
- Al-Harazi, O.; Al Insaif, S.; Al-Ajlan, M.A.; Kaya, N.; Dzimiri, N.; Colak, D. Integrated Genomic and Network-Based Analyses of Complex Diseases and Human Disease Network. J. Genet. Genom. 2016, 43, 349–367. [Google Scholar] [CrossRef] [PubMed]
- Caldera, M.; Buphamalai, P.; Müller, F.; Menche, J. Interactome-based approaches to human disease. Curr. Opin. Syst. Biol. 2017, 3, 88–94. [Google Scholar] [CrossRef]
- Doungpan, N.; Engchuan, W.; Chan, J.H.; Meechai, A. GSNFS: Gene subnetwork biomarker identification of lung cancer expression data. BMC Med. Genom. 2016, 9, 70. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gyorffy, B.; Lanczky, A.; Eklund, A.C.; Denkert, C.; Budczies, J.; Li, Q.; Szallasi, Z. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1809 patients. Breast Cancer Res. Treat. 2010, 123, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.M.; Stein, L. A network module-based method for identifying cancer prognostic signatures. Genome Biol. 2012, 13, R112. [Google Scholar] [CrossRef]
- Cao, B.W.; Patel, K.B.; Li, T.Y.; Yao, S.J.; Chung, C.H.; Wang, X.F. A subnetwork-based framework for prioritizing and evaluating prognostic gene modules from cancer transcriptome data. Iscience 2023, 26, 105915. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Franceschini, A.; Wyder, S.; Forslund, K.; Heller, D.; Huerta-Cepas, J.; Simonovic, M.; Roth, A.; Santos, A.; Tsafou, K.P.; et al. STRING v10: Protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015, 43, D447–D452. [Google Scholar] [CrossRef]
- Saha, A.; Jeon, M.; Tan, A.C.; Kang, J. iCOSSY: An Online Tool for Context-Specific Subnetwork Discovery from Gene Expression Data. PLoS ONE 2015, 10, e0131656. [Google Scholar] [CrossRef][Green Version]
- Saha, A.; Tan, A.C.; Kang, J. Automatic Context-Specific Subnetwork Discovery from Large Interaction Networks. PLoS ONE 2014, 9, e84227. [Google Scholar] [CrossRef][Green Version]
- Kahm, Y.J.; Kim, I.G.; Jung, U.; Lee, J.H.; Kim, R.K. Impact of KIF4A on Cancer Stem Cells and EMT in Lung Cancer and Glioma. Cancers 2023, 15, 5523. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Taniwaki, M.; Takano, A.; Ishikawa, N.; Yasui, W.; Inai, K.; Nishimura, H.; Tsuchiya, E.; Kohno, N.; Nakamura, Y.; Daigo, Y. Activation of KIF4A as a prognostic biomarker and therapeutic target for lung cancer. Clin. Cancer Res. 2007, 13, 6624–6631. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.T.; Hu, Y.Z.; Ding, H.; Wu, Q.; Pan, J.H.; Zhao, X.X.; Pan, Y.L. The overexpression of ZWINT in integrated bioinformatics analysis forecasts poor prognosis in breast cancer. Transl. Cancer Res. 2020, 9, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.R.; Shen, M.Y.; Zhang, Z.Y. ZW10 Binding Factor (ZWINT), a Direct Target of Mir-204, Predicts Poor Survival and Promotes Proliferation in Breast Cancer. Med. Sci. Monit. 2020, 26, e921659. [Google Scholar] [CrossRef]
- Xiong, Y.; Lu, J.; Fang, Q.L.; Lu, Y.Y.; Xie, C.R.; Wu, H.T.; Yin, Z.Y. UBE2C functions as a potential oncogene by enhancing cell proliferation, migration, invasion, and drug resistance in hepatocellular carcinoma cells. Biosci. Rep. 2019, 39, Bsr20182384. [Google Scholar] [CrossRef]
- Liu, Y.J.; Zhu, Z.C.; Xiong, Z.Q.; Zheng, J.; Hui, Z.L.; Qiu, J.F. Knockdown of protein tyrosine phosphatase receptor U inhibits growth and motility of gastric cancer cells. Int. J. Clin. Exp. Pathol. 2014, 7, 5750–5761. [Google Scholar]
- Gao, B.; Wu, X.J.; Bu, L.; Jiang, Q.W.; Wang, L.; Liu, H.N.; Zhang, X.M.; Wu, Y.Z.; Li, X.X.; Li, J.T.; et al. Atypical inflammatory kinase IKBKE phosphorylates and inactivates FoxA1 to promote liver tumorigenesis. Sci. Adv. 2024, 10, eadk2285. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Brueffer, C.; Vallon-Christersson, J.; Grabau, D.; Ehinger, A.; Hakkinen, J.; Hegardt, C.; Malina, J.; Chen, Y.L.; Bendahl, P.O.; Manjer, J.; et al. Clinical Value of RNA Sequencing-Based Classifiers for Prediction of the Five Conventional Breast Cancer Biomarkers: A Report From the Population-Based Multicenter Sweden Cancerome Analysis Network-Breast Initiative. JCO Precis. Oncol. 2018, 2, 1–18. [Google Scholar] [CrossRef]
