Candidate SNP Markers Significantly Altering the Affinity of the TATA-Binding Protein for the Promoters of Human Genes Associated with Primary Open-Angle Glaucoma
Abstract
:1. Introduction
2. Results
2.1. Assessment of the Phylostratigraphic Age Index (PAI) of the POAG-Related Genes
2.2. Data Mining Analysis of the POAG-Related Genes with ANDSystem
2.3. Verification of ANDSystem Outputs for POAG-Related Genes Against Results Obtained with Independent Data Mining Tools
2.4. Supervised Annotation of the Effects of Changes in the POAG-Related Genes’ Expression Levels on POAG Alleviation and Aggravation
2.5. In Silico Estimation of the Effects of SNPs in the POAG-Related Genes’ Promoters on TBP Affinity for These Genes’ Promoters
2.6. Selective In Vitro Verification of In Silico Estimates of the Effects of SNPs in Human Gene Promoters on TBP Affinity for These Promoters
2.7. Frequencies of the SNPs That Significantly Change TBP Affinity for the Promoters of the POAG-Related Genes and for the Promoters of All Human Genes
2.8. Assessing the Effects of SNP-Induced Increases and Decreases in the Expression Levels of the Older and Younger POAG-Related Genes on POAG Alleviation and Aggravation
2.9. Verification Results for the Proposed Candidate SNP Markers of POAG Using ClinVar Entries Related to Biomedical SNP Markers of Diseases
2.10. RNA-Seq Data on Domestic and Wild Animals for Verification of the Proposed Candidate SNP Markers That Change the Expression Levels of the POAG-Related Genes
3. Discussion
3.1. Why the 153 POAG-Related Genes?
3.2. POAG-Related Genes That Appeared Before and After Chordata Became Different as Lampreys Evolved the Camera-Type Eye
3.3. The 123 Older POAG-Related Genes Responsible for Pathogenesis and Apoptosis Play a Critical Role in How Misfolded Protein Aggregates Can Aggravate POAG
3.4. Normal Distribution of PAI Values of the 30 Younger POAG-Related Genes Involved in the Immune Response Has a Peak at Vertebrata, When Adaptive Immunity Appeared
3.5. Differences Between Domestic and Wild Animals in How POAG-Related Genes Alleviate or Aggravate POAG Fit in with Current Views of Natural Selection in Domestic and Wild Animals
3.6. POAG as a Symptom of the Human Self-Domestication Syndrome Is Consistent with POAG Aggravation by Anthropogenic Factors
3.7. Study Limitations
4. Materials and Methods
4.1. The Human Genes
4.2. In Silico Rating of the KEGG-Based Phylostratigraphic Age Index (PAI) of a Human Gene
4.3. Data Mining Analysis of Freely Available Publications and Databases Related to POAG
4.4. Biomedical Data on the Effect of Underexpression and Overexpression of the POAG-Related Genes on POAG Alleviation and Aggravation
4.5. In Silico Estimation of How SNPs in the POAG-Related Genes’ Promoters Change These Genes’ Expression Levels
4.6. Selective Verification of the In Silico Estimates of the Effect of SNPs in Human Gene Promoters on TBP Affinity for These Promoters Against the Norm
4.7. Verification Methods for the Proposed Candidate SNP Markers of POAG Using ClinVar Entries Related to Biomedical SNP Markers of Diseases
4.8. Differentially Expressed Genes (DEGs) in Domesticated Animals and Their Nearest Wild Counterparts
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAO | American Academy of Ophthalmology |
| APGS | Asia-Pacific Glaucoma Society |
| DEG | Differentially Expressed Gene |
| DT | digital tonometry |
| EGS | European Glaucoma Society |
| IOP | intraocular pressure |
| IQR | interquartile range as a height of a given box-and-whisker plot |
| JOAG | juvenile open-angle glaucoma |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| KO | KEGG Orthology |
| Mya | million years ago |
| NTG | normal tension glaucoma |
| OCT | optical coherence tomography |
| OHT | ocular hypertension |
| PAI | phylostratigraphic age index |
| PCG | primary congenital glaucoma |
| POAG | primary open-angle glaucoma |
| SAP | standard automated perimetry |
| SNP | single-nucleotide polymorphism |
| TBP | TATA-binding protein |
| WHO | World Health Organization |
References
- Weinreb, R.N.; Khaw, P.T. Primary open-angle glaucoma. Lancet 2004, 363, 1711–1720. [Google Scholar] [CrossRef] [PubMed]
- Umezurike, B.C.; Akhimien, M.O.; Udeala, O.; Green, U.G.; Okpechi-Agbo, U.; Ohaeri, M.U. Primary open angle glaucoma: The pathophysiolgy, mechanisms, future diagnostic and therapeutic directions. Ophthalmol. Res. Int. J. 2019, 10, 1–17. [Google Scholar] [CrossRef]
- Sekhar, G.C. Glaucoma definition: Implications for equitable care. Indian. J. Ophthalmol. 2021, 69, 1025–1026. [Google Scholar] [CrossRef] [PubMed]
- Iyer, J.V.; Boland, M.V.; Jefferys, J.; Quigley, H. Defining glaucomatous optic neuropathy using objective criteria from structural and functional testing. Br. J. Ophthalmol. 2021, 105, 789–793. [Google Scholar] [CrossRef] [PubMed]
- Kalyani, V.K.; Bharucha, K.M.; Goyal, N.; Deshpande, M.M. Comparison of diagnostic ability of standard automated perimetry, short wavelength automated perimetry, retinal nerve fiber layer thickness analysis and ganglion cell layer thickness analysis in early detection of glaucoma. Indian. J. Ophthalmol. 2021, 69, 1108–1112. [Google Scholar] [PubMed]
- Prum, B.E., Jr.; Rosenberg, L.F.; Gedde, S.J.; Mansberger, S.L.; Stein, J.D.; Moroi, S.E.; Herndon, L.W., Jr.; Lim, M.C.; Williams, R.D. Primary Open-Angle Glaucoma Preferred Practice Pattern(®) guidelines. Ophthalmology 2016, 123, P41–P111. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.P. Understanding the new primary open-angle glaucoma preferred practice pattern. Int. Ophthalmol. Clin. 1998, 38, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Sommer, A.; Tielsch, J.M.; Katz, J.; Quigley, H.A.; Gottsch, J.D.; Javitt, J.; Singh, K. Relationship between intraocular pressure and primary open angle glaucoma among white and black Americans. The Baltimore eye survey. Arch. Ophthalmol. 1991, 109, 1090–1095. [Google Scholar] [CrossRef] [PubMed]
- Erb, C.; Predel, H.G. Relevance of arterial hypertension in primary open-angle glaucoma. Klin. Monbl Augenheilkd. 2014, 231, 136–143. [Google Scholar]
- Kouassi Nzoughet, J.; Guehlouz, K.; Leruez, S.; Gohier, P.; Bocca, C.; Muller, J.; Blanchet, O.; Bonneau, D.; Simard, G.; Milea, D.; et al. A data mining metabolomics exploration of glaucoma. Metabolites 2020, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.J.; Hedberg-Buenz, A.; DeLuca, A.P.; Stone, E.M.; Alward, W.L.M.; Fingert, J.H. Primary congenital and developmental glaucomas. Hum. Mol. Genet. 2017, 26, R28–R36. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.; Jain, M.; Abdull, M.M.; Bastawrous, A. The association between cigarette smoking and primary open-angle glaucoma: A systematic review. Int. Ophthalmol. 2017, 37, 291–301. [Google Scholar] [CrossRef]
- Mahmoudinezhad, G.; Nishida, T.; Weinreb, R.N.; Baxter, S.L.; Chang, A.C.; Nikkhoy, N.; Walker, E.; Liebmann, J.M.; Girkin, C.A.; Moghimi, S. Associations of smoking and alcohol consumption with the development of open angle glaucoma: A retrospective cohort study. BMJ Open 2023, 13, e072163. [Google Scholar] [CrossRef] [PubMed]
- Roddy, G.W. Metabolic syndrome and the aging retina. Curr. Opin. Ophthalmol. 2021, 32, 280–287. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, J.M.; Lee, K.Y.; Kim, B.; Lee, M.Y.; Park, K.H. Relationships between obesity, nutrient supply and primary open angle glaucoma in koreans. Nutrients 2020, 12, 878. [Google Scholar] [CrossRef]
- Sbai, O.; Torrisi, F.; Fabrizio, F.P.; Rabbeni, G.; Perrone, L. Effect of the mediterranean diet (MeDi) on the progression of retinal disease: A narrative review. Nutrients 2024, 16, 3169. [Google Scholar] [CrossRef] [PubMed]
- Gildea, D.; Doyle, A.; O’Connor, J. The effect of exercise on intraocular pressure and glaucoma. J. Glaucoma 2024, 33, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Giacometti, H.H.A.R.; Coelho, L.F.; Iankilevich, L.G.; Valentin, L.S.S.; Ferreira, L.A.; Balbino, M.; Seixas, R.C.S. Prevalence of anxiety and depression among patients with glaucoma. Front. Psychol. 2024, 15, 1410890. [Google Scholar] [CrossRef] [PubMed]
- D’Esposito, F.; Gagliano, C.; Bloom, P.A.; Cordeiro, M.F.; Avitabile, A.; Gagliano, G.; Costagliola, C.; Avitabile, T.; Musa, M.; Zeppieri, M. Epigenetics in glaucoma. Medicina 2024, 60, 905. [Google Scholar] [CrossRef] [PubMed]
- Kanso, N.; Hashimi, M.; Amin, H.A.; Day, A.C.; Drenos, F. No evidence that vitamin D levels or deficiency are associated with the risk of open-angle glaucoma in individuals of european ancestry: A mendelian randomisation analysis. Genes 2024, 15, 1084. [Google Scholar] [CrossRef]
- Sharif, N.A. Elevated intraocular pressure and glaucomatous optic neuropathy: Genes to disease mechanisms, therapeutic drugs, and gene therapies. Pharmaceuticals 2023, 16, 870. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z. PubMed and beyond: A survey of web tools for searching biomedical literature. Database 2011, 2011, baq036. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Zhang, Z.; Shi, J.; He, B. Clinical application of NGS-based SNP haplotyping for the preimplantation genetic diagnosis of primary open angle glaucoma. Syst. Biol. Reprod. Med. 2019, 65, 258–263. [Google Scholar] [CrossRef]
- Hamel, A.R.; Yan, W.; Rouhana, J.M.; Monovarfeshani, A.; Jiang, X.; Mehta, P.A.; Advani, J.; Luo, Y.; Liang, Q.; Rajasundaram, S.; et al. Integrating genetic regulation and single-cell expression with GWAS prioritizes causal genes and cell types for glaucoma. Nat. Commun. 2024, 15, 396. [Google Scholar] [CrossRef]
- Fang Kho, P.; Lea, R.A.; Benton, M.C.; Eccles, D.; Haupt, L.M.; Hewitt, A.W.; Sherwin, J.C.; Mackey, D.A.; Griffiths, L.R. Expression QTL analysis of glaucoma endophenotypes in the Norfolk Island isolate provides evidence that immune-related genes are associated with optic disc size. J. Hum. Genet. 2018, 63, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Plotnikov, D.; Wang, H.; Shi, D.; Li, C.; Zhang, X.; Zhang, X.; Tang, S.; Shang, X.; Hu, Y.; et al. GWAS-by-subtraction reveals an IOP-independent component of primary open angle glaucoma. Nat. Commun. 2024, 15, 8962. [Google Scholar] [CrossRef] [PubMed]
- Petty, H.R. Frontiers of complex disease mechanisms: Membrane surface tension may link genotype to phenotype in glaucoma. Front. Cell Dev. Biol. 2018, 6, 32. [Google Scholar] [CrossRef] [PubMed]
- Wiggs, J.L.; Pasquale, L.R. Genetics of glaucoma. Hum. Mol. Genet. 2017, 26, R21–R27. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.J.; Zhang, X. Advances in the study of POAG-related genes and central nervous system diseases. Int. Eye Sci. 2021, 12, 436–441. [Google Scholar]
- Liu, Y.; Garrett, M.E.; Yaspan, B.L.; Bailey, J.C.; Loomis, S.J.; Brilliant, M.; Budenz, D.L.; Christen, W.G.; Fingert, J.H.; Gaasterland, D.; et al. DNA copy number variants of known glaucoma genes in relation to primary open-angle glaucoma. Investig. Ophthalmol. Vis. Sci. 2014, 55, 8251–8258. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, C.; Zheng, Y.; Yuan, X.L.; Chen, S.; Xu, Y.; Chen, L.J.; Pang, C.P.; Zhang, M.; Ng, T.K. Primary open-angle glaucoma risk prediction with ABCA1 and LOC102723944 variants and their genotype-phenotype correlations in southern Chinese population. Mol. Genet. Genom. 2023, 298, 1343–1352. [Google Scholar] [CrossRef]
- Fuse, N. Genetic bases for glaucoma. Tohoku J. Exp. Med. 2010, 221, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Amberger, J.; Bocchini, C.; Schiettecatte, F.; Scott, A.; Hamosh, A. OMIM.org: Online Mendelian Inheritance in Man (OMIM®), an online catalog of human genes and genetic disorders. Nucleic Acids Res. 2015, 43, D789–D798. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Xie, L.; Ye, J.; Liu, Y.; He, X. Screening of candidate genes for primary open angle glaucoma. Mol. Vis. 2012, 18, 2119–2126. [Google Scholar]
- Pradhan, S.; Sengupta, M.; Dutta, A.; Bhattacharyya, K.; Bag, S.K.; Dutta, C.; Ray, K. Indian genetic disease database. Nucleic Acids Res. 2011, 39, D933–D938. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.R.; Hem, V.; Katz, K.S.; Ovetsky, M.; Wallin, C.; Ermolaeva, O.; Tolstoy, I.; Tatusova, T.; Pruitt, K.D.; Maglott, D.R.; et al. Gene: A gene-centered information resource at NCBI. Nucleic Acids Res. 2015, 43, D36–D42. [Google Scholar] [CrossRef] [PubMed]
- Danford, I.D.; Verkuil, L.D.; Choi, D.J.; Collins, D.W.; Gudiseva, H.V.; Uyhazi, K.E.; Lau, M.K.; Kanu, L.N.; Grant, G.R.; Chavali, V.R.M.; et al. Characterizing the “POAGome”: A bioinformatics-driven approach to primary open-angle glaucoma. Prog. Retin. Eye Res. 2017, 58, 89–114. [Google Scholar] [CrossRef] [PubMed]
- Landrum, M.J.; Lee, J.M.; Riley, G.R.; Jang, W.; Rubinstein, W.S.; Church, D.M.; Maglott, D.R. ClinVar: Public archive of relationships among sequence variation and human phenotype. Nucleic Acids Res. 2014, 42, D980–D985. [Google Scholar] [CrossRef] [PubMed]
- Zerbino, D.; Wilder, S.; Johnson, N.; Juettemann, T.; Flicek, P. The Ensembl regulatory build. Genom. Biol. 2015, 16, 56. [Google Scholar] [CrossRef]
- Oliveira, M.B.; de Vasconcellos, J.P.C.; Ananina, G.; Costa, V.P.; de Melo, M.B. Association between IL1A and IL1B polymorphisms and primary open angle glaucoma in a Brazilian population. Exp. Biol. Med. 2018, 243, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Ponomarenko, M.; Mironova, V.; Gunbin, K.; Savinkova, L. Hogness Box. In Brenner’s Encyclopedia of Genetics, 2nd ed.; Maloy, S., Hughes, K., Eds.; Academic Press: San Diego, CA, USA, 2013; Volume 3, pp. 491–494. [Google Scholar] [CrossRef]
- Martianov, I.; Viville, S.; Davidson, I. RNA polymerase II transcription in murine cells lacking the TATA binding protein. Science 2002, 298, 1036–1039. [Google Scholar] [CrossRef] [PubMed]
- Mogno, I.; Vallania, F.; Mitra, R.D.; Cohen, B.A. TATA is a modular component of synthetic promoters. Genome Res. 2010, 20, 1391–1397. [Google Scholar] [CrossRef]
- Ponomarenko, M.; Rasskazov, D.; Arkova, O.; Ponomarenko, P.; Suslov, V.; Savinkova, L.; Kolchanov, N. How to use SNP_TATA_Comparator to find a significant change in gene expression caused by the regulatory SNP of this gene’s promoter via a change in affinity of the TATA-binding protein for this promoter. Biomed. Res. Int. 2015, 2015, 359835. [Google Scholar] [CrossRef] [PubMed]
- Shikhevich, S.; Chadaeva, I.; Khandaev, B.; Kozhemyakina, R.; Zolotareva, K.; Kazachek, A.; Oshchepkov, D.; Bogomolov, A.; Klimova, N.V.; Ivanisenko, V.A.; et al. Differentially expressed genes and molecular susceptibility to human age-related diseases. Int. J. Mol. Sci. 2023, 24, 3996. [Google Scholar] [CrossRef] [PubMed]
- Oshchepkov, D.; Chadaeva, I.; Kozhemyakina, R.; Zolotareva, K.; Khandaev, B.; Sharypova, E.; Ponomarenko, P.; Bogomolov, A.; Klimova, N.V.; Shikhevich, S.; et al. Stress reactivity, susceptibility to hypertension, and differential expression of genes in hypertensive compared to normotensive patients. Int. J. Mol. Sci. 2022, 23, 2835. [Google Scholar] [CrossRef] [PubMed]
- Bogomolov, A.; Filonov, S.; Chadaeva, I.; Rasskazov, D.; Khandaev, B.; Zolotareva, K.; Kazachek, A.; Oshchepkov, D.; Ivanisenko, V.A.; Demenkov, P.; et al. Candidate SNP markers significantly altering the affinity of TATA-binding protein for the promoters of human hub genes for atherogenesis, atherosclerosis and atheroprotection. Int. J. Mol. Sci. 2023, 24, 9010. [Google Scholar] [CrossRef] [PubMed]
- Varzari, A.; Tudor, E.; Bodrug, N.; Corloteanu, A.; Axentii, E.; Deyneko, I.V. Age-specific association of CCL5 gene polymorphism with pulmonary tuberculosis: A case-control study. Genet. Test. Mol. Biomark. 2018, 22, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Filonov, S.V.; Podkolodnyy, N.L.; Podkolodnaya, O.A.; Tverdokhleb, N.N.; Ponomarenko, P.M.; Rasskazov, D.A.; Bogomolov, A.G.; Ponomarenko, M.P. Human_SNP_TATAdb: A database of SNPs that statistically significantly change the affinity of the TATA-binding protein to human gene promoters: Genome-wide analysis and use cases. Vavilovskii Zhurnal Genet. I Sel. (Vavilov J. Genet. Breed.) 2023, 27, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Mustafin, Z.S.; Lashin, S.A.; Matushkin, Y.u.G. Phylostratigraphic analysis of gene networks of human diseases. Vavilovskii Zhurnal Genet. I Sel. (Vavilov J. Genet. Breed.) 2021, 25, 46–56. [Google Scholar] [CrossRef]
- Mustafin, Z.; Mukhin, A.; Afonnikov, D.; Matushkin, Y.; Lashin, S. OrthoWeb-web application for macroand microevolutionary analysis of genes. In Bioinformatics of Genome Regulation and Structure/Systems Biology (BGRS/SB-2020); Institute of Cytology and Genetics: Novosibirsk, Russia, 2020; pp. 228–229. [Google Scholar]
- Ivanisenko, V.A.; Demenkov, P.S.; Ivanisenko, T.V.; Mishchenko, E.L.; Saik, O.V. A new version of the ANDSystem tool for automatic extraction of knowledge from scientific publications with expanded functionality for reconstruction of associative gene networks by considering tissue-specific gene expression. BMC Bioinform. 2019, 20 (Suppl. 1), 34. [Google Scholar] [CrossRef] [PubMed]
- Mi, H.; Ebert, D.; Muruganujan, A.; Mills, C.; Albou, L.P.; Mushayamaha, T.; Thomas, P.D. PANTHER version 16: A revised family classification, tree-based classification tool, enhancer regions and extensive API. Nucleic Acids Res. 2021, 49, D394–D403. [Google Scholar] [CrossRef] [PubMed]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef] [PubMed]
- Warde-Farley, D.; Donaldson, S.L.; Comes, O.; Zuberi, K.; Badrawi, R.; Chao, P.; Franz, M.; Grouios, C.; Kazi, F.; Lopes, C.T.; et al. The GeneMANIA prediction server: Biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res. 2010, 38, W214–W220. [Google Scholar] [CrossRef]
- Stelzer, G.; Rosen, N.; Plaschkes, I.; Zimmerman, S.; Twik, M.; Fishilevich, S.; Stein, T.I.; Nudel, R.; Lieder, I.; Mazor, Y.; et al. The GeneCards suite: From gene data mining to disease genome sequence analyses. Curr. Protoc. Bioinform. 2016, 54, 1.30.1–1.30.33. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Kawashima, M.; Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 2023, 51, D587–D592. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Bell, E.A.; Boehnke, P.; Harrison, T.M.; Mao, W.L. Potentially biogenic carbon preserved in a 4.1 billion-year-old zircon. Proc. Natl. Acad. Sci. USA 2015, 112, 14518–14521. [Google Scholar] [CrossRef] [PubMed]
- Leander, B.S. Predatory protists. Curr. Biol. 2020, 30, R510–R516. [Google Scholar] [CrossRef]
- Maloof, A.C.; Porter, S.M.; Moore, J.L.; Dudas, F.O.; Bowring, S.A.; Higgins, J.A.; Fike, D.A.; Eddy, M.P. The earliest Cambrian record of animals and ocean geochemical change. Geol. Soc. Am. Bull. 2010, 122, 1731–1774. [Google Scholar] [CrossRef]
- Maloof, A.C.; Rose, C.V.; Beach, R.; Samuels, B.M.; Calmet, C.C.; Erwin, D.H.; Poirier, G.R.; Yao, N.; Simons, F.J. Possible animal-body fossils in pre-Marinoan limestones from South Australia. Nat. Geosci. 2010, 3, 653–659. [Google Scholar] [CrossRef]
- Shu, D.-G.; Luo, H.-L.; Conway Morris, S.; Zhang, X.-L.; Hu, S.-X.; Chen, L.; Han, J.; Zhu, M.; Li, Y.; Chen, L.-Z. Lower Cambrian vertebrates from south China. Nature 1999, 402, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Diogo, R. The Origin of Higher Clades: Osteology, Myology, Phylogeny and Evolution of Bony Fishes and the Rise of Tetrapods; CRC Press: New York, NY, USA, 2007. [Google Scholar]
- Datta, P.M. Earliest mammal with transversely expanded upper molar from the Late Triassic (Carnian) Tiki Formation, South Rewa Gondwana Basin, India. J. Vertebr. Paleontol. 2005, 25, 200–207. [Google Scholar] [CrossRef]
- Luo, Z.-X.; Yuan, C.-X.; Meng, Q.-J.; Ji, Q. A Jurassic eutherian mammal and divergence of marsupials and placentals. Nature 2011, 476, 442–445. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Hallstrom, B.M.; Janke, A. Coalescent-based genome analyses resolve the early branches of the euarchontoglires. PLoS ONE 2013, 8, e60019. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, H.J.; Ho, S.Y.; Barnes, I.; Groves, C. Estimating the phylogeny and divergence times of primates using a supermatrix approach. BMC Evol. Biol. 2009, 9, 259. [Google Scholar] [CrossRef] [PubMed]
- Dunn, R.H.; Rose, K.D.; Rana, R.S.; Kumar, K.; Sahni, A.; Smith, T. New euprimate postcrania from the early Eocene of Gujarat, India, and the strepsirrhine-haplorhine divergence. J. Hum. Evol. 2016, 99, 25–51. [Google Scholar] [CrossRef] [PubMed]
- Harrison, T. Catarrhine origins. In A Companion to Paleoanthropology; Blackwell Publ. Ltd.: New York, NY, USA, 2013; pp. 376–396. [Google Scholar]
- Hey, J. The ancestor’s tale: A pilgrimage to the dawn of evolution. J. Clin. Investig. 2005, 115, 1680. [Google Scholar] [CrossRef]
- Schrenk, F.; Kullmer, O.; Bromage, T. The earliest putative homo fossils. In Handbook of Paleoanthropology; Henke, W., Tattersall, I., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–19. [Google Scholar]
- Scerri, E.M.L.; Thomas, M.G.; Manica, A.; Gunz, P.; Stock, J.T.; Stringer, C.; Grove, M.; Groucutt, H.S.; Timmermann, A.; Rightmire, G.P.; et al. Did our species evolve in subdivided populations across Africa, and why does it matter? Trends Ecol. Evol. 2018, 33, 582–594. [Google Scholar] [CrossRef] [PubMed]
- Ban, N.; Lee, T.J.; Sene, A.; Choudhary, M.; Lekwuwa, M.; Dong, Z.; Santeford, A.; Lin, J.B.; Malek, G.; Ory, D.S.; et al. Impaired monocyte cholesterol clearance initiates age-related retinal degeneration and vision loss. JCI Insight 2018, 3, e120824. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Niu, L.; Li, L.; Song, M.; Zhang, Y.; Lei, Y.; Chen, Y.; Sun, X. ABCA1 Regulates IOP by modulating Cav1/eNOS/NO signaling pathway. Investig. Ophthalmol. Vis. Sci. 2020, 61, 33. [Google Scholar] [CrossRef]
- Bauer, M.; Karch, R.; Tournier, N.; Cisternino, S.; Wadsak, W.; Hacker, M.; Marhofer, P.; Zeitlinger, M.; Langer, O. Assessment of P-glycoprotein transport activity at the human blood-retina barrier with (R)-11C-verapamil PET. J. Nucl. Med. 2017, 58, 678–681. [Google Scholar] [CrossRef] [PubMed]
- Siegner, S.W.; Netland, P.A.; Schroeder, A.; Erickson, K.A. Effect of calcium channel blockers alone and in combination with antiglaucoma medications on intraocular pressure in the primate eye. J. Glaucoma 2000, 9, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, T.; Krohn, M.; Hofrichter, J.; Lange, C.; Stenzel, J.; Steffen, J.; Dunkelmann, T.; Paarmann, K.; Frohlich, C.; Uecker, A.; et al. ABC transporters B1, C1 and G2 differentially regulate neuroregeneration in mice. PLoS ONE 2012, 7, e35613. [Google Scholar] [CrossRef] [PubMed]
- Pattabiraman, P.P.; Pecen, P.E.; Rao, P.V. MRP4-mediated regulation of intracellular cAMP and cGMP levels in trabecular meshwork cells and homeostasis of intraocular pressure. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1636–1649. [Google Scholar] [CrossRef]
- Nalini, V.; Segu, R.; Deepa, P.R.; Khetan, V.; Vasudevan, M.; Krishnakumar, S. Molecular insights on post-chemotherapy retinoblastoma by microarray gene expression analysis. Bioinform. Biol. Insights 2013, 7, 289–306. [Google Scholar] [CrossRef]
