A Potential Use of Vidarabine: Alleviation of Functional Constipation Through Modulation of the Adenosine A2A Receptor-MLC Signaling Pathway and the Gut Microbiota
Abstract
:1. Introduction
2. Results
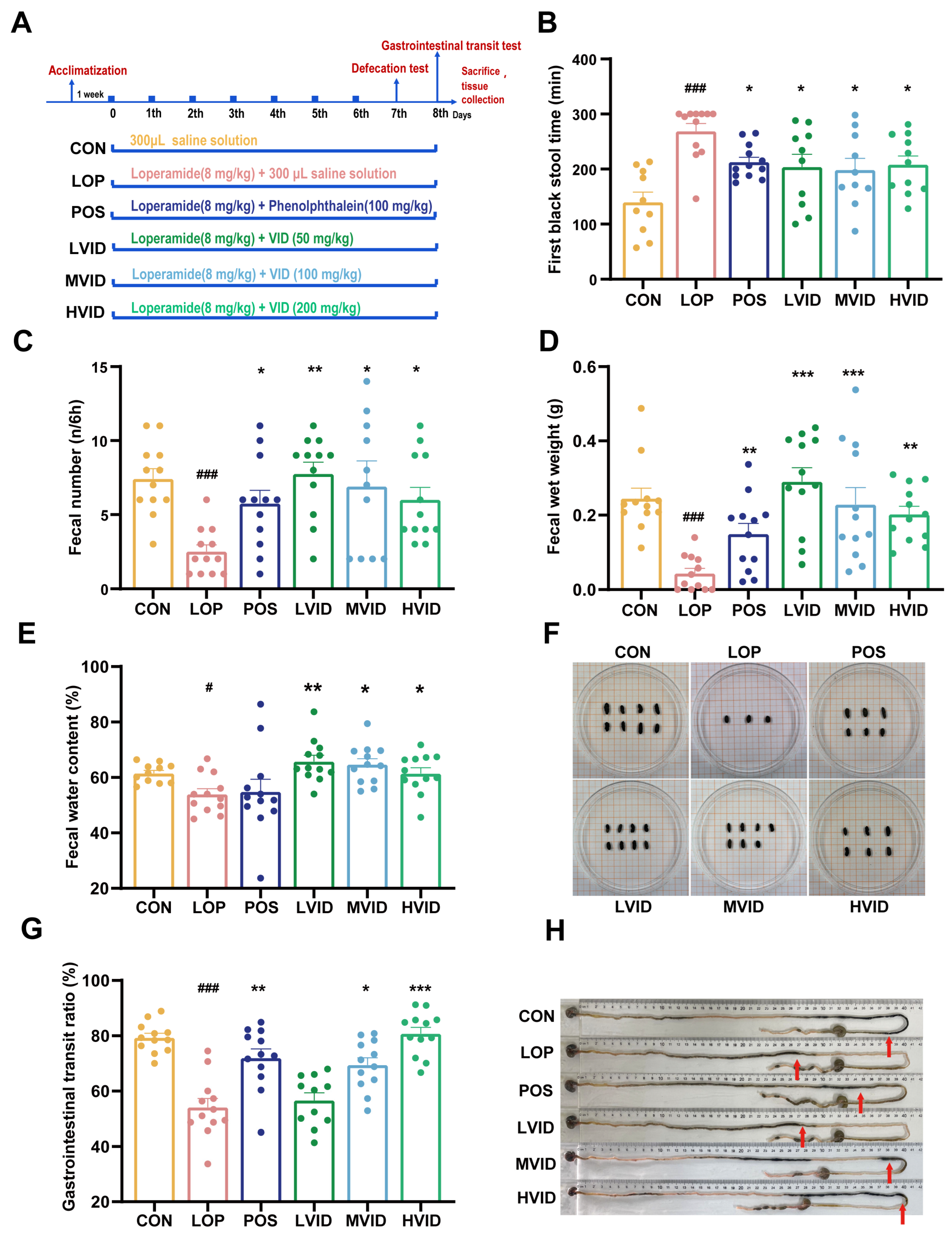
2.1. VID Attenuates Symptoms of Loperamide-Induced Constipation
2.2. VID Regulates the Expression of Aquaporins in the Colon
2.3. VID Modulates the Adenosine A2A Receptor Signaling Pathway and Neurotransmitter Expression
2.4. VID Reduces Colonic Inflammation and Enhances the Gut Barrier Function
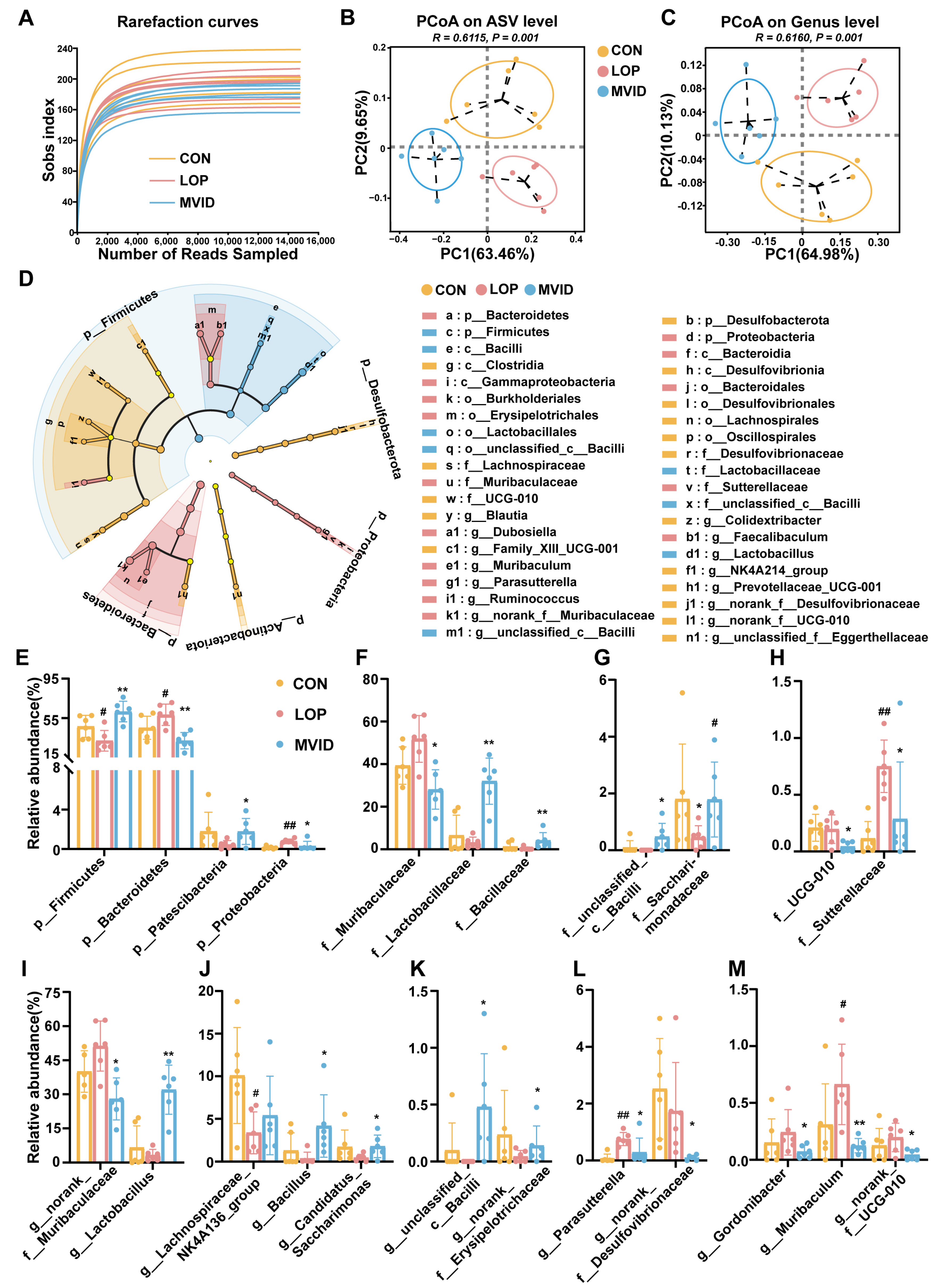
2.5. VID Remodels the Gut Microbiota in FC Mice
2.6. Correlation Analysis of Differential Microbial Taxa with Host Parameters
3. Discussion
4. Materials and Methods
4.1. Primary Materials
4.2. Design of Animal Experiments
4.3. Histopathologic Analysis and Immunohistochemistry
4.4. RNA Extraction, Reverse Transcription, and Real-Time Quantitative PCR
4.5. Western Blotting Analysis
4.6. Serum Neurotransmitter Assay
4.7. Analysis of Microbial Diversity of Cecum Contents
4.8. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| VID | Vidarabine |
| FC | Functional constipation |
| LVID | Low-VID-dose group |
| MVID | Medium-VID-dose group |
| HVID | High-VID-dose group |
| FBST | First black stool time |
| GTR | Gastrointestinal transit rate |
| FN | Fecal number |
| FW | Fecal wet weight |
| AChE | Acetylcholinesterase |
| VIP | Vasoactive intestinal polypeptide |
| ENS | Enteric nervous system |
| A2AR | Adenosine A2A receptor |
| smMLCK | Smooth muscle myosin light chain kinase |
| Calm | Calmodulin |
| MYL9 | Myosin light chain 9 |
| INF-γ | Interferon gamma |
| IL-1β | Interleukin-1β |
| IL-17 | Interleukin-17 |
| MCP-1 | Monocyte chemoattractant protein-1 |
| Muc2 | Mucin 2 |
| AQP3 | Aquaporin 3 |
| AQP4 | Aquaporin 4 |
| AQP8 | Aquaporin 8 |
| AQP9 | Aquaporin 9 |
References
- Lin, C.; He, H.; Kim, J.J.; Zheng, X.; Huang, Z.; Dai, N. Osmotic pressure induces translocation of aquaporin-8 by P38 and JNK MAPK signaling pathways in patients with functional constipation. Dig. Liver Dis. 2023, 55, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Randhwa, G.; Salloum, F.N.; Grider, J.R.; Murthy, K.S. Decreased smooth muscle function, peristaltic activity, and gastrointestinal transit in dystrophic (mdx) mice. Neurogastroenterol. Motil. 2020, 33, e13968. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Xu, C.; Fan, Y.; Li, Y.; Wang, Y.; Zhang, X.; Yu, S.; Wang, J.; Chai, R.; Zhao, Z.; et al. Effect of fecal microbiota transplantation in patients with slow transit constipation and the relative mechanisms based on the protein digestion and absorption pathway. J. Transl. Med. 2021, 19, 490. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Chen, Y.-Y.; Xu, D.-Q.; Yue, S.-J.; Fu, R.-J.; Yang, J.; Xing, L.-M.; Tang, Y.-P. Action Mode of Gut Motility, Fluid and Electrolyte Transport in Chronic Constipation. Front. Pharmacol. 2021, 12, 630249. [Google Scholar] [CrossRef]
- Boschetti, E.; Malagelada, C.; Accarino, A.; Malagelada, J.R.; Cogliandro, R.F.; Gori, A.; Bonora, E.; Giancola, F.; Bianco, F.; Tugnoli, V.; et al. Enteric neuron density correlates with clinical features of severe gut dysmotility. Am. J. Physiol. Liver Physiol. 2019, 317, G793–G801. [Google Scholar] [CrossRef]