- Gyorffy, B. Survival analysis across the entire transcriptome identifies biomarkers with the highest prognostic power in breast cancer. Comput. Struct. Biotechnol. J. 2021, 19, 4101–4109. [Google Scholar] [CrossRef]
- Aguirre-Gamboa, R.; Gomez-Rueda, H.; Martinez-Ledesma, E.; Martinez-Torteya, A.; Chacolla-Huaringa, R.; Rodriguez-Barrientos, A.; Tamez-Pena, J.G.; Trevino, V. SurvExpress: An online biomarker validation tool and database for cancer gene expression data using survival analysis. PLoS ONE 2013, 8, e74250. [Google Scholar] [CrossRef]
- Colak, D.; Elallali, A.; Kaya, I.; Alharazi, O. Identification of subnetwork markers with diagnostic and prognostic potential for women with breast cancer. Eur. J. Hum. Genet. 2024, 32, 267. [Google Scholar]


| GO Biological Process Term | p-Value | Genes |
|---|---|---|
| Subnetwork 1 | ||
| blood vessel development | 1.8 × 10−7 | NRP2, SLC12A6, SERPINF1, ID1, GATA6, NOTCH4, VEGFA, ZC3H12A, SEMA3C, ESM1 |
| angiogenesis | 2.0 × 10−7 | NRP2, SLC12A6, SERPINF1, ID1, GATA6, NOTCH4, VEGFA, ZC3H12A, ESM1 |
| gland development | 3.6 × 10−6 | TNFRSF11A, SERPINF1, GATA6, HOXD3, NOTCH4, VEGFA, SEMA3C, NKX2-3 |
| regulation of cell proliferation | 1.4 × 10−5 | NRP2, TNFRSF11A, SERPINF1, ID1, SPEG, GATA6, VEGFA, TXK, ESM1, NR4A3, NKX2-3, THPO |
| cell surface receptor signaling pathway | 1.8 × 10−5 | NRP2, ERH, NR4A3, ESM1, HOMER1, TNFRSF11A, ID1, GATA6, HOXD3, NOTCH4, VEGFA, SEMA3C, TXK, CRIM1, THPO |
| Subnetwork 2 | ||
| cell surface receptor signaling pathway | 5.2 × 10−4 | GPR83, STYK1, STAP1, SHARPIN, VIL1, SPRED1, PTPRU, KIT, PTPRO |
| enzyme-linked receptor protein signaling pathway | 7.9 × 10−4 | STYK1, STAP1, VIL1, SPRED1, PTPRU, KIT |
| cell migration | 2.1 × 10−3 | STYK1, STAP1, VIL1, PTPRU, KIT, PTPRO |
| movement of cell or subcellular component | 2.2 × 10−3 | STYK1, LYVE1, STAP1, VIL1, PTPRU, KIT, PTPRO |
| cell motility | 3.5 × 10−3 | STYK1, STAP1, VIL1, PTPRU, KIT, PTPRO |
| Subnetwork 3 | ||
| sister chromatid cohesion | 8.7 × 10−10 | CENPO, CENPQ, ZWINT, CENPP, KNTC1, RANGAP1, CENPI |
| CENP-A-containing chromatin organization | 7.1 × 10−6 | CENPO, CENPQ, CENPP, CENPI |
| DNA replication-independent nucleosome assembly | 1.4 × 10−5 | CENPO, CENPQ, CENPP, CENPI |
| histone exchange | 1.9 × 10−5 | CENPO, CENPQ, CENPP, CENPI |
| single-organism organelle organization | 2.5 × 10−4 | CENPO, TTBK2, CENPQ, ZWINT, CENPP, KNTC1, RANGAP1, CENPI |
| Subnetwork 4 | ||
| cell division | 3.4 × 10−12 | KIF14, KIF2C, FAM64A, KIF4A, CDCA8, PRC1, EVI5, ESPL1, UBE2C, KIF20A |
| mitotic nuclear division | 2.2 × 10−9 | KIF14, KIF2C, FAM64A, KIF4A, CDCA8, PRC1, ESPL1, UBE2C |
| chromosome segregation | 2.6 × 10−8 | KIF14, KIF2C, KIF4A, CDCA8, PRC1, ESPL1, UBE2C |
| organelle fission | 2.8 × 10−8 | KIF14, KIF2C, FAM64A, KIF4A, CDCA8, PRC1, ESPL1, UBE2C |
| cell cycle | 4.6 × 10−8 | KIF14, KIF2C, FAM64A, KIF4A, CDCA8, PRC1, EVI5, ESPL1, UBE2C, KIF20A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Harazi, O.; El Allali, A.; Kaya, N.; Colak, D. Identification of Diagnostic and Prognostic Subnetwork Biomarkers for Women with Breast Cancer Using Integrative Genomic and Network-Based Analysis. Int. J. Mol. Sci. 2024, 25, 12779. https://doi.org/10.3390/ijms252312779
Al-Harazi O, El Allali A, Kaya N, Colak D. Identification of Diagnostic and Prognostic Subnetwork Biomarkers for Women with Breast Cancer Using Integrative Genomic and Network-Based Analysis. International Journal of Molecular Sciences. 2024; 25(23):12779. https://doi.org/10.3390/ijms252312779
Chicago/Turabian StyleAl-Harazi, Olfat, Achraf El Allali, Namik Kaya, and Dilek Colak. 2024. "Identification of Diagnostic and Prognostic Subnetwork Biomarkers for Women with Breast Cancer Using Integrative Genomic and Network-Based Analysis" International Journal of Molecular Sciences 25, no. 23: 12779. https://doi.org/10.3390/ijms252312779
APA StyleAl-Harazi, O., El Allali, A., Kaya, N., & Colak, D. (2024). Identification of Diagnostic and Prognostic Subnetwork Biomarkers for Women with Breast Cancer Using Integrative Genomic and Network-Based Analysis. International Journal of Molecular Sciences, 25(23), 12779. https://doi.org/10.3390/ijms252312779