- Jiang, H.; Luo, J.; Lei, H. The roles of mouse double minute 2 (MDM2) oncoprotein in ocular diseases: A review. Exp. Eye Res. 2022, 217, 108910. [Google Scholar] [CrossRef] [PubMed]
- Hirooka, K.; Shiraga, F. Potential role for angiotensin-converting enzyme inhibitors in the treatment of glaucoma. Clin. Ophthalmol. 2007, 1, 217–223. [Google Scholar] [PubMed]
- Costagliola, C.; Di Benedetto, R.; De Caprio, L.; Verde, R.; Mastropasqua, L. Effect of oral captopril (SQ 14225) on intraocular pressure in man. Eur. J. Ophthalmol. 1995, 5, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Kondkar, A.A.; Sultan, T.; Azad, T.A.; Osman, E.A.; Almobarak, F.A.; Al-Obeidan, S.A. Association analysis of polymorphisms rs12997 in ACVR1 and rs1043784 in BMP6 genes involved in bone morphogenic protein signaling pathway in primary angle-closure and pseudoexfoliation glaucoma patients of Saudi origin. BMC Med. Genet. 2020, 21, 145. [Google Scholar] [CrossRef] [PubMed]
- Borras, T. Gene expression in the trabecular meshwork and the influence of intraocular pressure. Prog. Retin. Eye Res. 2003, 22, 435–463. [Google Scholar] [CrossRef]
- Vasu, K.; Ramachandiran, I.; Chechi, A.; Khan, K.; Khan, D.; Kaufman, R.; Fox, P.L. Translational control of murine adiponectin expression by an upstream open reading frame element. RNA Biol. 2023, 20, 737–749. [Google Scholar] [CrossRef]
- Lam, S.; Lindsey, J.; Carranza Leon, B.G.; Takkouche, S. Shedding light on eye disease in obesity: A review. Clin Obes. 2024, 14, e12616. [Google Scholar] [CrossRef] [PubMed]
- Denis, P.; Elena, P.P. Retinal vascular beta-adrenergic receptors in man. Ophtalmologie 1989, 3, 62–64. [Google Scholar] [PubMed]
- Hohberger, B.; Kunze, R.; Wallukat, G.; Kara, K.; Mardin, C.Y.; Lammer, R.; Schlotzer-Schrehardt, U.; Hosari, S.; Horn, F.; Munoz, L.; et al. Autoantibodies activating the β2-adrenergic receptor characterize patients with primary and secondary glaucoma. Front. Immunol. 2019, 10, 2112. [Google Scholar] [CrossRef] [PubMed]
- Dorfleutner, A.; Stehlik, C.; Zhang, J.; Gallick, G.E.; Flynn, D.C. AFAP-110 is required for actin stress fiber formation and cell adhesion in MDA-MB-231 breast cancer cells. J. Cell Physiol. 2007, 213, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.R.; Stubbs, E.B., Jr. TGF-β2 promotes oxidative stress in human trabecular meshwork cells by selectively enhancing NADPH oxidase 4 expression. Investig. Ophthalmol. Vis. Sci. 2021, 62, 4. [Google Scholar] [CrossRef] [PubMed]
- Asefa, N.G.; Kamali, Z.; Pereira, S.; Vaez, A.; Jansonius, N.; Bergen, A.A.; Snieder, H. Bioinformatic prioritization and functional annotation of GWAS-based candidate genes for primary open-angle glaucoma. Genes 2022, 13, 1055. [Google Scholar] [CrossRef] [PubMed]
- McVicar, C.M.; Ward, M.; Colhoun, L.M.; Guduric-Fuchs, J.; Bierhaus, A.; Fleming, T.; Schlotterer, A.; Kolibabka, M.; Hammes, H.P.; Chen, M.; et al. Role of the receptor for advanced glycation endproducts (RAGE) in retinal vasodegenerative pathology during diabetes in mice. Diabetologia 2015, 58, 1129–1137. [Google Scholar] [CrossRef]
- Pelletier, A.L.; Rojas-Roldan, L.; Coffin, J. Vision loss in older adults. Am. Fam. Physician 2016, 94, 219–226. [Google Scholar] [PubMed]
- Tzeng, T.F.; Liou, S.S.; Tzeng, Y.C.; Liu, I.M. Zerumbone, a phytochemical of subtropical ginger, protects against hyperglycemia-induced retinal damage in experimental diabetic rats. Nutrients 2016, 8, 449. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.; Moncaster, J.A.; Wang, L.; Hafeez, I.; Herz, J.; Tanzi, R.E.; Goldstein, L.E.; Guenette, S.Y. FE65 and FE65L1 amyloid precursor protein-binding protein compound null mice display adult-onset cataract and muscle weakness. FASEB J. 2015, 29, 2628–2639. [Google Scholar] [CrossRef] [PubMed]
- Stalhammar, G.; Damato, B.E.; Fili, M. Adenoma of the nonpigmented ciliary epithelium presenting as glaucoma. Am. J. Ophthalmol. Case Rep. 2023, 32, 101871. [Google Scholar] [CrossRef]
- Golanska, E.; Sieruta, M.; Gresner, S.M.; Pfeffer, A.; Chodakowska-Zebrowska, M.; Sobow, T.M.; Klich, I.; Mossakowska, M.; Szybinska, A.; Barcikowska, M.; et al. APBB2 genetic polymorphisms are associated with severe cognitive impairment in centenarians. Exp. Gerontol. 2013, 48, 391–394. [Google Scholar] [CrossRef]
- Jiang, A.; Gao, H.; Kelley, M.R.; Qiao, X. Inhibition of APE1/Ref-1 redox activity with APX3330 blocks retinal angiogenesis in vitro and in vivo. Vis. Res. 2011, 51, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Hayreh, S.S.; Zimmerman, M.B. Ocular neovascularization associated with central and hemicentral retinal vein occlusion. Retina 2012, 32, 1553–1565. [Google Scholar] [CrossRef]
- Aqrawi, L.A.; Chen, X.; Jensen, J.L.; Morthen, M.K.; Thiede, B.; Utheim, O.A.; Palm, O.; Tashbayev, B.; Utheim, T.P.; Galtung, H.K. Severity of clinical dry eye manifestations influences protein expression in tear fluid of patients with primary Sjogren’s syndrome. PLoS ONE 2018, 13, e0205762. [Google Scholar] [CrossRef] [PubMed]
- Castro, C.; Marta, A.; Marques, J.H.; Ferreira, A.; Jose, D.; Sousa, P.; Neves, I.; Meneres, M.J.; Barbosa, I. Ocular surface changes in primary open-angle glaucoma patients treated with topical antihypertensive drugs. J. Glaucoma. 2023, 32, e113–e120. [Google Scholar] [CrossRef]
- Omodaka, K.; Nishiguchi, K.M.; Yasuda, M.; Tanaka, Y.; Sato, K.; Nakamura, O.; Maruyama, K.; Nakazawa, T. Neuroprotective effect against axonal damage-induced retinal ganglion cell death in apolipoprotein E-deficient mice through the suppression of kainate receptor signaling. Brain Res. 2014, 1586, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Nowak, A.; Rozpędek, W.; Cuchra, M.; Wojtczak, R.; Siwak, M.; Szymanek, K.; Szaflik, M.; Szaflik, J.; Szaflik, J.; Majsterek, I. Association of the expression level of the neurodegeneration-related proteins with the risk of development and progression of primary open-angle glaucoma. Acta Ophthalmol. 2018, 96, e97–e98. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Vetrivel, L.; Verkman, A.S. Aquaporin deletion in mice reduces intraocular pressure and aqueous fluid production. J. Gen. Physiol. 2002, 119, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Mallick, S.; Sharma, M.; Kumar, A.; Du, Y. Cell-based therapies for trabecular meshwork regeneration to treat glaucoma. Biomolecules 2021, 11, 1258. [Google Scholar] [CrossRef] [PubMed]
- Springelkamp, H.; Iglesias, A.I.; Cuellar-Partida, G.; Amin, N.; Burdon, K.P.; van Leeuwen, E.M.; Gharahkhani, P.; Mishra, A.; van der Lee, S.J.; Hewitt, A.W.; et al. ARHGEF12 influences the risk of glaucoma by increasing intraocular pressure. Hum. Mol. Genet. 2015, 24, 2689–2699. [Google Scholar] [CrossRef] [PubMed]
- Keller, K.E.; Wirtz, M.K. Working your SOCS off: The role of ASB10 and protein degradation pathways in glaucoma. Exp. Eye Res. 2017, 158, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Keller, K.E.; Yang, Y.F.; Sun, Y.Y.; Sykes, R.; Acott, T.S.; Wirtz, M.K. Ankyrin repeat and suppressor of cytokine signaling box containing protein-10 is associated with ubiquitin-mediated degradation pathways in trabecular meshwork cells. Mol. Vis. 2013, 19, 1639–1655. [Google Scholar] [PubMed]
- Kasetti, R.B.; Maddineni, P.; Kiehlbauch, C.; Patil, S.; Searby, C.C.; Levine, B.; Sheffield, V.C.; Zode, G.S. Autophagy stimulation reduces ocular hypertension in a murine glaucoma model via autophagic degradation of mutant myocilin. JCI Insight 2021, 6, e143359. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Martins, M.; de Toledo, B.C.; Santos-Franca, P.L.; Oliveira-Valenca, V.M.; Vieira-Vieira, C.H.; Matos-Rodrigues, G.E.; Linden, R.; Norden, C.; Martins, R.A.P.; Silveira, M.S. De novo genesis of retinal ganglion cells by targeted expression of Klf4 in vivo. Development 2019, 146, dev176586. [Google Scholar] [CrossRef]
- Sen, N.E.; Arsovic, A.; Meierhofer, D.; Brodesser, S.; Oberschmidt, C.; Canet-Pons, J.; Kaya, Z.E.; Halbach, M.V.; Gispert, S.; Sandhoff, K.; et al. In human and mouse spino-cerebellar tissue, ataxin-2 expansion affects ceramide-sphingomyelin metabolism. Int. J. Mol. Sci. 2019, 20, 5854. [Google Scholar] [CrossRef] [PubMed]
- Rong, S.S.; Yu, X. Phenotypic and genetic links between body fat measurements and primary open-angle glaucoma. Int. J. Mol. Sci. 2023, 24, 3925. [Google Scholar] [CrossRef] [PubMed]
- Arsovic, A.; Halbach, M.V.; Canet-Pons, J.; Esen-Sehir, D.; Doring, C.; Freudenberg, F.; Czechowska, N.; Seidel, K.; Baader, S.L.; Gispert, S.; et al. Mouse ataxin-2 expansion downregulates CamKII and Other calcium signaling factors, impairing granule-Purkinje neuron synaptic strength. Int. J. Mol. Sci. 2020, 21, 6673. [Google Scholar] [CrossRef] [PubMed]
- Voigt, A.M.; Grabitz, S.; Hoffmann, E.M.; Schuster, A.K. Systemic diseases in primary open-angle glaucoma. Klin. Monbl Augenheilkd. 2024, 241, 170–176. [Google Scholar] [PubMed]
- Chapman, S.A.; Bonshek, R.E.; Stoddart, R.W.; O’Donoghue, E.; Goodall, K.; McLeod, D. Glycans of the trabecular meshwork in primary open angle glaucoma. Br. J. Ophthalmol. 1996, 80, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Bydlinski, N.; Maresch, D.; Schmieder, V.; Klanert, G.; Strasser, R.; Borth, N. The contributions of individual galactosyltransferases to protein specific N-glycan processing in Chinese hamster ovary cells. J. Biotechnol. 2018, 282, 101–110. [Google Scholar] [CrossRef]
- Slettedal, J.K.; Sandvik, L.; Ringvold, A. Significant lifespan difference between primary open-angle glaucoma and pseudoexfoliation glaucoma. Heliyon 2021, 7, e06421. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yang, X.; Liu, M.; Tang, H. B4GALT3 up-regulation by miR-27a contributes to the oncogenic activity in human cervical cancer cells. Cancer Lett. 2016, 375, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Deng, Q.; Lei, X.; Lu, W.; Zhao, Q.; Shen, Y. Overexpression of BMP4 protects retinal ganglion cells in a mouse model of experimental glaucoma. Exp. Eye Res. 2021, 210, 108728. [Google Scholar] [CrossRef]
- Chung, D.D.; Frausto, R.F.; Lin, B.R.; Hanser, E.M.; Cohen, Z.; Aldave, A.J. Transcriptomic profiling of posterior polymorphous corneal dystrophy. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3202–3214. [Google Scholar] [CrossRef] [PubMed]
- Skeie, J.M.; Nishimura, D.Y.; Wang, C.L.; Schmidt, G.A.; Aldrich, B.T.; Greiner, M.A. Mitophagy: An emerging target in ocular pathology. Investig. Ophthalmol. Vis. Sci. 2021, 62, 22. [Google Scholar] [CrossRef] [PubMed]
- Farrell, S.R.; Sargoy, A.; Brecha, N.C.; Barnes, S. Modulation of voltage-gated Ca2+ channels in rat retinal ganglion cells by gabapentin. Vis. Neurosci. 2014, 31, 47–55. [Google Scholar] [CrossRef]
- Chang, E.; Chen, X.; Kim, M.; Gong, N.; Bhatia, S.; Luo, Z.D. Differential effects of voltage-gated calcium channel blockers on calcium channel alpha-2-delta-1 subunit protein-mediated nociception. Eur. J. Pain. 2015, 19, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Openkova, Y.Y.; Korobeiynikova, E.N.; Rykin, V.S.; Vinkova, G.A. The analysis of status of biochemical indicators in blood serum and lacrimal fluid in patients with primary open-angle glaucoma. Klin. Lab. Diagn. 2013, 5, 8–11. [Google Scholar]
- Lerner, N.; Chen, I.; Schreiber-Avissar, S.; Beit-Yannai, E. Extracellular vesicles mediate anti-oxidative response-in vitro study in the ocular drainage system. Int. J. Mol. Sci. 2020, 21, 6105. [Google Scholar] [CrossRef]
- Gu, X.; Fliesler, S.J.; Zhao, Y.Y.; Stallcup, W.B.; Cohen, A.W.; Elliott, M.H. Loss of caveolin-1 causes blood-retinal barrier breakdown, venous enlargement, and mural cell alteration. Am. J. Pathol. 2014, 184, 541–555. [Google Scholar] [CrossRef] [PubMed]
- Simard, J.R.; Meshulam, T.; Pillai, B.K.; Kirber, M.T.; Brunaldi, K.; Xu, S.; Pilch, P.F.; Hamilton, J.A. Caveolins sequester FA on the cytoplasmic leaflet of the plasma membrane, augment triglyceride formation, and protect cells from lipotoxicity. J. Lipid Res. 2010, 51, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kwon, Y.J.; Lee, H.S.; Han, J.H.; Joung, B.; Kim, S.J. Fatty liver is an independent risk factor for elevated intraocular pressure. Nutrients 2022, 14, 4455. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, K.; Heo, D.W.; Kim, J.S.; Park, C.K.; Kim, C.S.; Kang, C. Expression-associated polymorphisms of CAV1-CAV2 affect intraocular pressure and high-tension glaucoma risk. Mol. Vis. 2015, 21, 548–554. [Google Scholar] [PubMed]
- Galbiati, F.; Volonte, D.; Gil, O.; Zanazzi, G.; Salzer, J.L.; Sargiacomo, M.; Scherer, P.E.; Engelman, J.A.; Schlegel, A.; Parenti, M.; et al. Expression of caveolin-1 and -2 in differentiating PC12 cells and dorsal root ganglion neurons: Caveolin-2 is up-regulated in response to cell injury. Proc. Natl. Acad. Sci. USA 1998, 95, 10257–10262. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Zhang, J.; Zhang, M.; Lan, H.; Yang, Q.; Li, C.; Zeng, L. MicroRNA-29b targeting of cell division cycle 7-related protein kinase (CDC7) regulated vascular smooth muscle cell (VSMC) proliferation and migration. Ann. Transl. Med. 2020, 8, 1496. [Google Scholar] [CrossRef] [PubMed]
- Omoti, A.E.; Edema, O.T. A review of the risk factors in primary open angle glaucoma. Niger. J. Clin. Pr. 2007, 10, 79–82. [Google Scholar]
- Cheng, A.N.; Jiang, S.S.; Fan, C.C.; Lo, Y.K.; Kuo, C.Y.; Chen, C.H.; Liu, Y.L.; Lee, C.C.; Chen, W.S.; Huang, T.S.; et al. Increased Cdc7 expression is a marker of oral squamous cell carcinoma and overexpression of Cdc7 contributes to the resistance to DNA-damaging agents. Cancer Lett. 2013, 337, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, K.; Dada, R.; Dada, T. Oxidative DNA damage and reduced expression of DNA repair genes: Role in primary open angle glaucoma (POAG). Ophthalmic Genet. 2017, 38, 446–450. [Google Scholar] [CrossRef]
- Rajic, J.; Dinic, S.; Uskokovic, A.; Arambasic Jovanovic, J.; Tolic, A.; Dordevic, M.; Dordevic, M.; Poznanovic, G.; Mihailovic, M.; Inic-Kanada, A.; et al. DNA methylation of miR-200 clusters promotes epithelial to mesenchymal transition in human conjunctival epithelial cells. Exp. Eye Res. 2020, 197, 108047. [Google Scholar] [CrossRef] [PubMed]
- Atencio, I.A.; Chen, Z.; Nguyen, Q.H.; Faha, B.; Maneval, D.C. p21WAF-1/Cip-1 gene therapy as an adjunct to glaucoma filtration surgery. Curr. Opin. Mol. Ther. 2004, 6, 624–628. [Google Scholar] [PubMed]
- Itoh, T.; Linn, S. The fate of p21CDKN1A in cells surviving UV-irradiation. DNA Repair 2005, 4, 1457–1462. [Google Scholar] [CrossRef]
- Eliseeva, N.V.; Ponomarenko, I.V.; Churnosov, M.I. Analysis of the functional role of polymorphism in the CDKN2B-AS1 gene GWAS-significant for primary open-angle glaucoma (an in-silico study). Vestn. Oftalmol. 2021, 137, 43–50. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Sharpless, N.E. Senescence in health and disease. Cell 2017, 169, 1000–1011. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Guo, R.; Huang, D.; Ji, J.; Gansevoort, R.T.; Snieder, H.; Jansonius, N.M. Co-occurrence of chronic kidney disease and glaucoma: Epidemiology and etiological mechanisms. Surv. Ophthalmol. 2023, 68, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Jakobs, T.C. Mice homozygous for a deletion in the glaucoma susceptibility locus INK4 show increased vulnerability of retinal ganglion cells to elevated intraocular pressure. Am. J. Pathol. 2016, 186, 985–1005. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Lim, S.Y.; Jeong, H.S.; Koo, K.A.; Sung, S.H.; Kim, Y.C. Oligonucleotide microarray analysis of apoptosis induced by 15-methoxypinusolidic acid in microglial BV2 cells. Br. J. Pharmacol. 2009, 157, 1053–1064. [Google Scholar] [CrossRef]
- Al Shweiki, M.R.; Oeckl, P.; Steinacker, P.; Barschke, P.; Dorner-Ciossek, C.; Hengerer, B.; Schonfeldt-Lecuona, C.; Otto, M. Proteomic analysis reveals a biosignature of decreased synaptic protein in cerebrospinal fluid of major depressive disorder. Transl. Psychiatry 2020, 10, 144. [Google Scholar] [CrossRef]
- Cumurcu, T.; Cumurcu, B.E.; Celikel, F.C.; Etikan, I. Depression and anxiety in patients with pseudoexfoliative glaucoma. Gen. Hosp. Psychiatry 2006, 28, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Elkjaer, M.L.; Nawrocki, A.; Kacprowski, T.; Lassen, P.; Simonsen, A.H.; Marignier, R.; Sejbaek, T.; Nielsen, H.H.; Wermuth, L.; Rashid, A.Y.; et al. CSF proteome in multiple sclerosis subtypes related to brain lesion transcriptomes. Sci. Rep. 2021, 11, 4132. [Google Scholar] [CrossRef]
- Jung, J.; Yoo, J.E.; Choe, Y.H.; Park, S.C.; Lee, H.J.; Lee, H.J.; Noh, B.; Kim, S.H.; Kang, G.Y.; Lee, K.M.; et al. Cleaved cochlin sequesters Pseudomonas aeruginosa and activates innate immunity in the inner ear. Cell Host Microbe. 2019, 25, 513–525.e6. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.; Schmutz, M.; Farukhi, S.; Mosaed, S. Baerveldt scleral patch graft abscess secondary to coagulase-negative staphylococcus. Case Rep. Ophthalmol. 2017, 8, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.K.; Peachey, N.S.; Crabb, J.W. Cochlin and glaucoma: A mini-review. Vis. Neurosci. 2005, 22, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Chong, R.S.; Lee, Y.S.; Chu, S.W.L.; Toh, L.Z.; Wong, T.T.L. Inhibition of monocyte chemoattractant protein 1 prevents conjunctival fibrosis in an experimental model of glaucoma filtration surgery. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3432–3439. [Google Scholar] [CrossRef] [PubMed]
- Roodnat, A.W.; Callaghan, B.; Doyle, C.; Vallabh, N.A.; Atkinson, S.D.; Willoughby, C.E. Genome-wide RNA sequencing of ocular fibroblasts from glaucomatous and normal eyes: Implications for glaucoma management. PLoS ONE 2024, 19, e0307227. [Google Scholar] [CrossRef]
- Hopfer, U.; Fukai, N.; Hopfer, H.; Wolf, G.; Joyce, N.; Li, E.; Olsen, B.R. Targeted disruption of Col8a1 and Col8a2 genes in mice leads to anterior segment abnormalities in the eye. FASEB J. 2005, 19, 1232–1244. [Google Scholar] [CrossRef] [PubMed]
- Desronvil, T.; Logan-Wyatt, D.; Abdrabou, W.; Triana, M.; Jones, R.; Taheri, S.; Del Bono, E.; Pasquale, L.R.; Olivier, M.; Haines, J.L.; et al. Distribution of COL8A2 and COL8A1 gene variants in Caucasian primary open angle glaucoma patients with thin central corneal thickness. Mol. Vis. 2010, 16, 2185–2191. [Google Scholar] [PubMed]
- Schmitt, L.; Marquardt, Y.; Amann, P.; Heise, R.; Huth, L.; Wagner-Schiffler, S.; Huth, S.; Baron, J.M. Comprehensive molecular characterization of microneedling therapy in a human three-dimensional skin model. PLoS ONE 2018, 13, e0204318. [Google Scholar] [CrossRef] [PubMed]
- Grehn, F. Surgery of primary open angle glaucoma. Klin. Monbl Augenheilkd. 2008, 225, 30–38. [Google Scholar] [CrossRef]
- Steinhart, M.R.; Cone, F.E.; Nguyen, C.; Nguyen, T.D.; Pease, M.E.; Puk, O.; Graw, J.; Oglesby, E.N.; Quigley, H.A. Mice with an induced mutation in collagen 8A2 develop larger eyes and are resistant to retinal ganglion cell damage in an experimental glaucoma model. Mol. Vis. 2012, 18, 1093–1106. [Google Scholar] [PubMed]
- Seet, L.F.; Toh, L.Z.; Chu, S.W.L.; Finger, S.N.; Chua, J.L.L.; Wong, T.T. Upregulation of distinct collagen transcripts in post-surgery scar tissue: A study of conjunctival fibrosis. Dis. Model. Mech. 2017, 10, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Sarnat-Kucharczyk, M.; Rokicki, W.; Zalejska-Fiolka, J.; Pojda-Wilczek, D.; Mrukwa-Kominek, E. Determination of serum ceruloplasmin concentration in patients with primary open angle glaucoma with cataract and patients with cataract only: A pilot study. Med. Sci. Monit. 2016, 22, 1384–1388. [Google Scholar] [CrossRef]
- Farkas, R.H.; Chowers, I.; Hackam, A.S.; Kageyama, M.; Nickells, R.W.; Otteson, D.C.; Duh, E.J.; Wang, C.; Valenta, D.F.; Gunatilaka, T.L.; et al. Increased expression of iron-regulating genes in monkey and human glaucoma. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1410–1417. [Google Scholar] [CrossRef]
- Bonet-Fernandez, J.M.; Aroca-Aguilar, J.D.; Corton, M.; Ramirez, A.I.; Alexandre-Moreno, S.; Garcia-Anton, M.T.; Salazar, J.J.; Ferre-Fernandez, J.J.; Atienzar-Aroca, R.; Villaverde, C.; et al. CPAMD8 loss-of-function underlies non-dominant congenital glaucoma with variable anterior segment dysgenesis and abnormal extracellular matrix. Hum. Genet. 2020, 139, 1209–1231. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Xiao, X.; Jia, X.; Li, S.; Li, M.; Guo, X.; Liu, X.; Zhang, Q. Mutation analysis of the genes associated with anterior segment dysgenesis, microcornea and microphthalmia in 257 patients with glaucoma. Int. J. Mol. Med. 2015, 36, 1111–1117. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.F.; Wu, X.H.; Engvall, E. Identification and characterization of CPAMD8, a novel member of the complement 3/alpha2-macroglobulin family with a C-terminal Kazal domain. Genomics 2004, 83, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Bakalash, S.; Kipnis, J.; Yoles, E.; Schwartz, M. Resistance of retinal ganglion cells to an increase in intraocular pressure is immune-dependent. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2648–2653. [Google Scholar]
- Shao, Z.; Ma, X.; Zhang, Y.; Sun, Y.; Lv, W.; He, K.; Xia, R.; Wang, P.; Gao, X. CPNE1 predicts poor prognosis and promotes tumorigenesis and radioresistance via the AKT singling pathway in triple-negative breast cancer. Mol. Carcinog. 2020, 59, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Hager, T.; Hoffmann, S.; Seitz, B. Unusual symptoms for tamoxifen-associated maculopathy. Ophthalmologe 2010, 107, 750–752. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Li, R.; Yin, K.; Wu, L. CPNE1, a potential therapeutic target in nasopharyngeal carcinoma, affects cell growth and radiation resistance. Radiat. Res. 2024, 201, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Hamada, N.; Azizova, T.V.; Little, M.P. Glaucomagenesis following ionizing radiation exposure. Mutat. Res. Rev. Mutat. Res. 2019, 779, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Ha, Y.; Liu, H.; Zhu, S.; Yi, P.; Liu, W.; Nathanson, J.; Kayed, R.; Loucas, B.; Sun, J.; Frishman, L.J.; et al. Critical role of the CXCL10/C-X-C chemokine receptor 3 axis in promoting leukocyte recruitment and neuronal injury during traumatic optic neuropathy induced by optic nerve crush. Am. J. Pathol. 2017, 187, 352–365. [Google Scholar] [CrossRef] [PubMed]
- Siwak, M.; Maslankiewicz, M.; Nowak-Zdunczyk, A.; Rozpędek, W.; Wojtczak, R.; Szymanek, K.; Szaflik, M.; Szaflik, J.; Szaflik, J.P.; Majsterek, I. The relationship between HDAC6, CXCR3, and SIRT1 genes expression levels with progression of primary open-angle glaucoma. Ophthalmic Genet. 2018, 39, 325–331. [Google Scholar] [CrossRef]
- Perepechaeva, M.L.; Grishanova, A.Y.; Rudnitskaya, E.A.; Kolosova, N.G. The mitochondria-targeted antioxidant SkQ1 downregulates aryl hydrocarbon receptor-dependent genes in the retina of OXYS rats with AMD-like retinopathy. J. Ophthalmol. 2014, 2014, 530943. [Google Scholar] [CrossRef]