- Deb, B.; Prichard, D.O.; Bharucha, A.E. Constipation and Fecal Incontinence in the Elderly. Curr. Gastroenterol. Rep. 2020, 22, 54. [Google Scholar] [CrossRef]
- Gottlieb, K.; Wacher, V.; Sliman, J.; Pimentel, M. Review article: Inhibition of methanogenic archaea by statins as a targeted management strategy for constipation and related disorders. Aliment Pharmacol. Ther. 2016, 43, 197–212. [Google Scholar] [CrossRef]
- Ikarashi, N.; Kon, R.; Sugiyama, K. Aquaporins in the colon as a new therapeutic target in diarrhea and constipation. Int. J. Mol. Sci. 2016, 17, 1172. [Google Scholar] [CrossRef]
- Huang, Y.-P.; Shi, J.-Y.; Luo, X.-T.; Luo, S.-C.; Cheung, P.C.; Corke, H.; Yang, Q.-Q.; Zhang, B.-B. How do probiotics alleviate constipation? A narrative review of mechanisms. Crit. Rev. Biotechnol. 2024, 1–17. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, R.; Li, D.; Zhao, L.; Zhu, L. Role of gut microbiota in functional constipation. Gastroenterol. Rep. 2021, 9, 392–401. [Google Scholar] [CrossRef]
- Ohkusa, T.; Koido, S.; Nishikawa, Y.; Sato, N. Gut Microbiota and Chronic Constipation: A Review and Update. Front. Med. 2019, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Joly, A.; Leulier, F.; De Vadder, F. Microbial Modulation of the Development and Physiology of the Enteric Nervous System. Trends Microbiol. 2021, 29, 686–699. [Google Scholar] [CrossRef]
- Kennedy, P.J.; Clarke, G.; Quigley, E.M.; Groeger, J.A.; Dinan, T.G.; Cryan, J.F. Gut memories: Towards a cognitive neurobiology of irritable bowel syndrome. Neurosci. Biobehav. Rev. 2012, 36, 310–340. [Google Scholar] [CrossRef] [PubMed]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar] [PubMed]
- Li, H.; Deng, J.; Yu, P.; Deng, L.; Ren, X. Gaining insight into irrational off-label use of vidarabine through analysis of a spontaneous reporting system in China. J. Clin. Pharm. Ther. 2020, 45, 1301–1311. [Google Scholar] [CrossRef] [PubMed]
- Honma, Y.; Niitsua, N. Vidarabine and 2-Deoxycoformycin as Antileukemic Agents Against Monocytic Leukemia. Leuk. Lymphoma 2000, 39, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, Y.; Suita, K.; Ohnuki, Y.; Mototani, Y.; Ishikawa, M.; Ito, A.; Nariyama, M.; Morii, A.; Kiyomoto, K.; Tsunoda, M.; et al. Vidarabine, an anti-herpes agent, prevents occlusal-disharmony-induced cardiac dysfunction in mice. J. Physiol. Sci. 2022, 72, 2. [Google Scholar] [CrossRef]
- Whitley, R.; Alford, C.; Hess, F.; Buchanan, R. Vidarabine. Drugs 1980, 20, 267–282. [Google Scholar] [CrossRef]
- Lambertucci, C.; Marucci, G.; Catarzi, D.; Colotta, V.; Francucci, B.; Spinaci, A.; Varano, F.; Volpini, R. A2A Adenosine Receptor Antagonists and their Potential in Neurological Disorders. Curr. Med. Chem. 2022, 29, 4780–4795. [Google Scholar] [CrossRef]
- Trinh, P.N.H.; Baltos, J.-A.; Hellyer, S.D.; May, L.T.; Gregory, K.J. Adenosine receptor signalling in Alzheimer’s disease. Purinergic Signal. 2022, 18, 359–381. [Google Scholar] [CrossRef]
- Vieira, C.; Ferreirinha, F.; Silva, I.; Duarte-Araújo, M.; Correia-De-Sá, P. Localization and function of adenosine receptor subtypes at the longitudinal muscle—Myenteric plexus of the rat ileum. Neurochem. Int. 2011, 59, 1043–1055. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Luo, L.; Yan, S.; Wen, T.; Bai, W.; Li, H.; Zhang, G.; Lu, X.; Liu, Y.; He, L. Clinical Features for Mild Hand, Foot and Mouth Disease in China. PLoS ONE 2015, 10, e0135503. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Cheng, F.; Xiao, F. Herpes zoster-induced acute urinary retention, limb paresis, and constipation in two immunocompetent patients. Clin. Auton. Res. 2022, 32, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Fang, F. Abdominal distention and constipation followed by herpes zoster infection in a 2-month-old female infant. J. Dermatol. 2015, 42, 809–811. [Google Scholar] [CrossRef] [PubMed]
- Antonioli, L.; Fornai, M.; Colucci, R.; Awwad, O.; Ghisu, N.; Tuccori, M.; Del Tacca, M.; Blandizzi, C. Differential recruitment of high affinity A1 and A2A adenosine receptors in the control of colonic neuromuscular function in experimental colitis. Eur. J. Pharmacol. 2011, 650, 639–649. [Google Scholar] [CrossRef]
- Jacobson, K.A.; Gao, Z.-G. Adenosine receptors as therapeutic targets. Nat. Rev. Drug Discov. 2006, 5, 247–264. [Google Scholar] [CrossRef]
- Neri, F.; Cavallari, G.; Tsivian, M.; Bianchi, E.; Aldini, R.; Cevenini, M.; Guidetti, E.; Piras, G.L.; Pariali, M.; Nardo, B. Effect of Colic Vein Ligature in Rats with Loperamide-Induced Constipation. J. Biomed. Biotechnol. 2012, 2012, 896162. [Google Scholar] [CrossRef]
- Wintola, O.A.; Sunmonu, T.O.; Afolayan, A.J. The effect of Aloe ferox Mill. in the treatment of loperamide-induced constipation in Wistar rats. BMC Gastroenterol. 2010, 10, 95. [Google Scholar] [CrossRef]
- Laforenza, U.; Miceli, E.; Gastaldi, G.; Scaffino, M.F.; Ventura, U.; Fontana, J.M.; Orsenigo, M.N.; Corazza, G.R. Solute transporters and aquaporins are impaired in celiac disease. Biol. Cell 2010, 102, 457–467. [Google Scholar] [CrossRef]
- Ouyang, L.; Yin, Y.; Liu, Y.; He, L.; Long, J.; Li, T.; He, X.; Li, J. Advances in research on the effects of aquaporins on animal health. Sci. Sin. Vitae 2020, 50, 427–437. [Google Scholar] [CrossRef]
- He, J.; Yang, B. Aquaporins in Renal Diseases. Int. J. Mol. Sci. 2019, 20, 366. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Ran, J.; Yang, B.; Mei, Z. Aquaporins in digestive system. Adv. Exp. Med. Biol. 2017, 969, 123–130. [Google Scholar] [CrossRef]
- Zheng, Y.-F.; Liu, C.-F.; Lai, W.-F.; Xiang, Q.; Li, Z.-F.; Wang, H.; Lin, N. The laxative effect of emodin is attributable to increased aquaporin 3 expression in the colon of mice and HT-29 cells. Fitoterapia 2014, 96, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, T.; Tajika, Y.; Ablimit, A.; Aoki, T.; Hagiwara, H.; Takata, K. Aquaporins in the digestive system. Med. Mol. Morphol. 2004, 37, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.S.; Ma, T.; Filiz, F.; Verkman, A.S.; Bastidas, J.A. Colon water transport in transgenic mice lacking aquaporin-4 water channels. Am. J. Physiol. Liver Physiol. 2000, 279, G463–G470. [Google Scholar] [CrossRef] [PubMed]