- Takeuchi, A.; Takeuchi, M.; Oikawa, K.; Sonoda, K.H.; Usui, Y.; Okunuki, Y.; Takeda, A.; Oshima, Y.; Yoshida, K.; Usui, M.; et al. Effects of dioxin on vascular endothelial growth factor (VEGF) production in the retina associated with choroidal neovascularization. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3410–3416. [Google Scholar] [CrossRef]
- Lopez-Garrido, M.P.; Blanco-Marchite, C.; Sanchez-Sanchez, F.; Lopez-Sanchez, E.; Chaques-Alepuz, V.; Campos-Mollo, E.; Salinas-Sanchez, A.S.; Escribano, J. Functional analysis of CYP1B1 mutations and association of heterozygous hypomorphic alleles with primary open-angle glaucoma. Clin. Genet. 2010, 77, 70–78. [Google Scholar] [CrossRef]
- Liu, X.L.; Jia, Q.J.; Wang, L.N.; Liu, Z.M.; Liu, H.; Duan, X.C.; Lyu, X.M. Roles of CYP2C19 gene polymorphisms in susceptibility to POAG and individual differences in drug treatment response. Med. Sci. Monit. 2016, 22, 310–355. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Yoshitomi, T.; Zorumski, C.F.; Izumi, Y. 24(S)-Hydroxycholesterol protects the ex vivo rat retina from injury by elevated hydrostatic pressure. Sci. Rep. 2016, 6, 33886. [Google Scholar] [CrossRef]
- Saadane, A.; Mast, N.; Trichonas, G.; Chakraborty, D.; Hammer, S.; Busik, J.V.; Grant, M.B.; Pikuleva, I.A. Retinal vascular abnormalities and microglia activation in mice with deficiency in cytochrome P450 46A1-mediated cholesterol removal. Am. J. Pathol. 2019, 189, 405–425. [Google Scholar] [CrossRef] [PubMed]
- Grusha, I.O.; Ismailova, D.S.; Gankovskaia, O.A. Risk factors of corneal damage in patients with thyroid eye disease. Vestn. Oftalmol. 2010, 126, 35–38. [Google Scholar] [PubMed]
- Grzybowski, A. Present knowledge on the effects of smoking tobacco on the eye diseases. Przegl Lek. 2008, 65, 724–727. [Google Scholar] [PubMed]
- Erichev, V.P.; Gankovskaia, L.V.; Koval’chuk, L.V.; Gankovskaia, O.A.; Dugina, A.E. Expression of the antimicrobial peptide beta-defensin-2 in the conjunctival epithelial cells in primary open-angle glaucoma and over time in the postoperative period. Vestn. Oftalmol. 2010, 126, 19–22. [Google Scholar] [PubMed]
- Sundermeier, T.R.; Sakami, S.; Sahu, B.; Howell, S.J.; Gao, S.; Dong, Z.; Golczak, M.; Maeda, A.; Palczewski, K. MicroRNA-processing enzymes are essential for survival and function of mature retinal pigmented epithelial cells in mice. J. Biol. Chem. 2017, 292, 3366–3378. [Google Scholar] [CrossRef] [PubMed]
- Cuellar-Partida, G.; Craig, J.E.; Burdon, K.P.; Wang, J.J.; Vote, B.J.; Souzeau, E.; McAllister, I.L.; Isaacs, T.; Lake, S.; Mackey, D.A.; et al. Assessment of polygenic effects links primary open-angle glaucoma and age-related macular degeneration. Sci. Rep. 2016, 6, 26885. [Google Scholar] [CrossRef]
- Hang, Q.; Zeng, L.; Wang, L.; Nie, L.; Yao, F.; Teng, H.; Deng, Y.; Yap, S.; Sun, Y.; Frank, S.J.; et al. Non-canonical function of DGCR8 in DNA double-strand break repair signaling and tumor radioresistance. Nat. Commun. 2021, 12, 4033. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Han, B.; Li, F.; Liu, H. Endothelin-1 concentration in aqueous humor predicts postoperative late low intraocular pressure in primary open-angle glaucoma after trabeculectomy. J. Glaucoma 2019, 28, 633–636. [Google Scholar] [CrossRef]
- Chaphalkar, R.M.; Stankowska, D.L.; He, S.; Kodati, B.; Phillips, N.; Prah, J.; Yang, S.; Krishnamoorthy, R.R. Endothelin-1 mediated decrease in mitochondrial gene expression and bioenergetics contribute to neurodegeneration of retinal ganglion cells. Sci. Rep. 2020, 10, 3571. [Google Scholar] [CrossRef] [PubMed]
- Daniel, S.; Renwick, M.; Chau, V.Q.; Datta, S.; Maddineni, P.; Zode, G.; Wade, E.M.; Robertson, S.P.; Petroll, W.M.; Hulleman, J.D. Fibulin-3 knockout mice demonstrate corneal dysfunction but maintain normal retinal integrity. J. Mol. Med. 2020, 98, 1639–1656. [Google Scholar] [CrossRef] [PubMed]
- Sein, J.; Galor, A.; Sheth, A.; Kruh, J.; Pasquale, L.R.; Karp, C.L. Exfoliation syndrome: New genetic and pathophysiologic insights. Curr. Opin. Ophthalmol. 2013, 24, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Cai, S.; Luo, X.; Li, Q.; Chen, Y.; Chen, Z.; Mao, Y.; Liu, G.; Yang, M.; Liu, X. Stop codon variant in EFEMP1 is associated with primary open-angle glaucoma due to impaired regulation of aqueous humor outflow. Exp. Eye Res. 2024, 241, 109859. [Google Scholar] [CrossRef] [PubMed]
- Saint-Jean, A.; Reguart, N.; Eixarch, A.; Adan, A.; Castella, C.; Sanchez-Dalmau, B.; Sainz-de-la-Maza, M. Ocular surface adverse events of systemic epidermal growth factor receptor inhibitors (EGFRi): A prospective trial. J. Fr. Ophtalmol. 2018, 41, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Harun-Or-Rashid, M.; Lindqvist, N.; Hallbook, F. Transactivation of EGF receptors in chicken Muller cells by α2A-adrenergic receptors stimulated by brimonidine. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3385–3394. [Google Scholar] [CrossRef] [PubMed]
- Urban, Z.; Agapova, O.; Hucthagowder, V.; Yang, P.; Starcher, B.C.; Hernandez, M.R. Population differences in elastin maturation in optic nerve head tissue and astrocytes. Investig. Ophthalmol. Vis. Sci. 2007, 48, 3209–3215. [Google Scholar] [CrossRef] [PubMed]
- NikhalaShree, S.; Karthikkeyan, G.; George, R.; Shantha, B.; Vijaya, L.; Ratra, V.; Sulochana, K.N.; Coral, K. Lowered decorin with aberrant extracellular matrix remodeling in aqueous humor and tenon’s tissue from primary glaucoma patients. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4661–4669. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Hernandez, R.; Serrano-Somavilla, A.; Ramos-Levi, A.; Sampedro-Nunez, M.; Lens-Pardo, A.; Munoz De Nova, J.L.; Trivino, J.C.; Gonzalez, M.U.; Torne, L.; Casares-Arias, J.; et al. Integrated miRNA and mRNA expression profiling identifies novel targets and pathological mechanisms in autoimmune thyroid diseases. EBioMedicine 2019, 50, 329–342. [Google Scholar] [CrossRef]
- Lorenzo, M.M.; Devlin, J.; Saini, C.; Cho, K.S.; Paschalis, E.I.; Chen, D.F.; Nascimento ESilva, R.; Chen, S.H.; Margeta, M.A.; Ondeck, C.; et al. The prevalence of autoimmune diseases in patients with primary open-angle glaucoma undergoing ophthalmic surgeries. Ophthalmol. Glaucoma 2022, 5, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Hines-Beard, J.; Bond, W.S.; Backstrom, J.R.; Rex, T.S. Virus-mediated EpoR76E gene therapy preserves vision in a glaucoma model by modulating neuroinflammation and decreasing oxidative stress. J. Neuroinflamm. 2016, 13, 39. [Google Scholar] [CrossRef] [PubMed]
- Mokbel, T.H.; Ghanem, A.A.; Kishk, H.; Arafa, L.F.; El-Baiomy, A.A. Erythropoietin and soluble CD44 levels in patients with primary open-angle glaucoma. Clin. Exp. Ophthalmol. 2010, 38, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Niu, L.; Liu, Y.; Pang, M.; Lu, W.; Xia, C.; Zhu, Y.; Yang, B.; Wang, Q. Study on the mechanism of Gegen Qinlian Decoction for treating type II diabetes mellitus by integrating network pharmacology and pharmacological evaluation. J. Ethnopharmacol. 2020, 262, 113129. [Google Scholar] [CrossRef] [PubMed]
- Abikoye, T.M.; Oluleye, T.S.; Aribaba, O.T.; Musa, K.O.; Idowu, O.O.; Onakoya, A.O. Is primary open-angle glaucoma a risk factor for diabetic retinopathy? Int. Ophthalmol. 2020, 40, 3233–3240. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, B.A.; Wnuk, A.; Przepiorska, K.; Lach, A.; Kajta, M. Posttreatment with ospemifene attenuates hypoxia- and ischemia-induced apoptosis in primary neuronal cells via selective modulation of estrogen receptors. Neurotox. Res. 2023, 41, 362–379. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.; Kocab, A.J.; Zacks, D.N.; Marshak-Rothstein, A.; Gregory-Ksander, M. A small peptide antagonist of the Fas receptor inhibits neuroinflammation and prevents axon degeneration and retinal ganglion cell death in an inducible mouse model of glaucoma. J. Neuroinflamm. 2019, 16, 184. [Google Scholar] [CrossRef]
- Gregory, M.S.; Hackett, C.G.; Abernathy, E.F.; Lee, K.S.; Saff, R.R.; Hohlbaum, A.M.; Moody, K.S.; Hobson, M.W.; Jones, A.; Kolovou, P.; et al. Opposing roles for membrane bound and soluble Fas ligand in glaucoma-associated retinal ganglion cell death. PLoS ONE 2011, 6, e17659. [Google Scholar] [CrossRef]
- Zhao, M.; Ma, P.; Xie, Q.; Bui, A.D.; Yonamine, S.; Hinterwirth, A.; Zhong, L.; Chen, C.; Doan, T.; Han, Y. Biomarkers for primary open-angle glaucoma progression. Exp. Eye Res. 2022, 219, 109025. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.A.; Goping, I.S.; Berry, F.; Walter, M.A. Dysfunction of the stress-responsive FOXC1 transcription factor contributes to the earlier-onset glaucoma observed in Axenfeld-Rieger syndrome patients. Cell Death Dis. 2014, 5, e1069. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, H.; Song, Y.; Liu, C.; Qian, X.; Zhang, D.; Jiang, X.; Zhang, S. Overexpression of Foxc1 ameliorates sepsis-associated encephalopathy by inhibiting microglial migration and neuroinflammation through the IκBα/NF-κB pathway. Mol. Med. Rep. 2022, 25, 107. [Google Scholar] [CrossRef] [PubMed]
- Liton, P.B.; Luna, C.; Challa, P.; Epstein, D.L.; Gonzalez, P. Genome-wide expression profile of human trabecular meshwork cultured cells, nonglaucomatous and primary open angle glaucoma tissue. Mol. Vis. 2006, 12, 774–790. [Google Scholar]
- Ju, Y.T.; Chang, A.C.; She, B.R.; Tsaur, M.L.; Hwang, H.M.; Chao, C.C.; Cohen, S.N.; Lin-Chao, S. Gas7: A gene expressed preferentially in growth-arrested fibroblasts and terminally differentiated Purkinje neurons affects neurite formation. Proc. Natl. Acad. Sci. USA 1998, 95, 11423–11428. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Hu, H.; Chen, Z.; Cai, X.; Zhang, Z.; Yang, Y.; Yu, N.; Zhang, J.; Xia, L.; Ge, J.; et al. BRCA1 silencing is associated with failure of DNA repairing in retinal neurocytes. PLoS ONE 2014, 9, e99371. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, N.; Hiramatsu, K.; Miyamoto, R.; Yasuda, K.; Suzuki, N.; Oshima, N.; Kiyonari, H.; Shiba, D.; Nishio, S.; Mochizuki, T.; et al. A murine model of neonatal diabetes mellitus in Glis3-deficient mice. FEBS Lett. 2009, 583, 2108–2113. [Google Scholar] [CrossRef] [PubMed]
- Scoville, D.W.; Kang, H.S.; Jetten, A.M. Transcription factor GLIS3: Critical roles in thyroid hormone biosynthesis, hypothyroidism, pancreatic beta cells and diabetes. Pharmacol. Ther. 2020, 215, 107632. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Willer, J.R.; Scherer, P.C.; Panzer, J.A.; Kugath, A.; Skordalakes, E.; Gregg, R.G.; Willer, G.B.; Balice-Gordon, R.J. Neural and synaptic defects in slytherin, a zebrafish model for human congenital disorders of glycosylation. PLoS ONE 2010, 5, e13743. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Comunale, M.A.; Rawat, S.; Casciano, J.C.; Lamontagne, J.; Herrera, H.; Ramanathan, A.; Betesh, L.; Wang, M.; Norton, P.; et al. Intrinsic hepatocyte dedifferentiation is accompanied by upregulation of mesenchymal markers, protein sialylation and core alpha 1,6 linked fucosylation. Sci. Rep. 2016, 6, 27965. [Google Scholar] [CrossRef]
- Ivanov, I.V.; Mappes, T.; Schaupp, P.; Lappe, C.; Wahl, S. Ultraviolet radiation oxidative stress affects eye health. J. Biophotonics 2018, 11, e201700377. [Google Scholar] [CrossRef] [PubMed]
- Laspas, P.; Zhutdieva, M.B.; Brochhausen, C.; Musayeva, A.; Zadeh, J.K.; Pfeiffer, N.; Xia, N.; Li, H.; Wess, J.; Gericke, A. The M1 muscarinic acetylcholine receptor subtype is important for retinal neuron survival in aging mice. Sci. Rep. 2019, 9, 5222. [Google Scholar] [CrossRef]
- Lubos, E.; Loscalzo, J.; Handy, D.E. Glutathione peroxidase-1 in health and disease: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal 2011, 15, 1957–1997. [Google Scholar] [CrossRef] [PubMed]
- Bell, S.; Maussion, G.; Jefri, M.; Peng, H.; Theroux, J.F.; Silveira, H.; Soubannier, V.; Wu, H.; Hu, P.; Galat, E.; et al. Disruption of GRIN2B impairs differentiation in human neurons. Stem Cell Rep. 2018, 11, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Muller, L.; Tokay, T.; Porath, K.; Kohling, R.; Kirschstein, T. Enhanced NMDA receptor-dependent LTP in the epileptic CA1 area via upregulation of NR2B. Neurobiol. Dis. 2013, 54, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Lyons, C.E.; Zhou, X.; Razzoli, M.; Chen, M.; Xia, W.; Ashe, K.; Zhang, B.; Bartolomucci, A. Lifelong chronic psychosocial stress induces a proteomic signature of Alzheimer’s disease in wildtype mice. Eur. J. Neurosci. 2022, 55, 2971–2985. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Wang, W.; Zhou, M.; Chen, S.; Zhang, X. Association of glutathione S-transferase polymorphisms (GSTM1 and GSTT1) with primary open-angle glaucoma: An evidence-based meta-analysis. Gene 2013, 526, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.S.; Kim, E.; Choi, H.; Lee, K.H.; Kim, K.J.; Lim, D.; Choi, S.Y.; Kim, Y.; Son, S.A.; Kim, J.S.; et al. Therapeutic potential of Peucedanum japonicum Thunb. and its active components in a delayed corneal wound healing model following blue light irradiation-induced oxidative stress. Antioxidants 2023, 12, 1171. [Google Scholar] [CrossRef]
- Fernando, N.; Wooff, Y.; Aggio-Bruce, R.; Chu-Tan, J.A.; Jiao, H.; Dietrich, C.; Rutar, M.; Rooke, M.; Menon, D.; Eells, J.T.; et al. Photoreceptor survival is regulated by GSTO1-1 in the degenerating retina. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4362–4374. [Google Scholar] [CrossRef]
- Emery, M.; Schorderet, D.F.; Roduit, R. Acute hypoglycemia induces retinal cell death in mouse. PLoS ONE 2011, 6, e21586. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, E.B.; Grosskreutz, C.L. Excitatory mechanisms in retinal ganglion cell death in primary open angle glaucoma (POAG). Clin. Neurosci. 1997, 4, 270–273. [Google Scholar] [PubMed]
- Cha, S.J.; Yoon, J.H.; Han, Y.J.; Kim, K. Knockdown of glutathione S-transferase leads to mislocalization and accumulation of cabeza, a drosophila homolog of FUS, in the brain. J. Neurogenet. 2023, 37, 20–24. [Google Scholar] [CrossRef]
- Chan, J.W.; Chan, N.C.Y.; Sadun, A.A. Glaucoma as neurodegeneration in the brain. Eye Brain 2021, 13, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.J.; Kim, K. Reduced oxidative stress suppresses neurotoxicity in the Drosophila model of TAF15-associated proteinopathies. Mol. Brain 2022, 15, 93. [Google Scholar] [CrossRef]
- Liu, A.; Wang, L.; Feng, Q.; Zhang, D.; Chen, K.; Yiming, G.H.; Wang, Q.; Hong, Y.; Whelchel, A.; Zhang, X.; et al. Low expression of GSTP1 in the aqueous humour of patients with primary open-angle glaucoma. J. Cell Mol. Med. 2021, 25, 3063–3079. [Google Scholar] [CrossRef]
- Yu, H.; Wark, L.; Ji, H.; Willard, L.; Jaing, Y.; Han, J.; He, H.; Ortiz, E.; Zhang, Y.; Medeiros, D.M.; et al. Dietary wolfberry upregulates carotenoid metabolic genes and enhances mitochondrial biogenesis in the retina of db/db diabetic mice. Mol. Nutr. Food Res. 2013, 57, 1158–1169. [Google Scholar] [CrossRef] [PubMed]
- Chitrala, K.N.; Hernandez, D.G.; Nalls, M.A.; Mode, N.A.; Zonderman, A.B.; Ezike, N.; Evans, M.K. Race-specific alterations in DNA methylation among middle-aged African Americans and Whites with metabolic syndrome. Epigenetics 2020, 15, 462–482. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.H.; Cho, Y.H.; Kim, Y.J.; Lee, S.Y.; Lee, J.G.; Kong, E.H.; Cho, B.M.; Tak, Y.J.; Hwang, H.R.; Lee, S.H.; et al. Metabolic syndrome as a risk factor for high intraocular pressure: The Korea National Health and Nutrition Examination Survey 2008–2010. Diabetes Metab. Syndr. Obes 2019, 12, 131–137. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Q.L.; Zhang, M.H. Identifying key genes in glaucoma based on a benchmarked dataset and the gene regulatory network. Exp. Ther. Med. 2017, 14, 3651–3657. [Google Scholar] [CrossRef] [PubMed]
- Ashok, A.; Chaudhary, S.; Kritikos, A.E.; Kang, M.H.; McDonald, D.; Rhee, D.J.; Singh, N. TGFβ2-hepcidin feed-forward loop in the trabecular meshwork implicates iron in glaucomatous pathology. Investig. Ophthalmol. Vis. Sci. 2020, 61, 24. [Google Scholar] [CrossRef] [PubMed]
- Sorkhabi, R.; Ghorbanihaghjo, A.; Javadzadeh, A.; Motlagh, B.F.; Ahari, S.S. Aqueous humor hepcidin prohormone levels in patients with primary open angle glaucoma. Mol. Vis. 2010, 16, 1832–1836. [Google Scholar]
- Yuan, H.; Li, H.; Yu, P.; Fan, Q.; Zhang, X.; Huang, W.; Shen, J.; Cui, Y.; Zhou, W. Involvement of HDAC6 in ischaemia and reperfusion-induced rat retinal injury. BMC Ophthalmol. 2018, 18, 300. [Google Scholar] [CrossRef]
- Zhang, L.; Sheng, S.; Qin, C. The role of HDAC6 in Alzheimer’s disease. J. Alzheimers Dis. 2013, 33, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, Y.; Guo, R.; Shen, W.; Qi, Y.; Wang, Q.; Guo, Z.; Qi, C.; Yin, H.; Wang, J. HES1 promotes extracellular matrix protein expression and inhibits proliferation and migration in human trabecular meshwork cells under oxidative stress. Oncotarget 2017, 8, 21818–21833. [Google Scholar] [CrossRef] [PubMed]
- Adachi, T.; Sakurai, T.; Kashida, H.; Mine, H.; Hagiwara, S.; Matsui, S.; Yoshida, K.; Nishida, N.; Watanabe, T.; Itoh, K.; et al. Involvement of heat shock protein a4/apg-2 in refractory inflammatory bowel disease. Inflamm. Bowel Dis. 2015, 21, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Qi, Y.; Zhang, J.; Guo, C.; Yuan, C. CDKN2B-AS1: An indispensable long non-coding RNA in multiple diseases. Curr. Pharm. Des. 2020, 26, 5335–5346. [Google Scholar] [CrossRef]
- Li, C.; Liu, D.; Yuan, Y.; Huang, S.; Shi, M.; Tao, K.; Feng, W. Overexpression of Apg-2 increases cell proliferation and protects from oxidative damage in BaF3-BCR/ABL cells. Int. J. Oncol. 2010, 36, 899–904. [Google Scholar] [PubMed]
- Sacca, S.C.; Pascotto, A.; Camicione, P.; Capris, P.; Izzotti, A. Oxidative DNA damage in the human trabecular meshwork: Clinical correlation in patients with primary open-angle glaucoma. Arch. Ophthalmol. 2005, 123, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Leung, Y.F.; Tam, P.O.; Lee, W.S.; Lam, D.S.; Yam, H.F.; Fan, B.J.; Tham, C.C.; Chua, J.K.; Pang, C.P. The dual role of dexamethasone on anti-inflammation and outflow resistance demonstrated in cultured human trabecular meshwork cells. Mol. Vis. 2003, 9, 425–439. [Google Scholar] [PubMed]
- Zhang, Z.; Tong, N.; Gong, Y.; Qiu, Q.; Yin, L.; Lv, X.; Wu, X. Valproate protects the retina from endoplasmic reticulum stress-induced apoptosis after ischemia-reperfusion injury. Neurosci. Lett. 2011, 504, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Kong, N.; Lu, X.; Li, B. Downregulation of microRNA-100 protects apoptosis and promotes neuronal growth in retinal ganglion cells. BMC Mol. Biol. 2014, 15, 25. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Huang, M. Etidronate protects chronic ocular hypertension induced retinal oxidative stress and promotes retinal ganglion cells growth through IGF-1 signaling pathway. Eur. J. Pharmacol. 2018, 841, 75–81. [Google Scholar] [CrossRef]
- Seet, L.F.; Toh, L.Z.; Finger, S.N.; Chu, S.W.L.; Wong, T.T. Valproic acid exerts specific cellular and molecular anti-inflammatory effects in post-operative conjunctiva. J. Mol. Med. 2019, 97, 63–75. [Google Scholar] [CrossRef]
- Boiko, E.V.; Pozniak, A.L.; Iakushev, D.I.; Maltsev, D.S.; Suetov, A.A.; Nuralova, I.V. Latent infections as a risk factor for posttrabeculectomy bleb failure. J. Glaucoma 2016, 25, 306–311. [Google Scholar] [CrossRef]
- Krupa, P.; Svobodova, B.; Dubisova, J.; Kubinova, S.; Jendelova, P.; Machova Urdzikova, L. Nano-formulated curcumin (Lipodisq™) modulates the local inflammatory response, reduces glial scar and preserves the white matter after spinal cord injury in rats. Neuroinflamm. 2019, 155, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.E.; Goldberg, J.L. Glaucoma 2.0: Neuroprotection, neuroregeneration, neuroenhancement. Ophthalmology 2012, 119, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Bungau, S.; Abdel-Daim, M.M.; Tit, D.M.; Ghanem, E.; Sato, S.; Maruyama-Inoue, M.; Yamane, S.; Kadonosono, K. Health benefits of polyphenols and carotenoids in age-related eye diseases. Oxid. Med. Cell Longev. 2019, 2019, 9783429. [Google Scholar] [CrossRef]
- Itakura, T.; Peters, D.M.; Fini, M.E. Glaucomatous MYOC mutations activate the IL-1/NF-κB inflammatory stress response and the glaucoma marker SELE in trabecular meshwork cells. Mol. Vis. 2015, 21, 1071–1084. [Google Scholar] [PubMed]
- Zhao, Y.; Li, X.; Gong, J.; Li, L.; Chen, L.; Zheng, L.; Chen, Z.; Shi, J.; Zhang, H. Annexin A1 nuclear translocation induces retinal ganglion cell apoptosis after ischemia-reperfusion injury through the p65/IL-1β pathway. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1350–1358. [Google Scholar] [CrossRef] [PubMed]
- Sugita, S.; Kawazoe, Y.; Imai, A.; Usui, Y.; Iwakura, Y.; Isoda, K.; Ito, M.; Mochizuki, M. Mature dendritic cell suppression by IL-1 receptor antagonist on retinal pigment epithelium cells. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3240–3249. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, I.; Nakazawa, M.; Ohguro, H. Autoimmune mechanisms in molecular pathology of glaucomatous optic neuropathy. Nippon Ganka Gakkai Zasshi J. Jpn. Ophthalmol. Soc. 2001, 105, 205–212. [Google Scholar] [CrossRef]
- Biswas, P.S.; Banerjee, K.; Kim, B.; Rouse, B.T. Mice transgenic for IL-1 receptor antagonist protein are resistant to herpetic stromal keratitis: Possible role for IL-1 in herpetic stromal keratitis pathogenesis. J. Immunol. 2004, 172, 3736–3744. [Google Scholar] [CrossRef] [PubMed]
- Kothari, M.T.; Mehta, B.K.; Asher, N.S.; Kothari, K.J. Recurrence of bilateral herpes simplex virus keratitis following bimatoprost use. Indian. J. Ophthalmol. 2006, 54, 47–48. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Hondur, G.; Tezel, G. Antioxidant treatment limits neuroinflammation in experimental glaucoma. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2344–2354. [Google Scholar] [CrossRef]
- Sakhnov, S.N.; Kharchenko, V.V. The diagnostic and prognostication of glaucoma. Klin. Lab. Diagn. 2018, 63, 246–249. [Google Scholar]
- Inoue-Mochita, M.; Inoue, T.; Kojima, S.; Futakuchi, A.; Fujimoto, T.; Sato-Ohira, S.; Tsutsumi, U.; Tanihara, H. Interleukin-6-mediated trans-signaling inhibits transforming growth factor-β signaling in trabecular meshwork cells. J. Biol. Chem. 2018, 293, 10975–10984. [Google Scholar] [CrossRef]
- Ulhaq, Z.S.; Soraya, G.V.; Hasan, Y.T.N.; Rachma, L.N.; Rachmawati, E.; Shodry, S.; Kusuma, M.A.S. Serum IL-6/IL-10 ratio as a biomarker for the diagnosis and severity assessment of primary-open angle glaucoma. Eur. J. Ophthalmol. 2022, 32, 2259–2264. [Google Scholar] [CrossRef] [PubMed]
- Paroli, M.P.; Del Giudice, E.; Giovannetti, F.; Caccavale, R.; Paroli, M. Management strategies of juvenile idiopathic arthritis-associated chronic anterior uveitis: Current perspectives. Clin. Ophthalmol. 2022, 16, 1665–1673. [Google Scholar] [CrossRef] [PubMed]
- Hamm, A.; Veeck, J.; Bektas, N.; Wild, P.J.; Hartmann, A.; Heindrichs, U.; Kristiansen, G.; Werbowetski-Ogilvie, T.; Del Maestro, R.; Knuechel, R.; et al. Frequent expression loss of Inter-alpha-trypsin inhibitor heavy chain (ITIH) genes in multiple human solid tumors: A systematic expression analysis. BMC Cancer 2008, 8, 25. [Google Scholar] [CrossRef]