- Okada, S.; Misaka, T.; Matsumoto, I.; Watanabe, H.; Abe, K. Aquaporin-9 is expressed in a mucus-secreting goblet cell subset in the small intestine. FEBS Lett. 2003, 540, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Segura-Anaya, E.; Martínez-Gómez, A.; Dent, M.A. Differences in the localization of AQP1 and expression patterns of AQP isoforms in rat and mouse sciatic nerve and changes in rat AQPs expression after nerve crush injury. IBRO Neurosci. Rep. 2021, 12, 82–89. [Google Scholar] [CrossRef]
- Yde, J.; Keely, S.; Wu, Q.; Borg, J.F.; Lajczak, N.; O’dwyer, A.; Dalsgaard, P.; Fenton, R.A.; Moeller, H.B. Characterization of AQPs in Mouse, Rat, and Human Colon and Their Selective Regulation by Bile Acids. Front. Nutr. 2016, 3, 46. [Google Scholar] [CrossRef]
- Korkutata, M.; Agrawal, L.; Lazarus, M. Allosteric Modulation of Adenosine A2A Receptors as a New Therapeutic Avenue. Int. J. Mol. Sci. 2022, 23, 2101. [Google Scholar] [CrossRef]
- Gao, Z. Adenosine A(2A) receptor and glia. Int. Rev. Neurobiol. 2023, 170, 29–48. [Google Scholar] [CrossRef]
- Canas, P.M.; Porciúncula, L.O.; Cunha, G.M.A.; Silva, C.G.; Machado, N.J.; Oliveira, J.M.A.; Oliveira, C.R.; Cunha, R.A. Adenosine A2AReceptor Blockade Prevents Synaptotoxicity and Memory Dysfunction Caused by β-Amyloid Peptides via p38 Mitogen-Activated Protein Kinase Pathway. J. Neurosci. 2009, 29, 14741–14751. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, M.D. The future of pharmacologic stress: Selective A2A adenosine receptor agonists. Am. J. Cardiol. 2004, 94, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Zizzo, M.; Mastropaolo, M.; Lentini, L.; Mulè, F.; Serio, R. Adenosine negatively regulates duodenal motility in mice: Role of A1 and A2A receptors. Br. J. Pharmacol. 2011, 164, 1580–1589. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J. Adenosine A2A receptor interactions with receptors for other neurotransmitters and neuromodulators. Eur. J. Pharmacol. 1999, 375, 101–113. [Google Scholar] [CrossRef]
- Uchida, S.; Kadowaki-Horita, T.; Kanda, T. Effects of the adenosine A2A receptor antagonist on cognitive dysfunction in Parkinson’s disease. Int. Rev. Neurobiol. 2014, 119, 169–189. [Google Scholar] [CrossRef]
- Khalif, I.L.; Quigley, E.M.; Konovitch, E.A.; Maximova, I.D. Alterations in the colonic flora and intestinal permeability and evidence of immune activation in chronic constipation. Dig. Liver Dis. 2005, 37, 838–849. [Google Scholar] [CrossRef]
- Cao, H.; Liu, X.; An, Y.; Zhou, G.; Liu, Y.; Xu, M.; Dong, W.; Wang, S.; Yan, F.; Jiang, K.; et al. Dysbiosis contributes to chronic constipation development via regulation of serotonin transporter in the intestine. Sci. Rep. 2017, 7, 10322. [Google Scholar] [CrossRef]
- Patel, R.M.; Myers, L.S.; Kurundkar, A.R.; Maheshwari, A.; Nusrat, A.; Lin, P.W. Probiotic Bacteria Induce Maturation of Intestinal Claudin 3 Expression and Barrier Function. Am. J. Pathol. 2011, 180, 626–635. [Google Scholar] [CrossRef]
- Docsa, T.; Sipos, A.; Cox, C.S.; Uray, K. The Role of Inflammatory Mediators in the Development of Gastrointestinal Motility Disorders. Int. J. Mol. Sci. 2022, 23, 6917. [Google Scholar] [CrossRef]
- Aube, A.C.; Blottiere, H.M.; Scarpignato, C.; Cherbut, C.; Roze, C.; Galmiche, J.P. Inhibition of acetylcholine induced intestinal motility by interleukin 1 beta in the rat. Gut 1996, 39, 470–474. [Google Scholar] [CrossRef]
- Main, C.; Blennerhassett, P.; Collins, S.M. Human recombinant interleukin 1β suppresses acetylcholine release from rat myenteric plexus. Gastroenterology 1993, 104, 1648–1654. [Google Scholar] [CrossRef]
- Buchholz, B.M.; Shapiro, R.A.; Vodovotz, Y.; Billiar, T.R.; Sodhi, C.P.; Hackam, D.J.; Bauer, A.J. Myocyte TLR4 enhances enteric and systemic inflammation driving late murine endotoxic ileus. Am. J. Physiol. Liver Physiol. 2015, 308, G852–G862. [Google Scholar] [CrossRef] [PubMed]
- Olsen, A.B.; Hetz, R.A.; Xue, H.; Aroom, K.R.; Bhattarai, D.; Johnson, E.; Bedi, S.; Cox, C.S.; Uray, K. Effects of traumatic brain injury on intestinal contractility. Neurogastroenterol. Motil. 2013, 25, 593-e463. [Google Scholar] [CrossRef] [PubMed]
- Indrio, F.; Riezzo, G.; Tafuri, S.; Ficarella, M.; Carlucci, B.; Bisceglia, M.; Polimeno, L.; Francavilla, R. Probiotic Supplementation in Preterm: Feeding Intolerance and Hospital Cost. Nutrients 2017, 9, 965. [Google Scholar] [CrossRef]
- Ford, C.L.; Wang, Y.; Morgan, K.; Boktor, M.; Jordan, P.; Castor, T.P.; Alexander, J.S. Interferon-gamma depresses human intestinal smooth muscle cell contractility: Relevance to inflammatory gut motility disturbances. Life Sci. 2019, 222, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Engel, D.R.; Koscielny, A.; Wehner, S.; Maurer, J.; Schiwon, M.; Franken, L.; Schumak, B.; Limmer, A.; Sparwasser, T.; Hirner, A.; et al. T helper type 1 memory cells disseminate postoperative ileus over the entire intestinal tract. Nat. Med. 2010, 16, 1407–1413. [Google Scholar] [CrossRef] [PubMed]
- Hori, M.; Nobe, H.; Horiguchi, K.; Ozaki, H.; Yuan, P.-Q.; Taché, Y.; Tajima, T.; Murata, T.; Aritake, K.; Urade, Y.; et al. MCP-1 targeting inhibits muscularis macrophage recruitment and intestinal smooth muscle dysfunction in colonic inflammation. Am. J. Physiol. Physiol. 2008, 294, C391–C401. [Google Scholar] [CrossRef]
- Krzystek-Korpacka, M.; Zawadzki, M.; Lewandowska, P.; Szufnarowski, K.; Bednarz-Misa, I.; Jacyna, K.; Witkiewicz, W.; Gamian, A. Distinct Chemokine Dynamics in Early Postoperative Period after Open and Robotic Colorectal Surgery. J. Clin. Med. 2019, 8, 879. [Google Scholar] [CrossRef]
- Pan, R.; Wang, L.; Xu, X.; Chen, Y.; Wang, H.; Wang, G.; Zhao, J.; Chen, W. Crosstalk between the Gut Microbiome and Colonic Motility in Chronic Constipation: Potential Mechanisms and Microbiota Modulation. Nutrients 2022, 14, 3704. [Google Scholar] [CrossRef]
- Guo, M.; Yao, J.; Yang, F.; Liu, W.; Bai, H.; Ma, J.; Ma, X.; Zhang, J.; Fang, Y.; Miao, Y.; et al. The Composition of Intestinal Microbiota and its Association with Functional Constipation of the Elderly Patients. Futur. Microbiol. 2020, 15, 163–175. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Yu, Q.; Tang, N.; Mei, C.; Zhang, H.; Wang, G.; Lu, J.; Chen, W. Characteristics of the Gut Microbiome and Serum Metabolome in Patients with Functional Constipation. Nutrients 2023, 15, 1779. [Google Scholar] [CrossRef] [PubMed]