- Kim, T.H.; Koo, J.H.; Heo, M.J.; Han, C.Y.; Kim, Y.I.; Park, S.Y.; Cho, I.J.; Lee, C.H.; Choi, C.S.; Lee, J.W.; et al. Overproduction of inter-α-trypsin inhibitor heavy chain 1 after loss of Gα13 in liver exacerbates systemic insulin resistance in mice. Sci. Transl. Med. 2019, 11, eaan4735. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kwon, Y.J.; Kim, S.J.; Joung, B. Metabolic syndrome as an independent risk factor for glaucoma: A nationally representative study. Diabetol. Metab. Syndr. 2023, 15, 177. [Google Scholar] [CrossRef] [PubMed]
- Blackler, G.; Akingbasote, J.; Cairns, E.; Howlett, C.; Kiser, P.; Barra, L. The effect of HLA-DRB1*04:01 on a mouse model of atherosclerosis. J. Transl. Autoimmun. 2023, 7, 100203. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Wang, J.; Zhang, Q.; Wang, Y.; Niu, L.; Shao, T. Overexpression of low-density lipoprotein receptors stimulated by vascular endothelial growth factor in fibroblasts from pterygium. Biomed. Pharmacother. 2017, 93, 609–615. [Google Scholar] [CrossRef]
- Wang, F.; Ge, Q.M.; Shu, H.Y.; Liao, X.L.; Liang, R.B.; Li, Q.Y.; Zhang, L.J.; Gao, G.P.; Shao, Y. Decreased retinal microvasculature densities in pterygium. Int. J. Ophthalmol. 2021, 14, 1858–1867. [Google Scholar] [CrossRef] [PubMed]
- Schlotzer-Schrehardt, U. Molecular pathology of pseudoexfoliation syndrome/glaucoma—New insights from LOXL1 gene associations. Exp. Eye Res. 2009, 88, 776–785. [Google Scholar] [CrossRef] [PubMed]
- Greene, A.G.; Eivers, S.B.; McDonnell, F.; Dervan, E.W.J.; O’Brien, C.J.; Wallace, D.M. Differential lysyl oxidase like 1 expression in pseudoexfoliation glaucoma is orchestrated via DNA methylation. Exp. Eye Res. 2020, 201, 108349. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.; Yaskolka Meir, A.; Bernhart, S.H.; Gepner, Y.; Shelef, I.; Schwarzfuchs, D.; Tsaban, G.; Zelicha, H.; Hopp, L.; Muller, L.; et al. DNA methylation signature in blood mirrors successful weight-loss during lifestyle interventions: The CENTRAL trial. Genome Med. 2020, 12, 97. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, L.G.N.; Young, D.; Chow, L.; Nicholas, J.; Lee, A.; Poon, M.C.; Dufour, A.; Agbani, E.O. Proteomics and metabolomics profiling of platelets and plasma mediators of thrombo-inflammation in gestational hypertension and preeclampsia. Cells 2022, 11, 1256. [Google Scholar] [CrossRef] [PubMed]
- Malishevskaia, T.N.; Dolgova, I.G. Options for correction of endothelial dysfunction and oxidative stress in patients with primary open-angle glaucoma]. Vestn. Oftalmol. 2014, 130, 67–70, 72–73. [Google Scholar] [PubMed]
- Ali, M.; McKibbin, M.; Booth, A.; Parry, D.A.; Jain, P.; Riazuddin, S.A.; Hejtmancik, J.F.; Khan, S.N.; Firasat, S.; Shires, M.; et al. Null mutations in LTBP2 cause primary congenital glaucoma. Am. J. Hum. Genet. 2009, 84, 664–671. [Google Scholar] [CrossRef]
- Inoue, T.; Ohbayashi, T.; Fujikawa, Y.; Yoshida, H.; Akama, T.O.; Noda, K.; Horiguchi, M.; Kameyama, K.; Hata, Y.; Takahashi, K.; et al. Latent TGF-β binding protein-2 is essential for the development of ciliary zonule microfibrils. Hum. Mol. Genet. 2014, 23, 5672–5682. [Google Scholar] [CrossRef] [PubMed]
- Kwek, X.Y.; Hall, A.R.; Lim, W.W.; Katwadi, K.; Soong, P.L.; Grishina, E.; Lin, K.H.; Crespo-Avilan, G.; Yap, E.P.; Ismail, N.I.; et al. Role of cardiac mitofusins in cardiac conduction following simulated ischemia-reperfusion. Sci. Rep. 2022, 12, 21049. [Google Scholar] [CrossRef]
- Okumichi, H.; Kiuchi, Y.; Baba, T.; Kanamoto, T.; Naito, T.; Nakakura, S.; Tabuchi, H.; Nii, H.; Sueoka, C.; Sugimoto, Y. The signs of ocular-surface disorders after switching from latanoprost to tafluprost/timolol fixed combination: A prospective study. Clin. Ophthalmol. 2017, 11, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Zhang, W.; Zhang, G.; Ding, X.; Kang, L.; Zhou, T.; Ji, M.; Guan, H. Age-related cataract: GSTP1 ubiquitination and degradation by Parkin inhibits its anti-apoptosis in lens epithelial cells. Biochim. Biophys. Acta Mol. Cell Res. 2023, 1870, 119450. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, T.; Sun, G.F.; Xiao, J.X.; Jiang, L.P.; Tou, F.F.; Qu, X.H.; Han, X.J. Metformin protects against retinal ischemia/reperfusion injury through AMPK-mediated mitochondrial fusion. Free Radic. Biol. Med. 2023, 205, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Nivison, M.P.; Ericson, N.G.; Green, V.M.; Bielas, J.H.; Campbell, J.S.; Horner, P.J. Age-related accumulation of phosphorylated mitofusin 2 protein in retinal ganglion cells correlates with glaucoma progression. Exp. Neurol. 2017, 296, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Cattin, M.E.; Wang, J.; Weldrick, J.J.; Roeske, C.L.; Mak, E.; Thorn, S.L.; DaSilva, J.N.; Wang, Y.; Lusis, A.J.; Burgon, P.G. Deletion of MLIP (muscle-enriched A-type lamin-interacting protein) leads to cardiac hyperactivation of Akt/mammalian target of rapamycin (mTOR) and impaired cardiac adaptation. J. Biol. Chem. 2015, 290, 26699–26714. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, D.; Li, X.; Zhang, Y.; Zhao, Y.; Xu, D.; Cheng, J.; Wang, J.; Li, W.; Lin, C.; et al. Comparative proteomics reveals genetic mechanisms of body weight in Hu sheep and Dorper sheep. J. Proteom. 2022, 267, 104699. [Google Scholar] [CrossRef] [PubMed]
- Helin-Toiviainen, M.; Ronkko, S.; Puustjarvi, T.; Rekonen, P.; Ollikainen, M.; Uusitalo, H. Conjunctival matrix metalloproteinases and their inhibitors in glaucoma patients. Acta Ophthalmol. 2015, 93, 165–171. [Google Scholar] [CrossRef]
- Markiewicz, L.; Pytel, D.; Mucha, B.; Szymanek, K.; Szaflik, J.; Szaflik, J.P.; Majsterek, I. Altered expression levels of MMP1, MMP9, MMP12, TIMP1, and IL-1β as a risk factor for the elevated IOP and optic nerve head damage in the primary open-angle glaucoma patients. Biomed. Res. Int. 2015, 2015, 812503. [Google Scholar] [CrossRef]
- De Groef, L.; Salinas-Navarro, M.; Van Imschoot, G.; Libert, C.; Vandenbroucke, R.E.; Moons, L. Decreased TNF levels and improved retinal ganglion cell survival in MMP-2 null mice suggest a role for MMP-2 as TNF sheddase. Mediat. Inflamm. 2015, 2015, 108617. [Google Scholar] [CrossRef] [PubMed]
- Ashworth Briggs, E.L.; Toh, T.; Eri, R.; Hewitt, A.W.; Cook, A.L. TIMP1, TIMP2, and TIMP4 are increased in aqueous humor from primary open angle glaucoma patients. Mol. Vis. 2015, 21, 1162–1172. [Google Scholar] [PubMed]
- Robertson, J.V.; Siwakoti, A.; West-Mays, J.A. Altered expression of transforming growth factor beta 1 and matrix metalloproteinase-9 results in elevated intraocular pressure in mice. Mol. Vis. 2013, 19, 684–695. [Google Scholar]
- Reddy, S.; Sahay, P.; Padhy, D.; Sarangi, S.; Suar, M.; Modak, R.; Rao, A. Tear biomarkers in latanoprost and bimatoprost treated eyes. PLoS ONE 2018, 13, e0201740. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.J.; Peng, Q.; Chen, C.; Humphrey, M.B.; Heinecke, J.; Zhang, S.X. Macrophage metalloelastase (MMP-12) deficiency mitigates retinal inflammation and pathological angiogenesis in ischemic retinopathy. PLoS ONE 2012, 7, e52699. [Google Scholar] [CrossRef] [PubMed]
- Vishal, M.; Sharma, A.; Kaurani, L.; Alfano, G.; Mookherjee, S.; Narta, K.; Agrawal, J.; Bhattacharya, I.; Roychoudhury, S.; Ray, J.; et al. Genetic association and stress mediated down-regulation in trabecular meshwork implicates MPP7 as a novel candidate gene in primary open angle glaucoma. BMC Med. Genom. 2016, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- New, M.; Van Acker, T.; Sakamaki, J.I.; Jiang, M.; Saunders, R.E.; Long, J.; Wang, V.M.; Behrens, A.; Cerveira, J.; Sudhakar, P.; et al. MDH1 and MPP7 Regulate Autophagy in Pancreatic Ductal Adenocarcinoma. Cancer Res. 2019, 79, 1884–1898. [Google Scholar] [CrossRef] [PubMed]
- Navneet, S.; Zhao, J.; Wang, J.; Mysona, B.; Barwick, S.; Ammal Kaidery, N.; Saul, A.; Kaddour-Djebbar, I.; Bollag, W.B.; Thomas, B.; et al. Hyperhomocysteinemia-induced death of retinal ganglion cells: The role of Muller glial cells and NRF2. Redox Biol. 2019, 24, 101199. [Google Scholar] [CrossRef] [PubMed]
- Junemann, A.; Rejdak, R.; Hohberger, B. Significance of homocysteine in glaucoma. Klin. Monbl. Augenheilkd. 2018, 235, 163–174. [Google Scholar]
- Borthakur, D.; Kumar, R.; Dada, R. Yoga: A natural solution to decrease disease burden in children of MTHFR deficient parents. Clin. Ter. 2023, 174, 28–32. [Google Scholar]
- Hsiao, T.H.; Lee, G.H.; Chang, Y.S.; Chen, B.H.; Fu, T.F. The incoherent fluctuation of folate pools and differential regulation of folate enzymes prioritize nucleotide supply in the zebrafish model displaying folate deficiency-induced microphthalmia and visual defects. Front. Cell Dev. Biol. 2021, 9, 702969. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.L.; Lukas, T.J.; Yuan, M.; Neufeld, A.H. Age-related increase in mitochondrial DNA damage and loss of DNA repair capacity in the neural retina. Neurobiol. Aging 2010, 31, 2002–2010. [Google Scholar] [CrossRef] [PubMed]
- Nakatake, S.; Murakami, Y.; Ikeda, Y.; Morioka, N.; Tachibana, T.; Fujiwara, K.; Yoshida, N.; Notomi, S.; Hisatomi, T.; Yoshida, S.; et al. MUTYH promotes oxidative microglial activation and inherited retinal degeneration. JCI Insight 2016, 1, e87781. [Google Scholar] [CrossRef] [PubMed]
- Chintala, S.K. Tissue and urokinase plasminogen activators instigate the degeneration of retinal ganglion cells in a mouse model of glaucoma. Exp. Eye Res. 2016, 143, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Orwig, S.D.; Chi, P.V.; Du, Y.; Hill, S.E.; Cavitt, M.A.; Suntharalingam, A.; Turnage, K.C.; Dickey, C.A.; France, S.; Fu, H.; et al. Ligands for glaucoma-associated myocilin discovered by a generic binding assay. ACS Chem. Biol. 2014, 9, 517–525. [Google Scholar] [CrossRef]
- Mookherjee, S.; Acharya, M.; Banerjee, D.; Bhattacharjee, A.; Ray, K. Molecular basis for involvement of CYP1B1 in MYOC upregulation and its potential implication in glaucoma pathogenesis. PLoS ONE 2012, 7, e45077. [Google Scholar] [CrossRef]
- Okubo, A.; Nakagawa, S.; Ogawa, S.; Ishii, K. Anterior chamber shallowing from the early stage of surgery and suprachoroidal effusion during clear corneal small-incision cataract surgery: A case report. Case Rep. Ophthalmol. 2022, 13, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Ye, C.; Gao, Z.; Hu, J.; Chen, C.; Xiao, R.; Lu, F.; Wei, K. Immune infiltration patterns and identification of new diagnostic biomarkers GDF10, NCKAP5, and RTKN2 in non-small cell lung cancer. Transl. Oncol. 2023, 29, 101618. [Google Scholar] [CrossRef]
- Kingston, E.J.; Zagora, S.L.; Symes, R.J.; Raman, P.; McCluskey, P.J.; Lusthaus, J.A. Infective necrotizing scleritis after XEN gel stent with mitomycin-c. J. Glaucoma 2022, 31, 129–132. [Google Scholar] [CrossRef]
- Wang, D.; Wei, Y.; Shi, L.; Khan, M.Z.; Fan, L.; Wang, Y.; Yu, Y. Genome-wide DNA methylation pattern in a mouse model reveals two novel genes associated with Staphylococcus aureus mastitis. Asian-Australas. J. Anim. Sci. 2020, 33, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Avotri, S.; Eatman, D.; Russell-Randall, K. Effects of resveratrol on inflammatory biomarkers in glaucomatous human trabecular meshwork cells. Nutrients 2019, 11, 984. [Google Scholar] [CrossRef] [PubMed]
- Rokicki, W.; Zaba, M.; Wyględowska-Promienska, D.; Kabiesz, A.; Reichman-Warmusz, E.; Brzozowa, M.; Majewski, W.; Wojnicz, R. Inducible and endothelial nitric synthetase expression and nitrotyrosine accumulation in iris vasculature of patients with primary open-angle glaucoma: A pilot study. Med. Sci. Monit. 2015, 21, 76–81. [Google Scholar] [PubMed]
- Liu, K.; Chen, B.; Zeng, F.; Wang, G.; Wu, X.; Liu, Y.; Li, G.; Yan, J.; Zhang, S. ApoE/NOS3 knockout mice as a novel cardiovascular disease model of hypertension and atherosclerosis. Genes 2022, 13, 1998. [Google Scholar] [CrossRef] [PubMed]
- Dada, T.; Bhai, N.; Midha, N.; Shakrawal, J.; Kumar, M.; Chaurasia, P.; Gupta, S.; Angmo, D.; Yadav, R.; Dada, R.; et al. Effect of mindfulness meditation on intraocular pressure and trabecular meshwork gene expression: A randomized controlled trial. Am. J. Ophthalmol. 2021, 223, 308–321. [Google Scholar] [CrossRef] [PubMed]
- Britto, J.M.; Lukehurst, S.; Weller, R.; Fraser, C.; Qiu, Y.; Hertzog, P.; Busfield, S.J. Generation and characterization of neuregulin-2-deficient mice. Mol. Cell Biol. 2004, 24, 8221–8226. [Google Scholar] [CrossRef] [PubMed]
- Jeanes, E.C.; Oliver, J.A.C.; Ricketts, S.L.; Gould, D.J.; Mellersh, C.S. Glaucoma-causing ADAMTS17 mutations are also reproducibly associated with height in two domestic dog breeds: Selection for short stature may have contributed to increased prevalence of glaucoma. Canine Genet. Epidemiol. 2019, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Lee, H.; Yang, C.H.; Ko, J.S.; Park, C.H.; Woo, R.S.; Kim, J.Y.; Sun, W.; Kim, J.H.; Ho, W.K.; et al. Bidirectional signaling of neuregulin-2 mediates formation of GABAergic synapses and maturation of glutamatergic synapses in newborn granule cells of postnatal hippocampus. J. Neurosci. 2015, 35, 16479–16493. [Google Scholar] [CrossRef]
- Malishevskaya, T.N.; Petrov, S.Y.; Petrov, S.A.; Vlasova, A.S.; Filippova, Y.E.; Markelova, O.I. Therapeutic possibilities of stimulating reparative neurogenesis in patients with glaucoma who have recovered from a coronavirus infection. Vestn. Oftalmol. 2023, 139, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.N.; Nagireddy, S.; Chakrabarti, S. Complex genetic mechanisms in glaucoma: An overview. Indian J. Ophthalmol. 2011, 59, S31–S42. [Google Scholar] [PubMed]
- Kowtharapu, B.S.; Murin, R.; Junemann, A.G.M.; Stachs, O. Role of corneal stromal cells on epithelial cell function during wound healing. Int. J. Mol. Sci. 2018, 19, 464. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra Rao, V.L.; Bowen, K.K.; Dhodda, V.K.; Song, G.; Franklin, J.L.; Gavva, N.R.; Dempsey, R.J. Gene expression analysis of spontaneously hypertensive rat cerebral cortex following transient focal cerebral ischemia. J. Neurochem. 2002, 83, 1072–1086. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Hu, H.Y.; Chu, D.; Chen, H.H.; Chang, C.K.; Chou, P. Patients with primary open-angle glaucoma may develop ischemic heart disease more often than those without glaucoma: An 11-year population-based cohort study. PLoS ONE 2016, 11, e0163210. [Google Scholar] [CrossRef]
- Krizsan-Agbas, D.; Pedchenko, T.; Smith, P.G. Neurotrimin is an estrogen-regulated determinant of peripheral sympathetic innervation. J. Neurosci. Res. 2008, 86, 3086–3095. [Google Scholar] [CrossRef] [PubMed]
- Fotesko, K.; Thomsen, B.S.V.; Kolko, M.; Vohra, R. Girl power in glaucoma: The role of estrogen in primary open angle glaucoma. Cell Mol. Neurobiol. 2022, 42, 41–57. [Google Scholar] [CrossRef]
- Kimura, A.; Namekata, K.; Guo, X.; Harada, C.; Harada, T. Neuroprotection, growth factors and Bdnf-TrkB signalling in retinal degeneration. Int. J. Mol. Sci. 2016, 17, 1584. [Google Scholar] [CrossRef] [PubMed]
- Harper, M.M.; Boese, E.A.; Kardon, R.H.; Ledolter, J.; Kuehn, M.H. High correlation between glaucoma treatment with topical prostaglandin analogs and BDNF immunoreactivity in human retina. Curr. Eye Res. 2021, 46, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Brun, P.; Brun, P.; Vono, M.; Venier, P.; Tarricone, E.; Deligianni, V.; Martines, E.; Zuin, M.; Spagnolo, S.; Cavazzana, R.; et al. Disinfection of ocular cells and tissues by atmospheric-pressure cold plasma. PLoS ONE 2012, 7, e33245. [Google Scholar] [CrossRef] [PubMed]
- Bosley, T.M.; Hellani, A.; Spaeth, G.L.; Myers, J.; Katz, L.J.; Moster, M.R.; Milcarek, B.; Abu-Amero, K.K. Down-regulation of OPA1 in patients with primary open angle glaucoma. Mol. Vis. 2011, 17, 1074–1079. [Google Scholar] [PubMed]
- Hu, X.; Dai, Y.; Zhang, R.; Shang, K.; Sun, X. Overexpression of optic atrophy type 1 protects retinal ganglion cells and upregulates parkin expression in experimental glaucoma. Front. Mol. Neurosci. 2018, 11, 350. [Google Scholar] [CrossRef] [PubMed]
- Acharya, M.; Mookherjee, S.; Bhattacharjee, A.; Thakur, S.K.; Bandyopadhyay, A.K.; Sen, A.; Chakrabarti, S.; Ray, K. Evaluation of the OPTC gene in primary open angle glaucoma: Functional significance of a silent change. BMC Mol. Biol. 2007, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Le Goff, M.M.; Lu, H.; Ugarte, M.; Henry, S.; Takanosu, M.; Mayne, R.; Bishop, P.N. The vitreous glycoprotein opticin inhibits preretinal neovascularization. Investig. Ophthalmol. Vis. Sci. 2012, 53, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Swarup, G.; Sayyad, Z. Altered functions and interactions of glaucoma-associated mutants of optineurin. Front. Immunol. 2018, 9, 1287. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.K. Retinal deimination in aging and disease. IUBMB Life 2009, 61, 504–509. [Google Scholar] [PubMed]
- Cafaro, T.A.; Santo, S.; Robles, L.A.; Crim, N.; Urrets-Zavalia, J.A.; Serra, H.M. Peptidylarginine deiminase type 2 is over expressed in the glaucomatous optic nerve. Mol. Vis. 2010, 16, 1654–1658. [Google Scholar]
- Zanon-Moreno, V.; Garcia-Medina, J.J.; Zanon-Viguer, V.; Moreno-Nadal, M.A.; Pinazo-Duran, M.D. Smoking, an additional risk factor in elder women with primary open-angle glaucoma. Mol. Vis. 2009, 15, 2953–2959. [Google Scholar]
- O’Connell, G.C.; Petrone, A.B.; Treadway, M.B.; Tennant, C.S.; Lucke-Wold, N.; Chantler, P.D.; Barr, T.L. Machine-learning approach identifies a pattern of gene expression in peripheral blood that can accurately detect ischaemic stroke. npj Genom. Med. 2016, 1, 16038. [Google Scholar] [CrossRef] [PubMed]
- Arslan, G.D.; Olgun, A.; Ozcan, D.; Gokcal, E.; Guven, D.; Asil, T. Assessment of cerebral vasomotor reactivity in patients with primary open-angle glaucoma and ocular hypertension using the breath-holding index. J. Glaucoma 2021, 30, 157–163. [Google Scholar] [CrossRef]
- Witters, P.; Honzik, T.; Bauchart, E.; Altassan, R.; Pascreau, T.; Bruneel, A.; Vuillaumier, S.; Seta, N.; Borgel, D.; Matthijs, G.; et al. Long-term follow-up in PMM2-CDG: Are we ready to start treatment trials? Genet. Med. 2019, 21, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Morava, E.; Wosik, H.; Karteszi, J.; Guillard, M.; Adamowicz, M.; Sykut-Cegielska, J.; Hadzsiev, K.; Wevers, R.A.; Lefeber, D.J. Congenital disorder of glycosylation type Ix: Review of clinical spectrum and diagnostic steps. J. Inherit. Metab. Dis. 2008, 31, 450–456. [Google Scholar] [CrossRef]
- Menniti, M.; Iuliano, R.; Amato, R.; Boito, R.; Corea, M.; Le Pera, I.; Gulletta, E.; Fuiano, G.; Perrotti, N. Serum and glucocorticoid-regulated kinase Sgk1 inhibits insulin-dependent activation of phosphomannomutase 2 in transfected COS-7 cells. Am. J. Physiol. Cell Physiol. 2005, 288, C148–C155. [Google Scholar] [CrossRef] [PubMed]
- Al Hussein Al Awamlh, S.; Wareham, L.K.; Risner, M.L.; Calkins, D.J. Insulin signaling as a therapeutic target in glaucomatous neurodegeneration. Int. J. Mol. Sci. 2021, 22, 4672. [Google Scholar] [CrossRef]
- Waugh, D.T. the contribution of fluoride to the pathogenesis of eye diseases: Molecular mechanisms and implications for public health. Int. J. Environ. Res. Public Health 2019, 16, 856. [Google Scholar] [CrossRef] [PubMed]
- Mumcu, U.Y.; Kocer, I.; Ates, O.; Alp, H.H. Decreased paraoxonase1 activity and increased malondialdehyde and oxidative DNA damage levels in primary open angle glaucoma. Int. J. Ophthalmol. 2016, 9, 1518–1520. [Google Scholar]
- Cmelo, J. Translaminar gradient and glaucoma. Cesk Slov. Oftalmol. 2017, 73, 52–56. [Google Scholar]
- Jang, S.Y.; Chae, M.K.; Lee, J.H.; Lee, E.J.; Yoon, J.S. MicroRNA-27 inhibits adipogenic differentiation in orbital fibroblasts from patients with Graves’ orbitopathy. PLoS ONE 2019, 14, e0221077. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Yang, Y.; Liu, Y.; Huang, L.; Gu, M.; Wu, Y.; Xu, L.; Sun, H.; Guo, W. Magnolol limits NFκB-dependent inflammation by targeting PPARγ relieving retinal ischemia/reperfusion injury. Int. Immunopharmacol. 2022, 112, 109242. [Google Scholar] [CrossRef] [PubMed]
- Ashok, A.; Kang, M.H.; Wise, A.S.; Pattabiraman, P.; Johnson, W.M.; Lonigro, M.; Ravikumar, R.; Rhee, D.J.; Singh, N. Prion protein modulates endothelial to mesenchyme-like transition in trabecular meshwork cells: Implications for primary open angle glaucoma. Sci. Rep. 2019, 9, 13090. [Google Scholar] [CrossRef] [PubMed]
- Perumal, N.; Yurugi, H.; Dahm, K.; Rajalingam, K.; Grus, F.H.; Pfeiffer, N.; Manicam, C. Proteome landscape and interactome of voltage-gated potassium channel 1.6 (Kv1.6) of the murine ophthalmic artery and neuroretina. Int. J. Biol. Macromol. 2024, 257, 128464. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.J.; Zhao, A.G.; Wang, X.L. Correlations of AFAP1, GMDS and PTGFR gene polymorphisms with intra-ocular pressure response to latanoprost in patients with primary open-angle glaucoma. J. Clin. Pharm. Ther. 2017, 42, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, M.; Higashide, T.; Takahashi, M.; Sugiyama, K. Association between genetic polymorphisms of the prostaglandin F2alpha receptor gene and response to latanoprost. Ophthalmology 2007, 114, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Matsou, A.; Anastasopoulos, E. Investigational drugs targeting prostaglandin receptors for the treatment of glaucoma. Expert. Opin. Investig. Drugs 2018, 27, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Cryan, L.M.; Fitzgerald, D.J.; O’Brien, C. Ocular prostaglandin production and morphology in mice lacking a single isoform of cyclooxygenase. Prostaglandins Leukot. Essent. Fat. Acids 2009, 81, 401–409. [Google Scholar] [CrossRef]
- Maihofner, C.; Schlotzer-Schrehardt, U.; Guhring, H.; Zeilhofer, H.U.; Naumann, G.O.; Pahl, A.; Mardin, C.; Tamm, E.R.; Brune, K. Expression of cyclooxygenase-1 and -2 in normal and glaucomatous human eyes. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2616–2624. [Google Scholar]
- Reitmair, A.; Lambrecht, N.W.; Yakubov, I.; Nieves, A.; Old, D.; Donde, Y.; Dinh, D.; Burk, R.; Sachs, G.; Im, W.B.; et al. Prostaglandin E2 receptor subtype EP2- and EP4-regulated gene expression profiling in human ciliary smooth muscle cells. Physiol. Genom. 2010, 42, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Gabelt, B.T.; Geiger, B.; Kaufman, P.L. The role of the actomyosin system in regulating trabecular fluid outflow. Exp. Eye Res. 2009, 88, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Webber, C.A.; Wang, J.; Xu, Y.; Martinez, J.A.; Liu, W.Q.; McDonald, D.; Guo, G.F.; Nguyen, M.D.; Zochodne, D.W. Activated RHOA and peripheral axon regeneration. Exp. Neurol. 2008, 212, 358–369. [Google Scholar] [CrossRef]
- Gao, Z.; Li, Q.; Zhang, Y.; Gao, X.; Li, H.; Yuan, Z. Ripasudil alleviated the inflammation of RPE cells by targeting the miR-136-5p/ROCK/NLRP3 pathway. BMC Ophthalmol. 2020, 20, 134. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R., Jr. Properties of FDA-approved small molecule protein kinase inhibitors: A 2021 update. Pharmacol. Res. 2021, 165, 105463. [Google Scholar] [CrossRef]