- Sugitani, Y.; Inoue, R.; Inatomi, O.; Nishida, A.; Morishima, S.; Imai, T.; Kawahara, M.; Naito, Y.; Andoh, A. Mucosa-associated gut microbiome in Japanese patients with functional constipation. J. Clin. Biochem. Nutr. 2021, 68, 187–192. [Google Scholar] [CrossRef]
- Kim, H.; Jeong, E.-J.; Park, C.; Lee, J.-S.; Kim, W.J.; Yu, K.-W.; Suh, H.J.; Ahn, Y.; Moon, S.-K. Modulation of gut microbiota ecosystem by a glucan-rich snail mucin heteropolysaccharide attenuates loperamide-induced constipation. Int. J. Biol. Macromol. 2023, 253, 126560. [Google Scholar] [CrossRef]
- Lin, Q.; Liu, M.; Erhunmwunsee, F.; Li, B.; Mou, Y.; Wang, S.; Zhang, G.; Tian, J. Chinese patent medicine shouhui tongbian capsule attenuated loperamide-induced constipation through modulating the gut microbiota in rat. J. Ethnopharmacol. 2022, 298, 115575. [Google Scholar] [CrossRef]
- Qi, B.; Zhang, Y.; Ren, D.; Qin, X.; Wang, N.; Yang, X. Fu Brick Tea Alleviates Constipation via Regulating the Aquaporins-Mediated Water Transport System in Association with Gut Microbiota. J. Agric. Food Chem. 2023, 71, 3862–3875. [Google Scholar] [CrossRef]
- Yi, X.; Zhou, K.; Jiang, P.; Deng, N.; Peng, X.; Tan, Z. Brain-bacteria-gut axis and oxidative stress mediated by intestinal mucosal microbiota might be an important mechanism for constipation in mice. 3 Biotech 2023, 13, 192. [Google Scholar] [CrossRef]
- Yuan, X.-Y.; Chen, Y.-S.; Liu, Z. Relationship among Parkinson’s disease, constipation, microbes, and microbiological therapy. World J. Gastroenterol. 2024, 30, 225–237. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, W.; Lu, H.; Cheng, T.; Wang, L.; Wang, G.; Zhang, H.; Chen, W. Lactic acid bacteria in relieving constipation: Mechanism, clinical application, challenge, and opportunity. Crit. Rev. Food Sci. Nutr. 2023, 1–24. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Y.; Ma, K.; Wang, G.; Tang, M.; Wang, R.; Xia, Z.; Xu, Z.; Sun, M.; Bao, X.; et al. Lactobacillus plantarum Lp3a improves functional constipation: Evidence from a human randomized clinical trial and animal model. Ann. Transl. Med. 2022, 10, 316. [Google Scholar] [CrossRef]
- Dang, C.; Zhao, K.; Xun, Y.; Feng, L.; Zhang, D.; Cui, L.; Cui, Y.; Jia, X.; Wang, S. In vitro Intervention of Lactobacillus paracasei N1115 Can Alter Fecal Microbiota and Their SCFAs Metabolism of Pregnant Women with Constipation and Diarrhea. Curr. Microbiol. 2022, 79, 212. [Google Scholar] [CrossRef]
- Riezzo, G.; Orlando, A.; D’attoma, B.; Linsalata, M.; Martulli, M.; Russo, F. Randomised double blind placebo controlled trial on Lactobacillus reuteri DSM 17938: Improvement in symptoms and bowel habit in functional constipation. Benef. Microbes 2018, 9, 51–60. [Google Scholar] [CrossRef]