- Greathouse, K.M.; Henderson, B.W.; Gentry, E.G.; Herskowitz, J.H. Fasudil or genetic depletion of ROCK1 or ROCK2 induces anxiety-like behaviors. Behav. Brain Res. 2019, 373, 112083. [Google Scholar] [CrossRef] [PubMed]
- Kapuganti, R.S.; Hayat, B.; Padhy, B.; Mohanty, P.P.; Alone, D.P. Dickkopf-1 and ROCK2 upregulation and associated protein aggregation in pseudoexfoliation syndrome and glaucoma. Life Sci. 2023, 326, 121797. [Google Scholar] [CrossRef]
- Gong, B.; Guo, Y.; Ding, S.; Liu, X.; Meng, A.; Li, D.; Jia, S. A Golgi-derived vesicle potentiates PtdIns4P to PtdIns3P conversion for endosome fission. Nat. Cell Biol. 2021, 23, 782–795. [Google Scholar] [CrossRef]
- Want, A.; Gillespie, S.R.; Wang, Z.; Gordon, R.; Iomini, C.; Ritch, R.; Wolosin, J.M.; Bernstein, A.M. Autophagy and mitochondrial dysfunction in tenon fibroblasts from exfoliation glaucoma patients. PLoS ONE 2016, 11, e0157404. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.; Bartenschlager, R. Hepatitis C virus’s next top models? Nat. Microbiol. 2016, 1, 15018. [Google Scholar] [CrossRef] [PubMed]
- Marino, P.F.; Rossi, G.C.M.; Campagna, G.; Capobianco, D.; Costagliola, C.; on behalf of Qualicos Study Group. Effects of citicoline, homotaurine, and vitamin E on contrast sensitivity and visual-related quality of life in patients with primary open-angle glaucoma: A preliminary study. Molecules 2020, 25, 5614. [Google Scholar] [CrossRef] [PubMed]
- Fuchshofer, R.; Stephan, D.A.; Russell, P.; Tamm, E.R. Gene expression profiling of TGFbeta2- and/or BMP7-treated trabecular meshwork cells: Identification of Smad7 as a critical inhibitor of TGF-beta2 signaling. Exp. Eye Res. 2009, 88, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Liu, B. Single nucleotide polymorphisms of metabolic syndrome-related genes in primary open angle glaucoma. Int. J. Ophthalmol. 2010, 3, 36–42. [Google Scholar]
- Yaman, D.; Takmaz, T.; Yuksel, N.; Dincer, S.A.; Sahin, F.I. Evaluation of silent information regulator T (SIRT) 1 and Forkhead Box O (FOXO) transcription factor 1 and 3a genes in glaucoma. Mol. Biol. Rep. 2020, 47, 9337–9344. [Google Scholar] [CrossRef]
- Khan, R.S.; Dine, K.; Das Sarma, J.; Shindler, K.S. SIRT1 activating compounds reduce oxidative stress mediated neuronal loss in viral induced CNS demyelinating disease. Acta Neuropathol. Commun. 2014, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Paper, W.; Kroeber, M.; Heersink, S.; Stephan, D.A.; Fuchshofer, R.; Russell, P.; Tamm, E.R. Elevated amounts of myocilin in the aqueous humor of transgenic mice cause significant changes in ocular gene expression. Exp. Eye Res. 2008, 87, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Meurer, L.; Ferdman, L.; Belcher, B.; Camarata, T. The SIX family of transcription factors: Common themes integrating developmental and cancer biology. Front. Cell Dev. Biol. 2021, 9, 707854. [Google Scholar] [CrossRef] [PubMed]
- Bormann, C.; Busch, C.; Rehak, M.; Scharenberg, C.T.; Furashova, O.; Ziemssen, F.; Unterlauft, J.D. Postoperative RNFL-changes after successful trabeculectomy: 2-year outcomes. Klin. Monbl Augenheilkd. 2023, 241, 772–779. [Google Scholar] [CrossRef]
- Luz-Madrigal, A.; Grajales-Esquivel, E.; McCorkle, A.; DiLorenzo, A.M.; Barbosa-Sabanero, K.; Tsonis, P.A.; Del Rio-Tsonis, K. Reprogramming of the chick retinal pigmented epithelium after retinal injury. BMC Biol. 2014, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- Hilgen, G.; Huebner, A.K.; Tanimoto, N.; Sothilingam, V.; Seide, C.; Garcia Garrido, M.; Schmidt, K.F.; Seeliger, M.W.; Lowel, S.; Weiler, R.; et al. Lack of the sodium-driven chloride bicarbonate exchanger NCBE impairs visual function in the mouse retina. PLoS ONE 2012, 7, e46155. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.D. Mouse models of SLC4-linked disorders of HCO3−-transporter dysfunction. Am. J. Physiol. Cell Physiol. 2018, 314, C569–C588. [Google Scholar] [CrossRef]
- Pang, R.; Peng, J.; Cao, K.; Sun, Y.; Pei, X.T.; Yang, D.; Lu, Z.L.; Wang, N. Association between contrast sensitivity function and structural damage in primary open-angle glaucoma. Br. J. Ophthalmol. 2023, 108, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Zanon-Moreno, V.; Asensio-Marquez, E.M.; Ciancotti-Oliver, L.; Garcia-Medina, J.J.; Sanz, P.; Ortega-Azorin, C.; Pinazo-Duran, M.D.; Ordovas, J.M.; Corella, D. Effects of polymorphisms in vitamin E-, vitamin C-, and glutathione peroxidase-related genes on serum biomarkers and associations with glaucoma. Mol. Vis. 2013, 19, 231–242. [Google Scholar]
- Kannan, R.; Stolz, A.; Ji, Q.; Prasad, P.D.; Ganapathy, V. Vitamin C transport in human lens epithelial cells: Evidence for the presence of SVCT2. Exp. Eye Res. 2001, 73, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, T.; Sakiyama, S.; Takashima, H.; Honda, R.; Shumba, M.N.; Nakamura, Y.; Kasahara, K.; Tamai, I. Toxicological implication of prostaglandin transporter SLCO2A1 inhibition by cigarette smoke in exacerbation of lung inflammation. Toxicol. Appl. Pharmacol. 2020, 405, 115201. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.; Banait, S. Through the smoke: An in-depth review on cigarette smoking and its impact on ocular health. Cureus 2023, 15, e47779. [Google Scholar] [CrossRef] [PubMed]
- Kraft, M.E.; Glaeser, H.; Mandery, K.; Konig, J.; Auge, D.; Fromm, M.F.; Schlotzer-Schrehardt, U.; Welge-Lussen, U.; Kruse, F.E.; Zolk, O. The prostaglandin transporter OATP2A1 is expressed in human ocular tissues and transports the antiglaucoma prostanoid latanoprost. Investig. Ophthalmol. Vis. Sci. 2010, 51, 2504–2511. [Google Scholar] [CrossRef] [PubMed]
- Haddadin, R.I.; Oh, D.J.; Kang, M.H.; Filippopoulos, T.; Gupta, M.; Hart, L.; Sage, E.H.; Rhee, D.J. SPARC-null mice exhibit lower intraocular pressures. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3771–3777. [Google Scholar] [CrossRef]
- Mathur, M.C.; Ratnam, P.V.; Saikumar, S.J.; John, M.; Ravishankar, S.; Dinesh, M.B.; Chandil, P.; Pahuja, K.; Cherlikar, V.; Wadhwani, S.; et al. Netarsudil monotherapy as the initial treatment for open-angle glaucoma and ocular hypertension in Indian patients: A real-world evaluation of efficacy and safety. Indian J. Ophthalmol. 2023, 71, 2500–2503. [Google Scholar] [CrossRef] [PubMed]
- Kirwan, R.P.; Wordinger, R.J.; Clark, A.F.; O’Brien, C.J. Differential global and extra-cellular matrix focused gene expression patterns between normal and glaucomatous human lamina cribrosa cells. Mol. Vis. 2009, 15, 76–88. [Google Scholar] [PubMed]
- Shi, H.; Yin, Z.; Koronyo, Y.; Fuchs, D.T.; Sheyn, J.; Davis, M.R.; Wilson, J.W.; Margeta, M.A.; Pitts, K.M.; Herron, S.; et al. Regulating microglial miR-155 transcriptional phenotype alleviates Alzheimer’s-induced retinal vasculopathy by limiting Clec7a/Galectin-3+ neurodegenerative microglia. Acta Neuropathol. Commun. 2022, 10, 136. [Google Scholar] [CrossRef] [PubMed]
- Sathiyanathan, P.; Tay, C.Y.; Stanton, L.W. Transcriptome analysis for the identification of cellular markers related to trabecular meshwork differentiation. BMC Genom. 2017, 18, 383. [Google Scholar] [CrossRef] [PubMed]
- Mormile, R. Primary open angle glaucoma in type 2 diabetes: Implications of the IL-10/STAT3-mediated anti-inflammatory response? Immunol. Lett. 2016, 179, 131–132. [Google Scholar] [CrossRef]
- Lozano, D.C.; Choe, T.E.; Cepurna, W.O.; Morrison, J.C.; Johnson, E.C. Early optic nerve head glial proliferation and Jak-Stat pathway activation in chronic experimental glaucoma. Investig. Ophthalmol. Vis. Sci. 2019, 60, 921–932. [Google Scholar] [CrossRef] [PubMed]
- Kurte, M.; Lopez, M.; Aguirre, A.; Escobar, A.; Aguillon, J.C.; Charo, J.; Larsen, C.G.; Kiessling, R.; Salazar-Onfray, F. A synthetic peptide homologous to functional domain of human IL-10 down-regulates expression of MHC class I and transporter associated with antigen processing 1/2 in human melanoma cells. J. Immunol. 2004, 173, 1731–1777. [Google Scholar] [CrossRef] [PubMed]
- Abi-Ayad, N.; Grange, J.D.; Watkin, E.; De Bats, M.; Fleury, J.; Kodjikian, L.; Gambrelle, J. Ring melanoma revealed by spontaneous hyphema. J. Fr. Ophtalmol. 2007, 30, 729–732. [Google Scholar] [CrossRef]
- Ge, Q.; Feng, F.; Liu, L.; Chen, L.; Lv, P.; Ma, S.; Chen, K.; Yao, Q. RNA-Seq analysis of the pathogenesis of STZ-induced male diabetic mouse liver. J. Diabetes Complicat. 2020, 34, 107444. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Liang, X.; Wen, Y.; Li, P.; Zhang, L.; Ma, M.; Cheng, S.; Du, Y.; Liu, L.; Ding, M.; et al. Integrative analysis of transcriptome-wide association study data and messenger RNA expression profiles identified candidate genes and pathways for inflammatory bowel disease. J. Cell Biochem. 2019, 120, 14831–14837. [Google Scholar] [CrossRef]
- Chaiwiang, N.; Poyomtip, T. Microbial dysbiosis and microbiota-gut-retina axis: The lesson from brain neurodegenerative diseases to primary open-angle glaucoma pathogenesis of autoimmunity. Acta Microbiol. Immunol. Hung. 2019, 66, 541–558. [Google Scholar] [CrossRef] [PubMed]
- Sears, N.C.; Boese, E.A.; Miller, M.A.; Fingert, J.H. Mendelian genes in primary open angle glaucoma. Exp. Eye Res. 2019, 186, 107702. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Huo, H.; Werid, G.M.; Ibrahim, Y.M.; Tang, L.; Wang, Y.; Chen, H. TBK1 mediates innate antiviral immune response against duck enteritis virus. Viruses 2022, 14, 1008. [Google Scholar] [CrossRef] [PubMed]
- Namburar, S.; Pillai, M.; Varghese, G.; Thiel, C.; Robin, A.L. Waste generated during glaucoma surgery: A comparison of two global facilities. Am. J. Ophthalmol. Case Rep. 2018, 12, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.; Shariq, M.; Dwivedi, D.; Krishnan, N.; Naumann, R.; Bhalla, U.S.; Ghosh, H.S. Adult brain neurons require continual expression of the schizophrenia-risk gene Tcf4 for structural and functional integrity. Transl. Psychiatry 2021, 11, 494. [Google Scholar] [CrossRef] [PubMed]
- Kaurani, L.; Vishal, M.; Kumar, D.; Sharma, A.; Mehani, B.; Sharma, C.; Chakraborty, S.; Jha, P.; Ray, J.; Sen, A.; et al. Gene-rich large deletions are overrepresented in POAG patients of Indian and Caucasian origins. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3258–3264. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Gong, X.; Johnson, S.T.; Corey, D.R.; Mootha, V.V. The TCF4 trinucleotide repeat expansion of fuchs’ endothelial corneal dystrophy: Implications for the anterior segment of the eye. Investig. Ophthalmol. Vis. Sci. 2023, 64, 16. [Google Scholar] [CrossRef] [PubMed]
- Kasetti, R.B.; Maddineni, P.; Kodati, B.; Nagarajan, B.; Yacoub, S. Astragaloside IV attenuates ocular hypertension in a mouse model of TGFβ2 induced primary open angle glaucoma. Int. J. Mol. Sci. 2021, 22, 12508. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Perkumas, K.M.; Qin, X.; Hauser, M.A.; Stamer, W.D.; Liu, Y. Expression profiling of human Schlemm’s canal endothelial cells from eyes with and without glaucoma. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6747–6753. [Google Scholar] [CrossRef]
- Xie, B.; Xiong, W.; Zhang, F.; Wang, N.; Luo, Y.; Chen, Y.; Cao, J.; Chen, Z.; Ma, C.; Chen, H. The miR-103a-3p/TGFBR3 axis regulates TGF-β-induced orbital fibroblast activation and fibrosis in thyroid-eye disease. Mol. Cell Endocrinol. 2023, 559, 111780. [Google Scholar] [CrossRef]
- Qin, M.; Yu-Wai-Man, C. Glaucoma: Novel antifibrotic therapeutics for the trabecular meshwork. Eur. J. Pharmacol. 2023, 954, 175882. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.J.; Huang, Y.L.; Ju, J.P.; Lu, C.L.; Chiu, A.W. Altered transcripts expression of matrix metalloproteinases and their tissue inhibitors in tenon capsule of patients with glaucoma. J. Glaucoma 2004, 13, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Wang, Y.; Wang, Y.; Liu, F.; Deng, S.; Xue, W.; Wang, Y. Ursolic acid ameliorated neuronal damage by restoring microglia-activated MMP/TIMP imbalance in vitro. Drug Des. Devel Ther. 2023, 17, 2481–2493. [Google Scholar] [CrossRef] [PubMed]
- Hill, L.J.; Mead, B.; Blanch, R.J.; Ahmed, Z.; De Cogan, F.; Morgan-Warren, P.J.; Mohamed, S.; Leadbeater, W.; Scott, R.A.; Berry, M.; et al. Decorin reduces intraocular pressure and retinal ganglion cell loss in rodents through fibrolysis of the scarred trabecular meshwork. Investig. Ophthalmol. Vis. Sci. 2015, 56, 3743–3757. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.S.; de Araujo, V.G.; Lani-Louzada, R.; Linden, R.; Ribas, V.T.; Petrs-Silva, H. Perspective on gene therapy for glaucoma In Glaucoma—Recent Advances and New Perspectives; Davey, P.G., Ed.; IntechOpen Ltd.: London, UK, 2022. [Google Scholar] [CrossRef]
- Stamer, W.D.; Perkumas, K.M.; Kang, M.H.; Dibas, M.; Robinson, M.R.; Rhee, D.J. Proposed mechanism of long-term intraocular pressure lowering with the bimatoprost implant. Investig. Ophthalmol. Vis. Sci. 2023, 64, 15. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, H.; Yaraee, R.; Faghihzadeh, S.; Ghassemi-Broumand, M.; Mahmoudi, M.; Babaei, M.; Naderi, M.; Safavi, M.; Ghazanfari, Z.; Rastin, M.; et al. Tear and serum MMP-9 and serum TIMPs levels in the severe sulfur mustard eye injured exposed patients. Int. Immunopharmacol. 2019, 77, 105812. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Fang, D.; Silver, P.B.; Wen, F.; Li, B.; Ren, X.; Lin, Q.; Caspi, R.R.; Su, S.B. The role of TLR2, TRL3, TRL4, and TRL9 signaling in the pathogenesis of autoimmune disease in a retinal autoimmunity model. Investig. Ophthalmol. Vis. Sci. 2010, 51, 3092–3099. [Google Scholar] [CrossRef] [PubMed]
- Shamsuddin, N.; Kumar, A. TLR2 mediates the innate response of retinal Muller glia to Staphylococcus aureus. J. Immunol. 2011, 186, 7089–7097. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Luo, Y.; Xu, H.; Huang, H.; Jiang, R.; Sun, X. Clinical analysis of infectious endophthalmitis following glaucoma filtration surgery. J. Ophthalmic Inflamm. Infect. 2024, 14, 11. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.L.; Mavlyutov, T.A.; Perlmutter, T.E.; Curry, S.M.; Harris, S.L.; Chauhan, A.K.; McDowell, C.M. Fibronectin extra domain A (FN-EDA) elevates intraocular pressure through Toll-like receptor 4 signaling. Sci. Rep. 2020, 10, 9815. [Google Scholar] [CrossRef] [PubMed]
- Poyomtip, T. Roles of Toll-like receptor 4 for cellular pathogenesis in primary open-angle glaucoma: A potential therapeutic strategy. J. Microbiol. Immunol. Infect. 2019, 52, 201–206. [Google Scholar] [CrossRef]
- Gao, L.; Ye, Z.; Liu, J.H.; Yang, J.A.; Li, Y.; Cai, J.Y.; Wang, Y.X.; Tong, S.A.; Deng, G.; Zhang, S.; et al. TMCO1 expression promotes cell proliferation and induces epithelial-mesenchymal transformation in human gliomas. Med. Oncol. 2022, 39, 90. [Google Scholar] [CrossRef] [PubMed]
- Drewry, M.D.; Challa, P.; Kuchtey, J.G.; Navarro, I.; Helwa, I.; Hu, Y.; Mu, H.; Stamer, W.D.; Kuchtey, R.W.; Liu, Y. Differentially expressed microRNAs in the aqueous humor of patients with exfoliation glaucoma or primary open-angle glaucoma. Hum. Mol. Genet. 2018, 27, 1263–1275. [Google Scholar] [CrossRef]
- Sunryd, J.C.; Cheon, B.; Graham, J.B.; Giorda, K.M.; Fissore, R.A.; Hebert, D.N. TMTC1 and TMTC2 are novel endoplasmic reticulum tetratricopeptide repeat-containing adapter proteins involved in calcium homeostasis. J. Biol. Chem. 2014, 289, 16085–16099. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Chen, H.; Wu, J.; Li, S.; Lin, W.; Wang, N.; Bai, L. Ferroptosis in glaucoma: A promising avenue for therapy. Adv. Biol. 2024, 8, e2300530. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Li, Y.; Luo, L. Effect of myricetin on primary open-angle glaucoma. Transl. Neurosci. 2018, 9, 132–141. [Google Scholar] [CrossRef]
- Tjandra, I.; Soeharso, P.; Artini, W.; Siregar, N.C.; Victor, A.A. Ganglion cells apoptosis in diabetic rats as early prediction of glaucoma: A study of Brn3b gene expression and association with change of quantity of NO, caspase-3, NF-κB, and TNF-α. Int. J. Ophthalmol. 2020, 13, 1872–1879. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, L. Association between rs4938723 polymorphism and the risk of primary open-angle glaucoma (POAG) in a Chinese population. J. Cell Biochem. 2019, 120, 12875–12886. [Google Scholar] [CrossRef] [PubMed]
- Nowak, A.; Przybylowska-Sygut, K.; Szymanek, K.; Szaflik, J.; Szaflik, J.; Majsterek, I. The relationship of TP53 and GRIN2B gene polymorphisms with risk of occurrence and progression of primary open-angle glaucoma in a Polish population. Pol. J. Pathol. 2014, 65, 313–321. [Google Scholar] [CrossRef]
- Irnaten, M.; O’Malley, G.; Clark, A.F.; O’Brien, C.J. Transient receptor potential channels TRPC1/TRPC6 regulate lamina cribrosa cell extracellular matrix gene transcription and proliferation. Exp. Eye Res. 2020, 193, 107980. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Teng, L.; Li, A.; Ge, J.; Laties, A.M.; Zhang, X. TRPC6 channel protects retinal ganglion cells in a rat model of retinal ischemia/reperfusion-induced cell death. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5751–5758. [Google Scholar] [CrossRef]
- Perniss, A.; Liu, S.; Boonen, B.; Keshavarz, M.; Ruppert, A.L.; Timm, T.; Pfeil, U.; Soultanova, A.; Kusumakshi, S.; Delventhal, L.; et al. Chemosensory cell-derived acetylcholine drives tracheal mucociliary clearance in response to virulence-associated formyl peptides. Immunity 2020, 52, 683–699.e11. [Google Scholar] [CrossRef]
- Wu, H.; Cui, Y.; He, C.; Gao, P.; Li, Q.; Zhang, H.; Jiang, Y.; Hu, Y.; Wei, X.; Lu, Z.; et al. Activation of the bitter taste sensor TRPM5 prevents high salt-induced cardiovascular dysfunction. Sci. China Life Sci. 2020, 63, 1665–1677. [Google Scholar] [CrossRef]
- Nizankowska, M.H.; Turno-Krecicka, A. Primary open angle glaucoma, age and age-related cardiovascular disease risk factors. Klin. Ocz. 1998, 100, 107–110. [Google Scholar]
- Maharaj, A.; Maudhoo, A.; Chan, L.F.; Novoselova, T.; Prasad, R.; Metherell, L.A.; Guasti, L. Isolated glucocorticoid deficiency: Genetic causes and animal models. J. Steroid Biochem. Mol. Biol. 2019, 189, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Tabak, S.; Schreiber-Avissar, S.; Beit-Yannai, E. Crosstalk between microRNA and oxidative stress in primary open-angle glaucoma. Int. J. Mol. Sci. 2021, 22, 2421. [Google Scholar] [CrossRef] [PubMed]
- Pickering, A.M.; Lehr, M.; Gendron, C.M.; Pletcher, S.D.; Miller, R.A. Mitochondrial thioredoxin reductase 2 is elevated in long-lived primate as well as rodent species and extends fly mean lifespan. Aging Cell 2017, 16, 683–692. [Google Scholar] [CrossRef]
- Zhang, X.; Xi, G.; Feng, P.; Li, C.; Kuehn, M.H.; Zhu, W. Intraocular pressure across the lifespan of Tg-MYOCY437H mice. Exp. Eye Res. 2024, 241, 109855. [Google Scholar] [CrossRef] [PubMed]
- Fujikawa, K.; Iwata, T.; Inoue, K.; Akahori, M.; Kadotani, H.; Fukaya, M.; Watanabe, M.; Chang, Q.; Barnett, E.M.; Swat, W. VAV2 and VAV3 as candidate disease genes for spontaneous glaucoma in mice and humans. PLoS ONE 2010, 5, e9050. [Google Scholar] [CrossRef] [PubMed]
- Tsou, Y.S.; Wang, C.Y.; Chang, M.Y.; Hsu, T.I.; Wu, M.T.; Wu, Y.H.; Tsai, W.L.; Chuang, J.Y.; Kao, T.J. Vav2 is required for Netrin-1 receptor-class-specific spinal motor axon guidance. Dev. Dyn. 2022, 251, 444–458. [Google Scholar] [CrossRef] [PubMed]
- Claes, M.; De Groef, L.; Moons, L. Target-derived neurotrophic factor deprivation puts retinal ganglion cells on death row: Cold hard evidence and caveats. Int. J. Mol. Sci. 2019, 20, 4314. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Xiang, X.; Liang, C.; Shi, L. Regulating Rac in the nervous system: Molecular function and disease implication of Rac GEFs and GAPs. Biomed. Res. Int. 2015, 2015, 632450. [Google Scholar] [CrossRef]
- Zhong, T.; Zhou, J.; Yan, T.; Qiu, J.; Wang, Y.; Lu, W. Pseudo-time series structural MRI revealing progressive gray matter changes with elevated intraocular pressure in primary open-angle glaucoma: A preliminary study. Acad. Radiol. 2024, 31, 3754–3763. [Google Scholar] [CrossRef] [PubMed]
- Jensen, N.S.; Wehland, M.; Wise, P.M.; Grimm, D. Latest knowledge on the role of vitamin D in hypertension. Int. J. Mol. Sci. 2023, 24, 4679. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hara, K.; Van Baaren, J.M.; Price, J.C.; Beecham, G.W.; Gallins, P.J.; Whitehead, P.L.; Wang, G.; Lu, C.; Slifer, M.A.; et al. Vitamin D receptor and Alzheimer’s disease: A genetic and functional study. Neurobiol. Aging 2012, 33, 1844.e1–1844.e9. [Google Scholar] [CrossRef]
- Korneva, A.; Schaub, J.; Jefferys, J.; Kimball, E.; Pease, M.E.; Nawathe, M.; Johnson, T.V.; Pitha, I.; Quigley, H. A method to quantify regional axonal transport blockade at the optic nerve head after short term intraocular pressure elevation in mice. Exp. Eye Res. 2020, 196, 108035. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xie, S.C.; Ma, Y.C. Low expression of microRNA-15b promotes the proliferation of retinal capillary endothelial cells and pericytes by up-regulating VEGFA in diabetic rats. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 6018–6025. [Google Scholar] [PubMed]
- Ebner, L.J.A.; Samardzija, M.; Storti, F.; Todorova, V.; Karademir, D.; Behr, J.; Simpson, F.; Thiersch, M.; Grimm, C. Transcriptomic analysis of the mouse retina after acute and chronic normobaric and hypobaric hypoxia. Sci. Rep. 2021, 11, 16666. [Google Scholar] [CrossRef]
- Tenge, V.R.; Knowles, J.; Johnson, J.L. The ribosomal biogenesis protein Utp21 interacts with Hsp90 and has differing requirements for Hsp90-associated proteins. PLoS ONE 2014, 9, e92569. [Google Scholar] [CrossRef]
- Gallenberger, M.; Kroeber, M.; Marz, L.; Koch, M.; Fuchshofer, R.; Braunger, B.M.; Iwata, T.; Tamm, E.R. Heterozygote Wdr36-deficient mice do not develop glaucoma. Exp. Eye Res. 2014, 128, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Katyal, S.; Li, Y.; El-Khamisy, S.F.; Russell, H.R.; Caldecott, K.W.; McKinnon, P.J. The genesis of cerebellar interneurons and the prevention of neural DNA damage require XRCC1. Nat. Neurosci. 2009, 12, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Mei, P.J.; Bai, J.; Miao, F.A.; Li, Z.L.; Chen, C.; Zheng, J.N.; Fan, Y.C. Relationship between expression of XRCC1 and tumor proliferation, migration, invasion, and angiogenesis in glioma. Investig. New Drugs 2019, 37, 646–657. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Zhang, Z.; Chen, P.; Zhong, Y.; Cai, X.; Hu, H.; Yang, Y.; Zhang, J.; Li, K.; Ge, J.; et al. Tetramethylpyrazine (TMP), an active ingredient of chinese herb medicine chuanxiong, attenuates the degeneration of trabecular meshwork through SDF-1/CXCR4 axis. PLoS ONE 2015, 10, e0133055. [Google Scholar] [CrossRef] [PubMed]
- Day, I.N. dbSNP in the detail and copy number complexities. Hum. Mutat. 2010, 31, 2–4. [Google Scholar] [CrossRef]
- Drachkova, I.; Savinkova, L.; Arshinova, T.; Ponomarenko, M.; Peltek, S.; Kolchanov, N. The mechanism by which TATA-box polymorphisms associated with human hereditary diseases influence interactions with the TATA-binding protein. Hum. Mutat. 2014, 35, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Arkova, O.V.; Drachkova, I.A.; Arshinova, T.V.; Rasskazov, D.A.; Suslov, V.V.; Ponomarenko, P.M.; Ponomarenko, M.P.; Kolchanov, N.A.; Savinkova, L.K. Prediction and verification of the influence of the rs367781716 SNP on the interaction of the TATA-binding protein with the promoter of the human АВСА9 gene. Russ. J. Genet. Appl. Res. 2016, 6, 785–791. [Google Scholar] [CrossRef]
- Ponomarenko, M.; Kleshchev, M.; Ponomarenko, P.; Chadaeva, I.; Sharypova, E.; Rasskazov, D.; Kolmykov, S.; Drachkova, I.; Vasiliev, G.; Gutorova, N.; et al. Disruptive natural selection by male reproductive potential prevents underexpression of protein-coding genes on the human Y chromosome as a self-domestication syndrome. BMC Genet. 2020, 21 (Suppl. 1), 89. [Google Scholar] [CrossRef] [PubMed]
- Sharypova, E.B.; Drachkova, I.A.; Kashina, E.V.; Rasskazov, D.A.; Ponomarenko, P.M.; Ponomarenko, M.P.; Kolchanov, N.A.; Savinkova, L.K. An experimental study of the effect of rare polymorphisms of human HBB, HBD and F9 promoter TATA boxes on the kinetics of interaction with the TATA-binding protein. Vavilov. J. Genet. Breed. 2018, 22, 145–152. [Google Scholar] [CrossRef]
- Ponomarenko, M.; Rasskazov, D.; Chadaeva, I.; Sharypova, E.; Drachkova, I.; Oshchepkov, D.; Ponomarenko, P.; Savinkova, L.; Oshchepkova, E.; Nazarenko, M.; et al. Candidate SNP markers of atherogenesis significantly shifting the affinity of TATA-binding protein for human gene promoters show stabilizing natural selection as a sum of neutral drift accelerating atherogenesis and directional natural selection slowing it. Int. J. Mol. Sci. 2020, 21, 1045. [Google Scholar] [CrossRef] [PubMed]
- Betzler, B.K.; Rim, T.H.; Sabanayagam, C.; Cheung, C.M.G.; Cheng, C.Y. High-density lipoprotein cholesterol in age-related ocular diseases. Biomolecules 2020, 10, 645. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.; Abid, F.; Ksiaa, I.; Zina, S.; Messaoud, R.; Khairallah, M. Bilateral acute angle-closure glaucoma following tramadol subcutaneous administration. BMC Ophthalmol. 2018, 18, 50. [Google Scholar] [CrossRef] [PubMed]
- Rezende Filho, F.M.; Jurkute, N.; de Andrade, J.B.C.; Marianelli, B.F.; de Lima, F.D.; Franca, M.C., Jr.; Sallum, J.M.F.; Yu-Wai-Man, P.; Barsottini, O.G.P.; Pedroso, J.L. Optic disc and retinal architecture changes in patients with spinocerebellar ataxia type 2. Mov. Disord. 2024, 39, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Lehmann, O.J.; Swaroop, A. Genetics and therapy for pediatric eye diseases. EBioMedicine 2021, 67, 103360. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Stasi, K.; Nagel, D.; Yang, X.; Ren, L.; Mittag, T.; Danias, J. Ceruloplasmin upregulation in retina of murine and human glaucomatous eyes. Investig. Ophthalmol. Vis. Sci. 2007, 48, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Murphy-Ryan, M.; Psychogios, A.; Lindor, N.M. Hereditary disorders of connective tissue: A guide to the emerging differential diagnosis. Genet. Med. 2010, 12, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Aldaas, K.; Challa, P.; Weber, D.J.; Fleischman, D. Infections and glaucoma. Surv. Ophthalmol. 2022, 67, 637–658. [Google Scholar] [CrossRef]
- Platzer, K.; Yuan, H.; Schutz, H.; Winschel, A.; Chen, W.; Hu, C.; Kusumoto, H.; Heyne, H.O.; Helbig, K.L.; Tang, S.; et al. GRIN2B encephalopathy: Novel findings on phenotype, variant clustering, functional consequences and treatment aspects. J. Med. Genet. 2017, 54, 460–470. [Google Scholar] [CrossRef]
- Da Silva, K.; Dowell, M.; Savatovsky, E.J.; Grosvenor, D.; Callender, D.; Campbell, M.H.; Hambleton, I.; Vanner, E.A.; Grajewski, A.L.; Chang, T.C. The burden of pediatric visual impairment and ocular diagnoses in Barbados. Int. J. Environ. Res. Public Health 2023, 20, 6554. [Google Scholar] [CrossRef] [PubMed]
- Campa, D.; Hashibe, M.; Zaridze, D.; Szeszenia-Dabrowska, N.; Mates, I.N.; Janout, V.; Holcatova, I.; Fabianova, E.; Gaborieau, V.; Hung, R.J.; et al. Association of common polymorphisms in inflammatory genes with risk of developing cancers of the upper aerodigestive tract. Cancer Causes Control 2007, 18, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.Y.; Lin, C.W.; Hsin, C.H.; Lee, C.Y.; Huang, J.Y.; Yang, S.F.; Lin, H.Y. Association of the nasopharyngeal carcinoma and the subsequent open glaucoma development: A nationwide cohort study. Int. J. Med. Sci. 2023, 20, 702–708. [Google Scholar] [CrossRef]
- Lozier, J.N.; Rosenberg, P.S.; Goedert, J.J.; Menashe, I. A case-control study reveals immunoregulatory gene haplotypes that influence inhibitor risk in severe haemophilia A. Haemophilia 2011, 17, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Al-Sharif, E.; AlEnezi, S.H.; Sharif, H.A.; Osman, E.A. Ocular bleeding in an undiagnosed hemophiliac neonate causing irreversible loss of vision: A case report with review of the literature. Eur. J. Ophthalmol. 2020, 30, NP62–NP65. [Google Scholar] [CrossRef]
- Cho, J.H.; Choi, J.S.; Chun, S.W.; Lee, S.; Han, K.J.; Kim, H.M. The IL-1B genetic polymorphism is associated with aspirin-induced peptic ulcers in a Korean ethnic group. Gut Liver 2016, 10, 362–368. [Google Scholar] [CrossRef]
- Sun, H.Y.; Luo, C.W.; Chiang, Y.W.; Yeh, K.L.; Li, Y.C.; Ho, Y.C.; Lee, S.S.; Chen, W.Y.; Chen, C.J.; Kuan, Y.H. Association between PM2.5 exposure level and primary open-angle glaucoma in Taiwanese adults: A nested case-control study. Int. J. Environ. Res. Public. Health 2021, 18, 1714. [Google Scholar] [CrossRef]
- Sanabria-Salas, M.C.; Hernandez-Suarez, G.; Umana-Perez, A.; Rawlik, K.; Tenesa, A.; Serrano-Lopez, M.L.; Sanchez de Gomez, M.; Rojas, M.P.; Bravo, L.E.; Albis, R.; et al. IL1B-CGTC haplotype is associated with colorectal cancer in admixed individuals with increased African ancestry. Sci. Rep. 2017, 7, 41920. [Google Scholar] [CrossRef] [PubMed]
- Mukamel, R.E.; Handsaker, R.E.; Sherman, M.A.; Barton, A.R.; Hujoel, M.L.A.; McCarroll, S.A.; Loh, P.R. Repeat polymorphisms underlie top genetic risk loci for glaucoma and colorectal cancer. Cell 2023, 186, 3659–3673.e23. [Google Scholar] [CrossRef] [PubMed]
- Sa-Ngasang, A.; Ohashi, J.; Naka, I.; Anantapreecha, S.; Sawanpanyalert, P.; Patarapotikul, J. Association of IL1B-31C/T and IL1RA variable number of an 86-bp tandem repeat with dengue shock syndrome in Thailand. J. Infect. Dis. 2014, 210, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Deshmukh, N.; Webel, A.; Johnson, S.; Suleiman, A.; Mohan, R.R.; Fraunfelder, F.; Singh, P.K. Viral infections and pathogenesis of glaucoma: A comprehensive review. Clin. Microbiol. Rev. 2023, 36, e0005723. [Google Scholar] [CrossRef]
- Kim, H.; Hysi, P.G.; Pawlikowska, L.; Poon, A.; Burchard, E.G.; Zaroff, J.G.; Sidney, S.; Ko, N.U.; Achrol, A.S.; Lawton, M.T.; et al. Common variants in interleukin-1-Beta gene are associated with intracranial hemorrhage and susceptibility to brain arteriovenous malformation. Cerebrovasc. Dis. 2009, 27, 176–182. [Google Scholar] [CrossRef]
- Razeghinejad, M.R.; Nowroozzadeh, M.H. Optic disk hemorrhage in health and disease. Surv. Ophthalmol. 2017, 62, 784–802. [Google Scholar] [CrossRef]
- Occhiutto, M.L.; de Melo, M.B.; Cabral de Vasconcellos, J.P.; Rodrigues, T.A.R.; Bajano, F.F.; Costa, F.F.; Costa, V.P. Association of APOE gene polymorphisms with primary open angle glaucoma in Brazilian patients. Ophthalmic Genet. 2021, 42, 53–61. [Google Scholar] [CrossRef]
- Liu, C.; Yuan, Z.Y.; Yuan, H.; Wu, K.X.; Cao, B.; Ren, K.Y.; Cui, M.J.; Liu, J.H.; Chen, H.X.; Pang, Y.W. Status of gene methylation and polymorphism in different courses of ulcerative colitis and their comparison with sporadic colorectal cancer. Inflamm. Bowel Dis. 2021, 27, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Gomes Dos Santos, A.; Watanabe, E.H.; Ferreira, D.T.; Oliveira, J.; Nakanishi, E.S.; Oliveira, C.S.; Bocchi, E.; Novaes, C.T.G.; Cruz, F.; Carvalho, N.B.; et al. A specific IL6 polymorphic genotype modulates the risk of Trypanosoma cruzi parasitemia while IL18, IL17A, and IL1B variant profiles and HIV Infection protect against cardiomyopathy in chagas disease. Front. Immunol. 2020, 11, 521409. [Google Scholar] [CrossRef]
- Chakravorty, M.; Ghosh, A.; Choudhury, A.; Santra, A.; Hembrum, J.; Roychoudhury, S. Interaction between IL1B gene promoter polymorphisms in determining susceptibility to Helicobacter pylori associated duodenal ulcer. Hum. Mutat. 2006, 27, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Dada, T.; Verma, S.; Gagrani, M.; Bhartiya, S.; Chauhan, N.; Satpute, K.; Sharma, N. Ocular and systemic factors associated with glaucoma. J. Curr. Glaucoma Pract. 2022, 16, 179–191. [Google Scholar]
- French, D.D.; Margo, C.E.; Harman, L.E. Ocular pseudoexfoliation and cardiovascular disease: A national cross-section comparison study. N. Am. J. Med. Sci. 2012, 4, 468–473. [Google Scholar] [CrossRef]
- Raji, M.M.; Tasneem, A.F.; Nayak, V.I.; Jafar, F.S.; Ahmed, Z.; Indraja, Y. The association between primary open-angle glaucoma and helicobacter pylori infection. J. Clin. Res. Ophthalmol. 2021, 8, 036–042. [Google Scholar] [CrossRef]
- El-Omar, E.M.; Carrington, M.; Chow, W.H.; McColl, K.E.; Bream, J.H.; Young, H.A.; Herrera, J.; Lissowska, J.; Yuan, C.C.; Rothman, N.; et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature 2000, 404, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Ezzati Amini, E.; Moradi, Y. Association between helicobacter pylori infection and primary open-angle glaucoma: A systematic review and meta-analysis. BMC Ophthalmol. 2023, 23, 374. [Google Scholar] [CrossRef] [PubMed]
- Hamajima, N.; Matsuo, K.; Saito, T.; Tajima, K.; Okuma, K.; Yamao, K.; Tominaga, S. Interleukin 1 polymorphisms, lifestyle factors, and Helicobacter pylori infection. Jpn. J. Cancer Res. 2001, 92, 383–389. [Google Scholar] [CrossRef]
- Vergroesen, J.E.; de Crom, T.O.E.; van Duijn, C.M.; Voortman, T.; Klaver, C.C.W.; Ramdas, W.D. MIND diet lowers risk of open-angle glaucoma: The Rotterdam study. Eur. J. Nutr. 2023, 62, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhou, B.; Li, S.; Yue, J.; Yang, H.; Wen, Y.; Zhan, S.; Wang, W.; Liao, M.; Zhang, M.; et al. Allele-specific induction of IL-1β expression by C/EBPβ and PU.1 contributes to increased tuberculosis susceptibility. PLoS Pathog. 2014, 10, e1004426. [Google Scholar] [CrossRef]
- Magesan, K.; Patnaik, G.; Majumder, P.D.; Biswas, J. Clinical profile, treatment, and visual outcome of scleritis: A single ophthalmologist experience. Oman J. Ophthalmol. 2022, 15, 153–158. [Google Scholar] [CrossRef]
- Das, A.P.; Saini, S.; Agarwal, S.M. A comprehensive meta-analysis of non-coding polymorphisms associated with precancerous lesions and cervical cancer. Genomics 2022, 114, 110323. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yuan, F. The association between interleukin-1β gene polymorphisms and the risk of breast cancer: A systematic review and meta-analysis. Arch. Med. Sci. 2021, 18, 1–10. [Google Scholar] [CrossRef]
- Wu, K.S.; Zhou, X.; Zheng, F.; Xu, X.Q.; Lin, Y.H.; Yang, J. Influence of interleukin-1 beta genetic polymorphism, smoking and alcohol drinking on the risk of non-small cell lung cancer. Clin. Chim. Acta 2010, 411, 1441–1446. [Google Scholar] [CrossRef]
- Tak, K.H.; Yu, G.I.; Lee, M.Y.; Shin, D.H. Association between polymorphisms of interleukin 1 family genes and hepatocellular carcinoma. Med. Sci. Monit. 2018, 24, 3488–3495. [Google Scholar] [CrossRef]
- Zhu, P.; Wu, X.; Zhou, J.; Wu, K.; Lu, Y. Gene polymorphisms of pro-inflammatory cytokines may affect the risk of Graves’ disease: A meta-analysis. J. Endocrinol. Investig. 2021, 44, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Amirian, E.; Liu, Y.; Scheurer, M.E.; El-Zein, R.; Gilbert, M.R.; Bondy, M.L. Genetic variants in inflammation pathway genes and asthma in glioma susceptibility. Neuro Oncol. 2010, 12, 444–452. [Google Scholar] [PubMed]
- Barlo, N.P.; van Moorsel, C.H.; Korthagen, N.M.; Heron, M.; Rijkers, G.T.; Ruven, H.J.; van den Bosch, J.M.; Grutters, J.C. Genetic variability in the IL1RN gene and the balance between interleukin (IL)-1 receptor agonist and IL-1β in idiopathic pulmonary fibrosis. Clin. Exp. Immunol. 2011, 166, 346–351. [Google Scholar] [CrossRef]
- De Iudicibus, S.; Franca, R.; Martelossi, S.; Ventura, A.; Decorti, G. Molecular mechanism of glucocorticoid resistance in inflammatory bowel disease. World J. Gastroenterol. 2011, 17, 1095–1108. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sun, X.; Luo, S.; Lin, J.; Xiao, Y.; Yu, H.; Huang, G.; Li, X.; Xie, Z.; Zhou, Z. The positivity rate of IA-2A and ZnT8A in the Chinese han population with type 1 diabetes mellitus: Association with rs1143627 and rs1143643 polymorphisms in the IL1B gene. Front. Pharmacol. 2021, 12, 729890. [Google Scholar] [CrossRef]
- Goracy, I.; Kaczmarczyk, M.; Ciechanowicz, A.; Lewandowska, K.; Jakubiszyn, P.; Bodnar, O.; Kopijek, B.; Brodkiewicz, A.; Cyrylowski, L. Polymorphism of interleukin 1b may modulate the risk of ischemic stroke in polish patients. Medicina 2019, 55, 558. [Google Scholar] [CrossRef]
- Ibanez, L.; Velli, P.S.; Font, R.; Jaen, A.; Royo, J.; Irigoyen, D.; Cairo, M.; De la Sierra, A.; Arranz, M.J.; Gallardo, D.; et al. HIV-infection, atherosclerosis and the inflammatory pathway: Candidate gene study in a Spanish HIV-infected population. PLoS ONE 2014, 9, e112279. [Google Scholar] [CrossRef]
- Rai, H.; Sinha, N.; Kumar, S.; Sharma, A.K.; Agrawal, S. Interleukin-1 gene cluster polymorphisms and their association with coronary artery disease: Separate evidences from the largest case-control study amongst North Indians and an updated meta-analysis. PLoS ONE 2016, 11, e0153480. [Google Scholar] [CrossRef]
- Strandberg, L.; Mellstrom, D.; Ljunggren, O.; Grundberg, E.; Karlsson, M.K.; Holmberg, A.H.; Orwoll, E.S.; Eriksson, A.L.; Svedberg, J.; Bengtsson, M.; et al. IL6 and IL1B polymorphisms are associated with fat mass in older men: The MrOS Study Sweden. Obes. (Silver Spring) 2008, 16, 710–713. [Google Scholar] [CrossRef]
- Tanimine, N.; Takei, D.; Tsukiyama, N.; Yoshinaka, H.; Takemoto, Y.; Tanaka, Y.; Kobayashi, T.; Tanabe, K.; Ishikawa, N.; Kitahara, Y.; et al. Identification of aggravation-predicting gene polymorphisms in Coronavirus disease 2019 patients using a candidate gene approach associated with multiple phase pathogenesis: A study in a Japanese city of 1 million people. Crit Care Explor. 2021, 3, e0576. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Carrillo, D.N.; Garza-Gonzalez, E.; Betancourt-Linares, R.; Monico-Manzano, T.; Antunez-Rivera, C.; Roman-Roman, A.; Flores-Alfaro, E.; Illades-Aguiar, B.; Fernandez-Tilapa, G. Association of IL1B-511C/-31T haplotype and Helicobacter pylori vacA genotypes with gastric ulcer and chronic gastritis. BMC Gastroenterol. 2010, 10, 126. [Google Scholar] [CrossRef] [PubMed]
- Sana, T.; Khan, M.; Jabeen, A.; Shams, S.; Hadda, T.B.; Begum, S.; Siddiqui, B.S. Urease and carbonic anhydrase inhibitory effect of xanthones from Aspergillus nidulans, an endophytic fungus of Nyctanthes arbor-tristis. Planta Med. 2023, 89, 377–384. [Google Scholar] [CrossRef]
- Borkowska, P.; Kucia, K.; Rzezniczek, S.; Paul-Samojedny, M.; Kowalczyk, M.; Owczarek, A.; Suchanek, R.; Medrala, T.; Kowalski, J. Interleukin-1beta promoter (-31T/C and -511C/T) polymorphisms in major recurrent depression. J. Mol. Neurosci. 2011, 44, 12–16. [Google Scholar] [CrossRef]
- Almonte, M.T.; Capellan, P.; Yap, T.E.; Cordeiro, M.F. Retinal correlates of psychiatric disorders. Ther. Adv. Chronic Dis. 2020, 11, 2040622320905215. [Google Scholar] [CrossRef]
- Kalmann, R.; Mourits, M.P. Prevalence and management of elevated intraocular pressure in patients with Graves’ orbitopathy. Br. J. Ophthalmol. 1998, 82, 754–757. [Google Scholar] [CrossRef]
- Ramos, B.R.; Mendes, N.D.; Tanikawa, A.A.; Amador, M.A.; dos Santos, N.P.; dos Santos, S.E.; Castelli, E.C.; Witkin, S.S.; da Silva, M.G. Ancestry informative markers and selected single nucleotide polymorphisms in immunoregulatory genes on preterm labor and preterm premature rupture of membranes: A case control study. BMC Pregnancy Childbirth 2016, 16, 30. [Google Scholar] [CrossRef]
- Walfisch, A.; Kessous, R.; Davidson, E.; Sergienko, R.; Beharier, O.; Sheiner, E. Increased risk for ophthalmic complications in patients with a history of preterm delivery. Am. J. Perinatol. 2016, 33, 708–714. [Google Scholar] [PubMed]
- Loft, N.D.; Skov, L.; Iversen, L.; Gniadecki, R.; Dam, T.N.; Brandslund, I.; Hoffmann, H.J.; Andersen, M.R.; Dessau, R.B.; Bergmann, A.C.; et al. Associations between functional polymorphisms and response to biological treatment in Danish patients with psoriasis. Pharmacogenom. J. 2018, 18, 494–500. [Google Scholar] [CrossRef]
- Li, S.H.; Cheng, C.Y. Risks of glaucoma among individuals with psoriasis: A population-based cohort study. Clin. Exp. Dermatol. 2024, 49, llae073. [Google Scholar] [CrossRef]
- Ding, X.; Mei, Y.; Mao, Z.; Long, L.; Han, Q.; You, Y.; Zhu, H. Association of immune and inflammatory gene polymorphism with the risk of IgA nephropathy: A systematic review and meta-analysis of 45 studies. Front. Immunol. 2021, 12, 683913. [Google Scholar] [CrossRef]
- van Dijk, E.H.C.; Soonawala, D.; Rooth, V.; Hoyng, C.B.; Meijer, O.C.; de Vries, A.P.J.; Boon, C.J.F. Spectrum of retinal abnormalities in renal transplant patients using chronic low-dose steroids. Graefes Arch. Clin. Exp. Ophthalmol. 2017, 255, 2443–2449. [Google Scholar] [CrossRef]
- Xie, X.; Li, J.; Gu, F.; Zhang, K.; Su, Z.; Wen, Q.; Sui, Z.; Zhou, P.; Yu, T. Genetic determinants for bacterial osteomyelitis: A focused systematic review of published literature. Front. Genet. 2021, 12, 654792. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.S.; Khanna, S. Actinomyces orbital osteomyelitis in the setting of multiple myeloma and bisphosphonate-related osteonecrosis. J. Neuroophthalmol. 2019, 39, 120–121. [Google Scholar] [CrossRef]
- Meng, L.; Zhen, Z.; Jiang, Q.; Li, X.H.; Yuan, Y.; Yao, W.; Zhang, M.M.; Li, A.J.; Shi, L. Predictive model based on gene and laboratory data for intravenous immunoglobulin resistance in Kawasaki disease in a Chinese population. Pediatr. Rheumatol. Online J. 2021, 19, 95. [Google Scholar] [CrossRef]
- Ng, L.H.; Lung, J.C. Bilateral juvenile open angle glaucoma in two Chinese children: Case report. Clin. Exp. Optom. 2008, 91, 403–410. [Google Scholar] [CrossRef]
- Kim, S.H.; Mok, J.W.; Kim, H.S.; Joo, C.K. Association of -31T>C and -511C>T polymorphisms in the interleukin 1 beta (IL1B) promoter in Korean keratoconus patients. Mol. Vis. 2008, 14, 2109–2116. [Google Scholar] [PubMed]
- Goel, S.; Ganger, A.; Gupta, V. Bilateral juvenile onset primary open-angle glaucoma among keratoconus patients. J. Glaucoma 2015, 24, e25–e27. [Google Scholar] [CrossRef] [PubMed]
- Krishna Priya, E.K.; Srinivas, L.; Rajesh, S.; Sasikala, K.; Banerjee, M. Pro-inflammatory cytokine response pre-dominates immuno-genetic pathway in development of rheumatoid arthritis. Mol. Biol. Rep. 2020, 47, 8669–8677. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Jeong, S.H.; Kim, H.; Park, E.C.; Jang, S.Y. Development of open-angle glaucoma in adults with seropositive rheumatoid arthritis in Korea. JAMA Netw. Open 2022, 5, e223345. [Google Scholar] [CrossRef] [PubMed]
- Okada, S.; Hata, K.; Shinagawa, T.; Noguchi, T.; Tanaka, T.; Kawai, K.; Nozawa, H.; Ishihara, S. A polymorphism in interleukin-1β gene is associated with the development of pouchitis in Japanese patients with ulcerative colitis. Digestion 2021, 102, 489–498. [Google Scholar] [CrossRef]
- Xie, M.S.; Zheng, Y.Z.; Huang, L.B.; Xu, G.X. Infliximab relieves blood retinal barrier breakdown through the p38 MAPK pathway in a diabetic rat model. Int. J. Ophthalmol. 2017, 10, 1824–1829. [Google Scholar]
- Reis, C.L.B.; Barbosa, M.C.F.; Machado, B.M.S.M.; Baratto, S.S.P.; de Lima, D.C.; Paza, A.O.; Filho, F.B.; Brancher, J.A.; Kuchler, E.C.; de Oliveira, D.S.B. Genetic polymorphisms in interleukin-6 and interleukin-1-beta were associated with dental caries and gingivitis. Acta Odontol. Scand. 2021, 79, 96–102. [Google Scholar] [CrossRef]
- Polla, D.; Astafurov, K.; Hawy, E.; Hyman, L.; Hou, W.; Danias, J. A pilot study to evaluate the oral microbiome and dental health in primary open-angle glaucoma. J. Glaucoma 2017, 26, 320–327. [Google Scholar] [CrossRef]
- He, Z.; Sun, Y.; Wu, J.; Xiong, Z.; Zhang, S.; Liu, J.; Liu, Y.; Li, H.; Jin, T.; Yang, Y.; et al. Evaluation of genetic variants in IL-1B and its interaction with the predisposition of osteoporosis in the northwestern Chinese Han population. J. Gene Med. 2020, 22, e3214. [Google Scholar] [CrossRef]
- Newman-Casey, P.A.; Talwar, N.; Nan, B.; Musch, D.C.; Pasquale, L.R.; Stein, J.D. The potential association between postmenopausal hormone use and primary open-angle glaucoma. JAMA Ophthalmol. 2014, 132, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Wade, N.B.; Chang, C.M.; Conti, D.; Millstein, J.; Skibola, C.; Nieters, A.; Wang, S.S.; De Sanjose, S.; Kane, E.; Spinelli, J.J.; et al. Infectious mononucleosis, immune genotypes, and non-Hodgkin lymphoma (NHL): An InterLymph Consortium study. Cancer Causes Control 2020, 31, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Dammacco, R.; Guerriero, S.; Alessio, G.; Dammacco, F. Natural and iatrogenic ocular manifestations of rheumatoid arthritis: A systematic review. Int. Ophthalmol. 2022, 42, 689–711. [Google Scholar] [CrossRef]
- Barseem, N.F.; Khattab, E.S.A.E.H.; Mahasab, M.M. IL-1β-31/IL1-RA genetic markers association with idiopathic generalized epilepsy and treatment response in a cohort of Egyptian population. Int. J. Neurosci. 2020, 130, 348–354. [Google Scholar] [CrossRef]
- Kulkarni, C.; Chaudhuri, U.R.; Jagathesan, A. Bilateral acute angle-closure glaucoma following treatment with topiramate for headache. Neurol. Ther. 2013, 2, 57–62. [Google Scholar] [CrossRef]
- Liu, Y.; Li, S.; Zhang, G.; Nie, G.; Meng, Z.; Mao, D.; Chen, C.; Chen, X.; Zhou, B.; Zeng, G. Genetic variants in IL1A and IL1B contribute to the susceptibility to 2009 pandemic H1N1 influenza A virus. BMC Immunol. 2013, 14, 37. [Google Scholar] [CrossRef]
- Dong, X.; Zeng, Y.; Liu, Y.; You, L.; Yin, X.; Fu, J.; Ni, J. Aloe-emodin: A review of its pharmacology, toxicity, and pharmacokinetics. Phytother. Res. 2020, 34, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Majumder, P.; Panda, S.K.; Ghosh, S.; Dey, S.K. Interleukin gene polymorphisms in chronic periodontitis: A case-control study in the Indian population. Arch. Oral. Biol. 2019, 101, 156–164. [Google Scholar] [CrossRef]
- Sun, K.T.; Shen, T.C.; Chen, S.C.; Chang, C.L.; Li, C.H.; Li, X.; Palanisamy, K.; Hsia, N.Y.; Chang, W.S.; Tsai, C.W.; et al. Periodontitis and the subsequent risk of glaucoma: Results from the real-world practice. Sci. Rep. 2020, 10, 17568. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, Y.; Wu, J.; Sun, Y.; Xiong, Z.; Niu, F.; Lei, L.; Du, S.; Chen, P.; Yang, Z. Genetic polymorphisms in IL1B predict susceptibility to steroid-induced osteonecrosis of the femoral head in Chinese Han population. Osteoporos. Int. 2019, 30, 871–877. [Google Scholar] [CrossRef]