- Wang, G.; Yang, S.; Sun, S.; Si, Q.; Wang, L.; Zhang, Q.; Wu, G.; Zhao, J.; Zhang, H.; Chen, W. Lactobacillus rhamnosus Strains Relieve Loperamide-Induced Constipation via Different Pathways Independent of Short-Chain Fatty Acids. Front. Cell. Infect. Microbiol. 2020, 10, 423. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Cao, S.; Xiao, H.; Hu, S.; Yao, K.; Huang, K.; Jiang, Z.; Wang, L. Lactobacillus reuteri 1 Enhances Intestinal Epithelial Barrier Function and Alleviates the Inflammatory Response Induced by Enterotoxigenic Escherichia coli K88 via Suppressing the MLCK Signaling Pathway in IPEC-J2 Cells. Front. Immunol. 2022, 13, 897395. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Hu, C.; Fang, H.; Zhu, L.; Gao, N.; Zhu, J. The Effects of Lactobacillus acidophilus on the Intestinal Smooth Muscle Contraction through PKC/MLCK/MLC Signaling Pathway in TBI Mouse Model. PLoS ONE 2015, 10, e0128214. [Google Scholar] [CrossRef] [PubMed]
- Yi, R.; Peng, P.; Zhang, J.; Du, M.; Lan, L.; Qian, Y.; Zhou, J.; Zhao, X. Lactobacillus plantarum CQPC02-Fermented Soybean Milk Improves Loperamide-Induced Constipation in Mice. J. Med. Food 2019, 22, 1208–1221. [Google Scholar] [CrossRef]
- Ren, B.; Fu, S.; Liu, Y.; Kang, J.; Wang, B.; Yao, Z.; Wang, H.; Sun, D. Dioscin ameliorates slow transit constipation in mice by up-regulation of the BMP2 secreted by muscularis macrophages. Iran. J. Basic Med. Sci. 2022, 25, 1132–1140. [Google Scholar] [CrossRef]
- Gao, X.; Hu, Y.; Tao, Y.; Liu, S.; Chen, H.; Li, J.; Zhao, Y.; Sheng, J.; Tian, Y.; Fan, Y. Cymbopogon citratus (DC.) Stapf aqueous extract ameliorates loperamide-induced constipation in mice by promoting gastrointestinal motility and regulating the gut microbiota. Front. Microbiol. 2022, 13, 1017804. [Google Scholar] [CrossRef]
- Choi, Y.J.; Kim, J.E.; Lee, S.J.; Gong, J.E.; Son, H.J.; Hong, J.T.; Hwang, D.Y. Dysbiosis of Fecal Microbiota From Complement 3 Knockout Mice with Constipation Phenotypes Contributes to Development of Defecation Delay. Front. Physiol. 2021, 12, 650789. [Google Scholar] [CrossRef]
- Zou, H.; Gao, H.; Liu, Y.; Zhang, Z.; Zhao, J.; Wang, W.; Ren, B.; Tan, X. Dietary inulin alleviated constipation induced depression and anxiety-like behaviors: Involvement of gut microbiota and microbial metabolite short-chain fatty acid. Int. J. Biol. Macromol. 2024, 259, 129420. [Google Scholar] [CrossRef]
- Zhao, P.; Meng, X.; Sun, M.; Qin, B.; Kong, S.; Xie, L.; Zhang, W.; Ding, X.; Zhang, C. Integrated metabolic profiles and microbial communities to reveal the beneficial effect of red pitaya on early constipation. Food Funct. 2024, 15, 5414–5428. [Google Scholar] [CrossRef]
- Li, R.; Li, M.; Li, B.; Chen, W.; Liu, Z. Cannabis sativa L. alleviates loperamide-induced constipation by modulating the composition of gut microbiota in mice. Front. Pharmacol. 2022, 13, 1033069. [Google Scholar] [CrossRef]
- Yang, Z.; Ye, S.; Xu, Z.; Su, H.; Tian, X.; Han, B.; Shen, B.; Liao, Q.; Xie, Z.; Hong, Y. Dietary synbiotic ameliorates constipation through the modulation of gut microbiota and its metabolic function. Food Res. Int. 2021, 147, 110569. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, M.; Shang, W.; Ma, Q.; Strappe, P.; Zhou, Z. Abundance of Probiotics and Butyrate-Production Microbiome Manages Constipation via Short-Chain Fatty Acids Production and Hormones Secretion. Mol. Nutr. Food Res. 2019, 63, e1801187. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Xu, C.; Xie, L.; Wang, Y.; Zhu, S.; An, J.; Li, Y.; Tian, Z.; Yan, Y.; Yu, S.; et al. Abnormal bile acid metabolism is an important feature of gut microbiota and fecal metabolites in patients with slow transit constipation. Front. Cell. Infect. Microbiol. 2022, 12, 956528. [Google Scholar] [CrossRef]