- Lai, H.Y.; Lai, I.C.; Fang, P.C.; Hsiao, C.C.; Hsiao, Y.T. Steroid-induced ocular hypertension in a pediatric patient with acute lymphoblastic leukemia: A case report. Children 2022, 9, 440. [Google Scholar] [CrossRef]
- Naranjo-Galvis, C.A.; de-la-Torre, A.; Mantilla-Muriel, L.E.; Beltran-Angarita, L.; Elcoroaristizabal-Martin, X.; McLeod, R.; Alliey-Rodriguez, N.; Begeman, I.J.; Lopez de Mesa, C.; Gomez-Marin, J.E.; et al. Genetic polymorphisms in cytokine genes in colombian patients with ocular toxoplasmosis. Infect. Immun. 2018, 86, e00597-17. [Google Scholar] [CrossRef]
- Suhardjo Utomo, P.T.; Agni, A.N. Clinical manifestations of ocular toxoplasmosis in Yogyakarta, Indonesia: A clinical review of 173 cases. Southeast. Asian J. Trop. Med. Public Health 2003, 34, 291–297. [Google Scholar]
- Wu, J.F.; Song, S.H.; Lee, C.S.; Chen, H.L.; Ni, Y.H.; Hsu, H.Y.; Wu, T.C.; Chang, M.H. Clinical predictors of liver fibrosis in patients with chronic hepatitis B virus infection from children to adults. J. Infect. Dis. 2018, 217, 1408–1416. [Google Scholar] [CrossRef] [PubMed]
- Gumus, K.; Yurci, A.; Mirza, E.; Arda, H.; Oner, A.; Topaktas, D.; Karakucuk, S. Evaluation of ocular surface damage and dry eye status in chronic hepatitis C at different stages of hepatic fibrosis. Cornea 2009, 28, 997–1002. [Google Scholar] [CrossRef]
- Byun, E.; Gay, C.L.; Portillo, C.J.; Pullinger, C.R.; Aouizerat, B.E.; Lee, K.A. Cytokine polymorphisms are associated with daytime napping in adults living with HIV. Sleep. Med. 2017, 32, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Wild, J.M.; Searle, A.E.; Dengler-Harles, M.; O’Neill, E.C. Long-term follow-up of baseline learning and fatigue effects in the automated perimetry of glaucoma and ocular hypertensive patients. Acta Ophthalmol. 1991, 69, 210–216. [Google Scholar] [CrossRef]
- Hudson, Z.D.; Miller, B.J. Meta-analysis of cytokine and chemokine genes in schizophrenia. Clin. Schizophr. Relat. Psychoses 2018, 12, 121–129B. [Google Scholar] [CrossRef]
- Lee, W.W.; Tajunisah, I.; Sharmilla, K.; Peyman, M.; Subrayan, V. Retinal nerve fiber layer structure abnormalities in schizophrenia and its relationship to disease state: Evidence from optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 2013, 54, 7785–7792. [Google Scholar] [CrossRef]
- Nishida, Y.; Hara, M.; Sakamoto, T.; Shinchi, K.; Kawai, S.; Naito, M.; Hamajima, N.; Kadota, A.; Suzuki, S.; Ibusuki, R.; et al. Influence of cigarette smoking and inflammatory gene polymorphisms on glycated hemoglobin in the Japanese general population. Prev. Med. Rep. 2016, 3, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Ou, K.; Li, Y.; Liu, L.; Li, H.; Cox, K.; Wu, J.; Liu, J.; Dick, A.D. Recent developments of neuroprotective agents for degenerative retinal disorders. Neural Regen. Res. 2022, 17, 1919–1928. [Google Scholar] [PubMed]
- Yin, Y.; Liu, Y.; Pan, X.; Chen, R.; Li, P.; Wu, H.J.; Zhao, Z.Q.; Li, Y.P.; Huang, L.Q.; Zhuang, J.H.; et al. Interleukin-1β promoter polymorphism enhances the risk of sleep disturbance in Alzheimer’s Disease. PLoS ONE 2016, 11, e0149945. [Google Scholar] [CrossRef] [PubMed]
- Onen, S.H.; Mouriaux, F.; Berramdane, L.; Dascotte, J.C.; Kulik, J.F.; Rouland, J.F. High prevalence of sleep-disordered breathing in patients with primary open-angle glaucoma. Acta Ophthalmol. Scand. 2000, 78, 638–641. [Google Scholar] [CrossRef]
- Yencilek, F.; Yildirim, A.; Yilmaz, S.G.; Altinkilic, E.M.; Dalan, A.B.; Bastug, Y.; Isbir, T. Investigation of interleukin-1β polymorphisms in prostate cancer. Anticancer. Res. 2015, 35, 6057–6061. [Google Scholar] [PubMed]
- Ahn, H.K.; Lee, H.S.; Park, J.Y.; Kim, D.K.; Kim, M.; Hwang, H.S.; Kim, J.W.; Ha, J.S.; Cho, K.S. Androgen deprivation therapy may reduce the risk of primary open-angle glaucoma in patients with prostate cancer: A nationwide population-based cohort study. Prostate Int. 2021, 9, 197–202. [Google Scholar] [CrossRef]
- Simeon, V.; Todoerti, K.; La Rocca, F.; Caivano, A.; Trino, S.; Lionetti, M.; Agnelli, L.; De Luca, L.; Laurenzana, I.; Neri, A.; et al. Molecular classification and pharmacogenetics of primary plasma cell leukemia: An initial approach toward precision medicine. Int. J. Mol. Sci. 2015, 16, 17514–17534. [Google Scholar] [CrossRef]
- Akaihata, M.; Somiya, H.; Shimomura, Y.; Hori, T.; Okumura, A. Surgical management of steroid-induced glaucoma in a child with leukemia. Pediatr. Int. 2022, 64, e15259. [Google Scholar] [CrossRef]
- Henderson, S.T.; Poirier, J. Pharmacogenetic analysis of the effects of polymorphisms in APOE, IDE and IL1B on a ketone body based therapeutic on cognition in mild to moderate Alzheimer’s disease; a randomized, double-blind, placebo-controlled study. BMC Med. Genet. 2011, 12, 137. [Google Scholar] [CrossRef] [PubMed]
- Bastani Viarsagh, S.; Zhang, M.E.; Shariflou, S.; Agar, A.; Golzan, S.M. Cognitive performance on the Montreal cognitive assessment test and retinal structural and functional measures in glaucoma. J. Clin. Med. 2022, 11, 5097. [Google Scholar] [CrossRef]
- Benke, K.S.; Carlson, M.C.; Doan, B.Q.; Walston, J.D.; Xue, Q.L.; Reiner, A.P.; Fried, L.P.; Arking, D.E.; Chakravarti, A.; Fallin, M.D. The association of genetic variants in interleukin-1 genes with cognition: Findings from the cardiovascular health study. Exp. Gerontol. 2011, 46, 1010–1019. [Google Scholar] [CrossRef]
- Tielsch, J.M.; Sommer, A.; Katz, J.; Royall, R.M.; Quigley, H.A.; Javitt, J. Racial variations in the prevalence of primary open-angle glaucoma. The Baltimore Eye Survey. JAMA 1991, 266, 369–374. [Google Scholar] [CrossRef]
- Choi, J.A.; Lee, S.N.; Jung, S.H.; Won, H.H.; Yun, J.S. Association of glaucoma and lifestyle with incident cardiovascular disease: A longitudinal prospective study from UK Biobank. Sci. Rep. 2023, 13, 2712. [Google Scholar] [CrossRef] [PubMed]
- Rizzato, C.; Canzian, F.; Rudnai, P.; Gurzau, E.; Stein, A.; Koppova, K.; Hemminki, K.; Kumar, R.; Campa, D. Interaction between functional polymorphic variants in cytokine genes, established risk factors and susceptibility to basal cell carcinoma of skin. Carcinogenesis 2011, 32, 1849–1854. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; VoPham, T.; Laden, F.; Rosner, B.A.; Wirostko, B.; Ritch, R.; Wiggs, J.L.; Qureshi, A.; Nan, H.; Pasquale, L.R. Cohort study of nonmelanoma skin cancer and the risk of exfoliation glaucoma. J. Glaucoma 2020, 29, 448–455. [Google Scholar] [CrossRef]
- Van Dyke, A.L.; Cote, M.L.; Wenzlaff, A.S.; Land, S.; Schwartz, A.G. Cytokine SNPs: Comparison of allele frequencies by race and implications for future studies. Cytokine 2009, 46, 236–244. [Google Scholar] [CrossRef]
- Ohia, S.E.; Njie-Mbye, Y.F.; Opere, C.A.; Kulkarni, M.; Barett, A. Ocular Health, Vision, and a Healthy Diet. In Inflammation, Advancing Age and Nutrition; Rahman, I., Bagchi, D., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 267–277. [Google Scholar] [CrossRef]
- Dhanireddy, S.; Yin, H.Y.; Dosakayala, N.; Kurochkin, P.; Gupta, N.; Cheng, A.M.S.; Fechtner, R.; Alpert, S. Severe inflammation and hyphema after micropulse diode transscleral cyclophotocoagulation. J. Glaucoma 2020, 29, e50–e52. [Google Scholar] [CrossRef] [PubMed]
- Maksymowych, W.P.; Rahman, P.; Reeve, J.P.; Gladman, D.D.; Peddle, L.; Inman, R.D. Association of the IL1 gene cluster with susceptibility to ankylosing spondylitis: An analysis of three Canadian populations. Arthritis Rheum. 2006, 54, 974–985. [Google Scholar] [CrossRef]
- Li, S.; Chen, M.; Zhang, Q.; Fang, M.; Xiong, W.; Bai, L. Ankylosing spondylitis and glaucoma in European population: A Mendelian randomization study. Front. Immunol. 2023, 14, 1120742. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Han, K.; Park, H.Y.L.; Lee, S.H.; Park, C.K. Metabolic health, obesity, and the risk of developing open-angle glaucoma: Metabolically healthy obese patients versus metabolically unhealthy but normal weight patients. Diabetes Metab. J. 2020, 44, 414–425. [Google Scholar] [CrossRef]
- Pampalakis, G.; Mitropoulos, K.; Xiromerisiou, G.; Dardiotis, E.; Deretzi, G.; Anagnostouli, M.; Katsila, T.; Rentzos, M.; Patrinos, G.P. New molecular diagnostic trends and biomarkers for amyotrophic lateral sclerosis. Hum. Mutat. 2019, 40, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Adduri, R.S.; Katamoni, R.; Pandilla, R.; Madana, S.N.; Paripati, A.K.; Kotapalli, V.; Bashyam, M.D. TP53 Pro72 allele is enriched in oral tongue cancer and frequently mutated in esophageal cancer in India. PLoS ONE 2014, 9, e114002. [Google Scholar] [CrossRef]
- Leonardi, A. Emerging drugs for ocular allergy. Expert. Opin. Emerg. Drugs 2005, 10, 505–520. [Google Scholar] [CrossRef] [PubMed]
- Schafer, D.P.; Stevens, B. Synapse elimination during development and disease: Immune molecules take centre stage. Biochem. Soc. Trans. 2010, 38, 476–481. [Google Scholar] [CrossRef]
- Peippo, M.; Ignatius, J. Pitt-Hopkins Syndrome. Mol. Syndr. 2012, 2, 171–180. [Google Scholar] [CrossRef]
- Bu, Q.; Zhu, H.; Cao, G.; Gong, G.; Su, Y.; Ge, Q.; Zhu, W.; Li, Z.; Pan, X. Targeting mechanics-induced trabecular meshwork dysfunction through YAP-TGFβ Ameliorates high myopia-induced ocular hypertension. Exp. Eye Res. 2024, 241, 109853. [Google Scholar] [CrossRef] [PubMed]
- Nagarsheth, M.; Singh, A.; Schmotzer, B.; Babineau, D.C.; Sugar, J.; Lee, W.B.; Iyengar, S.K.; Lass, J.H.; Fuchs’ Genetics Multi-Center Study Group. Relationship between Fuchs endothelial corneal dystrophy severity and glaucoma and/or ocular hypertension. Arch. Ophthalmol. 2012, 130, 1384–1388. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.L.; Paulose, S.A.; Yaghy, A.; Dalvin, L.A.; Constantinescu, A.B.; Lally, S.E.; Shields, J.A. Ocular surface squamous neoplasia managed with primary interferon α2b: A comparative analysis of 212 tumors in smokers versus nonsmokers. Cornea 2021, 40, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Rakusiewicz, K.; Kanigowska, K.; Hautz, W.; Ziolkowska, L. Choroidal thickness changes in children with chronic heart failure due to dilated cardiomyopathy. Int. Ophthalmol. 2021, 41, 2167–2177. [Google Scholar] [CrossRef] [PubMed]
- Betzler, B.K.; Siat, D.J.Y.; Agrawal, R.; Dorairaj, S.; Ang, B.C.H. Comparison of peripapillary choroidal thickness between primary open-angle glaucoma, normal tension glaucoma, and normal eyes: A systematic review and meta-analysis. Ophthalmol. Glaucoma 2024, 7, 359–371. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, H.; Shang, J.; Liu, G.; Xia, T.; Zhao, C.; Sun, G.; Dou, H. Comparative analysis of the blood transcriptomes between wolves and dogs. Anim. Genet. 2018, 49, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Albert, F.W.; Somel, M.; Carneiro, M.; Aximu-Petri, A.; Halbwax, M.; Thalmann, O.; Blanco-Aguiar, J.A.; Plyusnina, I.Z.; Trut, L.; Villafuerte, R.; et al. A comparison of brain gene expression levels in domesticated and wild animals. PLoS Genet. 2012, 8, e1002962. [Google Scholar] [CrossRef]
- Long, K.; Mao, K.; Che, T.; Zhang, J.; Qiu, W.; Wang, Y.; Tang, Q.; Ma, J.; Li, M.; Li, X. Transcriptome differences in frontal cortex between wild boar and domesticated pig. Anim. Sci. J. 2018, 89, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Adeola, A.C.; Xie, H.B.; Zhang, Y.P. Genomic and transcriptomic analyses reveal selection of genes for puberty in Bama Xiang pigs. Zool. Res. 2018, 39, 424–430. [Google Scholar]
- Hekman, J.P.; Johnson, J.L.; Edwards, W.; Vladimirova, A.V.; Gulevich, R.G.; Ford, A.L.; Kharlamova, A.V.; Herbeck, Y.; Acland, G.M.; Raetzman, L.T.; et al. Anterior pituitary transcriptome suggests differences in ACTH release in tame and aggressive foxes. Genes. Genomes Genet. 2018, 8, 859–873. [Google Scholar] [CrossRef] [PubMed]
- Sato, D.X.; Rafati, N.; Ring, H.; Younis, S.; Feng, C.; Blanco-Aguiar, J.A.; Rubin, C.J.; Villafuerte, R.; Hallbook, F.; Carneiro, M.; et al. Brain transcriptomics of wild and domestic rabbits suggests that changes in dopamine signaling and ciliary function contributed to evolution of tameness. Genome Biol. Evol. 2020, 12, 1918–1928. [Google Scholar] [CrossRef]
- Chadaeva, I.; Ponomarenko, P.; Kozhemyakina, R.; Suslov, V.; Bogomolov, A.; Klimova, N.; Shikhevich, S.; Savinkova, L.; Oshchepkov, D.; Kolchanov, N.A.; et al. Domestication explains two-thirds of differential-gene-expression variance between domestic and wild animals; the remaining one-third reflects intraspecific and interspecific variation. Animals 2021, 11, 2667. [Google Scholar] [CrossRef]
- Oshchepkov, D.; Ponomarenko, M.; Klimova, N.; Chadaeva, I.; Bragin, A.; Sharypova, E.; Shikhevich, S.; Kozhemyakina, R. A rat model of human behavior provides evidence of natural selection against underexpression of aggressiveness-related genes in humans. Front. Genet. 2019, 10, 1267. [Google Scholar] [CrossRef]
- Oshchepkov, D.; Chadaeva, I.; Kozhemyakina, R.; Shikhevich, S.; Sharypova, E.; Savinkova, L.; Klimova, N.V.; Tsukanov, A.; Levitsky, V.G.; Markel, A.L. Transcription factors as important regulators of changes in behavior through domestication of gray rats: Quantitative data from RNA sequencing. Int. J. Mol. Sci. 2022, 23, 12269. [Google Scholar] [CrossRef] [PubMed]
- Fallahshahroudi, A.; Lotvedt, P.; Belteky, J.; Altimiras, J.; Jensen, P. Changes in pituitary gene expression may underlie multiple domesticated traits in chickens. Hered. Edinb. 2019, 122, 195–204. [Google Scholar] [CrossRef]
- Ponomarenko, P.; Savinkova, L.; Drachkova, I.; Lysova, M.; Arshinova, T.; Ponomarenko, M.; Kolchanov, N. A step-by-step model of TBP/TATA box binding allows predicting human hereditary diseases by single nucleotide polymorphism. Dokl. Biochem. Biophys. 2008, 419, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Delgadillo, R.F.; Whittington, J.E.; Parkhurst, L.K.; Parkhurst, L.J. The TATA-binding protein core domain in solution variably bends TATA sequences via a three-step binding mechanism. Biochemistry 2009, 48, 1801–1809. [Google Scholar] [CrossRef]
- Hahn, S.; Buratowski, S.; Sharp, P.; Guarente, L. Yeast TATA-binding protein TFIID binds to TATA elements with both consensus and nonconsensus DNA sequences. Proc. Natl. Acad. Sci. USA 1989, 86, 5718–5722. [Google Scholar] [CrossRef]
- Berg, O.G.; von Hippel, P.H. Selection of DNA binding sites by regulatory proteins. Statistical-mechanical theory and application to operators and promoters. J. Mol. Biol. 1987, 193, 723–750. [Google Scholar] [CrossRef] [PubMed]
- Bucher, P. Weight matrix descriptions of four eukaryotic RNA polymerase II promoter elements derived from 502 unrelated promoter sequences. J. Mol. Biol. 1990, 212, 563–578. [Google Scholar] [CrossRef]
- Coleman, R.A.; Pugh, B.F. Evidence for functional binding and stable sliding of the TATA binding protein on nonspecific DNA. J. Biol. Chem. 1995, 270, 13850–13859. [Google Scholar] [CrossRef]
- Karas, H.; Knuppel, R.; Schulz, W.; Sklenar, H.; Wingender, E. Combining structural analysis of DNA with search routines for the detection of transcription regulatory elements. Comput. Appli. Biosci. 1996, 12, 441–446. [Google Scholar] [CrossRef]
- Ponomarenko, M.; Ponomarenko, J.; Frolov, A.; Podkolodny, N.; Savinkova, L.; Kolchanov, N.; Overton, G. Identification of sequence-dependent features correlating to activity of DNA sites interacting with proteins. Bioinformatics 1999, 15, 687–703. [Google Scholar] [CrossRef]
- Flatters, D.; Lavery, R. Sequence-dependent dynamics of TATA-box binding sites. Biophys. J. 1998, 75, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.L.; Nikolov, D.B.; Burley, S.K. Co-crystal structure of TBP recognizing the minor groove of a TATA element. Nature 1993, 365, 520–527. [Google Scholar] [CrossRef]
- Kim, Y.; Geiger, J.H.; Hahn, S.; Sigler, P.B. Crystal structure of a yeast TBP/TATA-box complex. Nature 1993, 365, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Waardenberg, A.; Basset, S.; Bouveret, R.; Harvey, R. CompGO: An R package for comparing and visualizing Gene Ontology enrichment differences between DNA binding experiments. BMC Bioinform. 2015, 16, 275. [Google Scholar] [CrossRef] [PubMed]
- Mustafin, Z.S.; Lashin, S.A.; Matushkin, Y.G.; Gunbin, K.V.; Afonnikov, D.A. Orthoscape: A cytoscape application for grouping and visualization KEGG based gene networks by taxonomy and homology principles. BMC Bioinform. 2017, 18, 1427. [Google Scholar] [CrossRef] [PubMed]
- Mustafin, Z.S.; Zamyatin, V.I.; Konstantinov, D.K.; Doroshkov, A.V.; Lashin, S.A.; Afonnikov, D.A. Phylostratigraphic analysis shows the earliest origination of the abiotic stress associated genes in A. thaliana. Genes. 2019, 10, 963. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Latka, M.; Turalska, M.; Glaubic-Latka, M.; Kolodziej, W.; Latka, D.; West, B.J. Phase dynamics in cerebral autoregulation. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H2272–H2279. [Google Scholar] [CrossRef]
- Kwak, S.G.; Kim, J.H. Central limit theorem: The cornerstone of modern statistics. Korean J. Anesth. 2017, 70, 144–156. [Google Scholar] [CrossRef]
- Gene Ontology Consortium; Aleksander, S.A.; Balhoff, J.; Carbon, S.; Cherry, J.M.; Drabkin, H.J.; Ebert, D.; Feuermann, M.; Gaudet, P.; Harris, N.L.; et al. The Gene Ontology knowledgebase in 2023. Genetics 2023, 224, iyad031. [Google Scholar] [PubMed]
- Husain, S.; Leveckis, R. Pharmacological regulation of HIF-1α, RGC death, and glaucoma. Curr. Opin. Pharmacol. 2024, 77, 102467. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Bai, Z.; Wang, F.; Han, Y.; Sun, H.; Xiaokereti, J.; Zhang, L.; Zhou, X.; Lu, Y.; Tang, B. Full-length transcriptome sequencing: An insight into the dog model of heart failure. Front. Cardiovasc. Med. 2021, 8, 712797. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, A.; Huang, J. Identification of gene changes induced by dexamethasone in the anterior segment of the human eye using bioinformatics analysis. Med. Sci. Monit. 2019, 25, 5501–5509. [Google Scholar] [CrossRef] [PubMed]
- Evangelho, K.; Mogilevskaya, M.; Losada-Barragan, M.; Vargas-Sanchez, J.K. Pathophysiology of primary open-angle glaucoma from a neuroinflammatory and neurotoxicity perspective: A review of the literature. Int. Ophthalmol. 2019, 39, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Graham, K.L.; Diefenbach, E.; McCowan, C.I.; White, A.J.R. A technique for shotgun proteomic analysis of the precorneal tear film in dogs with naturally occurring primary glaucoma. Vet. Ophthalmol. 2021, 24, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Dammak, A.; Sanchez Naves, J.; Huete-Toral, F.; Carracedo, G. New biomarker combination related to oxidative stress and inflammation in primary open-angle glaucoma. Life 2023, 13, 1455. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Harris, A.; Toris, C.; Tobe, L.; Lang, M.; Belamkar, A.; Ng, A.; Verticchio Vercellin, A.C.; Mathew, S.; Siesky, B. Effects of sex hormones on ocular blood flow and intraocular pressure in primary open-angle glaucoma: A review. J. Glaucoma 2018, 27, 1037–1041. [Google Scholar] [CrossRef] [PubMed]
- Blue Mountains Eye Study (BMES); Wellcome Trust Case Control Consortium 2 (WTCCC2). Genome-wide association study of intraocular pressure identifies the GLCCI1/ICA1 region as a glaucoma susceptibility locus. Hum. Mol. Genet. 2013, 22, 4653–4660. [Google Scholar] [CrossRef]
- Nie, Q.; Zhang, X. Transcriptional profiling analysis predicts potential biomarkers for glaucoma: HGF, AKR1B10 and AKR1C3. Exp. Ther. Med. 2018, 16, 5103–5111. [Google Scholar] [CrossRef]
- Song, X.; Li, P.; Li, Y.; Yan, X.; Yuan, L.; Zhao, C.; An, Y.; Chang, X. Strong association of glaucoma with atherosclerosis. Sci. Rep. 2021, 11, 8792. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Zhang, D. Apoptosis in glaucoma: A new direction for the treatment of glaucoma (Review). Mol. Med. Rep. 2024, 29, 82. [Google Scholar] [CrossRef]
- Pattabiraman, P.P.; Feinstein, V.; Beit-Yannai, E. Profiling the miRNA from exosomes of non-pigmented ciliary epithelium-derived identifies key gene targets relevant to primary open-angle glaucoma. Antioxidants 2023, 12, 405. [Google Scholar] [CrossRef]
- Pantalon, A.; Obada, O.; Constantinescu, D.; Feraru, C.; Chiselita, D. Inflammatory model in patients with primary open angle glaucoma and diabetes. Int. J. Ophthalmol. 2019, 12, 795–801. [Google Scholar]
- Vernazza, S.; Tirendi, S.; Bassi, A.M.; Traverso, C.E.; Sacca, S.C. Neuroinflammation in primary open-angle glaucoma. J. Clin. Med. 2020, 9, 3172. [Google Scholar] [CrossRef] [PubMed]
- Kaeslin, M.A.; Killer, H.E.; Fuhrer, C.A.; Zeleny, N.; Huber, A.R.; Neutzner, A. Changes to the aqueous humor proteome during glaucoma. PLoS ONE 2016, 11, e0165314. [Google Scholar] [CrossRef] [PubMed]
- Iomdina, E.N.; Tikhomirova, N.K.; Bessmertny, A.M.; Serebryakova, M.V.; Baksheeva, V.E.; Zalevsky, A.O.; Kotelin, V.I.; Kiseleva, O.A.; Kosakyan, S.M.; Zamyatnin, A.A., Jr.; et al. Alterations in proteome of human sclera associated with primary open-angle glaucoma involve proteins participating in regulation of the extracellular matrix. Mol. Vis. 2020, 26, 623–640. [Google Scholar]
- Horai, R.; Caspi, R.R. Microbiome and autoimmune uveitis. Front. Immunol. 2019, 10, 232. [Google Scholar] [CrossRef] [PubMed]
- Ziaastani, Z.; Kalantari-Khandani, B.; Niazi, M.J.; Kazemipour, A. Identification of critical genes and metabolic pathways in rheumatoid arthritis and osteoporosis toward drug repurposing. Comput. Biol. Med. 2024, 180, 108912. [Google Scholar] [CrossRef] [PubMed]
- Tezel, G. The immune response in glaucoma: A perspective on the roles of oxidative stress. Exp. Eye Res. 2011, 93, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Lin, Z.; Li, P.; Zhang, Z.; Jiang, J.; Yang, C. Investigation of potential crucial genes and key pathways in keratoconus: An analysis of gene expression Omnibus data. Biochem. Genet. 2023, 61, 2724–2740. [Google Scholar] [CrossRef] [PubMed]
- Schmalen, A.; Lorenz, L.; Grosche, A.; Pauly, D.; Deeg, C.A.; Hauck, S.M. Proteomic phenotyping of stimulated Muller cells uncovers profound pro-inflammatory signaling and antigen-presenting capacity. Front. Pharmacol. 2021, 12, 771571. [Google Scholar] [CrossRef] [PubMed]
- Maeda, Y. Pathogenesis of graft-versus-host disease: Innate immunity amplifying acute alloimmune responses. Int. J. Hematol. 2013, 98, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.A.; Ju, H.H.; Lee, J.; Kim, J.E.; Paik, S.Y.; Skiba, N.P.; Rao, P.V. Increased complement-associated inflammation in cytomegalovirus-positive hypertensive anterior uveitis patients based on the aqueous humor proteomics analysis. J. Clin. Med. 2022, 11, 2337. [Google Scholar] [CrossRef]
- Sato, K.; Ohira, M.; Imaoka, Y.; Imaoka, K.; Bekki, T.; Doskali, M.; Nakano, R.; Yano, T.; Tanaka, Y.; Ohdan, H. The aryl hydrocarbon receptor maintains antitumor activity of liver resident natural killer cells after partial hepatectomy in C57BL/6J mice. Cancer Med. 2023, 12, 19821–19837. [Google Scholar] [CrossRef]
- Yellen-Shaw, A.J.; Laughlin, C.E.; Metrione, R.M.; Eisenlohr, L.C. Murine transporter associated with antigen presentation (TAP) preferences influence class I-restricted T cell responses. J. Exp. Med. 1997, 186, 1655–1662. [Google Scholar] [CrossRef]
- Birney, E. The International Human Genome Project. Hum. Mol. Genet. 2021, 30, R161–R163. [Google Scholar] [CrossRef] [PubMed]
- 1000 Genomes Project Consortium; Abecasis, G.; Auton, A.; Brooks, L.; DePristo, M.; Durbin, R.; Handsaker, R.; Kang, H.; Marth, G.; McVean, G.; et al. An integrated map of genetic variation from 1092 human genomes. Nature 2012, 491, 56–65. [Google Scholar] [PubMed]
- Kasowski, M.; Grubert, F.; Heffelfinger, C.; Hariharan, M.; Asabere, A.; Waszak, S.; Habegger, L.; Rozowsky, J.; Shi, M.; Urban, A.; et al. Variation in transcription factor binding among humans. Science 2010, 328, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Haldane, J.B.S. The cost of natural selection. J. Genet. 1957, 55, 511–524. [Google Scholar] [CrossRef]
- Kimura, M. Evolutionary rate at the molecular level. Nature. 1968, 217, 624–626. [Google Scholar] [CrossRef] [PubMed]
- Belyaev, D.K. The Wilhelmine E. Key 1978 invitational lecture. Destabilizing selection as a factor in domestication. J Hered 1979, 70, 301–308. [Google Scholar] [CrossRef]
- Samet, H. A top-down quadtree traversal algorithm. IEEE Trans. Pattern Anal. Mach. Intell. 1985, 7, 94–98. [Google Scholar] [CrossRef]
- Sun, G.L.; Shen, W.; Wen, J.F. Triosephosphate isomerase genes in two trophic modes of euglenoids (euglenophyceae) and their phylogenetic analysis. J. Eukaryot. Microbiol. 2008, 55, 170–177. [Google Scholar] [CrossRef]
- Morozova, O.V.; Alekseeva, A.E.; Sashina, T.A.; Brusnigina, N.F.; Epifanova, N.V.; Kashnikov, A.U.; Zverev, V.V.; Novikova, N.A. Phylodynamics of G4P[8] and G2P[4] strains of rotavirus A isolated in Russia in 2017 based on full-genome analyses. Virus Genes 2020, 56, 537–545. [Google Scholar] [CrossRef]
- Theofanopoulou, C.; Gastaldon, S.; O’Rourke, T.; Samuels, B.D.; Martins, P.T.; Delogu, F.; Alamri, S.; Boeckx, C. Self-domestication in Homo sapiens: Insights from comparative genomics. PLoS ONE 2017, 12, e0185306. [Google Scholar] [CrossRef] [PubMed]
- Schwab, I.R. The evolution of eyes: Major steps. The Keeler lecture 2017: Centenary of Keeler Ltd. Eye 2018, 32, 302–313. [Google Scholar] [CrossRef]
- Randel, N.; Jekely, G. Phototaxis and the origin of visual eyes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371, 20150042. [Google Scholar]
- D’Aniello, S.; Bertrand, S. Cephalochordates. In Handbook of Marine Model Organisms in Experimental Biology; Rahman, I., Bagchi, D., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 341–355. [Google Scholar]
- Suzuki, D.G.; Grillner, S. The stepwise development of the lamprey visual system and its evolutionary implications. Biol. Rev. Camb. Philos. Soc. 2018, 93, 1461–1477. [Google Scholar] [CrossRef]
- Yam, G.H.; Gaplovska-Kysela, K.; Zuber, C.; Roth, J. Aggregated myocilin induces russell bodies and causes apoptosis: Implications for the pathogenesis of myocilin-caused primary open-angle glaucoma. Am. J. Pathol. 2007, 170, 100–109. [Google Scholar] [CrossRef]
- Kapitonov, V.V.; Jurka, J. RAG1 core and V(D)J recombination signal sequences were derived from Transib transposons. PLoS Biol. 2005, 3, e181. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.D.; Alder, M.N. The evolution of adaptive immune systems. Cell 2006, 124, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jamwal, K.; Distl, O. Tracking footprints of artificial and natural selection signatures in breeding and non-breeding cats. Sci. Rep. 2022, 12, 18061. [Google Scholar] [CrossRef]
- Zavos, C.; Kountouras, J.; Katsinelos, P.; Polyzos, S.A.; Deretzi, G.; Zavos, N.; Fragaki, M.; Diamantidis, M.D. Modern industrialization may increase primary open-angle glaucoma prevalence through easier transmission of Helicobacter pylori infection. Med. Hypotheses 2011, 76, 766–767. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Shao, M.; Li, S.; Lei, Y.; Cao, W.; Sun, X. The association between airborne particulate matter (PM2.5) exposure level and primary open-angle glaucoma. Ecotoxicol. Environ. Saf. 2024, 283, 116752. [Google Scholar] [CrossRef] [PubMed]
- Ciulla, L.; Moorthy, M.; Mathew, S.; Siesky, B.; Verticchio Vercellin, A.C.; Price, D.; Januleviciene, I.; Harris, A. Circadian rhythm and glaucoma: What do we know? J. Glaucoma 2020, 29, 127–132. [Google Scholar] [CrossRef] [PubMed]
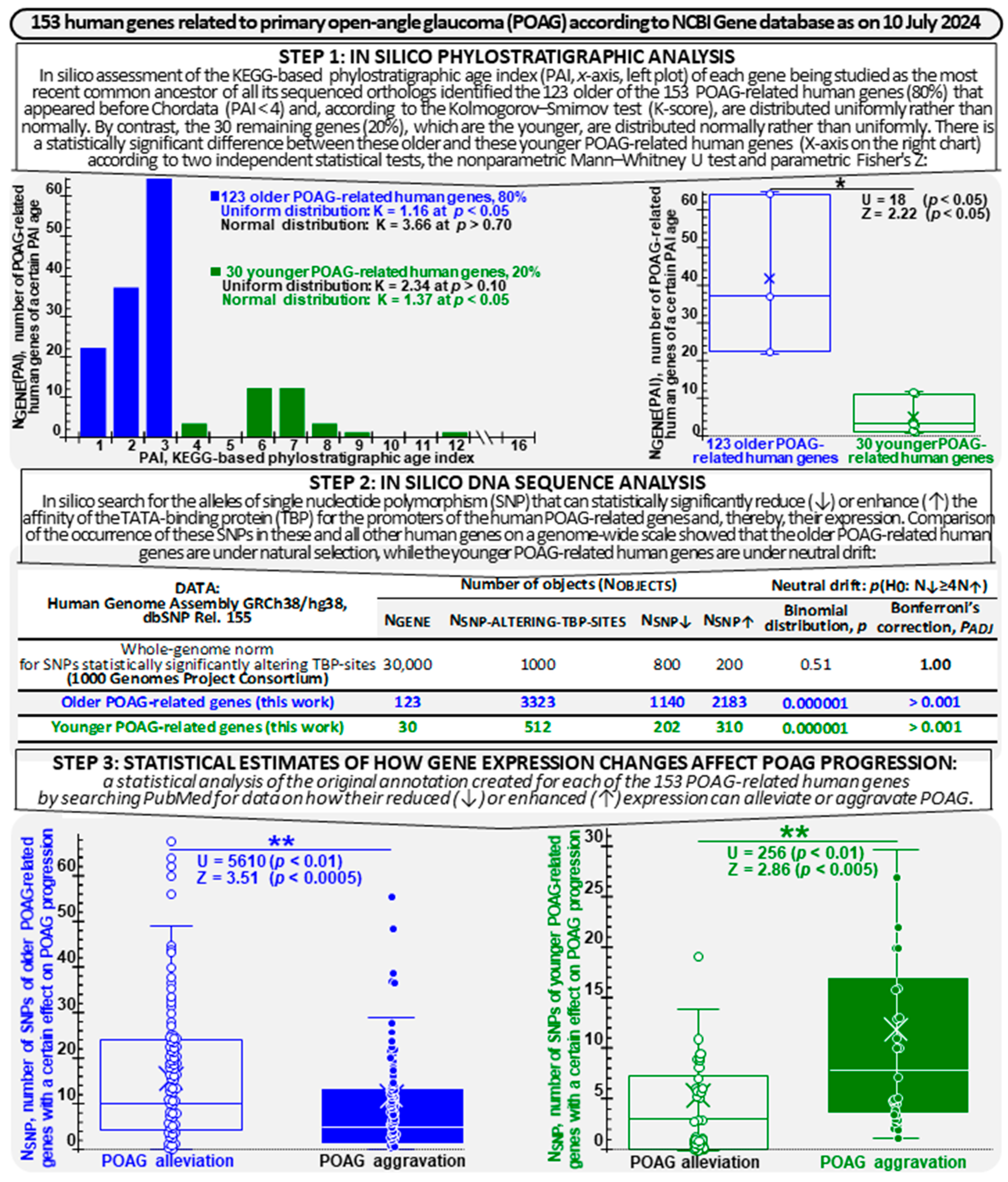
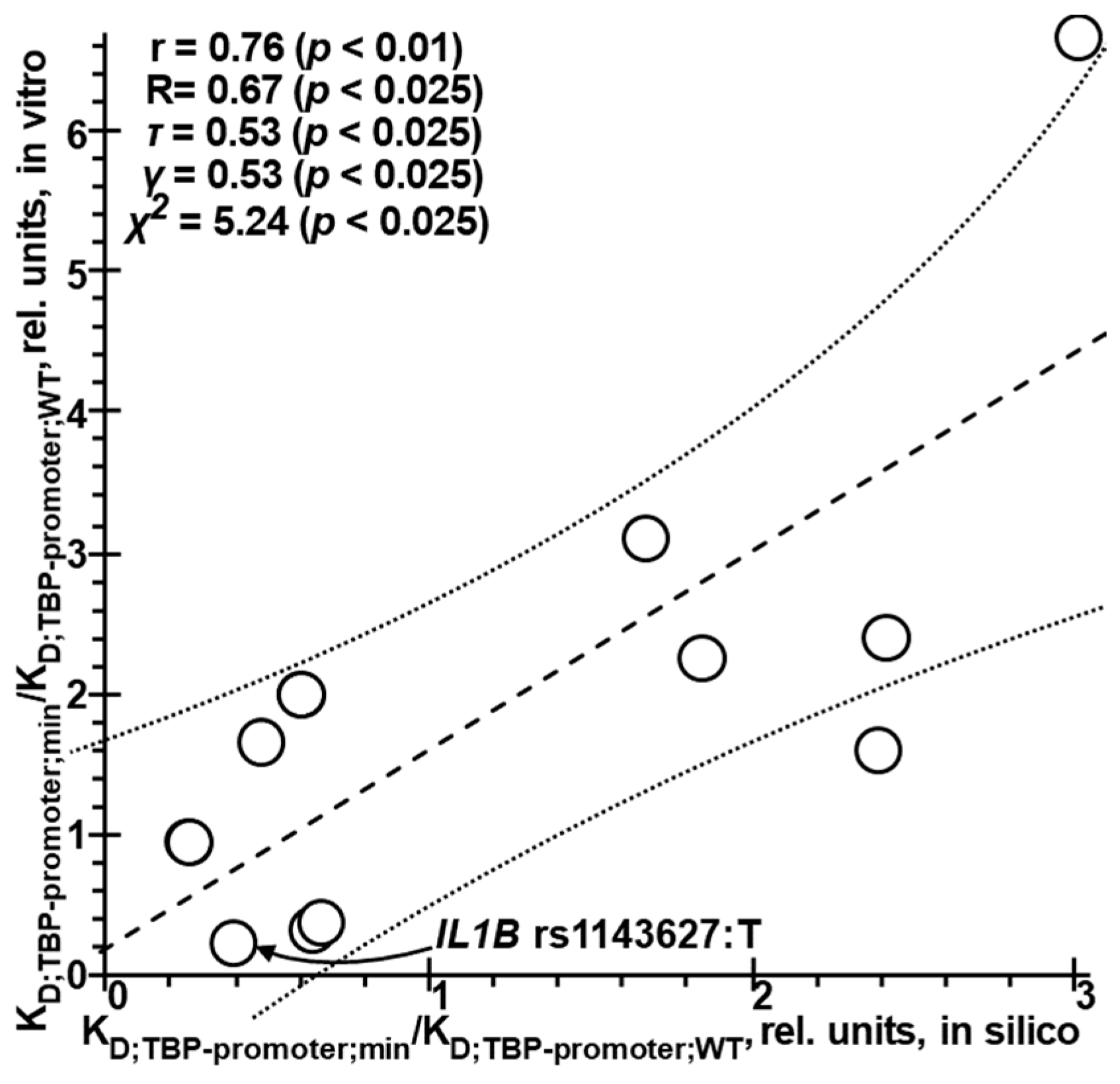
| Category | No | 123 Older POAG-Related Genes | 30 Younger POAG-Related Genes | ||||
|---|---|---|---|---|---|---|---|
| ID | Term | PADJ | ID | Term | PADJ | ||
| PANTHER [53] | |||||||
| Biological Process | 1 | GO0070887 | cellular response to chemical stimulus | 10−24 | GO0051241 | negative regulation of multicellular organismal process | 10−5 |
| 2 | GO0042221 | response to chemical | 10−23 | GO0009617 | response to bacterium | 10−5 | |
| Molecular Function | 3 | GO0042802 | identical protein binding | 10−7 | GO0005102 | signaling receptor binding | 10−5 |
| 4 | GO0019899 | enzyme binding | 10−7 | GO0030545 | signaling receptor regulator activity | 10−4 | |
| Cell Component | 5 | GO0005615 | extracellular space | 10−7 | GO0005576 | extracellular region | 10−3 |
| 6 | GO0031982 | vesicle | 10−6 | GO0042825 | transporter associated with antigen presentation (TAP) complex | 10−2 | |
| DAVID [54] | |||||||
| Biological Process | 7 | GO0001666 | response to hypoxia | 10−6 | GO0010575 | positive regulation of vascular endothelial growth factor production | 10−2 |
| 8 | GO0051045 | negative regulation of membrane protein ectodomain proteolysis | 10−6 | GO0032755 | positive regulation of interleukin-6 production | 10−2 | |
| Molecular Function | 9 | GO0002020 | protease binding | 10−5 | GO0005125 | cytokine activity | 10−4 |
| 10 | GO0042802 | identical protein binding | 10−4 | GO0005149 | interleukin-1 receptor binding | 0.05 | |
| Cell Component | 11 | GO0031012 | extracellular matrix | 10−6 | GO0005576 | extracellular region | 10−8 |
| 12 | GO0005615 | extracellular space | 10−4 | GO0005615 | extracellular space | 10−5 | |
| KEGG Pathway | 13 | hsa05205 | proteoglycans in cancer | 10−15 | hsa04933 | AGE-RAGE signaling pathway in diabetic complications | 10−4 |
| 14 | hsa05417 | lipid and atherosclerosis | 10−11 | hsa05332 | graft-versus-host disease | 10−2 | |
| STRING [55] | |||||||
| Biological Process | 15 | GO0010033 | response to organic substance | 10−21 | GO0009617 | response to bacterium | 10−7 |
| 16 | GO1901700 | response to oxygen-containing compound | 10−21 | GO0006953 | acute-phase response | 10−6 | |
| Molecular Function | 17 | GO0005515 | protein binding | 10−9 | GO0005102 | signaling receptor binding | 10−4 |
| 18 | GO0042802 | identical protein binding | 10−8 | GO0030545 | signaling receptor regulator activity | 10−4 | |
| Cell Component | 19 | GO0005576 | extracellular space | 10−6 | GO0005576 | extracellular region | 10−3 |
| 20 | GO0031982 | vesicle | 10−5 | GO0005615 | extracellular space | 10−2 | |
| KEGG Pathway | 21 | hsa05205 | proteoglycans in cancer | 10−17 | hsa04933 | AGE-RAGE signaling pathway in diabetic complications | 10−7 |
| 22 | hsa05200 | pathways in cancer | 10−12 | hsa05332 | graft-versus-host disease | 10−4 | |
| MetaScape [56] | |||||||
| Biological Process | 23 | GO0009725 | response to hormone | 10−20 | GO0009617 | response to bacterium | 10−7 |
| 24 | GO0009410 | response to xenobiotic stimulus | 10−19 | GO0007162 | negative regulation of cell adhesion | 10−4 | |
| KEGG Pathway | 25 | hsa05205 | proteoglycans in cancer | 10−23 | hsa04933 | AGE-RAGE signaling pathway in diabetic complications | 10−7 |
| 26 | hsa05200 | pathways in cancer | 10−18 | hsa05163 | human cytomegalovirus infection | 10−4 | |
| GeneMANIA [57] | |||||||
| Biological Process | 27 | GO2001233 | regulation of apoptotic signaling pathway | 10−10 | GO0071219 | cellular response to molecule of bacterial origin | 10−20 |
| 28 | GO0008285 | negative regulation of cell population proliferation | 10−21 | GO0071216 | cellular response to biotic stimulus | 10−19 | |
| ANDSystem [52] | # | PANTHER [53], DAVID [54], STRING [55], MetaScape [56], GeneMANIA [57]: Gene Ontology Terms and KEGG Pathways | Where ANDSystem [52] Agrees with PANTHER [53], DAVID [54], STRING [55], MetaScape [56], GeneMANIA [57] in Their Outcome Within PubMed [22] | |
|---|---|---|---|---|
| 123 older POAG-related genes studied in this work | ||||
| pathogenesis | 1 | GO0001666: response to hypoxia | hypoxia-caused ocular injuries speed POAG pathogenesis [594] | |
| 2 | GO0051045: negative regulation of membrane protein ectodomain proteolysis | within human disease models using dogs [595]: GO0051045 is one of the seventeen best GO-terms specifying heart failure pathogenesis | ||
| 3 | GO0002020: protease binding GO0042802: identical protein binding GO0005515: protein binding GO0019899: enzyme binding | within the bioinformatics meta-analysis of POAG-related transcriptome data along with GO-annotation [596]: protease binding and protein–protein interactions were found to accelerate POAG pathogenesis | ||
| 4 | GO0031012: extracellular matrix GO0005615: extracellular space | according to a comprehensive biomedical review [597]: extracellular matrix and space remodeling accelerate POAG pathogenesis | ||
| 5 | GO0010033: response to organic substance GO0042221: response to chemical | in human disease models using dog tears [598]: haptoglobin-based response to organic substances speeds POAG pathogenesis | ||
| 6 | GO1901700: response to oxygen-containing compound | oxidative stress can accelerate POAG pathogenesis [599] | ||
| 7 | GO0009725: response to hormone GO0070887: cellular response to chemical stimulus | during pregnancy and post-menopause, neuroprotective estrogen hormone therapy slows POAG pathogenesis [600] | ||
| 8 | GO0009410: response to xenobiotic stimulus | GO0009410 is a term specifying POAG pathogenesis [601] | ||
| 9 | GO0008285: negative regulation of cell population proliferation | within a cohort biomedical transcriptome meta-analysis [602]: GO0008285 is the best GO-term specifying POAG pathogenesis | ||
| 10 | hsa05205: proteoglycans in cancer hsa05200: pathways in cancer | in a cohort transcriptome meta-analysis [602]: hsa05200 is among the top five KEGG-pathways specifying POAG pathogenesis | ||
| 11 | hsa05417: lipid and atherosclerosis | in a cohort study [603]: atherosclerosis spurs POAG pathogenesis | ||
| apoptotic process | 12 | GO2001233: regulation of apoptotic signaling pathway | according to a comprehensive biomedical review [604]: retinal ganglion cell apoptosis contributes to POAG pathogenesis | |
| 13 | GO0031982: vesicle | apoptotic bodies are one of the types of extracellular vesicles [605] | ||
| 30 younger POAG-related genes studied in this work | ||||
| inflammatory response | 14 | GO0010575: positive regulation of vascular endothelial growth factor production | according to cohort clinical study [606]: vascular endothelial growth factor (VEGF) excess contributes to inflammation in POAG | |
| 15 | GO0032755: positive regulation of IL6 production | IL6 excess contributes to inflammatory response in POAG [258] | ||
| 16 | GO0005125: cytokine activity | in a cohort study [258]: cytokine IL6 contributes to inflammation | ||
| 17 | GO0005149: interleukin-1 receptor binding | IL1 binds to its receptor, raising the inflammatory response [607] | ||
| 18 | GO0006953: acute-phase response | GO0006953 is a GO-term specifying inflammation in POAG [608] | ||
| immune response | 19 | GO0005576: extracellular region GO0005615: extracellular space | within a cohort sclera sample study [609]: defects in the extracellular region and space can provoke an immune response in POAG | |
| 20 | GO0009617: response to bacterium | dysbiosis in the gut–retina axis triggers an immune response [610] | ||
| 21 | GO0005102: signaling receptor binding GO0030545: signaling receptor regulator activity | a retrospective meta-analysis [611]: GO0005102 and GO0030545 are GO-terms specifying the immune response in osteoporosis | ||
| 22 | GO0007162: negative regulation of cell adhesion | altered cell adhesion causes an immune response in POAG [612] | ||
| 23 | GO0071219: cellular response to molecule of bacterial origin | within meta-analysis of both KEGG and Omnibus Database [613]: bacterial origin molecules can cause a cellular immune response | ||
| 24 | GO0071216: cellular response to biotic stimulus | a biotic stimulus can provoke a cellular immune response [613] | ||
| 25 | hsa04933: AGE-RAGE signaling pathway in diabetic complications | within human POAG models using pig [614]: hsa04933 is among the top ten KEGG pathways specifying the immune response | ||
| 26 | hsa05332: graft-versus-host disease (GvHD) | immune response can contribute to GvHD pathogenesis [615] | ||
| 27 | hsa05163: human cytomegalovirus infection | immune response to cytomegalovirus can aggravate POAG [616] | ||
| 28 | GO0051241: negative regulation of multicellular organismal process | human anticancer therapy models using mice [617]: GO0051241 is among the top ten GO-terms specifying the immune response | ||
| 29 | GO0042825: TAP complex | the TAP complex can contribute to the immune response [618] | ||
| ClinVar Database [38] | Human_SNP_TATAdb Database [49] | ∑ + - | |||||
|---|---|---|---|---|---|---|---|
| # | NCBI Gene Symbol (Entrez Gene ID) | dbSNP ID:min [437] | Susceptibility to Human Disease | Δ: ↓ ↑ | ☼: ▼ ▲ | How Susceptibility to Human Disease, Biomedical Markers of Which Are the SNPs in Question, Can Aggravate or Alleviate POAG According to the Current State of the PubMed Database, as Cited Using [Refs] | |
| 1 | ABCA1 (19) | rs886063317:C, rs886063317:G | Tangier disease | ↓ | ▼ | Tangier disease (also known as familial high-density lipoprotein deficiency) is comorbid with POAG [443] | + |
| 2 | ABCB1 (5243) | rs1584915287:A | Tramadol response | ↑ | ▼ | a healthy man experienced glaucomatous vision impairment after injecting tramadol as a painkiller [444] | + |
| 3 | ATXN2 (6311) | rs695871:G | Spinocerebellar ataxia type 2 | ↑ | ▼ | near-threshold glaucomatous changes in the optic nerve in patients with Spinocerebellar ataxia type 2 [445] | + |
| 4 | BMP4 (652) | rs774069849:A | orofacial cleft, microphthalmia with brain and digit anomalies | ↑ | ▼ | these morpho-ontogenetic disorders of the human head development inevitably lead to developmental disorders of the eyes, as parts of the head colocalized along with all its other parts that can manifest as congenital, early-onset, pediatric, and juvenile forms of various glaucomas, including POAG [446] | + |
| 5 | CP (1356) | rs151304828:T | Ferroxidase deficiency | ↑ | ▼ | this biomedical SNP marker is a result of screening in healthy volunteers that after an in silico analysis of both the literature and factual biomedical data was labeled “Conflicting Pathogenicity Classifications”, while database Human_SNP_TATAdb documents—an in silico estimate of this SNP—was labeled “Ferroxidase excess “ rather than “Ferroxidase deficiency”, in line with a biomedical report [448] on this excess, which can be an antioxidant protector against inevitable damage of the optic nerve head sensing light flux | - |
| 6 | ELN (2006) | rs41410045:G, rs41410045:T, rs537200597:A, rs537200597:T | Supravalvar aortic stenosis, Cutis laxa, Williams syndrome | ↑ | ▼ | these four hereditary connective tissue disorders are capable of manifesting in morpho-ontogenetic eye defects such as a subluxation lentis leading to various congenital, early-onset, pediatric, and juvenile forms of glaucoma, including POAG [449] | + |
| 7 | FAS (355) | rs558072404:A | Autoimmune lymphoproliferative syndrome type 1 | ↑ | ▼ | this disease is a form of lymphoproliferative disorder resulting from infection-related post-traumatic and\or post-surgery complications, which can lead to various morphological changes in eyes that can elevate intraocular pressure, aggravating POAG [450] | + |
| 8 | GRIN2B (2904) | rs797044930:T | Intellectual disability | ↑ | ▼ | intellectual disability can manifest with movement disorders, cortical development malformations, and cortical visual impairment [451], which are comorbid with POAG [452] | + |
| 9 | IL1B (3553) | rs1143627:T | Gastric cancer at Helicobacter pylori, POAG, and 57 inflammation-related disorders | ↑ | ▼ | all these diseases are comorbid with POAG [452,453,454,455,456,457,458,459,460,461,462,463,464,465,466,467,468,469,470,471,472,473,474,475,476,477,478,479,480,481,482,483,484,485,486,487,488,489,490,491,492,493,494,495,496,497,498,499,500,501,502,503,504,505,506,507,508,509,510,511,512,513,514,515,516,517,518,519,520,521,522,523,524,525,526,527,528,529,530,531,532,533,534,535,536,537,538,539,540,541,542,543,544,545,546,547,548,549,550,551,552,553,554], as readers can find in Table S5, namely, row #9, penultimate column on the right (see Supplementary Materials) | + |
| 10 | PMM2 (5373) | rs751782324:A | PMM2-congenital glycosylation disorder | ↓ | ▼ | PMM2-congenital glycosylation disorder can aggravate POAG [330] | + |
| 11 | LDLR (3949) | rs1357531646:G, rs1568582310:C, rs747068848:C, rs879254501:C, rs879254502:C, rs879254503:G, rs879254505:C, rs879254506:C, rs879254507:G, rs879254511:C, rs969658891:A | Familial hypercholesterolemia | ↓ | ▼ | according to a cohort-based biomedical study [313]: familial hypercholesterolemia occurs in patients with POAG more often compared to those without POAG, which may be the aggravation of POAG | + |
| rs121908042:A, rs193922571:A, rs201102461:A, rs2077073153:T, rs2077269298:A, rs730882080:T, rs730882081:G, rs762139262:T, rs769383881:A, rs769383881:T, rs869320648:A, rs875989899:T, rs879254486:T, rs879254502:A, ars879254506:A | Familial hypercholesterolemia and pathogenic cardiovascular phenotype | ↑ | according to cohort biomedical studies: both familial hypercholesterolemia occurs in patients with POAG more often compared to those without POAG [313] as well as pathogenic cardiovascular phenotype, which can aggravate POAG [555] | ||||
| 12 | MFN2 (9927) | rs568548916:A, rs886045216:T, rs973376897:A | hereditary motor and sensory neuropathy with optic atrophy | ↑ | ▼ | according to a diseasome gene network encompassing human genes contributing simultaneously to amyotrophic lateral sclerosis and other diseases [556]: POAG along with hereditary motor and sensory neuropathy with optic atrophy is comorbid to amyotrophic lateral sclerosis | + |
| 13 | MUTYH (4595) | rs1645057147:C, rs752665489:G | hereditary cancer predisposition syndrome | ↓ | ▼ | hereditary cancer predisposition syndrome is associated with congenital hypertrophy of retinal pigment epithelium, which can cause advanced glaucomatous damage in the optic nerve that can aggravate POAG [557] | + |
| rs1060504202:A, rs1064795596:A, rs1338038953:A, rs1553127879:T, rs1553136984:A, rs1553137062:A, rs1570591700:A, rs1570591700:T, rs1570591736:A, rs2275602:T, rs587788237:A, rs753502884:T, rs755928199:A, rs755928199:C, rs755928199:T, rs758246147:A, rs766584437:A, rs766584437:T, rs767402084:A, rs767402084:C, rs774530388:T, rs876658588:A, rs878854188:G, rs878854188:T | ↑ | ||||||
| 14 | STAT3 (6774) | rs780393027:A, rs902564848:T | hyper-IgE recurrent infection syndrome | ↑ | ▼ | hyper-IgE recurrent infection syndrome 1 can cause both ocular allergy and allergic conjunctivitis, the treatment of which with corticosteroids has a side effect in the aggravation of POAG [558] | + |
| 15 | TAP1 (6890) | rs1408055208:T, rs202053684:T | MHC class I deficiency | ↑ | ▼ | MHC class I-knockout mice show a similarity to the very early stages of POAG development [559] | + |
| 16 | TCF4 (6925) | rs1555710523:T, rs17522826:T, rs2047109965:T, rs2061383201:A | Pitt–Hopkins syndrome | ↑ | ▼ | high myopia is both a symptom of Pitt–Hopkins syndrome [560] and a risk factor for POAG [561] | + |
| 17 | TP53 (7157) | rs1457582183:T, rs1597400604:A, rs34361146:A | Li–Fraumeni and cancer predisposition syndromes | ↑ | ▼ | tobacco smoking is a risk factor for POAG as well as for both Li–Fraumeni and hereditary cancer-predisposition syndromes [563] | + |
| 18 | TXNRD2 (10587) | rs182857388:T, rs886509891:T | primary dilated cardiomyopathy | ↑ | ▼ | choroidal thickness in children with chronic heart failure through dilated cardiomyopathy is decreased [564] and is observed alongside POAG [565] | + |
| (a) Animals | The Number of DEGs Whose Expression Changed in the Same Direction as Their Homologous Human Genes with a Given Effect on the Alleviation and Aggravation of POAG | ||||
|---|---|---|---|---|---|
| (b) Human | 123 Older POAG-Related Genes | 30 Younger POAG-Related Genes | |||
| Wild | Domestic | Wild | Domestic | ||
| The Effect of Changes in the Expression of the POAG-Related Genes on the Alleviation and Aggravation of POAG | Alleviation | 69 | 60 | 12 | 8 |
| Aggravation | 50 | 59 | 15 | 19 | |
| Binomial Distribution, p | <0.05 | >0.40 | >0.30 | <0.05 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zolotareva, K.; Dotsenko, P.A.; Podkolodnyy, N.; Ivanov, R.; Makarova, A.-L.; Chadaeva, I.; Bogomolov, A.; Demenkov, P.S.; Ivanisenko, V.; Oshchepkov, D.; et al. Candidate SNP Markers Significantly Altering the Affinity of the TATA-Binding Protein for the Promoters of Human Genes Associated with Primary Open-Angle Glaucoma. Int. J. Mol. Sci. 2024, 25, 12802. https://doi.org/10.3390/ijms252312802
Zolotareva K, Dotsenko PA, Podkolodnyy N, Ivanov R, Makarova A-L, Chadaeva I, Bogomolov A, Demenkov PS, Ivanisenko V, Oshchepkov D, et al. Candidate SNP Markers Significantly Altering the Affinity of the TATA-Binding Protein for the Promoters of Human Genes Associated with Primary Open-Angle Glaucoma. International Journal of Molecular Sciences. 2024; 25(23):12802. https://doi.org/10.3390/ijms252312802
Chicago/Turabian StyleZolotareva, Karina, Polina A. Dotsenko, Nikolay Podkolodnyy, Roman Ivanov, Aelita-Luiza Makarova, Irina Chadaeva, Anton Bogomolov, Pavel S. Demenkov, Vladimir Ivanisenko, Dmitry Oshchepkov, and et al. 2024. "Candidate SNP Markers Significantly Altering the Affinity of the TATA-Binding Protein for the Promoters of Human Genes Associated with Primary Open-Angle Glaucoma" International Journal of Molecular Sciences 25, no. 23: 12802. https://doi.org/10.3390/ijms252312802
APA StyleZolotareva, K., Dotsenko, P. A., Podkolodnyy, N., Ivanov, R., Makarova, A.-L., Chadaeva, I., Bogomolov, A., Demenkov, P. S., Ivanisenko, V., Oshchepkov, D., & Ponomarenko, M. (2024). Candidate SNP Markers Significantly Altering the Affinity of the TATA-Binding Protein for the Promoters of Human Genes Associated with Primary Open-Angle Glaucoma. International Journal of Molecular Sciences, 25(23), 12802. https://doi.org/10.3390/ijms252312802