- Callahan, B.J.; Mcmurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]




| Gene | Forward Primer | Reverse Sequence | Accession Number |
|---|---|---|---|
| IL-1β | TCCATGAGCTTTGTACAAGGA | AGCCCATACTTTAGGAAGACA | NM_008361.4 |
| INF-γ | ATCTGGAGGAACTGGCAAAA | TTCAAGACTTCAAAGAGTCTGAGGT | NM_008337.4 |
| IL-17 | TTTAACTCCCTTGGCGCAAAA | CTTTCCCTCCGCATTGACAC | NM_010552.3 |
| MCP-1 | AGCCATCCGACATTCTTC | GCCTATGCCTTCCACTTT | NM_015729.4 |
| AQP3 | GTCAACCCTGCCCGTGACTTTG | CGAAGACACCAGCGATGGAACC | NM_016689.2 |
| AQP4 | GCAGACAAGGTGCAACGTGGTT | GGCGGAAGGCAAAGCAGTATGG | NM_009700.3 |
| AQP8 | TGTGTAGTATGGACCTACCTGAG | ACCGATAGACATCCGATGAAGAT | NM_007474.2 |
| AQP9 | TGGTGTCTACCATGTTCCTCC | AACCAGAGTTGAGTCCGAGAG | NM_022026.3 |
| VIP | AGTGTGCTGTTCTCTCAGTCG | GCCATTTTCTGCTAAGGGATTCT | NM_011702.3 |
| AChE | CTCCCTGGTATCCCCTGCATA | GGATGCCCAGAAAAGCTGAGA | NM_001290010.1 |
| A2AR | GAAGCAGATGGAGAGCCAAC | GAGAGGATGATGGCCAGGTA | NM_001428350.1 |
| CALM | TGGGAATGGTTACATCAGTGC | CGCCATCAATATCTGCTTCTCT | NM_001313934.1 |
| smMLCK | AGAAGTCAAGGAGGTAAAGAATGATGT | CGGGTCGCTTTTCATTGC | NM_001408263.1 |
| MYL9 | ACAGCGCCGAGGACTTTTC | CAGCCATTCAGCACCATCCG | BC049974 |
| Muc 2 | AGGGCTCGGAACTCCAGAAA | CCAGGGAATCGGTAGACATCG | NM_023566.4 |
| Claudin-1 | ATCACCTTCGGGAGCTCAGGT | TGATGGGGGTCAAGGGGTCAT | NM_016674.4 |
| Claudin-5 | TGGACCACAACATCGTGACGG | TGCCTCCCGCCCTTAGACATA | NM_013805.4 |
| Claudin-7 | AACATCATCACAGCCCAGGCC | ATGTTTGGAGGTGGAGTGGCC | NM_016887.6 |
| RPL-19 | GAAGGTCAAAGGGAATGTGTTCA | CCTTGTCTGCCTTCAGCTTGT | NM_009078.2 |
| β-actin | GGCTGTATTCCCCTCCATCG | CCAGTTGGTAACAATGCCATGT | NM_007393 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.; Guo, K.; Liu, S.; Yang, W.; Sheng, J.; Tian, Y.; Peng, L.; Zhao, Y. A Potential Use of Vidarabine: Alleviation of Functional Constipation Through Modulation of the Adenosine A2A Receptor-MLC Signaling Pathway and the Gut Microbiota. Int. J. Mol. Sci. 2024, 25, 12810. https://doi.org/10.3390/ijms252312810
Gao X, Guo K, Liu S, Yang W, Sheng J, Tian Y, Peng L, Zhao Y. A Potential Use of Vidarabine: Alleviation of Functional Constipation Through Modulation of the Adenosine A2A Receptor-MLC Signaling Pathway and the Gut Microbiota. International Journal of Molecular Sciences. 2024; 25(23):12810. https://doi.org/10.3390/ijms252312810
Chicago/Turabian StyleGao, Xiaoyu, Kaifeng Guo, Shuangfeng Liu, Weixing Yang, Jun Sheng, Yang Tian, Lei Peng, and Yan Zhao. 2024. "A Potential Use of Vidarabine: Alleviation of Functional Constipation Through Modulation of the Adenosine A2A Receptor-MLC Signaling Pathway and the Gut Microbiota" International Journal of Molecular Sciences 25, no. 23: 12810. https://doi.org/10.3390/ijms252312810
APA StyleGao, X., Guo, K., Liu, S., Yang, W., Sheng, J., Tian, Y., Peng, L., & Zhao, Y. (2024). A Potential Use of Vidarabine: Alleviation of Functional Constipation Through Modulation of the Adenosine A2A Receptor-MLC Signaling Pathway and the Gut Microbiota. International Journal of Molecular Sciences, 25(23), 12810. https://doi.org/10.3390/ijms252312810






